Abstract
目的
探索非肌层浸润性膀胱癌(NMIBC)患者膀胱灌注治疗的最佳方案,并探讨影响灌注治疗后肿瘤复发的临床病理因素。评估现有NMIBC预后预测模型在我院NMIBC人群中的使用价值。
方法
自2016年起,将在本院行经尿道膀胱肿瘤电切术(TURBT)后确诊为中高危NMIBC的患者纳入研究。将患者随机分为卡介苗(BCG)19次、15次组及表柔比星(EPI,治疗18次)组,入组人数比例为2∶2∶1,进行膀胱灌注治疗。记录灌注前、中、后患者临床病理资料,采用无复发生存为终点指标,绘制生存曲线,评估比较上述3种方案的治疗效果,并对病理及临床资料进行分析,探索各项因素与灌注治疗后肿瘤复发的关系。采用一致性指数(c-index)评估西班牙泌尿肿瘤研究组(CUETO)预后预测模型及欧洲肿瘤研究及治疗组织(EORTC)预后预测模型对患者的预测准确性。
结果
本研究共纳入93例NMIBC患者(BCG19组35例,BCG15组37例,EPI组21例),中位随访时间33.46个月。其中22例患者肿瘤复发,8例患者出现肿瘤进展。生存曲线示,BCG组无复发生存优于EPI组(P=0.002),而BCG19和BCG15两组间的无复发生存的差异无统计学意义。虽然BCG组的并发症发生率高于EPI组患者(84.7% vs. 61.9%,P=0.022),但均未出现3~5级并发症。CUETO模型及EORTC模型的c-index高于单纯根据肿瘤T分期、核分级或EAU风险分层预测,且在BCG组中的c-index较在整体患者中更高。
结论
在本研究人群中,BCG膀胱灌注治疗的膀胱癌复发率较EPI膀胱灌注治疗低。CUETO模型及EORTC模型更适合对BCG膀胱灌注治疗的患者而非对整体患者进行预测。
Keywords: 膀胱癌, 卡介苗, 表柔比星, 膀胱灌注, 复发, 预后, 预测模型
Abstract
Objective
To explore the best treatment plan of intravesical instillation for patients with non-muscular invasive bladder cancer (NMIBC), to explore recurrence-related clinicopathological factors after intravesical instillation, and to evaluate the value of the prognosis and prediction models currently used for NMIBC patients.
Methods
Starting from 2016, patients who underwent transurethral resection of bladder tumor (TURBT) in our hospital and who received post-surgery diagnosis of having intermediate or high risks for NMIBC were enrolled in the study. They were randomly assigned to different group sat a ratio of 2∶2∶1 for receiving intravesical instillation therapy of Bacillus Calmette-Guérin (BCG) for 19 times, BCG for 15 times, and epirubicin (EPI) for 18 times. The clinicopathological data of the patients were recorded before, during and after instillation therapy, and survival curves were drawn to evaluate the effects of the three regimens, using recurrence-free survival as the endpoint. Clinicopathological data were analyzed to study the associations between various factors and post-instillation recurrence. The consistency index (c-index) was used to evaluate the predictive accuracy of the scoring model of the Spanish Urological Club for Oncological Treatment (CUETO) and the risk tables of European Organization for Research and Treatment of Cancer (EORTC).
Results
A total of 93 NMIBC patients (35 in the 19-time BCG group, 37 in the 15-time BCG group, and 21 in the EPI group) were included, with a median follow-up time of 33.46 months. Twenty-two patients experienced tumor recurrence and eight, tumor progression. The survival curve showed that the BCG group had better recurrence-free survival than the EPI group (P=0.002), while the difference in recurrence-free survival between 19-time BCG and 15-time BCG groups was not statistically significant. Higher general complication rate was seen in the BCG groups compared with the EPI group (84.7% vs. 61.9%, P=0.022), but there was no grade 3-5 adverse events in any group. The c-index of CUETO scoring model and EORTC risk tables was higher than that of the prediction based solely on T stage, nuclear grade, or EAU risk stratification. In addition, the c-index in the BCG group was higher than that in the whole cohort.
Conclusion
Among the subjects of this study, the recurrence rate of bladder cancer in the intravesical BCG instillation groups was lower than that of the epirubicin group. EORTC risk tables and CUETO scoring model exhibited higher predictive accuracies in BCG-treated patients than its performance for the whole NMIBC cohort.
