Abstract
Infection of susceptible mouse strains with BeAn, a less virulent strain of Theiler’s murine encephalomyelitis virus (TMEV), results in immune system-mediated demyelinating lesions in the central nervous system (CNS) similar to those in multiple sclerosis. Since macrophages appear to carry the major detectable antigen burden in vivo, and purification of sufficient cell numbers from the CNS for detailed analysis is difficult, macrophage-like cell lines provide an accessible system with which to study virus-macrophage interactions. The myeloid precursor cell line M1 differentiates in response to cytokines and expresses many characteristics of tissue macrophages. Incubation of TMEV with undifferentiated M1 cells produced neither infection nor apoptosis, whereas differentiated M1 (M1-D) cells developed a restricted virus infection and changes indicative of apoptosis. Virus binding and RNA replication as well as cellular production of alpha/beta interferons increased with differentiation. Although the amount of infectious virus was highly restricted, BeAn-infected M1-D cells synthesized and appropriately processed virus capsid proteins at levels comparable to those for permissive BHK-21 cells. Analysis of Bcl-2 protein family expression in undifferentiated and differentiated cells suggests that susceptibility of M1-D cells to apoptosis may be controlled, in part, by expression of the proapoptotic α isoform of Bax and Bak. These data suggest that macrophage differentiation plays a role in susceptibility to TMEV infection and apoptosis.
Theiler’s murine encephalomyelitis virus (TMEV), genus Cardiovirus, family Picornaviridae, is a natural enteric pathogen of mice and has been divided into two groups based on neurovirulence following intracerebral inoculation of susceptible mouse strains. Infection with BeAn, a less virulent strain of TMEV, results in a chronic, progressive demyelinating disease of the central nervous system (CNS). Persistence of BeAn within the CNS leads to immunopathologic damage of myelin, mediated by major histocompatibility complex (MHC) class II-restricted Th1 lymphocytes directed at a virus epitope(s) rather than host neuroantigens (8, 14, 15, 30, 31).
During TMEV persistence, the major virus antigen burden resides in CNS macrophages (Mφs) (24, 36), and the virus load in Mφs isolated from the CNS equals that in clarified CNS homogenates (9). However, only a small percentage of Mφs that infiltrate demyelinating lesions contain detectable virus antigen (24). We previously found that TMEV infection in two Mφ cell lines (RAW264.7 and P388D1) was restricted, and we postulated that Mφ susceptibility to infection depends on the differentiation/activation state of the Mφ (20). To examine the cellular basis of the restrictive BeAn virus replication in Mφs, we used the myeloid precursor cell line M1, derived from a spontaneous myeloid leukemia of SL mice (18, 19). M1 cells differentiate in vitro into either Mφ- or granulocyte-like cells in response to conditioned medium from embryonic cell cultures (18, 19), various cytokines (33, 34) including interleukin-6 (IL-6) (7, 16, 42), macrophage colony-stimulating factor (M-CSF) (46), and chemicals such as dexamethasone or 1α,23-dihydroxyvitamin D3 (22). Differentiated M1 cells upregulate several cell surface proteins, including Fc receptors (FcR), (49), tumor necrosis factor alpha receptors (29), and MHC class II proteins, and they acquire functions such as phagocytosis and lysozyme secretion (40). M1 myeloid precursor cells have been widely used as a model for Mφ differentiation and gene regulation.
Analysis of M1 cell infection with the highly virulent GDVII and the less virulent BeAn viruses revealed that only differentiated cells were susceptible to infection and apoptosis, and as previously reported for the restricted infection in BSC-1 cells (1), GDVII was a more efficient inducer of apoptosis than BeAn virus. However, since BeAn virus persists in mice and causes demyelination, subsequent studies focused on the less virulent BeAn virus. Interestingly, although BeAn infectious virus yields and viral RNA were lower in differentiated M1 (M1-D) cells than in permissive BHK-21 cells, large amounts of virus antigen were condensed within the cytoplasm of M1-D cells. The data reported here suggest that myeloid cells are susceptible to TMEV infection only when differentiated into Mφs and that these cells die by apoptosis thereafter.
MATERIALS AND METHODS
Cells, viruses, and reagents.
The M1 cell line, an immature myelomonocytic cell line derived from the SL mouse strain (kind gift from Selina Cheng Kang, Mount Sinai School of Medicine, and Barbara Hoffman, Temple University), was maintained in RPMI 1640 supplemented with 10% fetal bovine serum (GIBCO), 2 mM l-glutamine, 0.1 mg of kanamycin sulfate per ml, and nonessential amino acids (GIBCO) (complete medium). Conditioned medium from mouse L929 cells, which secrete M-CSF, was prepared by plating 0.5 × 106 cells in a T75 flask with 50 ml of Dulbecco modified Eagle medium (DMEM) supplemented as specified above for RPMI complete medium, harvesting the supernatant after 1 week, adding 50 ml fresh DMEM, and collecting the supernatant after an additional week. Conditioned medium from the IL-1-secreting mouse macrophage P388D1 cell line was prepared by plating 1.5 × 106 cells in 15 ml of RPMI complete medium, collecting the supernatant after 4 days, adding fresh medium, and harvesting cells after an additional 4 days. Supernatants from both time points were pooled, filtered, and stored at −70°C until use. M1 cells were induced to differentiate with conditioned medium containing 20% L929 supernatant, 17% P388D1 supernatant, and 0.25% 2-β-mercaptoethanol (43a).
The origin and passage history of the BeAn virus stock have been described elsewhere (38). Virus titers of clarified lysates from infected cells were determined by standard plaque assay on BHK-21 cells (38).
Virus infections.
After adsorption of virus at a multiplicity of infection (MOI) of 10 for 45 min at 24°C, M1 cells were washed with phosphate-buffered saline (pH 7.2), cultured with complete RPMI containing 5% fetal bovine serum, and incubated at 37°C in a 5% CO2 incubator for the indicated times.
UV irradiation of BeAn.
UV irradiation of BeAn virus was performed essentially as described elsewhere (21) except that the virus was exposed to UV for 1 or 20 min.
Flow cytometry.
