Abstract
目的
观察卒中后1个月内认知下降患者肠道菌群构成改变,并探讨差异菌类与认知功能等临床指标的相关性。
方法
采用横断面研究设计,分别选取卒中伴认知障碍 (PSCI组)、卒中不伴认知障碍(Non-PSCI组)和对照者(NC组)各12例。收集各组人群一般资料及临床指标。采用16S rRNA基因测序技术进行肠道菌群丰度、多样性及差异性分析,分析肠道菌群与临床指标的相关性及差异菌类对认知下降的鉴别效能。
结果
PSCI组简易精神状态检查(Mini-Mental State Examination, MMSE)和蒙特利尔认知评估(Montreal Cognitive Assessment, MoCA)得分低于Non-PSCI组(P<0.001),3组间其他一般资料和临床指标及肠道菌群Alpha多样性比较差异无统计学意义(P>0.05)。3组间肠道菌群在门、属、种水平构成上存在差异。门水平,PSCI组放线菌门相对丰度明显增加(LDA score>2)。属和种水平,3组排列前10的菌类以厚壁菌门多样性最高,相对丰度Non-PSCI组中疣微菌门呈增加趋势,PSCI组中放线菌门呈增加趋势。组间差异性分析显示各组存在不同的标志菌类,其中PSCI组放线菌门的Bifidobacterium属、Alloscardovia属和Alloscardovia omnicolens菌及厚壁菌门的Lactobacillus gasseri菌和Anaerostipes hadrus菌明显升高(LDA score>2),且相关性分析提示Anaerostipes hadrus菌与MoCA呈负相关,Bifidobacterium属与血尿酸呈正相关。Bifidobacterium属、Lactobacillus gasseri菌和Anaerostipes hadrus菌对区分有无认知下降有一定鉴别效能,曲线下面积分别为0.785、0.792、0.750(P<0.05)。
结论
卒中后早期认知下降患者肠道菌群结构发生改变,差异菌类与认知功能及相关危险因素存在一定相关性,为卒中后认知障碍的早期防治提供新的切入点。
Keywords: 肠道菌群, 认知功能障碍, 脑卒中, 16S rRNA, 微生物标记
Abstract
Objective
To observe the changes in the composition of gut microbiota in stroke patients showing cognitive impairment within one month after the stroke, and to explore the correlation between bacteria presenting dissimilarity and cognitive functions and other clinical indicators.
Methods
A cross-sectional study was conducted, involving 12 patients with post-stroke cognitive impairment (PSCI group), 12 stroke patients without cognitive impairment (Non-PSCI group), and 12 healthy volunteers in a normal control group (NC group). The demographic and clinical data were gathered. The abundance, diversity and dissimilarity of gut bacterial communities were determined by 16S rRNA gene sequencing. Then, we studied the correlation between gut microbiota and clinical characteristics and the effectiveness of using microbiome markers to identify cognitive decline.
Results
The Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores of the PSCI group were significantly lower than those the Non-PSCI group (P<0.001). There was no significant intergroup difference in the demographic data, the clinical data, and the Alpha diversity of gut microbiota among the three groups (P>0.05). Microbial composition analysis of the three groups revealed proportion alternations at the phylum, genus and species levels. At the phylum level, linear discriminant analysis (LDA) effect size (LEfSe) analysis suggested that the Actinomycetes had significantly increased relative abundance in the PSCI group (LDA score>2). At the genus and species levels, Firmicutes had the highest diversity among the top 10 bacteria in the three groups, while the relative abundance of Verrucomicrophyla presented an increasing trend in the Non-PSCI group and that of Actinobacteria showed an increasing trend in the PSCI group. Further LEfSe analysis revealed that there were different microbiome markers in each group, among which the Bifidobacterium, Alloscardovia, and Alloscardovia omnicolens of the phylum Actinomycetes and Lactobacillus gasseri and Anaerostipes hadrus of the phylum Firmicutes in the PSCI group increased significantly (LDA score>2). Correlation analysis indicated that Anaerostipes hadrus was negatively correlated with the MoCA scores, while Bifidobacterium was positively correlated with blood uric acid (UA). Bifidobacterium, Lactobacillus gasseri and Anaerostipes hadrus could be used to distinguish PSCI patients from Non-PSCI patients, presenting an area under the curve of 0.785, 0.792 and 0.750, respectively (P<0.05).
Conclusion
Stroke patients with cognitive impairment in the early stage showed composition changes in their gut microbiota, and the bacteria exhibiting dissimilarity were correlated, to some degree, with cognitive function and related risk factors, which could provide new clues for the early management of PSCI.
