Abstract
目的
探讨慢性失眠患者的睡眠脑电功率特征。
方法
回顾性分析符合ICD-10慢性失眠诊断标准并完成整夜多导睡眠监测(PSG)患者的睡眠脑电资料。比较失眠组和对照组睡眠结构及不同睡眠分期相对脑电功率特征的差异,并进一步分析脑电功率值与不同PSG指标的相关性。
结果
共计纳入45例研究对象,其中失眠患者25例(女性18例),年龄(36.2±10.7)岁;对照组20例(女性18例),年龄(36.1±7.6)岁。与对照组相比,失眠组患者NREM1期delta功率降低〔(38.0±6.1) vs. (43.2±5.8),P<0.05〕;NREM期及NREM1期、NREM2期beta功率增高〔NREM期:(5.4±2.3) vs. (3.8±1.4);NREM1期:(11.3±3.5) vs. (8.7±2.8);NREM2期:(5.7±2.3) vs. (4.4±1.4);P均<0.05〕。同时,失眠组患者NREM期delta功率与NREM3期睡眠时间呈正相关(r=0.527)。NREM期beta功率与NREM3期睡眠时间呈负相关(r=−0.767),与NREM1期、NREM2期睡眠时间呈正相关(r=0.486、0.589)。
结论
慢性失眠患者夜间NREM期睡眠中低频脑电功率减少,高频脑电功率增加,研究结果提示慢性失眠患者NREM期睡眠过程中大脑皮质觉醒水平增高。
Keywords: 失眠, 慢性失眠, 多导睡眠脑电图, 脑电图频谱分析
Abstract
Objective
To study the sleep electroencephalogram (EEG) power features of patients with chronic insomnia.
Methods
Retrospective analysis was performed with patients who met the ICD-10 diagnostic criteria for chronic insomnia, using polysomnography (PSG) to examine the overnight sleep EEG. The sleep architectures and relative EEG power across five frequency bands during overnight sleep were compared to study the differences between the insomnia and control groups. Furthermore, the correlation between EEG power and various PSG measures was also analyzed.
Results
Forty-five subjects were enrolled in the study, including 25 chronic insomniacs (18 females, aged [36.2±10.7] years) and 20 controls (18 females, aged [36.1±7.6] years). Compared to those of the control group, insomnia patients had significantly lower value of delta power ([38.0±6.1] vs. [43.2±5.8],P<0.05) in the NREM1 stage, and increased value of beta power during total NREM, NREM1 and NREM2 (NREM sleep [5.4±2.3] vs. [3.8±1.4], NREM1 [11.3±3.5] vs. [8.7±2.8], and NREM2 [5.7±2.3] vs. [4.4±1.4], allP<0.05). For correlation analyses, in the insomnia group, a significantly positive correlation was found between the delta value during NREM sleep and the duration of NREM3 sleep (r=0.527). The beta value during NREM sleep was found to be negatively correlated to the duration of NREM3 sleep (r=-0.767). A positive correlation was found between the beta value during NREM sleep and the duration of NREM1 and NREM2 sleep (r=0.486 and 0.589, respectively).
Conclusion
The results suggest that patients with chronic insomnia have decreased low-frequency EEG power, but increased high-frequency EEG power during NREM sleep. The findings indicate that cortex arousal level is elevated in chronic insomniacs during NREM sleep.