Staining for flow cytometry was done as described elsewhere (20). Briefly, nonspecific antibody binding to cells was blocked with 10 μl of goat serum and/or 2 μl of anti-CD32/16 (FcR) (PharMingen, San Diego, Calif.) where appropriate. TMEV antigens were detected by incubation with a 1:1,000 dilution of polyclonal rabbit anti-BeAn or normal rabbit serum for 30 min at 4°C, followed by a 1:200 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (Cappel/Organon Teknika, Durham, N.C.) for 30 min at 4°C. Cytoplasmic BeAn antigen was detected as described elsewhere (20). Commercially available antibodies were used for the following antigens: CD32/CD16b, CD11A, CD29, CD51, and CD54 from PharMingen; and CD11b(C3bi) and F4/80 from Caltag Laboratories (San Francisco, Calif.). Antibody 2F8 was a kind gift from Siamon Gordon (Oxford, United Kingdom) (23). The MHC class II antibody was from hybridoma 10-3.6.2 (American Type Culture Collection, Rockville, Md.) which detects MHC class II k, v, q, and s haplotypes. The secondary antibodies were FITC-conjugated goat anti-rat immunoglobulin G (IgG; Caltag) and biotinylated anti-hamster Ig and avidin-FITC (PharMingen). After staining, cells were fixed in 1% paraformaldehyde and analyzed on a FACScan (Becton Dickinson, Palo Alto, Calif.) or a Coulter Epics XL-MCL (Coulter Corporation, Miami, Fla.). Data were evaluated by using the Consort 30 and LYSIS I computer programs for the FACScan or the Coulter computer program specific for the Epics.
Immunoprecipitation of viral proteins.
Radiolabeled BeAn virus proteins were immunoprecipitated from cell lysates as described elsewhere (21) with 5 μl of polyclonal rabbit anti-BeAn antiserum, a concentration that is not limiting in these experiments.
[35S]methionine labeling and purification of BeAn virus.
BeAn virus was labeled with [35S]methionine as described previously (20). Purified virus from infected BHK-21 cell lysates was used in a standard binding assay (12). Briefly, washed cells were resuspended (106 cells/ml) in DMEM containing 20 mM HEPES plus 1% bovine serum albumin and incubated on ice for 1 h prior to the addition of 35S-labeled BeAn (20,000 particles/cell). At the indicated times, an aliquot of the suspension was removed, diluted in DMEM containing 20 mM HEPES, and microcentrifuged at 12,000 × g for 30 s. The supernatant and cell-associated radioactivity were determined with a Beckman (Palo Alto, Calif.) model LS5000TD scintillation counter, and the percentage of cell-associated counts per minute was calculated by using Cricket Graph III software.
Assay of viral RNA replication.
After virus adsorption, cells were washed and plated at 2 × 104 cells/100 μl/well in 96-microwell plates with complete medium containing actinomycin D (5 μg/ml) and [3H]uridine ([3H]UdR; 10 μCi/ml; specific activity, 15 to 25 Ci/mmol; ICN). Infected cells were incubated at 37°C for the indicated times and harvested on a PHD cell harvester (Cambridge Technologies, Watertown, Mass.), and radioactivity was determined with a Beckman model LS5000TD scintillation counter. Data were analyzed by using Cricket Graph III software.
Assay of caspase activity.
Caspases are members of the IL-1β converting enzyme family of cysteine proteases, which cleave at aspartic acid residues and are activated during apoptosis (3). Caspase protease activity was measured in cell lysates by using a modification of a described method (11). Briefly, 2 × 106 cells were lysed in 100 μl of buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 10 mM EGTA, and 10 μM digitonin. Cells were suspended, incubated for 10 min at 37°C, centrifuged to remove cellular debris, and assayed after 60 min of incubation with 1 μM CPP32 substrate peptide acetyl-Asp-Glu-Val-Asp conjugated to aminomethylcoumarin (DEVD-AMC). Fluorescence from cleaved AMC was detected with a 7625 Microplate fluorometer (Cambridge Technologies). Mean values of duplicate or triplicate samples are reported after subtraction of background values.
IFN-α/β assay.
Biologically active alpha and beta interferons (IFN-α/β) were measured essentially as described elsewhere (39). L929 cells (104/well of a 96-well plate) were incubated overnight at 37°C in a humidified 5% CO2 atmosphere and further incubated with serial twofold dilutions of supernatants from mock- or BeAn-infected lysates for 6 h at 37°C. Vesicular stomatitis virus (105 PFU in 50 μl) was added to each well, and incubation continued until control wells showed complete cytopathic effect (CPE). The IFN-α/β titer was taken as the reciprocal of the dilution in which 50% of the cells were protected from CPE. An IFN-α/β standard (Sigma, St. Louis, Mo.) was added to each plate to determine the concentration (units per milliliter).
Immunoblotting for Bcl-2, Bcl-XL and Bax expression.
Expression of Bcl-2, Bcl-XL, and Bax proteins was assessed by Western blotting. Cells were lysed in radioimmunoprecipitation assay buffer and clarified by low-speed centrifugation to remove nuclei and debris. Protein content was determined with Bio-Rad (Hercules, Calif.) DC protein assay kit according to the manufacturer’s instructions. Samples (20 μg/lane) were electrophoresed on a 12 or 15% polyacrylamide gel and transferred to a ProBlot membrane (Applied Biosystems, Foster City, Calif.). The membrane, blocked with Tris-buffered saline containing 5% nonfat dry milk and 0.02% Tween 20, was incubated with 1:1,000 dilution of polyclonal rabbit anti-Bcl-2, anti-Bcl-X, and anti-Bax antiserum (PharMingen) for 2 h at 4°C, followed by a 60-min incubation with a 1:1,000 dilution of peroxidase-labeled anti-rabbit IgG. Peroxidase-labeled proteins were detected with 3,3′-diaminobenzidine tetrahydrochloride (Amresco, Solon, Ohio) in the presence of 0.1% peroxide.
RPA.
RNA was isolated from M1 and M1-D cells by using TRIzol reagent (Life Technologies, Grand Island, N.Y.) according to the manufacturer’s instructions. The RiboQuant multiprobe RNase protection assay (RPA) (PharMingen) was used according to the manufacturer’s instructions to analyze RNA expression of bcl-2 family members, using the mAPO-2 template set from PharMingen (catalog no. 45354P). The probe was synthesized by using [α-35S]UTP instead of [α-32P]UTP.
