BACKGROUND:
The primary objective of this study was to characterize the pharmacological and behavioral activity of 2 novel compounds, DM497 [(E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide] and DM490 [(E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide], structural derivatives of PAM-2, a positive allosteric modulator of the α7 nicotinic acetylcholine receptor (nAChR).
METHODS:
A mouse model of oxaliplatin-induced neuropathic pain (2.4 mg/kg, 10 injections) was used to test the pain-relieving properties of DM497 and DM490. To assess possible mechanisms of action, the activity of these compounds was determined at heterologously expressed α7 and α9α10 nAChRs, and voltage-gated N-type calcium channel (CaV2.2) using electrophysiological techniques.
RESULTS:
Cold plate tests indicated that 10 mg/kg DM497 was able to decrease neuropathic pain in mice induced by the chemotherapeutic agent oxaliplatin. In contrast, DM490 induced neither pro- nor antinociceptive activity but inhibited DM497’s effect at equivalent dose (30 mg/kg). These effects are not a product of changes in motor coordination or locomotor activity. At α7 nAChRs, DM497 potentiated whereas DM490 inhibited its activity. In addition, DM490 antagonized the α9α10 nAChR with >8-fold higher potency than that for DM497. In contrast, DM497 and DM490 had minimal inhibitory activity at the CaV2.2 channel. Considering that DM497 did not increase the mouse exploratory activity, an indirect anxiolytic mechanism was not responsible for the observed antineuropathic effect.
CONCLUSIONS:
The antinociceptive activity of DM497 and the concomitant inhibitory effect of DM490 are mediated by opposing modulatory mechanisms on the α7 nAChR, whereas the involvement of other possible nociception targets such as the α9α10 nAChR and CaV2.2 channel can be ruled out.
KEY POINTS.
Question: Can positive allosteric modulators of α7 nicotinic acetylcholine receptors (nAChRs) relieve from neuropathic pain?
Findings: DM497, a positive allosteric modulator of the α7 nAChR, acutely relieves neuropathic pain evoked by oxaliplatin, while DM490, a negative allosteric modulator of the α7 nAChR, antagonized this effect.
Meaning: A positive modulation of the α7 nAChR by DM497 could be a valid strategy to counteract neuropathy evoked by chemotherapeutic drugs
Chronic pain is among the most common conditions for which adults seek medical treatment.1 Unfortunately, the most common pharmacological treatments for pain (ie, nonsteroidal anti-inflammatory drugs, opioids, antidepressants, and anticonvulsants) are not very effective, and in the case of opioids, they pose a high risk of dependence and overdose-induced death. Thus, there is an urgent need to develop nonopioid agents that alleviate chronic pain in an effective and safe manner.
In recent years, promising preclinical data using a variety of animal models showed that positive allosteric modulators (α7-PAMs) of α7 nicotinic acetylcholine receptors (nAChRs) exert potent antinociceptive and anti-inflammatory activities.2–5 These and other studies with selective α7 agonists described antinociceptive mechanisms triggered by activation/potentiation of α7 nAChRs expressed in both central and peripheral nervous systems.6,7 A central mechanism involves, for instance, the modulation of descending pain pathways,8 whereas the most important peripheral processes are those controlled by α7 nAChRs expressed in dorsal root ganglion neurons,9 immunological cells such as lymphocytes and macrophages,7 and glial cells.10
The inhibition of α9α10 nAChRs expressed in immunocompetent cells and lumbar dorsal root ganglion neurons is another important mechanism that reduces pain-inducing inflammation.7,11 Similarly, the inhibition of voltage-gated N-type calcium channel (CaV2.2) by different compounds has been considered an important antinociceptive mechanism.12,13 The antinociceptive activity of PAM-2 and DM489, 2 α7-PAMs, has been partially ascribed to inhibition of peripheral α9α10 nAChRs and CaV2.2 channels.14
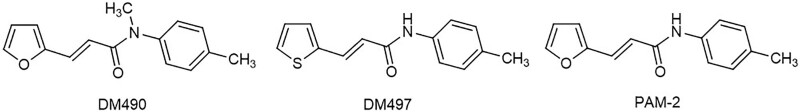
This study aimed to determine the behavioral activity of 2 novel compounds, DM490 [(E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide] and DM497 [(E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide] (see molecular structures in Figure 1), using the oxaliplatin-induced chronic pain model,5,15 a mouse model that resembles chemotherapeutic conditions in cancer patients. These 2 compounds have been recently synthesized based on the structure of PAM-2 [(E)-3-furan-2-yl-N-p-tolyl-acrylamide], an α7-PAM with known antinociceptive and anti-inflammatory properties.4,5 To determine the involved mechanisms, the pharmacological activity of DM497 and DM490 was assessed at heterologous rat α7 and α9α10 nAChRs expressed in Xenopus laevis oocytes using 2-electrode voltage clamp recordings.
Figure 1.
Molecular structures of the novel compounds, DM490 and DM497, compared to PAM-2. DM490 indicates (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide; DM497, (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide; PAM-2, (E)-3-furan-2-yl-N-p-tolyl-acrylamide.
In addition, both compounds were tested at transiently expressed human CaV2.2 channels, using the patch-clamp technique.
We propose that the antinociceptive activity of DM497 and the concomitant reduction of this activity elicited by DM490 are mediated by the respective positive and negative modulation of the α7 nAChR, whereas the involvement of other possible targets, such as the α9α10 nAChR and CaV2.2 channel, can be excluded.
METHODS
Materials
Acetylcholine chloride (ACh) and BAPTA-AM (1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid acetoxymethyl ester) were purchased from Sigma-Aldrich. Glucose and carboxymethyl cellulose were obtained from Sigma-Aldrich SRL. Oxaliplatin was obtained from Carbosynth. CGP 55845 hydrochloride (((2S)-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride)) was purchased from Tocris Bioscience. Ham’s F10 medium, horse serum, and hygromycin B were purchased from Thermo Fisher Scientific. Fetal bovine serum (FBS) was obtained from Bovogen and Thermo Fisher Scientific. Dulbecco's modified Eagle medium (DMEM), GlutaMAX, penicillin, and streptomycin were purchased from Invitrogen Life Technologies. DM490 and DM497 were synthesized by a method (manuscript in preparation) similar to that for PAM-2 and DM489.5 Salts and other compounds were of analytical grade.
Animals
Male CD-1 albino mice (Envigo), 2 to 3 months old (22–25 g), were housed in CeSAL (Centro Stabulazione Animali da Laboratorio, University of Florence) and used at least 1 week after their arrival. Mice (10/cage; size 26 × 41 cm) were fed a standard laboratory diet and tap water ad libitum and kept at 23 °C ± 1 °C with a 12-hour light/dark cycle, light at 7 am. All animal manipulations were performed according to the Directive 2010/63/EU of the European parliament and of the European Union council (September 22, 2010) on the protection of animals used for scientific purposes and have been reported according to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.16 Formal approval to conduct animal experiments was obtained from the animal subjects review board of the University of Florence, in compliance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health. All efforts were made to minimize animal suffering and to reduce the number of animals used. A randomization of animals between groups and treatments was performed. The investigators responsible for data analysis were blind to which animals represent treatments and controls.
