Abstract
Background:
Literature reports randomized trials have examined herbal drugs and other smoking cessation therapies such as aromatherapy acupuncture but no comprehensive overview of the overall results has been provided. The present systematic review and meta-analysis aimed to describe the overall effectiveness and safety of herbal medicines.
Methods:
This study was conducted from December 2020 to April 2021 by searching seven databases. Herbal drugs have been shown to help people quit smoking in randomized controlled studies. Two teams of researchers independently extracted the data.
Findings:
A total of 12 trials with 762 smokers were included in this study. The heterogeneity I2 was 43.6% with P=0.03 (Cochrane Q test) and χ2=15.77. The overall odds ratio (OR) at 95% confidence interval (CI) was 0.91 (0.68- 1.20) which shows a protective factor of herbal preparations and very low heterogeneity. The herbal treatments such as Vernonia cinerea, St. John’s Wort, and lavender essential oil were significantly related to a higher continuous abstinence rate (CAR) compared to the controls with risk ratio (RR): 2.13 (0.57-4.61) at week 8; RR: 2.72 (0.77-5.3) at week 12; and RR: 2.77 (0.37-1.13) at week 24. A 7-day point abstinence rate (PAR) at week 8 was RR: 1.24 (0.81-6.34) with 95% CI; RR: 2.09 (0.93-8.29) at week 12, and RR: 2.11 (0.3-3.08) at week 24. Black pepper and lime were better in craving reduction than the placebo group. This study found no significant difference between the treatment and control groups in adverse effects, despite some minor side effects with herbal drugs.
Conclusion:
The results of this study showed herbal treatments have the potential to help smokers quit the habit. Further well-designed trials comparing standardized herbal medicines with conventional therapy and placebo are recommended to reinforce this data.
Keywords: Smoking cessation, Herbal medicine, St. John’s Wort, Continuous abstinence rate, Point abstinence rate, Aromatherapy
Introduction
Several toxic compounds are found in the smoke from cigarettes.1-3 Smoking is a major cause of chronic respiratory illness i.e., chronic obstructive pulmonary disease (COPD),4 atherosclerotic cardiovascular disease, cancer, and cerebrovascular disorder,5 which are the most prevalent causes of death and morbidity.6-8 According to the World Health Organization (WHO), an estimated 6 million people die each year as a direct result of tobacco smoking. Smoking is directly responsible for the fatality of more than 5 million people, and secondhand smoke is a contributing factor in the death of over 600 000 people each year.9 Smoking addiction is a complicated problem which combines physical, mental, social, and other aspects10,11 predominantly as a result of nicotine, the psychoactive constituent of cigarettes.12,13 Smokers who stop smoking have a lower chance of developing smoking-related illnesses and dying as a result of these diseases.14,15 Countries all across the globe are pursuing strong anti-smoking efforts with the help of WHO.15 Nicotine replacement treatment, antidepressants, and psychological assistance are the most often suggested methods for quitting smoking.16 Despite the high cost, their efficacy is limited, and adverse effects (including nausea, anorexia, headache, constipation, sleepiness, or drowsiness) are possible.17-19 Thus, the new techniques to assist smokers to stop smoking are essential.
When it comes to smoking cessation therapy, herbal medication is a viable choice that might be more accessible and affordable with fewer negative effects. In recent years, clinical data supporting the use of herbal medication, including St. John’s Wort, Cytisus laburnum (cytisine), black pepper, or herbal tea, for smoking cessation have been documented.3,4 Although cytisine has been suggested as a low-cost alternative to plant-based medications for quitting smoking, it is not accessible in low- and middle-income nations. Vernonia cinerea (L.) Less is a popular herbal medication for smoking cessation in Thailand. V. cinerea (Asteraceae family), yadok khao (Thai name), Sahadevi, or small ironweed, is a weed found in tropical and subtropical parts of America, Africa, Australia, and Asia.5 In traditional medicine, V. cinerea has been utilized as an antipyretic, diuretic, anti-jaundice, antitussive, tonic, anti-hepatitis, and anti-hemorrhoid therapy.6,7 V. cinerea tea has been acknowledged as an alternative medication for smoking cessation therapy in Thailand when it was included in the National List of Herbal Medicine, Ministry of Public Health.8 Preclinical studies have shown the effectiveness of V. cinerea in helping people quit smoking. Other herbal medications like St. John’s Wort have been used for tobacco cessation. More herbal preparations like herbal tea, lavender oil, lime, black pepper, and angelica have been used for tobacco cessation.9 Many randomized controlled studies have analyzed the use of herbal remedies for the cessation of smoking.10,11 However, there is still a lack of critical evaluation of the overall results. The safety and effectiveness of V. cinerea herbal treatment have been investigated using meta-analysis and systematic review.14 No studies have been conducted so far comparing all the herbal preparations used for tobacco cessation. Therefore, this study aims to conduct a meta-analysis and systematic review to investigate the effectiveness and safety of various herbal medications used for the cessation of smoking.
