Abstract
Background:
Data from controlled laboratory experiments in adults indicate that the subjective effects of cannabis vary by administration method (e.g., combustible, vaporized). Whether the subjective effects of cannabis experienced in the natural ecology and among adolescents differ by cannabis administration method is unknown. In this observational study, adolescents’ retrospective reports of subjective effects after combustible, edible, and vaporized cannabis use were examined.
Methods:
Students from ten public schools in Los Angeles, CA, USA (M[SD] age = 16.1 [.43] years) who reported past 6-month use of combustible, edible, or vaporized cannabis (N = 584) were surveyed on subjective effects experienced after use (yes/no). They were provided with a 12 item self-report checklist of six positive (e.g., relaxed, energetic) and six negative (e.g., drowsy, lazy) subjective effects. For each method of administration, affirmative responses were summed in positive (range: 0–6) and negative (range: 0–6) effect composite scores.
Results:
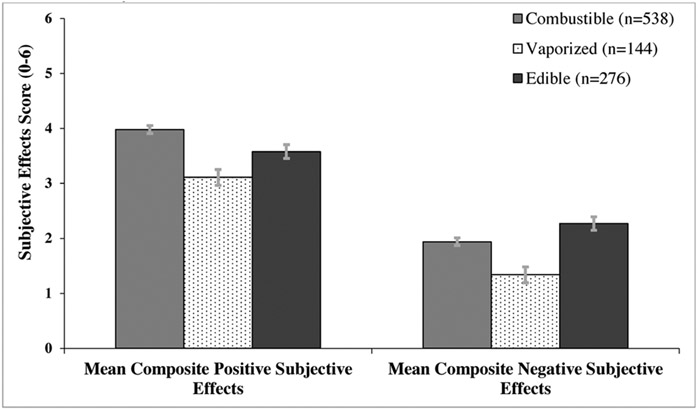
Generalized estimating equations adjusted for demographics and recent cannabis use revealed a graded pattern of differences in positive subjective effects across products, with highest scores for combustible (M[SD] = 3.98[1.76]), followed by edible (M[SD] = 3.58 [2.04]) and vaporized (M[SD] = 3.11 [2.21]) cannabis (all pairwise cross-product contrasts p < .01). Mean negative effect score was highest for edible (M[SD] = 2.27 [1.95]), followed by combustible (M[SD] = 1.94 [1.66]), and vaporized (M[SD] = 1.34 [1.73]) cannabis, respectively (all pairwise contrasts p < .02).
Conclusion:
Adolescents’ reports of subjective effects varied across cannabis administration methods. Combustible cannabis’ more desirable subjective effects profile might be indicative of higher abuse liability.
Keywords: Cannabis, Subjective effects, Adolescents, Combustible, Vaporized, Edible
1. Introduction
Cannabis use during adolescence is linked to harmful health consequences (Curran et al., 2016; Rasic et al., 2013; Renard et al., 2014). With expanding legalization of medical and recreational cannabis, there have been increases in the availability of alternative (non-combusted) methods of cannabis administration, such as vaporized (i.e., inhalable cannabis plant or oil aerosol emitted by an electronic heating device) and edible (i.e., cannabis-infused food or drinks) products (Borodovsky et al., 2016). Alternative cannabis administration methods may appeal to adolescents because of their availability in youth-friendly palatable preparations (e.g., cannabis-infused gummy bears and candy-flavored vaporizer solutions that include cannabis extracts) (MacCoun and Mello, 2015). While recent estimates suggest appreciable portions of youth use vaporized or edible cannabis products (e.g.; nationally, past 30-day vaping of cannabis rose from 4.9% in 2017 to 7.5% in 2018 among 12th grade students; survey data from California in 2017 indicates that more than 80% of youth who used cannabis in the past 30 days used edibles) (Borodovsky et al., 2017; Friese et al., 2017; Johnston et al., 2019; Morean et al., 2015; Peters et al., 2018) little is known about whether different methods of administration of cannabis affect key outcomes of relevance to basic addiction science and public health, such as differences in abuse liability and dependence on cannabis.
