Abstract
目的
确定去甲肾上腺素预防双胎产妇腰硬联合麻醉后低血压的90%有效输注剂量(90% effective dose, ED90),并与单胎产妇的ED90进行对比。
方法
于2020年11月–2021年6月在四川大学华西第二医院,纳入200例拟在腰硬联合麻醉下行剖宫产的产妇,采用随机对照研究,其中单胎、双胎产妇各100例。按随机数字表,产妇被随机分入以下5个组:0.025、0.050、0.075、0.100和0.125 μg/(kg·min)去甲肾上腺素组,每组包含单胎和双胎产妇各20例。在注射腰麻药物同时,开始去甲肾上腺素输注,直至胎儿娩出。主要结局指标为腰硬联合麻醉后至胎儿娩出期间产妇的低血压发生率。对产妇的低血压发生情况进行生存分析(定义未发生低血压为生存)。根据低血压发生率,通过probit回归分析分别推算去甲肾上腺素预防单胎和双胎产妇腰硬联合麻醉下剖宫产中,使90%产妇不发生低血压的去甲肾上腺素输注剂量(即ED90)及其95%置信区间(CI)。
结果
单胎产妇中、双胎产妇中,基线资料及麻醉手术数据差异无统计学意义(P>0.05)。以0.025、0.050、0.075、0.100和0.125 μg/(kg·min)输注去甲肾上腺素,单胎产妇中低血压发生率分别为50%(10/20)、35% (7/20)、20% (4/20)、10% (2/20)和5%(1/20),ED90为0.100 (95%CI: 0.082~0.130) μg/(kg·min);双胎产妇中,低血压发生率则分别为60%(12/20)、20%(4/20)、20% (4/20)、10% (2/20)和5% (1/20),ED90为0.098 (95%CI: 0.080~0.127) μg/(kg·min)。单胎产妇各剂量组间的生存曲线差异有统计学意义,双胎产妇各剂量组间的生存曲线差异有统计学意义(P<0.05)。单胎产妇、双胎产妇的反应性高血压发生率,均随去甲肾上腺素输注剂量的升高而增加(P<0.05),单胎产妇各剂量组间及双胎产妇各剂量组间,产妇其他不良反应、新生儿结局差异无统计学意义(P>0.05)。各剂量组单双胎产妇间的年龄、身高差异无统计学意义(P>0.05),但产妇的孕周、体质量、体质量指数(BMI)差异有统计学意义(P<0.05)。相同剂量组单、双胎产妇间,低血压、反应性高血压、心动过缓、头晕、恶心呕吐发生率差异无统计学意义(P>0.05)。各剂量组内单双胎产妇间生存曲线差异无统计学意义。单、双胎产妇对去甲肾上腺素的ED90差异无统计学意义。
结论
去甲肾上腺素预防双胎产妇与单胎产妇腰硬联合麻醉后低血压的ED90差异不明显,临床需考虑孕周、体质量、BMI因素的干扰。
Keywords: 剖宫产, 单胎妊娠, 双胎妊娠, 腰硬联合麻醉后低血压, 去甲肾上腺素量效关系, 随机对照研究
Abstract
Objective
To determine and compare the 90% effective dose (ED90) of prophylactic infusion of norepinephrine for preventing hypotension during combined spinal-epidural anesthesia for cesarean section in singleton versus twin pregnancies.
Methods
A randomized controlled trial was conducted, enrolling 200 pregnant women, 100 of which were of singleton pregnancies while the other 100 were of twin pregnancies, at West China Second University Hospital, Sichuan University between November 3, 2020 and June 2, 2021. All 200 subjects were to have Cesarean section under combined spinal-epidural anesthesia. By using a random number table, they were randomly assigned to five groups, receiving norepinephrine at the infusion dosage of 0.025, 0.050, 0.075, 0.100, and 0.125 μg/(kg·min), with 20 subjects of singleton pregnancy and 20 subjects of twin pregnancy in each group. Norepinephrine infusion started when the anesthesiologist initiated the spinal anesthetic injection and lasted until the delivery of the fetus. The primary outcome measure was the incidence of maternal hypotension during combined spinal-epidural anesthesia, up until the delivery of the fetus. Survival analysis, with survival being defined as not having hypotension, of the incidence of hypotension among the subjects was conducted. Probit regression was used to determine the ED90 of norepinephrine, as well as the corresponding 95% confidence interval (CI), for preventing hypotension during cesarean delivery under combined spinal-epidural anesthesia in women with singleton and twin pregnancies.
Results
There was no significant difference in the baseline data or the anesthesia and operation data between pregnant women of singleton pregnancy and those of twin pregnancy (P>0.05). In singleton pregnant women receiving 0.025, 0.05, 0.075, 0.1 and 0.125 μg/(kg·min) of norepinephrine, the incidence of hypotension was 50% (10/20), 35% (7/20), 20% (4/20), 10% (2/20) and 5% (1/20), respectively. The estimated ED90 of prophylactic norepinephrine for preventing hypotension during anesthesia was 0.100 (95% CI, 0.082-0.130) μg/(kg·min). In twin pregnant women receiving 0.025, 0.05, 0.075, 0.1 and 0.125 μg/(kg·min) of norepinephrine, the corresponding incidence of hypotension was 60% (12/20), 20% (4/20), 20% (4/20), 10% (2/20) and 5% (1/20). The estimated ED90 of norepinephrine for preventing hypotension during anesthesia was 0.098 (95% CI, 0.080-0.127) μg/(kg·min). Survival analysis showed significant difference in the incidence of hypotension among the five groups receiving different infusion doses in singleton pregnancy subjects, and the same is true of the twin pregnancy subjects (P<0.05). The incidence of reactive hypertension increased with increasing dosage of norepinephrine in both singleton pregnancy subjects and twin pregnancy subjects (P<0.05). There was no significant difference in the incidence of other maternal adverse reaction or in neonatal outcomes in singleton and twin pregnancy subjects receiving different dosage of norepinephrine (P>0.05). The gestational weeks, weight, and BMI were significantly different (P<0.05), while the other characteristics, including age and height, were comparable (P>0.05) between singleton and twin pregnancy subjects receiving norepinephrine at the same dosage. There was no significant difference in the incidence of hypotension, reactive hypertension, bradycardia, nausea and vomiting, and dizziness between singleton and twin pregnancy subjects receiving the same dose (P>0.05). Survival analysis displayed no significant difference in the incidence of hypotension between singleton and twin pregnancy subjects receiving norepinephrine at the same dosage (P>0.05). There was no significant difference in the ED90 of norepinephrine between women with singleton pregnancies and those with twin pregnancies (P>0.05).