Keywords: Gut microbiota, Cognitive impairment, Stroke, 16S rRNA, Microbiome marker
卒中后认知障碍(post-stroke cognitive impairment, PSCI)为卒中后出现并持续至6个月时仍有的以认知损害为特征的临床综合征[1],早期容易忽视以致进展为痴呆,并妨碍脑卒中的全面康复[2],增加脑卒中终身残疾率和死亡率[1]。因此,加强对PSCI高危人群的早期识别和干预对PSCI的管理具有重要意义。然而,至今我国尚无有关PSCI防治的指南,临床上还未发现可用于早期筛查PSCI的生物标志物[1]。近年来,不少研究发现肠道菌群与脑卒中的发生、发展及预后密切相关[3-4],可能是脑卒中新的危险因素。2020年有学者首次提出PSCI与肠道菌群及代谢产物的相关性[5],提示肠道菌群可能与PSCI发病有关。然而,目前有关PSCI肠道菌群变化的研究尚少,PSCI各阶段肠道菌群紊乱特征需要更多的研究论证。虽然最新PSCI管理专家共识指出PSCI的诊断要在卒中后3~6个月进行认知评估确定,但仍强调对卒中后早期认知下降的筛查和管理的重要性[1]。基于此,本文拟观察卒中后1个月内认知下降患者肠道菌群的变化特征,旨在从肠道菌群角度探索卒中早期认知下降的生物标志物,为PSCI的早期干预提供新思路。
1. 资料与方法
1.1. 研究对象
PSCI纳入标准:①首次卒中,病程≤1个月;②发病前无认知障碍,发病后简易精神状态检查(Mini-Mental State Examination, MMSE)及蒙特利尔认知评估表(Montreal Cognitive Assessment, MoCA)评定存在认知障碍[6];③1个月内无抗生素、益生元或益生菌使用史;④无其它胃肠道或肝胆胰等消化系统疾病和手术史;⑤患者或其家属签署知情同意书。卒中后无认知障碍纳入标准除以上第二点MMSE和MoCA评分正常,其余相同。
对照组纳入标准:同期据年龄和性别匹配因颈肩腰腿病于我科住院患者作为对照组。①无高血压病、糖尿病、冠心病、高脂血症、颈动脉粥样硬化斑块等病史;②MMSE和MoCA评分正常;③既往无任何原因造成的肢体运动障碍;④近1个月未服用抗生素、益生元或益生菌;⑤无慢性腹泻、便秘病史。
排除标准:①非动脉粥样硬化导致的卒中;②既往有脑外伤或其他颅内疾病;③其他原因所致认知障碍;④合并恶性肿瘤、严重自身免疫性疾病、免疫缺陷综合征、严重精神疾病者;⑤合并严重威胁生命的重大疾病;⑥有严重的视听障碍,无法配合研究。
本研究经医院伦理委员会批准〔伦审(研)2020年第384号〕,并遵循知情同意原则。选取2020年3–11月在我科住院且符合上述标准的卒中伴认知障碍患者(PSCI组)、卒中不伴认知障碍患者(Non-PSCI组)和对照者(NC组)各12例。收集一般资料,采用简化Fugl-Meyer量表(Fugl-Meyer assessment, FMA)[7]评定卒中患者偏瘫肢体运动功能。组间一般资料比较差异均无统计学意义(P>0.05)。见表1。
表 1. Analysis of demographic and clinical characteristics of the three groups (n=12).
