Abstract
Inner ear hair cells assemble mechanosensitive hair bundles on their apical surface that transduce sounds and accelerations. Each hair bundle is comprised of ~ 100 individual stereocilia that are arranged into rows of increasing height and width; their specific and precise architecture being necessary for mechanoelectrical transduction (MET). The actin cytoskeleton is fundamental to establishing this architecture, not only by forming the structural scaffold shaping each stereocilium, but also by composing rootlets and the cuticular plate that together provide a stable foundation supporting each stereocilium. In concert with the actin cytoskeleton, a large assortment of actin-binding proteins (ABPs) function to cross-link actin filaments into specific topologies, as well as control actin filament growth, severing, and capping. These processes are individually critical for sensory transduction and are all disrupted in hereditary forms of human hearing loss. In this review, we provide an overview of actin-based structures in the hair bundle and the molecules contributing to their assembly and functional properties. We also highlight recent advances in mechanisms driving stereocilia elongation and how these processes are tuned by MET.
Keywords: Actin, Actin binding protein, Stereocilia, Hair bundle, Deafness, Hearing loss
1. Introduction
The inner ear houses the auditory and vestibular end organs, which are responsible for the sensation of hearing and balance, respectively. Although these organs vary considerably in architectural complexity, from the 3D intricacy of the cochlea to the more planar maculae of the vestibular system, they all share a common transduction machinery necessary for sensory function. At the heart of sensory transduction are hair cells, which convert mechanical displacements into perturbations of hair cell receptor potential. To transduce mechanical stimuli originating from sound or accelerations, each individual hair cell assembles a complex sensory bundle consisting of approximately 100 stereocilia that elongates from its apical pole (Schwander et al., 2010). Within each hair bundle, stereocilia are precisely arranged into rows of graded heights and widths (Fig. 1A) and are interconnected by an array of extracellular links that merge them into a mechanically cohesive unit (Richardson and Petit 2019). Tip-link filaments, formed of proto-cadherin-15 (PCDH15) and cadherin-23 (CDH23), bridge between the tips of shorter stereocilia (row 2 + 3) to the shaft of the nearest taller stereocilia neighbor (Kazmierczak et al., 2007). Displacement of the hair bundle towards the tallest row tensions tip links and gates mechanoelectrical (MET) channels located on these shorter stereocilia rows (Qiu and Müller, 2022). The overall architecture of the hair bundle is essential for the MET apparatus to be optimally stimulated by sound and accelerations. Highlighting the exquisite precision of this system, this architecture ensures detection of sub-nanometer hair bundle displacements at the threshold of hearing.
Fig. 1.
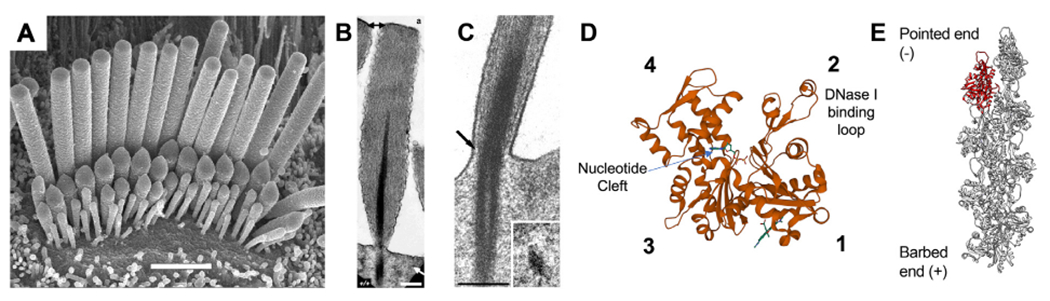
The actin cytoskeleton in hair cell stereocilia. (A) Scanning electron microscopy (SEM) of a cochlear hair cell, demonstrating the graded stereocilia sizes that contribute to the hair bundle architecture. Row 1 stereocilia are the tallest, with the shorter rows 2 + 3 having active MET channels gated by bundle deflection. Scale bar is 2 μm. (B) Transmission electron microscopy (TEM) of a sectioned stereocilia revealing its highly ordered core of actin filaments. The rootlet structure is darkly stained and penetrates into the stereocilia and cuticular plate. Scale bar is 250 nm. (C) TEM image of the stereocilia taper region demonstrating how the rootlet deforms during stereocilia deflection. (D) Structural model of an actin monomer (PDB: 1J6Z) with subdomains 1–4 and the nucleotide binding cleft labeled. The DNase I binding loop (D-loop) forms part of the interface between adjacent protomers in a filament. (E) Structural model of an actin filament (PDB: 6BNO) with an individual monomer highlighted in red. Actin filaments have structural polarity with a fast-growing barbed end and a pointed end where depolymerization occurs. Images reproduced with permission from: (A) Beurg M, et al. (2006) Journal of Neuroscience 26 (43) 10,992–11,000, DOI: 10.1523/JNEUROSCI.2188-06.2006, Copyright © 2006, Society for Neuroscience. (B) Mogensen et al. (2007) Cell Motil Cytoskeleton 64 (7): 496–508, DOI: 10.1002/cm.20199. Copyright © 2007, Wiley-Liss, Inc. (C) Furness et al. (2008) Journal of Neuroscience 28 (25): 6342–53, DOI: 10.1523/JNEUROSCI.1154-08.2008, Copyright © 2008, Society for Neuroscience. Molecular structures were rendered in VMD and Chimera.
Unlike their name implies, stereocilia are not true cilia assembled from microtubules. Instead, they are actin-based organelles that develop from microvillus-like precursors using a complex program of elongation and thickening (Barr-Gillespie, 2015). The actin cytoskeleton is central to this process, forming the structural core within each stereocilium defining its size, shape and mechanical properties (Fig. 1B). Stereocilia vary tremendously throughout the mammalian inner ear, ranging from a few microns long in the cochlea to over 100 μm long in the vestibular organs. Stereocilia further vary in size and length depending on their position within each sensory organ; this variation is particularly evident in the cochlea where stereocilia lengths scale along the tonotopic axis. Stereocilia lengths are further tuned within each hair bundle to establish the graded heights of the staircase architecture. This spectrum of stereocilia shapes and sizes are reproduced during development to within tight tolerances, revealing the presence of a precise and tunable cytoskeletal assembly template. How this template is specified at a molecular level remains an enduring question. A substantial number of proteins necessary for establishing stereocilia architecture have now been identified, providing the component parts-list for this mechanism. Perhaps unsurprisingly, this parts-list is dominated by actin and actin-associated proteins, confirming their indispensable role in stereocilia assembly and sensory transduction (see Table 1 and Fig. 2). In this review, we explore the fundamental properties of the actin cytoskeleton, and how these proteins are harnessed to enable the development, plasticity and long-term structural integrity of stereocilia; processes that are indispensable for sensory function.
Table 1.
Selected actin and actin-associated proteins in stereocilia.
| Protein | Gene | Human deafness |
|---|---|---|
| Beta cytoplasmic actin | ACTB | |
| Gamma cytoplasmic actin | ACTG1 | DFNA20/26 |
| Espin | ESPN | DFNB36 |
| Fascin 2 | FSCN2 | |
| Plastin-1 | PLS1 | |
| Xin-actin binding repeat containing 2 | XIRP2 | |
| Whirlin | WHRN | DFNB31 |
| Epidermal growth factor receptor pathway substrate 8 | EPS8 | DFNB36 |
| EPS8-like 2 | EPS8L2 | |
| G-protein signaling modulator 2 | GPSM2 | DFNB82 |
| G-protein subunit alpha i3 | GNAI3 | |
| Unconventional myosin 3 | MYO3A, MYO3B | DFNB30 |
| Unconventional myosin 6 | MYO6 | DFNA22, DFNB37 |
| Unconventional myosin 7 | MYO7A | DFNA11DFNB2USH1B |
| Unconventional myosin 15 | MYO15A | DFNB3 |
| Harmonin | USH1C | DFNB18, USH1C |
| Sans | USH1G | USH1G |
| Calcium + integrin binding family member 2 | CIB2 | DFNB48 |
| BAI1-associated protein 2-like 2 | BAIAP2L2 | |
| Twinfilin-2 | TWF2 | |
| Actin capping protein β2 | CAPZB2 | |
| Destrin (actin depolymerization factor) | DSTN | |
| Cofilin 1 | CFL1 | |
| TRIO and F-actin binding protein | TRIOBP | DFNB28 |
| Ankyrin repeat domain 24 | ANKRD24 | |
| Pejvakin | PJVK | DFNB59 |
| Taperin | TPRN | DFNB79 |
Fig. 2.
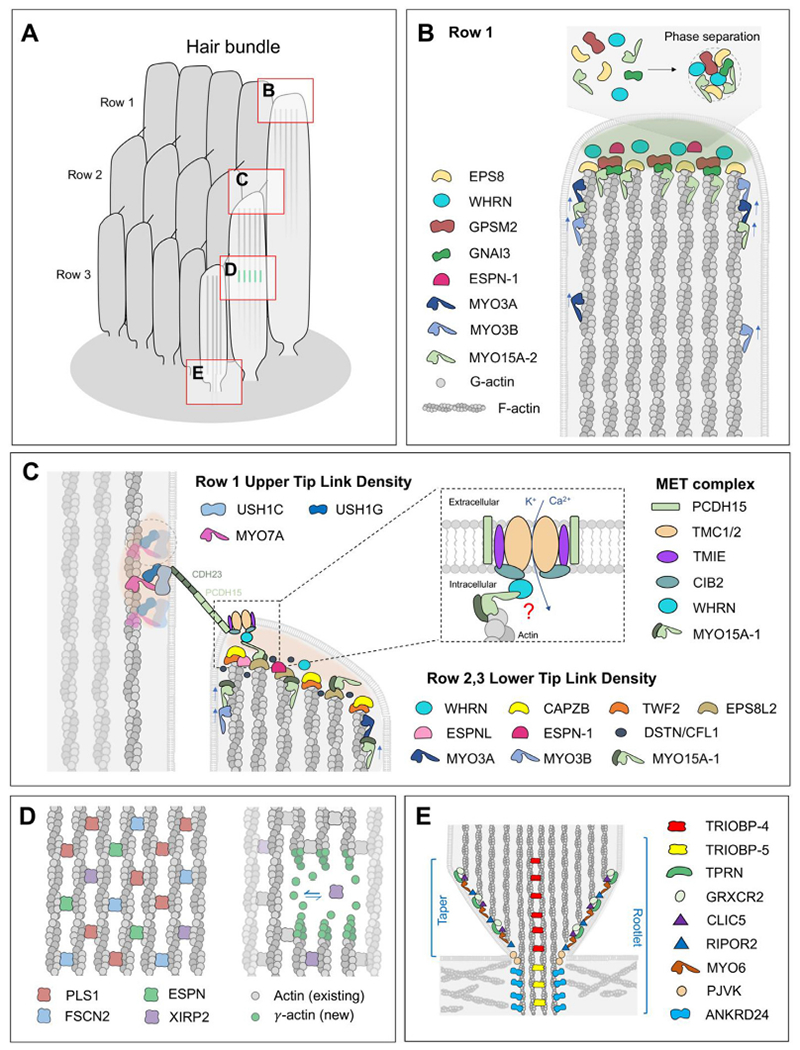
Distribution of actin-associated proteins in hair cell stereocilia. (A) Cartoon of a mammalian hair cell bundle with specific stereocilia zones expanded in panels B–E. Each stereocilium consists of thousands of parallel actin filaments that are cross-linked together and decorated by actin-associated proteins to form the structural core and provide signaling to control polymerization / depolymerization. (B) In the tallest row 1 stereocilia, MYO3A, MYO3B and MYO15A-2 traffic and deliver critical molecular cargoes to the tip. MYO3A / B transports ESPN-1 whilst MYO15A-2 delivers the elongation complex (EPS8, WHRN, GPSM2, GNAI3) that is hypothesized to form a biomolecular condensate as part of the tip density. Actin polymerization is concentrated at the stereocilia tip, where actin filament barbed ends are polarized and available for turnover during stereocilia development and maturation. (C) Mechanoelectrical transduction (MET) channels, comprised of TMC1/2, TMIE and CIB2 (LHFPL5 is not shown) are localized to the lower tip link density (LTLD) at the tips of shorter row 2 and 3 stereocilia that are also enriched with actin filament barbed ends. The tip link protein PCDH15 inserts into the LTLD. At the other end of the tip link is the upper tip link density (UTLD) that associates with CDH23 via MYO7A, USH1C (Harmonin) and USH1G (Sans). Barbed ends of row 2 actin filaments are capped with a combination of TWF2, EPS8L2 and CAPZB. Actin severing proteins DSTN1/CFL1 can uncover actin filament barbed ends to promote actin polymerization, and this process is stimulated by MET. CIB2 may interact with actin filaments via WHRN and MYO15A-1, although this interaction remains to be demonstrated in vivo. (D) Actin-binding proteins (PLS1, FSCN2, ESPN, XIRP2) extensively cross-link actin filaments to increase stiffness of the stereocilia core (left panel). Following mechanical trauma, XIRP2 and new γ-actin (ACTG1) monomers are incorporated to repair the stereocilia core. (E) TRIOBP-4 and TRIOBP-5 cross-link actin filaments to form a dense rootlet that stabilizes stereocilia in the actin meshwork of the cuticular plate. Additional proteins, including TPRN, GRXCR2, RIPOR2, CLIC5 and MYO6 localize to the taper region, where actin filament pointed ends terminate and the stereocilia diameter reduces.
2. Actin as the fundamental building block of stereocilia
Actin is the most abundantly expressed protein in eukaryotic cells and is the key building block of the filamentous actin cytoskeleton. The mammalian genome encodes six actin genes: four muscle (ACTA1, ACTC1, ACTA2, ACTG2) and two cytoplasmic actins (ACTB, ACTG1) (Vandekerckhove and Weber, 1978). The actin isoforms are highly conserved with an overall 95–99% sequence identity and share a similar capacity to reversibly polymerize into filaments (Kashina, 2020). Each actin monomer (42 kDa, 375 residues) consists of four distinct subdomains that together bind to a single adenine nucleotide (ATP, ADP-Pi or ADP) and has intrinsic ATPase activity (Fig. 1D). In the presence of mono and divalent cations, globular actin monomers (G-actin) above the critical concentration (Cc = ~ 0.1 μM for Mg-ATP-actin) spontaneously polymerize into filamentous actin (F-actin) polymers. This process of salt-induced actin polymerization is well characterized and initiates with actin monomers undergoing an energetically unfavorable nucleation to form a trimeric “seed” proto-filament, which can then be extended by the incorporation of additional Mg-ATP-actin monomers. Actin filaments form an apparent right-handed helix with two chains (see Fig. 1E). Each turn of the helix has approximately 13 actin monomers with an overall helical pitch of 36 nm and a helical rise of 2.76 nm per monomer (Pollard, 2016). F-actin exhibits structural polarity with Mg-ATP-actin monomers preferentially incorporated at the fast-growing barbed ends, so called for their appearance under TEM when decorated with the proteolytic S1 fragment of muscle myosin. As G-actin monomers are incorporated into F-actin, their ATPase activity acts as a built-in timer to hydrolyze ATP (the rate of hydrolysis of Mg-ATP-actin is 0.3 s−1) and measure the age of the monomer within the filament (Blanchoin and Pollard, 2002). Mg-ADP-actin monomers preferentially dissociate from the filament pointed end. The polymerization reaction continues until equilibrium, where the rate of pointed end monomer removal is equal to the rate of barbed end monomer addition (i.e., the barbed end association rate constant multiplied by the free actin monomer concentration). When both rates are balanced at equilibrium, actin filaments maintain a steady state length as monomers are continually added, and removed, in a process called treadmilling (Wegner 1976). The critical concentration (Cc) for this reversible polymerization is defined as the concentration of free actin monomer that co-exists with actin polymer at equilibrium (Cc = ~ 0.1 μM for Mg-ATP-actin). At [G-actin] concentrations more than Cc, the rate of barbed end actin monomer addition exceeds dissociation from the pointed end, and the filament can elongate. Treadmilling occurs in vitro, but also has been observed in cellular structures such as lamellipodia, filopodia and microvilli, confirming the importance of this basic biochemical phenomenon in cells (Carlier and Shekhar 2017).
To prevent spontaneous and uncontrolled actin polymerization within the cytosol, where [G-actin] >> Cc, actin filament polymerization is tightly controlled by actin-binding proteins (ABPs). ABPs influence almost every aspect of the polymerization cycle, from sequestering actin monomers, promoting actin seed nucleation, capping and stabilizing filament barbed or pointed ends, to severing existing filaments (Pollard, 2016). Actin severing proteins promote actin depolymerization, thereby controlling actin filament length and recycling actin monomers. Severing proteins can also promote actin polymerization, by uncovering new barbed ends available for elongation (Ono, 2007). Precise cellular control of these activities allows the assembly of actin filaments to be spatially coordinated within the cell. An additional class of ABPs can cross-link actin filaments into macromolecular networks, and these are particularly important for building larger cellular architectures such as the cytokinetic ring of dividing cells, contractile actin stress fibers, lamellipodia as well as stereocilia, a large actin structure of elaborate complexity. A large and constantly growing catalog of mutations have been identified in genes encoding ABPs that cause hereditary forms of human hearing loss (see Table 1), and many of these pathogenic alleles interfere with the structure and function of stereocilia.