Conclusion
There was no significant difference in the ED90 of norepinephrine for preventing hypotension during combined spinal-epidural anesthesia between women with singleton pregnancy and those with twin pregnancy. Interference of other factors, including gestational age, body mass, and BMI should be considered in clinical practice.
Keywords: Cesarean delivery, Singleton pregnancy, Twin pregnancy, Hypotension after combined spinal-epidural anesthesia, Norepinephrine dose-effect relationship, Randomized controlled study
众所周知,与单胎产妇相比,双胎产妇在妊娠期间经历的血流动力学变化更显著,尤其体现在心输出量这一指标上[1-6]。据报道,双胎产妇中剖宫产率可高达70%[7]。经典产科和麻醉教科书认为双胎产妇在椎管内麻醉下行剖宫产时出现低血压的可能性更大,程度更重[8-9]。可见维持围剖宫产期间双胎产妇的血流动力学稳定,尤其是维持母体心输出量处于较高水平,对于改善母婴结局具有重要意义。
目前广泛应用于预防椎管内麻醉后低血压的去氧肾上腺素,单纯激动α-肾上腺素能受体。其在升高血压的同时,反射性引起母体心动过缓,导致心输出量下降,干扰胎盘灌注。最近的研究表明,去甲肾上腺素(norepinephrine, NE)在预防剖宫产腰麻后低血压方面,可能比去氧肾上腺素更有优势[10-13]。NE除了可激活α-肾上腺素能受体外,还具有微弱的β-肾上腺素能受体活性,可有效对抗由于单纯α-肾上腺素能受体激活后引起的反射性心动过缓,保证心输出量。
已有学者报道了在单胎产妇中,预防腰麻后剖宫产中低血压的NE有效输注剂量[14-16],但预防双胎产妇在剖宫产期间低血压的NE剂量研究尚不多见。本研究旨在确定NE预防双胎产妇腰硬联合麻醉后低血压的90%有效输注剂量(90% effective dose, ED90),并与单胎产妇的ED90进行对比,探讨其差异并分析原因。
1. 对象与方法
1.1. 研究对象
本研究在实施前已获得中国注册临床试验伦理审查委员会批准(批准号:ChiECRCT20200339),并于中国临床试验注册中心完成注册(注册号:ChiCTR2000038261)。该临床试验于2020年11月–2021年6月在四川大学华西第二医院进行。
纳入标准:①孕周≥32周的双胎产妇;②足月(孕周≥37周)的单胎产妇;③年龄20~40岁;④拟行择期腰硬联合麻醉下剖宫产;⑤产妇自愿参与试验并签署知情同意书。
排除标准:①产妇具有严重的心脑血管疾病;②有妊娠高血压或糖尿病;③已知胎儿异常;④产妇对NE过敏或不耐受;⑤胎儿宫内生长受限;⑥近期使用单胺氧化酶抑制剂;⑦体质量指数>35 kg/m2;⑧身高>180 cm或<150 cm;⑧基础收缩压<100 mmHg(1 mmHg=0.133 kPa);⑨美国麻醉医师协会(ASA)分级≥Ⅲ级;⑩有任何其他椎管内麻醉禁忌症者。
1.2. 样本量计算与试验分组
根据既往文献报道的数据[14],我们设定双侧α=0.05,检验效能(1-β)=0.90,使用PASS软件(version 15.0.5, USA)计算总样本量为65例,考虑到存在失访的情况,参考同类型文献[15, 17],最终将所需样本量增加至100例。
共纳入220例产妇,7例单胎产妇因腰硬联合麻醉失败退出研究,13例双胎产妇因腰硬联合麻醉失败退出研究。最终共纳入200例产妇,包括单胎产妇100例,双胎产妇100例。使用SPSS软件(IBM SPSS Statistics 25.0)为单双胎产妇分别生成随机数字表,产妇随机分配进入以下5个组:0.025、0.05、0.075、0.100、0.125 μg/(kg·min) NE组,每组纳入单胎和双胎产妇各20例,按0.025、0.05、0.075、0.100、0.125 μg/(kg·min) ,分组输注NE。
1.3. 试验药物配制
采用完全相同的20 mL注射器配制试验所需要的NE溶液。为保持双盲,微量泵输注速度均设定为20 mL/h。为达到选定的NE输注速度,每个产妇的NE总量(mg)计算如下:①0.025 μg/(kg·min) NE组:体质量(kg)×1.5 μg;②0.05 μg/(kg·min) NE组:体质量(kg)×3 μg;③0.075 μg/(kg·min) NE组:体质量(kg)×4.5 μg;④0.100 μg/(kg·min) NE组:体质量(kg)×6 μg;⑤0.125 μg/(kg·min) NE组:体质量(kg)×7.5 μg。
1.4. 试验过程