3组一般人口学资料及临床指标分析(n=12)
| Clinical characteristic | PSCI group | Non-PSCI group | NC group | F/t/χ2 | P |
| BMI: Body mass index; UA: Uric acid; GHb: Glycated hemoglobin; HCY: Homocysteine; U-FMA: The Fugl-Meyer assessment for upper extremities; L-FMA: The Fugl-Meyer assessment for lower extremities; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment. | |||||
| Age/yr. | 60.75±12.45 | 65.58±12.76 | 60.58±9.53 | 0.710 | 0.499 |
| Sex (male/female) | 7/5 | 7/5 | 6/6 | 0.225 | 0.894 |
| BMI/(kg/m2) | 25.20±1.13 | 24.60±1.44 | 24.11±1.42 | 2.020 | 0.149 |
| Education/year | 9.92±1.73 | 9.42±2.75 | 10.25±1.55 | 0.490 | 0.617 |
| Duration/d | 21.83±7.30 | 17.42±5.49 | − | 1.676 | 0.108 |
| UA/(μmol/L) | 368.00±95.37 | 308.33±79.36 | 308.17±88.97 | 1.838 | 0.175 |
| GHb/% | 5.80±1.52 | 5.23±1.02 | 4.92±0.87 | 1.772 | 0.186 |
| HCY/(μmol/L) | 14.83±5.29 | 15.40±6.62 | 10.78±3.37 | 2.738 | 0.079 |
| U-FMA/score | 33.58±18.09 | 36.50±21.37 | − | −0.361 | 0.722 |
| L-FMA/score | 24.33±7.84 | 24.92±7.78 | − | −0.183 | 0.857 |
| MMSE/score | 19.75±1.49 | 24.25±1.55 | − | −7.275 | <0.001 |
| MoCA/score | 19.92±1.62 | 27.00±1.21 | − | −12.143 | <0.001 |
1.2. 研究方法
所有患者入组时完成认知功能评定,并于入组后48 h内采集粪便标本。
1.2.1. 认知功能评定
由1名高年资主治医师使用MMSE和MoCA进行评定。MMSE采用张明园版本[7],总分30分,≥24(初中及以上)/20(小学)/17(文盲)分为正常。MoCA采用福州版[7],总分为30分,受教育年限≤12年者加1分,≥26分为认知功能正常。
1.2.2. 粪便标本采集
粪便采集指定1名研究人员完成,采集前接受针对标本采集的无菌操作培训。具体步骤为:洗手消毒后戴上无菌手套,使用干净无菌勺子采集新鲜的、中后段、内部的粪便4~6 g,放入无菌冻存管中,取样完毕后立即盖上盖子旋紧,−80 ℃冰箱保存,保存期间切忌反复冻融。检测时足量干冰低温运送,从标本收集地成都运送至北京百迈客生物科技有限公司的分支机构青岛实验中心。
1.2.3. 微生物多样性全长16S rRNA测序
本研究与北京百迈客生物科技有限公司签约,由该公司完成粪便标本微生物多样性全长16S rRNA测序。步骤包括总DNA提取、PCR扩增和上机测序。下机原始数据即环形一致性序列(circular consensus sequencing, CCS)文件经3个预处理:CCS识别、CCS过滤和去除嵌合体,最后得到Effective-CCS序列。
1.2.4. 生物信息学分析
得到Effective-CCS序列后,由研究者使用百迈客医学微生物多样性分析平台(www.biocloud.net)进行数据分析。测序数据已上传至NCBI Sequence Read Archive database,数据集编号为PRJNA784356。包括:利用QIIME 软件(version 1.8.0)和R语言工具进行物种注释及分类学分析;使用Mothur软件进行Alpha多样性分析;以及通过LEfSe软件采用线性判别分析方法(LDA)进行组间差异显著性分析。
1.3. 统计学方法
计数资料采用χ²检验。计量资料满足正态分布且方差齐则采用 表示,多组间比较采用单因素方差分析,组间两两比较采用LSD-t 检验;两组间比较采用t检验。肠道菌群与临床指标的相关性采用Spearman相关性分析。差异菌类对认知下降的鉴别效能采用ROC曲线分析。P<0.05为差异有统计学意义。
表示,多组间比较采用单因素方差分析,组间两两比较采用LSD-t 检验;两组间比较采用t检验。肠道菌群与临床指标的相关性采用Spearman相关性分析。差异菌类对认知下降的鉴别效能采用ROC曲线分析。P<0.05为差异有统计学意义。
2. 结果
2.1. 一般资料和临床指标比较
组间一般资料及实验室指标比较差异均无统计学意义(P>0.05)。PSCI组和Non-PSCI组比较,上肢和下肢FMA评分差异无统计学意义(P>0.05),PSCI组的MMSE、MoCA评分低于Non-PSCI组(P<0.001)。见表1。
2.2. 肠道菌群结构特征分析结果
2.2.1. Alpha多样性分析
组间肠道菌群OUT水平Alpha多样性指数差异均无统计学意义(P>0.05)。见图1。
图 1.
