Abstract
Objective: Plant-based natural antioxidants have a wide variety of biological activities with significant therapeutic value. Mangifera indica has been used traditionally to treat a variety of ailments in animals and human, but little is defined about its biological or pharmacological effects. Therefore, the objective of the present study was to evaluate phytochemical, antioxidant, antipyretic and anti-inflammatory activities of aqueous-methanolic leaf extract of M. indica. Methods: To investigate the possible impact of aqueous-methanolic leaf extract of M. indica on oxidative stress, inflammation, and pyrexia, we used a combined in vitro and in vivo series of experiments on laboratory animals. Results: Results revealed significant antioxidant potential in 2,2-diphenylpicrylhydrazyl (DPPH) and nitric oxide (NO) scavenging assay, while significant but dose dependent antipyretic potential was documented in typhoid-paratyphoid A and B (TAB) vaccine and prostaglandin E (PGE) induced pyrexia models. Significant anti-inflammatory effects were observed in both acute and chronic inflammatory models of arachidonic acid and formalin. Phytochemical screening and high-performance liquid chromatography (HPLC) analysis of M. Indica confirmed the presence of mangiferin, quercetin, and isoquercetin. These phytoconstituents likely play a role in the observed biological activities. Our results show that M. indica has antioxidant, anti-inflammatory, and antipyretic effects, lending credence to its traditional use and advocating for its utilization as a viable contender in treating oxidative stress-associated ailments. Conclusion: It is concluded that Magnifera indica has various properties in the treatment of various diseases.
Keywords: Inflammation, pyrexia, antioxidant, Mangifera indica, prostaglandins
Introduction
Medicinal plants are gift from nature to humans. Since ancient times, herbs and spices have been used for medicinal purposes [1,2]. These plants are primarily used as a crucial component of the therapeutic pillars [2,3]. Unani, Hikmat, and Tibbe-Nabvi are well-known names for this school of therapy in Pakistan. More than 90,000 registered and unregistered hakims or tabibs use the 1000-1200 medicinal plants for therapeutic and curative purposes [2,4]. Since this practice is based on personal experiences instead of scientific evidence, it needs to be proven scientifically. Pakistan’s National Council for Tibb is working hard to ensure that medicinal plants in Pakistan are based on science and do not harm people [4,5].
Pain and inflammation are an increasing global public health concern [6,7]. Nonsteroidal anti-inflammatory agents (NSAIDs), corticosteroids, and opioids are commonly used to treat symptoms of pain and inflammation [6,8]. Still, it is crucial to ensure that drugs can be put on the skin’s surface and reach deeper skin tissues by penetration (for example, through massage or ultrasound) [9]. The symptoms of an infection, cancer or another disease can cause fever or pyrexia [10]. The body makes an environment that makes it hard for infectious pathogens or damaged tissues to live [2,10]. Pro-inflammatory mediators (cytokines such as interleukin 1, 6, and tumor necrosis factor-α) are typically produced rapidly by infected or injured tissue, which increases the production of prostaglandin E2 (PgE2) near the brain and triggers the hypothalamus to raise body temperature [2,11]. Antipyretic medications typically block PgE2 production to lower the elevated body temperature [11]. These manufactured compounds inhibit cyclooxygenase-2 (COX-2) in a way that cannot be undone, but they are dangerous for the brain, kidneys, heart muscles, and liver cells [2,7,12].
Mangifera indica, commonly known as mango (Chaunsa), belongs to the “Anacardiaceae” family, the plant kingdom native to the Indian subcontinent. Hundreds of developed assortments have been presented to other warm-weather countries worldwide [13]. Many countries have used mango extract from leaves, fruit pulp, roots, bark, stem, and seed kernel for curative purposes [14]. The different chemical constituents are present in the leaf of Mangifera indica, such as flavonoids, alkaloids, phenols, saponins, minerals, and vitamin C and B [15]. The leaf extract is being used for different biological activities, such as anti-diabetic [16], anti-microbial [17], immunomodulators [18], anti-allergic [19], hepatoprotective [20], cardioprotective [21], anti-inflammatory, and analgesic [22].
Mangiferin is a xanthone in various parts of the M. indica, such as the peel, leaves, stalks, kernel, and barks [23]. The aglycone 1,3,6,7-tetrahydroxy-xanthone hydrolysis with R-acetobromoglucose through O-glycosidic bond formation results in the synthesis of mangiferin [24]. It has hepatoprotective, anti-diabetic, anti-viral, anti-aging, anti-cancer, and immunomodulatory effects [23]. Mangiferin reduced lipid peroxidation induced by hydrogen peroxide in human white blood cells [25]. Mangiferin is used to cure various eye diseases [26].