Keywords: Bladder cancer, Bacillus Calmette–Guérin, Epirubicin, Intravesical instillation, Recurrence, Prognosis, Predictive model
膀胱癌是泌尿外科最常见的肿瘤之一,世界范围内,膀胱癌发病率居恶性肿瘤的第十一位[1]。我国膀胱癌发病率低于西方发达国家,但我国膀胱癌的发病率和死亡率近十年来呈现上升趋势,加强我国膀胱癌防治,控制膀胱癌发病和死亡水平,已成为我国恶性肿瘤防控工作的重要任务[2]。
膀胱癌患者中,大约75%为非肌层浸润性膀胱癌(NMIBC:Ta或T1期)[3]。目前经尿道膀胱肿瘤电切术(TURBT)为NMIBC的主要治疗手段,单纯的TURBT术后有高达70%的患者会出现肿瘤复发,10%~20%的患者可能会进展为肌层浸润性膀胱癌(MIBC)[4]。术后膀胱内灌注治疗,可以降低肿瘤复发及进展的风险,改善患者预后。目前,欧洲泌尿外科协会及美国泌尿外科学会均推荐卡介苗(BCG)作为中/高危NMIBC术后膀胱灌注的首选药物[5-6]。数项研究显示:针对白种人中高危NMIBC患者,相比单纯TURBT以及TURBT后使用化学药物膀胱灌注治疗,TURBT术后使用BCG灌注治疗在预防肿瘤复发及进展方面都有显著的优越性[7-8]。然而,在既往的临床试验中,由于较高的并发症发生率,BCG膀胱灌注治疗在中国膀胱癌患者中的使用受到限制,且尚未得出最佳的灌注治疗方案。此外,即使术后规律膀胱灌注BCG治疗,患者的膀胱癌复发率仍达到了32.6%,进展率达13.4%[9]。本研究进行了一项随机对照试验,以评估不同方案的BCG及表柔比星(EPI)膀胱灌注治疗的疗效和安全性;同时还评价了不同预后模型在本组患者的预测价值,以及影响NMIBC预后的各项临床病理因素。
1. 对象与方法
1.1. 研究对象
对象来源于四川大学华西医院进行的一项前瞻性、随机、对照、非盲性研究。自2016年起,临床诊断考虑为NMIBC,且无术中膀胱穿孔、术后严重血尿等灌注禁忌症的患者,TURBT术后24 h内膀胱灌注EPI 50 mg,根据手术发现及术后病理报告,最终被确诊为中/高危NMIBC的患者,纳入本研究。本研究由国家药品监督管理局药品审评中心(注册号为:CTR20150840)和四川大学华西医院临床试验伦理分委员会批准〔2015年临床试验(上市)审(19)号〕,并全程监督。
纳入标准:①年龄20~75岁;②膀胱镜下肉眼可见肿瘤完全切除;③根据欧洲泌尿外科学会(EAU)指南NMIBC风险分层[5],经病理检查证实为中/高危NMIBC。
排除标准:① 美国东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)体能状态评分大于1分;② 活动性肺结核或正在接受抗结核治疗的患者;③ 免疫缺陷或接受免疫抑制治疗的患者;④ 严重并发症(如严重心脑血管疾病)或存在其他类型癌症的患者;⑤ 既往诊断为MIBC的患者;⑥ 在前4周内接受过可能影响研究结果的治疗(如化疗、放疗或免疫治疗)的患者;⑦ 术中及术后出现严重并发症的患者(如膀胱穿孔、术后严重血尿、膀胱刺激等);⑧ 因怀孕、严重残疾、严重心理问题等原因不适合接受治疗或不能参加试验的患者。
1.2. 治疗方案及分组
术后患者随机分为3种灌注治疗方案组〔BCG 19次(BCG19)组、BCG 15次(BCG15)组和EPI组〕,术后14 d内,以掷硬币随机化的方法,按照2∶2∶1的比例进行分组(DAS for IWRS version 5.0, BioVoice & BioGuider,Beijing,China)。灌注治疗自术后2周开始,持续约1年。分组治疗方案如下:①BCG19组:诱导期BCG 120 mg每周灌注1次,连续6次,后每两周灌注1次,连续3次;维持期每月灌注1次,共10次;②BCG15组:诱导期BCG 120 mg每周灌注1次,连续6次;维持期术后第3、6、12个月每月前3周每周灌注1次,共9次;③EPI组:EPI 50 mg每周灌注1次,共8次;后每月灌注1次,共10次。
1.3. 数据收集
收集记录的临床变量包括:性别、年龄和吸烟史。根据手术记录及术后病理报告(所有病理报告由我院病理科出具)收集患者肿瘤大小、位置、数量以及肿瘤T分期(8th American Joint Committee on Cancer TNM classification system)[10],核分级(Grade,2004 World Health Organization grading system),是否存在原位癌(carcinoma in situ, CIS)。
1.4. 随访及终点指标
对于中危患者,手术后3个月和6个月进行膀胱镜检查,然后每半年检查1次,直到术后第5年。对于高危患者,术后2年内每3个月进行1次膀胱镜检查,然后每半年检查1次,直到术后5年。每次灌注前进行尿液常规检查。对患者进行随访,直到患者肿瘤复发或进展。每次随访时按照不良事件通用术语标准4.0〔Common terminology criteria for adverse events (CTCAE) version 4.0〕记录不良反应。
肿瘤风险分层(中危、高危)定义参照EAU指南[5]。肿瘤复发的定义为患者TURBT术后再次发现膀胱肿瘤;肿瘤进展的定义为肿瘤进展为MIBC。
患者既往复发频率的定义为自患者第一次发现NMIBC起,肿瘤1年内复发的最高频率。患者在本次行TURBT前第一次发现膀胱肿瘤,且结合术中所见和术后病理诊断为NMIBC,记为原发;随访期间再次发现NMIBC,记为复发。以患者第一次发现NMIBC为起点,随访既往复发间隔,取间隔最短者,分为复发≤1次每年和复发>1次每年。起始时间为患者行TURBT手术当天。终点指标为无复发生存,包括无复发生存期(RFI)及无复发生存率,即从手术日到肿瘤复发的间期及其生存率。根据EAU[5]的定义记录BCG治疗失败和不耐受。