RESULTS
In vitro differentiation of M1 cells.
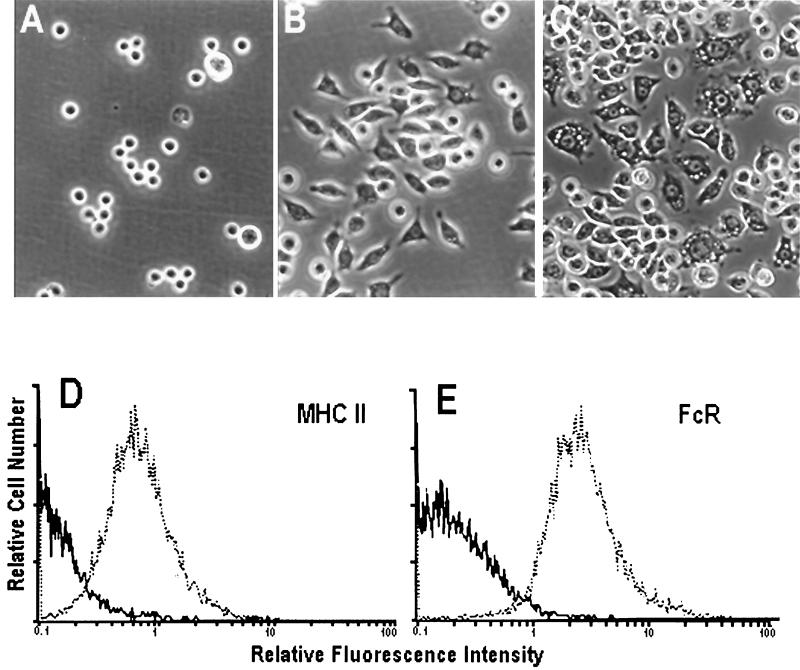
M1 cells differentiate to Mφ-like cells in vitro in response to a number of stimuli, including IL-6, M-CSF, and IL-1 (7, 33, 34, 40, 46). When M1 cells were exposed to conditioned medium containing cytokines, cell morphology changed from round, nonadherent cells to bipolar, flattened adherent cells (Fig. 1A and B). Flow cytometry confirmed this change in morphology, with an ∼2-fold increase in both size (shift in forward scatter from 24 to 41 U) and granularity (shift in side scatter from 10.5 to 21 U). Treatment of M1-D cells with IFN-γ (100 U/ml; Genzyme, Cambridge, Mass.) resulted in even larger, more adherent cells with numerous cytoplasmic vacuoles (Fig. 1C). These cells increased an additional 1.5-fold in size and granularity (data not shown). Thus, M1 cells grown in conditioned medium were not terminally differentiated and remained responsive to an additional cytokine signal.
FIG. 1.
(A to C) Morphological changes in M1 cells incubated with or without conditioned medium and IFN-γ. Photomicrographs were taken with a Nikon inverted-phase microscope using a 20× objective. (A) M1 cells; (B) M1-D cells differentiated in the presence of conditioned medium; (C) M1-D cells treated with γ-IFN. (D and E) Flow cytometry of M1 cells stained with monoclonal antibodies to MHC class II and FcR before (solid line) and after (dotted line) treatment with conditioned medium. Control samples showed overlapping histograms for M1 and M1-D cells.
Several Mφ-specific markers, including MHC class II proteins (Fig. 1D), FcR (CD32/CD16) (40) (Fig. 1E), F4/80 (28), Mac-1 (43), and 2F8 (10) (Table 1), were expressed concomitant with the morphological changes in M1 cells grown in conditioned medium. Of the other surface proteins tested, the integrin β1 chain (CD29) was upregulated, the integrin αv chain (CD51) was not expressed in either the undifferentiated or differentiated population, and I-CAM (CD54) was highly expressed on both cell populations (Table 1). Together, these data indicate that M1 cells undergo differentiation to Mφ-like cells in the presence of conditioned medium. This differentiated state has been stable for 12 months with or without continuous exposure to conditioned medium.
TABLE 1.
Expression of cell surface markers on M1 cells before and after differentiation
| Cell surface molecule | Expressiona
|
|
|---|---|---|
| Untreated | Differentiated | |
| CD11a (integrin αL, LFA-1) | + | + |
| CD11b (integrin αM, Mac-1) | − | + |
| CD29 (integrin α1 chain) | − | + |
| CD51 (integrin αv chain) | − | − |
| CD54 (ICAM-1) | + | + |
| MHC class II | − | + |
| FcR | − | + |
| F4/80 | − | + |
| 2F8 (Mφ scavenger receptor) | − | + |
Cells were incubated in conditioned medium to induce differentiation and analyzed by flow cytometry as described in Materials and Methods. +, positive staining compared to controls; −, no difference in staining compared to controls.
Virus-induced apoptosis of M1-D cells.
When M1 and M1-D cells were infected with BeAn virus at an MOI of 10, only the M1-D cells showed CPE after 18 h, with adherent cells rounding and detaching from the plate. Phase contrast microscopy revealed cell shrinkage and prominent surface blebbing consistent with active cell death by apoptosis. Indeed, DNA staining with 4′,6-diamidino-2-phenylindole (DAPI) revealed condensed nuclei and distinct apoptotic bodies in M1-D cells (Fig. 2B), whereas the nuclear morphology in M1 cells remained intact with clear chromatin structure (Fig. 2A). Counterstaining of these cells delineated virus antigen in 70 to 90% of M1-D cells (see Fig. 4A), which was present as a compact mass in condensed cells (Fig. 2B). Analysis of DNA cleavage into nucleosome-sized bands, a hallmark of apoptosis, showed clear fragmentation in DNA isolated from supernatants of BeAn-infected M1-D cells but not in uninfected M1-D or infected undifferentiated M1 cells (Fig. 2C).
FIG. 2.