Female Xenopus laevis were sourced from Nasco, and a maximum of 3 frogs were kept in purpose-built 15-L aquarium at 20 °C to 26 °C with 12-hour light/dark cycle. Oocytes were obtained from 5-year-old frogs anesthetized with 1.7 mg/mL ethyl 3-aminobenzoate methanesulfonate (pH 7.4 with NaHCO3). For recovery, postsurgery animals were placed in fresh water at level below the nostrils. Frogs were allowed to recover for a minimum of 4 months between surgeries. Terminal anesthesia with 5 mg/mL ethyl 3-aminobenzoate methanesulfonate was performed after the sixth surgery. All procedures were approved by the Animal Ethics Committees from University of Wollongong and University of Sydney (project number AE2003 and 2016/970, respectively).
Lipophilicity and Brain Penetration Properties of DM497 and DM490
To predict whether DM497 and DM490 could cross the blood-brain barrier, the lipophilicity (ie, LogP) and brain penetration (LogBBB = Log[brain]/[plasma]) parameters were calculated using Biovia Discovery Studio software (Dassault Systèmes Co). The scale for LogBBB ranges from values ≥0.7 (very high brain penetration), between 0 and 0.7 (high penetration), between −0.01 and −0.52 (medium penetration), and ≤−0.52 (low penetration). This software has been previously used to estimate LogBBB values for other α7-PAMs.5,17
Effect of DM497 and DM490 on Oxaliplatin-Induced Neuropathic Pain
To develop neuropathic pain, mice (n = 10/condition) were injected (intraperitoneally [i.p.]) with 2.4 mg/kg oxaliplatin (dissolved in 5% glucose solution [vehicle]), during 5 days per week for 2 weeks.14,15,18 On day 15, pain threshold was evaluated using the cold plate test. On the same day, dedicated groups of oxaliplatin-treated mice were administered orally DM497 (1, 3, 10, or 30 mg/kg), DM490 (30 mg/kg), or DM497+DM490 (30 mg/kg each), and the response latency subsequently determined every 15 minutes for a total time of 60 to 75 minutes, using the cold plate test. Each drug was suspended in 1% carboxymethyl cellulose (ie, vehicle). Control animals received an equivalent volume of vehicle.
Effect of DM490 on Pain Threshold
To assess whether DM490 changes the pain threshold, mice (n = 10/condition) were orally administered a single dose of 30 mg/kg DM490 (acute treatment) or a daily dose of 30 mg/kg DM490 for 7 consecutive days (repeated treatment), or vehicle. The response latency was assessed either right after drug administration (acute treatment) or on day 8 (repeated treatment), using both cold and hot plate tests, each recorded every 15 minutes for a total time of 1 hour.
Cold Plate Test
Thermal allodynia was assessed using the cold plate test as described before.19,20 Pain-related behavior (licking of the hind paw) was determined by recording the time (seconds) of the first sign of licking of the hind paw. Measurements were taken every 15 minutes for a total time of 60 to 75 minutes. The cutoff time of the response latency was set at 30 seconds.18,19
Hot Plate Test
With minimal animal-handler interaction, mice were taken from home cages and placed onto the surface of the hot plate (Ugo Basile) maintained at a constant temperature of 49 °C ± 1 °C. The time (in seconds) of the first sign of pain-related behavior, including lifting or licking of the hind paw (ie, response latency), was recorded every 15 minutes for a total time of 1 hour. The cutoff time of the licking latency was set at 40 seconds.21
Rota-Rod Test
To determine the integrity of motor coordination in mice after drug treatment, the rota-rod test was used as described previously.22,23 Mice (n = 5/condition) were administered (i.p.) a single dose of 30 mg/kg DM490 or DM497 (acute treatment), a daily dose of 30 mg/kg DM490 for 7 consecutive days (repeated treatment), or vehicle. The integrity of motor coordination was assessed considering the number of falls from the rod during the total time of the test (30 seconds). Mice scoring <3 and >6 falls in the pretest were rejected (20%).21,22
Hole-Board Test
To determine the spontaneous motility (board) and exploratory activity (hole) of mice, the hole-board test was used.14,24 Mice (n = 5/condition) were administered (i.p.) a single dose of 30 mg/kg DM490 or DM497 (acute treatment), a daily dose of 30 mg/kg DM490 for 7 consecutive days (repeated treatment), or vehicle. Locomotor activity (board) and exploratory activity (hole) were each recorded right after acute administration or on day 8 (after repeated treatment) during 5 minutes, respectively.
Activity of DM497 and DM490 at α7 and α9α10 nAChRs Heterologously Expressed in Xenopus Laevis Oocytes
Rat (r) α7 and α9α10 nAChRs were heterologously expressed in X. laevis oocytes, as described previously.5 Oocytes were incubated at 18 °C in sterile ND96 buffer (in mM): 96 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4, supplemented with 5% FBS, 0.1 mg/mL gentamicin, and 100 U/mL penicillin-streptomycin. Two-electrode voltage clamp recordings were performed 2–7 days after cRNA microinjection using a GeneClamp 500B amplifier and pClamp9 software interface (Molecular Devices) at a holding potential of –80 mV and room temperature (RT, 21 °C–23 °C). Voltage-recording and current-injecting electrodes were pulled from GC150T-7.5 borosilicate glass (Harvard Apparatus) and filled with 3 M KCl, giving resistances of 0.3–1 MΩ.
Oocytes expressing α9α10 nAChRs were incubated with 100 μM BAPTA-AM at 18 °C for ~3 hours before recording to minimize the activation of X. laevis oocyte endogenous calcium-activated chloride channels. Oocytes expressing α7 or α9α10nAChRs were, respectively, washed (2 mL/min) with ND96 or ND115 buffer (in mM): 115 NaCl, 2.5 KCl, 1.8 CaCl2, 10 HEPES, pH 7.4, followed by ACh application at different concentrations (ie, 30, 100, and 300 µM for α7; or 10 and 100 µM for α9α10). The 30 µM ACh concentration corresponds to the rα7 ACh 20%-maximal effective concentration (EC20) based on reported ACh half-maximal effective concentration (EC50),5 and the 10 µM ACh concentration corresponds to the rα9α10 ACh EC50.25 Oocytes were subsequently incubated with DM497 or DM490 (1–300 µM; prepared in ND96/ND115 + 0.1% FBS) for 5 minutes with the perfusion system stopped, followed by coapplication of ACh and the compound with flowing bath solution. Incubation with 0.1% FBS was performed to ensure that the FBS and the pressure of the perfusion system had no effect on nAChRs.