Keywords: Insomnia, Chronic insomnia, Polysomnography, EEG spectral analysis
失眠是最常见的睡眠障碍之一,约有30%的人有过失眠经历,其中约10%会发展成为慢性失眠[1–2]。慢性失眠不仅表现为持续性入睡困难、易醒、早醒等夜间睡眠异常,还可能出现疲劳乏力、白天嗜睡、记忆力受损、学习和工作能力下降等日间功能受损表现。目前认为大脑皮质高觉醒是失眠的一个重要维持因素[3],大脑皮质过度觉醒可能导致失眠患者在睡眠起始和睡眠维持过程中感觉和信息处理异常,进而干扰正常睡眠。
脑电功率分析作为一种对脑电活动进行定量分析的方法,已经被广泛应用于失眠研究领域。脑电功率分析利用快速傅立叶变换,将脑电信号由时域转换到频域。与传统睡眠分期相比,脑电功率分析可以从另一维度发掘脑电信号中的变化。delta功率是delta频率范围内脑电波的功率值,与睡眠深度相关,delta功率增高睡眠加深。beta功率值增高则被认为是皮质觉醒的标志。既往研究对失眠患者的脑电功率特征进行了探索,由于不同研究间失眠患者的纳入标准以及频率带划分标准不同,研究结果存在较大差异。有部分研究发现,与正常对照相比,失眠患者beta功率增高,delta功率降低[4–7],但也有部分研究并未发现此种差异[8–9]。同时,失眠患者脑电功率特征变化是否与多导睡眠监测(PSG)不同参数相关亦不清楚。本研究拟通过对慢性失眠患者的脑电功率进行分析,研究慢性失眠患者夜间睡眠脑电功率谱特征及其与PSG指标的相关关系。
1. 对象与方法
1.1. 研究对象
依据是否存在失眠分为失眠组和对照组。失眠组为2018年1月–2020年10月因失眠主诉就诊于睡眠门诊并完成PSG监测的患者。对照组为同期通过广告招募的健康大学生、医护人员和其他健康志愿者。失眠组和正常组年龄和性别匹配。本研究采用回顾性分析,已获得四川大学华西医院生物医学伦理委员会批准,批准号2016年审(90)号。
1.1.1. 纳入标准
失眠组:①以失眠为主诉并符合ICD-10慢性失眠诊断标准;②年龄18~70岁;③睡眠呼吸暂停低通气指数(AHI)<5次/h;④肢体周期性运动指数<15次/h。
对照组:①无失眠相关主诉;②年龄18~70岁;③AHI<5次/h;④肢体周期性运动指数<15次/h。
1.1.2. 排除标准
①服用镇静催眠药者;②合并发作性睡病等其他睡眠障碍者;③合并重大精神疾病者;④合并严重心肺疾病、肝肾疾病等重大躯体疾病者;⑤合并可能导致脑电异常的疾病,如帕金森、癫痫等;⑥PSG信号干扰较多影响睡眠分期判读和脑电功率分析者。
1.2. 方法
1.2.1. 资料收集
由睡眠医学中心工作人员采集,包括:年龄、性别、体质量、体质量指数(BMI)、Epworth嗜睡量表(ESS)评分、用药史等一般资料。
1.2.2. PSG监测
在睡眠实验室进行整夜PSG监测,数据采集时间>8 h。使用Alice6多功能PSG监测系统(美国飞利浦伟康公司)进行信号采集。采集信号包括:脑电图、眼动电图、心电图、下颌肌及双侧胫前肌肌电图、热敏与压力式口鼻气流、动脉血氧饱和度、胸腹运动。PSG采集结果由高级技术人员根据美国睡眠医学会睡眠及其相关事件判读手册[10]对睡眠分期和事件进行判读。
1.2.3. 脑电功率分析
选择所有受试对象的中央区脑电(C3/A1或C4/A2)作为采样点,采样频率为500 Hz,双侧乳突为参考电极。以欧洲数据格式(EDF)将PSG数据导出,进一步导入脑电功率分析软件进行分析。设置低通滤波为40 Hz,高通滤波为0.5 Hz。脑电图以30 s为一帧,将睡眠期转换前后10 s的脑电以及包含体动、呼吸、腿动相关的觉醒脑电等伪迹排除分析。选择汉宁窗进行以5 s为1个小周期的脑电频谱分析,取6个连续小周期的平均值作为30 s的平均脑电功率。脑电频率带划分标准为:slow-oscillation(0.5~1.0 Hz)、delta波(1.0~4.0 Hz)、theta波(4.0~8.0 Hz)、alpha波(8.0~13.0 Hz)、beta波(13.0~30.0 Hz)。W期脑电功率:取清醒卧床安静且无伪迹干扰的第一个睡眠期出现前的2 min脑电波进行分析。相对脑电功率:单个频带功率与总频带功率的比值,能减少个体间的差异对脑电功率的影响[11]。
1.2.4. 统计学方法
首先对统计分析数据进行正态性检验。正态分布的数据以
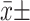 s表示,并采用独立样本t检验进行组间比较;非正态分布数据用中位数(P25, P75)表示,组间比较采用曼-惠特尼秩和检验。率的比较使用χ2检验。分析NREM期beta、delta相对功率与PSG指标的相关性时,采用秩相关分析。α双侧=0.05。
s表示,并采用独立样本t检验进行组间比较;非正态分布数据用中位数(P25, P75)表示,组间比较采用曼-惠特尼秩和检验。率的比较使用χ2检验。分析NREM期beta、delta相对功率与PSG指标的相关性时,采用秩相关分析。α双侧=0.05。
2. 结果
2.1. 人口学特征、PSG睡眠指标及ESS评分
失眠组与对照组的年龄、性别、睡眠潜伏期、REM睡眠潜伏期、睡后觉醒时间、NREM1期睡眠时间、NREM2期睡眠时间、REM期睡眠时间以及ESS评分的组间差异均无统计学意义。而失眠组总睡眠时间、NREM3期睡眠时间、NREM期睡眠时间以及睡眠效率低于对照组(均P<0.05)。见表1。
表 1. Comparison of demographics, overnight PSG measurements and ESS scores between controls and insomniacs.