Methods
Study selection and research strategy
Prior to starting the study, the review procedure was registered in PROSPERO with registration number: CRD4202125439. Research publications were found in AMED, PubMed, Cochrane Central Register of Clinical Trials, CINAHL, EMBASE, Cochrane Library, Thai LIS-Thai Library Integrated System, WHO Trial Registry, ACI (ASEAN Citation Index), and Clinicaltrial.gov databases from their inception at December 2020 to April 2021. Search phrases included “Tobacco treatment” OR “Tobacco Dependence” OR “Tobacco Relapse” OR “Tobacco Abusers” OR “Smoking Cessation” OR “Cigarette Craving” OR “Smoking withdrawal symptoms” OR “Nicotine Replacement” AND (“Herbal Medicine” OR “Herbal” OR “Traditional Medicine” OR “Alternative Medicine” OR “Complementary Medicine” OR “Unani” OR “Ayurveda”. An offline and online search was conducted and references of full-text articles were obtained to find studies that were not included in the aforesaid databases.
Inclusion criteria
There was no language restriction. Articles from the selected publications that reported the effect of herbal medications on smoking cessation in patients were selected. This review included randomized controlled trials (RCTs) and quasi-RCTs. Participants of all ages with tobacco dependence were included. There were no restrictions on gender or ethnicity. Oral herbal medicines in any form were included. No restrictions were imposed on the kind of herbal medication, the dose, or the length of therapy.
Exclusion criteria
Herbal injections and non-herbal therapies were not included in this study. Trials comparing herbal medicine with other forms of herbal medication were not included in this study. In vitro and animal research, as well as in silico drug trials, were not included in the study since they are not considered preclinical investigations. Letters to the editor and editorials were not included.
Study abstracts and titles were checked to see whether herbal medicine effects had been studied in detail. Then, the researchers separately evaluated full-text publications from the potential studies. When appropriate, uncertainties and disagreements about eligibility were addressed via conversations.
Data extraction and quality assessment
The data were extracted in compliance with the CONSORT criteria for reporting herbal medicinal treatments.16 The following data were extracted: research design, participants number, age of participants, smoking habit, “Fagerstrom Test for Nicotine Dependence” (FTND), intervention features, and outcome assessment. At week 12, the continuous abstinence rate (CAR) and the 7-day point prevalence abstinence rate (PAR) are the key outcomes. Adverse events (AE) at weeks 2, 4, 8, and 12 as secondary outcome were reported. Disagreements among the reviewers were resolved by consensus and debate.
Statistical analysis and outcome measure
In this meta-analysis, data from all trials were combined to calculate the overall effect size with a 95% confidence interval. The risk ratio (RR) was used to calculate the pooled effects. The I2 test was used to analyze statistical heterogeneity across trials, and the effect size was calculated using odds ratio (OR) and risk ratio (RR). Moreover, SPSS version 21 was used to perform meta-analyses (Chicago, New York).
Results
A total of 247 papers were detected from the selected databases and two articles were identified from other sources (contacting a content expert for further articles) resulting in 249 articles suitable to be screened for inclusion in the study. After removing duplicates, 219 articles remained for a full-text review based on titles and abstracts. The systematic review and meta-analysis comprised 30 RCTs, 18 of which were removed due to incomplete articles and animal studies. Figure 1 depicts the study design. The OR description of the 12 studies in the forest plot showed heterogeneity of I2 = 43.6% (Higgins I2 test statistics) which was low (P = 0.03) (Cochrane Q test) and χ2 equaled 15.77. The overall OR (95% CI) was 0.91 (0.68-1.20) indicating a protective factor for herbal preparations (Figure 2).
Figure 1.