Whole-Cell Patch Clamp Recording of CaV2.2 Channels Expressed in HEK293T Cells
Human CaV2.2 channels were expressed in human embryonic kidney 293T (HEK293T) cells as described previously.14 Whole-cell patch clamp recordings were performed at RT within 24 to 48 hours after transfection. Cells were constantly superfused using a gravity flow perfusion system (AutoMate Scientific) with extracellular solution containing (in mM): 110 NaCl, 10 BaCl2, 1 MgCl2, 5 CsCl, 30 TEA-Cl, 10 glucose, 10 HEPES (pH 7.35 with TEA-OH; ~310 mOsmol/kg). Fire-polished borosilicate patch pipettes (2–3 MΩ) were filled with intracellular solution containing (in mM): 125 K-Gluconate, 5 NaCl, 2 MgCl2, 5 EGTA, and 10 HEPES (pH 7.2 with KOH; ~290 mOsmol/kg). Inward Ba2+ currents were elicited by a test depolarization to −10 mV (50 ms duration) from a holding potential of −80 mV applied at 0.1 Hz. Solutions containing DM497 or DM490 were prepared in the external solution.
Statistical Analysis of Data
The concentration-response relationships were analyzed using the Prism 7 software (GraphPad) according to the Hill equation. At least 8 animals for each experimental group were used. Experiments were conducted by researchers blinded to the treatments. One-way analysis of variance (ANOVA) and post hoc analysis with Bonferroni adjustments for multiple comparisons were assessed using the Origin software. Values of P < .05 were considered statistically significant.
RESULTS
Brain Permeability Prediction for DM497 and DM490
Both DM497 and DM490 have LogBBB values of 0.39 and 0.30, respectively (Supplemental Digital Content 1, Table 1, http://links.lww.com/AA/E278). Based on Biovia’s scale (see Methods), these values correspond to high brain permeability. In comparison, PAM-2 has a lower brain penetration (LogBBB = 0.095). The improvement in LogBBB of DM497 and DM490 reflects the increase of lipophilicity (LogP = 3.52 and 3.17, respectively) with respect to PAM-2 (LogP = 2.965) (Supplemental Digital Content, Table 1, http://links.lww.com/AA/E278). This is due to the replacement of the polar furanyl oxygen atom of PAM-2 with the less electronegative thienyl sulfur atom in DM497, and to the loss of an H-bond donor moiety in DM490 due to the methylation of the secondary amide group of PAM-2 (Figure 1).
DM497 Decreases Oxaliplatin-Induced Neuropathic Pain in Mice, Whereas DM490 Inhibits This Effect
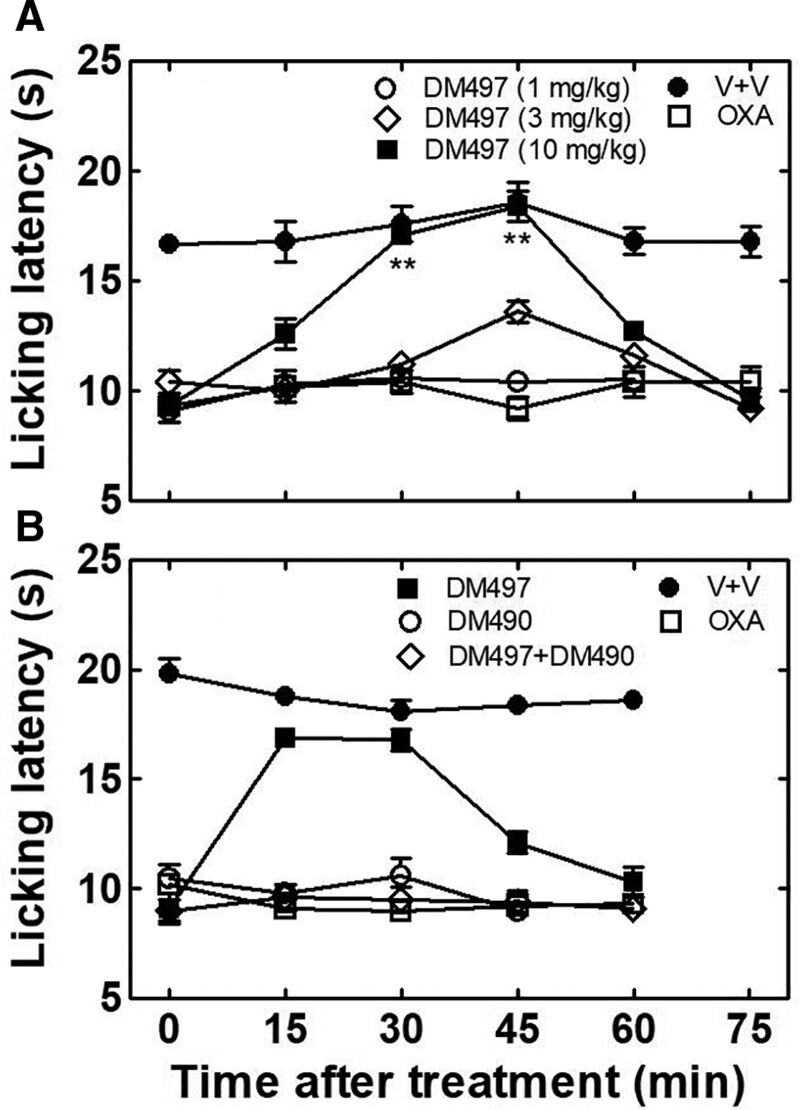
The pain-relieving properties of DM497 and DM490 were evaluated using the oxaliplatin-induced neuropathic pain model.14,15 Treatment (i.p.) with 2.4 mg/kg oxaliplatin progressively developed neuropathic pain in mice as evaluated by the cold plate test, a measurement of thermal allodynia, on day 15, where the licking latency decreased to an average value of 10.0 ± 0.5 seconds (oxaliplatin + vehicle-treated mice) compared to vehicle-treated animals (17.2 ± 0.7 seconds; P < .01) as indicated by 1-way ANOVA and Bonferroni post hoc analyses (Figure 2A). The same behavioral test showed that a single acute administration of 10 mg/kg DM497, but not 1 or 3 mg/kg, possessed antinociceptive activity compared to oxaliplatin-treated animals, with maximal effect in the 30 to 45 minutes interval (P < .01), without inducing any activity when administered alone (Figure 2A). A higher dose of DM497 (30 mg/kg) shortened the onset of activity from 30 minutes to 15 minutes compared to that at 10 mg/kg DM497, without changing the extent of the antinociceptive effect (Figure 2B, cold plate test), suggesting that the maximal effect was attained at 10 mg/kg. Interestingly, 1-way ANOVA and Bonferroni’s post hoc analyses of the licking latency (mean ± standard error of the mean [SEM]) indicated that 30 mg/kg DM490 decreased the antinociceptive effect elicited by 30 mg/kg DM497 in DM497 + DM490-treated mice (P < .01) in comparison to animals treated only with DM497, without inducing any activity on oxaliplatin-treated animals in the cold plate test (Figure 2B).