Evidence for apoptosis in M1-D cells. (A and B) Fluorescence microscopy of M1 (A) and M1-D (B) cells infected at an MOI of 10 and stained for BeAn virus (green) and nuclear morphology (red) 20 h after infection. (C) Ethidium bromide staining of DNA isolated from supernatants of 6 × 106 M1 and M1-D cells either uninfected (U) or BeAn virus infected (I) 20 h after infection. DNA laddering is seen only in samples from infected M1-D cells. (D) Caspase activity measured by DEVD-AMC cleavage in lysates from 2 × 106 M1 and M1-D cells uninfected or BeAn virus infected. Mean ± standard deviation of triplicate samples is shown. Data are representative of four experiments.
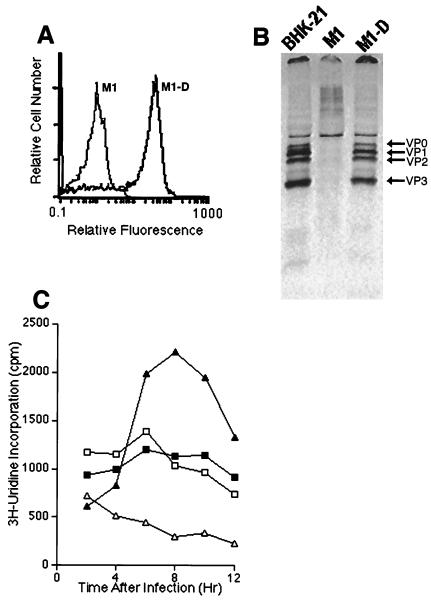
FIG. 4.
Characteristics of BeAn virus infection in M1-D cells. (A) Flow cytometry of M1 and M1-D cells 20 h after BeAn infection. Cells were permeabilized with 0.3% saponin and stained for cytoplasmic virus antigen with a 1:1,000 dilution of polyclonal rabbit anti-BeAn antiserum followed by a 1:200 dilution of goat anti-rabbit Ig-FITC. Although the infected M1-D cells were apoptotic, they contained large amounts of virus antigen. (B) Immunoprecipitation of BeAn virus capsid proteins with 5 μl of polyclonal rabbit anti-BeAn antiserum from 106 cells lysed 20 h after infection from permissive BHK-21, M1, and M1-D cells. Virus capsid proteins are labeled. Densitometric scans revealed similar percentages of each capsid protein immunoprecipitated from BHK-21 and M1-D cells. (C) Incorporation of [3H]UdR into BeAn virus RNA before (open symbols) and after (closed symbols) infection of M1 (□, ■) and M1-D (▵, ▴) cells. Cells (2 × 104) were incubated with [3H]UdR in the presence of actinomycin D and harvested at the indicated times. Mean of triplicates is shown; standard deviation was less than 10 for all samples. All experiments were repeated at least three times.
Caspase activity, which is elevated during apoptosis, was at similar levels in uninfected and infected M1 cells (Fig. 2D). The background caspase activity in uninfected M1-D cells was consistently lower (three experiments), while activity from BeAn-infected M1-D cells increased 15- to 17-fold (Fig. 2D). Time course studies indicated a detectable increase in caspase activity beginning by 6 h, reaching peak levels by 8 to 10 h after infection (data not shown). This contrasts with the detection of morphological changes or DNA degradation, which were observed only after 12 h. Together, these data indicate that precursor Mφs became susceptible to BeAn virus infection during differentiation and that infection results in apoptosis.
As previously demonstrated with BSC-1 cells (a primate kidney cell line) (21), infection with the highly virulent GDVII virus resulted in significant apoptosis in M1-D cells at a 1-log-lower MOI than for BeAn (data not shown).
BeAn virus binding.
Flow cytometry revealed BeAn virus binding to the surface of M1-D cells (0.5 to 1.0 log increase in mean fluorescence intensity) but not to M1 cells (background fluorescence) (Fig. 3A). In direct analysis of virus binding with 35S-labeled BeAn (Fig. 3B), the maximum binding to M1-D cells after 60 min was similar to that of permissive BHK-21 cells (40 to 50%) and approximately fivefold greater than that for the undifferentiated M1 cells (10%), consistent with the flow cytometry result. BeAn binding to M1-D cells rapidly increased with time, while binding to M1 cells remained close to background levels (5 to 10%), as measured in a variant, BeAn-uninfectable BHK-21 cell line developed in this laboratory (unpublished observations). These data suggest upregulation of the molecule(s) that binds BeAn virus in differentiated Mφs.
FIG. 3.
Surface binding of BeAn virus to M1 and M1-D cells. (A) Flow cytometry of cells incubated with BeAn virus for 45 min at 4°C and stained with a 1:1,000 dilution of polyclonal rabbit anti-BeAn virus antiserum followed by a 1:200 dilution of goat anti-rabbit Ig-FITC. Solid lines indicate staining with normal rabbit serum; dotted lines indicate staining with anti-BeAn antiserum. (B) 35S-labeled BeAn virus binding to M1 (□) and M1-D (◊) cells. Cells (106/ml) were incubated on ice for 1 h prior to the addition of 35S-labeled BeAn (20,000 particles/cell). At the indicated times, an aliquot of the suspension was removed and centrifuged at 12,000 × g for 30 s. The supernatant and cell-associated radioactivity were determined, and the percentage of cell-associated counts per minute was calculated by using Cricket Graph III software.
Expression of BeAn virus proteins.