产妇入手术室后,连接心电监护,包括心电图、脉搏血氧饱和度和无创血压。麻醉开始前,每3 min测1次血压,以连续测量差异小于10%的3次血压和心率的平均值作为基础血压和基础心率。麻醉实施前,开放2条18G静脉通路,一条用于补液,另一条用于输注NE。所有患者均不接受静脉输液预负荷。
产妇左侧卧位,选择第三与第四腰椎间隙进行穿刺。利用负压试验确定18G硬膜外针抵达硬膜外腔后,置入25G腰麻针。确认有脑脊液自腰麻针流出后,向蛛网膜下腔注入2.5 mL 0.5%等比重布比卡因。随后通过硬膜外针置入硬膜外导管并固定。最后将产妇转为平卧位,设置手术床向左倾斜15°。蛛网膜下腔给药10 min后测定麻醉平面,阻滞平面未达T6的受试者被认为麻醉失败并剔除试验。
蛛网膜下腔给药的同时,启动NE输注,同时快速度输注乳酸钠林格液,补液量最多不超过1.5 L。当胎儿(双胎产妇则为第二个胎儿)娩出后,停止输注NE,此后的补液速度由管理手术的麻醉医生调节。从麻醉开始至胎儿娩出期间,每1 min测1次血压和心率。当发生低血压时,静脉推注10 μg NE补救[18]。当发生反应性高血压时,暂停NE输注,待收缩压恢复至正常范围时再次启动输注。当心率低于50 min−1并伴有低血压时,给予0.5 mg阿托品补救;当心率低于50 min−1并伴有高血压时,停止输注NE,待心率恢复至高于50 min−1时,再次启动输注。
胎儿娩出以后,由不知晓患者分组情况的助产士于分娩后第1 min和第5 min对新生儿进行Apgar评分。采集0.5 mL脐动脉内的血液进行血气分析。
1.5. 结局指标
主要结局指标为每组产妇低血压的发生率,据此使用probit回归分析推算单胎和双胎产妇中NE预防腰硬联合麻醉后低血压的ED90。
次要结局指标包括:反应性高血压、心动过缓的发生率,术中补救性NE、阿托品的使用率,断脐带时的输液量,产妇恶心、呕吐和头晕的发生率,新生儿1 min、5 min Apgar评分,脐动脉血血气分析,新生儿重症监护室(NICU)转入率。记录每组接受麻醉医生干预血流动力学异常的比例,补救性使用NE,暂停药物输注和补救性使用阿托品其中的任何一项处理均视为麻醉干预。
1.6. 定义
ED90:90%有效剂量。对本研究而言, ED90为使90%产妇不发生低血压的NE输注剂量。
生存:对本研究而言,生存分析中的生存指的是产妇不发生低血压。
低血压:收缩压低于基础收缩压的80%或小于90 mmHg。
反应性高血压:收缩压高于基础值的120%或大于140 mmHg。
心动过缓:心率低于50 min-1。
1.7. 统计学方法
使用SPSS25.0和GraphPad Prism 8.0分析数据。计数资料用例数(百分率)表示。同一剂量组单双胎产妇之间的比较采用卡方检验,单、双胎产妇各自不同剂量组间比较采用卡方趋势检验进行统计分析,若组间差异有统计学意义,则使用卡方检验或Fisher检验进行两两比较。计量资料的正态性判断使用Shapiro-Wilk检验。正态分布数据以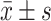 表示,同一剂量组单、双胎产妇之间的比较采用t检验,单双胎产妇各自不同剂量组间比较采用单因素方差分析。非正态分布数据以中位数(P25~P75)表示,同一剂量组单、双胎产妇之间的比较采用Mann-Whitney U检验,单、双胎产妇各自不同剂量组间比较采用Kruskale-Wallis检验。麻醉后前20 min内收缩压随时间的波动用曲线下面积(AUC)表示,并用单因素方差分析比较组间差异。Kaplan-Meier生存曲线用于分析低血压的发生情况,并用log-rank检验比较组间差异。P<0.05为差异有统计学意义。使用R语言(4.1.1)软件中的probit回归分析(probit regression)推算NE的ED90值及其95%可信区间(CI)。
表示,同一剂量组单、双胎产妇之间的比较采用t检验,单双胎产妇各自不同剂量组间比较采用单因素方差分析。非正态分布数据以中位数(P25~P75)表示,同一剂量组单、双胎产妇之间的比较采用Mann-Whitney U检验,单、双胎产妇各自不同剂量组间比较采用Kruskale-Wallis检验。麻醉后前20 min内收缩压随时间的波动用曲线下面积(AUC)表示,并用单因素方差分析比较组间差异。Kaplan-Meier生存曲线用于分析低血压的发生情况,并用log-rank检验比较组间差异。P<0.05为差异有统计学意义。使用R语言(4.1.1)软件中的probit回归分析(probit regression)推算NE的ED90值及其95%可信区间(CI)。
2. 结果
2.1. 纳入产妇基线资料
在单胎产妇中,各剂量组的年龄、身高、体质量、体质量指数、孕周、麻醉平面、基础血压、基础心率及手术数据差异均无统计学意义(P>0.05)。同样,在双胎产妇中,各剂量组产妇的上述指标差异无统计学意义(P>0.05)。
在相同剂量组中,单双胎产妇之间的年龄、身高差异无统计学意义(P>0.05),孕周、体质量、BMI差异有统计学意义(P<0.05)(表1)。
表 1. Baseline data of the pregnant women.