Figure 2.
Effect of DM497 and DM490 on oxaliplatin-induced neuropathic pain in mice. Mice (n = 10/condition) were treated (i.p.) with 2.4 mg/kg oxaliplatin to induce neuropathic pain. On day 15, the response to a thermal stimulus was evaluated by the cold plate test. The licking latency (mean ± SEM) for the first signs of pain-related behavior was recorded every 15 min for 60 to 75 min. A, Mice were then orally administered DM497 at different doses [1 (○), 3 (◊), and 10 () mg/kg] or vehicle, and the response to a thermal stimulus subsequently evaluated by the cold plate test. One-way ANOVA and Bonferroni’s post hoc analyses indicated that oxaliplatin + vehicle induced neuropathic pain (□) during the whole testing time (P < .01 versus vehicle + vehicle-treated mice [●]), and that 10, but not 1 (○) or 3 (◊), mg/kg DM497 () decreased oxaliplatin-induced neuropathic pain during the 30–45 min period (**P < .01). B, Oxaliplatin-treated mice were subsequently orally administered 30 mg/kg DM497 (), 30 mg/kg DM490 (○), or DM490 + DM497 (30 mg/kg each) (◊). Statistical analyses indicated that although the antinociceptive effect of 30 mg/kg DM497 () was not different to that of 10 mg/kg DM497 ([ ] in [A]), an earlier onset was observed, lasting during the 15–45 min period (P < .01). DM490 (30 mg/kg) inhibited the antinociceptive activity elicited by 30 mg/kg DM497 (◊) (P < .01), without changing the effect of oxaliplatin (○), compared to oxaliplatin + vehicle-treated mice (□). ANOVA indicates analysis of variance; DM490, (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide; DM497, (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide; OXA, oxaliplatin; SEM, standard error of the mean; V, vehicle.
Neither Acute Nor Repeated Administration of DM490 Changes the Pain Threshold
To determine whether DM490 induces pain or not after acute and repeated treatments, both cold and hot plate tests were used. Acute or repeated administration (7 days) of 30 mg/kg DM490 did not change the pain threshold for heat or cold stimuli. One-way ANOVA and Bonferroni’s post hoc analyses of the licking latency (mean ± SEM) indicated that neither acute nor repeated treatment induces significant effect (P > .05) compared to vehicle-treated animals (Supplemental Digital Content 2, Table 2, http://links.lww.com/AA/E279).
Effect of DM490 and DM497 on Mouse Motor Coordination, Spontaneous Motility, and Exploratory Activity
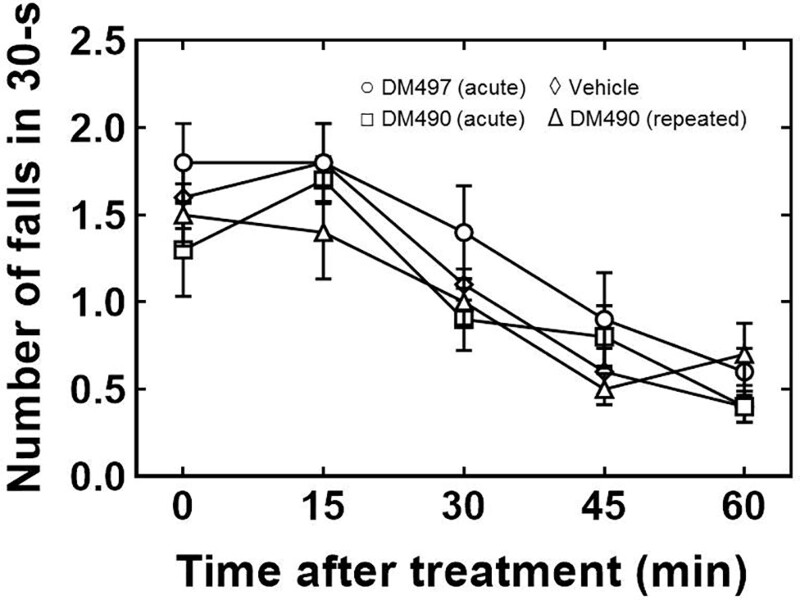
The effect of DM490 and DM497 on mouse motor coordination was evaluated using the rota-rod test. Since previous experiments showed that oxaliplatin perturbs motor coordination,14 mice were not pretreated with this drug. The results showed that the acute administration of DM490 or DM497 at the highest dose used in the cold plate tests (30 mg/kg), or the repeated treatment with DM490 at the same dose, did not modify the endurance time (ie, the number of falls per 30 seconds), counted every 15 minutes for 1 hour (Figure 3). One-way ANOVA and Bonferroni’s post hoc analyses showed no statistical difference with vehicle-treated animals (P > .05), indicating that mouse motor coordination was not altered after acute or repeated treatment.
Figure 3.
Effect of DM497 and DM490 on mouse motor coordination determined by the rota-rod test. Mice (n = 5/condition) were orally administered a single dose of 30 mg/kg DM490 (□) or DM497 (○) (acute treatment), a daily dose of 30 mg/kg DM490 for 7 consecutive days (Δ) (repeated treatment), or vehicle (◊). The endurance time (ie, number of falls per 30 s; counted every 15 min for 1 h) was determined right after the acute treatment or on day 8, after repeated (7 d) treatment. One-way ANOVA and Bonferroni’s post hoc analyses showed no statistical difference with vehicle-treated animals (P < .05), indicating that no drug alters mice motor coordination after acute or repeated treatment. ANOVA indicates analysis of variance; DM490, (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide; DM497, (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide.
The spontaneous motility and exploratory activity of the animals in the presence of DM490 and DM497 were also assessed using the hole-board test. As oxaliplatin reduces locomotor activity, mice were not pretreated with the drug.14 The results showed that the acute administration of DM490 or DM497 (30 mg/kg) or the repeated treatment with DM490 at the same dose modify neither the number of crossings through the board’s surface attained in 5 minutes (spontaneous motility) nor the number of times the mouse inserts its head in the holes during 5 minutes (exploratory activity) (Supplemental Digital Content 3, Table 3, http://links.lww.com/AA/E280). One-way ANOVA and Bonferroni post hoc analyses showed no statistical difference with vehicle-treated animals (P > .05), indicating that both drugs affected neither locomotor nor exploratory activity after acute or repeated treatment.
