Abstract
目的
探讨新生大鼠长时间吸入七氟烷是否导致注意缺陷多动障碍(attention-deficit/hyperactivity disorder,ADHD)相关的反常活动和记忆损害。
方法
将出生后5 d(postnatal day 5,P5)的SD大鼠随机分为七氟烷组和对照组,分别采用吸入3%的七氟烷和吸入相同流量纯氧2 h、4 h建立七氟烷吸入麻醉模型及处理对照组。采用旷场实验(open field test,OFT)、5项选择连续反应时间任务实验(5-choice serial reaction time task,5-CSRTT)、场景恐惧(fear-conditioning,FC)实验、水迷宫(morris water maze,MWM)实验评估青少年期大鼠(~P25)和成年大鼠(~P65)的认知、记忆、焦虑及ADHD相关行为等变化。
结果
在OFT中,七氟烷2 h、4 h组大鼠P21、P61活动水平和探索行为指标与对照组的差异均无统计学意义;5-CSRTT实验结果显示,七氟烷组在P25和P65时ADHD相关行为(不成熟反应次数、正确率、遗漏率)与对照组比较,差异也无统计学意义;在FC实验中,与对照组相比,七氟烷4 h暴露组大鼠僵直时间缩短,僵直潜伏期时间延长(P=0.029);MWM实验结果显示,与对照组相比,七氟烷4 h组大鼠训练过程中逃逸潜伏期在第二天和第三天延长(P<0.05),七氟烷组平均游泳速度在P69和P76时差异无统计学意义,七氟烷4 h组的目标象限停留时间缩短(P=0.039),目标象限移动距离百分比降低(P=0.048)。
结论
新生大鼠七氟烷吸入麻醉4 h后,可致记忆损害但并未增加发生ADHD相关反常活动的风险。
Keywords: 七氟烷, 注意缺陷多动障碍, 认知, 记忆
Abstract
Objective
To investigate whether long-term exposure to inhaled sevoflurane, a volatile anesthetic, causes abnormal activities and memory impairment related to attention-deficit/hyperactivity disorder (ADHD) in neonatal rats.
Methods
On postnatal day 5 (P5), Sprague-Dawley rats were randomly assigned to two sevoflurane subgroups and two control subgroups and underwent experimental intervention. The two sevoflurane (SEVO) subgroups were exposed to 3% sevoflurane for 2 h and 4 h respectively, while the two control subgroups were given pure oxygen for the same amount and duration. Behavioral tests, including open-field test (OFT), five-choice serial reaction time task (5-CSRTT), fear-conditioning (FC) and Morris water maze (MWM), were applied to evaluate changes in cognition, memory, anxiety and ADHD-related behavioral changes in the rats in adolescence (-P25) and in adulthood (-P65).
Results
In OFT, the SEVO 2 h and SEVO 4 h subgroups displayed activity level and exploratory behaviors similar to those of the control subgroups on P21 and P61, with no statistically significant difference identified in the data. 5-CSRTT results on P25 and P65 indicated no statistically significant difference between the SEVO subgroups and the control subgroups in regard to ADHD-related abnormal behaviors, including number of immature reaction, rate of correct response and omission rate. In the FC experiment, SEVO 4 h group had a shorter freezing period and longer period of freezing latency (P=0.029) in comparison to the control groups. The results of the MWM test showed that the escape latency period of rats in the SEVO 4 h group was significantly prolonged on the second day and the third day, compared to the control groups (P<0.05). The average swimming speed of SEVO groups did no exhibit any statistically significant difference on P69 or P76. The time the SEVO 4 h group spent in the target quadrant was significantly shorter than that of the control group (P=0.039) and percentage of distance traveled in the target quadrant was significantly reduced compared to that the control group (P=0.048).
Conclusion
The findings suggest that four hours of inhaled sevoflurane exposure in neonate rats may cause memory impairment, but does no increase risks for ADHD-related abnormal activities.