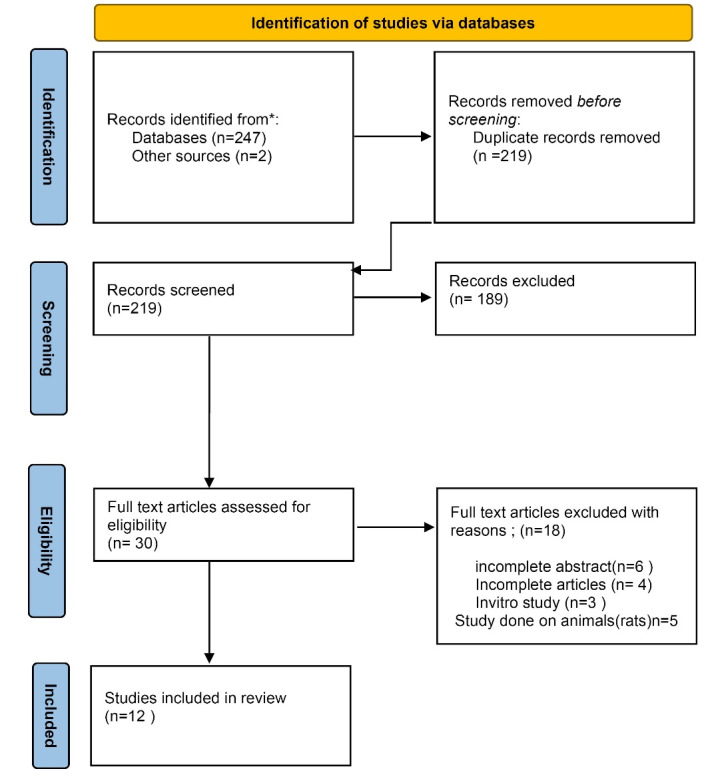
Study design
Figure 2.
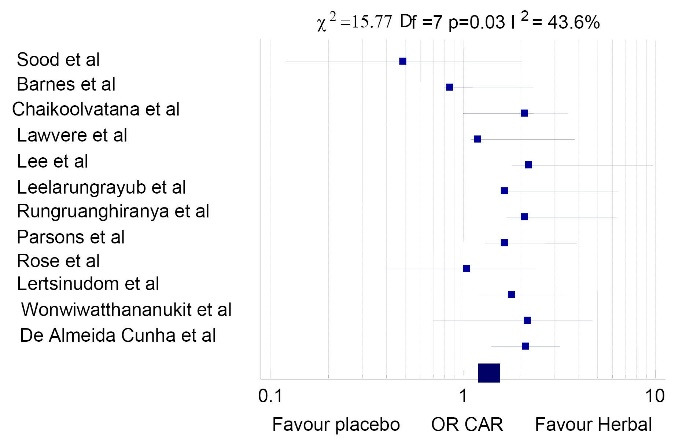
Description of odds ratio of herbal preparations in the studies included
Study characteristics
The effectiveness of herbal smoking cessation was examined in all 12 trials that met the inclusion criteria (Table 1), including 762 smokers. There was a wide variance in the age of the participants in all included studies. Most of the respondents were men (60–100%), which was linked to the high prevalence of smoking among men. The number of years of smoking reported in seven studies ranged from 24 to 56.1. The FTND score, which ranged from 3–9 nicotine dependence, was observed in all studies except one study.23 Different herbal remedies were studied in various dose forms, kinds, and dosages.
Table 1. Demographic details of the investigated studies .