Subjective responses to drugs are a key component in deciphering the likelihood of future problematic use of psychoactive compounds (Carter and Griffiths, 2009). There are positive (e.g., pleasant feelings) as well as negative effects (e.g., difficulty concentrating, anxiety), and the quality and severity of effects may be critical for mediating risk of several adverse health outcomes, including cannabis use disorder, impaired driving and psychiatric disorders (de Wit and Phillips, 2012). Furthermore, subjective responses are indicators of the basic psychopharmacological mechanisms underlying the biobehavioral effects of drugs of abuse (Fischman and Foltin, 1991; Treloar Padovano and Miranda, 2018).
While of importance to addiction science and prevention, evidence of subjective effects across cannabis products are limited. Controlled laboratory experiments in adult cannabis users show that acute positive subjective effects indicative of abuse potential as well as negative effects that may deter future use (e.g., difficulty concentrating, anxiety) can vary dependent on whether cannabis is delivered by combustible, vaporized, or edible administration methods (Lee et al., 2016; Newmeyer et al., 2017; Spindle et al., 2018). Differences in pharmacokinetics across methods of administration may alter the corresponding subjective effects of different products (e.g., higher blood concentration of THC resulting from vaporized versus combustible might make the former method more reinforcing) (Spindle et al., 2018). Although possessing strong internal validity, such experiments are limited in ecological validity due to being conducted in controlled laboratory environments and with researcher-provided cannabis products that may not be entirely representative of products available in the natural ecology (Steigerwald et al., 2018).
Surveys of retrospectively reported subjective effects experienced during drug use episodes in the natural ecology have been used successfully and associated with relevant health outcomes (e.g., cannabisuse disorder), yet have been circumscribed and includes only adult established users (Fergusson et al., 2003; Lee et al., 2017, 2016; Zeiger et al., 2010, 2012). For the purposes of estimating the abuse liability of cannabis products, it is important to understand subjective effects early in the cannabis use uptake trajectory, which typically occurs in adolescence—a period in which the developing brain may be particularly vulnerable to the effects of cannabinoids (Volkow et al., 2014). Whether the subjective effects of combustible, edible, and vaporized cannabis differ among adolescents is unknown.
This cross-sectional observational study compared the subjective effects of combustible, edible, and vaporized cannabis based on adolescents’ retrospective reports of experiences using each product. The primary aim was to examine broad dimensions of subjective effects measured by composite indicators of positive (e.g., energetic, relaxed) and negative (e.g., drowsy, unable to concentrate) subjective effects.
2. Methods
2.1. Participants and procedure
Study data were drawn from the Health and Happiness Study, a survey of substance use and mental health of students from 10 high schools in the Los Angeles metropolitan area (described previously; (Leventhal et al., 2015). All 9th grade students enrolled in the participating schools in 2013 were eligible (N = 4100), with a total of 3396 (82.8%) who assented and provided parental consent to participate in the study. Students completed paper-and-pencil surveys in their classrooms on site biennially. Those absent at data collections completed shortened surveys by telephone, Internet, or mail. Questions on edible and vaporized cannabis subjective effects were first included in Spring 2015 (10th grade), which are the data used in this report; questions on combustible cannabis subjective effects were included from the start. Of the 3177 students who completed the 10th grade survey, 584 (18.4%) had used at least one method of administration form of cannabis in the past 6 months and reported subjective effects over this time frame, constituting the analytic sample for this study. The study was approved by the University of Southern California Institutional Review Board.
2.2. Measures
2.2.1. Sociodemographic characteristics
Participants self-reported their gender, age, race/ethnicity (response options: White, Black, Hispanic, Asian, Multiethnic or Multiracial, or other), highest education of either parent (some college or less vs. college degree or higher), and eligibility for free/subsidized school lunch program (yes/no; yes indicates family income ≤ 185% of federal poverty limit). Education and income variables were combined to create a socioeconomic status (SES) composite variable, for which high SES was defined as youth who reported their parents attended college or higher education level and being ineligible for free/subsidized school lunch program as reported in prior work (Peters et al., 2018).