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is an essential bioflavonoid in more than twenty plant materials. It cannot be produced naturally in the human body [27]. It has an anti-inflammatory effect. Quercetin is used to treat metabolic ailments, hypertension, prevent cardiac hypertrophy, and chronic heart disease (CHD), and it can control obesity because it can block glucose uptake from the blood, inhibit fat production and accumulation in cells, trigger the apoptosis of existing fat cells, and decrease cholesterol [28].
Isoquercetin (quercetin-3-O-β-D-glucopyranoside) is a natural flavonoid found in various parts of the plant, including herbs, fruits, and vegetables [29,30]. The conjugation of different glucose moieties makes them more soluble in water, and isoquercetin is easier to get [31].
Materials and methods
Drugs, chemicals, and instruments
Diclofenac sodium was purchased from SAMI Pharmaceuticals (Pvt) Ltd. KRC, SD, Pakistan and Carbpol-940 from Duksan Pure Chemicals Co., Ltd., South Korea. Methanol was purchased from Merck, Germany. Sodium benzoate, triethanolamine, and glycerol were purchased from Sigma-Aldrich, Pakistan. Distilled water was purchased from SARCO Chemicals, Mtn, Pakistan. The thermometer was purchased from Unex Diagnostics, LHR, PU, Pakistan. Analytical grade (all other) chemicals and reagents were used in this experiment for phytochemistry and antioxidant assays.
Collection of plant materials and extract preparation
M. indica was taken from a Multan, South Punjab, at the Muhammad Institute of Medical and Allied Sciences. An expert taxonomist identified the plant at the Department of Agronomy, MNS University of Agriculture, Multan (R.R.Steward, F.W. Pak. 625-3). The fresh leaves of the plant were left for shade drying. Dirt and debris were cleared before the grinding of dried leaves by the special herb grinder to the coarsely powdered form. The airtight jar was used for the preservation of the powdered plant. For extract, preparation from powdered material was done by a standard reported method, including a maceration procedure in an aqueous-methanolic (70:30) mixture. The evaporation of crude extract pool to a thick paste as stock solutions was done on a rotatory evaporator at 37°C under low pressure [32-35]. The estimated 10% yield of extract was taken using the formula (Equation (1)).
Its 20%, 10%, and 5% dilutions were stored in airtight jars in a lab refrigerator at -2°C.
Animals
Male local bread rabbits and albino rats were used for experimentation in the laboratory of pharmacology, Muhammad Institute of Medical and Allied Sciences, Multan, Pakistan, after approval from the institutional ethical committee (No. 25/DPT/MIMAS/Oct/21) following the NIH guidelines for the use of animals in experimentation [36,37].
Preliminary phytochemical evaluation
Aqueous-methanolic (30:70) leaf extract of M. indica was evaluated for the possible presence of vital phytochemical classes using standard protocol [32].
High-performance liquid chromatography (HPLC) analysis of aqueous-methanolic leaf extract of M. indica
The polyphenols in the aqueous-methanolic leaf extract of M. indica were measured with HPLC. A binary gradient solvent system was used in HPLC, paired with a C-18 column with dimensions (250 4.6 mm), capable of separating one polyphenol (mangiferin) and two flavonoids (quercetin and isoquercetin) in 36 minutes at a flow rate of 0.0008 µL/min, and a film thickness of 5 µm, with an oven set at 30°C. The replicability for separation of components was good with run-to-run. Mangiferin, quercetin, and isoquercetin were prepared as reference (purity > 99%), obtained from Aldrich (St. Louis, USA), and the dilutions were prepared with methanol to achieve 50 µg/mL. Samples were distinguished by comparing the sample retention times to standards [32]. The separation factor and resolution were used to evaluate the efficiency of separated components using HPLC.
Antioxidant activity
Antioxidant activity was analyzed using 2,2-diphenylpicrylhydrazyl (DPPH) and Nitric oxide (NO) assay.
DPPH assay
As mentioned earlier, the DPPH test has been carried out [5]. The diluted sample with methanol was mixed with an aqueous-methanolic leaf extract (30:70) from M. indica to make a final volume of 5 mL with different concentrations (4 mL). Then, for 40 minutes, this mixture was stored in the dark. The stated solution’s 517 nm absorbance was measured using a spectrophotometer. Each study was done three times, and the percentage of inhibition in vitamin C equivalency was measured [38-41]. Equation (2) was used to compute the percentage of DPPH scavenging potential:
Nitric oxide radical scavenging assay
The extract was constructed using a 10 mg/mL aqueous-methanolic leaf extract of M. indica. The extract was then repetitively diluted with distilled water to yield concentrations ranging from 100 to 1,000 µg/mL, and the same procedure was applied to standard (Gallic acid). For experiments, solutions were kept at 4 degrees Celsius. For the reaction, a freshly prepared Griess reagent was used. 0.5 mL of 10 mM sodium nitroprusside in phosphate-buffered saline was combined with 1 mL of each extract concentration (100-1000 µg/mL) and incubated at 25 degrees Celsius for three hours. An equal volume of freshly produced Griess reagent was added to the extract. The control samples were created in the same manner as the test samples, minus the extracts, and with an equal volume of buffer. The color tubes contained the specified amounts of extracts but lacked sodium nitroprusside. A volume of 150 uL of the reaction mixture was transferred to a 96-well plate. The absorbance at 546 nm was measured using a UV-Vis microplate reader (Alibaba, Hangzhou, China), as described in our previous correspondence [38-41]. The extract and standard inhibition percentage were calculated and recorded using the following formula (Equation 3), and the percentage of nitrite radical scavenging activity of extracts and gallic acid was calculated.