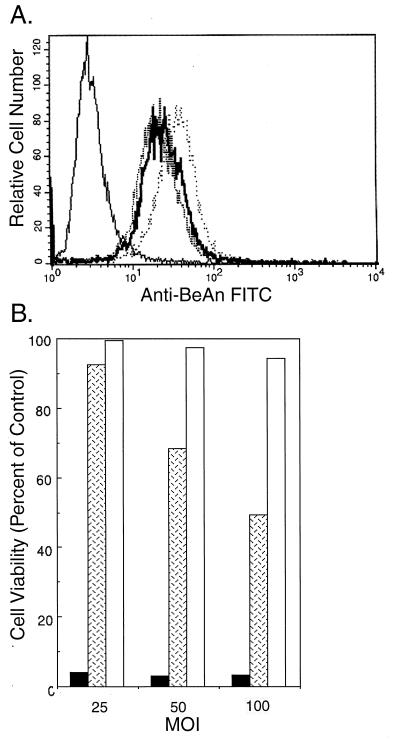
To obtain a relative measure of the virus antigen observed by fluorescence microscopy in BeAn-infected M1-D cells (Fig. 2B), cytoplasmic BeAn virus antigen was measured by flow cytometry 20 h after infection. When M1 and M1-D cells were permeabilized with 0.3% saponin and stained with a polyclonal rabbit anti-BeAn serum, only M1-D cells expressed large amounts (37.5-fold increase) of virus antigen (mean fluorescence intensities of M1 and M1-D cells, 0.8 and 30 relative fluorescence units, respectively [Fig. 4A]). Immunoprecipitation of virus proteins from BeAn-infected permissive BHK-21, M1, and M1-D cells with a polyclonal rabbit anti-BeAn serum (Fig. 4B) revealed similar amounts of radioactive virus capsid proteins from 106 cell equivalents of M1-D and permissive BHK-21 cells. Densitometric scans of the two immunoprecipitates revealed similar levels of intensity for each virus capsid protein in the two populations (data not shown). No virus antigen was precipitated in infected undifferentiated M1 cells (Fig. 4B) or in uninfected M1-D cells (data not shown). These data indicate that M1-D cells were infected and virus capsid proteins were generated at levels comparable to those in permissive BHK-21 cells.
BeAn virus RNA replication and production of infectious virus.
BeAn virus RNA replication was measured by incorporation of [3H]UdR in actinomycin D-treated M1 and M1-D cells; uninfected cells were assayed simultaneously to obtain background incorporation (Fig. 4C). Only BeAn-infected M1-D cells incorporated significant amounts of radioactivity above uninfected controls. The amount of radioactivity incorporated in M1 cells did not differ significantly in infected or uninfected cells, although background levels were higher than in M1-D cells. Compared to permissive BHK-21 cells, which routinely incorporate 30,000 to 50,000 cpm at the peak of virus RNA replication, M1-D cells were highly restricted, never exceeding 3,500 cpm with four different preparations of M1-D cells in six experiments. Nevertheless, virus RNA replication was, on average, 5.9-fold greater in infected M1-D than in M1 cells. Virus RNA levels in BHK-21 cells peaked between 10 and 12 h, increasing only slightly by 24 h (not shown), whereas the levels in M1-D cells peaked between 8 and 10 h and declined thereafter. It is possible that virus RNA is degraded in differentiated M1-D cells upon induction of apoptosis and activation of RNases (5, 6). Virus titers in infected cell populations at 20 h were low, ranging from 2 to 10 PFU/cell for M1-D cells and background levels for M1 cells. Thus, virus production in M1-D cells was present but highly restricted compared to permissive BHK-21 cells, which produce 70 PFU/cell 6 h after infection (20).
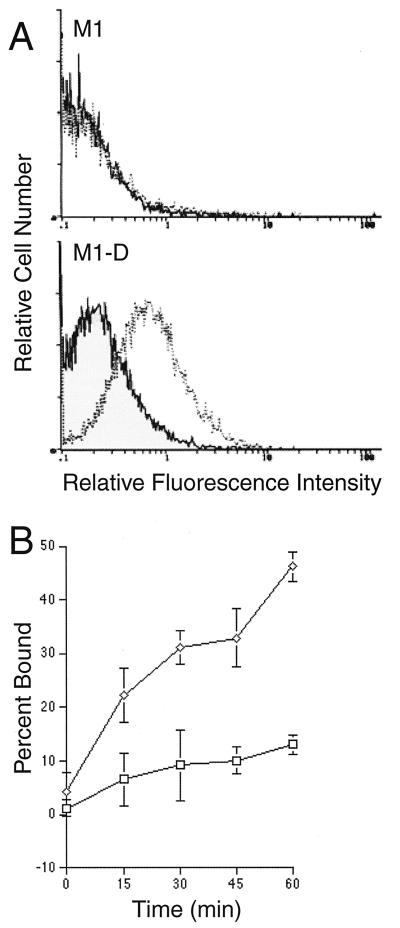
To demonstrate that virus replication is required for the induction of apoptosis, M1-D cells were infected with UV-inactivated BeAn virus. Cell surface binding of UV-inactivated BeAn virus to M1-D cells was similar to that of untreated virus (Fig. 5A). In fact, UV treatment for 20 min appeared to enhance virus binding somewhat. When assayed for cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (21) at high MOI (Fig. 5B), less than 5% of M1-D cells infected with untreated BeAn virus remained viable. UV inactivation for 1 min reduced cell death, with greater than 90% of the cells remaining viable at an MOI of 25; however, at an MOI of 100, cell viability was 50%. UV inactivation for 20 min essentially destroyed the virus’s ability to induce apoptosis even at an MOI of 100, with no change in the ability of the virus to bind to the surface of M1-D cells. These data indicate that virus RNA replication is necessary for the induction of apoptosis by BeAn virus.
FIG. 5.
UV-inactivated BeAn virus infection of M1-D cells. BeAn virus was UV inactivated for 1 or 20 min. (A) Flow cytometry of virus bound to the surface of unlabeled M1-D cells as in Fig. 3A. Solid thin line, uninfected control cells; solid thick line, untreated BeAn virus; dashed line, 1-min exposure to UV; dotted line, 20-min UV exposure. (B) MTT assay measuring cell viability 20 h after infection with untreated BeAn virus (black bars), 1-min UV exposure (hatched bars), and 20-min UV exposure (gray bars). Triplicate samples were averaged, and the results are expressed as percentages of values for uninfected control cells.
IFN-α/β secretion.
To test whether constitutive secretion of IFN-α/β contributed to resistance to (M1) or restriction of (M1-D) BeAn virus infection, levels of these cytokines were measured in supernatants of uninfected M1 and M1-D cells (Table 2). Supernatants from M1 precursor cells contained undetectable amounts of IFN-α/β (less than 10 U/ml), whereas ∼500 U/ml was detected in supernatants from M1-D cells. Secretion of IFN-α/β could be induced in both cell populations with IFN-γ treatment (100 U/ml). Thus, M1-D cells may already secrete sufficient levels prior to BeAn infection to slow virus RNA replication and allow cells time to respond by apoptosis. The morphological characteristics and increased IFN-α/β levels after IFN-γ treatment of M1-D cells resemble those of resident Mφs which can also be activated after an additional cytokine signal (4).