As actin filaments polymerize and assemble into larger macro-molecular structures, filament mechanics become foundational to the emergent properties of the polymer network. A key mechanical property of biological polymers, such as actin filaments, is their persistence length, which is a measure of bending stiffness and the energetic cost of deformation. Polymers much shorter than the persistence length can be modelled as rigid, whereas polymers much longer than the persistence length are flexible and are described by random-walk statistical models. Individual actin filaments have a persistence length of ~18 μm, meaning that they are flexible on the cellular scale (Gittes et al., 1993). The bending stiffness of actin filaments and networks can be dynamically tuned, and one important mechanism is through the availability of cations in solution. Two divalent cation-binding sites have been identified in the actin monomer, with one controlling “polymerization” and the other “stiffness” (Kang et al., 2012). Binding of divalent cations to the “stiffness” site located in proximity to the actin D-loop increases actin filament torsional stiffness and persistence length (Hocky et al., 2016; Kang et al., 2012). Actin filament cross-linking by ABPs also massively increases persistence length and allows the assembly of more complex viscoelastic networks (between a viscous fluid and elastic solid) that enable cells to generate and respond to mechanical forces. Depending on the physical properties of crosslinking ABPs, including their dissociation constant for actin binding (KD) and inter-filament cross-link spacing, ABPs can generate diverse network topologies with tunable mechanical properties (Claessens et al., 2006). Stereocilia are an extraordinary example of how individual actin filaments can be organized and cross-linked by ABPs to create a large, ordered cellular structure (Fig. 2).
Further diversity in actin filament properties originates from the expression of specific actin isoforms, which can co-polymerize into filaments and create subunit heterogeneity (Bergeron et al., 2010). Only beta (ACTB) and gamma (ACTG1) cytoplasmic actin isoforms are detected in sensory hair cells (Andrade, 2015; Furness et al., 2005; Perrin and Ervasti, 2010; Slepecky and Savage, 1994). These ACTB and ACTG1 isoforms are 99% identical, differing by only four amino acid substitutions in the first ten N-terminal residues. Despite the overall amino acid identity between ACTB and ACTG1 isoforms, biochemical assays using purified proteins reveal these two actin isomers have significantly different polymerization kinetics, with ACTG1 having notably slower nucleation and filament elongation kinetics as compared with ACTB (Bergeron et al., 2010). There is extensive evidence supporting distinct functions for ACTB and ACTG1 in cells, with differences in sub-cellular localization, post-translational modifications and isoform specific interactions with ABPs (Kashina, 2020). The cumulative effects of different actin isoform activity are emphasized in mutant animal models. Knockout Actb(−/−) mice are embryonically lethal at E8.5 (Bunnell et al., 2011; Shawlot et al., 1998), whereas Actg1(−/−) mice are viable, albeit with increased perinatal lethality (Belyantseva et al., 2009). Remarkably, Actg1(−/−) mice have a progressive hearing loss phenotype that results from degeneration of the stereocilia core, arguing that ACTB1 cannot compensate for the loss of ACTG1. Conversely, conditional deletion of Actb1 specifically from hair cells also leads to postnatal degeneration of the stereocilia core and hearing loss, highlighting the need for both ACTB and ACTG1 in hair cells (Perrin et al., 2010).
Further insight into the function of actin isoforms comes from the identification of pathogenic ACTB and ACTG1 alleles that co-segregate with hereditary hearing loss in humans. Dominantly inherited mutations in ACTB and ACTG1 have been respectively reported in Baraitser-Winter cerebrofrontofacial syndromes BRWS1 and BRWS2, pleiotropic diseases characterized by cranio-facial abnormalities, defective CNS development, coloboma and sensorineural hearing loss, amongst other phenotypes (Cuvertino et al., 2017; Yates et al., 2017). Mutations in ACTG1 separately cause a phenotypically milder autosomal dominant, non-syndromic hereditary hearing loss DFNA20/26, characterized by a post-lingual progressive sensorineural hearing loss (Morell et al., 2000; Zhu et al., 2003). A number of pathogenic variants have been reported since the first confirmation of a causative deafness-causing mutation (p.T89I) in ACTG1 (Zhu et al., 2003). Missense ACTG1 variants do not cluster in specific hot spots but are distributed throughout the actin monomer. Deciphering the biochemical effects of ACTB and ACTG1 mutations has proven challenging, as mutant actins studied in, or purified from, recombinant expression systems are contaminated with wild-type actin to varying degrees. To address this, one approach has been to introduce mutations into the endogenous wild-type actin gene in yeast. Using this technique, DFNA20/26 mutations have been found to have varying effects upon increased nucleotide exchange rate and actin monomer conformational flexibility (Bryan and Rubenstein, 2009; Morín et al., 2009). DFNA20/26 mutations in ACTG1 can interfere with F-actin binding to ABPs, such as the severing protein cofilin, and the actin nucleator Arp2/3 complex (Bryan and Rubenstein, 2009; Kruth and Rubenstein, 2012) and have been reported to prevent polymerization into filaments (Miyajima et al., 2020). Newer approaches using actins tagged with cleavable profilin / beta-thymosin-4 fusions allow isoforms and mutants to be easily purified away from contaminating endogenous actin and will greatly accelerate structural and biochemical analyses (Ceron et al., 2022; Hatano et al., 2018). Although mouse models carrying Actg1 variants have not been reported to date, it is clear from the broad spectrum of molecular phenotypes identified in vitro that DFNA20/26 hearing loss likely has multiple etiologies.
3. Actin filaments form the para-crystalline stereocilia core
The stereocilia core acts as a scaffold to shape and support the overlaying plasma membrane and provides the necessary rigidity for the hair bundle to be coherently stimulated by sounds and accelerations (Tilney et al., 1980). Pioneering ultra-structural studies using transmission electron microscopy (TEM) showed that stereocilia consist of thousands of parallel actin filaments, that are so precisely arranged, they exhibit para-crystalline order along the stereocilia long axis (DeRosier et al., 1980; Tilney et al., 1980). Individual actin filaments span the entire length of the stereocilia core and are all orientated unidirectionally with their fast-growing barbed-ends towards the tip (Flock and Cheung, 1977; Tilney et al., 1980). As a result of this exquisite structural order the stereocilia core can behave as a “light pipe”, which has inadvertently confounded the interpretation of fluorescence microscopy images (Egelman, 1981; Kohllöffel, 1977). Hair cells express cytoplasmic actins ACTB and ACTG1, and both proteins are distributed throughout the stereocilia actin core (Andrade, 2015; Belyantseva et al., 2009; Furness et al., 2005; Höfer et al., 1997; Patrinostro et al., 2018; Perrin et al., 2010), with some indication that ACTG1 might be more concentrated at the stereocilia tips (Patrinostro et al., 2018). These studies indicate that the bulk of stereocilia actin filaments might be heterogenous co-polymers of ACTB and ACTG1. Significant differences in actin isoform distribution emerge following mechanical damage to the actin core. Noise trauma to the cochlea causes breaks (or “gaps”) in the stereocilia actin core, and ACTG1 specifically accumulates in these damaged regions, potentially to repair the core (Belyantseva et al., 2009). In Actg1(−/−) knockout mice, ACTB is recruited to these breaks instead of ACTG1, but this is unable to prevent overall stereocilia degeneration (Belyantseva et al., 2009; Perrin et al., 2010). The relative expression of ACTG1 relative to ACTB decreases in geriatric mice at 22 months of age, suggesting that attenuation of this repair process might contribute to age-related hearing loss (Andrade, 2015).
Stereocilia lengths typically exceed the persistence length of naked actin filaments, and thus assembly of each stereocilium depends upon ABPs to cross-link filaments and increase their flexural rigidity. Mass-spectrometry of hair bundles have catalogued a set of major cross-linking proteins, including fascin-2 (FSCN2), espin (ESPN), plastin-1 (PLS1) and Xin actin binding repeat containing 2 (XIRP2) (Krey et al., 2016; Krey et al., 2015; Shin et al., 2010). Together, these proteins contribute to assembling the mature stereocilia core with a 10–13 nm inter-filament spacing (Song et al., 2020; Tilney et al., 1980). The structural and mechanical properties of cross-linked actin filaments are determined by the biophysical and biochemical properties of actin crosslinkers, including cross-linker size, flexibility, dissociation constant (KD) and binding kinetics (kon and koff) (Bathe et al., 2008; Claessens et al., 2006). In this section we will discuss the properties of these key actin cross-linkers and how they contribute to the mature stereocilia structure.
ESPN is the most critical actin cross-linking for hair bundle formation, evidenced by the severe stereocilia phenotype in the jerker mouse that prevents normal stereocilia widening and leads to rapid hair bundle degeneration and deafness (Rzadzinska et al., 2005; Sekerková et al., 2011; Sjöström and Anniko, 1992; Zheng et al., 2000). Mutations in ESPN cause autosomal recessive human hearing loss, DFNB36 (Naz et al., 2004). The mouse Espn gene encodes multiple ESPN protein isoforms (ranging from ~ 25 kDa to 110 kDa) that all share a common, calcium insensitive actin-filament binding module in their COOH-terminus (Bartles et al., 1998; Sekerková et al., 2004). Actin filaments crosslinked by ESPN form unipolar parallel bundles with ~ 12 nm inter-filament spacing, with ESPN molecules sparsely bound every 20–50 actin monomers (Kitajiri et al., 2010; Purdy et al., 2007). ESPN isoforms are detected along the length of stereocilia, consistent with their function building the para-crystalline core (Salles et al., 2009; Zheng et al., 2000) All ESPN isoforms additionally contain a WH2 domain that can bind to actin monomers but does not stimulate Arp2/3 actin nucleation activity (Loomis et al., 2006). Longer ESPN isoforms (ESPN-1, ESPN-2B, ESPN-3A) bind to profilin (Sekerková et al., 2004), and the longest isoforms ESPN-1 and ESPN-2 contain an additional actin filament binding site (Chen et al., 1999) that is autoinhibited by the ankyrin repeat domain in ESPN-1 (Zheng et al., 2014), and also have anti-capping activity (Zheng et al., 2022). The localization of specific ESPN isoforms has not been determined, except for ESPN-1, which concentrates at the tips of all stereocilia rows in a similar pattern to MYO3A (Ebrahim et al., 2016; Zheng et al., 2014). Interestingly, the auto-inhibition of ESPN-1 is relieved by binding to the tail domain of either MYO3A or MYO3B, providing an important connection to the stereocilia length regulation machinery (Liu et al., 2016). Consistent with this inhibition, Espn-1 mutant hair cells in the vestibular organs have over-elongated stereocilia and lack the graded staircase architecture (Ebrahim et al., 2016). Stereocilia lengths are curiously unaffected in cochlear hair cells of Espn-1 mutant mice, showing that there are additional components to this mechanism (Ebrahim et al., 2016).
Plastin-1 (PLS1, also known as I-fimbrin) is another major stereocilia ABP; it crosslinks actin filaments into parallel bundles with ~ 12 nm inter-filament spacing (Bretscher, 1981; Volkmann et al., 2001). PLS1 has two actin binding domains (ABD) with each ABD containing two calponin homology domains, in addition to a regulatory domain with EF-hand motifs that confer Ca2+ sensitive actin binding (Namba et al., 1992). Mutations in PLS1 are associated with autosomal dominant hearing loss (Diaz-Horta et al., 2019; Morgan et al., 2019) and Pls1(−/−) mouse models similarly exhibit a progressive hearing loss (Krey et al., 2016; Taylor et al., 2015). PLS1 is an abundant crosslinker detected in stereocilia and is localized along the entire stereocilia shaft in both auditory and vestibular hair cells (Krey et al., 2016; Shin et al., 2013; Taylor et al., 2015; Tilney et al., 1989). The stereocilia of Pls1(−/−) knockout hair cells are moderately shorter and thinner than wild-type hair cell stereocilia (Krey et al., 2016). Actin filaments within Pls1(−/−) stereocilia are packed hexagonally in the presence of FSCN2 and ESPN, rather than in a random liquid order normally observed in wild-type stereocilia with PLS1 (Krey et al., 2016). Because the binding sites for FSCN2 and ESPN are not distributed at equal 60° intervals along the actin filament helix it is energetically expensive to deform actin filaments to fit into a hexagonal lattice, and this limits the size of actin filament bundles due to internal strain (Claessens et al., 2008). PLS1 thus allows stereocilia cores to widen by biasing actin filaments towards liquid packing, releasing the internal strain within the actin paracrystal that would otherwise be limiting (Krey et al., 2016).
The third major crosslinker of the stereocilia paracrystal is fascin 2 (FSCN2), which is a paralog of FSCN1 and FSCN3 (Lin-Jones and Burnside, 2007). Fascin proteins have four β-trefoil domains with the two major actin-binding sites located in β-trefoil 1 and 3 (Jansen et al., 2011). By homology to FSCN1, FSCN2 is expected to form densely packed, parallel actin bundles with ~ 8 nm inter-filament spacing (Aramaki et al., 2016; Jansen et al., 2011; Sedeh et al., 2010). FSCN1 assembles stiff actin bundles with a high persistence length of ~ 170 μm at a 2:1 actin to fascin stoichiometry (Takatsuki et al., 2014), and is able to promote the formation of filopodia in heterologous cell line models (Vignjevic et al., 2006). FSCN2 is the only fascin gene expressed by mammalian hair cells and its expression increases as stereocilia undergo differential elongation, suggesting a role in the initial assembly of the hair bundle (Shin et al., 2010). FSCN2 is localized along the stereocilia paracrystal and is additionally concentrated at the stereocilia tips of mouse (Perrin et al., 2013; Shin et al., 2010). Hair cells in the zebrafish otocyst express the ortholog fascin 2b that is similarly localized along the length of the stereocilia paracrystal (Chou et al., 2011). Consistent with a role in regulating the stereocilia paracrystal, over-expression of FSCN2 increases stereocilia width and length, and is able to displace endogenous PLS1 and ESPN (Chou et al., 2011; Roy and Perrin, 2018). Furthermore, a missense substitution in Fscn2 (p.R109H) causes postnatal shortening of stereocilia and results in progressive hearing loss (Perrin et al., 2013; Shin et al., 2010). Zebrafish fascin 2b and mouse FSCN2 both have moderate affinities for actin filaments with KD estimates ranging from 0.4 to 5 μM, respectively (Perrin et al., 2013; Chou et al., 2011). Interestingly, the p.R109H mutation does not significantly affect FSCN2 binding to actin filaments but does potently inhibit actin filament cross-linking activity (Perrin et al., 2013). These data suggest that filament bundling by FSCN2 is critical to stabilizing actin filaments in the paracrystal and prevents their depolymerization in postnatal stereocilia (Perrin et al., 2013). Despite FSCN2 extensively cross-linking actin filaments in vitro, fluorescence recovery after photobleaching (FRAP) experiments in hair cells reveal a significant mobile fraction of fascin 2b / FSCN2 with half-lives of ~8 min in mouse and ~1 min in zebrafish (Roy and Perrin 2018; Hwang et al., 2015) These rates of exchange are several orders of magnitude faster than turnover of actin monomers in the stereocilia core (Hwang et al., 2015) This dynamic exchange of fascin cross-linkers in the paracrystal may facilitate the localized exchange of actin monomers and filaments that are required for stereocilia remodeling, repair and ongoing proteostasis (Hwang et al., 2015; Roy and Perrin, 2018). Interestingly, fascin paralogs are sensitive to post-translational modifications and phosphorylation of fascin 2b on serine 38 reduces its actin binding affinity and localization to filopodia and stereocilia (Chou et al., 2011; Ono et al., 1997). It remains to be determined if a balance of kinase and phosphatase activities modulate FSCN2 in vivo to promote actin remodeling in precise stereocilia subdomains.