对照组与失眠组人口学特征、PSG指标及ESS评分比较
| Item | Controls
(n=20) |
Insomniacs
(n=25) |
t | P |
PSG: Polysomnography; TST: Total sleep time; SOL: Sleep onset latency; WASO: Wake after sleep onset; NREM1: Duration of non-rapid eye movement sleep stage 1; NREM2: Duration of non-rapid eye movement sleep stage 2; NREM3: Duration of non-rapid eye movement sleep stage 3; NREM: Time of whole non-rapid eye movement sleep; REM: Rapid eye movement sleep time; SE: Sleep efficiency; ESS: Epworth Sleepiness Scale.a:
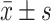 ; b: median (P25, P75).
; b: median (P25, P75).
| ||||
| Age/yr.a | 36.1±7.6 | 36.2±10.7 | 1.61 | 0.114 |
| Sex(male/female) | 2/18 | 7/18 | 1.27 | 0.026 |
| TST/mina | 446.6±44.5 | 398.5±91.0 | 2.32 | 0.026 |
| SOL/mina | 15.5±17.5 | 32.8±45.2 | −0.03 | 0.980 |
| REM SOL/minb | 7.5 (4.0, 20.8) | 10.5 (6.5, 34.8) | 313.00 | 0.150 |
| WASO/mina | 46.3±33.9 | 72.9±61.0 | −1.85 | 0.071 |
| NREM/mina | 354.5±29.7 | 319.6±70.5 | 2.24 | 0.032 |
| NREM1/mina | 70.5±43.0 | 64.4±24.2 | 0.57 | 0.571 |
| NREM2/mina | 256.4±39.4 | 237.8±75.6 | 1.06 | 0.296 |
| NREM3/minb | 24.8 (8.0, 36.5) | 1.5 (0.0, 28.5) | 152.00 | 0.024 |
| REM/mina | 91.7±30.2 | 81.1±34.5 | 1.09 | 0.282 |
| SE/%b | 89.7 (84.0, 92.5) | 84.1 (72.8, 90.2) | 157.00 | 0.034 |
| ESSb | 7.5 (2.5, 10.8) | 5.0 (2.0, 8.5) | 197.00 | 0.223 |
2.2. 失眠组与对照组相对脑电功率的组间比较
失眠组delta功率在NREM1期更低(P<0.05),在其余睡眠期无组间差异;beta功率在整个NREM期、NREM1期及NREM2期均高于对照组(P均<0.05),在W期、NREM3期及REM期组间差异无统计学意义。失眠组与对照组的slow-oscillation、theta、alpha频率带功率在所有睡眠期差异均无统计学意义。见表2。
表 2. Comparison of the relative EEG power across different frequency bands between controls and insomniacs during the W, NREM and REM sleep stages.