采用欧洲癌症研究与治疗组织(EORTC)风险评分表[11]和西班牙泌尿肿瘤研究组(CUETO)风险评分表[9]预测NMIBC术后肿瘤复发率,对患者进行危险分层。
1.5. 统计学方法
使用log-rank检验评估两组或多个亚组之间的生存差异。采用单因素和多因素Cox回归分析,确定与NMIBC患者肿瘤复发相关的临床病理因素。使用单因素方差分析及卡方检验比较组间差异。同时采用卡方检验分析分类变量。当n≥40、T≥5时,采用Pearson卡方检验;当n≥40且1≤T<5时,采用连续校正卡方检验;如n<40或T<0,则使用Fisher精确检验。使用一致性指数(c-index)[12]评价EORTC模型和CUETO模型的预测能力,c-index越高,则预测准确性越高。P<0.05为差异有统计学意义。
2. 结果
2.1. 基线比较
见表1。共纳入NMBIC患者93例(BCG19组、BCG15组、EPI组分别为35例、37例、21例),平均年龄(62.96±8.16)岁,中位随访时间33.46个月(四分位间距: 18.30~44.80个月)。除肿瘤T分期外,BCG组和EPI组的基线临床病理因素差异均无统计学意义。8例患者在TURBT术后出现肿瘤进展,后行根治性膀胱切除术。
表 1. Clinicopathological data of patients with NMIBC.
非肌层浸润性膀胱癌患者的临床病理资料
| Clinicopathological
variable |
BCG group
(n=72) |
EPI group
(n=21) |
Total
(n=93) |
P |
| BCG: Bacillus Calmette–Guérin; EPI: Epirubicin; NMIBC: Non-muscle invasive bladder cancer; Recurrent≤1 rec/year: Prior recurrence rate of less than one per year; Recurrent>1 rec/year: Prior recurrence rate of more than one per year; EAU: European Association of Urology; EORTC: European Organization for Research and Treatment of Cancer; CUETO: Spanish Urological Club for Oncological Treatment. | ||||
| Gender/case | 0.390 | |||
| Male | 57 | 19 | 76 | |
| Female | 15 | 2 | 17 | |
| Preoperative age/case | 0.930 | |||
| ≤60 yr. | 24 | 8 | 32 | |
| 60−70 yr. | 34 | 10 | 44 | |
| >70 yr. | 14 | 3 | 17 | |
| Smoking history/case | 0.089 | |||
| No | 43 | 8 | 51 | |
| Yes | 29 | 13 | 42 | |
| Prior recurrence rate/case | 0.232 | |||
| Primary | 44 | 13 | 57 | |
| Recurrent≤1 rec/year | 18 | 2 | 20 | |
| Recurrent>1 rec/year | 10 | 6 | 16 | |
| T stage/case | 0.002 | |||
| Ta | 51 | 21 | 72 | |
| T1 | 21 | 0 | 21 | |
| Grade/case | 0.331 | |||
| Low | 32 | 12 | 44 | |
| High | 40 | 9 | 49 | |
| Tumor size/case | 0.465 | |||
| <3 cm | 38 | 9 | 47 | |
| ≥3 cm | 34 | 12 | 46 | |
| Amount of tumors/case | 0.793 | |||
| ≤3 | 49 | 13 | 62 | |
| >3 | 23 | 8 | 31 | |
| EAU risk stage/case | 0.453 | |||
| Intermediate | 27 | 10 | 37 | |
| High | 45 | 11 | 56 | |
| Recurrence/case | 0.006 | |||
| No | 60 | 11 | 71 | |
| Yes | 12 | 10 | 22 | |
| EORTC risk table/case | 0.218 | |||
| 1−4 | 28 | 5 | 33 | |
| 5−9 | 39 | 16 | 55 | |
| 10−17 | 5 | 0 | 5 | |
| CUETO scoring model/case | 0.962 | |||
| 0−4 | 21 | 7 | 28 | |
| 5−6 | 26 | 8 | 35 | |
| 7−9 | 20 | 5 | 25 | |
| 10−16 | 5 | 1 | 6 | |
2.2. BCG和EPI的治疗效果
经历1年以上的随访后,12例BCG组患者(16.7%)肿瘤复发,其中9例(12.5%)出现在BCG灌注治疗期间;6例BCG组患者(8.3%)发生肿瘤进展。EPI组的复发和进展例数分别为10例(47.6%)和2例(9.5%)。共有8例患者出现肿瘤进展。其中EPI组灌注治疗期间复发6例(28.6%)。与EPI组相比,BCG组的无复发生存更优(P=0.002),而BCG19和BCG15两亚组间无复发生存的差异无统计学意义。见图1。
图 1.