Antipyretic activity
The antipyretic activity was estimated by using TAB vaccine-induced pyrexia and prostaglandin E (PGE)-induced pyrexia model in rabbits.
TAB-vaccine induced pyrexia
In this procedure, the rabbits were segregated into four groups, with five animals in each group. The control group was administered 2 ml/kg of normal saline. A clinical thermometer was used to measure the mean rectal temperature of a group of rabbits at an hourly interval for four hours. 0.5 ml/rabbit of typhoid vaccine (0.5 mL/rabbit) was injected intravenously into the marginal ear vein of rabbits. Aqueous-methanolic leaf extract of M. indica was given orally at doses of 100 and 200 mg/kg/h after TAB vaccination administration in the presence of severe fever [2,42]. The rectal temperature was then recorded every hour for the next four hours. Paracetamol (100 mg/kg/po) was utilized for comparison.
PGE-induced pyrexia
The rabbits were sorted into four groups of five each. For 24 hours, they were kept at a constant temperature of 24°C-25°C. The animals were fasted overnight. Pyrexia was induced by S.C. injection of 100 g/kg PGE1 (misoprostol). After recording the baseline rectal temperature with a temperature sensor, after 1 hour of PGE1 injection, the aqueous-methanolic leaf extract of M. indica was administered orally through oral gavages. Normal saline was given to Group-1 at a rate of 2 ml/kg, aspirin 150 mg/kg was given to Group-2, while Group-3 and Group-4 were given aqueous-methanolic leaf extract of M. indica at a dose of 100 and 200 mg/kg.
Anti-inflammatory activity
In this study, we opted for two well-recognized inflammatory models. Models were used for acute inflammation (arachidonic acid-induced inflammation) and chronic inflammation (formalin-induced paw edema).
Arachidonic acid-induced inflammatory model
In brief, this model is primarily used to assess the anti-inflammatory potential of plant extracts and pharmaceutical drugs by causing cutaneous inflammation. Arachidonic acid provides useful information about anti-inflammatory drugs used to treat topical inflammation. Applying arachidonic acid to a specific area of the skin promotes inflammation via eicosanoids such as leukotriene C4 (LTC4), prostaglandin-E2 (PGE2), and thromboxane A2. Because eicosanoids trigger histamine release via mast cell destruction. This inflammation is characterized by edema, severe erythema, and neutrophil accumulation. Arachidonic acid 0.01 mL topical was given to the left ear of rats while the right ear was left as control [43]. Rats were divided into 5 group: group-1 animals were used as controls, group-2 animals were given diclofenac gel 1% w/w, group-3 animals were treated with diclofenac gel 2.3% w/w, group-4 animals were treated with aqueous methanolic leaf extract of M. indica 10%, and group-5 were animals were treated with M. indica 20%. The percent decrease in edema was calculated with the help of this formula (Equation (4)):
Formalin-induced edema model
In brief, formalin (5%) 0.1 mL sub planter injections were given to the left hind paw of rats while the right hind paw was left as control. Rats were divided into 5 groups: group-1 animal were sued as controls, group-2 animals were given diclofenac gel 1% w/w, group-3 animals were treated with diclofenac gel 2.3% w/w, group-4 animals were treated with aqueous methanolic leaf extract of M. indica 10%, and group-5 were animals treated with M. indica 20% [44]. The percent decrease in edema was calculated with the help of this formula (Equation (5)):
Statistical analysis
The results were communicated using the GraphPad Prism version 8 software as mean ± SEM and investigated using one-way ANOVA followed by Dunnett’s multiple comparison test. The confidence interval was 95%, and P < 0.05 considered as significant.
Results
Preliminary phytochemical evaluation
Preliminary phytochemical evaluation of M. indica showed the presence of vital phytochemcial classes such as, alkaloids, terpenoids, phytosterols, flavonoids and flavonones as shown in Table 1.
Table 1.