| Author | Study groups herbal medication or other medication given | Type of study | Standard intervention | N |
Age
Mean±SD |
Male | Female | Smoking duration | Smoking frequency | FTND score |
|---|---|---|---|---|---|---|---|---|---|---|
| Leelarungrayub et al20 |
Vernonia cinerea
Placebo Exercise + V. cinerea Exercise |
RCT | Medicinal urinary and cotinine level and other parameters | 120 | 49.9 ± 9.02 56.1 ± 15.42 46.1 ± 11.35 49.1 ± 15.9 |
NA | NA | 18 21 13 12 |
10 9 15 14 |
6 3 4 8 |
| Sood et al21 | St. John’s Wort 300 mg 600 mg Placebo |
Randomized placebo-controlled three-arm clinical trial | Behavior intervention and dose-ranging | 118 | 39.3 ± 13 38.3 ± 11.9 35.6 ± 12.2 |
13 22 18 |
26 18 21 |
21.7 ± 12.3 21.7 ± 12.3 16.6 ± 11.6 |
19.4 ± 7.3 19.1 ± 7.2 21.0 ± 5.2 |
4.7 ± 2.3 5.0 ± 2 5.2 ± 1.7 |
| Barne et al22 | St. John’s Wort | Randomized uncontrolled clinical trial | Behavior and herbal extract tablets | 28 | 42.8 | 23 | 5 | 11 | 17 | 9 |
| Chaikoolvatana et al23 | Vernonia cinerea jelly | Quasi-experimental study | VC jelly medication and motivation | 50 | 25.3 27.96 |
50 | NA | 35 11.4 |
12.5 12.2 |
NA |
| De Almeida Cunha et al24 | Lavender Placebo Nicotine |
Single blind RCT | Medication and behavior monitoring | 60 | 48 ± 11.26 | 42 | 18 | 15 | 20 | 6 3 7 |
| Lawvere et al25 | St. John’s Wort | Single-arm phase II study | Medication cessation counseling | 37 | 39.42 | 24 | 13 | 10 | 17.08 | 7 |
| Lee et al26 | Medicinal herbal tea Placebo |
RCT | Medicinal and urinary cotinine level analysis | 100 | 39.71 ± 9.7 40.25 ± 10.2 |
NA | NA | 11.5 ± 7.9 53.7 ± 8.6 |
20.3 ± 7.4 19.2 ± 4.8 |
4.9 4.8 |
| Rungruanghiranya et al27 | Fresh lime Nicotine gum |
RCT | Medicinal and counseling | 53 | 47.23 ± 17.93 39.75 ± 13.37 |
41 49 |
6 4 |
30.83 ± 18.24 23.04 ± 12.0 |
12.4 ± 6.22 17.79 ± 9.10 |
5.13 ± 2.28 5.74 ± 2.40 |
| Parsons et al28 | St. John’s Wort Chromium |
RCT | Medicinal and behavioral support | 143 | 44.0 ± 10.9 43.2 ± 11.2 45.6 ± 13.7 49.4 ± 13.3 |
13 16 14 10 |
23 19 23 25 |
27 ± 11 27.3 ± 11.9 30.1 ± 13.7 33.1 ± 13.3 |
20 21 20 22 |
5.4 ± 1.7 5.3 ± 2 5.4 ± 2 5.7 ± 2.2 |
| Rose et al29 | Essential oil black pepper vapor Menthol cartridge inhalation Empty cartridge placebo |
RCT | Medicinal and behavioral support | 48 | 35.8 ± 9.05 | 16 16 16 |
NA | 19.2 ± 10.02 | 31.3 ± 9.05 | 5.6 |
| Lertsinudom et al30 |
Vernonia cinerea pastilles Placebo |
RCT | Medicinal and behavioral | 111 | 41.1 ± 15.3 39.5 ± 14.7 |
54 57 |
NA | 20 20 |
7 10 |
4.82 4.71 |
| Wongwiwatthananukit et al31 |
Vernonia cinerea tea bag placebo | RCT | Medicinal and monitoring | 64 | 40.13 ± 11.86 41.7 ± 11.19 |
32 0 |
25 7 |
23.44 ± 11.79 23.81 ± 9.52 |
19.88 ± 10.5 18.83 ± 10.3 |
5.22 ± 2.56 5.41 ± 1.86 |
Tea was utilized in two trials,18,20 while other studies used juice23, capsule,21 or lozenge22 as treatment (Table 1). In three studies, the dose form was liquid (juice or tea)20,23 whereas the other two studies had a solid dose form.16,17 Other studies used lavender, black pepper, and aromatherapy. Counseling was used as a control in one investigation.24 All individuals received counseling as part of their therapy. All RCTs had interventions lasting between two and eight weeks. However, there were only two reports that offered quantitative descriptions and quantitative testing for herbal formulations utilized in the included trials. For 12 studies, researchers examined PAR and CAR at intervals ranging from 2 to 24 weeks.10,11 Decline rate and cessation rate were assessed in all studies.13,24 The various studies with herbal remedies were compared to a control group to evaluate the adverse effects.10,11 A total of ten clinical studies were conducted to demonstrate the effectiveness of each treatment in terms of carbon monoxide (CO)11 or urine cotinine levels.10
The meta-analysis of the clinical effects of herbal preparations on smoking cessation showed that the use of herbal treatment was related to a significantly higher cessation rate compared to the control group for both CAR (RR: 2.72 (0.77-5.3)) and PAR (RR: 2.09 (0.93-8.29)); 95% CI at week 12 for both CAR and PAR. The pooled effect of herbal studies was statistically significant when CAR and PAR were analyzed at weeks 2, 4, and 8. Cigarette cravings were also reduced by the usage of herbal preparations especially with black pepper, lavender, and lime while in other groups, it did not vary considerably as compared to the control group (Table 2). The findings of the subgroup analysis were similar to those of the main study. Even when herbal preparations were included in the study, the impact of smoking cessation remained unaffected. In contrast to the placebo group, there was a statistical difference in PAR and CAR. In any of the studies that were included, no major adverse events were recorded. Treatment and control groups didn’t vary significantly in the incidence of adverse events. Herbal preparations had slightly higher acceptance and tolerability rates than the control group. The risk of experiencing symptoms such as numbness in the tongue, disorientation, or an aversion to the smell or taste of cigarette smoke rose among those who received the treatment.