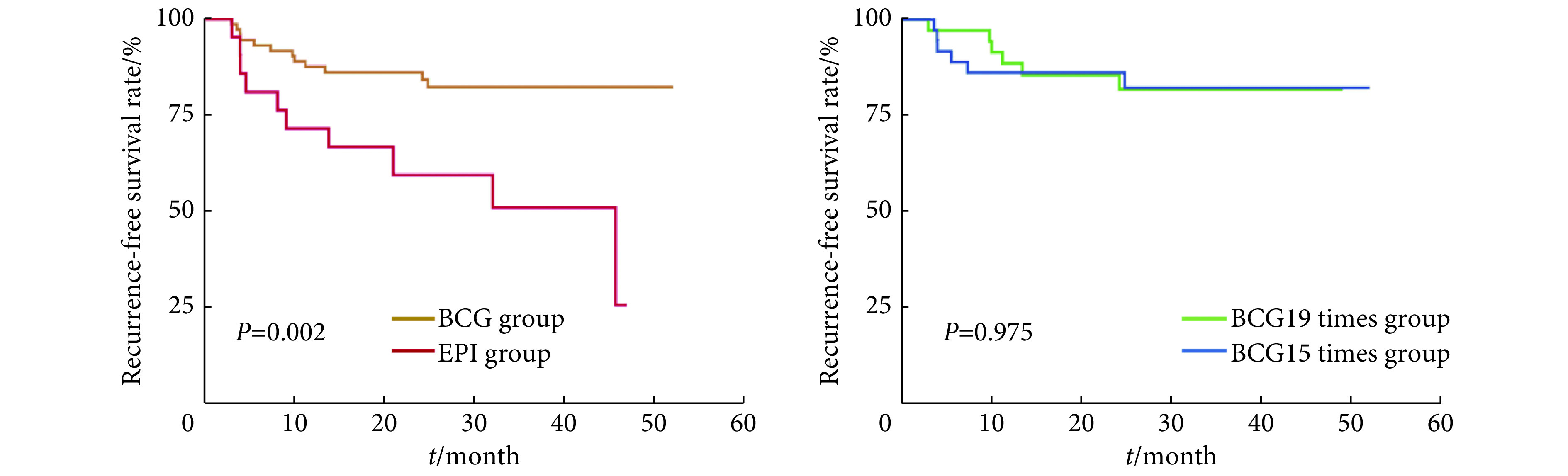
Survival curves of recurrence-free survival in different instillation regimens
不同灌注方案的无复发生存曲线
BCG: Bacillus Calmette–Guérin; EPI: Epirubicin.
2.3. 安全性评价
见表2。BCG组的并发症发生率高于EPI组(84.7% vs. 61.9%,P=0.022);但BCG组大部分并发症均无需干预治疗,两组均未发生严重不良事件(3~5级)。膀胱炎是膀胱内灌注治疗最常见的并发症,BCG组的发生率高于EPI组(75.0% vs. 52.4%,P=0.047);发热是BCG治疗后特有的并发症(16.7%),但所有患者均无需治疗。血尿在BCG组和EPI组均有发生,但组间差异无统计学意义(43.1% vs. 42.9%,P=0.987)。BCG组出现呼吸道感染、高血压、高血糖等罕见并发症的患者较多,但与EPI组比较差异无统计学意义(29.2% vs. 9.5%,P=0.122)。6例患者因不能耐受并发症而未完成BCG全周期治疗(数据不剔除,按照患者实际复发或进展时间计入BCG组进行统计),均发生在维持期(BCG19组3例,BCG15组3例;5例因膀胱炎,1例因血尿);对此6例患者进行持续随访,其中仅有1例患者在治疗结束后2年复发。BCG19和BCG15两亚组间并发症发生率差异无统计学意义。
表 2. Complications for intravesical therapy.
