Abstract
Background:
The effect of nicotine on nausea, vomiting, and postoperative pain has been investigated in studies on animals and humans. This study aimed to evaluate the effect of nicotine patch on decreasing nausea, vomiting, and pain in laparoscopic cholecystectomy.
Methods:
The study sample consisted of 100 non-smoking patients undergoing laparoscopic cholecystectomy under general anesthesia in a triple-blind clinical trial. One hour after the start of surgery, patients were randomly assigned to receive 17.5-mg nicotine or placebo patches. The patches located on the right arm were left for 24 hours. The visual analogue scale (VAS) for pain and N/V score for the severity of nausea and vomiting were measured at intervals of 0, 6, 12, and 24 hours.
Findings:
The results showed there was no statistically significant difference between the groups in terms of pain intensity as well as nausea and vomiting at different time periods after surgery (P>0.05). A total of 36 patients in the nicotine group and 24 patients in the placebo group received meperidine. There was also no statistically significant difference between the two groups in terms of analgesics (P=0.096) and antiemetics (P=0.1). Moreover, the frequency of severe nausea and vomiting during the study in the nicotine group was higher than in the placebo group (4 vs. 1) but this difference was not statistically significant (P>0.05).
Conclusion:
Receiving a 17.5-mg nicotine patch had a similar effect to receiving placebo in controlling postoperative pain, nausea, and vomiting in non-smokers. Nicotine use had no effect on reducing analgesia.
Keywords: Nicotine, Nausea and vomiting, Pain, Cholecystectomy, Laparoscopy
Introduction
Postoperative nausea and vomiting (PONV) is an undesirable complication that is highly prevalent despite medical advances. The frequency of this complication in different surgeries is 40-80% and it affects the level of activity and satisfaction of the patient. Factors such as pregnancy, menstrual cycle, gender, history of nausea and vomiting or motion sickness, smoking, duration of anesthesia, drug use, and finally the type of surgery such as laparoscopy are effective factors in the occurrence of this complication.1-3
Laparoscopy is used to diagnose and treat many diseases. Pneumoperitoneum produced during laparoscopy can irritate the vagus nerve and increase the risk of nausea and vomiting. PONV causes delayed discharge, dehydration, wound opening, pulmonary aspiration, patient dissatisfaction, and increased treatment costs.2,3 Prevention and treatment of postoperative pain4 and control of complications such as nausea and vomiting play an important role in early mobility, improve the quality of surgery, and lead to patient satisfaction, early discharge, and reduced costs.5
Lately, preoperative risk assessment and following standard prophylactic antiemetic protocols are recommended to decrease the incidence of nausea and vomiting.6 Recently, drugs such as metoclopramide,7,8 ondansetron,9,10 and dexamethasone11,12 are used to reduce PONV. Nonsteroidal anti-inflammatory drugs, cyclooxygenase inhibitors, local anesthetics, opioids, steroids, and clonidine are used for systemic pain control. Despite known side effects, narcotics are still the main drugs used for pain control. Therefore, reducing the consumption of narcotics and their side effects is a key point to control pain.13 Smoking has been shown to significantly reduce PONV. This may be due to the effect of smoking on the induction of cytochrome P450 enzyme.
Furthermore, prolonged exposure to nicotine in cigarettes reduces the sensitivity of central nicotine receptors, which increases the tolerance to the emetogenic effects of anesthesia and surgery. Among the 4000 ingredients in cigarettes, the effect of nicotine on reducing nausea has been investigated in few studies.14 It is unclear whether the administration of a low dose of preoperative nicotine can reduce PONV. Although the analgesic effects of nicotine are known,15 there have been limited recent studies on the effects of nicotine on pain control after surgery and their results are somewhat contradictory.16,17 Animal studies have shown that nicotine agonists have analgesic properties through activating the central inhibitory pathways of pain as well as environmental actions.18 Therefore, this study aimed to evaluate the effect of nicotine patches on controlling postoperative nausea, vomiting, and pain.
Methods
This triple-blind randomized clinical trial study was conducted during 2017-2018 in Imam Khomeini hospital in Ardabil. Written informed consent was obtained from all patients. A total of 100 patients undergoing laparoscopic cholecystectomy under general anesthesia aged 18 to 65 years who were non-smokers, with American Society of Anesthesiologists Classification (ASA class) I/II were selected for the study.