TABLE 2.
IFN-α/β production in M1 and M1-D cells before and after IFN-γ treatment
| Cells | IFN-α/β (U/ml)a |
|---|---|
| M1 | None detected |
| M1-D | 493 ± 13 |
| M1 + IFN-γ | 145 ± 16 |
| M1-D + IFN-γ | 4,572 ± 289 |
Supernatants from untreated or IFN-γ-treated M1 or M1-D cells were collected 48 h after treatment and assayed for IFN-α/β (see Materials and Methods). Data are means and standard deviations of triplicate samples and are representative of three separate experiments. The limit of detection was 10 U/ml.
Bcl-2, Bcl-XL, and Bax expression.
To determine whether Bcl-2 proteins, which regulate apoptosis (17), play a role in the sensitivity of M1-D cells to infection and apoptosis, Western blotting of lysates from cells before and after infection and IFN-γ treatment was performed with antibodies to Bcl-2, Bcl-X, and Bax (Fig. 6). Bcl-2 was expressed in M1 cells independent of infection or IFN-γ treatment; however, in M1-D cells, Bcl-2 expression was lost and not regained upon infection or IFN-γ treatment. The antiapoptotic Bcl-XL showed the opposite pattern of expression, i.e., high levels of M1-D cells and low levels of M1 cells regardless of infection or IFN-γ treatment. Expression of the proapoptotic Bcl-XS protein, an alternatively spliced variant of Bcl-X, was not observed (not shown). In contrast, the proapoptotic α isoform of Bax, whose expression has been shown to correlate with accelerated apoptosis (32), was found only in M1-D cells. Bax-β, whose function is unclear, was ubiquitously expressed under all conditions. However, in two independently derived populations of M1-D, IFN-γ treatment resulted in the loss of Bax-α expression. The significance of this finding is unknown. These data indicate that expression of the Bcl-2 family of proteins is modulated during differentiation or activation of M1 cells, consistent with their increased susceptibility to apoptosis (25).
FIG. 6.
Detection of Bcl-2 proteins by Western blot analysis of M1 and M1-D lysates. Proteins (20 μg/lane) were loaded onto 12% (A and B) or 15% (C) polyacrylamide gels, transferred to ProBlot membranes, and stained with 1:1,000 dilution of polyclonal rabbit antisera to Bcl-2 (A), Bcl-X (B), and Bax (C). The secondary antibody was peroxidase-labeled goat anti-rabbit Ig (1:1,000 dilution). Only undifferentiated M1 cells expressed high levels of Bcl-2. There was no change in expression of these proteins after infection (I). Differentiated M1-D cells expressed high levels of Bcl-XL. Bax-α was expressed only in M1-D cells, and expression was lost with IFN-γ treatment. Bax-β appeared to be ubiquitous. L929 cells are shown as a control. Western blots were repeated with two independently derived M1-D cell populations. Positions of molecular size markers (M) are shown on the left. U, uninfected.
To confirm the immunoblot results, RPA with the 35S-labeled mAPO-2 template set (PharMingen) was performed. Of the seven bcl-2 family members that are screened in this template set, only bcl-2, bclX, bak, and bax showed significant changes in mRNA expression (Fig. 7). Consistent with the above results, bcl-2 mRNA was expressed in M1 cells and bclX was expressed in M1-D cells. In addition to bax mRNA upregulation observed with immunoblotting, bak mRNA levels appear to be upregulated when assayed by RPA. Both bak and bax proteins have been reported to be proapoptotic (1). Bak protein expression was not tested by immunoblotting.
FIG. 7.
RPA for the bcl-2 family. α-35S-labeled mAPO-2 probe (PharMingen) was used to protect seven bcl-2 family RNAs in M1 and M1-D cells. The radioactively labeled probe was used to identify each band as marked.
DISCUSSION
Our data using an in vitro model of Mφ differentiation are consistent with the hypothesis that the state of Mφ differentiation influences the susceptibility of these cells to infection and apoptosis. Previous experiments using the highly virulent GDVII virus and transformed macrophage cell lines revealed that the M1 cells were resistant to infection, whereas two other macrophage cell lines were susceptible (20). The M1 cell line allowed us to ask the specific question of whether direct differentiation of a myeloid precursor cell line in vitro affected the cells’ ability to be infected. Preliminary experiments using both GDVII and BeAn virus to infect M1-D cells showed results similar to previously published data using BSC-1 cells (21).
After exposure to conditioned medium, the myeloid precursor M1 cells acquired characteristics similar to those of resident Mφs (Fig. 1; Table 1). At this stage of differentiation, M1-D cells were observed to bind BeAn virus, become infected, and undergo apoptosis. The data suggest that virus entry into undifferentiated M1 cells is blocked at the receptor level. Proof that M1 cells can support BeAn virus infection and die by apoptosis awaits transfection of BeAn virus RNA into these cells, although to date, transfection efficiencies (<1.0%) have been too low for analysis.
Infection at an MOI of 10 statistically guarantees that each cell is infected with at least one infectious virus particle. The flow cytometry data for infected M1-D cells indicate that 70 to 90% of the cells contain virus antigen. This discrepancy could be due to (i) a low estimate of the antigen-containing cells by flow cytometry or (ii) a resistant subpopulation of cells. Microscopic examination of infected M1-D cells indicates that nearly 90% of the cells contain virus antigen. Since these cells are neither clonal nor synchronized in their cell cycle, there may be a subpopulation of M1-D cells that are resistant to infection. This observation is being investigated further.