Xin-actin-binding-repeat-containing protein 2 (XIRP2) is another major class of actin crosslinker localized to the stereocilia paracrystal. XIRP2 binds and crosslink actin filaments via its 28 Xin domains (Pacholsky et al., 2004). Two major isoform classes are encoded from the Xirp2 gene and detected in the inner ear. Two long isoforms have been identified, containing the N-terminal Xin repeats and a C-terminal LIM (Lin11, Isl-1, and Mec-3) domain. A short isoform, also known as XEPLIN, splices out the large exon 7 containing Xin repeats and encodes only the C-terminal LIM domain (Francis et al., 2015; Scheffer et al., 2015). XIRP2 isoforms display different localizations in hair cells, with the short isoform accumulating primarily in stereocilia, whilst the longer isoforms are detected in the cuticular plate, peri–cuticular necklace and stereocilia (Francis et al., 2015; Scheffer et al., 2015). A CRISPR induced mutation in Xirp2 causes a progressive high frequency hearing loss in mouse that becomes evident 6 weeks after birth (Francis et al., 2015). Consistent with XIRP2 localization in stereocilia and a late onset hearing loss phenotype, hair cells in Xirp2 mutants initially develop a normal hair bundle with functioning MET currents, but start to exhibit stereocilia degeneration by P7, with the shorter rows being particularly severely affected (Francis et al., 2015; Scheffer et al., 2015). The degeneration process is associated with structural disorganization and loss of the normal, periodic spacing of actin filaments (Scheffer et al., 2015). These data argue that XIRP2 is either required for the normal development of the stereocilia paracrystal or is involved in its maintenance. In support of the latter interpretation, a preliminary report has found that XIRP2 is recruited to sites of paracrystal damage following acoustic trauma, similar to ACTG1, and that this is dependent upon the C-terminal LIM domain (Wagner et al., 2021). LIM domain containing proteins are specifically recruited to actin filaments under tension (Sun et al., 2020), and XIRP2 may similarly function to recognize damaged actin filaments and potentially catalyze their repair.
4. Tapers, rootlets and the cuticular plate
Analogous to a tall building requiring deep structural foundations for stability, stereocilia project actin-based rootlets down into the hair cell body where they are anchored within the cuticular plate, which is an actin filament meshwork at the apical pole of the hair cell. The assembly and sculpting of these regions are critical for MET and dependent upon the unique properties of actin filaments. As stereocilia approach their contact point with the hair cell, stereocilia diameters narrow to form a taper region, which allows stereocilia to mechanically pivot as they are deflected (Flock et al., 1977). In order to taper the stereocilia diameter, the paracrystal reduces from several hundreds of parallel actin filaments, to fewer than 50 (Song et al., 2020; Tilney et al., 1980). Actin filaments in the taper region remain parallel with filaments in the main paracrystal and their pointed ends terminate in close juxtaposition to the stereocilia membrane (Song et al., 2020). Within this tapered region, the osmiophilic rootlet is visualized as an intensely stained rod using transmission electron microscopy (TEM) (Fig. 1C). The rootlet extends bidirectionally, with the upper rootlet typically penetrating between a third to a half of the stereocilia length as measured from the cuticular plate insertion site (Furness et al., 2008). The lower rootlet core penetrates into the cuticular plate with its length scaling with the size of its corresponding stereocilium, such that the row 1 stereocilia have the longest lower rootlets (Furness et al., 2008; Kitajiri et al., 2010).
Rootlets are formed from actin filaments that have the same polarity as the stereocilia paracrystal, with barbed ends orientated towards the tips, but are spaced significantly closer together at an 8 – 9 nm inter-filament distance (Itoh and Nakashima, 1980; Song et al., 2020). Using tannic acid negative staining, longitudinal TEM images show a distinct banding pattern in the rootlet filaments with a periodicity of ~36 nm, which is identical to actin’s half-pitch helical distance (Itoh, 1982). The organization of actin filaments in such a tight register suggests that significantly different crosslinking proteins are utilized within the rootlet. Similar to stereocilia, both ACTB and ACTG1 isoforms are detected in rootlets (Furness et al., 2008, 2005), however, their distinct functions in rootlets, if any, have not been explored. As the lower rootlets penetrate into the cuticular plate, radial fibers extend from the rootlets and anchor into the surrounding cuticular plate (Furness et al., 2008; Itoh and Nakashima, 1980; Itoh, 1982; Slepecky and Chamberlain, 1982). These filaments are not decorated by the muscle myosin S1-fragment in TEM micrographs, indicating they are unlikely to be actin filaments (Slepecky and Chamberlain, 1982). The cuticular plate itself is comprised of randomly orientated actin filaments, containing both ACTB and ACTG1, that are cross-linked to form a gel at the apical pole of the hair cell (DeRosier and Tilney, 1989; Furness et al., 2008; Slepecky and Chamberlain, 1982). The rootlet and cuticular plate are independently complex cellular structures, and we refer the reader to more in-depth treatment (Pacentine et al., 2020; Pollock and McDermott, 2015).
The molecules required to assemble stereocilia rootlets have only been recently identified. Central amongst these is TRIOBP (TRIO and F-actin binding protein) that is mutated in DFNB28 hereditary human hearing loss (Riazuddin et al., 2006; Seipel et al., 2001; Shahin et al., 2006). The Triobp gene in mouse encodes three splice isoforms, TRIOBP-1, TRIOBP-4 and TRIOBP-5 (Kitajiri et al., 2010). TRIOBP-5 encodes the full-length protein, whilst the shorter TRIOBP-4 and TRIOBP-1 isoforms are formed from its N-terminal and C-terminal exons, respectively (Kitajiri et al., 2010). Within hair cells, TRIOBP-4 protein is primarily concentrated within the upper rootlet in the stereocilia core, whereas TRIOBP-5 is restricted to the lower rootlet within the cuticular plate (Katsuno et al., 2019; Kitajiri et al., 2010). The simultaneous loss of TRIOBP-4 and TRIOBP-5 in a mutant mouse model completely blocks the development of rootlets (Kitajiri et al., 2010). In a more selective ablation of just TRIOBP-5, hair cell rootlets are dysmorphic (Katsuno et al., 2019). Supporting the role of TRIOBP catalyzing stereocilia rootlet formation, purified TRIOBP-4 binds actin filaments in vitro via its R1 motif and uniquely cross-links filaments into bundles that are significantly denser than cross-linked by ESPN (Bao et al., 2013). The inter-filament spacing in TRIOBP-4 bundles is 8.2 ± 1.4 nm (Kitajiri et al., 2010), similar to the diameter of the actin filament itself, implying that actin filaments are so tightly packed there are likely steric constraints on proteins cross-linking from within the bundle. TRIOBP may therefore wrap around the filaments to bundle them, although this mechanism awaits biochemical confirmation (Kitajiri et al., 2010).
In addition to revealing a key structural assembly pathway for rootlets, Triobp −4 / −5 null mice allowed the physiological function of rootlets to be experimentally tested for the first time. Rootlets do not form in Triobp −4 / −5 null mice and stereocilia bending stiffness is reduced several-fold as a result, leaving the hair bundle susceptible to mechanical overstimulation (Kitajiri et al., 2010). Stereocilia bending stiffness is similarly reduced in Triobp – 5 null animals with dysmorphic rootlets, confirming their key function in establishing the rigidity of hair bundles (Babahosseini et al., 2022). Intriguingly, genome-wide association studies (GWAS) have uncovered a statistically significant association between single-nucleotide polymorphisms (SNPs) in TRIOBP and presbycusis risk, teasing that a change in actin bundling and rootlet stiffness may clinically impact resilience to acoustic trauma (Hoffmann et al., 2016; Trpchevska et al., 2022). Consistent with this, a common effect of acoustic trauma is to physically damage stereocilia rootlets (Liberman, 1987; Tilney et al., 1982).
Additional proteins cooperate with TRIOBP to help cross-link actin filaments to correctly form stereocilia rootlets. The ankyrin repeat protein ANKRD24 forms a ring at the stereocilia insertion point on the hair cell surface, and bridges between the extracellular membrane and TRIOBP-5 in the rootlet (Krey et al., 2022). The association of ANKRD24 with TRIOBP-5 continues along the entire length of the lower rootlet, forming a boundary that separates the rootlet from the surrounding cuticular plate (Krey et al., 2022). In Ankrd24 knock out hair cells, TRIOBP-5 is not efficiently recruited to the rootlets and this correlates with rootlets being abnormally patterned in the cuticular plate, as well as being significantly thicker (Krey et al., 2022). Consistent with the TRIOBP-5 / ANKRD24 complex being required for normal rootlet development, Ankrd24 knock out hair cells are more susceptible to noise trauma and mechanical overstimulation (Krey et al., 2022). Pejvakin (PJVK), a member of the gasdermin protein family, also co-localizes with the lower rootlet structure and can interact with TRIOBP-1 in vitro (Kazmierczak et al., 2017). Whilst endogenous TRIOBP-1 is not detected in the stereocilia rootlet (Babahosseini et al., 2022), it contains the common C-terminal exons of the longer TRIOBP-5 isoform, and thus PJVK likely interacts with TRIOBP-5 in the lower rootlet in vivo. Despite this specific association with the major rootlet actin cross-linker TRIOBP, mutant Pjvk mice do not display any gross rootlet deformities, although they are deaf and exhibit degeneration of shorter stereocilia with active MET currents (Kazmierczak et al., 2017). The correct targeting of TRIOBP-5 is further dependent upon LIM domain protein 7 (LMO7), which is concentrated uniformly throughout the hair cell cuticular plate (Du et al., 2019). Lmo7 knock-out mice have reduced recruitment of TRIOBP-5 to rootlets, and rootlets are both shorter and disorganized, which ultimately results in hearing loss (Du et al., 2019).
Proteins resident at the stereocilia taper region are intimately connected with rootlet formation. Taperin (TPRN) concentrates to the tapered zone of the stereocilia as it contacts the hair cell cuticular plate (Rehman et al., 2010), and super-resolution fluorescence imaging further localizes it to the center of the stereocilia core, within the rootlet proper (Zhao et al., 2016). Tprn knockout mice have hearing loss, recapitulating DFNB79 human deafness, and exhibit abnormally curved lower rootlets that have a hollow core within them (Chen et al., 2016; Men et al., 2019). The changes to the lower rootlet likely reflect altered cross-linking of actin filaments in this region. A series of protein interactions are required for TPRN’s critical localization at the base of stereocilia. TPRN forms a complex with chloride intracellular channel protein 5 (CLIC5), myosin 6 (MYO6) and protein tyrosine phosphatase receptor Q (PTPRQ) that provides an anchor to the plasma membrane and actin filaments (Salles et al., 2014). RIPOR2 (also known as FAM65B) is also involved in correctly localizing TPRN. RIPOR2 localizes to the base of stereocilia, where it forms a membrane-associated ring-like structure at the taper (Diaz-Horta et al., 2014; Zhao et al., 2016). Although TPRN does not directly bind to RIPOR2, TPRN is mis-localized along the stereocilia shaft in Ripor2 knock-out hair cells, which exhibit stereocilia degeneration and hearing loss (Zhao et al., 2016). Similar to RIPOR2, glutaredoxin domain-containing cysteine-rich protein 2 (GRXCR2) also concentrates at the base of stereocilia where it interacts directly with CLIC5 and TPRN (Liu et al., 2018; Li et al., 2021). TPRN is either absent, or mis-localized along the stereocilia shaft of Grxcr2 knockout mice which have dysmorphic stereocilia and develop hearing loss (Avenarius et al., 2018; Liu et al., 2018). Whilst the rootlet morphology in Gxrcr2 and Ripor2 mutant mice has not been reported, due to the mislocalization of TPRN in these models, we speculate that rootlets will similarly be disrupted. The restriction of TPRN to the stereocilia base thus appears to be an important theme in regulating the stereocilia actin cytoskeleton. TPRN shares a 34% similarity with phostensin (Rehman et al., 2010), a known pointed-end actin capping protein (Lai et al., 2009), and additionally drives cytoskeletal remodeling when overexpressed in COS-7 cells (Liu et al., 2018), suggesting that it can directly regulate the actin cytoskeleton.
5. Mechanisms driving stereocilia growth
Nascent stereocilia emerge from the lawn of microvilli that are present on the apical surface of a presumptive hair cell. Microvilli have a core of ~ 30 parallel bundled actin filaments but are significantly shorter and thinner than stereocilia. The transformation of these microvilli-like precursors into the mature stereocilia paracrystalline core is one of the marvels of inner ear biology and requires extraordinary remodeling of the actin cytoskeleton. Nascent stereocilia undergo four phases of elongation and thickening to form the mature staircase architecture with graded stereocilia heights (Tilney et al., 1988; Krey et al., 2020; Kaltenbach et al., 1994; Krey et al., 2023). During these growth phases, actin monomers are concentrated and polymerized into the paracrystalline core at the distal stereocilia tips (Drummond et al., 2015; Narayanan et al., 2015; Schneider et al., 2002). Whilst stereocilia originate from microvilli-like precursors, there are major differences in the stability of their respective core actin filaments. The microvillar core continually treadmills, a process where new actin monomers are added to the microvillar tip, and simultaneously removed from the base at an equal rate, to maintain a steady-state length (Loomis et al., 2003). In contrast, stereocilia do not appear to treadmill at an appreciable rate, and their core actin filaments are remarkably stable (Narayanan et al., 2015; Drummond et al., 2015; Zhang et al., 2012); however, stereocilia actin exchange is observed in zebrafish (Hwang et al., 2015). Fine control of actin polymerization at the stereocilia tip is thus likely responsible for setting the ultimate length of each stereocilium. How stereocilia heights are set remains a major question.
At the heart of the stereocilia growth mechanism is the unconventional molecular motor myosin 15 (MYO15A), which traffics to the stereocilia tip compartment where actin polymerization occurs (Belyantseva et al., 2003). Mutations in MYO15A cause autosomal recessive hearing loss DFNB3 (Friedman et al., 1995; Wang et al., 1998). A missense substitution in the mouse ortholog of Myo15a causes hearing loss in the shaker 2 mutant mouse by preventing stereocilia elongation, leaving the resulting hair bundles too short to be effectively stimulated (Probst et al., 1998). Multiple isoforms of MYO15A are expressed by hair cells, however it is the short isoform (MYO15A-2, aka MYO15A-S) that is expressed by embryonic hair cells as stereocilia initially start to elongate (Fang et al., 2015). In agreement with this, EGFP-tagged MYO15A-2 is also sufficient to rescue stereocilia growth in shaker 2 hair cells cultured in vitro (Belyantseva et al., 2005). A longer isoform (MYO15A-1, aka MYO15A-L) is expressed by postnatal hair cells as early at P4.5, but is dispensable for the initial phase of stereocilia elongation (Fang et al., 2015; Krey et al., 2020). A third isoform (MYO15A-3) has been identified in inner ear mRNA, and a splice-site mutation uniquely affecting this isoform has been reported in a DFNB3 pedigree (Abu Rayyan et al., 2020; Ranum et al., 2019; Rehman et al., 2016). These findings indicate that MYO15A-3 may also be required for hearing, but its function remains unknown.
How does MYO15A-2 drive stereocilia elongation? One key function is delivering the molecular components required for elongation to the growing stereocilia tip. The ATPase motor domain of MYO15A-2 is kinetically optimized for long-range trafficking along actin filaments, and as a barbed end directed motor can move along stereocilia actin filaments towards the tips (Jiang et al. 2021; Bird et al., 2014; Belyantseva et al., 2003). Live imaging of zebrafish hair cells reveals there is a continual turnover of myosin 15a (equivalent to mouse MYO15A-2) into the tip compartment, with a half-life of 12 h measured using FRAP (Hwang et al., 2015). MYO15A interacts with the elongation complex (EC) consisting of four proteins: whirlin (WHRN), epidermal growth factor receptor pathway substrate 8 (EPS8), G-protein signaling modulator 2 (GPSM2) and G-protein subunit alphai3 (GNAI3). Each EC protein is normally concentrated at the tips of the tallest row 1 stereocilia and are all mislocalized in Myo15a(sh2/sh2) hair cells, demonstrating their dependence upon MYO15A for targeting (Belyantseva et al., 2005; Delprat et al., 2005; Manor et al., 2011; Mauriac et al., 2017; Mburu et al., 2003; Tadenev et al., 2019; Tarchini et al., 2016; Zampini et al., 2011). Furthermore, knock-out mouse models of individual EC proteins all phenocopy the short stereocilia phenotype observed in Myo15a(sh2/sh2) hair cells (Manor et al., 2011; Mauriac et al., 2017; Mburu et al., 2003; Tarchini et al., 2016). Detailed measurement of protein distributions reveals that MYO15A-2 and EPS8 localize first to the elongating hair bundle during embryogenesis, and later recruit the WHRN-GPSM2-GNAI3 module as row 1 undergoes postnatal differential elongation (Tadenev et al., 2019; Krey et al., 2023). This data supports a model where MYO15A-2 delivers the EC to stereocilia tips, and where the EC proteins subsequently stimulate growth of the paracrystalline actin core in addition to specifying row identity.