Patients with a history of recent myocardial infarction, severe arrhythmia, recent stroke, Parkinson’s disease, skin dermatitis, diabetes, and uncontrolled hypertension as well as those with a pain score above 8 based on pain assessment criteria, requiring laparotomy during surgery, and not being able to tolerate nicotine patch for 24 hours were excluded from the study. Patients, researchers, and analyzers were blind to the type of drug received. Drugs were prepared using quadruple blocks with random sequence allocation (2 nicotine patches and 2 placebo patches) in sealed envelopes (sequentially numbered, sealed, opaque envelopes) by someone unaware of the study protocol.
To maintain a random sequence, the envelopes were numbered in the same way on the outer surface of the packets. Based on the order of entry of the participants, one of the envelopes was opened and the assigned group of that participant was determined. One hour before surgery, patients in the nicotine group used nicotine patches containing 17.5 mg of nicotine for 24 hours located on their right arm, and in the control group, placebo (nicotine-free) patches were used.
The patches were placed on the upper arm and covered with sterile gauze and adhesive tape (Nicotell, 17 mg/24 h patch). The patches were kept for 24 hours after surgery. Upon arrival in the operating room, (30 min before induction), an IV infusion of 500 ml Ringer’s solution was started.
All patients were anesthetized with midazolam 1 mg, fentanyl 2 μg/kg, propofol 1.5 to 2.5mgkg, and 0.5-0.6 mg/kg atracurium. Anesthesia was maintained with remifentanil (0.1-1 μg/kg/min) and propofol (0.1-0.2 mg/kg/min) in 100% oxygen. When the HR and BP were increased 20% above the patient’s preoperative reference level, 100 μg fentanyl was given. The patients were mechanically ventilated to maintain the end-expiratory CO2 value between 34 and 36 mm Hg. Reverse Trendelenburg position was maintained at 25˚-35˚ during the operation. Pneumoperitoneum was established by insufflation of carbon dioxide gas at 12-15 mm Hg pressure. At the end of skin closure, infusion of propofol was discontinued and neuromuscular blockade was reversed with 2.5 mg/IV neostigmine and 1.25 mg/IV atropine.
Throughout the surgery, the patient’s blood pressure, ECG, HR, as well as inspiratory and expiratory CO2 and O2 were checked. All patients were monitored for any events of nausea, vomiting, and pain for the first 24 hours after surgery, by one of the blinded authors. The use of rescue antiemetic medication was recorded. Rescue antiemetic medication consisted of 10 mg metoclopramide IV administered for N/V scores 2 and 3. The intensity of postoperative pain was studied as a quantitative variable by visual analogue scale (VAS) ranging from 0 (no pain) to 10 (unbearable pain). If any patient experienced VAS > 4, 25 mg meperidine IV was administered as an anesthetic agent.
All patients were compared on demographic characteristics. Primary outcomes of patients included measuring pain VAS score and incidence of PONV using the N/V score at 0, 6, 12, and 24 hours after surgery. The adverse effects such as severe pruritus, respiratory disorders, headache, and allergic reactions were evaluated in the recovery ward.
The sample size was estimated at 50 patients per group based on a study power of 80%, type I error of 5%, and a difference of 30% in the incidence of PONV within 24 hours in controls.19 The data were analyzed using SPSS software (version 22). Qualitative data were reported with percentage and quantitative data with mean and standard deviation. Due to the normal distribution of data verified with the Kolmogorov-Smirnov test, student t test was used for quantitative data analysis and chi-square test and Fisher’s exact test were used for analyzing the qualitative data. Repeated measures ANOVA was used to examine quantitative variables at different time intervals. P value less than 0.05 was considered significant.
Results
A total of 100 patients were randomly divided into two groups (each with 50 patients). There was no loss to follow-up in groups (Figure 1).
Figure 1.
Consort flow diagram of patients in the trial study
There was no significant difference between the two groups with respect to age, gender, medical history, surgery history, and duration of surgery (Table 1).