Table 2. Comparison of outcomes .
| Type | Follow-up duration (wk) | Abstinence rate | N; Risk ratio (95% CI) | P | |
| Herbal preparations |
Placebo and other non-herbal
Preparations |
||||
| CAR | 2 | 151/416 | 51/344 | 760; 1.15 (0.41-3.19) | 0.022* |
| 4 | 148/416 | 48/344 | 760; 1.77 (0.52-4.11) | 0.013* | |
| 8 | 130/416 | 41/344 | 760; 2.13 (0.57-4.61) | 0.011* | |
| 10 | 9/32 | 2/32 | 64; 1.71 (0.41-1.17) | 0.037* | |
| 12 | 166/416 | 38/344 | 760; 2.72 (0.77-5.3) | 0.014* | |
| 24 | 16/32 | 1/32 | 64; 2.77 (0.37-1.13) | 0.003* | |
| PAR | 2 | 153/416 | 52/344 | 760; 1.14 (0.42-3.19) | 0.026* |
| 4 | 150/416 | 50/344 | 760;1.17 (0.61-4.55) | 0.016* | |
| 8 | 137/416 | 43/344 | 760; 1.24 (0.81-6.34) | 0.022* | |
| 10 | 11/28 | 1/28 | 64;1.66 (0.31-2.16) | 0.031* | |
| 12 | 169/416 | 37/344 | 760; 2.09 (0.93-8.29) | 0.018* | |
| 24 | 18/28 | 2/28 | 64;2.11 (0.31-3.08) | 0.004* | |
| Craving reduction | End of study | 156/416 | 34/344 | 760; 2.13 (0.91-8.12) | 0.0014* |
* Significant.
Table 3 shows various side effects of the herbal medications used. There were mild symptoms like insomnia, mild GI disturbance, nausea, and dry mouth. The quit rate was highest in the study24 using lavender (68%).
Table 3. Other study determinants .
| Author | Salivary cotinine (after therapy) | Urine cotinine (after therapy) | Dropouts | Quit rate | Adverse effects |
| Leelarungrayub et al20 | 4.76 ppm | 7.1ppm | 5% | 15% | Decreased locomotor activity and exploratory behavior subsided by exercise |
| Sood et al21 | < 8 ppm | - | 43% | 15% | Mild GI disturbance, insomnia, headache |
| Barnes et al22 | < 10 ng/mL | < 10 ppm | 12% | 37% | Mild GI disturbance |
| Chaikoolvatana et al23 | 1.89 | 5 ppm | 10% | 40% | Dry mouth, insomnia |
| De Almeida Cunha et al24 | - | - | 5% | 68% | Reduced craving, improved anxiety |
| Lawvere et al25 | 3.35 ng/ml | - | 10% | 37.5% | Mild symptoms like change in bowel movement, constipation, dizziness |
| Lee et al26 | 1.89 ppm | 24.5 mmol/L | 14% | 38% | Reduced craving |
| Rungruanghiranya et al27 | 4.3 ppm | 5.6 ppm | 11% | 55.3% | Tooth sensitivity, sore mouth, dyspepsia |
| Parsons et al28 | 2.2 | 3.4 | 66% | 54% | Nausea, vomiting, dry mouth, mood swings |
| Rose et al29 | - | - | 3% | 73% | Reduced craving, mild airway irritation |
| Lertsinudom et al30 | 5.7 ppm | - | 2% | 35.9% | Numb tongue, stomachache, dry mouth |
| Wongwiwatthananukit et al31 |
- | 6.3 ppm | 20.3% | 54% | Tongue numbness, abdominal discomfort, craving reduction |
Discussion
This systematic review and meta-analysis was conducted on 12 clinical trials investigating the effect of herbal medication on tobacco cessation. The present meta-analysis is the first to include all herbal preparations used in clinical trials and analyze the side effects. This meta-analysis reflected a positive recommendation for herbal remedies using V. cinerea, St. John’s Wort, and lavender oil for tobacco cessation treatment. The effects of herbal medicines on PAR and CAR parameters were examined based on the fact that effective quitting was validated by objective measurement.15,24 After eight weeks of therapy, the data showed quitting smoking was more beneficial than placebo and was conclusive after 12 weeks. There was a significant degree of agreement in the included studies in terms of methodological quality (I2 = 43.6%). In this meta-analysis, herbal smoking cessation products from various studies conducted so far were summarized for their therapeutic advantages. Patients who took herbal medicine had the same level of side effects as those who took placebo, but there were no really major side effects reported in most of the studies done so far. As a result, active smokers may find it more pleasant and bearable than placebo. The evidence that herbal medications have a smoking cessation impact is trustWort hy and consistent in different researches; however, certain limitations should be acknowledged. Among the included studies, there were variations in the product compositions (juice, tea, capsules, or