2.2.2. Cannabis use
Questions regarding cannabis use were developed from the Youth Risk Behavior Surveillance Survey (Eaton et al., 2008) and the Monitoring the Future Questionnaire (Johnston et al., 2018). Students were provided the instructional stem question, “Have you ever used the following substances?,” followed by a list of substances, including 3 separate items for combustible, edible, and vaporized cannabis, assessed independently (response options: “no”; “yes, but not in the last 6 months”; or “yes, in the last 6 months.”). There were also items assessing “In the last 30 days, how many total days have you used…?” (response options: 0, 1–2, 3–5, 6–9, 10–19, 20–29, and 30 days) for each method of cannabis administration, which was used to generate a binary past 30-day use indicator. Combustible cannabis use was phrased as “smoking marijuana.” Edible cannabis use was listed as “marijuana or THC food or drinks.” Vaporized cannabis use was worded as “electronic device to vape liquid THC or hash oil.”
2.2.3. Subjective effects
Questions regarding subjective effects of cannabis use were derived from the Modified Lyons Battery for Subjective Effects (MLBSE) (Lyons et al., 1997). The measure provides a checklist instructing participants to indicate whether use of the respective cannabis product over the previous 6 months resulted in any of 6 positive (i.e., Pleasant/Happy, Relaxed, Energetic, Increased Sex Drive, Creative, Social) and 6 negative (i.e., Drowsy, Unable to Concentrate, Dizzy, Out of Control, Lazy, Upset Stomach) effects, each rated yes/no (e.g., “Vaping marijuana made me feel pleasant/happy” [yes/no]). Composite scores were computed by summing the number of positive (range: 0–6) and negative (range: 0–6) effects, which were used as the primary outcomes for the study, consistent with previous studies (Lyons et al., 1997; Zeiger et al., 2010). Secondary outcomes included each of the 12 individual subjective effect items. The MLBSE for combustible cannabis has demonstrated adequate psychometric properties in previous adolescent and young adult samples (Haberstick et al., 2011).
2.3. Statistical analysis
Following descriptive results, the primary analysis used generalized estimating equation (GEE) regression models (Zeger et al., 1988) to evaluate comparisons across the three methods of administration (combustible vs. vaporized vs. edible) in subjective effect outcomes. With this approach, 1–3 outcome observations were available for each participant depending on which of the 3 cannabis products they used, and within-student clustering of the data was modeled in GEEs by an exchangeable correlation matrix. Linear regression GEE models were used for the positive and negative composite score outcomes. Binary logistic regression GEE models were used for the secondary analysis of individual subjective effect items. All models included gender, race/ethnicity, SES, age at baseline, school, number of days of cannabis use in past 30-day, and age of onset of cannabis use. Analyses utilized all observations with available subjective effect outcome data. Missing covariate data were managed with multiple imputation (Rubin, 2004). Adjusted B-weights (Bs) and Odds Ratios (ORs) with 95% Confidence Intervals (CIs) are reported from the linear and logistic regression models, respectively, indicative of estimates of differences in outcomes for each pairwise comparison between the three methods of cannabis administration. Analyses were conducted using SPSS version 23 (IBM SPSS Inc, Armonk, NY, USA). Additional sensitivity analyses were conducted and are detailed below. Raw p-values from two-tailed tests were considered statistically significant after correction for multiple testing using the Benjamini-Hochberg procedure to maintain a study-wide false discovery rate of 0.05 (Benjamini and Hochberg, 1995).
3. Results
3.1. Participants
The analytic sample of 584 students reporting past 6-month use of at least one form of cannabis (Mean age [SD] = 16.13[.43] years, 55% female, 65.7% low SES]) was racially/ethnically diverse (Hispanic, 56.3%; White, 17.4%; Asian, 7.3%; Black, 5.4%; Multiethnic/Multiracial 6.1%; Other, 7.5%). Demographics and past 30-day use of each respective product are reported in Table 1 within the subsamples of users of each cannabis product (combustible, N = 538; edible, N = 276; or vaporized, N = 144; groups are overlapping due to presence of poly-product users of more than 1 product). Patterns of dual and tri-product use in the sample are reported in Supplemental Fig. 1. To describe differences between student users in the sample and the remainder the cohort, we conducted descriptive comparisons which found that past 6-month cannabis users (vs. those excluded from sample) had a higher proportion of past 30-days users of any form of cannabis, low-SES as well as different distributions of race/ethnicity (see Supplemental Table 1).
Table 1.