Table 1. Baseline comparative patient characteristics .
| Variable |
Nicotine (n=50)
Mean±SD |
Placebo (n=50)
Mean±SD |
P value |
| Age (y) | 50.74 ± 10.27 | 47.06 ± 11.75 | 0.09a |
| Gender (M/F) | 20/30 | 23/27 | 0.68b |
| Medical history, N (%) | 15 (30) | 13 (26) | 0.82b |
| Surgery history, N (%) | 6 (12) | 4 (8) | 0.74b |
| Duration of surgery | 46.36 ± 7.52 | 44.06 ± 8.19 | 0.14a |
| Intraoperative opioid analgesics (fentanyl μg) | 150 ± 10 | 140 ± 15 | 0.09a |
aStudent t test with equal variances.
bChi- Square; Fisher’s exact test.
The trend of pain reduction in both nicotine and placebo groups at different postoperative time periods was significant (P < 0.001) (Figure 2), but the difference in the intensity of pain between the two groups at different study time periods was not significant (Table 2).
Figure 2.
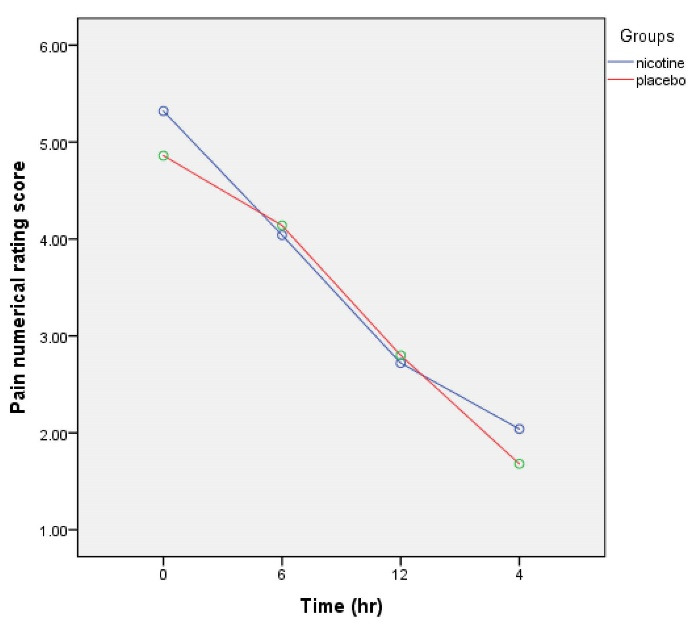
Numerical pain scale score with and without nicotine
Table 2. Measurement of postoperative pain .
| Evaluation (h) | Nicotine (n=50) | Placebo (n=50) | P value a | ||
| Mean±SD | Median | Mean±SD | Median | ||
| 0 | 5.32 ± 1.46 | 5 | 4.86 ± 1.60 | 5 | 0.13 |
| 6 | 4.04 ± 1.67 | 4 | 4.14 ± 1.32 | 4 | 0.74 |
| 12 | 2.72 ± 1.34 | 2 | 2.80 ± 1.22 | 2 | 0.75 |
| 24 | 2.04 ± 1.12 | 2 | 1.68 ± 0.86 | 2 | 0.07 |
a Student t test with equal variances
The rescue medication usage did not differ between the two groups (P = 0.096). In the nicotine group, 18 patients received 25 mg of meperidine and 8 patients received 50 mg of meperidine. In the placebo group, 15 patients received 25 mg of meperidine and 9 patients received 50 mg of meperidine (Table 3).
Table 3. Assessment of rescue medication .
| Meperidine | Nicotine (n=50) | Placebo (n=50) | P value |
| 0 mg | 24 (48%) | 26 (52%) | 0.096a |
| 25 mg | 18 (36%) | 15 (30%) | |
| 50 mg | 8 (16%) | 9 (18%) |
aChi-Square; Fisher’s exact test.
There was no statistically significant difference between nicotine and placebo groups in the rate of nausea and vomiting in any of the time periods including 0, 6, 12, and 24 hours after surgery (Table 4). The number of patients with grade 3 nausea and vomiting at different time periods was higher in the nicotine group than in the placebo group (4 vs. 1), but this difference was not statistically significant (Table 4).