lozenges), extraction procedures (boiling, infusion, or dry powder), dosage schedules (ranging from 3-9 g/d), as well as the herbal preparations used. Despite these differences, the overall results were consistent. The overall OR (95% CI) was 0.91 (0.68-1.20) indicating a protective factor of herbal preparations. There was a favorable effect seen with herbal medication and also the quit rate showed marked improvement after the herbal medication follow-up (Figure 2). V. cinerea and St. John’s Wort were the most commonly used herbs for smoking cessation. A meta-analysis found in literature14 was conducted in 2017 which included studies on V. cinerea and reported similar findings of tobacco cessation.
The present study had some limitations. First, the preclinical findings of pharmacological activity, mechanism of action, and clinical research of herbal medications are still needed to be proved. Second, for the therapeutic use of herbal medicines, standardization of techniques and bioactive content is critical.16 Several variables, including location, season, and preparation, impact the bioactive content of herbal medicine; therefore, diverse therapeutic outcomes may be possible as reported in the present study. Lack of standardization or reporting of the number of bioactive substances in herbal preparations may have contributed to the lack of clinical efficacy in the included studies. Standardized extracts or dry powders of herbal remedies for smoking cessation should include bioactive ingredients like flavonoids (chrysoeriol, apigenin, quercetin, and luteolin) and hirsutinolides as a biomarker to validate product quality. Third, long-term follow-up randomized clinical trials are needed to establish the potential efficacy of herbal medication and evaluate its benefits in the long run. The present study included trials, with an intervention or investigation period of about 24 weeks. Herbal smoking cessation treatments have only been studied for a short time (3 months) in CAR and PAR, while contemporary pharmaceuticals have been studied for at least six months.17,18 Moreover, no studies have compared herbal medicine treatment with first-line drugs or nicotine replacement therapy in terms of effectiveness and safety. According to the PROSPERO guidelines for systematic review and meta-analysis, a generally accepted strategy for deriving conclusions about clinical evidence of therapies, the present research reviewed existing clinical evidence of herbal therapy for smoking cessation. When new RCTs are available, it is necessary to update these findings. Additional long-term clinical investigations are required to compare the standardized product against current medications and to report on the results of these studies as well.
Conclusion
Recent research shows that herbal medications are more effective and safer than placebo. In addition to helping the local economy, the usage of V. cinerea and St. John’s Wort, as the most commonly used herbal treatments, may make smoking cessation medication more accessible to those who are currently addicted. It is easier to access as no prescription is required and it can be made locally in any nation without the need for imported ingredients, unlike current pharmaceuticals. If proven effective on all aforementioned limitations, herbal medicines can play an important role in tobacco cessation. Hence, herbal therapy should be explored as an alternative to first-line smoking cessation drugs, particularly for individuals who cannot afford them. They can play a significant role in reducing the burden on the health system in the contemporary world. In the past few years, the use of herbal drugs has increased substantially due to their easy availability and general perception among masses of their being safe and free from side effects. Therefore, to validate their efficacy for smoking cessation, further well-designed trials are recommended to compare standardized herbal products with standard medication and placebo for at least twelvemonths.