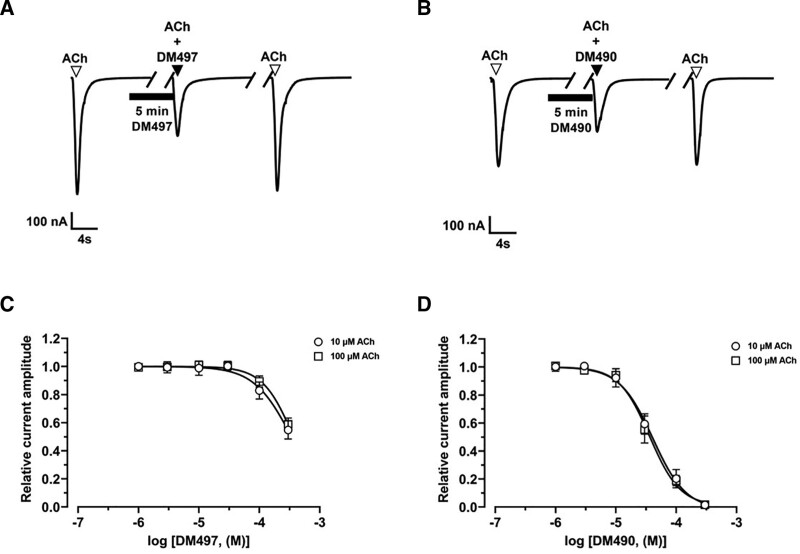
DM497 Potentiates, Whereas DM490 Inhibits, ACh-Activated Rat α7 nAChR Currents
Since our behavioral studies showed that DM490 decreases the antinociceptive activity of DM497 at equivalent doses (Figure 2), we determined the pharmacological activity of each drug at rα7 nAChRs heterologously expressed in X. leavis oocytes using the 2-electrode voltage clamp recording technique.
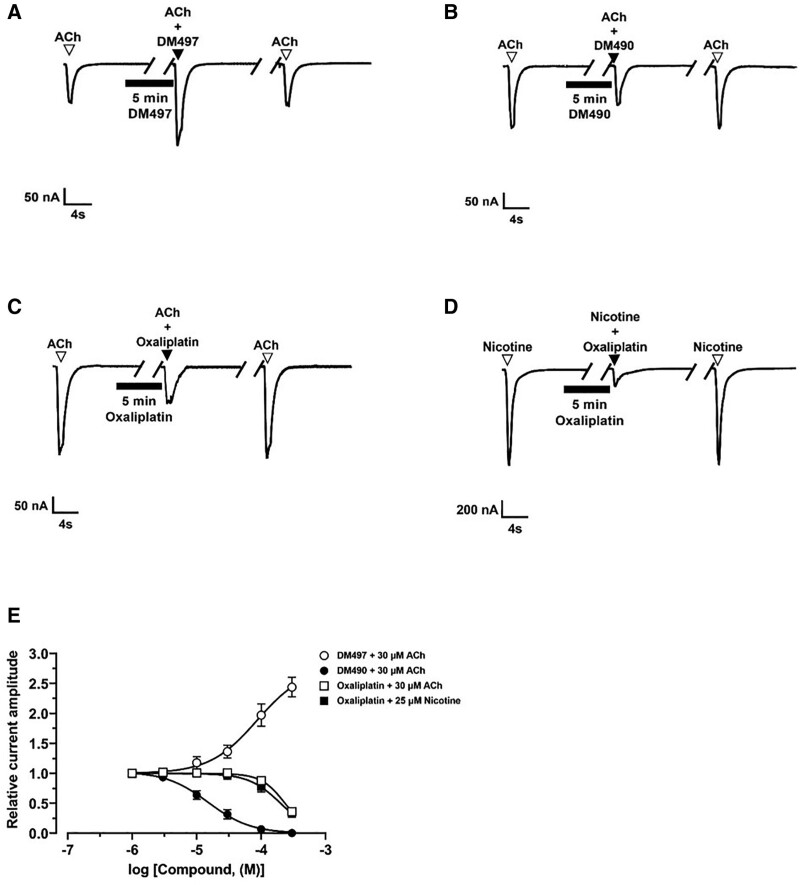
In the presence of 30 µM ACh (ie, rα7 ACh EC20), 100 µM DM497 potentiated ACh-evoked currents by ~100% (Figure 4A), whereas 10 µM DM490 inhibited the current amplitude by ~40% (Figure 4B). The concentration-response relationships indicated that the inhibitory potency of DM490 (IC50 = 15.1 ± 1.8 µM) was higher than the enhancing potency of DM497 (EC50 = 86 ± 10 µM) (Supplemental Digital Content 4, Table 4, http://links.lww.com/AA/E281). The fitted nH values for DM497 (near 2) and DM490 (<1.5) suggested that the observed potentiating and inhibitory effects are mediated by cooperative and noncooperative mechanisms, respectively.
Figure 4.
Effect of DM497 and DM490 on ACh-activated rα7 nAChRs. Representative ACh (30 µM)-evoked rα7 currents obtained at −80 mV in the presence of 100 µM DM497 (A) and 10 µM DM490 (B), respectively. Representative ACh (30 µM)- (C) and nicotine (25 µM)-evoked rα7 currents (D) in the presence of 300 µM oxaliplatin. ▼, ACh alone; ▼, coapplication of ACh + compound after 5 min incubation (▬) with compound alone; ▼, ACh alone after washout. E, Concentration-response relationships for DM497 (○) (goodness of fit [r2] = 0.96), DM490 (●) (r2 = 0.98), and oxaliplatin (ACh: [□], nicotine [ ]; r2 = 0.98 for each curve). Current amplitudes were normalized to the response elicited by the respective ACh (mean ± SD; n = 7–12) or nicotine (mean ± SD; n = 8–11) concentration. The calculated EC50, Emax, IC50, and nH values are summarized in Supplemental Digital Content, Table 4, http://links.lww.com/AA/E281. ACh indicates acetylcholine; DM490, (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide; DM497, (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide; nAChR, nicotinic acetylcholine receptor.
To assess whether the proinflammatory and pronociceptive effects of oxaliplatin are mediated by direct interaction with α7 nAChRs, the activity of this compound was also determined at ACh- and nicotine-evoked currents (Figure 4C, D). Oxaliplatin had relatively low potency (IC50 200 µM) at ACh (30 µM)- and nicotine (25 µM)-evoked rα7 nAChR currents (Figure 4E; Supplemental Digital Content, Table 4, http://links.lww.com/AA/E281). This indicates that although this chemotherapeutic agent can downregulate α7 nAChRs,26 the effect was not triggered by direct inhibition of the channel activity.
DM497 and DM490 Inhibit α9α10 nAChRs by a Noncompetitive Mechanism
The activity of DM497 and DM490 was also determined at heterologous rα9α10 nAChRs using 2-electrode voltage clamp recordings. In the presence of 10 µM ACh, DM497 (300 μM) inhibited ACh-evoked currents mediated by α9α10 (Figure 5A) by 40%, a value similar as that elicited by DM490 (~45%) at a lower concentration (30 μM) (Figure 5B). The IC50 values indicated that DM490 (41 ± 4 µM) was more potent than DM497 (340 ± 21 µM) (Figure 5C; Supplemental Digital ContentTable 4, http://links.lww.com/AA/E281). This difference was also observed at 100 µM ACh, with IC50 values of 36 ± 4 µM and 369 ± 21 µM, respectively (Supplemental Digital Content, Table 4, http://links.lww.com/AA/E281). As the calculated IC50 values for each ligand were not modified by a 10-fold change in the ACh concentration, we can conclude that the α9α10 nAChR inhibition was mediated by a noncompetitive mechanism.