Keywords: Sevoflurane, ADHD, Cognition, Memory
尽管吸入麻醉在临床上的应用已超过170年,但其作用机制却仍未完全明确[1-3]。吸入麻醉药后在产生催眠、意识丧失、记忆遗忘、肌肉松弛作用的同时,也带来了相应的副反应,常见的副反应如呼吸、循环抑制等,甚至还可能产生神经系统毒性[1, 4-5]。目前已有动物实验指出,新生小鼠使用吸入麻醉药4~6 h后,可致剂量依赖性的神经凋亡[6-10]和出现记忆认知障碍[11-14]。多数动物模型研究[15-18]发现,发生认知功能障碍的全身麻醉时间大于4 h。而大部分儿科手术时间小于3 h[19],这是否对儿童而言全身麻醉比较安全?尽管现在的临床观点认为[20],在生命早期接受常规儿科手术麻醉其带来的神经毒性可以忽略不计,但仍需警惕。无论如何,动物实验的结果与人类临床研究结果不应盲目相互套用,需开展进一步的高质量研究。一项对59例婴儿行心脏手术后的回顾性队列研究发现[21],新生儿吸入麻醉时间〔(4.4±3.1) h〕与生后12月所测试的认知功能障碍存在负相关;但另外一项针对95例婴儿接受心脏手术后的队列研究结果[22]并未发现上述情况。因此,目前新生儿使用吸入麻醉药后将来是否会产生心理认知障碍,尚无定论。
注意缺陷多动障碍(attention-deficit/hyperactivity disorder,ADHD)即多动症,是一类以注意力缺陷和行为冲动为特征的儿童期常见的心理障碍[23-24],幼年发生后,症状可一直持续到成年。多个回顾性队列研究揭示了婴儿期接受全身麻醉与多动症之间的关系[25-28]。然而ADHD是一种异质性疾病,其致病因素尚未完全清楚[23-24]。随着对ADHD研究的逐渐深入,许多学者认为ADHD的致病因素是多方面原因综合导致,如遗传、化学、毒物及环境因素等[23-24]。因此,全身麻醉是否影响ADHD的发生目前存在争议[29-31]。七氟烷是临床上使用最为普遍的吸入麻醉药之一,尤其在小儿麻醉中使用普遍。动物实验表明新生大鼠暴露于七氟烷可引发神经退行性变,如认知及记忆障碍等[32-34]。但也有研究发现,新生大鼠无论是单次还是多次暴露于七氟烷都不会在成年表现出任何注意障碍[35]。因此,本研究建立新生大鼠长时间七氟烷吸入麻醉模型,通过对青少年期大鼠和成年大鼠进行五5项选择连续反应时间任务实验、旷场实验、场景恐惧实验、水迷宫等实验,评估早期长时间七氟烷吸入麻醉对青少年期大鼠和成年大鼠的认知、记忆、焦虑及ADHD相关行为等影响,从而间接探讨人类婴儿期接受七氟烷吸入麻醉后,是否可导致青少年期及成年期ADHD相关的反常活动和记忆损害。
1. 材料与方法
1.1. 实验动物及分组
健康Sprague-Dawley孕鼠4只,鼠龄9周,体质量200~290 g,购自四川大学实验动物中心。孕鼠分笼饲养。以孕鼠子代9~11只为一组,每一组为一笼,在恒温动物房饲养,温度维持在(22±2) ℃,湿度维持40%~70%,予以每天12 h的灯光(8∶00-20∶00),无强光及强声刺激,可自由摄食和饮水。所有实验均遵循《医学实验动物管理实施细则》(中国卫生和计划生育委员会)及《实验动物使用和管理指南》(美国国立卫生研究院)进行。
本实验将源于同一母鼠的子代随机分组为对照(control, CTL)组和七氟烷(sevoflurane,SEVO)组,SEVO组和CTL组再根据暴露于七氟烷和相同流量纯氧的时间不同(2 h和4 h)分别分为两个亚组:SEVO 2 h组(n=9);SEVO 4 h组(n=11);CTL 2 h组(n=9);CTL 4 h(n=11)。
1.2. 新生大鼠七氟烷吸入麻醉模型的建立和对照组大鼠的处理
子代大鼠生后5 d(postnatal,P5),七氟烷组幼鼠接受3%(容积百分比)七氟烷(Abbott,中国上海)最低肺泡有效浓度(minimum alveolar concentration,MAC)≈1.2 MAC[15]吸入麻醉建立模型,同时将对照组幼鼠置于相同流量(≈2 L/min)纯氧中持续吸入相同的时间。大鼠放入透明的气密塑料室(25 cm×15 cm×12 cm)中,在塑料室下放置加热垫,保持温度在36~38 ℃之间,同时在塑料室中放置钠石灰,以维持室内CO2分压(PCO2)<5 mmHg(1 mmHg=0.133 Pka)。七氟烷浓度由RGM监测器(Datex-Ohmeda,美国)监测。