Other viruses in which Mφ differentiation determines susceptibility to infection have been described: visna virus infects Mφs but not monocytes (13), equine infectious anemia virus RNA is found only in resident Mφs and not in peripheral blood monocytes (41), herpes simplex virus type 1 infects differentiated human U937 monocytoid cells but not undifferentiated cells (45), and Puumula virus produces more infectious virus in differentiated U937 than in undifferentiated cells (44). However, our system reveals an interesting paradox paralleling TMEV persistence in the CNS of susceptible mice. Although virus antigen was readily observed in the cytoplasm of the infected M1-D cells (Fig. 2, 4A, and 4B), viral RNA and production of infectious virus particles were highly restricted. This finding suggests that abundant virus capsid proteins are synthesized without assembly into infectious virus particles. There are several possible explanations for this observation, including insufficient transcription of viral RNA molecules for packaging into viral particles, defective assembly of capsid proteins into packaging intermediates, i.e., protomers and/or pentamers, and/or a block in virus RNA replication and virion assembly by an as yet unidentified cellular mechanism, perhaps related to apoptosis. Experiments to define the relevant mechanisms are being pursued in our laboratory. Preliminary experiments suggest that virus assembly in M1-D cells is altered.
It is also possible that M1 cells are inherently resistant to BeAn virus-induced apoptosis due to a high level of Bcl-2 expression (Fig. 5). Lotem and Sachs (25) have also reported that M1 cells express Bcl-2 and resist apoptosis induction by adriamycin and cycloheximide. Recently, Van Der Vliet et al. (47) showed that neutrophils in human peripheral blood had high Bax/Bcl-2 ratios, while monocytes and lymphocytes had relatively low ratios, and that susceptibility to anti-Fas-induced apoptosis correlated with the ratios. These data suggest that monocytes, in vivo, are inherently more resistant to apoptosis than neutrophils.
We examined protein expression of only 3 of the 15 known Bcl-2 family members based on previous data (23). Our results showing that undifferentiated cells express Bcl-2, while differentiated cells express Bcl-XL, are consistent with those of Lotem and Sachs (25). However, in contrast to that study, we observed upregulation, not downregulation, of Bax-α protein expression. This discrepancy may be explained by the difference in either differentiation protocols or detection methods. In addition, RPA confirmed the immunoblot results, including upregulation of bax. An additional proapoptotic RNA, bak, appeared to be upregulated by RPA analysis as well. Our data are consistent with an increased susceptibility to apoptosis after differentiation, although we cannot exclude a potential role for other members of the Bcl-2 family. However, we can exclude a regulatory role for Bcl-2, since overexpression of bcl-2 in M1-D cells did not affect the ability of the virus to induce apoptosis (data not shown).
A possible mechanism for the maintenance of an inflammatory response despite low virus titers is suggested by the fact that virus capsid proteins were abundant in apoptotic M1-D cells. It is possible that virus antigens contained in apoptotic corpses are phagocytized by macrophages and made available for presentation to T cells. Bellone et al. (5) recently reported that antigen-specific cytotoxic T cells were activated by peritoneal macrophages that phagocytized antigen-expressing apoptotic cells. An earlier study (35) reported the detection of Sindbis virus glycoproteins in the surface blebs of apoptotic infected HeLa cells. Recently dendritic cells have been shown to acquire antigen from apoptotic cells and present them to both class I and class II-restricted T cells (2, 37). Therefore, it seems possible that phagocytosis of virus protein-containing Mφs by MHC class II-expressing Mφs or CNS microglia could maintain an immune response with very few productively infected cells but with sufficient virus antigen to trigger a T-cell response. This possibility is being tested in assays using apoptotic M1-D cells as phagocytic targets for peritoneal macrophages from TMEV-susceptible mice.
ACKNOWLEDGMENTS
This work supported by NIH grant NS21913 and the Leiper Foundation.
We thank Shannon Hertzler for the radioactive virus binding data and Shiaolan Yang and George Twaddle for helpful discussions.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family:arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 3.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 4.Auger M J, Ross J A. The biology of the macrophage. In: Lewis C E, McGee J O, editors. The natural immune system: the macrophage. New York, N.Y: IRL Press; 1992. pp. 3–74. [Google Scholar]
- 5.Bellone M, Iezzl G, Rovere P, Galati G, Ronchetti A, Protti M P, Davoust J, Rugarli C, Manfredi A A. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol. 1997;159:5391–5399. [PubMed] [Google Scholar]
- 6.Castelli J C, Hassel B A, Wood K A, Li X, Amemiya K, Dalakas M C, Torrence P F, Youle R J. A study of the interferon antiviral mechanism:apoptosis activation by the 2-5A system. J Exp Med. 1997;186:967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlu C, Lee F. IL-6 is a differentiation factor for M1 and WEHI-3B myeloid leukemic cells. J Immunol. 1989;142:1909–1915. [PubMed] [Google Scholar]
- 8.Clatch R J, Lipton H L, Miller S D. Characterization of Theiler’s murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986;138:920–926. [PubMed] [Google Scholar]
- 9.Clatch R J, Miller S D, Metzner R, Dal Canto M C, Lipton H L. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- 10.daSilva R P, Platt N, deVilliers W J S, Gordon S. Membrane molecules and macrophage endocytosis: scavenger receptor and macrosialin as markers of plasma-membrane and vacuolar functions. Biochem Soc Trans. 1996;24:221–224. doi: 10.1042/bst0240220. [DOI] [PubMed] [Google Scholar]
- 11.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 12.Fotladis C, Kilpatrick D R, Lipton H L. Comparison of the binding characteristics to BHK-21 cells of viruses representing the two Theiler’s virus neurovirulence groups. Virology. 1991;182:365–370. doi: 10.1016/0042-6822(91)90683-3. [DOI] [PubMed] [Google Scholar]
- 13.Gendelman H E, Narayan O, Kennedy-Stoskopf S, Kennedy P G E, Ghotbi Z, Clements J E, Stanley J, Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986;58:67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerety S J, Karpus W J, Cubbon A R, Goswaml R G, Rundell M K, Peterson J D, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 15.Gerety S J, Rundell K M, Dal Canto M C, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 16.Gothelf Y, Raber J, Chen L, Schattner A, Chebath J, Revel M. Terminal differentiation of myeloleukemic M1 cells induced by IL-6: role of endogenous interferon. Lymphokine Cytokine Res. 1991;10:369–375. [PubMed] [Google Scholar]