Figure 5.
Effect of DM497 and DM490 on ACh-activated rα9α10 nAChRs. Representative ACh (10 µM)-evoked rα9α10 currents obtained at −80 mV in the presence of 300 µM DM497 (A) or 30 µM DM490 (B). ▼, ACh alone; ▼, coapplication of ACh + compound after 5-min incubation (▬) with compound alone; ▼, ACh alone after washout. Concentration-response relationships for DM497 (C) and DM490 (D) at 10 µM and 100 µM ACh, respectively (r2 = 0.96–0.98). Current amplitudes (mean ± SD; n = 7–9) were normalized to the response elicited by ACh alone. The calculated IC50 and nH values are summarized in Supplemental Digital Content, Table 4, http://links.lww.com/AA/E281. Ach indicates acetylcholine; DM490, (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide; DM497, (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide; nAChR, nicotinic acetylcholine receptor.
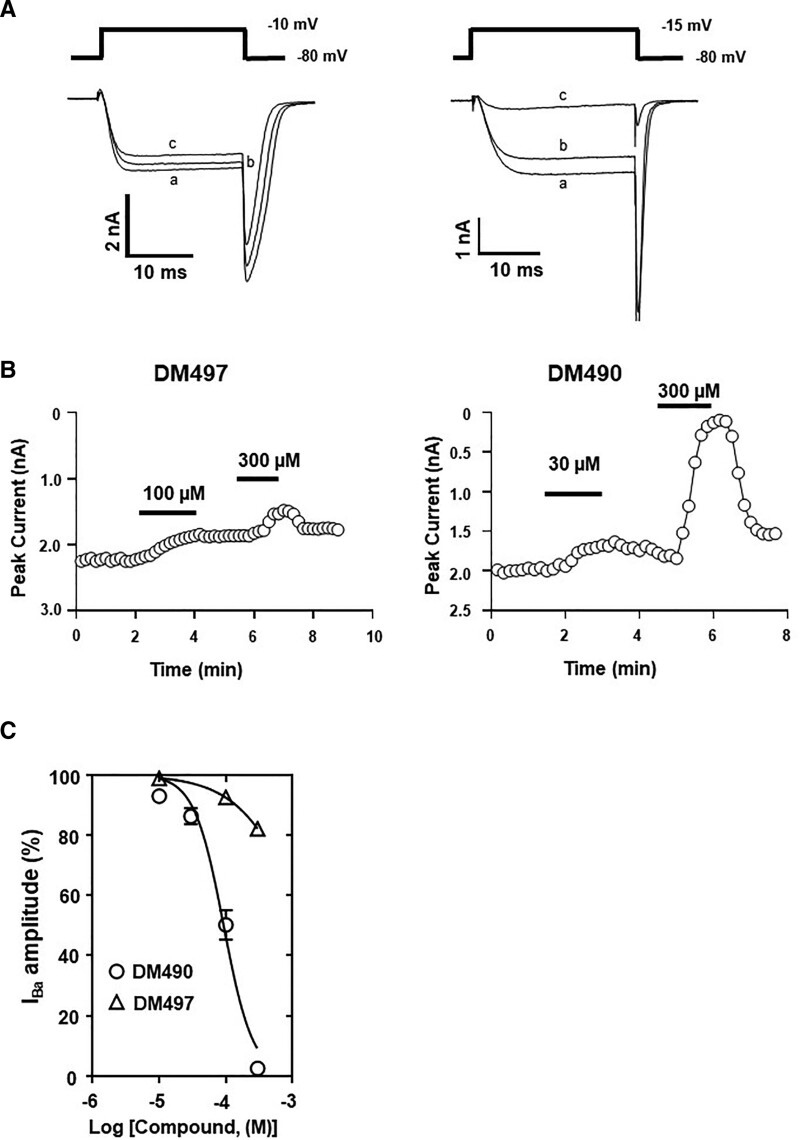
DM497 and DM490 Inhibit CaV2.2 Channels With Relatively Low Potency
Considering previous studies showing that PAM-2 and DM489 directly inhibit human CaV2.2 channels,14 we investigated the activity of DM497 and DM490 on this channel. The results indicated that DM497 only partially inhibits whole-cell Ba2+ currents by ~20% (Figure 6A), whereas DM490 inhibits the currents in a concentration-dependent manner (Figure 6B). The concentration-response curve for DM490 (Figure 6C) showed a relatively low potency for this ligand (IC50 = 93 ± 16 µM) (Supplemental Digital Content, Table 4, http://links.lww.com/AA/E281).
Figure 6.
Inhibitory activity of DM497 and DM490 at hCaV2.2 channels. A, Representative depolarization-activated Ba2+ currents (IBa) elicited from a holding potential of −80 mV to a test potential of either −10mV or −15 mV (25 ms duration; 0.1 Hz) from HEK293T cells expressing hCaV2.2 channels in the absence (a) and presence of 100 µM (b) and 300 µM (c) DM497, respectively, or in the presence of 30 µM (b) and 300 µM (c) DM490, respectively. B, Time-dependent plot of IBa amplitudes before, during, and on washout of DM497 or DM490. C, Concentration-response relationship for the inhibitory activity of DM497 (∆) (r2 = 0.95) and DM490 (○) (r2 = 0.97) at hCaV2.2 channels (mean ± SEM; n = 4). The calculated IC50 and nH values are summarized in Supplemental Digital Content, Table 4, http://links.lww.com/AA/E281. DM490 indicates (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide; DM497, (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide; hCaV2.2, human CaV2.2; HEK293T, human embryonic kidney 293T; SEM, standard error of the mean.
DISCUSSION
The primary objective of this study was to determine the effects of DM497 and DM490 on the pronociceptive activity of the chemotherapeutic agent oxaliplatin in mice. To determine potential molecular mechanisms, electrophysiological studies were also performed.