膀胱灌注治疗相关并发症
| Complication | BCG group/case (%) | EPI group/case (%), n=21 | P1 | P2 | ||
| Total (n=72) | 19 times group (n=35) | 15 times group (n=37) | ||||
| BCG: Bacillus Calmette–Guérin; EPI: Epirubicin; CTCAE: Common Terminology Criteria for Adverse Events; Others: Rare complications including respiratory infection, hypertension and hyperglycemia. P1: BCG 19 times group vs. BCG 15 times group; P2: BCG group vs. EPI group. | ||||||
| Urocystitis | 54 (75.0) | 27 (77.1) | 27 (73.0) | 11 (52.4) | 0.683 | 0.047 |
| Hematuresis | 31 (43.1) | 17 (48.6) | 14 (37.8) | 9 (42.9) | 0.358 | 0.987 |
| Fever | 12 (16.7) | 5 (14.3) | 7 (18.9) | 0 (0.0) | 0.598 | 0.062 |
| Others | 21 (29.2) | 9 (25.7) | 12 (32.4) | 2 (9.5) | 0.353 | 0.122 |
| CTCAE grade | ||||||
| 1 | 53 (73.6) | 29 (82.9) | 24 (64.9) | 13 (61.9) | − | − |
| 2 | 8 (11.1) | 2 (5.7) | 6 (16.2) | 0 (0.0) | − | − |
| 3−5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | − | − |
| Total adverse events | 61 (84.7) | 31 (88.6) | 30 (81.1) | 13 (61.9) | 0.226 | 0.022 |
2.4. 临床病理因素及治疗方案对肿瘤复发的影响
见表3。在93例TURBT术后患者中,单因素分析显示:只有既往肿瘤复发频率>1次/年及肿瘤直径≥3 cm与无复发生存降低有关(P分别为0.001及0.009)。同时,单独在BCG组(n=72)中考虑这些因素时,肿瘤T分期和核分级较低的患者无复发生存升高,但差异无统计学意义;图2中,无论是在总体人群还是BCG组中,肿瘤既往复发频率≤1次/年的患者的无复发生存都高于>1次/年的患者(P<0.001)。进一步纳入性别、手术前年龄、吸烟史、肿瘤复发频率,肿瘤T分期、核分级,肿瘤大小、数量及治疗方式行多因素Cox回归分析发现,在总人群及BCG组中,肿瘤既往复发频率>1次/年都是无复发生存的危险因素(P<0.001)。在BCG组中,肿瘤直径≥3 cm亦为患者无复发生存降低的独立危险因素(P=0.046)。治疗方案的比较,使用BCG膀胱灌注治疗为无复发生存的保护因素(P=0.020)。在总人群及BCG组中肿瘤T分期与患者无复发生存无明显相关性。见表4。
表 3. Univariate Cox regression analysis of recurrence-free survival in the NMIBC cohort and BCG subgroup.
NMIBC队列与BCG组无复发生存的单因素Cox回归
| Clinicopathological variable | Total patients (n=93) | BCG group (n=72) | |||
| P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | ||
| CI: Confidence interval. NMIBC, BCG, EPI, recurrent≤1 rec/year, recurrent>1 rec/year, EAU: Note the same as table 1 . | |||||
| Gender (female) | |||||
| Male | 0.538 | 1.466 (0.434−4.958) | 0.718 | 0.786 (0.213−2.905) | |
| Age (≤60 yr.) | |||||
| 61−70 yr. | 0.255 | 0.592 (0.240−1.459) | 0.546 | 0.682 (0.197−2.360) | |
| >70 yr. | 0.310 | 0.512 (0.140−1.865) | 0.647 | 0.682 (0.132−3.514) | |
| Smoking history (no) | |||||
| Yes | 0.071 | 2.226 (0.933−5.314) | 0.473 | 1.514 (0.488−4.695) | |
| Prior recurrence rate (primary) | |||||
| Recurrent≤1 rec/year | 0.062 | 3.282 (0.944−11.412) | 0.033 | 10.827 (1.209−96.991) | |
| Recurrent>1 rec/year | 0.001 | 15.121 (5.297−43.167) | 0.001 | 53.346 (6.522−436.343) | |
| T stage (Ta) | |||||
| T1 | 0.892 | 1.072 (0.394−2.915) | 0.246 | 1.972 (0.626−6.215) | |
| Grade (low grade) | |||||
| High grade | 0.920 | 0.958 (0.415−2.212) | 0.359 | 1.754 (0.528−5.825) | |
| Tumor size (<3 cm) | |||||
| ≥3 cm | 0.009 | 3.758 (1.385−10.195) | 0.034 | 5.170 (1.131−23.632) | |
| Amount of tumors (≤3) | |||||
| >3 | 0.344 | 1.509 (0.644−3.540) | 0.397 | 1.644 (0.520−5.197) | |
| EAU risk stage (intermediate risk) | |||||
| High risk | 0.504 | 1.345 (0.564−3.210) | 0.108 | 3.482 (0.762−15.912) | |
| Treatment group (BCG 19 times) | |||||
| BCG 15 times | 0.956 | 0.969 (0.312−3.005) | 0.975 | 1.018 (0.328−3.158) | |
| Treatment group (BCG) | |||||
| EPI | 0.019 | 3.378 (1.225−9.313) | − | − | |
图 2.