Demographic Characteristics among Past 6-month Users of Combustible, Edible, and Vaporized Cannabisa.
| Combustible (N = 538) |
Edible (N = 276) |
Vaporized (N = 144) |
|
|---|---|---|---|
| Female gender | 289 (53.7) | 139 (50.4) | 64 (44.4) |
| Age, M (SD) | 16.15 (.44) | 16.15 (.44) | 16.11 (.46) |
| SESb,c | |||
| High | 159 (34.2) | 84 (35.3) | 50 (41.3) |
| Low | 306 (65.8) | 154 (64.7) | 71 (58.7) |
| Race/Ethnicityd | |||
| Asian | 38 (7.2) | 16 (5.9) | 8 (5.7) |
| Black | 28 (5.3) | 12 (4.5) | 6 (4.3) |
| Hispanic | 301 (57.0) | 151 (57.2) | 75 (53.2) |
| White | 93 (17.6) | 45 (16.7) | 30 (21.3) |
| Multiethnic or multiracial | 30 (5.7) | 20 (7.4) | 13 (9.2) |
| Othere | 38 (7.2) | 22 (8.2) | 9 (6.4) |
| Past 30-day use of any form of cannabisf | 358 (67.4) | 173 (63.1) | 83 (57.6) |
Note. aData provided for past 6-month users for each method of cannabis administration; the three groups are not mutually exclusive due to poly-use of multiple products (see supplemental Fig. 1). Total number of users of at least one methods of administration N = 584. Data are expressed as No. (%) unless otherwise indicated. bHigh SES is defined as parental education equal or higher than some college degree and family income higher than 185% the US poverty line (i.e., respondents who are not eligible for free or reduced lunch). Low SES is defined as the other respondents.
3.2. Differences in Cannabis subjective effects, by method of administration
3.2.1. Positive effects
There was a graded pattern whereby the mean positive effects composite scores significantly differed in each pairwise comparison across the three administration methods (Ps ≤ .01), with highest scores for combustible (M[SD] = 3.98[1.76]), followed by edible (M[SD] = 3.58 [2.04]) and vaporized (M[SD] = 3.11 [2.21]) cannabis, respectively in covariate-adjusted linear regression models (see Fig. 1).
Fig. 1.
Mean Composite Positive and Negative Subjective Effects Scores of Cannabis in the Past 6 Months, By Method of Administration.
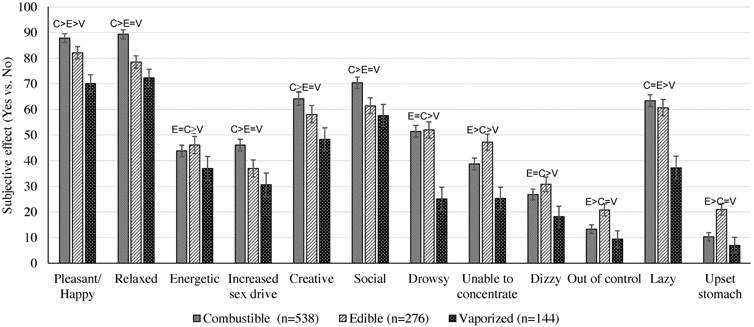
Regarding specific positive subjective effects, across products, ‘relaxed’ and ‘pleasant/happy’ were more commonly reported than ‘energetic’, ‘increased sex drive’, ‘creative’ and ‘social’. Secondary analyses of the prevalence of reporting each of the 6 different positive effects, by administration method, and results of adjusted logistic regression models contrasting the odds of reporting each effect, are depicted in Fig. 2. With the exception of positive subjective effect item ‘energized’, item-level analyses showed significantly or non-significantly greater odds reporting each of the positive subjective effects for combustible followed by edible, and then vaporized (see Table 2).
Fig. 2.
Subjective Cannabis Effects in Past 6 Months, by Method of Cannabis Administrationa.
Note. aPercentages and standard errors of subjective effects by different methods of cannabis administration. Differences across methods of cannabis administration were adjusted for school effects, demographic characteristics of respondents (i.e., age, gender, race/ethnicity, and SES), and past 30-day use of cannabis (yes or no). Notations above bars represent pairwise contrast results, with > symbol indicating statistically significant difference in subjective effect between methods of administration, and = symbol indicating non-statistically significant difference in subjective effect between methods of administration.