How the EC complex drive stereocilia growth is unclear; none of the EC proteins are known to directly stimulate actin polymerization. EPS8 is the most compelling candidate in this regard and can influence actin polymerization in heterologous cell lines through activating the Rac GTPase (Offenhäuser et al., 2004). To date there is limited evidence for Rac GTPases being present in stereocilia, leaving this mechanism unsubstantiated (Grimsley-Myers et al., 2009). EPS8 has also been demonstrated to have “leaky” capping activity that can allow the regulated addition of actin monomers to the filament barbed end (Disanza et al., 2004). If leaky capping activity of EPS8 is key for stereocilia elongation, then the loss of EPS8 in a mouse model might be expected to cause stereocilia over-elongation, rather than the short stereocilia phenotype observed experimentally (Manor et al., 2011). Another proposed actin regulatory mechanism is through WHRN binding and regulating the bundling activity of ESPN1 (Wang et al., 2012). Whilst the EC proteins are unquestionably required for stereocilia growth, how they mechanistically contribute to stereocilia elongation and actin polymerization is still a major unanswered question.
New insight into the stereocilia growth mechanism comes from the discovery that MYO15A can directly stimulate actin polymerization, in addition to its known role trafficking within stereocilia. Using a reconstituted system of purified proteins, the actin-binding motor domain of MYO15A potently nucleates actin seeds in vitro, bypassing the rate limiting step of actin polymerization (Gong et al., 2022). The MYO15A motor domain nucleates by bridging across two actin monomers, whilst simultaneously preserving structural plasticity within the D-loop at the interprotomer contact (Gong et al., 2022). A missense substitution (p.D1647G) in the MYO15A motor domain, that causes hearing loss in mouse (Bowl et al., 2017), locks the actin protomer’s D-loop into a single conformation and prevents it from flexing (Gong et al., 2022). A preliminary report shows that the p.D1647G substitution inhibits MYO15A’s ability to catalyze actin nucleation in vitro, arguing that the change in D-loop flexibility is a key regulatory effect of MYO15A upon actin at the stereocilia tip (Moreland et al., 2021). The potential involvement for actin nucleation in stereocilia growth is counterintuitive, as actin filament barbed ends already exist at the stereocilia tips (Flock and Cheung, 1977; McGrath et al., 2021). One possibility is that MYO15A nucleates short actin polymers that could anneal to existing stereocilia barbed ends to extend the actin core (Gong et al., 2022). Actin filament extension by polymer addition has been demonstrated in vitro (Murphy et al., 1988) and in vivo (Okreglak and Drubin, 2010), but whether this occurs in stereocilia remains speculative.
Given the activities of MYO15A and the EC proteins upon actin polymerization, how might these be integrated together at the stereocilia tip? Fascinating additional insight into this question comes from the discovery that MYO15A and EC proteins undergo liquid–liquid phase separation (LLPS) and self-assemble into biomolecular condensates (Lin et al., 2021; Shi et al., 2022). LLPS is driven by multi-valent interactions between constituent proteins and underpins the formation of membrane-less cytosolic, nuclear and synaptic particles that are involved in a range of cellular processes (Wang et al., 2021). Purified WHRN protein undergoes LLPS at high protein concentrations in vitro (> 5 μM) and recruits EPS8 and the tail MyTH4 domain of MYO15A as client proteins into the resulting spherical biomolecular condensates (Lin et al., 2021). GPSM2 and GNAI3 catalyze the formation of MYO15A / WHRN / EPS8 biomolecular condensates and lowers the protein concentration required for LLPS to ~ 1 μM in vitro (Shi et al., 2022), providing a molecular mechanism for how GPSM2 and GNAI3 stabilize the MYO15A + EC complex on the tallest row 1 stereocilia (Tadenev et al., 2019). Though the demonstration of LLPS in vitro is somewhat obscure, there is evidence for this phenomenon occurring in stereocilia. The MYO15A + EC complex spatially coincides with the tip density, which is an osmiophilic protein-dense structure visualized by TEM at the stereocilia tips (Furness and Hackney, 1985; Rzadzinska et al., 2004). The tip-density is severely diminished, or absent, in Myo15a and Whrn mouse mutants in vivo (Mogensen et al., 2007; Rzadzinska et al., 2004), and the introduction of hearing loss mutations into purified MYO15A or GPSM2 inhibits LLPS in vitro (Lin et al., 2021; Shi et al., 2022). Although further investigation is now needed to confirm if LLPS truly occurs in vivo, these data point to the biomolecular condensation of MYO15A + EC proteins as a potential feature of their combined activity at the stereocilia tips. MYO15A / EC biomolecular condensates can cross-link actin filaments to form large bundles in vitro (Lin et al., 2021; Shi et al., 2022) and we speculate that other emergent activities are waiting to be discovered.
A parallel system to regulate elongation of the stereocilia actin core has been identified that uses a separate myosin molecular motor. The myosin-3 paralogs (MYO3A and MYO3B) are expressed by cochlear hair cells in mouse as early as E16.5 and accumulate at the distal tips of all stereocilia rows (Lelli et al., 2016; Merritt et al., 2012; Salles et al., 2009; Schneider et al., 2006). MYO3A and MYO3B are both barbed-end directed motors that are kinetically tuned for trafficking along actin filaments, consistent with them moving towards and accumulating at the tips of filopodia and stereocilia (Dosé et al., 2007; Komaba et al., 2003; Les Erickson et al., 2003; Merritt et al., 2012; Salles et al., 2009). Mutant knock-out Myo3a(−/−), or Myo3b(−/−) mice exhibit subtle, to no hearing loss (Ebrahim et al., 2016; Lelli et al., 2016), whereas double knock-out Myo3a(−/−):Myo3b(−/−) mice have severe hearing loss and suffer from a high rate of embryonic lethality (Ebrahim et al., 2016; Lelli et al., 2016). This data indicates that Myo3a and Myo3b share some degree of functional redundancy and can compensate for each other. Interestingly, knock-in Myo3a hypomorphic alleles cause a more severe hearing loss than Myo3a knock-outs (Li et al., 2018; Walsh et al., 2011) and are potentially better models of DFNB30 human deafness caused by mutations of MYO3A (Walsh et al., 2002).
The hair bundles of Myo3a(−/−):Myo3b(−/−) mice are highly dysmorphic with all stereocilia rows having a similar length, so that the graded staircase architecture is lost (Lelli et al., 2016). Thus, stereocilia rows over-elongate in the absence of both MYO3A and MYO3B, but not when only one paralog is absent (Lelli et al., 2016). How do the myosin-3 paralogs influence the size of the stereocilia actin paracrystal? MYO3A and MYO3B both independently bind isoform 1 of espin (ESPN-1) and espin-like (ESPNL) and traffic these proteins to the distal tips of filopodia in heterologous cells, where they co-operate to stimulate actin polymerization and filopodia growth (Ebrahim et al., 2016; Merritt et al., 2012; Salles et al., 2009). ESPN-1 accumulates at the tips of all stereocilia rows, whereas ESPNL is restricted to the shorter stereocilia rows 2 + 3 with active MET currents (Ebrahim et al., 2016; Lelli et al., 2016; Salles et al., 2009). Curiously, both ESPN and ESPNL are still localized to the stereocilia tips of Myo3a(−/−):Myo3b(−/−) double knock-out hair cells, indicating there may yet be additional, hair cell specific mechanisms that target ESPN-1 and ESPNL to the stereocilia tip compartment (Lelli et al., 2016). Supporting the role of ESPN-1 at the stereocilia tip regulating growth of the actin paracrystal, shorter row stereocilia in Espn-1 knock out hair cells over-elongate, eliminating the staircase architecture as in Myo3a(−/−):Myo3b(−/−) hair cells (Ebrahim et al., 2016; Lelli et al., 2016). Both MYO3A and MYO3B can relieve ESPN-1 autoinhibition, consistent with myosin-3 paralogs activating ESPN-1 at the tip (Liu et al., 2016). The over-elongation phenotype is only seen in the extrastriolar region of Espn-1 KO hair cells of the utricle and saccule, and not within cochlea hair cells (Ebrahim et al., 2016). Espnl knockout hair cells do not exhibit stereocilia over-elongation, and instead display a specific loss of row 3 stereocilia in the cochlea (Ebrahim et al., 2016). These differing phenotypes of Espn-1 and Espnl knockout mice demonstrate that MYO3A and MYO3B likely influence stereocilia architecture by engaging different effector proteins in a hair cell specific manner. Additional proteins that bind the myosin 3 paralogs include MORN4 and coronin 1A (CORO1A), though their potential involvement in regulating stereocilia size remains to be explored (Lelli et al., 2016; Li et al., 2019; Mecklenburg et al., 2015).
6. Tuning of stereocilia architecture by mechanoelectrical transduction (MET)
Initial evidence for MET influencing stereocilia architecture used a conditional knockout of the tip-link associated protein Sans (USH1G) to postnatally disrupt tip-links and block MET (Caberlotto et al., 2011a,b). This approach circumvented the otherwise severe hair bundle phenotype caused by the loss of tip-links earlier in development (Alagramam et al., 2011; Caberlotto et al., 2011a,b; Kazmierczak et al., 2007). Following the delayed loss of tip-links in postnatal Sans knock-out hair cells, the stereocilia tips of row 2 + 3 stereocilia lost their normal prolate shape and were shorter in height, implying a significant remodeling of the actin cytoskeleton (Caberlotto et al., 2011b). Using an in vitro model of the mouse cochlea cultured at P4, chelating extracellular Ca2+ with BAPTA reveals that row 2 + 3 stereocilia begin shrinking within 1 h of tip-link breakage, whilst row 1 is unaffected (Vélez-Ortega et al., 2017). What is the cellular signaling that leads to this rapid change of the actin cytoskeleton, specifically in the shorter mechanosensitive rows? The loss of tip-links decouples shorter stereocilia from both transduction-associated mechanical forces in addition to MET currents. To separate these two possible stimuli, acute pharmacological inhibition of MET currents using benzamil or tubocurarine, that preserve tip-links intact, also induce shortening of row 2 and 3 stereocilia specifically (Fig. 3A)(Vélez-Ortega et al., 2017). Remarkably, row 2 and 3 stereocilia regrow after MET blocker washout, arguing that these stereocilia are not only highly plastic, but also tune their size in response to MET (Vélez-Ortega et al., 2017). Targeted intracellular perfusion of BAPTA into hair cells in vitro triggers a shortening and thinning of row 2 + 3 stereocilia, further arguing that it is Ca2+ influx, as part of the MET current, that is a key signal for remodeling the actin cytoskeleton (Vélez-Ortega et al., 2017). Genetic approaches to determine the contribution of MET in vivo have revealed different contributions to hair bundle architecture (Kawashima et al., 2011; Beurg et al., 2018; Krey et al., 2020; Krey et al., 2023). Stereocilia have abnormal widths and heights in mutant Tmie or Tmc1:Tmc2 double KO (dKO) hair cells that retain tip links but lack MET currents (Kawashima et al., 2011; Beurg et al., 2018; Krey et al., 2020; Krey et al., 2023). Several changes are evident in Tmie and Tmc1:Tmc2 dKO hair cells compared to controls; row 1 stereocilia are thinner and shorter, and row 2 stereocilia are thinner but do not shorten (Krey et al., 2020; Krey et al., 2023). These different effects of pharmacological (Vélez-Ortega et al., 2017) versus genetic (Krey et al., 2020) perturbation of MET upon stereocilia architecture are potentially due to timing of MET inhibition. Pharmacological inhibition was performed at P4 onwards in vitro (Vélez-Ortega et al., 2017), whereas in the case of Tmie and Tmc1:Tmc2 dKO mutant hair cells (Krey et al., 2020), MET is already absent by P4 and likely significantly earlier (Kawashima et al., 2011; Cunningham et al., 2020). Taken together, this exciting body of work experimentally supports the longstanding postulate that MET and Ca2+ influx is critical to establishing the size of stereocilia and the overall architecture of the hair bundle (Tilney and Tilney, 1988).
Fig. 3.
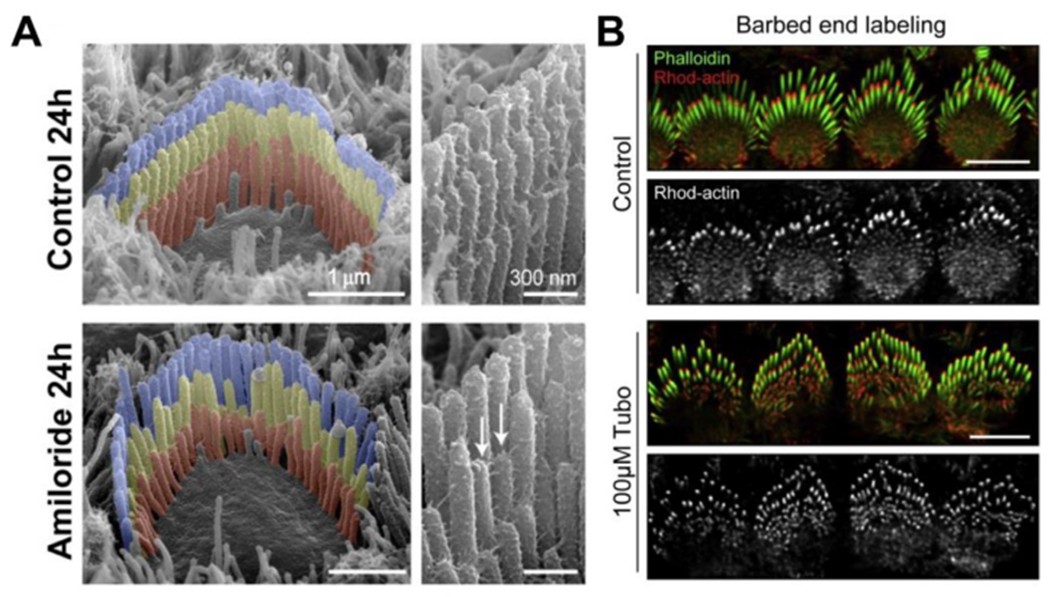
MET currents adaptively shape the stereocilia cytoskeleton. (A) Scanning electron microscopy (SEM) of hair bundles treated with a pharmacological inhibitor of the MET channel (amiloride), leaving tip-links intact. After 24 h of treatment, row 2 and 3 stereocilia with active MET channels have abnormal architecture, indicating a significant remodeling of the actin cytoskeleton. (B) Fluorescence confocal imaging of permeabilized hair cells incubated with rhodamine-labeled actin monomers to reveal the presence of barbed ends available to support polymerization. Free barbed ends are normally concentrated at the tips of row 2, but this is reduced following pharmacological blockade of MET currents using tubocurarine. Scale bars are 5 μm. Images reproduced with permission from: (A) Vélez-Ortega et al. (2017) eLife 6:e24661, DOI:10.7554/eLife.24661. (B) McGrath et al. (2021) Current Biology 31 (6): 1141–1153, DOI: 10.1016/j.cub.2020.12.006.
How is Ca2+ sensed in the shorter stereocilia rows, and what effector molecules drive plasticity of the stereocilia actin cytoskeleton? The prolific nature of Ca2+ as an intracellular messenger has the potential to couple to the actin cytoskeleton through multiple independent pathways. The loss of Ca2+ influx causes a general redistribution of MYO15A and EC proteins within the hair bundle. In mutant Tmie and Tmc1:Tmc2 dKO hair cells, or in wild-type hair cells treated with pharmacological MET blockers, EPS8, GNAI3, WHRN and MYO15A-2 (MYO15A-S) are less concentrated on the tallest row 1 and begin accumulating at row 2 + 3 stereocilia (Krey et al., 2020; Krey et al., 2023). As MYO15A is required to traffic these molecules in stereocilia, one question is how Ca2+ could alter the row targeting of MYO15A-2? The MYO15A motor domain can bind the EF-hand protein calmodulin (e.g., CALM1) as a light-chain and this may allow for Ca2+ regulated motility (Bird et al., 2014). If Ca 2+ were to inhibit MYO15A-2 this would be a mechanism to limit trafficking of the EC into row 2 + 3 in wild-type hair cells with active MET channels (Krey et al., 2020).
Another candidate Ca2+ sensor well positioned to sample MET associated Ca2+ influx to stereocilia is calcium and integrin binding protein 2 (CIB2). CIB2 binds Ca2+ via three functional EF-hand motifs and, along with its paralog CIB3, is an integral component of the MET channel complex on the shorter stereocilia rows (Giese et al., 2017; Liang et al., 2021). Mutations in CIB2 cause DFNB48 human hearing loss and mutant mouse models reveal dysregulation of row 2 and 3 stereocilia architecture, consistent with phenotypes from genetic / pharmacological disruption of MET (Giese et al., 2017; Michel et al., 2017; Riazuddin et al., 2012; Wang et al., 2017). In addition to being a component of the MET complex, CIB2 can interact with WHRN, providing a potential mechanism to couple Ca2+ sensing with the activity of the elongation complex (EC) (Riazuddin et al., 2012). Calmodulin, a prototypical Ca2+ sensor, is also abundant in stereocilia, including being concentrated at the stereocilia tips where can integrate into the MET complex (Furness et al., 2002; Jeong et al., 2022; Walker et al., 1993). Calmodulin can bind to several actin regulatory proteins (Izadi et al., 2018), including the actin nucleator cordon-blue (COBL) that is needed to build microvilli in the intestinal brush border (Grega-Larson et al., 2015), in addition to binding as a regulatory light chain to the various myosin motors in stereocilia (Heissler and Sellers, 2014). Ca2+ may additionally regulate ABPs that are directly involved in forming the stereocilia paracrystal. Plastin-1 (PLS1) has two EF hand domains that confer Ca2+ sensitivity to its ABD. Actin cross-linking by PLS1 is inhibited by elevated Ca2+ (Schwebach et al., 2017), and this could result in localized changes in actin filament structure allowing different complements of ABPs / myosin motors to bind and engage the filament. These potential sources of Ca2+ sensitive signaling remain to be explored in hair cells.