产妇基线资料
| Characteristic | Group△ | Dose of NE/(μg/[kg·min]), n=20 | P | ||||
| 0.025 | 0.05 | 0.075 | 0.10 | 0.125 | |||
| BMI: Body mass index; SBP: Systolic blood pressure; HR: Heart rate. △1: Singleton pregnancy; 2: Twin pregnancy. *P<0.05, vs. group 1 at the same dose level. 1 mmHg=0.133 kPa. | |||||||
| Age/yr. | 1 | 31.0±3.1 | 29.0±3.1 | 30.9±3.5 | 30.8±2.7 | 31.0±2.5 | 0.38 |
| 2 | 32.0±2.1 | 30.6±3.5 | 32.8±3.4 | 31.8±3.6 | 33.0±3.2 | 0.16 | |
| Height/cm | 1 | 161.0±4.6 | 159.4±3.9 | 159.4±3.7 | 159.8±3.1 | 161.7±5.2 | 0.23 |
| 2 | 159.0±4.4 | 160.0±5.5 | 160.0±4.6 | 160.3±6.5 | 161.1±5.7 | 0.86 | |
| Body mass/kg | 1 | 64.1±7.5 | 68.3±6.9 | 68.1±5.5 | 65.1±6.2 | 66.8±6.6 | 0.19 |
| 2 | 70.4±7.2* | 72.5±10.3 | 68.4±7.7 | 69.6±6.9* | 71.3±5.7* | 0.68 | |
| BMI/(kg/cm2) | 1 | 24.7±2.7 | 26.9±2.3 | 26.8±1.7 | 25.8±2.7 | 25.6±2.4 | 0.27 |
| 2 | 27.7±2.6* | 28.4±3.3 | 26.8±2.9 | 27.1±2.3 | 27.5±2.3* | 0.37 | |
| Gestational age/week | 1 | 39.3±0.6 | 39.2±0.7 | 39.2±0.8 | 39.3±0.7 | 39.3±0.5 | 0.96 |
| 2 | 36.6±1.0* | 36.6±0.8* | 36.3±1.5* | 36.9±0.6* | 36.4±1.5* | 0.52 | |
| Induction-delivery interval/min | 1 | 22.1±4.2 | 22.3±6.1 | 22.1±5.2 | 20.4±3.3 | 20.9±4.1 | 0.63 |
| 2 | 22.3±4.3 | 22.4±4.7 | 22.6±5.5 | 21.7±3.7 | 23.4±4.7 | 0.86 | |
| Baseline SBP/mmHg | 1 | 114.0±9.8 | 118.4±7.5 | 114.3±7.3 | 116.0±7.8 | 116.9±7.5 | 0.40 |
| 2 | 119.7±8.3 | 122.2±7.2 | 120.7±8.5 | 118.2±8.9 | 119.6±8.1 | 0.64 | |
| Baseline HR/min−1 | 1 | 85.2±7.3 | 84.3±7.3 | 87.0±5.4 | 83.6±8.2 | 85.0±9.6 | 0.88 |
| 2 | 83.4±9.0 | 86.8±8.9 | 90.3±11.9 | 84.3±11.0 | 85.1±14.7 | 0.34 | |
2.2. 单、双胎产妇麻醉后血流动力学变化
2.2.1. 单胎各剂量组间的比较
从麻醉开始到胎儿娩出期间,每剂量组产妇的补液量差异无统计学意义(P>0.05)。0.025、0.05、0.075、0.100和0.125 μg/(kg·min) NE组产妇:其低血压的发生率分别是50% (10/20)、35%(7/20)、20%(4/20)、10%(2/20)和5%(1/20),各剂量组间产妇低血压发生率差异有统计学意义(P<0.05);反应性高血压发生率在各剂量组间差异有统计学意义(P<0.05)。心动过缓的发生率、需麻醉医师干预血流动力学异常的产妇比例在各剂量组间差异无统计学意义(P>0.05)(表2)。
表 2. Maternal outcomes.
产妇结局
| Maternal outcome | Group△ | Dose of NE/(μg/[kg·min]), n=20 | P | ||||
| 0.025 | 0.05 | 0.075 | 0.10 | 0.125 | |||
| △1: Singleton pregnancy; 2: Twin pregnancy. | |||||||
| Hypotension/case (%) | 1 | 10 (50) | 7 (35) | 4 (20) | 2 (10) | 1 (5) | <0.01 |
| 2 | 12 (60) | 4 (20) | 4 (20) | 2 (10) | 1 (5) | <0.01 | |
| Reactive hypertension/case (%) | 1 | 0 (0) | 0 (0) | 2 (10) | 2 (10) | 9 (45) | <0.01 |
| 2 | 2 (10) | 2 (10) | 1 (5) | 5 (25) | 7 (35) | <0.01 | |
| Bradycardia/case (%) | 1 | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0.48 |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | − | |
| Nausea or vomiting/case (%) | 1 | 1 (5) | 1 (5) | 3 (15) | 2 (10) | 1 (5) | 0.80 |
| 2 | 1 (5) | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 0.22 | |
| Dizziness/case (%) | 1 | 2 (10) | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 0.07 |
| 2 | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0.13 | |
| Physician intervention needed/case (%) | 1 | 10 (50) | 7 (35) | 6 (30) | 4 (20) | 10 (50) | 0.66 |
| 2 | 14 (70) | 6 (30) | 5 (25) | 7 (35) | 8 (40) | 0.11 | |
| Rescue vasopressor bolus/case (%) | 1 | 10 (50) | 7 (35) | 4 (20) | 2 (10) | 1 (5) | <0.01 |
| 2 | 12 (60) | 4 (20) | 4 (20) | 2 (10) | 1 (5) | <0.01 | |
| Atropine rescue bolus/case (%) | 1 | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0.48 |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | − | |
| Intravenous fluid /mL, median (P25-P75) | 1 | 534.6 (420.0-595.8) | 537.6 (492.5-609.8) | 517.8 (467.5-589.0) | 559.9 (460.6-550.5) | 521.1 (495.5-598.3) | 0.44 |
| 2 | 587.0 (454.9-709.0) | 572.5 (461.5-659.0) | 556.6 (444.8-666.5) | 625.6 (517.5-728.0) | 585.8 (452.3-662.0) | 0.74 | |
单胎产妇在麻醉后前20 min内的收缩压和心率随时间变化趋势见图1。血压-时间AUC,在各组间差异有统计学意义(P<0.05)。心率-时间AUC,在各组间差异无统计学意义(P>0.05)。对各组单胎产妇是否发生低血压进行生存分析,生存曲线差异有统计学意义(P<0.05)(图2)。根据各组单胎产妇低血压的发生率,由probit回归分析得出,NE预防腰硬联合麻醉后低血压的ED90为0.100 (95%CI:0.082~0.130) μg/(kg·min)(图3)。
图 1.