The cold plate results showed that a single administration of 10 mg/kg, but not 1 or 3 mg/kg, DM497 reduces oxaliplatin-induced neuropathic pain in mice, and that a higher dose (30 mg/kg) shortened the onset without changing the extent of this activity. These results in general agreed with previous studies with PAM-2 and DM489 using the same oxaliplatin model.5 It is interesting that our study also showed that DM490 inhibits the antineuropathic effect of DM497 at equivalent doses. In contrast, DM490 (alone) affected neither the pronociceptive activity of oxaliplatin nor the pain threshold for heat or cold stimuli, indicating no behavioral activity per se. Additional behavioral tests indicated that the activities elicited by DM497 and DM490 are not the result of, or are not perturbed by, spurious motor effects. Our hole-board tests also indicated that DM497 and DM490 do not increase the exploratory behavior of mice, suggesting a lack of anxiolytic or stimulatory activity,25 and thus, discarding the possibility that the observed antinociceptive activity is mediated by an indirect anxiolytic or stimulatory effect.27
The observed behavioral effects, on the other hand, are consistent with the electrophysiological results showing that DM497 potentiates, whereas DM490 inhibits, α7 nAChRs. Thus, DM497 behaves as an α7-PAM, whereas DM490 acts as a novel negative allosteric modulator of α7 nAChRs (α7-NAM), inhibiting the receptor with relatively higher potency. Our results also ruled out the possibility that oxaliplatin per se induces the observed behavioral effects through direct inhibition of the α7 nAChR. New data from our laboratory showed that oxaliplatin increases the production of proinflammatory molecules in brain and spinal cord of treated animals (unpublished observations).
To assess whether α7-PAMs with higher brain penetration induce maximal antinociceptive activity at lower doses, the LogBBB value for DM497 was calculated and compared to that for DM489 and PAM-2 using the same software.5 The observed correlation for the respective brain penetration (DM497 [0.39] > DM489 [0.17] > PAM-2 [0.09]) was opposite to their active doses, where α7-PAMs with lower brain penetration such as PAM-2-induced antinociceptive activity at lower doses (3 mg/kg) than those with higher brain penetration such as DM497 and DM489 (10 mg/kg each)5 (this work). A possible explanation is that lipophilic compounds such as DM497 (LogP = 3.52) are more likely sequestered in fatty tissues and/or bound to transporter proteins, decreasing the free (active) population, compared to relatively hydrophilic compounds such as PAM-2 (2.96).5 This also suggests that DM497 might be acting at peripheral sites. An obvious target is the α7 nAChR expressed in immunocompetent cells, such as macrophages7 and glial cells,10 and in dorsal root ganglion neurons.9 Although we did not determine the activity of DM497 at those potential sites, new results from our laboratory showed that PAM-2 inhibits oxaliplatin-induced proinflammatory activity in glial cells (unpublished observations), supporting the anti-inflammatory role of glial α7 nAChRs.
The unexpected lack of correlation between brain penetration and active doses suggests the involvement of other mechanisms. To understand this dichotomy, electrophysiological studies were performed at a variety of potential membrane targets. Our results on rα7 nAChRs combined with previous experiments under the same conditions5 allowed us to make comparisons between the potency or maximal efficacy and the active doses of these compounds. The relatively lower potency of DM497 (IC50 = 86 µM) compared to that for PAM-2 (IC50 = 12 µM) correlates with the observed active doses, whereas the higher efficacy of DM497 (196% ± 14%) with respect to that for PAM-2 (135% ± 4%) shows an opposite correlation with the observed active doses. A possible explanation is that compounds with higher lipophilicity interact less with the receptor. However, DM490 (3.17), which is relatively less lipophilic than DM497 (3.52), inhibits the rα7 nAChR with relatively higher potency (IC50 = 15.1 µM) compared to the enhancing potency of DM497.
Considering that both α9α10 nAChR and CaV2.2 channel are involved in the modulation of chronic pain7,11–13 and are potential targets for α7-PAMs,5 subsequent electrophysiological experiments were performed with DM497 and DM490, and the results were compared to previous studies.5,28 The inhibitory potencies (IC50s) obtained in the α9α10 nAChR under the same experimental conditions follow the sequence: DM489 (39 µM) ~ DM490 (41 µM) > PAM-2 (174 µM) > DM497 (340 µM), suggesting that DM489 and DM490 might have a discreet effect at this nAChR subtype. However, DM490 does not decrease oxaliplatin-induced chronic pain, indicating that the antinociceptive activity of DM497 (this work), and likely other α7-PAMs,5 is not mediated by inhibition of peripheral α9α10 nAChRs. The results on the CaV2.2 channel showed that DM490 (IC50 = 93 µM) has weak inhibitory activity, whereas DM497 produced minimal effect. Considering that DM490, an α7-NAM with no antinociceptive activity, inhibited the CaV2.2 channel with potency similar as that for PAM-2 (IC50 = 89 µM), an α7-PAM with relatively strong antinociceptive activity,5 we can rule out the involvement of this calcium channel in the antinociceptive activity of α7-PAMs.
In conclusion, the antinociceptive activity of DM497 and the concomitant reduction of the activity elicited by DM490 are mediated by positive and negative modulation of the α7 nAChR, respectively, whereas the involvement of other possible targets such as the α9α10 nAChR and CaV2.2 channel can be ruled out.
DISCLOSURES
Name: Hugo R. Arias, PhD.
Contribution: This author developed the concept of the study, wrote the overall manuscript, performed data analyses, and contributed to critical comments on the manuscript and discussion.
Name: Han-Shen Tae, PhD.
Contribution: This author performed the electrophysiological experiments, wrote the Methods and Results, performed the data analyses, and contributed to critical comments on the manuscript and discussion.
Name: Laura Micheli, PhD.
Contribution: This author performed the animal studies.
Name: Arsalan Yousuf, PhD.
Contribution: This author performed the electrophysiological experiments.
Name: Dina Manetti, PhD.
Contribution: This author synthesized the compounds.
Name: Maria Novella Romanelli, PhD.
Contribution: This author synthesized the compounds.
Name: Carla Ghelardini, PhD.
Contribution: This author contributed to critical comments on the manuscript and discussion.
Name: David J. Adams, PhD.
Contribution: This author performed data analyses and contributed to critical comments on the manuscript and discussion.
Name: Lorenzo Di Cesare Mannelli, PhD.
Contribution: This author wrote the Methods and Results, performed the data analyses, and contributed to critical comments on the manuscript and discussion.
This manuscript was handled by: Jianren Mao, MD, PhD.