Following the loss of MET currents, in addition to shortening of the actin paracrystal there is evidence for a change in actin filament structure at the tips of row 2 stereocilia. Uncapped actin filament barbed ends, detected by the incorporation of fluorescently labeled actin monomers (i.e., a barbed end assay), are normally detected at the tips of all stereocilia rows but are particularly enriched at the tips of row 2 stereocilia (McGrath et al., 2021). In Tmie(−/−) cochleae that lack MET currents, or in wild-type cochleae treated with the MET channel blocker tubocurarine (Fig. 3B), free actin barbed ends no longer accumulate at the tips of row 2 stereocilia (McGrath et al., 2021). These data indicate that stereocilia shortening in the absence of MET is correlated with a loss of actin barbed ends that would be potentially available to extend the core. Actin severing proteins that can generate barbed ends, including WDR1 (also known as AIP1), DSTN (also known as ADF) and cofilin-1 (CFL1) are required to maintain the lengths of the shorter stereocilia rows, hinting at their involvement in the MET regulation pathway (McGrath et al., 2021; Narayanan et al., 2015). DSTN and CFL1 are concentrated at the tips of row 2 stereocilia, and this localization is lost in Tmie(−/−) knockout mice, confirming their dependence upon MET currents for normal activity (McGrath et al., 2021). The availability of actin barbed ends at the tips of row 2 stereocilia is also significantly reduced in mutant Dstn(−/−):Cfl1(fl/+) mice that have one functional allele of Cfl1, arguing that both actin severing proteins are critical for generating barbed ends in response to MET (McGrath et al., 2021). Actin severing proteins may cooperate with other factors such as Molecules Interacting with CasL (MICAL) that can oxidize actin filaments and potentiate their cleavage by CFL1 (Grintsevich et al., 2016; Hung et al., 2010). Whether MICAL is localized in stereocilia is yet to be determined, however a recent study identified MICAL as a cargo protein of Sisyphus (Myo10), the MYO15A ortholog in D.melanogastor, teasing that it may also be trafficked to the stereocilia tips by MYO15A (Rich et al., 2021).
In parallel with severing proteins, the availability of barbed ends is influenced by the presence of multiple capping proteins that stabilize filaments and prevent polymerization and depolymerization. A number of cappers concentrate at the tips of the shorter mechanosensitive rows, including heterodimeric capping protein CAPZB isoform 2 (CAPZB2), twinfilin-2 (TWF2), EPS8L2, and to a lesser extent EPS8 (Avenarius et al., 2017; Furness et al., 2013; Peng et al., 2009; Rzadzinska et al., 2009). Conditional deletion of Capzb specifically from hair cells causes severe hearing loss in mouse, and although mutant hair bundles appear normal at P1, stereocilia shorten and thin by P21 (Avenarius et al., 2017). TWF2 interacts directly with CAPZB to potentiate capping activity (Johnston et al., 2018) and is also concentrated on row 2 stereocilia (Peng et al., 2009; Rzadzinska et al., 2009). Overexpression of TWF2 in hair cells by biolistic transfection shortens stereocilia lengths in rows 2 and 3, consistent with increased capping activity (Peng et al., 2009). EPS8L2 is a member of the EPS8 family of proteins and shares the same C-terminal domain that binds to actin filaments and has barbed end capping activity (Disanza et al., 2004). Knockout Eps8l2 hair cells exhibit a slowly progressing hearing loss that is caused by the thinning, over-elongation and degeneration of the shorter stereocilia rows (Furness et al., 2013). Thus, in parallel with actin severing proteins generating new barbed ends at the tips of shorter rows, there is an opposing mechanism to cap and stabilize barbed ends. The normal tip localizations of CAPZB2, TWF2 and EPS8L2 on row 2 stereocilia are all disrupted in the hair bundles of Tmie(−/−) mutant mice, further demonstrating that transduction, and likely Ca2+ influx, is central to controlling their activity (Krey et al., 2020, 2023).
Recent reports have described an additional ABP complex that localizes to the shorter stereocilia rows 2 and 3 with MET currents. Brain-specific angiogenesis inhibitor 1-associated protein 2-like protein 2 (BAIAP2L2) contains an inverse-BAR domain that supports formation of membrane curvature, in addition to a WASP-homology domain 2 (WH2) domain that can bind to actin filaments (Pykäläinen et al., 2011). Hair cells in Baiap2l2 KO cochleae have reduced MET current amplitudes by P11 and stereocilia rows 2 and 3 degenerate resulting in progressive hearing loss (Carlton et al., 2021; Yan et al., 2022). BAIAP2L2 forms a tri-partite complex with the tail domain of MYO15A and EPS8, and its localization to stereocilia tips is lost in either Myo15a(sh2/sh2) or Eps8(−/−) hair cells, showing the necessity of this complex in vivo (Carlton et al., 2021; Halford et al., 2022). Even though the largest MYO15A-1 isoform co-localizes on the shorter stereocilia row tip with BAIAP2L2, MYO15A-1 is dispensable for the normal distribution of BAIAP2L2, implying the involvement of either MYO15A-2 or MYO15A-3 (Halford et al., 2022). BAIAP2L2 is absent from the hair bundles of Tmie KO and Tmc1:Tmc2 dKO transduction mutants, or in hair cells loaded with intracellular BAPTA-AM, strongly arguing that influx of Ca2+ is necessary for its recruitment to stereocilia tips (Halford et al., 2022). Consistent with this, BAIAP2L2 interacts with CIB2 in vitro, and is mislocalized in Cib2(−/−) hair cells (Yan et al., 2022). BAIAP2L2 may therefore be closely associated with the MET channel via CIB2 and retained by the influx of Ca2+ ions through the mechanotransduction pore. How BAIAP2L2 then influences the stereocilia actin cytoskeleton, potentially through its WH2 domain, remains to be explored.
7. Conclusions + outlook
The ability of hair cells to build a mechano-sensitive bundle using the actin cytoskeleton is a magnificent feat of biology fundamental for the sense of hearing and balance. From a large body of work encompassing genetics, cell biology and biochemistry, a plethora of ABPs orchestrating this complex process have been identified, with mutations in many of these proteins causing hereditary forms of human hearing loss. A major question is how these molecules are all biochemically integrated and coordinated within the hair bundle. For example, how are factors driving elongation of stereocilia actin filaments balanced with mechanisms promoting shortening? Or more specifically, how are the activities of MYO15A, EC proteins, MYO3A / B, along with actin severing and barbed end capping proteins kept in equilibrium to precisely control actin filament growth and stereocilia length? Influx of Ca2+ through the MET channel has emerged as a key physiological signal coordinating these processes, creating a feedback mechanism that adaptively changes bundle architecture in response to MET. Significant unresolved questions remain, including how the absolute length and width of stereocilia is specified, and how the stereocilia cytoskeleton responds to oxidative stress, mechanical noise trauma and aging.
Exploring these processes will require uncovering the full spectrum of ABP activities in hair cells. It will also necessitate, in our opinion, in situ high-resolution structural mapping of actin filaments within the stereocilia core. Actin filaments are structurally heterogenous and state-of-the-art technical refinements to cryo-EM and image processing are providing unprecedented resolution (approaching 2 Å) to identify the conformational landscape of individual protomers (Chou and Pollard, 2019; Galkin et al., 2015; Oosterheert et al., 2022; Reynolds et al., 2022). Actin filaments are themselves mechanosensitive and externally applied forces can induce localized conformational changes that regulate the binding and activity of specific ABPs (Jégou and Romet-Lemonne, 2021). Changes to actin’s nucleotide-bound state (ADP vs ADP•Pi) also mediate filament bending and deformation, arguing that actin filaments can actively alter their mechanical properties (Reynolds et al., 2022). We speculate that similar structural plasticity is present within the stereocilia paracrystal, and that localized differences in actin filament conformation underlie key aspects of stereocilia biology. As an example, the LIM domain motif recognizes a stretched conformation of actin that allows it to bind selectively to tensed filaments (Hoffmann et al., 2014; Sun et al., 2020; Winkelman et al., 2020). Within the cuticular plate and stereocilia, several LIM domain containing ABPs are present, including XIRP2 and LMO7 (Du et al., 2019; Scheffer et al., 2015). Actin filament mechanosensation may explain why XIRP2 is recruited to sites of stereocilia core damage (Francis et al., 2015; Scheffer et al., 2015), especially following noise trauma where stereocilia core actin filaments are known to be mechanically disrupted (Wagner et al., 2021). Evidence for conformational changes at the level of individual actin protomers is currently lacking; however, visualization of the stereocilia paracrystal by electron cryo-tomography is rapidly progressing, and advances in direct electron detectors combined with image motion correction are expected to ultimately yield atomistic detail of stereocilia actin filaments (Elferich et al., 2021; Metlagel et al., 2019; Song et al., 2020). The integration of actin filament nanoscale architecture alongside exhaustive cataloging of ABP activities will help decipher how stereocilia dimensions are established, how they respond to MET and displacement forces, and potentially reveal therapeutic opportunities for stimulating repair of these critical mechanosensory organelles.
Acknowledgments
We would like to thank Zane Moreland for critical reading of this manuscript. The authors are supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (R01 DC018827 to JEB). The funding body had no role in the preparation of this manuscript, or the decision to publish.
Footnotes
CRediT authorship contribution statement
Jinho Park: Conceptualization, Writing – original draft, Writing – review & editing. Jonathan E. Bird: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition.
Data availability
No data was used for the research described in the article.
References
- Abu Rayyan A, Kamal L, Casadei S, Brownstein Z, Zahdeh F, Shahin H, Canavati C, Dweik D, Jaraysa T, Rabie G, Carlson RJ, Gulsuner S, Lee MK, Avraham KB, Walsh T, King M-C, Kanaan MN, 2020. Genomic analysis of inherited hearing loss in the Palestinian population. Proc. Natl. Acad. Sci. U.S.A 117, 20070–20076. doi: 10.1073/pnas.2009628117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam K, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP, 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One 6, e19183. doi: 10.1371/journal.pone.0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade LR, 2015. Evidence for changes in beta- and gamma-actin proportions during inner ear hair cell life. Cytoskeleton (Hoboken) 72, 282–291. doi: 10.1002/cm.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki S, Mayanagi K, Jin M, Aoyama K, Yasunaga T, 2016. Filopodia formation by crosslinking of F-actin with fascin in two different binding manners. Cytoskeleton (Hoboken) 73, 365–374. doi: 10.1002/cm.21309. [DOI] [PubMed] [Google Scholar]
- Avenarius MR, Krey JF, Dumont RA, Morgan CP, Benson CB, Vijayakumar S, Cunningham CL, Scheffer DI, Corey DP, Müller U, Jones SM, Barr-Gillespie PG, 2017. Heterodimeric capping protein is required for stereocilia length and width regulation. J. Cell Biol 216, 3861–3881. doi: 10.1083/jcb.201704171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius MR, Jung J-Y, Askew C, Jones SM, Hunker KL, Azaiez H, Rehman AU, Schraders M, Najmabadi H, Kremer H, Smith RJH, Géléoc GSG, Dolan DF, Raphael Y, Kohrman DC, 2018. Grxcr2 is required for stereocilia morphogenesis in the cochlea. PLoS One 13, e0201713. doi: 10.1371/journal.pone.0201713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babahosseini H, Belyantseva IA, Yousaf R, Tona R, Hadi S, Inagaki S, Wilson E, Kitajiri S-I, Frolenkov GI, Friedman TB, Cartagena-Rivera AX, 2022. Unbalanced bidirectional radial stiffness gradients within the organ of Corti promoted by TRIOBP. Proc. Natl. Acad. Sci. U.S.A 119, e2115190119. doi: 10.1073/pnas.2115190119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Bielski E, Bachhawat A, Taha D, Gunther LK, Thirumurugan K, Kitajiri S, Sakamoto T, 2013. R1 motif is the major actin-binding domain of TRIOBP-4. Biochemistry 52, 5256–5264. doi: 10.1021/bi400585h. [DOI] [PubMed] [Google Scholar]
- Barr-Gillespie P-G, 2015. Assembly of hair bundles, an amazing problem for cell biology. Mol. Biol. Cell 26, 2727–2732. doi: 10.1091/mbc.E14-04-0940 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles JR, Zheng L, Li A, Wierda A, Chen B, 1998. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J. Cell Biol 143, 107–119. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathe M, Heussinger C, Claessens MMAE, Bausch AR, Frey E, 2008. Cytoskeletal bundle mechanics. Biophys. J 94, 2955–2964. doi: 10.1529/biophysj.107.119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Friedman TB, 2003. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc. Natl. Acad. Sci. U.S.A 100, 13958–13963. doi: 10.1073/pnas.2334417100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB, 2005. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat. Cell Biol 7, 148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Perrin BJ, Sonnemann KJ, Zhu M, Stepanyan R, McGee J, Frolenkov GI, Walsh EJ, Friderici KH, Friedman TB, Ervasti JM, 2009. Gamma-actin is required for cytoskeletal maintenance but not development. Proc. Natl. Acad. Sci. U.S.A 106, 9703–9708. doi: 10.1073/pnas.0900221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron SE, Zhu M, Thiem SM, Friderici KH, Rubenstein PA, 2010. Ion-dependent polymerization differences between mammalian beta- and gamma-nonmuscle actin isoforms. J. Biol. Chem 285, 16087–16095. doi: 10.1074/jbc.M110.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Cui R, Goldring AC, Ebrahim S, Fettiplace R, Kachar B, 2018. Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat. Commun 9, 2185. doi: 10.1038/s41467-018-04589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JE, Takagi Y, Billington N, Strub M-P, Sellers JR, Friedman TB, 2014. Chaperone-enhanced purification of unconventional myosin 15, a molecular motor specialized for stereocilia protein trafficking. Proc. Natl. Acad. Sci. U.S.A 111, 12390–12395. doi: 10.1073/pnas.1409459111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Pollard TD, 2002. Hydrolysis of ATP by polymerized actin depends on the bound divalent cation but not profilin. Biochemistry 41, 597–602. doi: 10.1021/bi011214b. [DOI] [PubMed] [Google Scholar]
- Bowl MR, Simon MM, Ingham NJ, Greenaway S, Santos L, Cater H, Taylor S, Mason J, Kurbatova N, Pearson S, Bower LR, Clary DA, Meziane H, Reilly P, Minowa O, Kelsey L, International Mouse Phenotyping Consortium, Tocchini-Valentini GP, Gao X, Bradley A, Skarnes WC, Moore M, Beaudet AL, Justice MJ, Seavitt J, Dickinson ME, Wurst W, de Angelis MH, Herault Y, Wakana S, Nutter LMJ, Flenniken AM, McKerlie C, Murray SA, Svenson KL, Braun RE, West DB, Lloyd KCK, Adams DJ, White J, Karp N, Flicek P, Smedley D, Meehan TF, Parkinson HE, Teboul LM, Wells S, Steel KP, Mallon A-M, Brown SDM, 2017. A large scale hearing loss screen reveals an extensive unexplored genetic landscape for auditory dysfunction. Nat. Commun 8, 886. doi: 10.1038/s41467-017-00595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, 1981. Fimbrin is a cytoskeletal protein that crosslinks F-actin in vitro. Proc. Natl. Acad. Sci. U.S.A 78, 6849–6853. doi: 10.1073/pnas.78.11.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan KE, Rubenstein PA, 2009. Allele-specific effects of human deafness gammaactin mutations (DFNA20/26) on the actin/cofilin interaction. J. Biol. Chem 284, 18260–18269. doi: 10.1074/jbc.M109.015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM, 2011. ,β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 22, 4047–4058. doi: 10.1091/mbc.E11-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E, Michel V, Boutet de Monvel J, Petit C, 2011a. Coupling of the mechanotransduction machinery and stereocilia F-actin polymerization in the cochlear hair bundles. Bioarchitecture 1, 169–174. doi: 10.4161/bioa.1.4.17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E, Michel V, Foucher I, Bahloul A, Goodyear RJ, Pepermans E, Michalski N, Perfettini I, Alegria-Prévot O, Chardenoux S, Do Cruzeiro M, 2011b. Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc. Natl. Acad. Sci. U.S.A 108, 5825–5830. doi: 10.1073/pnas.1017114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M-F, Shekhar S, 2017. Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat. Rev. Mol. Cell Biol 18, 389–401. doi: 10.1038/nrm.2016.172. [DOI] [PubMed] [Google Scholar]