Phytoconstituents present in aqueous-methanolic leaf extract of the Mangifera indica
| Serial | Test | Observations | Result |
|---|---|---|---|
| Number | |||
| 1 | Alkaloid | Ppt | Positive |
| 2 | Saponins | 1 cm froth | Positive |
| 3 | Tannins | Light purple | Positive |
| 4 | Anthraquinones | Pink | Positive |
| 5 | Coumarins | Yellow fluorescence | Positive |
| 6 | Phenols | Light purple | Positive |
| 7 | Flavanoids | Light yellow colour | Positive |
• Ppt: Precipitates; cm: Centimeter.
HPLC analysis
HPLC of aqueous-methanolic leaf extract of M. indica was displayed in Figure 1. Based on retention time to standards, the main phytochemicals found were mangiferin (A), quercetin (B), and isoquercetin (C).
Figure 1.

HPLC of aqueous-methanolic leaf extract of M. indica indicating the presence of mangiferin (A), quercetin (B) and isoquercetin (C) in comparison to retention time.
Antioxidant assay
DPPH assay
DPPH activity of ascorbic acid and M. indica is shown in Figure 2.
Figure 2.

Antioxidant potential of aqueous-methanolic leaf extract of M. indica using DPPH assay concerning ascorbic acid. DPPH: 2,2-diphenylpicrylhydrazyl.
NO scavenging assay
NO scavenging assay of Gallic acid and M. indica is shown in Figure 3.
Figure 3.

Antioxidant potential of aqueous-methanolic leaf extract of M. indica using NO scavenging assay concerning ascorbic acid. NO: Nitric oxide.
Antipyretic activity
PGE-induced pyrexia model
At a dose of 100 mg/kg b.w., the aqueous-methanolic leaf extract of M. indica demonstrated considerable (P≤0.05) antipyretic efficacy, with a significant drop in body temperature lasting up to 4 hours after delivery. At a 200 mg/kg dose, it demonstrated highly significant (P≤0.000) efficacy compared with the standard antipyretic drug aspirin, as shown in Figure 4.
Figure 4.

Antipyretic potential of aqueous-methanolic leaf extract of M. indica against PGE-induced pyrexia in rabbits. PGE: Prostaglandin E.
TAB vaccine-induced pyrexia model
Once the extract was given to rabbits with an established TAB vaccine-induced hyperthermia, the fever was considerably lowered. The rabbits’ body temperatures returned to normal when they were given 100 and 200 mg/kg of the extract orally. Figure 5 shows that the response at higher doses was identical to that of the standard antipyretic drug paracetamol.
Figure 5.

Antipyretic potential of aqueous-methanolic leaf extract of M. indica against typhoid-paratyphoid A and B (TAB) vaccine-induced pyrexia in rabbits.
Anti-inflammatory activity
Acute (arachidonic acid-induced ear edema) and chronic (formalin-induced paw edema) inflammatory models were tested, and the results were explained respectively.
Arachidonic acid induced edema model
Anti-inflammatory potential of aqueous-methanolic leaf extract of M. indica against arachidonic acid-induced inflammation in rats is shown in Figure 6.
Figure 6.

Anti-inflammatory potential of aqueous-methanolic leaf extract of M. indica against arachidonic acid-induced inflammation in rats. * = Significant variation as compared to week zero (P < 0.05). ** = Significant variation as compared to week zero (P < 0.01). NS = Non-significant variation as compared to week zero (P > 0.05).
Formalin-induced paw edema model
Anti-inflammatory potential of aqueous-methanolic leaf extract of M. indica against formalin induced inflammation in rats is shown in Figure 7.
Figure 7.
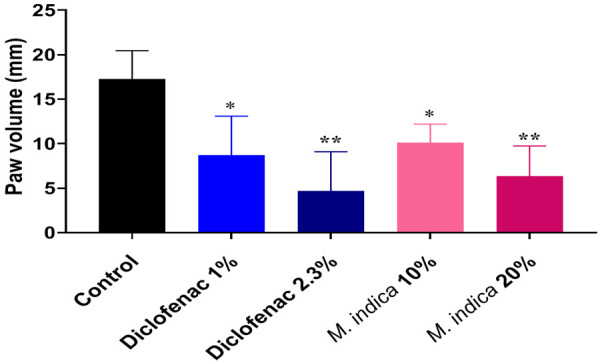
Anti-inflammatory potential of aqueous-methanolic leaf extract of M. indica against formalin induced inflammation in rats. * = Significant variation as compared to week zero (P < 0.05). ** = Significant variation as compared to week zero (P < 0.01). NS = Non-significant variation as compared to week zero (P > 0.05).
Proposed mechanism of action
Mangiferin has anti-inflammatory and analgesic activity by decreasing the synthesis of PGE-2 and COX-2 protein induced by LPS but does not modify the transcription of COX-2 [45]. It can also decrease the plasma levels of IL-1β, TNF-α, IL-6, and MCP-1 (monocyte chemoattractant protein-1) [46,47].