Table 2.
Differences in Subjective Cannabis Effects in Past 6 Months, by Method of Administrationa.
| Outcomes | Pairwise contrast of subjective effect by method of administration | |||||
|---|---|---|---|---|---|---|
| Combustible vs. Vaporized | Edible vs. Vaporized | Combustible vs. Edible | ||||
| Estimateb,c (95% CI) | P Value | Estimateb,c (95% CI) | P Value | Estimateb,c (95% CI) | P Value | |
| Subjective effect composite scores (primary outcomes)b | ||||||
| Positive effects composite | .68 (.36, 1.00) | < .001* | .41 (.09, .73) | .01* | .27 (.04, .50) | .02* |
| Negative effects composite | .56 (.27, .84) | < .001* | .92 (.62, 1.22) | < .001* | −.37 (−.59, −.15) | .001* |
| Item-level ratings (secondary outcomes)c | ||||||
| Positive effects | ||||||
| Pleasant/Happy | 3.50 (2.22, 5.52) | < .001* | 2.32 (1.52, 3.53) | < .001* | 1.51 (1.02, 2.25) | .04 |
| Relaxed | 3.72 (2.36, 5.87) | < .001* | 1.90 (1.22, 2.96) | <.001* | 1.96 (1.30, 2.94) | .001* |
| Energetic | 1.44 (.93, 2.26) | .10 | 1.74 (1.11, 2.73) | .02* | .83 (.62, 1.11) | .21 |
| Increased sex drive | 1.83 (1.18, 2.85) | .01* | 1.38 (.88, 2.17) | .16 | 1.33 (.99, 1.77) | .06 |
| Creative | 2.03 (1.30, 3.18) | .002* | 1.60 (1.03, 2.51) | .04 | 1.27 (.92, 1.74) | .14 |
| Social | 1.70 (1.11, 2.61) | .02* | 1.25 (.83, 1.88) | .28 | 1.35 (.98, 1.88) | .07 |
| Negative effects | ||||||
| Drowsy | 3.27 (2.12, 5.04) | < .001* | 3.47 (2.22, 5.42) | < .001* | .94 (.70, 1.26) | .68 |
| Unable to concentrate | 1.95 (1.28, 2.97) | .002* | 2.89 (1.87, 4.48) | < .001* | .67 (.50, .91) | .01* |
| Dizzy | 1.61 (.98, 2.65) | .06 | 1.92 (1.17, 3.17) | .01* | .84 (.61, 1.15) | .28 |
| Out of control | 1.64 (.81, 3.30) | .17 | 3.12 (1.53, 6.38) | .002* | .53 (.37, .76) | .001* |
| Lazy | 3.35 (2.21, 5.07) | < .001* | 2.93 (1.89, 4.52) | < .001* | 1.14 (.84, 1.55) | .39 |
| Upset stomach | 1.48 (.70, 3.13) | .31 | 3.36 (1.57, 7.17) | .002* | .44 (.30, .65) | < .001* |
Note. aEstimates from generalized estimating equation (GEE) models predicting subjective effects from administration method, adjusted for school effects, respondents demographics (i.e., age, gender, race/ethnicity, and SES), number of days of cannabis use in past 30-day, and age of onset of cannabis use. bFor composite scores of positive and negative effects, linear regression models were used and unstandardized linear regression parameters (Bs) are shown. cFor individual subjective effects, binary logistic regression models were used odds ratios (ORs) are shown. * Indicates statistical significance after correction using the Benjamini-Hochberg procedure.
3.2.2. Negative effects
In adjusted linear regression models, mean negative effects composite scores significantly differed in each pairwise comparison across the three administration methods (Ps ≤.02), with highest scores for edible (M[SD] = 2.27 [1.95]), followed by combustible (M[SD] = 1.94 [1.66]), and vaporized (M[SD] = 1.34 [1.73]) cannabis, respectively (see Fig. 1). Regarding specific negative subjective effects, across products, ‘unable to concentrate’ was more commonly reported than ‘drowsy’, ‘out of control’, ‘lazy’, ‘upset stomach’ and ‘dizzy’. Analyses of each of the individual six negative effects found results generally consistent with the overall negative effect composite (see Table 2), with greater odds of experiencing a negative effect for edible, followed by combustible, and then vaporized, respectively, although some of the item-level comparisons were not statistically significant (see Fig. 2 and Table 2).