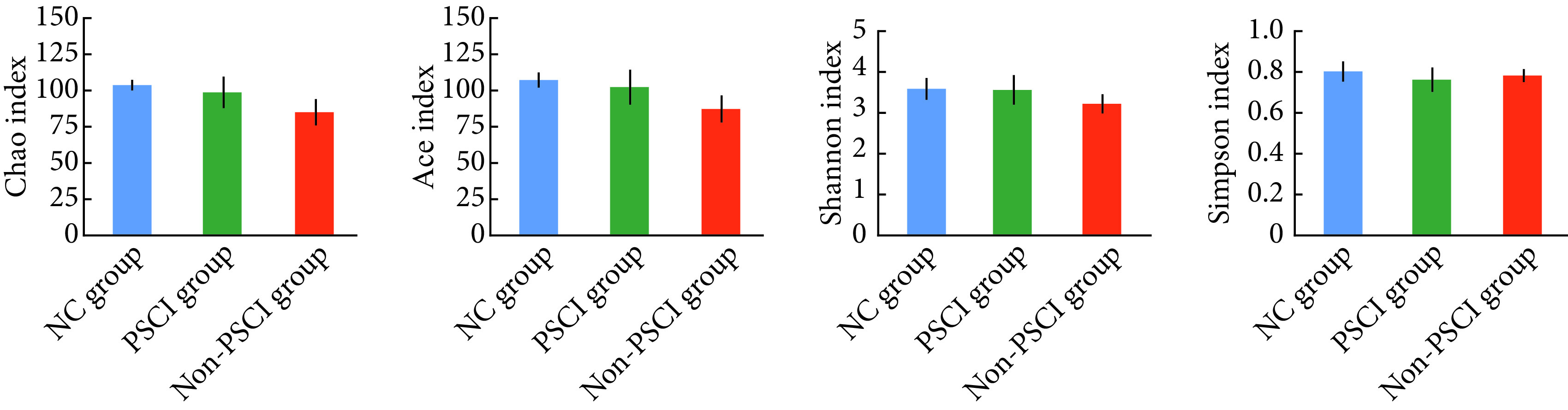
Intergroup comparison of the Alpha diversity analysis of the three groups (n=12)
组间Alpha多样性指数比较(n=12)
2.2.2. 组间肠道菌群构成及丰度分析
2.2.2.1. 门水平比较结果
以单个样本为横坐标、菌门相对丰度百分比为纵坐标作门水平样品间物种分布柱状图(图2)。再以组别为横坐标、菌门相对丰度百分比为纵坐标作组间物种分布柱状图(图3A)。从图中可见样品间及组间菌门构成及相对丰度存在差异,组间比较PSCI组放线菌门高于其余两组(LDA score>2)(图3B、图3C)。
图 2.
Gut microbiota composition of all samples in the three groups at the phylum level (n=12)
3组所有样品门水平肠道菌群构成(n=12)
A1-A12: PSCI group; B1-B12: Non-PSCI group; C1-C12: NC group.
图 3.

Taxonomic differences of gut microbiota at the phylum level (n=12)
组间门水平肠道菌群构成比较(n=12)
A: Bar chart of species composition; B: Bar chart of LDA value from LEfSe analyses (LDA score>2); C: The phylogenetic distribution diagram from LEfSe analyses (The circles represent the taxonomic levels from kingdom [inside] to phylum [outside]. The larger diameter of the small circle, the higher relative abundance. Yellow circles indicate the phyla with no significant difference, while the red one indicates the phylum with higher abundance in the PSCI group).
2.2.2.2. 属水平比较结果
图4为所有样品物种分布柱状图,图5A为3组间物种分布柱状图,从图中可见样品间及组间菌属构成存在差异,所属菌门亦不相同,其中Streptococcus、Blautia、Faecalibacterium、Subdoligranulum、Eubacterium为厚壁菌门;Escherichia、Klebsiella为变形菌门;Bacteroides为拟杆菌门;Bifidobacterium为放线菌门;Akkermansia为疣微菌门。进一步使用LEfSe分析进行组间比较,PSCI组Bifidobacterium属和Alloscardovia属高于其余两组;Non-PSCI组Clostridium_sensu_stricto_12属和Lachnoclostridium属高于其余两组;NC组Butyricimonas属高于其余两组(LDA score>2)(图5B、图5C)。
图 4.
Gut microbiota composition of all samples in the three groups at the genus level (n=12)
3组所有样品属水平肠道菌群构成(n=12)
A1-A12: PSCI group; B1-B12: Non-PSCI group; C1-C12: NC group.
图 5.