Changes in systolic blood pressure (SBP, A) and heart rate (HR, B) in the first 20 min after anesthesia initiated (n=20)
产妇麻醉后前20 min内收缩压(A)、心率(B)变化(n=20)
图 2.
Kaplan-Meier survival curves for the pregnancy women, showing the proportion of subjects not having hypotension during the first 20 min after anesthesia initiated (n=20)
产妇麻醉后前20 min内不发生低血压的生存曲线(n=20)
图 3.
Dose-response curve of norepinephrine infusions for preventing hypotension after combined spinal and epidural anesthesia in singleton or twin pregnancies, calculated using probit regression method
去甲肾上腺素预防单、双胎产妇腰硬联合麻醉后低血压的ED90
2.2.2. 双胎各剂量组间的比较
从麻醉开始到胎儿娩出期间,每组双胎产妇的补液量差异无统计学意义(P>0.05)。0.025、0.050、0.075、0.100和0.125 μg/(kg·min)NE组双胎产妇,其低血压的发生率分别是60%(12/20)、20% (4/20)、20% (4/20)、10%(2/20)和5%(1/20),各剂量组间双胎产妇低血压发生率、反应性高血压发生率差异有统计学意义(P<0.05)。无产妇发生心动过缓。需麻醉医师干预血流动力学异常的产妇比例差异无统计学意义(P>0.05)(表2)。
双胎产妇在麻醉后前20 min内的收缩压和心率随时间变化趋势见图1。血压-时间AUC,在各剂量组间差异有统计学意义(P<0.05)。心率-时间AUC,在各剂量组间差异无统计学意义(P>0.05)。对各组双胎产妇是否发生低血压进行生存分析,差异有统计学意义(P<0.05)(图2)。根据各组双胎产妇低血压发生率,由probit回归分析得出,NE预防腰硬联合麻醉后低血压的ED90为0.098 (95%CI:0.080~0.127) μg/(kg·min)(图3)。
2.2.3. 单、双胎产妇各剂量组间比较
各剂量组内,单、双胎产妇间低血压,反应性高血压,心动过缓,补救性使用NE,补救性使用阿托品的发生率均差异无统计学意义(P>0.05)(表2)。各剂量组内,单、双胎产妇间生存曲线差异无统计学意义(图4)。单、双胎产妇ED90的95%CI高度重合。
图 4.

Kaplan-Meier survival curves for singleton and twin pregnancy women receiving the same dose, showing the proportion of subjects not having hypotension during the first 20 min after anesthesia initiated (n=20)
相同剂量组内,单双胎产妇麻醉后前20 min内不发生低血压的生存曲线(n=20)
A-E: 0.025,0.050,0.075,0.1,and 0.125 μg/(kg·min) NE, respectively.
2.3. 产妇不良反应
无论是在单胎产妇中还是在双胎产妇中,各剂量组产妇头晕、恶心呕吐发生率差异无统计学意义(P>0.05)。各剂量组内,单胎产妇和双胎产妇间头晕、恶心呕吐发生率差异无统计学意义(P>0.05)(表2)。
2.4. 新生儿情况
无论是在单胎产妇中还是在双胎产妇中,各剂量组新生儿NICU转入率、Apgar评分、脐动脉血pH、脐动脉血CO2分压、O2分压、碱剩余,均差异无统计学意义(P>0.05)。相同剂量组内,单双胎新生儿的体质量、身长差异有统计学意义(P<0.05)。部分剂量组内,单双胎新生儿的脐动脉血pH、CO2分压、碱剩余差异有统计学意义,但相应值均在临床检验的正常范围内,此统计学差异无临床意义(表3)。
表 3. Neonatal outcomes.
新生儿结局
| Neonatal outcomes | Group△ | Dose of NE/ (μg/[kg·min])# | P | ||||
| 0.025 | 0.05 | 0.075 | 0.10 | 0.125 | |||
| NICU: Neonatal intensive care unit.△1: Singleton pregnancy; 2: Twin pregnancy. * P<0.05, vs. group 1 at the same dose level. # n=20 for singleton pregnancy, n=40 for twin pregnancy. | |||||||
| (Male/female)/case | 1 | 10/10 | 13/7 | 10/10 | 8/12 | 13/7 | 0.44 |
| 2 | 20/20 | 19/21 | 21/19 | 18/22 | 21/19 | 0.95 | |
| Body mass/g | 1 | 3444.0±355.7 | 3408.0±360.9 | 3325.5±378.2 | 3340.0±350.0 | 3400.0±437.5 | 0.85 |
| 2 | 2546.0±310.4* | 2511.8±303.0* | 2493.3±286.9* | 2522.8±247.1* | 2405.5±354.5* | 0.64 | |
| Height/cm | 1 | 50.4±1.2 | 49.9±1.6 | 49.3±2.0 | 50.0±1.1 | 49.6±2.1 | 0.30 |
| 2 | 46.3±1.7* | 46.4±1.8* | 46.5±1.8* | 47.2±1.7* | 46.3±2.3* | 0.57 | |
| NICU admission/case (%) | 1 | 0 (0) | 0 (0) | 2 (10) | 0 (0) | 0 (0) | 0.20 |
| 2 | 8 (20) | 2 (5) | 10 (25) | 4 (10) | 10 (25) | 0.05 | |
| Apgar <7 at 1 min/case (%) | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | − |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | − | |
| Apgar <7 at 5 min/case (%) | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | − |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | − | |
| Umbilical artery pH | 1 | 7.289±0.028 | 7.278±0.049 | 7.292±0.042 | 7.302±0.029 | 7.302±0.029 | 0.31 |
| 2 | 7.291±0.023 | 7.297±0.020 | 7.290±0.020 | 7.279±0.035* | 7.291±0.029 | 0.31 | |