对照组与失眠组在W期、NREM期及REM期不同频率范围的相对脑电功率比较
| Frequency band | Controls
(n=20) |
Insomniacs
(n=25) |
t | P |
| W: Wake stage before sleep onset; NREM: Non-rapid eye movement sleep; NREM1: Non-rapid eye movement sleep stage 1; NREM2: Non-rapid eye movement sleep stage 2; NREM3: Non-rapid eye movement sleep stage 3; REM: Rapid eye movement sleep. | ||||
| W | ||||
| Slow-oscillation | 14.7±13.3 | 13.2±9.1 | 0.34 | 0.738 |
| Delta | 23.3±9.5 | 23.8±10.1 | −0.21 | 0.833 |
| Theta | 14.9±5.4 | 15.5±7.4 | −0.27 | 0.792 |
| Alpha | 34.3±17.7 | 32.1±18.5 | 0.41 | 0.685 |
| Beta | 12.7±4.8 | 15.2±7.0 | −0.66 | 0.515 |
| NREM | ||||
| Slow-oscillation | 25.1±4.2 | 25.1±6.4 | 0.01 | 0.989 |
| Delta | 50.6±3.6 | 48.0±5.6 | 1.24 | 0.223 |
| Theta | 12.4±2.0 | 12.5±2.8 | −0.11 | 0.915 |
| Alpha | 8.1±2.8 | 8.2±3.5 | −0.06 | 0.951 |
| Beta | 3.8±1.4 | 5.4±2.3 | −2.81 | 0.008 |
| NREM1 | ||||
| Slow-oscillation | 12.7±4.8 | 16.0±8.7 | −1.61 | 0.115 |
| Delta | 43.2±5.8 | 38.0±6.1 | 2.99 | 0.005 |
| Theta | 20.9±4.0 | 19.3±6.1 | 1.38 | 0.176 |
| Alpha | 14.5±6.2 | 15.5±7.5 | 0.50 | 0.617 |
| Beta | 8.7±2.8 | 11.3±3.5 | −2.74 | 0.009 |
| NREM2 | ||||
| Slow-oscillation | 23.4±4.7 | 24.0±7.1 | −3.42 | 0.732 |
| Delta | 49.3±3.8 | 48.2±5.0 | 0.83 | 0.411 |
| Theta | 13.7±2.5 | 13.3±3.0 | 0.51 | 0.612 |
| Alpha | 9.2±3.0 | 8.8±4.3 | 0.73 | 0.468 |
| Beta | 4.4±1.4 | 5.7±2.3 | −2.33 | 0.025 |
| NREM3 | ||||
| Slow-oscillation | 32.6±5.9 | 31.1±6.2 | 0.69 | 0.494 |
| Delta | 54.2±4.3 | 55.9±6.3 | −0.90 | 0.375 |
| Theta | 8.0±1.8 | 8.1±2.4 | −0.21 | 0.838 |
| Alpha | 4.0±2.0 | 3.3±1.5 | 1.06 | 0.300 |
| Beta | 1.2±0.34 | 1.6±1.2 | 0.46 | 0.653 |
| REM | ||||
| Slow-oscillation | 15.9±5.5 | 16.9±6.3 | −0.54 | 0.592 |
| Delta | 43.7±4.1 | 42.7±5.9 | 0.60 | 0.554 |
| Theta | 20.8±4.9 | 19.0±4.0 | 1.33 | 0.191 |
| Alpha | 11.8±3.5 | 12.5±6.1 | −0.15 | 0.884 |
| Beta | 7.7±1.5 | 8.9±3.1 | −1.43 | 0.159 |
2.3. NREM期delta和beta相对功率与PSG指标的相关性
在失眠组中,NREM期delta功率与NREM3期睡眠时间正相关,beta功率与NREM1、NREM2期睡眠时间呈正相关,与NREM3期睡眠时间负相关(r=0.527、0.486、0.589、−0.767,P均<0.05)。对照组未发现NREM期beta、delta功率与PSG指标之间具有相关关系。见表3。
表 3. Correlations between the relative power of NREM-stage delta and beta-frequency EEG and PSG parameters (r) .