为确保长期暴露于七氟烷中不会引起缺氧,在正式实验前,将P5的子代大鼠以3%的七氟烷麻醉2 h和4 h(n=10),对照组的P5幼鼠置于相同流量(~2 L/min)纯氧中持续吸入相同的时间(n=10)后采血进行血气分析。血气分析的具体方法为,七氟烷组和对照组吸入时间结束后,以安乐死方式立即从主动脉中采集血样,通过血气分析仪(Radiometer ABL800 FLEX,美国克利夫兰)测量血液样品的pH、PCO2和O2分压(PO2)值。所有暴露于七氟烷或氧气的大鼠,血液样本的生理参数均保持在正常范围内:CTL组:pH=7.33±0.01,PCO2=(43±4) mmHg和PO2=(96±9) mmHg; SEVO 2 h组pH=7.12±0.03,PCO2=(44±8) mmHg和PO2=(92±6) mmHg;SEVO 4 h组pH=7.05±0.02,PCO2=(50±6) mmHg和PO2=(90±18) mmHg。两组上述血气分析参数差异无统计学意义。在正式实验开始、结束后均无实验动物死亡。
建立七氟烷吸入麻醉模型后在大鼠不同时期进行行为学实验测试。不同行为学实验测试时间见图1。所有行为学实验的观察指标均由实验室另一位不知晓分组情况的研究人员完成。所有行为学测试均在8∶00至14∶00之间进行。
图 1.

The experiment protocol
实验方案
The rats in the SEVO subgroups were anesthetized with 3% sevoflurane at P5 for durations of 2 and 4-hour, respectively. Multiple behavioral tests were performed in the same rats at different postnatal ages. The first test of open-field (purple bar) was performed on P21 and repeated on P61. Fear-conditioning (blue bar) training was conducted on P26 and tested on P27. The training of five-choice serial reaction time task (5-CSRTT) (green bar) began on P20 and lasted until P24 and the test was conducted on P25 for the first time. Then the rats were tested again on P65. Before the second 5-CSRTT, reinforced training was conducted from P60 to P64. For Morris water maze (yellow bar), the training was done from P66 to P68 and the test conducted on P69 for the first time and then again on P76 to evaluate long-term memory. *The day for test; P: Postnatal.
1.3. 旷场实验
采用旷场实验分别评估子代大鼠P21(处于青春期)和P61(处于成年期)的活动水平和探索行为。将大鼠放入塑料方盒(120 cm×120 cm×120 cm)中,中心区域定义为中心100 cm×100 cm区域。测试前将大鼠放进实验环境1 h以适应周围环境。总测试时间为15 min,并通过视频跟踪系统(Smart 2.5,Panlab,DL Naturgene Life Science,Inc.,北京)测定移动距离和平均移动速度。分析停留在中心区域的时间百分比和在中心区域移动距离百分比,每次实验结束后,将大鼠放回笼子里,以体积分数75%乙醇清洗中心区域。
1.4. 5项选择连续反应时间任务实验(5-choice serial reaction time task,5-CSRTT)