- 17.Hawkins C J, Vaux D L. The role of the Bcl-2 family of apoptosis regulatory proteins in the immune system. Semin Immunol. 1997;9:25–33. doi: 10.1006/smim.1996.0052. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1969;74:223–234. doi: 10.1002/jcp.1040740303. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa Y. Further studies on the differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1970;76:175–184. doi: 10.1002/jcp.1040760207. [DOI] [PubMed] [Google Scholar]
- 20.Jelachich M L, Bandyopadhyay P, Blum K, Lipton H L. Theiler’s virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 21.Jelachich M L, Lipton H L. Theiler’s murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasukabe T, Okabe-Kado J, Honma Y, Hozuml M. Interleukin-4 inhibits the differentiation of mouse myeloid leukemia M1 cells induced by dexamethasone, D-factor/leukemia inhibitory factor and interleukin-β, but not by 1α,25-dihydroxyvitamin D3. FEBS Lett. 1991;291:181–184. doi: 10.1016/0014-5793(91)81278-g. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 24.Lipton H L, Twaddle G, Jelachich M L. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotem J, Sachs L. Regulation of bcl-2, bcl-XL, and bax in the control of apoptosis by hematopoietic cytokines and dexamethasone. Cell Growth Differ. 1995;6:647–653. [PubMed] [Google Scholar]
- 26.Mangan D F, Wahl S M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and proinflammatory cyokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 27.Mangan D F, Welch G R, Wahl S M. Lipopolysaccharide, tumor necrosis factor-a, and IL-1b prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 28.McKnight A J, Mcfarlane A J, Dir P, Turley L, Willis A C, Gordon S. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane hormone receptor family. J Biol Chem. 1997;271:486–489. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 29.Michishita M, Yoshido Y, Uchino H, Nagata K. Induction of tumor necrosis factor-a and its receptors during differentiation in myeloid leukemic cells along the monocytic pathway: a possible regulatory mechanism for TNF-a production. J Biol Chem. 1990;265:8751–8759. [PubMed] [Google Scholar]
- 30.Miller S D, Clatch R J, Pevear D C, Trotter J L, Lipton H L. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Cross-specificity among TMEV substrains and related picornaviruses, but not myelin proteins. J Immunol. 1987;138:3776–3784. [PubMed] [Google Scholar]
- 31.Miller S D, Gerety S J, Kennedy M K, Peterson J D, Trotter J L, Touhy V K, Waltenbaugh C, Dal Canto M C, Lipton H L. Class-II restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1989;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 33.Onozaki K, Tamatani T, Hashimoto T, Matsushima K. Growth inhibition and augmentation of mouse myeloid leukemic cell line differentiation by interleukin-1. Cancer Res. 1987;47:2397–2402. [PubMed] [Google Scholar]
- 34.Onozaki K, Urawa H, Tamatani T, Iwamura Y, Hashimoto T, Baba T, Suzuki H, Yamada M, Yamamoto S, Oppenheim J J, Matsushima K. Synergistic interactions of interleukin 1, interferon-b, and tumor necrosis factor in terminally differentiating a mouse myeloid leukemic cell line (M1): evidence that interferon-b is an autocrine differentiating factor. J Immunol. 1997;140:112–119. [PubMed] [Google Scholar]
- 35.Rosen A, Casciola-Rosen L, Ahearn J. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995;181:1557–1561. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi C P, Delcroix M, Huitinga I, McAllister A, van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler’s virus infection. J Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rovere P, Vallinoto C, Bondanza A, Crosti M C, Ciardi-Castagnoll P, Rugarli C, Manfredl A A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 38.Rozhon E J, Kratochvil J D, Lipton H L. Analysis of genetic variation in Theiler’s virus during persistent infection in the mouse central nervous system. Virology. 1983;128:16–32. doi: 10.1016/0042-6822(83)90315-x. [DOI] [PubMed] [Google Scholar]
- 39.Rubinstein S, Famillietti P C, Pestka S. Convenient assay for interferons. J Virol. 1981;37:755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruhl S, Pluznik D H. Dissociation of early and late markers of murine myeloid differentiation by interferon-gamma and interleukin-6. J Cell Physiol. 1993;155:130–138. doi: 10.1002/jcp.1041550117. [DOI] [PubMed] [Google Scholar]
- 41.Sellon D C, Perry S T, Coggins L, Fuller F J. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J Virol. 1992;66:5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shabo Y, Lotem J, Rubinstein M, Revel M, Clark S C, Wolf S F, Kamen R, Sachs L. The myeloid blood cell differentiation-inducing protein MGI-2A is interleukin-6. Blood. 1988;72:2070–2073. [PubMed] [Google Scholar]
- 43.Springer T, Galfre G, Secher D S, Milstein C. A Mac-1 macrophage differentiation antigen identified by a monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 43a.Steinberg, T. (Washington University School of Medicine, St. Louis, Mo.). Personal communication.
- 44.Temonen M, Lankinen H, Vapalahti O, Ronni T, Julkunen I, Vaheri A. Effect of interferon-α and cell differentiation on Puumala virus infection in human monocyte/macrophages. Virology. 1995;206:8–15. doi: 10.1016/s0042-6822(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 45.Tenney D J, Morahan P S. Effects of differentiation of human macrophage-like U937 cells on intrinsic resistance to herpes simplex type 1. J Immunol. 1987;139:3076–3083. [PubMed] [Google Scholar]
- 46.Tauda H, Neckers L M, Pluznik D H. Colony stimulating factor-induced differentiation of murine M1 myeloid leukemia cells is permissive in early G1 phase. Proc Natl Acad Sci USA. 1986;83:4317–4321. doi: 10.1073/pnas.83.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Der Vliet H J, Wever P C, Van Diepen F N, Yong S L, Ten Berge I J. Quantification of Bax/Bcl2-ratios in peripheral blood lymphocytes, monocytes and granulocytes and their relation to susceptibility to anti-Fas (anti-CD95)-induced apoptosis. Clin Exp Immunol. 1997;110:324–328. doi: 10.1111/j.1365-2249.1997.tb08335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]
- 49.Yodoi J, Masuda T, Miyama M, Maeda M, Ichikawa Y. Interaction of lymphocytes and macrophage cell line cells (M1 cells). 1. Functional maturation and appearance of Fc receptors in M1 cells. Cell Immunol. 1978;39:5–17. doi: 10.1016/0008-8749(78)90077-1. [DOI] [PubMed] [Google Scholar]