Taxonomic differences of gut microbiota at the genus level (n=12)
组间属水平肠道菌群构成比较(n=12)
A: Bar chart of species composition; B: Bar chart of LDA value from LEfSe analyses (LDA score>2); C: The phylogenetic distribution diagram from LEfSe analyses (The circles represent the taxonomic levels from kingdom [inside] to genus [outside]. Each small circle at different taxonomic levels represents a certain taxon, and the larger diameter of the circle, the higher relative abundance. Yellow circles indicate the genera with no significant difference, while red/green/blue ones indicate the genus with higher abundance in the NC/PSCI/non-PSCI groups respectively).
2.2.2.3. 种水平比较结果
图6为所有样品物种分布柱状图,图7A为3组间物种分布柱状图,从图中可见样品间及组间菌种构成存在差异,所属菌门亦不相同,其中 Klebsiella_pneumoniae、Faecalibacterium_prausnitzii、Streptococcus_salivarius、uncultured_bacterium_g_Subdoligranulum、uncultured_bacterium_g_[Eubacterium]_coprostanoligenes_group为厚壁菌门;Escherichia_coli为变形菌门;Bacteroides_vulgatus为拟杆菌门;Bifidobacterium_pseudocatenulatum为放线菌门;Akkermansia_muciniphila为疣微菌门;uncultured_bacterium_o_Mollicutes_RF39为柔膜菌门。进一步使用LEfSe分析进行组间比较,PSCI组Alloscardovia_omnicolens菌、Anaerostipes_hadrus菌和Lactobacillus_gasseri菌高于其余两组;Non-PSCI组Lactobacillus_salivarius菌和uncultured_bacterium_g_ Clostridium_sensu_stricto_12菌高于其余两组;NC组Butyricimonas_virosa菌、Desulfovibrio_piger菌、Bacteroides_finegoldii菌和Alistipes_ihumii菌高于其余两组(LDA score>2)(图7B、图7C)。
图 6.

Gut microbiota composition of all samples in the three groups at the species level (n=12)
3组所有样品种水平肠道菌群构成(n=12)
A1-A12: PSCI group; B1-B12: Non-PSCI group; C1-C12: NC group.
图 7.

Taxonomic differences of gut microbiota at the species level (n=12)
组间种水平肠道菌群构成比较(n=12)
A, B, and C denote the same as those in Fig 5.
2.3. 差异菌类与临床指标的相关性分析
差异菌类与PSCI组临床指标的相关性分析(图8A)显示,Anaerostipes_hadrus菌与MoCA、糖化血红蛋白均呈负相关(r<0, P<0.05),Clostridium_sensu_stricto_12属、uncultured bacterium_g_ Clostridium_sensu_stricto_12菌与上肢FMA呈负相关(r<0, P<0.05), Lactobacillus_salivarius菌与同型半胱氨酸(HCY)呈负相关(r<0, P<0.01),Lachnoclostridium属与体质量指数、年龄呈负相关(r<0, P<0.05),Bifidobacterium属与血尿酸、病程呈正相关(r>0, P<0.05)。
图 8.

Correlation analysis of microbiome markers and clinical features in PSCI group (A), and ROC curves based on microbiome markers in PSCI group (B)
属和种水平差异菌类与PSCI组临床指标相关性分析(A)以及基于PSCI差异菌类的ROC曲线(B)
* P<0.05; ** P<0.01. GHb: Glycated hemoglobin; MoCA: Montreal Cognitive Assessment; CRP: C-reaction protein; LDL-C: Low-density lipoprotein cholesterol; HCY: Homocysteine; MBP: Mean blood pressure; MMSE: Mini-Mental State Examination; U-FMA: The Fugl-Meyer assessment of the upper extremities; BMI: Body mass index; FIM: Functional independence measure; L-FMA: The Fugl-Meyer assessment of the lower extremities; ADL: Activity of daily living; UA: Uric acid.