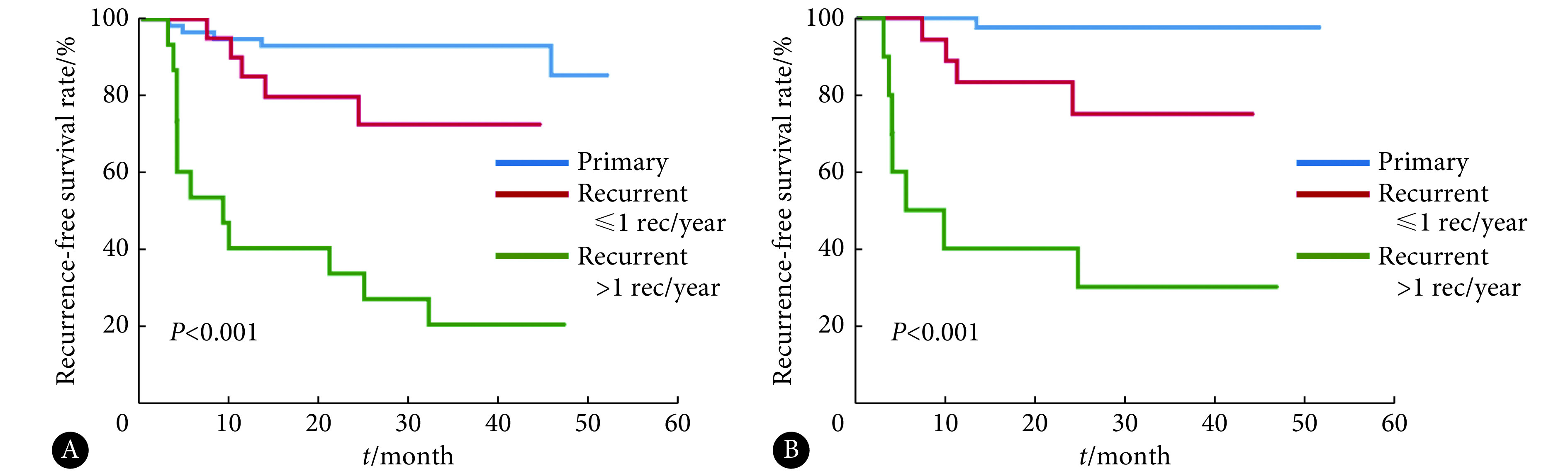
Survival curves of relapse-free survival with different frequencies of previous tumor recurrence
不同既往肿瘤复发频率的无复发生存曲线
A: Total; B: BCG group. BCG: Bacillus Calmette–Guérin.
表 4. Multivariate Cox regression of recurrence-free survival in the NMIBC cohort and BCG group.
NMIBC队列与BCG亚组无复发生存的多因素Cox分析
| Clinicopathological variable | Total patients (n=93) | BCG group (n=72) | |||
| P | Hazard ratio (95%CI) | P | Hazard ratio (95%CI) | ||
| CI: Confidence interval. NMIBC, BCG, EPI, recurrent≤1 rec/year, recurrent>1 rec/year: Note the same as table 1. | |||||
| Gender (female) | |||||
| Male | 0.275 | 0.409 (0.082−2.035) | 0.688 | 0.689 (0.121−4.044) | |
| Age (≤60 yr.) | |||||
| 60−70 yr. | 0.916 | 1.061 (0.353−3.191) | 0.860 | 1.145 (0.254−5.164) | |
| >70 yr. | 0.945 | 1.060 (0.207−5.438) | 0.812 | 0.786 (0.107−5.754) | |
| Smoking history (no) | |||||
| Yes | 0.355 | 1.536 (0.618−3.817) | 0.700 | 1.339 (0.303−5.923) | |
| Prior recurrence rate (primary) | |||||
| Recurrent≤1 rec/year | 0.036 | 3.863 (1.095−13.636) | 0.087 | 6.876 (0.754−62.671) | |
| Recurrent>1 rec/year | 0.001 | 13.347 (4.645−38.356) | 0.001 | 53.506 (6.452−443.698) | |
| T stage (Ta) | |||||
| T1 | 0.648 | 1.337 (0.384−4.649) | 0.484 | 1.570 (0.444−5.548) | |
| Grade (low grade) | |||||
| High grade | 0.230 | 1.787 (0.692−4.613) | 0.256 | 2.099 (0.584−7.547) | |
| Tumor size (<3 cm) | |||||
| ≥3 cm | 0.435 | 1.642 (0.478−5.572) | 0.046 | 4.998 (1.027−24.333) | |
| Amount of tumors (≤3) | |||||
| >3 | 0.402 | 1.520 (0.517−4.051) | 0.777 | 0.793 (0.159−3.956) | |
| Treatment group (BCG) | |||||
| EPI | 0.020 | 2.851 (1.182−6.881) | − | − | |
2.5. EORTC及CUETO预后模型的预测价值
在本研究整体患者中,以及在BCG组患者中,EORTC模型和CUETO模型的c-index均高于单纯根据肿瘤T分期、核分级或EAU风险分层预测(表4)。同时,EORTC模型和CUETO模型在BCG组中的c-index较在整体患者中更高(表5)。
表 5. Predictive value of prognostic models.