| Umbilical artery PCO2/mmHg | 1 | 53.8±5.9 | 56.1±7.4 | 55.6±6.6 | 52.4±5.6 | 55.4±7.0 | 0.40 |
| 2 | 50.7±4.6 | 52.1±2.4* | 53.1±5.8 | 53.9±7.2 | 51.3±7.0 | 0.41 | |
| Umbilical artery PO2/mmHg | 1 | 15.6±3.9 | 15.0±6.3 | 15.0±4.0 | 14.4±5.0 | 14.7±5.1 | 0.95 |
| 2 | 13.8±3.8 | 14.7±2.8 | 12.4±4.7 | 14.2±2.6 | 14.4±4.6 | 0.40 | |
| Base excess/mmol L−1 | 1 | −0.8±2.1 | 0.3±1.6 | 0.2±1.6 | −0.7±1.4 | −0.4±1.6 | 0.17 |
| 2 | −2.3±2.1* | −0.9±1.3* | −1.2±2.2* | −1.6±1.9 | −1.5±1.8 | 0.24 | |
3. 讨论
本研究探讨了在单胎与双胎产妇中,NE预防剖宫产腰硬联合麻醉后低血压的90%有效输注剂量。在单胎产妇,NE预防低血压的ED90为0.100 (95%CI:0.082~0.130) μg/(kg·min);在双胎产妇,ED90为0.098 (95%CI: 0.080~0.127) μg/(kg·min)。NE在单、双胎产妇的ED90的95%CI高度重合,提示NE预防单胎与双胎产妇腰硬联合麻醉下剖宫产中低血压的ED90差异无统计学意义[19]。
NGAN KEE等[20]的研究显示,与单胎产妇相比,多胎妊娠产妇在腰麻期间并没有表现出更明显的血流动力学波动;并且在剖宫产术中两组产妇对用于预防低血压的间羟胺的总需求量差异也无统计学意义。然而,这项研究联合了单次推注与连续输注的给药方法,尤其是输注速度根据血压人为频繁调整,这一给药方式在实际临床工作中罕有应用,因此在很大程度上限制了其结论的推广。本研究采用了根据体质量计算NE输注剂量的方案,也是国内外剖宫产指南中最为推荐的给药方式[21]。在确保NE用于剖宫产产妇安全性的前提下[22],本研究设置了更为宽泛的剂量梯度〔0.025~0.125 μg/(kg·min),前人的剂量梯度仅为0.04~0.07 μg/(kg·min)[15]〕,使得ED90位于试验设置的剂量梯度范围之内,从而提高了统计的效力。
经典的产科麻醉教科书认为:由于双胎产妇的子宫体积更大,下腔静脉受压程度更大,使双胎产妇腰麻后发生低血压的风险和严重程度均更高[8-9]。然而这一结论主要源于理论推导,缺乏高质量证据的支持。MEI等[17]在探寻罗哌卡因用于单胎和多胎产妇剖宫产的量效关系时发现,单胎和多胎产妇腰麻后低血压的发生率差异无统计学意义,用于治疗低血压的NE使用剂量在单双胎产妇中也差异无统计学意义。另一项比较腰麻药在双胎和单胎产妇椎管内扩散情况的研究中,尽管观察到双胎产妇的麻醉平面比单胎产妇高两个节段,但两组的低血压发生率和需要麻黄碱治疗低血压的产妇比例差异无统计学意义[23]。此外,一项比较三胎/四胎产妇和单胎产妇的椎管内麻醉的回顾性研究也发现单胎和多胎产妇的低血压发生率差异无统计学意义[24]。本研究中,在相同剂量组内,单、双胎产妇之间的低血压、心动过缓发生率差异无统计学意义,对单、双胎产妇低血压发生情况进行生存分析,差异也无统计学意义。NE用于预防单、双胎产妇剖宫产腰硬联合麻醉后低血压的ED90的95%CI高度重合,表明产妇在腰麻下剖宫产手术中维持血压稳定所需的NE输注剂量差异亦无统计学意义。相同剂量组内,单、双胎产妇低血压发生率,生存曲线及NE预防单胎与双胎产妇腰麻后低血压的量效曲线(图3)差异无统计学意义,均提示单胎和双胎产妇在围手术麻醉期的血流动力学变化程度基本相似。因此,本研究及其他研究均证实传统理论认为的双胎产妇在椎管内麻醉后更容易发生低血压的观点可能是不恰当的。本研究纳入的单双胎产妇的孕周差异较大,可能是使得NE在这两个人群中的ED90差异无统计学意义的原因之一。本研究中,45%双胎产妇为早产产妇,双胎早产产妇子宫体积的增大程度可能与足月单胎产妇子宫体积增大的程度相似,因此对下腔静脉的压迫程度差别较小,双胎产妇的回心血流量并未因下腔静脉受压而比单胎产妇下降更多,从而使得两者麻醉后的血流动力学波动及对血管活性药物的需求差异无统计学意义。然而,目前大多数关于单、双胎产妇对比的麻醉学研究纳入的单、双胎产妇孕周差异均较大,其原因可能与双胎产妇接受早产剖宫产在临床工作中多见有关[25]。因此,本研究所纳入的双胎产妇是临床实际工作情况的反映,研究结果具有临床适用性。此外,本研究NE的给药方式采用根据产妇的公斤体质量持续输注。双胎产妇体质量较单胎产妇更重,其中一部分来自双胎胎儿总体质量较单胎胎儿重。这可能使得在接受相同剂量输注NE的产妇中,双胎产妇体内NE的血药浓度较单胎产妇高,从而降低了双胎产妇低血压的发生几率。但根据体质量持续输注血管活性药物是目前国际产科麻醉指南推荐的给药方法[21]。
近期研究报道,NE预防单胎产妇剖宫产腰麻后低血压的ED90为0.054(95%CI: 0.044~0.079) μg/(kg·min),双胎产妇的ED90为0.058(95%CI: 0.046~0.085) μg/(kg·min)[26],显著低于本研究的结果。这种差异可能来自研究方案的不同。在SHENG等[26]的研究中,麻醉前,产妇接受了10 mL/kg的晶体液输注;麻醉后,产妇进一步接受了胶体液的输注。本研究中,仅在椎管内麻醉时,产妇开始接受晶体液输注。显然,麻醉前的液体输注,极大程度上减少了产妇对缩血管药物的需求[27-28]。但国内外的产科麻醉指南均不推荐在实施椎管内麻醉前预先输注液体,而是提倡在实施椎管内麻醉的同时输注液体,以减少总液体用量,避免肺水肿等不良反应[29]。因此本研究结果更为可靠。这亦与2021年XU等[30]发表的研究结果一致。然而,由于并非所有产妇对NE的反应都与本研究的受试者相同,因此仍有必要根据术中血压变化调整输注速度。
本研究存在一些局限,首先,我们仅根据产妇麻醉后的收缩压和心率变化判断血流动力学波动。虽然心率可作为心输出量的替代指标[31],但是测量全身外周血管阻力和心输出量可提供更有说服力的数据。其次,本研究的量效曲线关系是从健康妊娠产妇人群获得,因此应慎重考虑将此研究结果用于预防先兆子痫或心功能异常的产妇剖宫产腰麻后低血压。最后,本研究所纳入的单胎产妇和双胎产妇的孕周差异较大,这一混杂因素可能使单双胎产妇之间的比较不太合理。后续研究在理论上可设计为,在招募每一个双胎产妇时,同时招募孕周、体质量相当的单胎产妇,以减小单双胎产妇之间基线资料的统计学差异,从而比较单双胎产妇之间的药物量效曲线关系[20]。
综上,本研究显示,NE预防单胎和双胎产妇剖宫产腰硬联合麻醉后低血压的有效剂量差异无统计学意义。在单胎产妇,NE预防剖宫产腰硬联合麻醉后低血压的ED90为0.100 μg/(kg·min);在双胎产妇,NE预防剖宫产腰硬联合麻醉后低血压的ED90为0.098 μg/(kg·min)。本研究为产科麻醉中NE在双胎产妇的合理应用提供了思路,同时为NE用于双胎产妇的后续研究提供了证据支持。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
成都市科技局技术创新研发项目(No. 2019-YFYF-00108-SN)资助
Contributor Information
海英 银 (Hai-ying YIN), Email: yinhaiying@stu.scu.edu.cn.
瀚 黄 (Han HUANG), Email: han.huang@scu.edu.cn.
References