- Carlton AJ, Halford J, Underhill A, Jeng J-Y, Avenarius MR, Gilbert ML, Ceriani F, Ebisine K, Brown SDM, Bowl MR, Barr-Gillespie PG, Marcotti W, 2021. Loss of Baiap2l2 destabilizes the transducing stereocilia of cochlear hair cells and leads to deafness. J. Physiol. (Lond.) 599, 1173–1198. doi: 10.1113/JP280670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceron RH, Carman PJ, Rebowski G, Boczkowska M, Heuckeroth RO, Dominguez R, 2022. A solution to the long-standing problem of actin expression and purification. Proc. Natl. Acad. Sci. U.S.A 119, e2209150119. doi: 10.1073/pnas.2209150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Li A, Wang D, Wang M, Zheng L, Bartles JR, 1999. Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization junctional plaque. Mol. Biol. Cell 10, 4327–4339. doi: 10.1091/mbc.10.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang Q, Zhu G-H, Hu P, Zhou Y, Wang T, Lai R-S, Xiao Z-A, Xie D-H, 2016. Progressive hearing loss and degeneration of hair cell stereocilia in taperin gene knockout mice. Biochem. Biophys. Res. Commun 479, 703–707. doi: 10.1016/j.bbrc.2016.09.148. [DOI] [PubMed] [Google Scholar]
- Chou SZ, Pollard TD, 2019. Mechanism of actin polymerization revealed by cryo-EM structures of actin filaments with three different bound nucleotides. Proc. Natl. Acad. Sci. U.S.A 116, 4265–4274. doi: 10.1073/pnas.1807028115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S-W, Hwang P, Gomez G, Fernando CA, West MC, Pollock LM, Lin-Jones J, Burnside B, McDermott BM, 2011. Fascin 2b is a component of stereocilia that lengthens actin-based protrusions. PLoS One 6, e14807. doi: 10.1371/journal.pone.0014807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens MMAE, Bathe M, Frey E, Bausch AR, 2006. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat. Mater 5, 748–753. doi: 10.1038/nmat1718. [DOI] [PubMed] [Google Scholar]
- Claessens MMAE, Semmrich C, Ramos L, Bausch AR, 2008. Helical twist controls the thickness of F-actin bundles. Proc. Natl. Acad. Sci. U.S.A 105, 8819–8822. doi: 10.1073/pnas.0711149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Qiu X, Wu Z, Zhao B, Peng G, Kim Y-H, Lauer A, Müller U, 2020. TMIE defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron 107, 126–143. doi: 10.1016/jneuron.2020.03.033 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvertino S, Stuart HM, Chandler KE, Roberts NA, Armstrong R, Bernardini L, Bhaskar S, Callewaert B, Clayton-Smith J, Davalillo CH, Deshpande C, Devriendt K, Digilio MC, Dixit A, Edwards M, Friedman JM, Gonzalez-Meneses A, Joss S, Kerr B, Lampe AK, Langlois S, Lennon R, Loget P, Ma DYT, McGowan R, Des Medt M, O’Sullivan J, Odent S, Parker MJ, Pebrel-Richard C, Petit F, Stark Z, Stockler-Ipsiroglu S, Tinschert S, Vasudevan P, Villa O, White SM, Zahir FR, Study DDD, Woolf AS, Banka S, 2017. ACTB loss-of-function mutations result in a pleiotropic developmental disorder. Am. J. Hum. Genet 101, 1021–1033. doi: 10.1016/jajhg.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprat B, Michel V, Goodyear R, Yamasaki Y, Michalski N, El-Amraoui A, Perfettini I, Legrain P, Richardson G, Hardelin J-P, Petit C, 2005. Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum. Mol. Genet 14, 401–410. doi: 10.1093/hmg/ddi036. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG, 1989. The structure of the cuticular plate, an in vivo actin gel. J. Cell Biol 109, 2853–2867. doi: 10.1083/jcb.109.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG, Egelman E, 1980. Actin in the inner ear: the remarkable structure of the stereocilium. Nature 287, 291–296. doi: 10.1038/287291a0. [DOI] [PubMed] [Google Scholar]
- Diaz-Horta O, Subasioglu-Uzak A, Grati M, DeSmidt A, Foster J, Cao L, Bademci G, Tokgoz-Yilmaz S, Duman D, Cengiz FB, Abad C, Mittal R, Blanton S, Liu XZ, Farooq A, Walz K, Lu Z, Tekin M, 2014. FAM65B is a membrane-associated protein of hair cell stereocilia required for hearing. Proc. Natl. Acad. Sci. U.S.A 111, 9864–9868. doi: 10.1073/pnas.1401950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Horta O, Bademci G, Tokgoz-Yilmaz S, Guo S, Zafeer F, Sineni CJ, Duman D, Farooq A, Tekin M, 2019. Novel variant p.E269K confirms causative role of PLS1 mutations in autosomal dominant hearing loss. Clin. Genet 96, 575–578. doi: 10.1111/cge.13626. [DOI] [PubMed] [Google Scholar]
- Disanza A, Carlier M-F, Stradal TEB, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G, 2004. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat. Cell Biol 6, 1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- Dosé AC, Ananthanarayanan S, Moore JE, Burnside B, Yengo CM, 2007. Kinetic mechanism of human myosin IIIA. J. Biol. Chem 282, 216–231. doi: 10.1074/jbc.M605964200. [DOI] [PubMed] [Google Scholar]
- Drummond MC, Barzik M, Bird JE, Zhang D-S, Lechene CP, Corey DP, Cunningham LL, Friedman TB, 2015. Live-cell imaging of actin dynamics reveals mechanisms of stereocilia length regulation in the inner ear. Nat. Commun 6, 6873. doi: 10.1038/ncomms7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T-T, Dewey JB, Wagner EL, Cui R, Heo J, Park J-J, Francis SP, Perez-Reyes E, Guillot SJ, Sherman NE, Xu W, Oghalai JS, Kachar B, Shin J-B, 2019. LMO7 deficiency reveals the significance of the cuticular plate for hearing function. Nat. Commun 10, 1117. doi: 10.1038/s41467-019-09074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S, Avenarius MR, Grati M, Krey JF, Windsor AM, Sousa AD, Ballesteros A, Cui R, Millis BA, Salles FT, Baird MA, Davidson MW, Jones SM, Choi D, Dong L, Raval MH, Yengo CM, Barr-Gillespie PG, Kachar B, 2016. Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like. Nat. Commun 7, 10833. doi: 10.1038/ncomms10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman E, 1981. Problem of light piping in immunofluorescence studies. Nature 294. doi: 10.1038/294674a0, 674–674. [DOI] [PubMed] [Google Scholar]
- Elferich J, Clark S, Ge J, Goehring A, Matsui A, Gouaux E, 2021. Molecular structures and conformations of protocadherin-15 and its complexes on stereocilia elucidated by cryo-electron tomography. Elife 10. doi: 10.7554/eLife.74512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Indzhykulian AA, Mustapha M, Riordan GP, Dolan DF, Friedman TB, Belyantseva IA, Frolenkov GI, Camper SA, Bird JE, 2015. The 133-kDa N-terminal domain enables myosin 15 to maintain mechanotransducing stereocilia and is essential for hearing. Elife 4. doi: 10.7554/eLife.08627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Cheung HC, 1977. Actin filaments in sensory hairs of inner ear receptor cells. J. Cell Biol 75, 339–343. doi: 10.1083/jcb.75.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Flock B, Murray E, 1977. Studies on the sensory hairs of receptor cells in the inner ear. Acta Otolaryngol. 83, 85–91. doi: 10.3109/00016487709128817. [DOI] [PubMed] [Google Scholar]
- Francis SP, Krey JF, Krystofiak ES, Cui R, Nanda S, Xu W, Kachar B, Barr-Gillespie PG, Shin J-B, 2015. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J. Neurosci 35, 1999–2014. doi: 10.1523/JNEUROSCI.3449-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TB, Liang Y, Weber JL, Hinnant JT, Barber TD, Winata S, Arhya IN, Asher JH, 1995. A gene for congenital, recessive deafness DFNB3 maps to the pericentromeric region of chromosome 17. Nat. Genet 9, 86–91. doi: 10.1038/ng0195-86. [DOI] [PubMed] [Google Scholar]
- Furness DN, Hackney CM, 1985. Cross-links between stereocilia in the guinea pig cochlea. Hear. Res 18, 177–188. doi: 10.1016/0378-5955(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Furness DN, Karkanevatos A, West B, Hackney CM, 2002. An immunogold investigation of the distribution of calmodulin in the apex of cochlear hair cells. Hear. Res 173, 10–20. doi: 10.1016/s0378-5955(02)00584-1. [DOI] [PubMed] [Google Scholar]
- Furness DN, Katori Y, Mahendrasingam S, Hackney CM, 2005. Differential distribution of beta- and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear. Res 207, 22–34. doi: 10.1016/j.heares.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM, 2008. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J. Neurosci 28, 6342–6353. doi: 10.1523/JNEUROSCI.1154-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Johnson SL, Manor U, Rüttiger L, Tocchetti A, Offenhauser N, Olt J, Goodyear RJ, Vijayakumar S, Dai Y, Hackney CM, Franz C, Di Fiore PP, Masetto S, Jones SM, Knipper M, Holley MC, Richardson GP, Kachar B, Marcotti W, 2013. Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc. Natl. Acad. Sci. U.S.A 110, 13898–13903. doi: 10.1073/pnas.1304644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, Vos MR, Schröder GF, Egelman EH, 2015. Near-atomic resolution for one state of F-actin. Structure 23, 173–182. doi: 10.1016/j.str.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese APJ, Tang Y-Q, Sinha GP, Bowl MR, Goldring AC, Parker A, Freeman MJ, Brown SDM, Riazuddin S, Fettiplace R, Schafer WR, Frolenkov GI, Ahmed ZM, 2017. CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat. Commun 8, 43. doi: 10.1038/s41467-017-00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes F, Mickey B, Nettleton J, Howard J, 1993. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol 120, 923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Jiang F, Moreland ZG, Reynolds MJ, de Los Reyes SE, Gurel P, Shams A, Heidings JB, Bowl MR, Bird JE, Alushin GM, 2022. Structural basis for tunable control of actin dynamics by myosin-15 in mechanosensory stereocilia. Sci. Adv 8, eabl4733. doi: 10.1126/sciadv.abl4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega-Larson NE, Crawley SW, Erwin AL, Tyska MJ, 2015. Cordon bleu promotes the assembly of brush border microvilli. Mol. Biol. Cell 26, 3803–3815. doi: 10.1091/mbc.E15-06-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley-Myers CM, Sipe CW, Géléoc GSG, Lu X, 2009. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J. Neurosci 29, 15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grintsevich EE, Yesilyurt HG, Rich SK, Hung R-J, Terman JR, Reisler E, 2016. F-actin dismantling through a redox-driven synergy between Mical and cofilin. Nat. Cell Biol 18, 876–885. doi: 10.1038/ncb3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfer D, Ness W, Drenckhahn D, 1997. Sorting of actin isoforms in chicken auditory hair cells. J. Cell Sci 110 (Pt 6), 765–770. doi: 10.1242/jcs.110.6.765. [DOI] [PubMed] [Google Scholar]
- Halford J, Bateschell M, Barr-Gillespie PG, 2022. Ca2+ entry through mechanotransduction channels localizes BAIAP2L2 to stereocilia tips. Mol. Biol. Cell 33, br6. doi: 10.1091/mbc.E21-10-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T, Alioto S, Roscioli E, Palani S, Clarke ST, Kamnev A, Hernandez-Fernaud JR, Sivashanmugam L, Chapa-Y-Lazo B, Jones AME, Robinson RC, Sampath K, Mishima M, McAinsh AD, Goode BL, Balasubramanian MK, 2018. Rapid production of pure recombinant actin isoforms in Pichia pastoris. J. Cell Sci 131. doi: 10.1242/jcs.213827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler SM, Sellers JR, 2014. Myosin light chains: teaching old dogs new tricks. Bioarchitecture 4, 169–188. doi: 10.1080/19490992.2015.1054092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocky GM, Baker JL, Bradley MJ, Sinitskiy AV, De La Cruz EM, Voth GA, 2016. Cations stiffen actin filaments by adhering a key structural element to adjacent subunits. J. Phys. Chem. B 120, 4558–4567. doi: 10.1021/acs.jpcb.6b02741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Moreau F, Moes M, Luthold C, Dieterle M, Goretti E, Neumann K, Steinmetz A, Thomas C, 2014. Human muscle LIM protein dimerizes along the actin cytoskeleton and cross-links actin filaments. Mol. Cell. Biol 34, 3053–3065. doi: 10.1128/MCB.00651-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TJ, Keats BJ, Yoshikawa N, Schaefer C, Risch N, Lustig LR, 2016. A large genome-wide association study of age-related hearing impairment using electronic health records. PLoS Genet. 12, e1006371. doi: 10.1371/journal.pgen.1006371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R-J, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJH, Terman JR, 2010. Mical links semaphorins to F-actin disassembly. Nature 463, 823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P, Chou S-W, Chen Z, McDermott BM, 2015. The stereociliary paracrystal is a dynamic cytoskeletal scaffold in vivo. Cell Rep. 13, 1287–1294. doi: 10.1016/jcelrep.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nakashima T, 1980. Structure of the hair rootlets on cochlear sensory cells by tannic acid fixation. Acta Otolaryngol. 90, 385–390. doi: 10.3109/00016488009131739. [DOI] [PubMed] [Google Scholar]
- Itoh M, 1982. Preservation and visualization of actin-containing filaments in the apical zone of cochlear sensory cells. Hear. Res 6, 277–289. doi: 10.1016/0378-5955(82)90060-0. [DOI] [PubMed] [Google Scholar]
- Izadi M, Hou W, Qualmann B, Kessels MM, 2018. Direct effects of Ca2+/calmodulin on actin filament formation. Biochem. Biophys. Res. Commun 506, 355–360. doi: 10.1016/j.bbrc.2018.07.159. [DOI] [PubMed] [Google Scholar]
- Jégou A, Romet-Lemonne G, 2021. Mechanically tuning actin filaments to modulate the action of actin-binding proteins. Curr. Opin. Cell Biol 68, 72–80. doi: 10.1016/j.ceb.2020.09.002. [DOI] [PubMed] [Google Scholar]
- Jansen S, Collins A, Yang C, Rebowski G, Svitkina T, Dominguez R, 2011. Mechanism of actin filament bundling by fascin. J. Biol. Chem 286, 30087–30096. doi: 10.1074/jbc.M111.251439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Clark S, Goehring A, Dehghani-Ghahnaviyeh S, Rasouli A, Tajkhorshid E, Gouaux E, 2022. Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature 610, 796–803. doi: 10.1038/s41586-022-05314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AB, Hilton DM, McConnell P, Johnson B, Harris MT, Simone A, Amarasinghe GK, Cooper JA, Goode BL, 2018. A novel mode of capping protein-regulation by twinfilin. Elife 7. doi: 10.7554/eLife.41313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Falzarano PR, Simpson TH, 1994. Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J. Comp. Neurol 350, 187–198. doi: 10.1002/cne.903500204. [DOI] [PubMed] [Google Scholar]
- Kang H, Bradley MJ, McCullough BR, Pierre A, Grintsevich EE, Reisler E, De La Cruz EM, 2012. Identification of cation-binding sites on actin that drive polymerization and modulate bending stiffness. Proc. Natl. Acad. Sci. U.S.A 109, 16923–16927. doi: 10.1073/pnas.1211078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashina AS, 2020. Regulation of actin isoforms in cellular and developmental processes. Semin. Cell Dev. Biol 102, 113–121. doi: 10.1016/j.semcdb.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno T, Belyantseva IA, Cartagena-Rivera AX, Ohta K, Crump SM, Petralia RS, Ono K, Tona R, Imtiaz A, Rehman A, Kiyonari H, Kaneko M, Wang Y-X, Abe T, Ikeya M, Fenollar-Ferrer C, Riordan GP, Wilson EA, Fitzgerald TS, Segawa K, Omori K, Ito J, Frolenkov GI, Friedman TB, Kitajiri S, 2019. TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing. JCI Insight 4. doi: 10.1172/jci.insight.128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ, 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Invest 121, 4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B, 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Kazmierczak M, Kazmierczak P, Peng AW, Harris SL, Shah P, Puel J-L, Lenoir M, Franco SJ, Schwander M, 2017. Pejvakin, a candidate stereociliary rootlet protein, regulates hair cell function in a cell-autonomous manner. J. Neurosci 37, 3447–3464. doi: 10.1523/JNEUROSC1.27n-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB, 2010. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 141, 786–798. doi: 10.1016/j.cell.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohllöffel LU, 1977. Interaction of light with the organ of Corti. I. Light guide effects in cochlear hair cells. Arch Otorhinolaryngol. 218, 87–103. doi: 10.1007/BF00469737. [DOI] [PubMed] [Google Scholar]