5-CSRTT用于衡量动物等待视觉刺激并保持冲动反应的能力,是测试实验动物注意力和多动行为的经典行为学模型[31]。分别于子代大鼠P20~P24进行5-CSRTT训练,并在P25第一次测试,P60~P64进行强化训练,P65第二次测试。在实验测试前饲予大鼠50%~60%日常晚上的食物。大鼠分别放在盒子中进行训练,自动记录正确鼻触行为与食物奖励的条件反射。检测指标包括错误反应次数、不成熟反应次数、正确反应次数(正确率)、无反应次数(遗漏率)。大鼠鼻触正确的信号孔后,就可以获得食物奖励,记为一次正确反应。如果信号灯亮期间,大鼠没有鼻触任何信号孔,则视为一次遗漏。如果鼻触了错误的信号孔,则记为一次错误反应。如果大鼠间歇期鼻触信号孔,则记为一次不成熟反应(反映大鼠的冲动行为)。在训练中,提示灯的持续时间为8 s,随后允许大鼠存在响应时间为12 s。在测试中,提示灯的持续时间为5 s,然后是8 s的响应时间。正确率(%)=正确反应次数/(正确反应次数+错误反应次数+不成熟反应次数+无反应次数);遗漏率(%)=无反应次数/(正确反应次数+错误反应次数+不成熟反应次数+无反应次数)[36]。
1.5. 场景恐惧试验
场景恐惧试验于P26训练,P27开始测试。该测试在带有不锈钢网格地板的黑色腔室中进行,该腔室能对测试老鼠造成电击。在对大鼠进行训练时,将其置于一个提示噪声(80 dB,持续3 s)(条件刺激)与足部的有害电击刺激(电击,0.8 mA,持续2 s)(非条件刺激)环境,使实验动物在听觉线索与电击之间、电击与环境之间学会关联记忆。在发出条件提示音后,随机电击5至10 s,并重复学习过程10次,使其产生情景恐惧记忆,通过表现出僵直行为来应对恐惧环境。第二天(P27),在没有任何其他刺激的情况下(或者其他训练内容),在有提示音的情况下记录僵直时间和僵直潜伏期。
1.6. 水迷宫
采用水迷宫实验检测大鼠的空间学习和记忆力。从P66~P68开始训练,P76开始测试。迷宫水池的直径为120 cm,分为四个象限。水池中装有不透明的水,将平台(直径10 cm)放在水池的一个象限中,平台的顶部在水面以下1 cm处。具有平台的象限称为目标象限。在训练过程中,将老鼠在四个象限之一面向池壁轻轻释放。对于每个实验,老鼠有90 s的时间来找到平台。如果大鼠在90 s的截止时间内未找到平台,则将其轻轻地引导至平台,并在其上停留30 s。每天训练大鼠重复3次实验,记录逃逸潜伏期(找到平台的时间)。在测试过程中,将平台移开,将大鼠放置在池中,而不是从目标象限中移出。测试持续90 s。记录测试大鼠的目标象限停留时间、目标象限移动距离百分比和平均游泳速度。通过视频跟踪系统(Smart 2.5,Panlab,DL Naturgene Life Science,Inc.,北京)分析大鼠移动轨迹。
1.7. 统计学方法
采用Prism 8(Graph-Pad软件)进行统计分析。所有符合正态分布的计量资料以
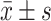 表示。计数资料以百分位数表示。样本量的计算是以场景恐惧实验(3%SEVO 4 h组)的僵直时间差值等于14 s有实验意义,在α=0.05、β=0.10时,计算得出最小样本量为7.28。由于受限于每只孕鼠的幼崽数,并考虑可能潜在的幼崽死亡风险,故本研究选择了9至11的样本量。计量资料的正态性检验采用Shapiro-Wilk检验,组间比较采用两独立样本t检验。计数资料比较采用非参数检验Kolmogorov-Smirnor检验。P<0.05为差异有统计学意义。
表示。计数资料以百分位数表示。样本量的计算是以场景恐惧实验(3%SEVO 4 h组)的僵直时间差值等于14 s有实验意义,在α=0.05、β=0.10时,计算得出最小样本量为7.28。由于受限于每只孕鼠的幼崽数,并考虑可能潜在的幼崽死亡风险,故本研究选择了9至11的样本量。计量资料的正态性检验采用Shapiro-Wilk检验,组间比较采用两独立样本t检验。计数资料比较采用非参数检验Kolmogorov-Smirnor检验。P<0.05为差异有统计学意义。
2. 结果
2.1. 新生大鼠长时间七氟烷吸入麻醉后对青春期、成年期活动水平和探索行为的影响
由图2可见,在P21,与对照组比较,七氟烷组大鼠所有行为,包括总移动距离、平均移动速度、停留在中心区域的时间百分比和在中心区域移动距离百分比差异均无统计学意义。在P61,七氟烷组大鼠所有行为项目指标与对照组比较差异仍无统计学意义。上述结果表明,出生后长时间七氟烷吸入麻醉不会改变青春期和成年期大鼠的活动水平功能以及产生焦虑行为。
图 2.