预后模型的预测价值
| Prognostic grading
systems |
Total patients (n=93) | BCG group (n=72) | |||
| c-index | 95% CI | c-index | 95% CI | ||
| CI: Confidence interval; c-index: Concordance index. BCG, EAU, CUETO, EORTC: Note the same as table 1. | |||||
| T stage | 0.526 | 0.514−0.538 | 0.592 | 0.575−0.609 | |
| Grade | 0.534 | 0.521−0.547 | 0.590 | 0.575−0.605 | |
| EAU risk stage | 0.572 | 0.561−0.583 | 0.635 | 0.623−0.647 | |
| CUETO scoring model | 0.766 | 0.753−0.779 | 0.812 | 0.800−0.827 | |
| EORTC risk table | 0.741 | 0.729−0.753 | 0.817 | 0.805−0.829 | |
3. 讨论
NMIBC的复发受多种因素的影响,包括肿瘤大小、肿瘤数量、既往复发频率、肿瘤T分期、核分级、有无原位癌等[11],综合考虑这些因素可以判断患者是否属于复发进展的高危人群。在EAU风险分层中,将NMIBC患者分为低、中、高危3组,并分别推荐不同的治疗策略和随访方案[5]。然而,这一危险分层方式并不能准确反映患者预后,有47.8%的患者经历至少一次复发[11]。
自MORALES首次报道卡介苗灌溉治疗膀胱癌以来[13],该方法的疗效已得到广泛证实,成为欧美国家中高危NMIBC的标准治疗方法[6]。据研究表明,NMIBC患者TURBT术后肿瘤复发主要有以下4种机制:① 尿路上皮其他部位肿瘤细胞的种植或播散;② 手术时已经存在的微小肿瘤病灶;③ TURBT时未能完全切除肿瘤;④ 新发的肿瘤。前3种机制代表了当TURBT治疗时,肿瘤细胞或组织已经存在,第4种严格来说为第二原发肿瘤,合称为肿瘤复发。膀胱内化学药物灌注治疗对上述第4种机制产生的第二原发肿瘤预防效果不佳[14],而BCG灌注治疗可诱导非特异性免疫反应,引起Th1细胞介导的免疫应答和抗肿瘤活性,从而降低肿瘤远期进展及复发风险[15]。
与接受化学药物膀胱灌注治疗的患者相比,接受BCG灌注治疗的患者肿瘤复发率更低[7, 16];然而,并发症的发生率较高[17]。由于BCG治疗的不耐受和相关禁忌症,有关在中国患者中使用BCG的研究较少。但随着医疗技术的改进,BCG灌注治疗逐渐被患者所接受。我们首次报道了一项针对中国患者的随机对照试验,比较了BCG和EPI对NMIBC患者的疗效。结果表明,尽管有6例患者对BCG治疗表现出不耐受,但该药物在预防肿瘤复发方面具有明显优势。虽然BCG灌注治疗的并发症发生率较EPI灌注治疗高,但大多为患者可耐受的1~2级并发症。在分析BCG组和EPI组的基线临床病理资料时发现,两组患者肿瘤T分期存在明显差异,EPI组未分配到T1期患者,由于本研究标本容量相对较小,随机化分组时可能出现此差异,但我们认为,这对结果的解读影响不大:本研究的多因素Cox回归在总人群及BCG组中校正并排除了肿瘤T分期对无复发生存的影响,且并未涉及用多因素Cox回归探讨EPI组中肿瘤T分期的影响。多因素Cox回归分析发现,在总人群及BCG组中,肿瘤既往复发频率>大于1次/年(P<0.001)为无复发生存的独立危险因素,但统计结果显示95%CI过宽(分别为4.645~38.356及6.452~443.698),此统计现象考虑由样本量相对较小以及患者个体差异较大造成。我们考虑在后续研究中进一步扩大样本量,提高数据的可信度。
仅6周的BCG诱导灌注治疗对于中高危NMIBC患者的治疗是不够的,目前的证据支持1~3年的维持灌注治疗[18]。最近一项包含1 951例患者的荟萃分析表明,与单纯诱导治疗相比,长期维持治疗没有增加副作用的发生率,但长期维持治疗(1年以上)治疗效果并不优于1年维持治疗[19]。然而,膀胱内维持灌注治疗的最佳次数和频率仍不清楚[5]。因此,我们对两种1年期BCG维持灌注治疗方案进行了比较,发现15次方案和19次方案在肿瘤复发率和副作用率方面无明显差异。
JESUS等[9]研究报道:即使接受BCG灌注治疗,高达32.6%的患者仍会出现肿瘤复发。即使在接受BCG全程维持灌注治疗后,仍有15.7%和26.3%的患者分别出现早期和晚期复发[20]。因此,我们针对NMIBC的预后预测方面做了大量的工作,以更合理地对患者进行危险分层,确定更加适合的个体疾病监测方案。EORTC评分模型基于接受几种不同灌注方案的患者而制定,用于预测NMIBC患者的复发率及预后(复发及进展的内部验证的c-index分别为0.66和0.75)[7]。之后,CUETO评分模型被制定用于预测BCG灌注治疗患者的预后(复发及进展的c-index分别为0.636和0.687)[9]。与肿瘤危险分层相比较,CUETO模型和EORTC模型纳入了肿瘤既往复发情况(一项重要的预后因素)。此外,在本研究中,这两种模型的预测能力明显优于肿瘤危险分层,尤其是在BCG组的患者中,提示CUETO模型和EORTC模型可能更适合用于NMIBC患者的危险分层和预后预测。
本研究存在一些局限性。一方面,样本容量相对较小,无法进行进一步的亚组分析;另一方面,由于随访时间较短,阳性事件的发生数量有限,无法评估肿瘤进展。但由于所有患者均完成了1年以上的随访,并详细记录了并发症相关情况,所以目前的结果可以较好地反映BCG灌注治疗在中国NMIBC人群中的可行性。
Funding Statement
国家自然科学基金项目(No. 81672552)和四川大学华西医院学科卓越发展1·3·5工程项目(No. ZY2016104)资助
Contributor Information
为潇 杨 (Wei-xiao YANG), Email: 498763562@qq.com.