- Komaba S, Inoue A, Maruta S, Hosoya H, Ikebe M, 2003. Determination of human myosin III as a motor protein having a protein kinase activity. J. Biol. Chem 278, 21352–21360. doi: 10.1074/jbc.M300757200. [DOI] [PubMed] [Google Scholar]
- Krey JF, Sherman NE, Jeffery ED, Choi D, Barr-Gillespie PG, 2015. The proteome of mouse vestibular hair bundles over development. Sci. Data 2, 150047. doi: 10.1038/sdata.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Krystofiak ES, Dumont RA, Vijayakumar S, Choi D, Rivero F, Kachar B, Jones SM, Barr-Gillespie PG, 2016. Plastin 1 widens stereocilia by transforming actin filament packing from hexagonal to liquid. J. Cell Biol 215, 467–482. doi: 10.1083/jcb.201606036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Chatterjee P, Dumont RA, O’Sullivan M, Choi D, Bird JE, Barr-Gillespie PG, 2020. Mechanotransduction-dependent control of stereocilia dimensions and row identity in inner hair cells. Curr. Biol 30, 442–454. doi: 10.1016/jcub.2019.11.076, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Liu C, Belyantseva IA, Bateschell M, Dumont RA, Goldsmith J, Chatterjee P, Morrill RS, Fedorov LM, Foster S, Kim J, 2022. ANKRD24 organizes TRIOBP to reinforce stereocilia insertion points. J. Cell Biol 221. doi: 10.1083/jcb.202109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Chatterjee P, Halford J, Cunningham CL, Perrin BJ, Barr-Gillespie PG, 2023. Control of stereocilia length during development of hair bundles. PLoS Biol. 21, e3001964. doi: 10.1371/journal.pbio.3001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth KA, Rubenstein PA, 2012. Two deafness-causing (DFNA20/26) actin mutations affect Arp2/3-dependent actin regulation. J. Biol. Chem 287, 27217–27226. doi: 10.1074/jbc.M112.377283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai N-S, Wang T-F, Wang S-L, Chen C-Y, Yen J, Huang H-I, Li C, Huang K-Y, Liu S-Q, Lin T-H, Huang H.-b., 2009. Phostensin caps to the pointed end of actin filaments and modulates actin dynamics. Biochem. Biophys. Res. Commun 387, 676–681. doi: 10.1016/j.bbrc.2009.07.086. [DOI] [PubMed] [Google Scholar]
- Lelli A, Michel V, Boutet de Monvel J, Cortese M, Bosch-Grau M, Aghaie A, Perfettini I, Dupont T, Avan P, El-Amraoui A, Petit C, 2016. Class III myosins shape the auditory hair bundles by limiting microvilli and stereocilia growth. J. Cell Biol 212, 231–244. doi: 10.1083/jcb.201509017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Les Erickson F, Corsa AC, Dose AC, Burnside B, 2003. Localization of a class III myosin to filopodia tips in transfected HeLa cells requires an actin-binding site in its tail domain. Mol. Biol. Cell 14, 4173–4180. doi: 10.1091/mbc.E02-10-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wen Z, Zhang G, Zhang A, Fu X, Gao J, 2018. Knock-in mice with Myo3a Y137C mutation displayed progressive hearing loss and hair cell degeneration in the inner ear. Neural Plast. 2018, 4372913. doi: 10.1155/2018/4372913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu H, Raval MH, Wan J, Yengo CM, Liu W, Zhang M, 2019. Structure of the morn4/myo3a tail complex reveals MORN repeats as protein binding modules. Structure 27, 1366–1374. doi: 10.1016/j.str.2019.06.004, e3. [DOI] [PubMed] [Google Scholar]
- Li J, Liu C, Zhao B, 2021. N-Terminus of GRXCR2 interacts with CLIC5 and is essential for auditory perception. Front. Cell Dev. Biol. 9, 671364. doi: 10.3389/fcell.2021.671364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Qiu X, Dionne G, Cunningham CL, Pucak ML, Peng G, Kim Y-H, Lauer A, Shapiro L, Müller U, 2021. CIB2 and CIB3 are auxiliary subunits of the mechanotransduction channel of hair cells. Neuron 109, 2131–2149. doi: 10.1016/jneuron.2021.05.007, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, 1987. Chronic ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear. Res 26, 65–88. doi: 10.1016/0378-5955(87)90036-0. [DOI] [PubMed] [Google Scholar]
- Lin L, Shi Y, Wang M, Wang C, Lu Q, Zhu J, Zhang R, 2021. Phase separation-mediated condensation of Whirlin-Myo15-Eps8 stereocilia tip complex. Cell Rep. 34, 108770. doi: 10.1016/jcelrep.2021.108770. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J, Burnside B, 2007. Retina-specific protein fascin 2 is an actin cross-linker associated with actin bundles in photoreceptor inner segments and calycal processes. Invest. Ophthalmol. Vis. Sci 48, 1380–1388. doi: 10.1167/iovs.06-0763. [DOI] [PubMed] [Google Scholar]
- Liu H, Li J, Raval MH, Yao N, Deng X, Lu Q, Nie S, Feng W, Wan J, Yengo CM, Liu W, Zhang M, 2016. Myosin III-mediated cross-linking and stimulation of actin bundling activity of Espin. Elife 5. doi: 10.7554/eLife.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Luo N, Tung C-Y, Perrin BJ, Zhao B, 2018. GRXCR2 regulates taperin localization critical for stereocilia morphology and hearing. Cell Rep. 25, 1268–1280. doi: 10.1016/j.celrep.2018.09.063, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis PA, Zheng L, Sekerková G, Changyaleket B, Mugnaini E, Bartles JR, 2003. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol 163, 1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis PA, Kelly AE, Zheng L, Changyaleket B, Sekerková G, Mugnaini E, Ferreira A, Mullins RD, Bartles JR, 2006. Targeted wild-type and jerker espins reveal a novel, WH2-domain-dependent way to make actin bundles in cells. J. Cell Sci 119, 1655–1665. doi: 10.1242/jcs.02869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B, 2011. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr. Biol 21, 167–172. doi: 10.1016/jcub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriac SA, Hien YE, Bird JE, Carvalho SD-S, Peyroutou R, Lee SC, Moreau MM, Blanc J-M, Geyser A, Medina C, Thoumine O, Beer-Hammer S, Friedman TB, Rüttiger L, Forge A, Nürnberg B, Sans N, Montcouquiol M, 2017. Defective Gpsm2/Gαi3 signalling disrupts stereocilia development and growth cone actin dynamics in Chudley-McCullough syndrome. Nat. Commun 8, 14907. doi: 10.1038/ncomms14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, Rump A, Hardisty RE, Blanchard S, Coimbra RS, Perfettini A, Parkinson N, Mallon A-M, Glenister P, Rogers MJ, Paige AJ, Moir L, Clay J, Rosenthal A, Liu XZ, Blanco G, Steel KP, Petit C, Brown SDM, 2003. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet 34, 421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- McGrath J, Tung C-Y, Liao X, Belyantseva IA, Roy P, Chakraborty O, Li J, Berbari NF, Faaborg-Andersen CC, Barzik M, Bird JE, Zhao B, Balakrishnan L, Friedman TB, Perrin BJ, 2021. Actin at stereocilia tips is regulated by mechanotransduction and ADF/cofilin. Curr. Biol 31, 1141–1153. doi: 10.1016/j.cub.2020.12.006 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenburg KL, Freed SA, Raval M, Quintero OA, Yengo CM, O’Tousa JE, 2015. Invertebrate and vertebrate class III myosins interact with MORN repeat-containing adaptor proteins. PLoS One 10, e0122502. doi: 10.1371/journal.pone.0122502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y, Li X, Tu H, Zhang A, Fu X, Wang Z, Jin Y, Hou C, Zhang T, Zhang S, Zhou Y, Li B, Li J, Sun X, Wang H, Gao J, 2019. Tprn is essential for the integrity of stereociliary rootlet in cochlear hair cells in mice. Front. Med 13, 690–704. doi: 10.1007/s11684-018-0638-8. [DOI] [PubMed] [Google Scholar]
- Merritt RC, Manor U, Salles FT, Grati M, Dose AC, Unrath WC, Quintero OA, Yengo CM, Kachar B, 2012. Myosin IIIB uses an actin-binding motif in its espin-1 cargo to reach the tips of actin protrusions. Curr. Biol 22, 320–325. doi: 10.1016/j.cub.2011.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlagel Z, Krey JF, Song J, Swift MF, Tivol WJ, Dumont RA, Thai J, Chang A, Seifikar H, Volkmann N, Hanein D, Barr-Gillespie PG, Auer M, 2019. Electron cryo-tomography of vestibular hair-cell stereocilia. J. Struct. Biol 206, 149–155. doi: 10.1016/j.jsb.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Booth KT, Patni P, Cortese M, Azaiez H, Bahloul A, Kahrizi K, Labbé M, Emptoz A, Lelli A , Dégardin J, Dupont T, Aghaie A, Oficjalska-Pham D, Picaud S, Najmabadi H, Smith RJ, Bowl MR, Brown SD, Avan P, Petit C, El-Amraoui A, 2017. CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol. Med 9, 1711–1731. doi: 10.15252/emmm.201708087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima H, Moteki H, Day T, Nishio S-Y, Murata T, Ikezono T, Takeda H, Abe S, Iwasaki S, Takahashi M, Naito Y, Yamazaki H, Kanda Y, Kitajiri S-I, Usami S-I, 2020. Novel ACTG1 mutations in patients identified by massively parallel DNA sequencing cause progressive hearing loss. Sci. Rep 10, 7056. doi: 10.1038/s41598-020-63690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen MM, Rzadzinska A, Steel KP, 2007. The deaf mouse mutant whirler suggests a role for whirlin in actin filament dynamics and stereocilia development. Cell Motil. Cytoskel 64, 496–508. doi: 10.1002/cm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morín M, Bryan KE, Mayo-Merino F, Goodyear R, Mencía A, Modamio-Høybjør S, del Castillo I, Cabalka JM, Richardson G, Moreno F, Rubenstein PA, Moreno-Pelayo MA, 2009. In vivo and in vitro effects of two novel gamma-actin (ACTG1) mutations that cause DFNA20/26 hearing impairment. Hum. Mol. Genet 18, 3075–3089. doi: 10.1093/hmg/ddp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland ZG, Jiang F, Aguilar C, Barzik M, Gong R, Shams A, Faaborg-Andersen C, Werth JC, Harley R, Sutton DC, Cole SM, Parker A, Morse S, Wilson E, Takagi Y, Sellers JR, Brown SDM, Friedman TB, Alushin GM, Bowl MR, Bird JE, 2021. Myosin-driven nucleation of actin filaments drives stereocilia development critical for hearing. BioRxiv doi: 10.1101/2021.07.09.451618. [DOI] [Google Scholar]
- Morell RJ, Friderici KH, Wei S, Elfenbein JL, Friedman TB, Fisher RA, 2000. A new locus for late-onset, progressive, hereditary hearing loss DFNA20 maps to 17q25. Genomics 63, 1–6. doi: 10.1006/geno.1999.6058. [DOI] [PubMed] [Google Scholar]
- Morgan A, Koboldt DC, Barrie ES, Crist ER, García García G, Mezzavilla M, Faletra F, Mihalic Mosher T, Wilson RK, Blanchet C, Manickam K, Roux A-F., Gasparini P, Dell’Orco D, Girotto G, 2019. Mutations in PLS1, encoding fimbrin, cause autosomal dominant nonsyndromic hearing loss. Hum. Mutat 40, 2286–2295. doi: 10.1002/humu.23891. [DOI] [PubMed] [Google Scholar]
- Murphy DB, Gray RO, Grasser WA, Pollard TD, 1988. Direct demonstration of actin filament annealing in vitro. J. Cell Biol 106, 1947–1954. doi: 10.1083/jcb.106.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y, Ito M, Zu Y, Shigesada K, Maruyama K, 1992. Human T cell L-plastin bundles actin filaments in a calcium-dependent manner. J. Biochem 112, 503507. doi: 10.1093/oxfordjournals.jbchem.a123929. [DOI] [PubMed] [Google Scholar]
- Narayanan P, Chatterton P, Ikeda A, Ikeda S, Corey DP, Ervasti JM, Perrin BJ, 2015. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat. Commun 6, 6855. doi: 10.1038/ncomms7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz S, Griffith AJ, Riazuddin S, Hampton LL, Battey JF, Khan SN, Riazuddin S, Wilcox ER, Friedman TB, 2004. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J. Med. Genet 41, 591–595. doi: 10.1136/jmg.2004.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhäuser N, Borgonovo A, Disanza A, Romano P, Ponzanelli I, Iannolo G, Di Fiore PP, Scita G, 2004. The eps8 family of proteins links growth factor stimulation to actin reorganization generating functional redundancy in the Ras/Rac pathway. Mol. Biol. Cell 15, 91–98. doi: 10.1091/mbc.E03-06-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okreglak V, Drubin DG, 2010. Loss of Aip1 reveals a role in maintaining the actin monomer pool and an in vivo oligomer assembly pathway. J. Cell Biol 188, 769–777. doi: 10.1083/jcb.200909176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, Matsumura F, 1997. Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin. J. Biol. Chem 272, 2527–2533. doi: 10.1074/jbc.272.4.2527. [DOI] [PubMed] [Google Scholar]
- Ono S, 2007. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int. Rev. Cytol 258, 1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Oosterheert W, Klink BU, Belyy A, Pospich S, Raunser S, 2022. Structural basis of actin filament assembly and aging. Nature 611, 374–379. doi: 10.1038/s41586-022-05241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacentine I, Chatterjee P, Barr-Gillespie PG, 2020. Stereocilia rootlets: actin-based structures that are essential for structural stability of the hair bundle. Int. J. Mol. Sci 21. doi: 10.3390/ijms21010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholsky D, Vakeel P, Himmel M, Löwe T, Stradal T, Rottner K, Fürst DO, van der Ven PFM, 2004. Xin repeats define a novel actin-binding motif. J. Cell Sci 117, 5257–5268. doi: 10.1242/jcs.01406. [DOI] [PubMed] [Google Scholar]
- Patrinostro X, Roy P, Lindsay A, Chamberlain CM, Sundby LJ, Starker CG, Voytas DF, Ervasti JM, Perrin BJ, 2018. Essential nucleotide- and protein-dependent functions of Actb/β-actin. Proc. Natl. Acad. Sci. U.S.A 115, 7973–7978. doi: 10.1073/pnas.1807895115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Belyantseva IA, Hsu PD, Friedman TB, Heller S, 2009. Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J. Neurosci 29, 15083–15088. doi: 10.1523/JNEUROSCI.2782-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Ervasti JM, 2010. The actin gene family: function follows isoform. Cytoskeleton (Hoboken) 67, 630–634. doi: 10.1002/cm.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Sonnemann KJ, Ervasti JM, 2010. β-actin and γ-actin are each dispensable for auditory hair cell development but required for Stereocilia maintenance. PLoS Genet. 6, e1001158. doi: 10.1371/journal.pgen.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Strandjord DM, Narayanan P, Henderson DM, Johnson KR, Ervasti JM, 2013. β-Actin and fascin-2 cooperate to maintain stereocilia length. J. Neurosci 33, 8114–8121. doi: 10.1523/JNEUROSCI.0238-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, 2016. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol 8. doi: 10.1101/cshperspect.a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock LM, McDermott BM, 2015. The cuticular plate: a riddle, wrapped in a mystery, inside a hair cell. Birth Defects Res. C Embryo Today 105, 126–139. doi: 10.1002/bdrc.21098. [DOI] [PubMed] [Google Scholar]
- Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, Morell RJ, Touchman JW, Lyons RH, Noben-Trauth K, Friedman TB, Camper SA, 1998. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science 280, 1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- Purdy KR, Bartles JR, Wong GCL, 2007. Structural polymorphism of the actinespin system: a prototypical system of filaments and linkers in stereocilia. Phys. Rev. Lett 98, 058105. doi: 10.1103/PhysRevLett.98.058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pykäläinen A, Boczkowska M, Zhao H, Saarikangas J, Rebowski G, Jansen M, Hakanen J, Koskela EV, Peränen J, Vihinen H, Jokitalo E, Salminen M, Ikonen E, Dominguez R, Lappalainen P, 2011. Pinkbar is an epithelial-specific BAR domain protein that generates planar membrane structures. Nat. Struct. Mol. Biol 18, 902–907. doi: 10.1038/nsmb.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Müller U, 2022. Sensing sound: cellular specializations and molecular force sensors. Neuron 110, 3667–3687. doi: 10.1016/j.neuron.2022.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranum PT, Goodwin AT, Yoshimura H, Kolbe DL, Walls WD, Koh J-Y, He DZZ, Smith RJH, 2019. Insights into the biology of hearing and deafness revealed by single-cell RNA sequencing. Cell Rep. 26, 3160–3171. doi: 10.1016/j.celrep.2019.02.053, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Morell RJ, Belyantseva IA, Khan SY, Boger ET, Shahzad M, Ahmed ZM, Riazuddin S, Khan SN, Riazuddin S, Friedman TB, 2010. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am. J. Hum. Genet 86, 378–388. doi: 10.1016/j.ajhg.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Bird JE, Faridi R, Shahzad M, Shah S, Lee K, Khan SN, Imtiaz A, Ahmed ZM, Riazuddin S, Santos-Cortez RLP, Ahmad W, Leal SM, Riazuddin S, Friedman TB, 2016. Mutational spectrum of MYO15A and the molecular mechanisms of DFNB3 human deafness. Hum. Mutat 37, 991–1003. doi: 10.1002/humu.23042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MJ, Hachicho C, Carl AG, Gong R, Alushin GM, 2022. Bending forces and nucleotide state jointly regulate F-actin structure. Nature 611, 380–386. doi: 10.1038/s41586-022-05366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Khan SN, Ahmed ZM, Ghosh M, Caution K, Nazli S, Kabra M, Zafar AU, Chen K, Naz S, Antonellis A, Pavan WJ, Green ED, Wilcox ER, Friedman PL, Morell RJ, Riazuddin S, Friedman TB, 2006. Mutations in TRIOBP, which encodes a putative cytoskeletal-organizing protein, are associated with nonsyndromic recessive deafness. Am. J. Hum. Genet 78, 137–143. doi: 10.1086/499164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Belyantseva IA , Giese APJ, Lee K, Indzhykulian AA , Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, Hegde RS, Ali RA, Anwar S, Andrade-Elizondo PB, Sirmaci A, Parise LV, Basit S, Wali A, Ayub M, Ansar M, Ahmad W, Khan SN, Akram J, Tekin M, Riazuddin Sheikh, Cook T, Buschbeck EK, Frolenkov GI, Leal SM, Friedman TB, Ahmed ZM, 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet 44, 1265–1271. doi: 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SK, Baskar R, Terman JR, 2021. Propagation of F-actin disassembly via Myosin15-Mical interactions. Sci. Adv 7. doi: 10.1126/sciadv.abg0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Petit C, 2019. Hair-bundle links: genetics as the gateway to function. Cold Spring Harb. Perspect. Med 9. doi: 10.1101/cshperspect.a033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Perrin BJ, 2018. The stable actin core of mechanosensory stereocilia features continuous turnover of actin cross-linkers. Mol. Biol. Cell 29, 1856–1865. doi: 10.1091/mbc.E18-03-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B, 2004. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J. Cell Biol 164, 887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska A, Schneider M, Noben-Trauth K, Bartles JR, Kachar B, 2005. Balanced levels of Espin are critical for stereociliary growth and length maintenance. Cell Motil. Cytoskel 62, 157–165. doi: 10.1002/cm.20094. [DOI] [PubMed] [Google Scholar]
- Rzadzinska AK, Nevalainen EM, Prosser HM, Lappalainen P, Steel KP, 2009. MyosinVIIa interacts with Twinfilin-2 at the tips of mechanosensory stereocilia in the inner ear. PLoS One 4, e7097. doi: 10.1371/journal.pone.0007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FT, Merritt RC, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dosé AC, Kachar B, 2009. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat. Cell Biol 11, 443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FT, Andrade LR, Tanda S, Grati M, Plona KL, Gagnon LH, Johnson KR, Kachar B, Berryman MA, 2014. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken) 71, 61–78. doi: 10.1002/cm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer DI, Zhang D-S, Shen J, Indzhykulian A, Karavitaki KD, Xu YJ, Wang Q, Lin JJ-C, Chen Z-Y, Corey DP, 2015. XIRP2, an actin-binding protein essential for inner ear hair-cell stereocilia. Cell Rep 10, 1811–1818. doi: 10.1016/j.celrep.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ME, Belyantseva IA, Azevedo RB, Kachar B, 2002. Rapid renewal of auditory hair bundles. Nature 418, 837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- Schneider ME, Dosé AC, Salles FT, Chang W, Erickson FL, Burnside B, Kachar B, 2006. A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J. Neurosci 26, 10243–10252. doi: 10.1523/JNEUROSCI.2812-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Kachar B, Müller U, 2010. Review series: the cell biology of hearing. J. Cell Biol 190, 9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwebach CL, Agrawal R, Lindert S, Kudryashova E, Kudryashov DS, 2017. The roles of actin-binding domains 1 and 2 in the calcium-dependent regulation of actin filament bundling by human plastins. J. Mol. Biol 429, 2490–2508. doi: 10.1016/j.jmb.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Sedeh RS, Fedorov AA, Fedorov EV, Ono S, Matsumura F, Almo SC, Bathe M, 2010. Structure, evolutionary conservation, and conformational dynamics of Homo sapiens fascin-1, an F-actin crosslinking protein. J. Mol. Biol 400, 589–604. doi: 10.1016/j.jmb.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seipel K, O’Brien SP, Iannotti E, Medley QG, Streuli M, 2001. Tara, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization. J. Cell Sci 114, 389–399. doi: 10.1242/jcs.114.2.389. [DOI] [PubMed] [Google Scholar]
- Sekerková G, Zheng L, Loomis PA, Changyaleket B, Whitlon DS, Mugnaini E, Bartles JR, 2004. Espins are multifunctional actin cytoskeletal regulatory proteins in the microvilli of chemosensory and mechanosensory cells. J. Neurosci 24, 5445–5456. doi: 10.1523/JNEUROSCI.1279-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerková G, Richter C-P, Bartles JR, 2011. Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet. 7, e1002032. doi: 10.1371/journal.pgen.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin H, Walsh T, Sobe T, Abu Sa’ed J, Abu Rayan A, Lynch ED, Lee MK, Avraham KB, King M-C, Kanaan M, 2006. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. Am. J. Hum. Genet 78, 144–152. doi: 10.1086/499495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Fohn LE, Behringer RR, 1998. Restricted beta-galactosidase expression of a hygromycin-lacZ gene targeted to the beta-actin locus and embryonic lethality of beta-actin mutant mice. Transgenic Res. 7, 95–103. doi: 10.1023/a:1008816308171. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lin L, Wang C, Zhu J, 2022. Promotion of row 1-specific tip complex condensates by Gpsm2-Gαi provides insights into row identity of the tallest stereocilia. Sci. Adv 8, eabn4556. doi: 10.1126/sciadv.abn4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J-B, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, Johnson KR, 2010. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J. Neurosci 30, 9683–9694. doi: 10.1523/JNEUROSCI.1541-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J-B, Krey JF, Hassan A, Metlagel Z, Tauscher AN, Pagana JM, Sherman NE, Jeffery ED, Spinelli KJ, Zhao H, Wilmarth PA, Choi D, David LL, Auer M, Barr-Gillespie PG, 2013. Molecular architecture of the chick vestibular hair bundle. Nat. Neurosci 16, 365–374. doi: 10.1038/nn.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström B, Anniko M, 1992. Cochlear structure and function in a recessive type of genetically induced inner ear degeneration. ORL J Otorhinolaryngol. Relat. Spec 54, 220–228. doi: 10.1159/000276302. [DOI] [PubMed] [Google Scholar]
- Slepecky N, Chamberlain SC, 1982. Distribution and polarity of actin in the sensory hair cells of the chinchilla cochlea. Cell Tissue Res. 224, 15–24. doi: 10.1007/BF00217262. [DOI] [PubMed] [Google Scholar]
- Slepecky NB, Savage JE, 1994. Expression of actin isoforms in the guinea pig organ of Corti: muscle isoforms are not detected. Hear. Res 73, 16–26. doi: 10.1016/0378-5955(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Song J, Patterson R, Metlagel Z, Krey JF, Hao S, Wang L, Ng B, Sazzed S, Kovacs J, Wriggers W, He J, Barr-Gillespie PG, Auer M, 2020. A cryotomography-based volumetric model of the actin core of mouse vestibular hair cell stereocilia lacking plastin 1. J. Struct. Biol 210, 107461. doi: 10.1016/j.jsb.2020.107461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Phua DYZ, Axiotakis L, Smith MA, Blankman E, Gong R, Cail RC, Espinosa de Los Reyes S, Beckerle MC, Waterman CM, Alushin GM, 2020. Mechanosensing through direct binding of tensed F-actin by LIM domains. Dev. Cell 55, 468–482. doi: 10.1016/j.devcel.2020.09.022, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadenev ALD, Akturk A, Devanney N, Mathur PD, Clark AM, Yang J, Tarchini B, 2019. GPSM2-GNAI specifies the tallest stereocilia and defines hair bundle row identity. Curr. Biol 29, 921–934. doi: 10.1016/j.cub.2019.01.051, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki H, Bengtsson E, Månsson A, 2014. Persistence length of fascin-cross-linked actin filament bundles in solution and the in vitro motility assay. Biochim. Biophys. Acta 1840, 1933–1942. doi: 10.1016/j.bbagen.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Tarchini B, Tadenev ALD, Devanney N, Cayouette M, 2016. A link between planar polarity and staircase-like bundle architecture in hair cells. Development 143, 3926–3932. doi: 10.1242/dev.139089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Bullen A, Johnson SL, Grimm-Günter E-M, Rivero F, Marcotti W, Forge A, Daudet N, 2015. Absence of plastin 1 causes abnormal maintenance of hair cell stereocilia and a moderate form of hearing loss in mice. Hum. Mol. Genet 24, 37–49. doi: 10.1093/hmg/ddu417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, 1988. The actin filament content of hair cells of the bird cochlea is nearly constant even though the length, width, and number of stereocilia vary depending on the hair cell location. J. Cell Biol 107, 2563–2574. doi: 10.1083/jcb.107.6.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Derosier DJ, Mulroy MJ, 1980. The organization of actin filaments in the stereocilia of cochlear hair cells. J. Cell Biol 86, 244–259. doi: 10.1083/jcb.86.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Saunders JC, Egelman E, DeRosier DJ, 1982. Changes in the organization of actin filaments in the stereocilia of noise-damaged lizard cochleae. Hear. Res 7, 181–197. doi: 10.1016/0378-5955(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Cotanche DA, 1988. Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J. Cell Biol 106, 355–365. doi: 10.1083/jcb.106.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney MS, Tilney LG, Stephens RE, Merte C, Drenckhahn D, Cotanche DA, Bretscher A, 1989. Preliminary biochemical characterization of the stereocilia and cuticular plate of hair cells of the chick cochlea. J. Cell Biol 109, 1711–1723. doi: 10.1083/jcb.109.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trpchevska N, Freidin MB, Broer L, Oosterloo BC, Yao S, Zhou Y, Vona B, Bishop C, Bizaki-Vallaskangas A, Canlon B, Castellana F, Chasman DI, Cherny S, Christensen K, Concas MP, Correa A, Elkon R, Team, Estonian Biobank Research, Mengel-From J, Gao Y, Giersch ABS, Girotto G, Gudjonsson A, Gudnason V, Heard-Costa NL, Hertzano R, Hjelmborg JVB, Hjerling-Leffler J, Hoffman HJ, Kaprio J, Kettunen J, Krebs K, Kähler AK, Lallemend F, Launer LJ, Lee I-M, Leonard H, Li C-M, Lowenheim H, Magnusson PKE, van Meurs J, Milani L, Morton CC, Mäkitie A, Nalls MA, Nardone GG, Nygaard M, Palviainen T, Pratt S, Quaranta N, Rämü J, Saarentaus E, Sardone R, Satizabal CL, Schweinfurth JM, Seshadri S, Shiroma E, Shulman E, Simonsick E, Spankovich C, Tropitzsch A, Lauschke VM, Sullivan PF, Goedegebure A, Cederroth CR, Williams FMK, Nagtegaal AP, 2022. Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss. Am. J. Hum. Genet 109, 1077–1091. doi: 10.1016/j.ajhg.2022.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Ortega AC, Freeman MJ, Indzhykulian AA., Grossheim JM, Frolenkov GI, 2017. Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells. Elife 6. doi: 10.7554/eLife.24661 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J, Weber K, 1978. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol 126, 783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Kojima SI, Aratyn Y, Danciu O, Svitkina T, Borisy GG, 2006. Role of fascin in filopodial protrusion. J. Cell biology 174, 863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann N, DeRosier D, Matsudaira P, Hanein D, 2001. An atomic model of actin filaments cross-linked by fimbrin and its implications for bundle assembly and function. J. Cell Biol 153, 947–956. doi: 10.1083/jcb.153.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EL, Im J-S, Nakahata MI, Imbery TE, Li S, Chen DB, Noy Y, Archer DW, Xu W, Hashisaki GT, Avraham KB, Shin J-B, 2021. Repair of noise-induced damage to stereocilia F-actin cores is facilitated by XIRP2. BioRxiv doi: 10.1101/2021.08.10.455815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Hudspeth AJ, Gillespie PG, 1993. Calmodulin and calmodulin-binding proteins in hair bundles. Proc. Natl. Acad. Sci. U.S.A 90, 2807–2811. doi: 10.1073/pnas.90.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Walsh V, Vreugde S, Hertzano R, Shahin H, Haika S, Lee MK, Kanaan M, King M-C, Avraham KB, 2002. From flies’ eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc. Natl. Acad. Sci. U.S.A 99, 7518–7523. doi: 10.1073/pnas.102091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh VL, Raviv D, Dror AA , Shahin H, Walsh T, Kanaan MN, Avraham KB, King M-C, 2011. A mouse model for human hearing loss DFNB30 due to loss of function of myosin IIIA. Mamm. Genome 22, 170–177. doi: 10.1007/s00335-010-9310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB, 1998. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science 280, 1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- Wang L, Zou J, Shen Z, Song E, Yang J, 2012. Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II. Hum. Mol. Genet 21, 692–710. doi: 10.1093/hmg/ddr503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li J, Yao X, Li W, Du H, Tang M, Xiong W, Chai R, Xu Z, 2017. Loss of CIB2 causes profound hearing loss and abolishes mechanoelectrical transduction in mice. Front. Mol. Neurosci 10, 401. doi: 10.3389/fnmol.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang Lei, Dai T, Qin Z, Lu H, Zhang Long, Zhou F, 2021. Liquid-liquid phase separation in human health and diseases. Signal Transduct. Target. Ther 6, 290. doi: 10.1038/s41392-021-00678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A, 1976. Head to tail polymerization of actin. J. Mol. Biol 108, 139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Winkelman JD, Anderson CA, Suarez C, Kovar DR, Gardel ML, 2020. Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proc. Natl. Acad. Sci. U.S.A 117, 25532–25542. doi: 10.1073/pnas.2004656117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Zong W, Du H, Zhai X, Ren R, Liu S, Xiong W, Wang Y, Xu Z, 2022. BAIAP2L2 is required for the maintenance of mechanotransducing stereocilia of cochlear hair cells. J. Cell. Physiol 237, 774–788. doi: 10.1002/jcp.30545. [DOI] [PubMed] [Google Scholar]
- Yates TM, Turner CL, Firth HV, Berg J, Pilz DT, 2017. Baraitser-Winter cerebrofrontofacial syndrome. Clin. Genet 92, 3–9. doi: 10.1111/cge.12864. [DOI] [PubMed] [Google Scholar]
- Zampini V, Rüttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, Di Fiore PP, Knipper M, Masetto S, Marcotti W, 2011. Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells. PLoS Biol. 9, e1001048. doi: 10.1371/journal.pbio.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D-S, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP, 2012. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481, 520–524. doi: 10.1038/nature10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Müller U, 2016. Murine Fam65b forms ring-like structures at the base of stereocilia critical for mechanosensory hair cell function. Elife 5. doi: 10.7554/eLife.14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Sekerková G, Vranich K, Tilney LG, Mugnaini E, Bartles JR, 2000. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377–385. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Beeler DM, Bartles JR, 2014. Characterization and regulation of an additional actin-filament-binding site in large isoforms of the stereocilia actin-bundling protein espin. J. Cell Sci 127, 1306–1317. doi: 10.1242/jcs.143255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Adam SA, García-Anoveros J, Mitchell BJ, Bartles JR, 2022. Espin overexpression causes stereocilia defects and provides an anti-capping effect on actin polymerization. Cytoskeleton (Hoboken) 79, 64–74. doi: 10.1002/cm.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Yang T, Wei S, DeWan AT, Morell RJ, Elfenbein JL, Fisher RA, Leal SM, Smith RJH, Friderici KH, 2003. Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am. J. Hum. Genet 73, 1082–1091. doi: 10.1086/379286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