Table 4. Occurrence of postoperative nausea and vomiting .
| Evaluation (h) | Nicotine (n=50) | Placebo (n=50) | P valuea | ||||||
| No nausea, No vomiting | Nausea present, No vomiting | Nausea present, Vomiting, present | Vomiting>2 episodes in 30 min | No nausea, No vomiting | Nausea present, No vomiting | Nausea present, Vomiting, present | Vomiting>2 episodes in 30 min | ||
| 0 | 22(44%) | 21(42%) | 7(14%) | 0 | 20(40%) | 21(42%) | 9(18%) | 0 | 0.7 |
| 6 | 24(48%) | 18(36%) | 5(10%) | 3(6%) | 24(48%) | 22(44%) | 4(8%) | 0 | 0.3 |
| 12 | 32(64%) | 16(32%) | 1(2%) | 1(2%) | 39(78%) | 9(18%) | 2(4%) | 0 | 0.2 |
| 24 | 42(84%) | 6(12%) | 2(4%) | 0 | 41(82%) | 8(16%) | 0 | 1(2%) | 0.3 |
| Antiemetics | 19 (38) | 16 (32) | 0.1 | ||||||
a Chi- square; Fisher’s exact test.
Discussion
The present triple-blind controlled clinical trial was conducted on 100 patients aged 18 to 65 years with a mean age of 50.74 ± 10.27 in the nicotine group and 47.06 ± 11.75 in the placebo group. The nicotine group included 20 males and 30 females while the placebo group consisted of 23 males and 27 females, indicating that the gender distribution was not statistically significant. Given that the duration of surgery can affect the amount of pain and postoperative complications, the mean duration of surgery in the nicotine and placebo groups was 46.36 ± 7.52 and 46.06 ± 8.19 minutes respectively, which was not significantly different (P = 0.14).
There was a significant decrease in pain intensity over time (P < 0.001). However, the amount of pain in the nicotine group compared to the control group was not statistically significant at different time periods.
The results of various studies have shown that the rate of PONV in non-smokers is one-eighth that of smokers. The mechanism is not well understood but researchers believe that the emergence of a desensitization state to nausea and vomiting in smokers is probably the cause of this phenomenon.20 In the present study, comparing the two nicotine and placebo groups indicated that the association of nicotine and PONV was not statistically significant. Two clinical trial studies were conducted to evaluate the effectiveness of nicotine patch on postoperative complications.14,21 The findings of both studies were consistent with the results of the present study. In the study by Habib et al, 90 non-smokers underwent prostatectomy under general anesthesia.21 These patients received a 7-mg nicotine patch in 24 hours, similar to the present study, and were compared with the placebo group. They found that there was no statistical difference in the incidence of PONV and the need for antiemetic medication. Even the maximum verbal rating scale scores of nausea were higher in the nicotine group than in the placebo group.21
In the study conducted by Czarnetzki et al, the 24-hour cumulative incidence of nausea and vomiting was higher in the nicotine group than in the placebo group, and PONV episodes occurred earlier in the nicotine group.14 On the other hand, Ionescu et al. confirmed the effect of nicotine patch to prevent PONV. They concluded that the smoking group (receiving nicotine patch versus the non-smoking group) had a significant reduction in the incidence of PONV.22 This difference can be due to the observational type of study and biases of observation and selection of samples cannot be rejected.
Research has shown that smoking has a protective effect on PONV.23-27 Probably, the existence of antiemetics in cigarette smoke is the reason for this effect. PONV-mediated receptors include dopamine (D2), cholinergic, histamine (H1), and 5HT3. The antiemetic effect of cigarette smoke can be due to the inhibition of one of these receptors.28 Two influential components for the acute effects of smoking are nicotine and carbon monoxide. Nicotine may inhibit the function of the serotonin receptor and thereby affect nausea and vomiting.29 On the other hand, nicotine may increase gastrointestinal stimulation.28 Chronic smoking may reduce PONV. This effect may be due to the chronic effect of smoking on nicotine receptors in the nervous system, and these changes manifest themselves in the form of increased resistance to stimulants of nausea and vomiting. Smoking also increases the metabolism of some drugs by improving the activity of cytochrome P450. This accelerates the metabolism of some anesthetics (especially inhaled anesthetics) that may play a role in PONV.30,31
A possible explanation for the reduced likelihood of PONV in smokers may be the greater resistance of these people to PONV-promoting stimuli. As a result, these people are more likely to become chronic smokers and addicted to tobacco. Therefore, nicotine acts as an indicator of natural resistance to PONV stimulants, not as an antiemetic.14
The use of nicotine patch did not control postoperative pain or reduce drug use after laparoscopic cholecystectomy. Lack of sufficient blood nicotine concentration using a nicotine patch may be the reason for this result in the present study. Failure to examine blood nicotine concentrations may be one of the limitations of the present study. Chronic exposure to nicotine during surgery, with acute downregulation of the nicotine receptor system, may lead to tolerance to the central analgesic effects of the drug.32 Since the use of inhaled anesthetics such as isoflurane has a competitive effect on nicotine receptors, confounding the study results, all patients in this study underwent pure intravenous general anesthesia to avoid this effect. Due to the possibility of confounding and intervening factors and use of various anesthetics in the present study, different time periods including 0, 6, 12, and 24 hours were taken to measure the consequences.