- 1.KULEVA M, YOUSSEF A, MARONI E, et al Maternal cardiac function in normal twin pregnancy: A longitudinal study. Ultrasound Obstet Gynecol. 2011;38(5):575–580. doi: 10.1002/uog.8936. [DOI] [PubMed] [Google Scholar]
- 2.CAMPBELL D M, CAMPBELL A J Arterial blood pressure--The pattern of change in twin pregnancies. Acta Genet Med Gemellol. 1985;34(3/4):217–223. doi: 10.1017/s0001566000004773. [DOI] [PubMed] [Google Scholar]
- 3.VEILLE J C, MORTON M J, BURRY K J Maternal cardiovascular adaptations to twin pregnancy. Am J Obstet Gynecol. 1985;153(3):261–263. doi: 10.1016/s0002-9378(85)80109-5. [DOI] [PubMed] [Google Scholar]
- 4.ROBSON S C, HUNTER S, BOYS R J, et al Hemodynamic changes during twin pregnancy. A Doppler and M-mode echocardiographic study. Am J Obstet Gynecol. 1989;161(5):1273–1278. doi: 10.1016/0002-9378(89)90682-0. [DOI] [PubMed] [Google Scholar]
- 5.THOMSEN J K, FOGH-ANDERSEN N, JASZCZAK P Atrial natriuretic peptide, blood volume, aldosterone, and sodium excretion during twin pregnancy. Acta Obstet Gynecol Scand. 1994;73(1):14–20. doi: 10.3109/00016349409013386. [DOI] [PubMed] [Google Scholar]
- 6.KAMETAS N Maternal cardiac function in twin pregnancy. Obstetric Gynecol. 2013;102(4):806–815. doi: 10.1016/s0029-7844(03)00807-x. [DOI] [PubMed] [Google Scholar]
- 7.BATENI Z H, CLARK S L, SANGI-HAGHPEYKAR H, et al. Trends in the delivery route of twin pregnancies in the United States, 2006-2013. Eur J Obstet Gynecol Reprod Biol, 2016, 205: 120−126[2021-09-04]. https://sci-hub.mksa.top/10.1016/j.ejogrb.2016.08.031. doi: 10.1016/j.ejogrb.2016.08.031.
- 8.HUGHES S C, LEVINSON G, ROSEN M A ed. Anesthesia for obstetrics: Anesthesia for abnormal positions and presentations, shoulder dystocia, and multiple births. Philadelphia: Lippincott Williams and Wilkins, 2002: 287−295.
- 9.CHESTNUT D H ed. Obstetric anesthesia: Abnormal presentation and multiple gestation. St. Louis: Mosby, 1999: 694−710.
- 10.SHARKEY A M, SIDDIQUI N, DOWNEY K, et al Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat spinal-induced hypotension in Cesarean deliveries: Randomized controlled trial. Anesth Analg. 2019;129(5):1312–1318. doi: 10.1213/ane.0000000000003704. [DOI] [PubMed] [Google Scholar]
- 11.NGAN KEE W D, LEE S W, NG F F, et al Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for Cesarean delivery. Anesthesiology. 2015;122(4):736–745. doi: 10.1097/aln.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 12.NGAN KEE W D, LEE S W Y, NG F F, et al Prophylactic norepinephrine infusion for preventing hypotension during spinal anesthesia for Cesarean delivery. Anesth Analg. 2018;126(6):1989–1994. doi: 10.1213/ane.0000000000002243. [DOI] [PubMed] [Google Scholar]
- 13.MOHTA M, GARG A, CHILKOTI G T, et al A randomised controlled trial of phenylephrine and noradrenaline boluses for treatment of postspinal hypotension during elective Caesarean section. Anaesthesia. 2019;74(7):850–855. doi: 10.1111/anae.14675. [DOI] [PubMed] [Google Scholar]
- 14.FU F, XIAO F, CHEN W, et al. A randomised double-blind dose-response study of weight-adjusted infusions of norepinephrine for preventing hypotension during combined spinal-epidural anaesthesia for Caesarean delivery. Br J Anaesth, 2020, 124(3): e108−e114[2021-09-14]. https://www.bjanaesthesia.org/action/showPdf?pii=S0007-0912%2819%2931000-1. doi: 10.1016/j.bja.2019.12.019.