Quercetin has anti-inflammatory activity by inhibiting PGE-2, cytokine, and iNOS (inducible nitric oxide synthase) by inhibiting NF-kappa B and TNF-α [48,49]. Isoquercetin proved to be, to some extent, better than quercetin for anti-inflammatory activity by inhibiting COX-2, mRNA, and inflammatory cell exudation [50] (Figure 8).
Figure 8.

The proposed mechanism of action of active constituents of M. indica (mangiferin, quercetin and isoquercetin) on various parameters.
Discussion
Terpenoids and flavonoids were found to be the most abundant chemical components in the aqueous-methanolic leaf extract of M. indica, according to the early results of a phytochemical investigation (Table 1). Since prostaglandins are responsible for edema, flavonoids can suppress them [51]. In most cases, the antipyretic effect of nonsteroidal anti-inflammatory medications is achieved by inhibiting the synthesis of prostaglandins inside the hypothalamus [52]. The drug being tested may be able to reduce fever because it has flavonoid molecules in it. Because certain flavonoids are potent cyclooxygenase or lipoxygenase inhibitors [53], it is possible to conclude that the aqueous-methanolic leaf extract of M. indica lowered the body temperature by blocking the production of prostaglandins in the same way that aspirin does. M. indica’s antipyretic activity may be due to its phytoconstituents, flavonoids, as it has been reported to reduce the availability of prostaglandins.
Body thermal regulation demands a careful balance between heat generation and heat loss in the hypothalamus, which centrally maintains the set point at which body temperature is maintained [54]. The elements stated above raise this set point in fever. Antipyretic medications are known to work either centrally on the brain’s temperature control center or peripherally via vasodilation and heat dissipation [2]. They reset the hypothalamic thermostat and lower fever quickly by increasing heat dissipation (sweating, cutaneous vasodilation) [52]. They also work by preventing the production of prostaglandin E2 [55]. The results indicate that the aqueous-methanolic leaf extract of M. indica has an antipyretic property comparable to that of aspirin (standard drug) in rabbits with PGE1-induced elevations in body temperature. As a result, inhibition of prostaglandin synthesis could be a plausible mechanism of M. indica’s antipyretic activity as aspirin [7], and there are multiple mediators or processes underlying fever pathogenesis. Any of these mediators may be inhibited from producing antipyresis. We conclude from the above study that M. indica has antipyretic potential against both pyrexia models in rabbits.
The arachidonic acid model is primarily used to assess the anti-inflammatory potential of plant extracts and pharmaceutical drugs by causing cutaneous inflammation. Arachidonic acid (acute inflammatory model) provides valuable information about anti-inflammatory drugs used in the treatment of topical inflammation. Applying arachidonic acid to a specific area of the skin promotes inflammation via eicosanoids such as leukotriene C4 (LTC4), prostaglandin-E2 (PGE2), and thromboxane A2 [43]. Eicosanoids trigger histamine release via mast cell destruction. This inflammation is characterized by edema, severe erythema, and neutrophil accumulation. Formalin-induced paw edema model is used to assess the chronic anti-inflammatory efficacy of various medications. This model is identical to human arthritis [44]. Formalin causes inflammation that happens in two stages. The first stage is neurogenic and is controlled by substance P and bradykinin. Prostaglandins, serotonin, histamine, and bradykinin are all involved in the later phase [56]. Drugs such as opioids decrease both phases, but NSAIDs and corticosteroids inhibit the second phase. HPLC analysis of an aqueous-methanolic leaf extract of M. Indica confirmed the presence of three vital anti-inflammatory phytoconstituents: mangiferin, quercetin, and isoquercetin (Figure 1). The antipyretic and anti-inflammatory roles of these phytoconstituents are now well established [57], and the reported mechanism of action behind these phytoconstituents’ antipyretic and anti-inflammatory properties is explained in Figure 6. The anti-inflammatory and antipyretic activity of these phytoconstituents corresponds to their antioxidant and antihistaminic properties.
Conclusions
Results demonstrated that the aqueous-methanolic leaf extract of M. indica has, antioxidant, anti-inflammatory, and antipyretic activities that may be due to different phytochemical constituents present in M. indica like mangiferin, quercetin, and isoquercetin. This report is the first of its kind and shows the advantage of acting on different pharmacological and biochemical pathways controlling inflammation and pyrexia.
Acknowledgements
Hereby we extend our gratitude to the laboratory staff and staff of Muhammad Institute of Medical and Allied Sciences, Multan, Pakistan, for their cooperation throughout the study.
Disclosure of conflict of interest
None.