In a review of 9 studies conducted by Mishriky and Habib,31 it was reported that the reduction in pain score at 24 hours was neither clinically nor statistically significant, which was similar to the results of the present study. Moreover, in the study by Turan et al, intraoperative use of nicotine patch and opioids did not reduce the amount of postoperative pain after gynecological surgery.32 Nevertheless, Martins et al33 analyzed 17 patients under laparoscopic cholecystectomy and observed that despite the low pain score for the nicotine group at 24 hours, nicotine was not effective in controlling pain at the time intervals of the study. In addition, it has been shown in various studies that nicotine can cause hypoxia in tissues as a vasoconstrictor and also by increasing the concentration of calcium ions. Besides, as a result of short-term increase in muscle contractions, it can cause fatigue and increase pain.34 The nicotine patch was associated with a significant reduction in morphine consumption over the first 24 hours but there was no difference in pain intensity, despite this decrease.
One of the limitations of the present study was not investigating the effect of different doses of nicotine patches as well as different nicotine use methods such as inhalation. Other limitations were not examining the history of smoking and not measuring nicotine levels in patient blood. Nicotine side effects and nicotine-induced hemodynamic changes also need to be investigated. It is recommended to conduct more studies with larger sample sizes and different doses of nicotine patch to investigate the effect of nicotine on hemodynamic changes at different time periods after surgery and even a few days later. The effect of nicotine administration on smokers for postoperative analgesia also needs to be analyzed.
Conclusion
Preoperative transcutaneous administration of low-dose nicotine in non-smokers did not decrease the incidence of PONV and pain intensity within the first 24 hours after surgery. Nicotine patch did not result in a significant reduction in postoperative morphine consumption after laparoscopic cholecystectomy.
Acknowledgments
This study reports the results of a student thesis. The authors would like to thank the Research Deputy of Ardabil University of Medical Sciences for supporting and funding this research.
Citation: Seyedsadeghi M, Arabzadeh A, Entezariasl M, Shahbazzadegan B, Dindar S, Isazadehfar K. The effect of nicotine patch on reducing nausea, vomiting, and pain following laparoscopic cholecystectomy: A randomized clinical trial. Addict Health. 2023;15(1):39-44. doi:10.34172/ahj.2023.1364
Footnotes
Authors’ Contribution
Conceptualization: Mirsalim Seyedsadeghi, Khatereh Isazadehfar, Masood Entezariasl.
Data Curation: Amirahmad Arabzadeh, Sajjad Dindar.
Formal Analysis: Khatereh Isazadehfar, Masood Entezariasl.
Investigation: Mirsalim Seyedsadeghi, Amirahmad Arabzadeh, Sajjad Dindar.
Methodology: Khatereh Isazadehfar.
Project Administration: Mirsalim Seyedsadeghi, Masood Entezariasl.
Resources: Amirahmad Arabzadeh.
Supervision: Mirsalim Seyedsadeghi.
Validation: Khatereh Isazadehfar, Bita Shahbazzadegan.
Writing – Original Draft: Khatereh Isazadehfar, Bita Shahbazzadegan.
Writing – Review & Editing: khatereh Isazadehfar, Bita Shahbazzadegan, Mirsalim Seyedsadeghi, Masood Entezariasl.
Competing Interests
The authors declared no conflict of interest.