Acknowledgments
We thank the staff at Rajendra Institute of Medical Sciences for their cooperation and all those who participated in the studies included.
Citation: Mitra R, Rai A, Kumar A, Mitra JK. Role of herbal medication in tobacco cessation treatment: A systematic review and meta-analysis. Addict Health. 2023;15(1):63–70. doi:10.34172/ahj.2023.1290
Footnotes
Authors’ Contribution
Conceptualization: Ruchi Mitra.
Data Curation:Ruchi Mitra.
Formal Analysis: Arpita Rai.
Investigation: Ruchi Mitra.
Methodology: Arpita Rai.
Project Administration: Jeewan Kumar Mitra.
Resources: Ruchi Mitra.
Supervision: Jeewan Kumar Mitra.
Validation: Ruchi Mitra, Arpita Rai.
Visualization: Ansul Kumar, Jeewan Kumar Mitra.
Writing—Original Draft: Ruchi Mitra.
Writing—Review & Editing: Arpita Rai.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
References
- 1. World Health Organization (WHO). WHO Global Report on Trends in Prevalence of Tobacco Smoking 2015. Available from: http://apps.who.int/iris/bitstream/10665/156262/1/9789241564922_eng.pdf. Accessed January 28, 2017.
- 2.Lando HA. Promoting tobacco cessation in low- and middle-income countries. J Smok Cessat. 2016;11(2):66–9. doi: 10.1017/jsc.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarghami M, Taghizadeh F, Sharifpour A, Alipour A. Efficacy of smoking cessation on stress, anxiety, and depression in smokers with chronic obstructive pulmonary disease: a randomized controlled clinical trial. Addict Health. 2018;10(3):137–47. doi: 10.22122/ahj.v10i3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard P, Gilles H, Alavi Z, Christine L, Maryline LB, Ronan G, et al. Screening for chronic obstructive pulmonary disease in smoking cessation clinic in France. Addict Health. 2016;8(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Sonibare MA, Aremu OT, Okorie PN. Antioxidant and antimicrobial activities of solvent fractions of Vernonia cinerea (L.) Less leaf extract. Afr Health Sci. 2016;16(2):629–39. doi: 10.4314/ahs.v16i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasopthum A, Pouyfung P, Sarapusit S, Srisook E, Rongnoparut P. Inhibition effects of Vernonia cinerea active compounds against cytochrome P450 2A6 and human monoamine oxidases, possible targets for reduction of tobacco dependence. Drug Metab Pharmacokinet. 2015;30(2):174–81. doi: 10.1016/j.dmpk.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Dogra NK, Kumar S. A review on ethno-medicinal uses and pharmacology of Vernonia cinerea Less. Nat Prod Res. 2015;29(12):1102–17. doi: 10.1080/14786419.2014.981814. [DOI] [PubMed] [Google Scholar]
- 8.Jitruknatee A, Tosanguan K, Doangjai Y, Theantawee W, Martro J. A review on the selection of drugs in Thai heath care at national, pharmaceutical industries and public hospital levels. J Health Sci. 2020;29:S31–S44. [Google Scholar]
- 9.Promputta C, Anupunpisit V, Panyarachun B, Sawatpanich T, Watthanachaiyingcharoen R, Paeratakul O, et al. Effect of Vernonia cinerea in improvement of respiratory tissue in chronic nicotine treatment. J Med Assoc Thai. 2012;95 Suppl 12:S47–55. [PubMed] [Google Scholar]
- 10.Baumeister RF. Addiction, cigarette smoking, and voluntary control of action: Do cigarette smokers lose their free will? Addict Behav Rep. 2017;5:67–84. doi: 10.1016/j.abrep.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moosazadeh M, Ziaaddini H, Mirzazadeh A, Ashrafi-Asgarabad A, Haghdoost AA. Meta-analysis of smoking prevalence in Iran. Addict Health. 2013.5(3-4):140-53. [PMC free article] [PubMed]
- 12. Benowitz NL. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Annu Rev Pharmacol Toxicol. 2009.49:57–71. 10.1146/annurev.pharmtox.4113006.094742. [DOI] [PMC free article] [PubMed]