Abbreviations
- DPPH
2,2-diphenylpicrylhydrazyl
- NO
nitric oxide
- TAB
typhoid-paratyphoid A and B
- PGE
prostaglandin E
- HPLC
high-performance liquid chromatography
- NSAIDs
nonsteroidal anti-inflammatory agents
- CHD
chronic heart disease
- LTC4
leukotriene C4
- PGE2
prostaglandin-E2
References
- 1.Shaito A, Thuan DTB, Phu HT, Nguyen THD, Hasan H, Halabi S, Abdelhady S, Nasrallah GK, Eid AH, Pintus G. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front Pharmacol. 2020;11:422. doi: 10.3389/fphar.2020.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan IA, Aziz A, Manzoor Z, Munawar SH, Sarwar HS, Afzal A, Raza MA. Study on antipyretic activity of Rumex vesicarius leaves extract in albino rabbits. Vet World. 2014;7:44–48. [Google Scholar]
- 3.Shinwari ZK. Medicinal plants research in Pakistan. J Med Plant Res. 2010;4:161–76. [Google Scholar]
- 4.Saeed M, Muhammad N, Khan H. Assessment of heavy metal content of branded Pakistani herbal products. Tropical J Pharm Res. 2011;10:499–506. [Google Scholar]
- 5.Saeed M, Muhammad N, Khan H, Khan SA. Analysis of toxic heavy metals in branded Pakistani herbal products. J Chem Soc Pak. 2010;32:471. [Google Scholar]
- 6.Bukhari KA, Khan IA, Ishaq S, Iqbal MO, Alqahtani AM, Alqahtani T, Menaa F. Formulation and evaluation of diclofenac potassium gel in sports injuries with and without phonophoresis. Gels. 2022;8:612. doi: 10.3390/gels8100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz A, Khan IA, Munawar SH, Shaheed SU. Antipyretic study of methanolic bark extract of Plumeria rubra, linn. in various pyrexia-induced models. Int J Res Dev Pharm Life Sci. 2013;4:33–37. [Google Scholar]
- 8.Wade AG, Crawford GM, Young D, Corson S, Brown C. Comparison of diclofenac gel, ibuprofen gel, and ibuprofen gel with levomenthol for the topical treatment of pain associated with musculoskeletal injuries. J Int Med Res. 2019;47:4454–4468. doi: 10.1177/0300060519859146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75:539–53. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay D, Arunachalam G, Ghosh L, Rajendran K, Mandal AB, Bhattacharya SK. Antipyretic activity of Alstonia macrophylla wall ex A. DC: an ethnomedicine of Andaman Islands. J Pharm Pharm Sci. 2005;8:558–64. [PubMed] [Google Scholar]
- 11.Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–6. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 12.Luo C, He ML, Bohlin L. Is COX-2 a perpetrator or a protector? Selective COX-2 inhibitors remain controversial. Acta Pharmacol Sin. 2005;26:926–33. doi: 10.1111/j.1745-7254.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal MO, Naeem M, Mumtaz A, Ahmed MM, Ahmad A, Riaz R, Mesbah Z, Munawar N. Biochemical evaluation and medicinal ability of Jatropha mollissima in hepatic disorders. Am J Transl Res. 2022;14:7178. [PMC free article] [PubMed] [Google Scholar]
- 14.Núñez-Sellés AJ. Antioxidant therapy: myth or reality? J Braz Chem Soc. 2005;16:699–710. [Google Scholar]
- 15.Okwu DE, Ezenagu V. Evaluation of the phytochemical composition of mango (Mangifera indica Linn) stem bark and leaves. Int J Chem Sci. 2008;6:705–16. [Google Scholar]
- 16.Aderibigbe AO, Emudianughe TS, Lawal BA. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother Res. 2001;15:456–8. doi: 10.1002/ptr.859. [DOI] [PubMed] [Google Scholar]
- 17.Akinpelu DA, Onakoya TM. Antimicrobial activities of medicinal plants used in folklore remedies in South-Western. Afr J Biotechnol. 2006;5:1078–1081. [Google Scholar]
- 18.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (Mango) Pharmacogn Rev. 2010;4:42–48. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera DG, Balmaseda IH, León AÁ, Hernández BC, Montiel LM, Garrido GG, Hernández RD, Cuzzocrea S. Anti-allergic properties of Mangifera indica L. extract (Vimang) and contribution of its glucosylxanthone mangiferin. J Pharm Pharmacol. 2006;58:385–92. doi: 10.1211/jpp.58.3.0014. [DOI] [PubMed] [Google Scholar]