Ethical Approval
This study was approved by the ethics committee of Ardabil University of Medical Sciences under the code IR.ARUMS.Rec.1396.82 and registered in the Iranian Registry of Clinical Trials (identifier: IRCT2017092336328N1; https://www.irct.ir/trial/27192)
References
- 1. Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Miller’s Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010.
- 2. Brunton LL, Chabner BA, Knollmann BC. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw Hill; 2002. p. 879-81.
- 3.Wilhelm SM, Dehoorne-Smith ML, Kale-Pradhan PB. Prevention of postoperative nausea and vomiting. Ann Pharmacother. 2007;41(1):68–78. doi: 10.1345/aph.1H398. [DOI] [PubMed] [Google Scholar]
- 4.Alizadeh S, Mahmoudi GA, Solhi H, Sadeghi-Sedeh B, Behzadi R, Kazemifar AM. Post-operative analgesia in opioid dependent patients: comparison of intravenous morphine and sublingual buprenorphine. Addict Health. 2015;7(1-2):60–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Soltani Mohammadi S, Seyedi M. Effects of gabapentin on early postoperative pain, nausea and vomiting in laparoscopic surgery for assisted reproductive technologies. Pak J Biol Sci. 2008;11(14):1878–80. doi: 10.3923/pjbs.2008.1878.1880. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson SJ, Jiwanmall M, Cherian NE, Kamakshi S, Williams A. Reduction in post-operative nausea and vomiting (PONV) by preoperative risk stratification and adherence to a standardized anti emetic prophylaxis protocol in the day-care surgical population. J Family Med Prim Care. 2021;10(2):865–70. doi: 10.4103/jfmpc.jfmpc_1692_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88(5):659–68. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- 8.Entezariasl M, Khoshbaten M, Isazadehfar K, Akhavanakbari G. Efficacy of metoclopramide and dexamethasone for postoperative nausea and vomiting: a double-blind clinical trial. East Mediterr Health J. 2010;16(3):300–3. [PubMed] [Google Scholar]
- 9.Teshome D, Fenta E, Hailu S. Preoperative prevention and postoperative management of nausea and vomiting in resource limited setting: a systematic review and guideline. Int J Surg Open. 2020;27:10–7. doi: 10.1016/j.ijso.2020.10.002. [DOI] [Google Scholar]
- 10.Leksowski K, Peryga P, Szyca R. Ondansetron, metoclopramid, dexamethason, and their combinations compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a prospective randomized study. Surg Endosc. 2006;20(6):878–82. doi: 10.1007/s00464-005-0622-7. [DOI] [PubMed] [Google Scholar]
- 11.Numazaki M, Fujii Y. Reduction of emetic symptoms during cesarean delivery with antiemetics: propofol at subhypnotic dose versus traditional antiemetics. J Clin Anesth. 2003;15(6):423–7. doi: 10.1016/s0952-8180(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Xie X, Gu X. Dexamethasone for preventing postoperative nausea and vomiting after mastectomy. Medicine (Baltimore) 2020;99(30):e21417. doi: 10.1097/md.0000000000021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose MA, Kam PC. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57(5):451–62. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 14.Czarnetzki C, Schiffer E, Lysakowski C, Haller G, Bertrand D, Tramèr MR. Transcutaneous nicotine does not prevent postoperative nausea and vomiting: a randomized controlled trial. Br J Clin Pharmacol. 2011;71(3):383–90. doi: 10.1111/j.1365-2125.2010.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ditre JW, Heckman BW, Zale EL, Kosiba JD, Maisto SA. Acute analgesic effects of nicotine and tobacco in humans: a meta-analysis. Pain. 2016;157(7):1373–81. doi: 10.1097/j.pain.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flood P, Daniel D. Intranasal nicotine for postoperative pain treatment. Anesthesiology. 2004;101(6):1417–21. doi: 10.1097/00000542-200412000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Cheng SS, Yeh J, Flood P. Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg. 2008;106(1):264–9, table of contents. doi: 10.1213/01.ane.0000287653.77372.d9. [DOI] [PubMed] [Google Scholar]