Supplementary Material
GLOSSARY
- ACh =
- acetylcholine
- α7-PAMs =
- a7-postive allosteric modulators
- ANOVA =
- analysis of variance
- ARRIVE =
- Animal Research: Reporting of In Vivo Experiments
- BAPTA-AM =
- 1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid acetoxymethyl ester
- CaV2.2 =
- voltage-gated N-type calcium channel
- CeSAL =
- Centro Stabulazione Animali da Laboratorio
- CGP =
- (2S)-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride
- DM489 =
- (E)-3-(furan-2-yl)-1-(indolin-1-yl)prop-2-en-1-one)
- DM490 =
- (E)-3-(furan-2-yl)-N-methyl-N-(p-tolyl)acrylamide
- DM497 =
- (E)-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide
- DMEM =
- Dulbecco's modified Eagle medium
- EC20 = =
- ligand concentration that produces 20%-maximal potentiation
- EC50 = =
- ligand concentration that produces half-maximal potentiation
- Emax = =
- maximal potentiation
- FBS =
- fetal bovine serum
- HEK293T =
- human embryonic kidney 293T
- IC50 = =
- ligand concentration that produces half-maximal inhibition
- nAChR =
- nicotinic acetylcholine receptor
- NAM =
- negative allosteric modulator
- nH = =
- Hill coefficient
- PAM =
- positive allosteric modulator
- PAM-2 =
- (E)-3-Furan-2-yl-N-p-tolyl-acrylamide
- r2 =
- goodness of fit;
- RT =
- room temperature
- SEM =
- standard error of the mean
- TEA-OH =
- tetraethylammonium hydroxide
Funding: This work was supported by the IMI2 project NeuroDerisk (821528), by the “Knowpain. Identification of Key gene polymorphisms and epigenetic alterations for the developmeNt Of neW Pharmacological Approaches In the treatment of oxaliplatin-related Neurotoxicity in patients with colorectal cancer” project, and by the Italian Ministry of Instruction, University and Research (MIUR), Italy (to C.G. and L.D.C.M.), from University of Florence, Italy (to M.N.R. and D.M.), the Australian Research Council (Discovery Project Grant DP150103990) (to D.J.A.), and by OVPR Pilot/Seed Grants (Oklahoma State University Center for Health Sciences) (to H.R.A.).
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
H. R. Arias and H.-S. Tae contributed equally and share first authorship.
REFERENCES
- 1.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freitas K, Carroll FI, Damaj MI. The antinociceptive effects of nicotinic receptors α 7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 2013;344:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas M, Alzarea S, Papke RL, Rahman S. The α7 nicotinic acetylcholine receptor positive allosteric modulator prevents lipopolysaccharide-induced allodynia, hyperalgesia and TNF-α in the hippocampus in mice. Pharmacol Rep. 2019;71:1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagdas D, Targowska-Duda KM, López JJ, Perez EG, Arias HR, Damaj MI. The antinociceptive and antiinflammatory properties of 3-furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of α7 nicotinic acetylcholine receptors in mice. Anesth Analg. 2015;121:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias HR, Ghelardini C, Lucarini E, et al. E-3-Furan-2-yl-N-p-tolyl-acrylamide and its derivative DM489 decrease neuropathic pain in mice predominantly by α7 nicotinic acetylcholine receptor potentiation. ACS Chem Neurosci. 2020;11:3603–3614. [DOI] [PubMed] [Google Scholar]
- 6.Gao B, Hierl M, Clarkin K, et al. Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 2010;149:33–49. [DOI] [PubMed] [Google Scholar]
- 7.Hone AJ, McIntosh JM. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018;592:1045–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umana IC, Daniele CA, Miller BA, et al. Nicotinic modulation of descending pain control circuitry. Pain. 2017;158:1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci. 2004;113:32–42. [DOI] [PubMed] [Google Scholar]
- 10.Parada E, Egea J, Buendia I, et al. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. 2013;19:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero HK, Christensen SB, Di Cesare Mannelli L, et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc Natl Acad Sci. 2017;114: E1825–E1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel R, Montagut-Bordas C, Dickenson AH. Calcium channel modulation as a target in chronic pain control. Br J Pharmacol. 2018;175:2173–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias HR, Tae H-S, Micheli L, et al. Coronaridine congeners decrease neuropathic pain in mice and inhibit α9α10 nicotinic acetylcholine receptors and CaV2.2 channels. Neuropharmacology. 2020;175:108194. [DOI] [PubMed] [Google Scholar]
- 15.Baptista-de-Souza D, Di Cesare Mannelli L, Zanardelli M, et al. Serotonergic modulation in neuropathy induced by oxaliplatin: effect on the 5HT2C receptor. Eur J Pharmacol. 2014;735:141–149. [DOI] [PubMed] [Google Scholar]
- 16.McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP: implementing guidelines on reporting research using animals (ARRIVE etc.). Br J Pharmacol. 2015;172:3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagdas D, Sevdar G, Gul Z, et al. (E)-3-furan-2-yl-N-phenylacrylamide (PAM-4) decreases nociception and emotional manifestations of neuropathic pain in mice by α7 nicotinic acetylcholine receptor potentiation. Neurol Res. 2021;43:1056–1068. [DOI] [PubMed] [Google Scholar]
- 18.Micheli L, Di Cesare Mannelli L, Rizzi A, et al. Intrathecal administration of nociceptin/orphanin FQ receptor agonists in rats: a strategy to relieve chemotherapy-induced neuropathic hypersensitivity. Eur J Pharmacol. 2015;766:155–162. [DOI] [PubMed] [Google Scholar]
- 19.Failli P, Bani D, Bencini A, et al. A novel manganese complex effective as superoxide anion scavenger and therapeutic agent against cell and tissue oxidative injury. J Med Chem. 2009;52:7273–7283. [DOI] [PubMed] [Google Scholar]
- 20.Micheli L, Rajagopalan R, Lucarini E, et al. Pain relieving and neuroprotective effects of non-opioid compound, DDD-028, in the rat model of paclitaxel-induced neuropathy. Neurotherapeutics. 2021;18:2008–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micheli L, Vasarri M, Barletta E, et al. Efficacy of posidonia oceanica extract against inflammatory pain: in vivo studies in mice. Mar Drugs. 2021;19:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerrini G, Ciciani G, Crocetti L, et al. Identification of a New Pyrazolo[1,5- a]quinazoline Ligand Highly Affine to γ-Aminobutyric Type A (GABA A) receptor subtype with anxiolytic-lik and antihyperalgesic activity. J Med Chem. 2017;60:9691–9702. [DOI] [PubMed] [Google Scholar]
- 23.Bedini A, Di Cesare Mannelli L, Micheli L, et al. Functional selectivity and antinociceptive effects of a novel KOPr agonist. Front Pharmacol. 2020;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micheli L, Parisio C, Lucarini E, et al. VEGF-A/VEGFR-1 signalling and chemotherapy-induced neuropathic pain: therapeutic potential of a novel anti-VEGFR-1 monoclonal antibody. J Exp Clin Cancer Res. 2021;40:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halai R, Clark RJ, Nevin ST, Jensen JE, Adams DJ, Craik DJ. Scanning mutagenesis of alpha-conotoxin Vc1.1 reveals residues crucial for activity at the alpha9alpha10 nicotinic acetylcholine receptor. J Biol Chem. 2009;284:20275–20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Cesare Mannelli L, Pacini A, Matera C, et al. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation. Neuropharmacology. 2014;79:37–48. [DOI] [PubMed] [Google Scholar]
- 27.Vukovic R, Kumburovic I, Joksimovic Jovic J, et al. N-Acetylcysteine protects against the anxiogenic response to cisplatin in rats. Biomolecules. 2019;9:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arias HR. Positive and negative modulation of nicotinic receptors. Adv Protein Chem Struct Biol. 2010;80:153–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.