- 15.WEI C, QIAN J, ZHANG Y, et al Norepinephrine for the prevention of spinal-induced hypotension during Caesarean delivery under combined spinal-epidural anaesthesia: Randomised, double-blind, dose-finding study. Eur J Anaesthesiol. 2020;37(4):309–315. doi: 10.1097/eja.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 16.HASANIN A M, AMIN S M, AGIZA N A, et al Norepinephrine infusion for preventing postspinal anesthesia hypotension during Cesarean delivery: A randomized dose-finding trial. Anesthesiology. 2019;130(1):55–62. doi: 10.1097/ALN.0000000000002483. [DOI] [PubMed] [Google Scholar]
- 17.MEI Z, NGAN KEE W D, SHENG Z M, et al. Comparative dose-response study of hyperbaric ropivacaine for spinal anesthesia for Cesarean delivery in singleton versus twin pregnancies. J Clin Anesth, 2020, 67: 110068[2021-09-29]. https://sci-hub.mksa.top/10.1016/j.jclinane.2020.110068. doi: 10.1016/j.jclinane.2020.110068.
- 18.NGAN KEE W D A random-allocation graded dose-response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for Cesarean delivery. Anesthesiology. 2017;127(7):934–941. doi: 10.1097/ALN.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 19.WU B, SHAN J, ZHOU Q, et al Determination of the ED95 of a single bolus dose of dexmedetomidine for adequate sedation in obese or nonobese children and adolescents. Br J Anaesth. 2021;126(3):684–691. doi: 10.1016/j.bja.2020.11.037. [DOI] [PubMed] [Google Scholar]
- 20.NGAN KEE W D, KHAW K S, NG F F, et al A prospective comparison of vasopressor requirement and hemodynamic changes during spinal anesthesia for Cesarean delivery in patients with multiple gestation versus singleton pregnancy. Anesth Analg. 2007;104(2):407–411. doi: 10.1213/01.ane.0000252461.97656.3e. [DOI] [PubMed] [Google Scholar]
- 21.KINSELLA S M, CARVALHO B, DYER R A, et al International consensus statement on the management of hypotension with vasopressors during Caesarean section under spinal anaesthesia. Anaesthesia. 2018;73(1):71–92. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 22.CHEN D, QI X, HUANG X, et al. Efficacy and safety of different norepinephrine regimens for prevention of spinal hypotension in Cesarean section: A randomized trial. Biomed Res Int, 2018, 2018: 2708175[2021-09-26]. https://www.hindawi.com/journals/bmri/2018/2708175/. doi: 10.1155/2018/2708175.
- 23.JAWAN B, LEE J H, CHONG Z K, et al Spread of spinal anaesthesia for Caesarean section in singleton and twin pregnancies. Br J Anaesth. 1993;70(6):639–641. doi: 10.1093/bja/70.6.639. [DOI] [PubMed] [Google Scholar]
- 24.BEHFOROUZ N, DOUNAS M, BENHAMOU D Epidural anaesthesia for Caesarean delivery in triple and quadruple pregnancies. Acta Anaesthesiol Scand. 1998;42(9):1088–1091. doi: 10.1111/j.1399-6576.1998.tb05381.x. [DOI] [PubMed] [Google Scholar]
- 25.ROBERTS C L, ALGERT C S, MORRIS J M, et al Trends in twin births in New South Wales, Australia, 1990–1999. Int J Gynaecol Obstet. 2002;78(3):213–219. doi: 10.1016/s0020-7292(02)00147-9. [DOI] [PubMed] [Google Scholar]
- 26.SHENG Z, SUN H, LIU J, et al Comparative dose--Response study on norepinephrine infusion for preventing hypotension during spinal anaesthesia for Caesarean delivery in singleton versus twin pregnancies: A randomized, double-blind, controlled, dose-finding trial. Eur J Anaesthesiol. 2021;38(8):895–897. doi: 10.1097/eja.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 27.MCDONALD S, FERNANDO R, ASHPOLE K, et al Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective Cesarean delivery: A randomized controlled trial. AnesthAnalg. 2011;113(4):803–810. doi: 10.1213/ANE.0b013e31822c0f08. [DOI] [PubMed] [Google Scholar]
- 28.KAUFNER L, KAREKLA A, HENKELMANN A, et al Crystalloid coloading vs. colloid coloading in elective Caesarean section: Postspinal hypotension and vasopressor consumption, a prospective, observational clinical trial. J Anesth. 2019;33(1):40–49. doi: 10.1007/s00540-018-2581-x. [DOI] [PubMed] [Google Scholar]
- 29.APFELBAUM J M, HAWKINS J L, AGARKAR M, et al Practice Guidelines for Obstetric Anesthesia: An updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270–300. doi: 10.1097/aln.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 30.XU W, DRZYMALSKI D M, AI L, et al. The ED50 and ED95 of prophylactic norepinephrine for preventing post-spinal hypotension during Cesarean delivery under combined spinal-epidural anesthesia: A prospective dose-finding study. Front Pharmacol, 2021, 12: 691809[2021-10-14]. https://www.frontiersin.org/articles/10.3389/fphar.2021.691809/full. doi: 10.3389/fphar.2021.691809.
- 31.DYER R A, REED A R, VAN DYK D, et al Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective Cesarean delivery. Anesthesiology. 2009;111(4):753–765. doi: 10.1097/ALN.0b013e3181b437e0. [DOI] [PubMed] [Google Scholar]