The results of open-field test on P21 (A) and P61 (B) (nSEVO=9, nCTL=11)
大鼠P21(A)和P61(B)的旷场实验结果(nSEVO=9,nCTL=11)
Aa, Ba: The representative traces of rats in the open-field test; Ab, Bb: Total distance traveled of rats during the test; Ac, Bc: Mean velocity of rats during the open-field test; Ad, Bd: The time of rats stayed in the center part during the test; Ae, Be: The percentage of distance traveled of rats in the center part of the open-field during the test. CTL: Control; Sevo: Sevoflurane.
2.2. 新生大鼠长时间七氟烷吸入麻醉后对青春期、成年期ADHD相关行为的影响
由图3可见,SEVO 2 h、4 h组在P25和P65时ADHD相关行为(不成熟反应次数、正确率、遗漏率)与对照组比较,差异均无统计学意义。结果表明,七氟烷吸入麻醉2 h、4 h,不会导致青春期及成年期大鼠多动症相关行为。
图 3.

The results of 5-CSRTT (nSEVO=9, nCTL=11)
5项选择连续反应时间任务实验结果(nSEVO=9,nCTL=11)
Single long-time exposure to sevoflurane did not change ADHD relevant behaviors of rats at P25 and P65, respectively. A: The schematic diagram of 5-CSRTT. Premature activity was defined as the nose-poke behavior before cue light. After cue light, the behaviors of rats were divided into correct (nose-poke to the hole with cue light), incorrect (nose-poke to the other holes without cue light) and omission (no response to the cue light); B: Numbers of premature activity of rats at adolescent age (P25); C: Percentage of correct responses of rats at adolescent age (P25); D: Percentage of omission responses of rats at adolescent age (P25); E: Numbers of premature activity of rats at adult age (P65); F: Percentage of correct responses of rats at adult age (P65); G: Percentage of omission responses of rats at adult age (P65).
2.3. 新生大鼠长时间七氟烷吸入麻醉后对青春期大鼠记忆力的影响
由图4可见。与对照组相比,SEVO2 h组大鼠青春期时僵直时间和僵直潜伏期差异无统计学意义。而SEVO 4 h组大鼠与对照组相比,僵直时间缩短,僵直潜伏期延长〔(81.4±16.6) s vs.(35.2±9.6) s〕,差异有统计学意义(P=0.029),提示场景恐惧记忆明显受损。
图 4.
The results of fear-conditioning test at P27 (nSEVO=9, nCTL=11)
P27场景恐惧实验结果(nSEVO=9,nCTL=11)
A: Freezing time of rats was tested on P27; B: Freezing latency time of rats was tested on P27. * P<0.05.
2.4. 新生大鼠长时间七氟烷吸入麻醉后对大鼠成年后空间学习和记忆力的影响
通过P66~P68训练后,P69进行Morris水迷宫测试(短期记忆)和P76(长期记忆)测试大鼠的空间学习和记忆力。在3 d的训练过程中,与对照组相比,SEVO 4 h组大鼠的逃避潜伏期在第2天和第3天延长( P <0.05, 图5A)。SEVO组和CTL组大鼠在水迷宫中的活动轨迹见图5B、5F。P69的短期记忆测试,与对照组相比,SEVO 2 h组大鼠各项指标差异无统计学意义(图5D、5E)。而SEVO 4 h组大鼠的目标象限停留时间短于对照组〔(26.3±3.3) s vs.(35.0±1.9) s, P=0.039,图5D〕,目标象限移动距离百分比低于对照组〔(25.7±4.1)% vs. (33.1±2.7)%, P=0.048,图5E〕。然而,P76的长期记忆测试,七氟烷组和对照组各项指标差异无统计学意义(图5H、5I)。七氟烷组和对照组之间的平均游泳速度差异亦无统计学意义(图5C、5G)。
图 5.