3.3. Sensitivity analysis
Use of the three products was unevenly distributed throughout the sample (e.g., 25% of sample provided subjective effect data on vaporized cannabis, whereas 92% provided combustible cannabis subject effect data). It is therefore possible that individual differences in factors that contribute to cannabis use subjective effect expression (e.g., genetics, other substance use, personality traits) and promote liability to use one cannabis product versus another may confound differences in subjective effects by administration method observed. Among tri-product users of cannabis by all three methods (N = 113), however, confounding by inter-individual differences is constrained because participants are equivalently represented across the three methods of administration and each participant reflect their own ‘control’ akin to a cross-over design. We tested whether the results differed between tri-product users (N = 113) and dual/single product users (N = 471) with formal tri-product use status x administration method interactions. No significant interactions were found for any pairwise contrasts, indicating no evidence that the pattern of differences in subjective effects by cannabis administration differed between the tri-product user group for whom confounding is constrained and the remainder of the sample.
4. Discussion
This survey of 584 adolescent cannabis product users provides new evidence that adolescents’ report of their subjective responses to cannabis in the natural ecology varied across combustible, edible, and vaporized administration methods. Study main findings are: (1) Combustible cannabis generally resulted in a more desirable subjective effect profile, producing the most positive effects and a moderate number of negative effects, on average; (2) Edible cannabis produced more aversive subjective effects than the other two products in this sample; and (3) Vaporized cannabis produced fewer effects (both positive and negative) than the other two products.
Previous studies are restricted to laboratory cannabis administration experiments in adults (Chait and Zacny, 1992; Newmeyer et al., 2017; Spindle et al., 2018), with unknown generalizability to adolescent populations in the community. Studies addressing user-reported cannabis experiences in the natural ecology typically ask poly-product users’ opinions of whether they prefer the high or taste of one product versus another (Etter, 2015; Lee et al., 2016; Malouff et al., 2014). To our knowledge, the present study is the first to directly assess and characterize the subjective effects of alternative cannabis product use in youth, providing data on the incidence of 12 different subjective effects for each of three cannabis administration methods.
The vaporized cannabis findings reported here do not align with a previous laboratory drug administration experiment in adults, which found that both positive and negative subjective effects of vaporized cannabis were either greater than or equal to those from combustible cannabis (Spindle et al., 2018), but are similar to results from an internet survey of adults conducted in 2013–2014, which found that vaping cannabis produced fewer positive effects than smoking cannabis (Etter, 2015). These findings may indicate differences induced by observational vs. laboratory-based research. When studies are conducted in the laboratory, participants are administered products with known cannabinoid composition using standardized researcher-provided vaporizers. By contrast, cannabinoid-infused oils and liquids, which are self-made or obtained illegally by youth, might have variable cannabinoid composition. Further, vaporizers sold on the open market vary in terms of the efficiency by which they aerosolize the product, which, in turn, can affect the rate and level of absorption of psychoactive cannabinoids to the user (Lanz et al., 2016). Combustion, which occurs at a much higher temperature than aerosolization, consistently produces similar levels of bioavailable cannabinoids (Fabritius et al., 2013). It is possible that youth in the current sample might have been experiencing attenuated effects due to less efficient vaping devices available to them, especially in 2015 as compared to the advances in vaporizer technology to the present day.
Our findings provide some of the first subjective effect data to align with and advance other streams of evidence indicating greater risk of adverse response to cannabis edible products. Following the legalization of recreational cannabis in Colorado in 2009, cannabis intoxication-related visits to the emergency care nearly doubled (Kim and Monte, 2016), which may reflect increased consumption of edible products. Common symptoms in cannabis intoxication-involved emergency room visits include dizziness/vertigo, feeling out of control and other dissociative experiences, and upset stomach/vomiting (Bui et al., 2015; Cao et al., 2016). Secondary outcomes of specific subjective effect items revealed edible products were more likely than combustible or vaporized products to produce many of these symptoms, including feeling unable to concentrate, out of control, and upset stomach. Overconsumption may be more common with edible cannabis than other cannabis products; when ingesting cannabis edibles, it is difficult for the user to titrate the amount of tetrahydrocannabinol (THC) being consumed because peak drug effect onset typically occurs 1.5 to 3h after intake (Vandrey et al., 2017). Users sometimes consume additional products shortly after experiencing modest effects from an initial dose of edible cannabis, which later could result in more robust psychoactive effects when the peak drug effect arises (Russell et al., 2018).