NREM期delta、beta相对功率与PSG指标的相关性(r值)
| Item | Controls | Insomniacs | |||
| Delta | Beta | Delta | Beta | ||
| Data are presented as Spearman’s rank correlation coefficient. NREM: Non-rapid eye movement sleep stage; TST: Total sleep time; SOL: Sleep onset latency; WASO: Wake after sleep onset; NREM1: Duration of non-rapid eye movement sleep stage 1; NREM2: Duration of non-rapid eye movement sleep stage 2; NREM3: Duration of non-rapid eye movement sleep stage 3; REM: Rapid eye movement sleep time; SE: Sleep efficiency; ESS: Epworth Sleepiness Scale; * P<0.05. | |||||
| TST | −0.182 | 0.215 | 0.040 | 0.342 | |
| SOL | −0.224 | 0.111 | −0.074 | −0.346 | |
| WASO | 0.189 | −0.287 | 0.006 | −0.320 | |
| NREM1 | 0.003 | −0.108 | −0.392 | 0.486* | |
| NREM2 | −0.074 | 0.352 | −0.098 | 0.589* | |
| NREM3 | 0.054 | −0.238 | 0.527* | −0.767* | |
| REM | −0.172 | 0.167 | 0.124 | 0.323 | |
| SE | −0.050 | 0.078 | 0.047 | 0.336 | |
| ESS | −0.072 | 0.071 | −0.234 | −0.148 | |
3. 讨论
本研究发现,与对照组相比,失眠组NREM1期delta功率更低,在整个NREM期、NREM1期及NREM2期beta功率更高。进一步分析脑电功率与PSG指标的相关性发现,delta功率与NREM3期睡眠时间正相关。beta功率与NREM2期、NREM1期睡眠时间正相关,与NREM3期睡眠时间负相关。
既往研究发现,失眠患者在W期、NREM期以及REM期低频率带功率显著低于对照,而高频率带功率更高[7, 12–19],这与本研究结果一致。然而,多数研究只对部分睡眠期的脑电功率进行分析,并未充分描述睡前清醒期以及各个睡眠分期的脑电功率特征,也尚无研究分析脑电功率值与PSG指标之间的相关性。本研究在详细描述慢性失眠患者夜间睡眠脑电功率谱特征的基础上,同时对功率值与临床常用PSG指标之间的相关性进行了创新性探索,进一步证实失眠患者存在皮质高觉醒,且觉醒程度与患者不同睡眠期持续时间相关。
失眠患者在浅睡期beta频率带功率增高,NREM期beta功率与浅睡时长呈正相关,与深睡时长呈负相关。而既往研究已经证实beta功率与感觉处理、认知过程以及大脑皮质过度觉醒有关[5, 17, 20]。提示失眠患者睡眠起始阶段及浅睡眠期大量高频脑电活动增多,信息处理过程不能及时终止,皮质觉醒水平增高,从而不能更好区分睡眠和清醒状态,导致睡眠主观感受变差[21]。同时,失眠患者夜间睡眠过程中的高觉醒状态,又可反过来导致beta频率带功率增高,浅睡眠增多,进一步影响睡眠的主观体验。delta功率增高与睡眠深度有关[22],我们发现失眠患者NREM1期delta相对功率更低[23-24],delta功率与深睡时长呈正相关。因此,失眠患者在夜间睡眠中不仅存在大脑皮质过度觉醒,浅睡眠时间增多,其浅睡眠期睡眠深度与健康人群相比可能更差,加重其夜间睡眠紊乱及知觉异常。此外,在对照组中NREM期beta、delta功率值与PSG指标之间无相关关系,提示夜间睡眠过程中大脑皮质的高觉醒状态可能只存在于慢性失眠患者中[7, 17, 20]。
在NREM3期和REM期两组脑电功率的差异无统计学意义,可能是由于失眠患者NREM3期睡眠减少或缺失导致可用于分析的脑电波减少;并且REM期脑电活动会受到快速眼球运动的干扰,而快速眼球运动对脑电活动的干扰程度以及其具体影响的脑电频率范围仍需要进一步明确[5, 20]。此外,也可能提示失眠患者在深睡眠中,不存在大脑皮质的过度觉醒。
本研究未发现NREM期beta、delta功率与总睡眠时间、入睡潜伏期、入睡后觉醒时间、睡眠效率之间的相关性。考虑可能的原因如下:第一,本研究样本量仍然较小,因此可能导致以上指标之间的相关性未能被揭示。第二,本研究的PSG指标仅是进行一夜睡眠监测采集的结果,而首夜效应和逆首夜效应可能导致PSG指标的个体内变异[25],从而可能掩盖了上述指标之间的相关性。本研究还存在一定的不足,首先本研究是横断面观察性研究,无法确定失眠与beta、delta功率改变的因果关系,将来需要纵向研究来探讨两者之间的关系。其次,本研究未对失眠进行具体分型,不能说明所有类型失眠患者的脑电功率谱特征,因此需要在今后的研究里扩大样本量进一步对不同类型失眠患者的脑电活动进行分析。
综上,慢性失眠患者夜间NREM期睡眠中大脑皮质觉醒程度增高,睡眠深度变浅,睡眠质量变差。delta和beta功率的变化与失眠患者夜间睡眠异常有关,delta功率降低,睡眠深度变浅;beta功率增高,皮质觉醒水平增高。delta和beta功率的改变可能部分揭示了慢性失眠的神经生理学机制,今后的研究需要扩大样本量纵向观察和验证睡眠脑电活动的变化与失眠患者睡眠改变间的因果关系。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
国家自然科学基金(No. 82120108002、No. 82170100)资助
Contributor Information
媛 时 (Yuan SHI), Email: 1254996496@qq.com.