- 18.Hong D, Conell-Price J, Cheng S, Flood P. Transdermal nicotine patch for postoperative pain management: a pilot dose-ranging study. Anesth Analg. 2008;107(3):1005–10. doi: 10.1213/ane.0b013e318163204f. [DOI] [PubMed] [Google Scholar]
- 19.Tramèr MR. Tramèr MRA rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviewsPart IEfficacy and harm of antiemetic interventions, and methodological issues. Acta Anaesthesiol Scand. 2001;45(1):4–13. doi: 10.1034/j.1399-6576.2001.450102.x. [DOI] [PubMed] [Google Scholar]
- 20.Erhardt S, Schwieler L, Engberg G. Excitatory and inhibitory responses of dopamine neurons in the ventral tegmental area to nicotine. Synapse. 2002;43(4):227–37. doi: 10.1002/syn.10044. [DOI] [PubMed] [Google Scholar]
- 21.Habib AS, White WD, El Gasim MA, Saleh G, Polascik TJ, Moul JW, et al. Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesth Analg. 2008;107(3):999–1004. doi: 10.1213/ane.0b013e31816f2616. [DOI] [PubMed] [Google Scholar]
- 22.Ionescu D, Badescu C, Acalovschi I. Nicotine patch for the prevention of postoperative nausea and vomiting: a prospective randomised trial. Clin Drug Investig. 2007;27(8):559–64. doi: 10.2165/00044011-200727080-00004. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigo C. The effects of cigarette smoking on anesthesia. Anesth Prog. 2000;47(4):143–50. [PMC free article] [PubMed] [Google Scholar]
- 24.Ionescu D, Bãdescu C, Maican D, Acalovschi I. Does smoking have an influence on postoperative nausea and vomiting? South Afr J Anaesth Analg. 2007;13(4):29–32. doi: 10.1080/22201173.2007.10872495. [DOI] [Google Scholar]
- 25.Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78(1):7–16. doi: 10.1213/00000539-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Pierre S, Benais H, Pouymayou J. Apfel’s simplified score may favourably predict the risk of postoperative nausea and vomiting. Can J Anaesth. 2002;49(3):237–42. doi: 10.1007/bf03020521. [DOI] [PubMed] [Google Scholar]
- 27.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91(1):109–18. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney BP. Why does smoking protect against PONV? Br J Anaesth. 2002;89(6):810–3. doi: 10.1093/bja/aef269. [DOI] [PubMed] [Google Scholar]
- 29.Breitinger HG, Geetha N, Hess GP. Inhibition of the serotonin 5-HT3 receptor by nicotine, cocaine, and fluoxetine investigated by rapid chemical kinetic techniques. Biochemistry. 2001;40(28):8419–29. doi: 10.1021/bi0106890. [DOI] [PubMed] [Google Scholar]
- 30.Guengerich FP. Cytochrome P450 2E1 and its roles in disease. Chem Biol Interact. 2020;322:109056. doi: 10.1016/j.cbi.2020.109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishriky BM, Habib AS. Nicotine for postoperative analgesia: a systematic review and meta-analysis. Anesth Analg. 2014;119(2):268–75. doi: 10.1213/ANE.0b013e3182a8fa7b. [DOI] [PubMed] [Google Scholar]
- 32.Turan A, White PF, Koyuncu O, Karamanliodlu B, Kaya G, Apfel CC. Transdermal nicotine patch failed to improve postoperative pain management. Anesth Analg. 2008;107(3):1011–7. doi: 10.1213/ane.0b013e31816ba3bb. [DOI] [PubMed] [Google Scholar]
- 33.Martins Filho ED, de Melo Vasconcelos CF, de Santa Cruz Oliveira F, da Fonseca Pereira A, Ferraz ÁAB. Evaluation of nicotine patch in pain control of patients undergoing laparoscopic cholecystectomy. Rev Col Bras Cir. 2018;45(3):e1756. doi: 10.1590/0100-6991e-20181756. [DOI] [PubMed] [Google Scholar]
- 34.Pirouzi S, Ghanbari A, Moslemi Haghighi F, Ghafarinejad F, Pouya F, Motiallah T. The prevalence of musculoskeletal pain in male cigarette smoking students at Shiraz University of Medical Sciences, Iran. Addict Health. 2011;3(3-4):125–9. [PMC free article] [PubMed] [Google Scholar]