- 20.Das J, Ghosh J, Roy A, Sil PC. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFκB pathways. Toxicol Appl Pharmacol. 2012;260:35–47. doi: 10.1016/j.taap.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt L, Joshi V. Mangifera indica L. leaf extract alleviates doxorubicin induced cardiac stress. J Intercult Ethnopharmacol. 2017;6:284–289. doi: 10.5455/jice.20170701075019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil R, Patil R, Ahirwar B, Ahirwar D. Current status of Indian medicinal plants with antidiabetic potential: a review. Asian Pac J Trop Biomed. 2011;1:S291–8. [Google Scholar]
- 23.Dar A, Faizi S, Naqvi S, Roome T, Zikr-ur-Rehman S, Ali M, Firdous S, Moin ST. Analgesic and antioxidant activity of mangiferin and its derivatives: the structure activity relationship. Biol Pharm Bull. 2005;28:596–600. doi: 10.1248/bpb.28.596. [DOI] [PubMed] [Google Scholar]
- 24.Faizi S, Zikr-Ur-Rehman S, Ali M, Naz A. Temperature and solvent dependent NMR studies on mangiferin and complete NMR spectral assignments of its acyl and methyl derivatives. Magn Reson Chem. 2006;44:838–44. doi: 10.1002/mrc.1854. [DOI] [PubMed] [Google Scholar]
- 25.Jagetia GC, Venkatesha VA. Effect of mangiferin on radiation-induced micronucleus formation in cultured human peripheral blood lymphocytes. Environ Mol Mutagen. 2005;46:12–21. doi: 10.1002/em.20124. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Hou Y, Liu Y, Yu X, Li B, Cui H. Determination of mangiferin in rat eyes and pharmacokinetic study in plasma after oral administration of mangiferin-hydroxypropyl-beta-cyclodextrin inclusion. J Ocul Pharmacol Ther. 2010;26:319–24. doi: 10.1089/jop.2010.0024. [DOI] [PubMed] [Google Scholar]
- 27.Lakhanpal P, Rai DK. Quercetin: a versatile flavonoid. Internet J Med Updat. 2007;2:22–37. [Google Scholar]
- 28.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–9. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 29.Appleton J. Evaluating the bioavailability of isoquercetin. Nat Med J. 2010;2:1–6. [Google Scholar]
- 30.Zhang R, Yao Y, Wang Y, Ren G. Antidiabetic activity of isoquercetin in diabetic KK-Ay mice. Nutr Metab (Lond) 2011;8:85. doi: 10.1186/1743-7075-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulke A, Eckert GP, Schubert-Zsilavecz M, Wurglics M. Isoquercitrin provides better bioavailability than quercetin: comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie. 2012;67:991–6. [PubMed] [Google Scholar]
- 32.Ain QU, Abid MUH, Hashim M, Ishaq S, Manzoor A, Perwasha P, Mizgan GE, Iqbal MO, Munawar SH, Manzoor Z, Khan IA. Anticoagulant and thrombolytic activities of leaf extract of Mangifera Indica in smokers. Tob Regul Sci. 2022;8:1189–1201. [Google Scholar]
- 33.Khan IA, Lodhi AH, Munawar SH, Manzoor A, Manzoor Z, Raza MA. Formulation and evaluation of rutin-allicin gelagainst diabetic foot ulcer. Lat Am J Pharm. 2020;39:725–9. [Google Scholar]
- 34.Khan IA, Aziz A, Sattar M, Munawar SH, Manzoor Z, Raza MA, Ghayoor F, Abdul H. Evaluation of wound healing potential of Rumex vesicarius L. Leaf extract and fractions in rabbit. Afr J Tradit Complement Altern Med. 2015;12:60–4. [Google Scholar]
- 35.Aziz A, Saqib F, Khan IA, Ashraf MM, Ashraf MN, Raza MA. Dermatological evaluation of anti-irritant and anti-inflammatory effect of plumerin-R isolated from the latex of Plumeria rubra Linn. Lat Am J Pharm. 2018;37:317–20. [Google Scholar]
- 36.National Institute of Health. Guide for the Care and Use of Laboratory Animals. Washington DC: NIH Publishing; 1996. [Google Scholar]
- 37.Iqbal MO, Khan IA, Manzoor A, Arshad S, Sial AS, Dar E, Shaikh AR. Cardioprotective effect of hydroalcoholic leaf extract of Jatropha mollissima on isoproterenol-induced myocardial infarction in rats. Pharmacogn Mag. 2021;17:251. [Google Scholar]