2.4. 差异菌类对认知下降的诊断价值
利用ROC曲线分析PSCI组差异菌类鉴别有无认知下降的效能,如图8B显示,Bifidobacterium属、Lactobacillus_gasseri菌和Anaerostipes_hadrus菌的曲线下面积(area under the curve, AUC)分别为0.785、0.792、0.750(P<0.05),说明这3种菌类对卒中早期筛查认知功能下降具有一定诊断效能。
3. 讨论
PSCI可分为卒中后认知障碍非痴呆(post-stroke cognitive impairment no dementia, PSCIND)和卒中后痴呆(post-stroke dementia, PSD)两个阶段[1]。PSCIND较为隐匿,若未及时有效治疗有较高进展为PSD的风险[8],严重影响卒中患者的预后,给社会及家庭带来沉重负担[9]。最新专家共识提出PSCI的诊断需在卒中后3~6个月确定[1],因此,在卒中后早期对认知下降患者进行有效识别和干预,对减少PSCI的发生进展具有重要意义。肠道菌群是肠道微生态系统的重要组成成分,可借助“菌群-肠-脑轴”与大脑进行双向互动[10],即通过迷走神经、内分泌、代谢及免疫等途径进行信息交流,从而影响大脑功能和行为[11-13]。近年不少研究发现肠道菌群不仅与脑卒中的危险因素如高血压、糖尿病及动脉粥样硬化等密切相关[14-15],还直接影响卒中的发生、发展及预后[3-4],被认为是卒中新的危险因素。随着研究不断深入,2020年首次报道与卒中后无认知障碍比较,PSCI肠道菌群多样性及相对丰度发生改变[5]。PSCI肠道菌群紊乱特征尚不明确,尤其是卒中后早期认知下降患者肠道菌群的改变及其对PSCI早期识别、干预和预后判断的价值需要进一步深入研究。因此,本研究着眼于卒中后早期认知下降的管理,对卒中伴或不伴认知障碍及对照人群的肠道菌群结构特征进行对比分析,探讨肠道菌群改变与临床指标的相关性,以及差异菌类对卒中后早期认知下降的诊断效能,以期发现卒中后早期认知下降的生物标志物,为PSCI的早期防治提供新靶点。
结果发现,3组间肠道菌群在门、属和种水平构成均有差异,但组间Alpha多样性无显著差异。目前,关于肠道菌群多样性在各种原因所致认知受损中的变化仍无结论,如LING等[16]发现卒中后合并认知障碍和抑郁患者与卒中后无认知障碍和抑郁患者比较,Alpha多样性无明显差异;ZHANG等[17]报道HIV伴认知损害患者肠道菌群多样性无明显改变。肠道菌群紊乱包括肠道菌群结构、代谢产物及局部分布的改变[18],由于卒中后早期认知下降患者与其余两组间肠道菌群构成及丰度存在明显的异质性,菌群改变可能为某些致病菌增加和有益菌减少,亦或代谢产物、菌群分布的改变,故其多样性不一定必然降低,且有研究报道某些疾病好转后其多样性反而降低[19]。关于卒中后早期认知下降肠道菌群多样性的改变尚需更多研究证据证实。
人体肠道菌群的优势菌门为厚壁菌门和拟杆菌门,其次为变形菌门、放线菌门和疣微菌门等[20]。本研究在门水平上3组的优势菌门仍均为厚壁菌门、变形菌门、拟杆菌门,占各组50%以上,与其余两组比较,PSCI组的放线菌门相对丰度明显增加。在属和种水平,3组排列前10的物种其多样性和相对丰度各有特点,以厚壁菌门多样性最高,相对丰度对照者以厚壁菌门、变形菌门、拟杆菌门占优势,而脑卒中两组中这3个菌门虽占优势但有减少趋势,PSCI组放线菌门呈现明显升高趋势;Non-PSCI组疣微菌门呈现明显升高趋势。组间差异性分析显示,各组均存在不同的标志菌类,其中PSCI组放线菌门的Bifidobacterium属、Alloscardovia属和Alloscardovia_omnicolens菌以及厚壁菌门的Anaerostipes_hadrus菌和Lactobacillus_gasseri菌明显高于其余两组,且Bifidobacterium属、Anaerostipes_hadrus菌和Lactobacillus_gasseri菌对区分有无认知功能下降具一定识别效能。3组在门、属及种的分布情况表明,卒中后早期认知下降患者门水平上放线菌门相对丰度明显升高,属和种水平亦均发现有放线菌门的标志物种,且其标志菌类——放线菌门的Bifidobacterium属对区分卒中后早期伴或不伴认知下降有一定鉴别效能。因此提示,放线菌门的相对富集可能在卒中早期认知损害中起着重要的作用。然而,关于放线菌门与认知功能的关系报道尚少见,结论亦不一致。有动物研究发现在大气细颗粒物暴露后认知功能受伤的小鼠中,放线菌门相对丰度减少,并且与认知功能呈正相关[21];另有研究报道阿尔茨海默病患者肠道菌群中放线菌门减少,其相对丰度与MMSE评分呈正相关[22];还有研究发现补充Bifidobacterium有助于改善老年人认知功能[23-25]。相反,也有研究报道阿尔茨海默病患者肠道菌群中放线菌门轻度富集[26]。因此,关于放线菌门在卒中后认知损害的变化趋势及作用仍需进一步研究论证。