The current study has some limitations. First, data available on the amounts or composition (e.g., THC:CBD ratios, strain of cannabis, cannabis-tobacco mixes) of the cannabis consumed by participants was not available. It is possible that different products may covary with cannabinoid compositions or volume of use per episode, which may explain (i.e., mediate) cross-product differences in the subjective effects in this study. Second, even though the sample was socioeconomically and ethnically diverse, the convenience sampling method from a single geographic region may limit the generalizability of results, particularly as the types of cannabis products available may vary between locations where cannabis is legal versus illegal. In Los Angeles, California, US in 2015 when this study was conducted, medical (but not recreational) cannabis use was legal, and legal medical cannabis dispensaries had been widespread across the city for many years at that point (Thomas and Freisthler, 2016). Third, the current study utilized self-report measures for subjective effects and cannabis consumption and relied on retrospective reports, which opens the possibility that reporting errors and biases could influence the results. Because honesty of reporting is a common concern with self-report methods, and in order to examine validity of participant responses, the survey included a question about the use of a non-existent, fake drug named “Derbisol”. For this wave of participants, the reported prevalence of “Derbisol” use over the past 6 months was 0.5%, suggesting that dishonesty or reporting errors in the current sample might not be of significant influence on the results. Another limitation to note is that adolescents who use two to three products may not be able to accurately remember or discern how their experiences might differ by product, especially if using different products simultaneously. Finally, because some youth might use cannabis concurrently with alcohol and tobacco products and students were not instructed to report on experiences using the respective cannabis product in the absence of other substances, the extent to which co-occurring use of cannabis and other substances may have influenced the subjective effect results is unclear.
4.1. Conclusion
Adolescents’ report of their subjective responses to cannabis in the natural ecology varied significantly across combustible, edible, and vaporized administration methods. Whether these results align with differences in the probability of cannabis use disorder following adolescent use of cannabis by combustible, edible, or vaporized methods remains to be seen. Further research identifying the extent to which certain product constituents and use patterns underlie cross-product differences in cannabis subjective effects may inform precise regulatory policies and prevention strategies targeting the spectrum of cannabis products used among youth in the current milieu.
Supplementary Material
Role of funding source
This work was supported by grant K01DA042950, K24DA048160, and R01 DA033296 from the National Institute for Drug Abuse at NIH, and grant 27-IR-0034 from the California Tobacco Related Disease Research Program (TRDRP). The sponsors had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2019.107716.
References
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57 (1), 289–300. [Google Scholar]
- Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ, 2016. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int. J. Drug Policy 36, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovsky JT, Lee DC, Crosier BS, Gabrielli JL, Sargent JD, Budney AJ, 2017. US cannabis legalization and use of vaping and edible products among youth. Drug Alcohol Depend. 177, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui QM, Simpson S, Nordstrom K, 2015. Psychiatric and medical management of marijuana intoxication in the emergency department. West J. Emerg. Med 16 (3), 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Srisuma S, Bronstein AC, Hoyte CO, 2016. Characterization of edible marijuana product exposures reported to United States poison centers. Clin. Toxicol. (Phila) 54 (9), 840–846. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, 2009. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 105, S14–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait L, Zacny JP, 1992. Reinforcing and subjective effects of oral Δ 9-THC and smoked marijuana in humans. Psychopharmacology 107 (2-3), 255–262. [DOI] [PubMed] [Google Scholar]
- Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH, 2016. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci 17 (5), 293. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ, 2012. Do initial responses to drugs predict future use or abuse? Neurosci. Biobehav. Rev 36 (6), 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, 2008. Youth risk behavior surveillance–United States, 2007. Morbidity and mortality weekly report. Surveillance Summaries (Washington, DC: 2002) 57 (4), 1–131. [PubMed] [Google Scholar]
- Etter JF, 2015. Electronic cigarettes and cannabis: an exploratory study. Eur. Addict. Res 21 (3), 124–130. [DOI] [PubMed] [Google Scholar]