- 38.Khan IA, Hussain M, Syed SK, Saadullah M, Alqahtani AM, Alqahtani T, Aldahish AA, Asiri S, Zeng LH. Pharmacological justification for the medicinal use of Plumeria rubra Linn. in cardiovascular disorders. Molecules. 2021;27:251. doi: 10.3390/molecules27010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan IA, Hussain M, Munawar SH, Iqbal MO, Arshad S, Manzoor A, Sha MA, Abbas K, Shakeel W, Syed SK. Jasminum sambac: a potential candidate for drug development to cure cardiovascular ailments. Molecules. 2021;26:5664. doi: 10.3390/molecules26185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan IA, Hussain M, Hussain N, Alqahtani AM, Alqahtani T. Cardioprotective effect of Rumex vesicarius Linn. leaf extract against catecholamine-induced cardiotoxicity. Molecules. 2022;27:3383. doi: 10.3390/molecules27113383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iqbal MO, Ahmed MM, Arshad S, Javaid U, Khan IA, Manzoor M, Andleeb S, Riaz R, Munawar SH, Manzoor Z, Mumtaz A. Nephroprotective effects of Alhagi camelorum against cisplatin-induced nephrotoxicitfy in albino wistar rats. Molecules. 2022;27:941. doi: 10.3390/molecules27030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aziz A, Khan IA, Ahmed MB, Hussain S. Evaluation of antipyretic activity of Thuja occidentalis Linn. in PGE1 and TAB-Vaccine induced pyrexia models in rabbits. Int J Pharm Sci. 2014;2:481–484. [Google Scholar]
- 43.Aked DM, Foster SJ. Leukotriene B4 and prostaglandin E2 mediate the inflammatory response of rabbit skin to intradermal arachidonic acid. Br J Pharmacol. 1987;92:545–52. doi: 10.1111/j.1476-5381.1987.tb11355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prabhu VV, Kuruvilla CA, Guruvayoorappan C. Potentiating effect of 1, 2-diazole a plant alkaloid on carrageenan and formalin induced paw edema in experimental mice. Int J Pharm Pharm Sci. 2012;4:380–3. [Google Scholar]
- 45.Bhatia HS, Candelario-Jalil E, de Oliveira AC, Olajide OA, Martínez-Sánchez G, Fiebich BL. Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch Biochem Biophys. 2008;477:253–8. doi: 10.1016/j.abb.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Jia L, Sun P, Gao H, Shen J, Gao Y, Meng C, Fu S, Yao H, Zhang G. Mangiferin attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting TLR4/p65 and TGF-β1/Smad2/3 pathway. J Pharm Pharmacol. 2019;71:1017–1028. doi: 10.1111/jphp.13077. [DOI] [PubMed] [Google Scholar]
- 47.Morais TC, Arruda BR, de Sousa Magalhães H, Trevisan MT, de Araújo Viana D, Rao VS, Santos FA. Mangiferin ameliorates the intestinal inflammatory response and the impaired gastrointestinal motility in mouse model of postoperative ileus. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:531–8. doi: 10.1007/s00210-015-1095-4. [DOI] [PubMed] [Google Scholar]
- 48.Cho YH, Kim NH, Khan I, Yu JM, Jung HG, Kim HH, Jang JY, Kim HJ, Kim DI, Kwak JH, Kang SC, An BJ. Anti-inflammatory potential of quercetin-3-O-β-D-(“2”-galloyl)-glucopyranoside and quercetin isolated from diospyros kaki calyx via suppression of MAP signaling molecules in LPS-induced RAW 264.7 macrophages. J Food Sci. 2016;81:C2447–C2456. doi: 10.1111/1750-3841.13497. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrasekharan NV, Dai H, Roos KLT, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimoto T, Furukawa M, Yamamoto S, Horie T, Watanabe-Kohno S. Flavonoids: potent inhibitors of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 1983;116:612–8. doi: 10.1016/0006-291x(83)90568-5. [DOI] [PubMed] [Google Scholar]
- 52.Demain AL, Vaishnav P. Natural products for cancer chemotherapy. Microb Biotechnol. 2011;4:687–99. doi: 10.1111/j.1751-7915.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy LA, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 54.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacol. 6th edition. Churchill Livingstone Edinb; 2007. [Google Scholar]
- 55.Cheng WY, Wu SL, Hsiang CY, Li CC, Lai TY, Lo HY, Shen WS, Lee CH, Chen JC, Wu HC, Ho TY. Relationship between San-Huang-Xie-Xin-Tang and its herbal components on the gene expression profiles in HepG2 cells. Am J Chin Med. 2008;36:783–97. doi: 10.1142/S0192415X08006235. [DOI] [PubMed] [Google Scholar]
- 56.Singh G, Bhatti R, Mannan R, Singh D, Kesavan A, Singh P. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacology. 2019;27:949–60. doi: 10.1007/s10787-018-0486-9. [DOI] [PubMed] [Google Scholar]
- 57.Olorunfemi OJ, Nworah DC, Egwurugwu JN, Hart VO. Evaluation of anti-inflammatory, analgesic and antipyretic effect of Mangifera indica leaf extract on fever-induced albino rats (Wistar) Br J Pharmacol Toxicol. 2012;3:54–7. [Google Scholar]