蓉 任 (Rong REN), Email: 498880651@qq.com.
References
- 1.OHAYON M Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.MORIN C M, LEBLANC M, DALEY M, et al Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 3.RIEMANN D, SPIEGELHALDER K, FEIGE B, et al The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 4.BUYSSE D J, GERMAIN A, HALL M L, et al EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31(2):1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KRYSTAL A D, EDINGER J D, WOHLGEMUTH W K, et al NREM Sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640. doi: 10.1093/sleep/25.6.626. [DOI] [PubMed] [Google Scholar]
- 6.FERNANDEZ-MENDOZA J, LI Y, VGONTZAS A N, et al Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep. 2016;39(5):1029–1036. doi: 10.5665/sleep.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PERLIS M L, SMITH M T, ANDREWS P J, et al Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 8.KANG S G, MARIANI S, MARVIN S A, et al Sleep EEG spectral power is correlated with subjective-objective discrepancy of sleep onset latency in major depressive disorder. Prog Neuropsychopharmacology Biol Psychiatry. 2018;85:122–127. doi: 10.1016/j.pnpbp.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 9.WU Y M, PIETRONE R, CASHMERE J D, et al EEG power during waking and NREM sleep in primary insomnia. J Clin Sleep Med. 2013;9(10):1031–1037. doi: 10.5664/jcsm.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BERRY R B, BROOKS R, GAMALDO C, et al AASM scoring manual updates for 2017 (Version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MISRA A Ethnic-specific criteria for classification of body mass index: A perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol Ther. 2015;17(9):667–671. doi: 10.1089/dia.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SCIENTIFIC E, IRELAND P, CLINIC L, et al EEG power spectra in sleep-onset insomina. Electroencephalogr Clin Nuerophysiol. 1986;63(5):408–413. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 13.MERICA H, GAILLARD J M The EEG of the sleep onset period in insomnia: A discriminant analysis. Physiol Behav. 1992;52(2):199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- 14.LAMAECHE C H, OGILVIE R D Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20(9):724–733. [PubMed] [Google Scholar]
- 15.CORSI-CABRERA M, FIGUEREDO-RODRIGUEZ P, DEL RIO-PORTILLA Y, et al Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35(4):501–511. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MERICA H Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10(5):1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 17.PERLIS M L, KEHR E L, SMITH M T, et al Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10(2):93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 18.PERRIER J, CLOCHON P, BERTRAN, et al Specific EEG sleep pattern in the prefrontal cortex in primary insomnia. PLoS One. 2015;10(1):e0116864[2021-01-22]. https://doi.org/10.1371/journal.pone.0116864. doi: 10.1371/journal.pone.0116864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RIEDNER B A, GOLDSTEIN M R, PLANTE D T, et al Regional patterns of elevated alpha and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: A pilot study. Sleep. 2016;39(4):801–812. doi: 10.5665/sleep.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SPIEGELHALDER K, REGEN W, FEIGE B, et al Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91(3):329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 21.CHOI H, JEONG J, KIM H, et al Implication of fast activities of spectral analysis in subjective sleep complaints of elderly women. J Geriatr Psychiatry Neurol. 2019;32(1):24–30. doi: 10.1177/0891988718813711. [DOI] [PubMed] [Google Scholar]
- 22.KURYLENKO O, SEMKIV M, RUCHALA J, et al Slow-oscillation activity is reduced and high frequency activity is elevated in older adults wi th insomnia. J Clin Sleep Med. 2020;16(9):1445–1454. doi: 10.5664/jcsm.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SVETNIK V, SNYDER E S, MA J, et al EEG spectral analysis of NREM sleep in a large sample of patients with insomnia and good sleepers: Effects of age, sex and part of the night. J Sleep Res. 2017;26(1):92–104. doi: 10.1111/jsr.12448. [DOI] [PubMed] [Google Scholar]
- 24.ANDRILLON T, SOLELHAC G, BOUCHEQUET P, et al Revisiting the value of polysomnographic data in insomnia: More than meets the eye. Sleep Med. 2020;66:184–200. doi: 10.1016/j.sleep.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 25.HERBERT V, PRETT D, EMALEY R, et al Predictors of nightly subjective-objective sleep discrepancy in poor sleepers over a seven-day period. Brain Sci. 2017;7(3):29. doi: 10.3390/brainsci7030029. [DOI] [PMC free article] [PubMed] [Google Scholar]