- Fabritius M, Chtioui H, Battistella G, Annoni JM, Dao K, Favrat B, Fornari E, Lauer E, Maeder P, Giroud C, 2013. Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal. Bioanal. Chem 405 (30), 9791–9803. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA, 2003. Early reactions to cannabis predict later dependence. Arch. Gen. Psychiatry 60 (10), 1033–1039. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, 1991. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br. J. Addict 86 (12), 1563–1570. [DOI] [PubMed] [Google Scholar]
- Friese B, Slater MD, Battle RS, 2017. Use of marijuana edibles by adolescents in California. J. Primary Prev 38 (3), 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, Hewitt JK, 2011. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction 106 (1), 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2018. Monitoring the Future National Survey Results on Drug Use, 1975-2017: Overview, Key Findings on Adolescent Drug Use. [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2019. Monitoring the Future National Survey Results on Drug Use, 1975–2018: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research. [Google Scholar]
- Kim HS, Monte AA, 2016. Colorado Cannabis legalization and its effect on emergency care. Ann. Emerg. Med 68 (1), 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz C, Mattsson J, Soydaner U, Brenneisen R, 2016. Medicinal Cannabis: in vitro validation of vaporizers for the smoke-free inhalation of Cannabis. PLoS One 11 (1), e0147286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Cadigan JM, Patrick ME, 2017. Differences in reporting of perceived acute effects of alcohol use, marijuana use, and simultaneous alcohol and marijuana use. Drug Alcohol Depend. 180, 391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Crosier BS, Borodovsky JT, Sargent JD, Budney AJ, 2016. Online survey characterizing vaporizer use among cannabis users. Drug Alcohol Depend. 159, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J, 2015. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 314 (7), 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, True WR, Tsuang MT, 1997. How do genes influence marijuana use? The role of subjective effects. Addiction 92 (4), 409–417. [PubMed] [Google Scholar]
- MacCoun RJ, Mello MM, 2015. Half-baked—the retail promotion of marijuana edibles. N. Engl. J. Med 372 (11), 989–991. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Rooke SE, Copeland J, 2014. Experiences of marijuana-vaporizer users. Subst. Abuse 35 (2), 127–128. [DOI] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Krishnan-Sarin S, 2015. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics 136 (4), 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA, 2017. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 175, 67–76. [DOI] [PubMed] [Google Scholar]
- Peters EN, Bae D, Barrington-Trimis JL, Jarvis BP, Leventhal AM, 2018. Prevalence and sociodemographic correlates of adolescent use and polyuse of combustible, vaporized, and edible Cannabis products. JAMA Net. Open 1 (5), e182765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasic D, Weerasinghe S, Asbridge M, Langille DB, 2013. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend. 129 (1-2), 49–53. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs M-O, Le Pen G, Jay TM, 2014. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci 8, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 2004. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons. [Google Scholar]
- Russell C, Rueda S, Room R, Tyndall M, Fischer B, 2018. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int. J. Drug Policy 52, 87–96. [DOI] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R, 2018. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Net. Open 1 (7), e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald S, Wong PO, Khorasani A, Keyhani S, 2018. The form and content of cannabis products in the United States. J. Gen. Intern. Med 33 (9), 1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Freisthler B, 2016. Examining the locations of medical marijuana dispensaries in Los angeles. Drug Alcohol Rev. 35 (3), 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar Padovano H, Miranda R Jr., 2018. Subjective cannabis effects as part of a developing disorder in adolescents and emerging adults. J. Abnorm. Psychol 127 (3), 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, Cone EJ, 2017. Pharmacokinetic profile of oral Cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J. Anal. Toxicol 41 (2), 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR, 2014. Adverse health effects of marijuana use. N. Engl. J. Med 370 (23), 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS, 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1049–1060. [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ, Stallings MC, Young SE, Rhee SH, 2010. Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend. 109 (1-3), 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ, Stallings MC, Young SE, Rhee SH, 2012. Subjective effects for alcohol, tobacco, and marijuana association with cross-drug outcomes. Drug Alcohol Depend. 123, S52–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.