The results of Morris water maze (nSEVO=9, nCTL=11)
水迷宫实验结果(nSEVO=9,nCTL=11)
A: The learning curves of training from P66 to P68 (*P<0.05, vs. CTL group); B: The representative traces of rats in water maze on P69; C: The mean swimming speed of rats during the test at P69; D: The time of rats stayed in the target quadrant during the test on P69 (*P<0.05); E: The percentage of traveled distance in the target quadrant during the test on P69 (*P<0.05); F: The representative traces of rats in water maze on P76; G: The mean swimming speed of rats during the test on P76; H: The time of rats stayed in the target quadrant during the test on P76, as long-term memory after training; I: The percentage of traveled distance in the target quadrant during the test on P76. ★The target quadrant.
3. 讨论
吸入麻醉药在临床上广泛使用,其中七氟烷在小儿麻醉中的应用尤甚。有研究指出[32-34],新生儿长时间暴露于吸入麻醉气体后可致认知障碍、记忆缺陷。由吸入麻醉药所致的神经凋亡、退行性变会在长时间、重复多次暴露后愈加明显,症状可持续青少年期甚至到成年后[37-41]。本研究采用了长时间七氟烷吸入麻醉建立大鼠模型,并对大鼠序贯进行不同行为学实验,将不同的实验参数进行综合分析,以期更加客观描述啮齿类动物的行为学变化与吸入麻醉之间的关系。
本研究结果发现,新生大鼠七氟烷吸入麻醉后,场景恐惧实验、水迷宫实验结果均提示大鼠青少年期和成年期发生学习记忆损害,但是5-CSRTT实验结果显示七氟烷组大鼠在2 h与4 h吸入麻醉后,其不成熟反应、正确率、遗漏率无明显变化(P>0.05),这提示大鼠生后长时间七氟烷吸入麻醉后并未增加发生注意缺陷多动障碍的风险。
旷场实验主要用于观察大鼠自主运动能力、自发性探索行为、焦虑行为及抑郁行为等[42-43],广泛应用于评价围术期大/小鼠认知功能障碍[44- 45]。JI等[45]研究发现,成年大鼠暴露于3%七氟烷4 h后的1、4、12周,旷场实验结果显示其大鼠的活动水平和探索行为与对照组相比,差异无统计学意义,该研究认为七氟烷吸入暴露后不会损害成年大鼠的学习与记忆能力。本实验采用的是幼鼠暴露于3%七氟烷2 h、4 h,旷场实验分别评估大鼠P21(处于青春期)和P61(处于成年期)的活动水平和探索行为,结果显示,与对照组相比,七氟烷组大鼠所有行为,包括总移动距离、平均移动速度、停留在中心区域的时间百分比和在中心区域移动距离百分比差异均无统计学意义。这些结果表明,新生鼠长时间(4 h)七氟烷吸入麻醉不会改变青春期和成年期大鼠的活动水平以及探索行为。
场景恐惧实验为测试大鼠海马记忆能力的方法之一[46]。僵直时间反应及僵直潜伏期表明了大鼠再次处于同样环境时对习得性恐惧记忆引起的行为学改变[47]。在本实验中,大鼠吸入3%七氟烷4 h后,于P27测试时,与对照组相比,大鼠僵直时间缩短,僵直潜伏期延长,且差异有统计学意义(P<0.05),表明大鼠的场景恐惧记忆受损。同样,GUI等[48]研究发现,出生后7 d大鼠接受3%七氟烷吸入2 h后,于第31天进行场景恐惧实验,与对照组相比,七氟烷组的僵直行为百分比明显降低,证实七氟烷吸入暴露后引起新生大鼠恐惧记忆减退。
水迷宫主要用于研究啮齿动物空间学习能力的行为[49]。BI等[15]发现对生后7 d大鼠进行3%七氟烷吸入4 h后,其逃逸潜伏期延长,跨平台次数降低,这些行为学指标均提示了大鼠的学习记忆能力损害。本研究发现,成年大鼠(P60)水迷宫所测试的学习和记忆功能损害。尽管P76进行水迷宫测试时并未发现差异有统计学意义。我们推测,P69和P76行为学实验的结果差异可能与短期记忆与长期记忆不同有关,其中涉及到各个脑区和分子机制等[50]。另外,由于大鼠的游泳速度和活动能力并未表现出异常,所以这些提示学习和记忆功能损害的指标,如逃逸潜伏期、目标象限停留时间等发生改变,可能并不是由躯体运动功能障碍所致。