差异菌类与临床指标的相关性分析发现,Non-PSCI的标志菌类Clostridium_sensu_stricto_12属与上肢运动功能呈负相关,提示卒中后影响运动和认知的菌类可能存在差异。此外,PSCI标志菌类Anaerostipes_hadrus菌与MoCA评分呈负相关,ROC曲线显示Anaerostipes_ hadrus菌对区分卒中后早期伴或不伴认知障碍有一定甄别能力,推测该菌可能参与卒中后认知损害。关于Anaerostipes_hadrus与认知功能关系的研究罕见,ZEEVI等[27]提出Anaerostipes_hadrus中存在编码复合肌醇分解代谢-丁酸盐生物合成途径的区域,其存在与宿主代谢相关疾病风险低相关,本研究中亦发现Anaerostipes_hadrus与糖化血红蛋白呈负相关,又提示该菌可能是代谢性疾病的保护因子,与前面的推测存在矛盾。但本研究同时发现,对于卒中危险因素,Bifidobacterium属与UA呈正相关,Lachnoclostridium属与体质量指数呈负相关及Lactobacillus_salivarius菌与HCY呈负相关。这些菌类均在脑卒中患者中相对富集,对脑卒中发生既有起正性作用的菌类,也有起负性作用的菌类。最新PSCI管理专家共识[1]指出控制脑卒中的危险因素,减少脑卒中的发生,是预防PSCI的根本方式,因此细菌与卒中危险因素的作用可能间接地参与卒中后认知损害的发生、发展。由此看来,由于卒中后早期认知下降患者肠道菌群紊乱涉及到门至种多个水平及多菌类的改变,而非单一菌类单一趋势丰度水平的改变;不同菌类对同一环境因子的影响不同,同一菌类对不同环境因子的影响亦可能不同。多水平多菌类丰度的改变以及菌类间、菌类与环境因子间的相互作用综合可能才是导致疾病发生发展的原因。
综上所述,本研究表明,卒中后早期认知下降患者肠道菌群在门、属和种水平构成发生改变,且标志菌类与认知功能及卒中相关危险因素存在一定的相关性,对卒中早期伴或不伴认知障碍具有一定识别价值。尽管本研究存在一定的局限性,如样本量偏小、未进行随访观察,但相关研究工作为今后PSCI早期筛查和诊治提供了一定的参考。下一步的研究中将在现有证据的基础上,扩大样本量,随访观察卒中后早期肠道菌群的改变对卒中后3~6个月PSCI的预测价值,以及早期根据肠道菌群的改变进行针对性的干预对后期PSCI发生率的影响,为PSCI的早期筛查、准确预测、康复干预和预后判断提供新的视角。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
四川省科技厅重点研发项目(No. 2021YFS0132)和四川省医学科研课题计划(No. S19052)资助
Contributor Information
亚梅 李 (Ya-mei LI), Email: 306592581@qq.com.
茜 余 (Qian YU), Email: yqswc11@163.com.
References
- 1.汪凯, 董强, 郁金泰, 等 卒中后认知障碍管理专家共识2021. 中国卒中杂志. 2021;16(4):376–389. doi: 10.3969/j.issn.1673-5765.2021.04.011. [DOI] [Google Scholar]
- 2.ROHDE D, GAYNOR E, LARGE M, et al The impact of cognitive impairment on poststroke outcomes: A 5-year follow-up. J Geriatr Psychiatry Neurol. 2019;32(5):275–281. doi: 10.1177/0891988719853044. [DOI] [PubMed] [Google Scholar]
- 3.SINGH V, ROTH S, LLOVERA G, et al Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DURGAN D J, LEE J, MCCULLOUGH L D, et al Examining the role of the microbiota-gut-brain axis in stroke. Stroke. 2019;50(8):2270–2277. doi: 10.1161/STROKEAHA.119.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LIU Y, KONG C, GONG L, et al The association of post-stroke cognitive impairment and gut microbiota and its corresponding metabolites. J Alzheimers Dis. 2020;73(4):1455–1466. doi: 10.3233/JAD-191066. [DOI] [PubMed] [Google Scholar]
- 6.KHAW J, SUBRAMANIAM P, ABD AZIZ N A, et al Current update on the clinical utility of MMSE and MoCA for stroke patients in Asia: A systematic review. Int J Environ Res Public Health. 2021;18(17):8962. doi: 10.3390/ijerph18178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.王玉龙. 康复功能评定学. 第3版. 北京: 人民卫生出版社, 2018.