- 13.Kotirum S, Ismail SB, Chaiyakunapruk N. Efficacy of Tongkat Ali (Eurycoma longifolia) on erectile function improvement: systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2015;23(5):693–8. doi: 10.1016/j.ctim.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Habibagahi R, Navabi N, Alsadat Hashemipour M, Hashemzehi A. Does smoking cessation improve oral health-related quality of life? A pilot study. Addict Health. 2020;12(3):167–74. doi: 10.22122/ahj.v12i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puttarak P, Sawangjit R, Chaiyakunapruk N. Efficacy and safety of Derris scandens (Roxb.) Benth for musculoskeletal pain treatment: a systematic review and meta-analysis of randomized controlled trials. J Ethnopharmacol. 2016;194:316–23. doi: 10.1016/j.jep.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Sterne JA, Savovic J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29–31. [Google Scholar]
- 17.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. J Clin Epidemiol. 2006;59(11):1134–49. doi: 10.1016/j.jclinepi.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62. doi: 10.1016/s0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 19.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Leelarungrayub D, Pratanaphon S, Pothongsunun P, Sriboonreung T, Yankai A, Bloomer RJ. Vernonia cinerea Less. supplementation and strenuous exercise reduce smoking rate: relation to oxidative stress status and beta-endorphin release in active smokers. J Int Soc Sports Nutr. 2010;7:21. doi: 10.1186/1550-2783-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood A, Ebbert JO, Prasad K, Croghan IT, Bauer B, Schroeder DR. A randomized clinical trial of St. John’s Wort for smoking cessation. J Altern Complement Med. 2010;16(7):761–7. doi: 10.1089/acm.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes J, Barber N, Wheatley D, Williamson EM. A pilot randomised, open, uncontrolled, clinical study of two dosages of St John’s Wort (Hypericum perforatum) herb extract (LI-160) as an aid to motivational/behavioural support in smoking cessation. Planta Med. 2006;72(4):378–82. doi: 10.1055/s-2005-916219. [DOI] [PubMed] [Google Scholar]
- 23.Chaikoolvatana A, Thanawirun C, Chaikoolvatana C, Puchcharanapaponthorn P, Suwanakoot P, Saisingha N. Use of Vernonia cinerea jelly candies for smoking cessation, Ubon Ratchathani region, Thailand. EnvironmentAsia. 2018;11(2):172–91. doi: 10.14456/ea.2018.32. [DOI] [Google Scholar]
- 24.de Almeida Cunha NB, Orozco CM, de Morais Pordeus LC, Fernandes Braga JE. Effects of essential oil of Lavandula angustifolia in patients with cigarette craving. J Med Ther. 2018;2(3):1–6. doi: 10.15761/jmt.1000133. [DOI] [Google Scholar]
- 25.Lawvere S, Mahoney MC, Cummings KM, Kepner JL, Hyland A, Lawrence DD, et al. A phase II study of St. John’s Wort for smoking cessation. Complement Ther Med. 2006;14(3):175–84. doi: 10.1016/j.ctim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, Lee JH. Effects of medicinal herb tea on the smoking cessation and reducing smoking withdrawal symptoms. Am J Chin Med. 2005;33(1):127–38. doi: 10.1142/s0192415x05002722. [DOI] [PubMed] [Google Scholar]
- 27.Rungruanghiranya S, Ekpanyaskul C, Sakulisariyaporn C, Watcharanat P, Akkalakulawas K. Efficacy of fresh lime for smoking cessation. J Med Assoc Thai. 2012;95 Suppl 12:S76–82. [PubMed] [Google Scholar]
- 28.Parsons A, Ingram J, Inglis J, Aveyard P, Johnstone E, Brown K, et al. A proof of concept randomised placebo controlled factorial trial to examine the efficacy of St John’s Wort for smoking cessation and chromium to prevent weight gain on smoking cessation. Drug Alcohol Depend. 2009;102(1-3):116–22. doi: 10.1016/j.drugalcdep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Rose JE, Behm FM. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug Alcohol Depend. 1994;34(3):225–9. doi: 10.1016/0376-8716(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 30.Lertsinudom S, Sawanyawisuth K, Srisoi S, Areemit J, Hansuri N, Tawinkan N, et al. Vernonia cinerea pastilles is effective for smoking cessation. J Tradit Complement Med. 2021;11(2):90–4. doi: 10.1016/j.jtcme.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wongwiwatthananukit S, Benjanakaskul P, Songsak T, Suwanamajo S, Verachai V. Efficacy of Vernonia cinerea for smoking cessation. J Health Res. 2009;23(1):31–6. [Google Scholar]