响 李 (Xiang LI), Email: xiangli.87@163.com.
References
- 1.MATTIUZZI C, LIPPI G Currentcancer epidemiology. J Epidemiol Glob Health. 2019;9(4):217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.韩苏军, 张思维, 陈万青, 等 中国膀胱癌发病现状及流行趋势分析. 癌症进展. 2013;11(1):89–95. doi: 10.3969/j.issn.1672-1535.2013.01.021. [DOI] [Google Scholar]
- 3.BURGER M, CATTO J W F, DALBAGNI G, et al Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 4.KAUFMAN D S, SHIPLEY W U, FELDMAN A S Bladder cancer. Lancet. 2009;374(9685):239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 5.BABJUK M, BURGER M, COMPÉRAT E M, et al European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and CarcinomaIn Situ) - 2019 Update . Eur Urol. 2019;76(5):639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 6.CHANG S S, BOORJIAN S A, CHOU R, et al Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 7.SYLVESTER R J, BRAUSI M A, KIRKELS W J, et al Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766–773. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.PAN J, LIU M, ZHOU X Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with non-muscle invasive bladder cancer? An update and cumulative meta-analysis. Front Med. 2014;8(2):241–249. doi: 10.1007/s11684-014-0328-0. [DOI] [PubMed] [Google Scholar]
- 9.FERNANDEZ-GOMEZ J, MADERO R, SOLSONA E, et al Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guérin: the CUETO scoring model. J Urol. 2009;182(5):2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 10.NICHOLLS R J, ZINICOLA R, HABOUBI N Extramural spread of rectal cancer and the AJCC cancer staging manual 8th edition, 2017. Ann Oncol. 2019;30(8):1394–1395. doi: 10.1093/annonc/mdz147. [DOI] [PubMed] [Google Scholar]
- 11.SYLVESTER R J, VAN DER MEIJDEN A P, OOSTERLINCK W, et al Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–475. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 12.HARRELL F E, Jr, LEE K L, MARK D B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.MORALES A, EIDINGER D, BRUCE A W Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–183. doi: 10.1016/S0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 14.AKAZA H, KURTH K H, WILLIAMS R, et al Intravesical chemotherapy and immunotherapy for superficial tumors basic mechanism of action and future direction. Urol Oncol. 1998;4(4/5):121–129. doi: 10.1016/S1078-1439(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 15.SMALDONE M C, GAYED B A, TOMASZEWSKI J J, et al Strategies to enhance the efficacy of intravescical therapy for non-muscle invasive bladder cancer. Minerva Urol Nefrol. 2009;61(2):71–89. [PubMed] [Google Scholar]
- 16.SHANG P F, KWONG J, WANG Z P, et al. Intravesical Bacillus Calmette-Guérin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev, 2011, 5: CD006885[2020-11-12]. https://doi.org/10.1002/14651858.CD006885.pub2
- 17.JÄRVINEN R, KAASINEN E, SANKILA A, et al Long-term efficacy of maintenance bacillus Calmette-Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up . Eur Urol. 2009;56(2):260–265. doi: 10.1016/j.eururo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.SYLVESTER R J Maintenance Bacillus Calmette-Guérin therapy: the search for the optimum treatment schedule continues. Eur Urol. 2015;68(2):263–264. doi: 10.1016/j.eururo.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 19.HUANG Z, LIU H, WANG Y, et al Determining optimal maintenance schedules for adjuvant intravesical Bacillus Calmette-Guérin immunotherapy in non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Curr Med Res Opin. 2017;33(8):1379–1387. doi: 10.1080/03007995.2017.1326889. [DOI] [PubMed] [Google Scholar]
- 20.CAMBIER S, SYLVESTER R J, COLLETTE L, et al EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance Bacillus Calmette-Guérin. Eur Urol. 2016;69(1):60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]