- 8.MIJAJLOVIC M D, PAVLOVIC A, BRAININ M, et al Post-stroke dementia-a comprehensive review. BMC Medicine. 2017;15(1):11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NARASIMHALU K, ANG S, DE SILVA D A, et al The prognostic effects of poststroke cognitive impairment no dementia and domain-specific cognitive impairments in nondisabled ischemic stroke patients. Stroke. 2011;42(4):883–888. doi: 10.1161/STROKEAHA.110.594671. [DOI] [PubMed] [Google Scholar]
- 10.COLLINS S M, SURETTE M, BERCIK P The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 11.GIAU V V, WU S Y, JAMERLAN A, et al Gut microbiota and their neuroinflammatory implications in Alzheimer's disease. Nutrients. 2018;10(11):1765. doi: 10.3390/nu10111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WANG Y, KASPER L H The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CRUMEYROLLE-ARIAS M, JAGLIN M, BRUNEAU A, et al Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 14.PEVSNER-FISCHER M, BLACHER E, TATIROVSKY E, et al The gut microbiome and hypertension. Curr Opin Nephrol Hypertens. 2017;26(1):1–8. doi: 10.1097/MNH.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 15.BAOTHMAN O A, ZAMZAMI M A, TAHER I, et al. The role of Gut microbiota in the development of obesity and diabetes. Lipids Health Dis, 2016, 15: 108[2021-09-15]. https://lipidworld.biomedcentral.com/articles/10.1186/s12944-016-0278-4. doi: 10.1186/s12944-016-0278-4.
- 16.LING Y, GU Q, ZHANG J, et al Structural change of gut microbiota in patients with post-stroke comorbid cognitive impairment and depression and its correlation with clinical features. J Alzheimers Dis. 2020;77(4):1595–1608. doi: 10.3233/JAD-200315. [DOI] [PubMed] [Google Scholar]
- 17.ZHANG F, YANG J, JI Y, et al. Gut Microbiota dysbiosis is not independently associated with neurocognitive impairment in people living with HIV. Front Microbiol, 2019, 9: 3352[2021-09-15]. https://doi.org/10.3389/fmicb.2018.03352.
- 18.李兰娟. 感染微生态学. 北京: 人民卫生出版社, 2012.
- 19.ZHANG Y, BOBE G, REVEL J S, et al. Improvements in metabolic syndrome by xanthohumol derivatives are linked to altered gut microbiota and bile acid metabolism. Mol Nutr Food Res, 2020, 64(1): e1900789[2021-09-15]. https://doi.org/10.1002/mnfr.201900789.
- 20.HARRIS K, KASSIS A, MAJOR G, et al. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J Obes, 2012, 2012: 879151[2021-09-15]. https://doi.org/10.1155/2012/879151.
- 21.吴燕芳, 尹晋, 曹娃, 等 大气细颗粒物暴露对成年雄性小鼠认知功能及肠道菌群组成的影响. 卫生研究. 2021;50(5):821–826. doi: 10.19813/j.cnki.weishengyanjiu.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 22.周达成, 王明, 蒋辉 老年阿尔茨海默病患者肠道微生态改变及其与认知功能的关系探讨. 中国微生态学杂志. 2020;32(6):700–704. doi: 10.13381/j.cnki.cjm.202006018. [DOI] [Google Scholar]
- 23.INOUE T, KOBAYASHI Y, MORI N, et al Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;9(6):843–853. doi: 10.3920/BM2017.0193. [DOI] [PubMed] [Google Scholar]
- 24.XIAO J, KATSUMATA N, BERNIER F, et al Probiotic Bifidobacterium breve in Improving cognitive functions of older adults with suspected mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J Alzheimers Dis. 2020;77(1):139–147. doi: 10.3233/JAD-200488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.KOBAYASHI Y, KUHARA T, OKI M, et al Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef Microbes. 2019;10(5):511–520. doi: 10.3920/BM2018.0170. [DOI] [PubMed] [Google Scholar]
- 26.ZHUANG Z Q, SHEN L L, LI W W, et al Gut microbiota is altered in patients with Alzheimer's disease. J Alzheimers Dis. 2018;63(4):1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 27.ZEEVI D, KOREM T, GODNEVA A, et al Structural variation in the gut microbiome associates with host health. Nature. 2019;568(7750):43–48. doi: 10.1038/s41586-019-1065-y. [DOI] [PubMed] [Google Scholar]