5-CSRTT实验主要检验啮齿动物模型发生多动症时的主要临床特点,如注意力不集中和过早活动等[36]。在本次研究中,尽管我们发现,新生大鼠(P5)七氟烷吸入麻醉4 h后,在P27和P69时行为学实验中表现出学习记忆缺陷,但是5-CSRTT的主要结局并未表现出明显差异。因此,我们推测多动症相关行为的发生,可能不完全源于学习和记忆损害。其他干扰因素,如手术应激、自身的基础疾病都可能参与该病的发生、发展。
本研究仍存在一些局限性,首先,在相同的麻醉时间下,反复吸入麻醉药可能比单次持续吸入麻醉具有更强的神经毒性[51]。因此,重复吸入七氟烷麻醉可能比单次吸入更具有意义,但我们在本次研究中在研究设计阶段未予考虑,未来需要进一步的动物实验以验证其危险性;其次,由于每只大鼠都进行了多次行为学实验,尽管将不同测试参数综合分析更能客观描述啮齿类动物的行为学变化,但是5-CSRTT结果可能会受到其它行为学实验的干扰,影响实验结果;另外,由于啮齿动物寿命短,中枢神经系统的发育速度远快于人类,因此很难证明啮齿动物吸入麻醉4 h是否等效于人类的吸入麻醉时间;最后,尽管有动物实验研究[52]表明,新生大鼠七氟烷吸入麻醉发生的行为学改变存在性别差异,但是本实验是将同窝子代随机分组后进行实验干预,因此暂未考虑大鼠性别差异对实验结果的影响,所以在未来我们会进一步补充动物实验加以证实。
综上,基于以上讨论,以及本实验研究结果,我们发现出生后5 d大鼠吸入七氟烷麻醉达4 h可致记忆损害,但并未增加注意缺陷多动障碍风险。我们采用多个行为学实验对大鼠的学习记忆能力进行综合分析,结果具有一定的客观性和创新性。在未来的研究中,我们会补充组织病理学和电生理等方面的探究与认知记忆功能相关的脑区,如海马、基底核等,明确七氟烷暴露后,这些脑区的突触结构及功能,以及相关神经损伤相关蛋白分子水平的变化。
Funding Statement
中国博士后科学基金面上基金(No. 2019M663507)、四川大学专职博后研发基金(No. 2020SCU12033)、四川大学华西医院专职博士后研发基金(No. 19HXBH064)、四川省科技厅应用基础研究项目(No. 2020YJ0052)和四川省健委科研课题(No. 20PJ012)资助
Contributor Information
明凯 陈 (Ming-kai CHEN), Email: chenmingkai_scu@foxmail.com.
羽 李 (Yu LI), Email: liyu1936@163.com.
References
- 1.HEMMINGS H C, AKABAS M H, GOLDSTEIN P A, et al Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26(10):503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 2.FRANKS N P Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(Suppl 1):S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FRANKS N P General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 4.JEVTOVIC-TODOROVIC V, BRAMBRICK A General anesthesia and young brain: what is new? J Neurosurg Anesthesiol. 2018;30(3):217–222. doi: 10.1097/ANA.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PEROUANSKY M, HEMMINGS H C Neurotoxicity of general anesthetics: cause for concern? Anesthesiology. 2009;111(6):1365–1371. doi: 10.1097/ALN.0b013e3181bf1d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LIU Y F, LIU C L, ZENG M T, et al Influence of sevoflurane exposure on mitogen-activated protein kinases and Akt/GSK-3β/CRMP-2 signaling pathways in the developing rat brain. Exp Ther Med. 2018;15(2):2066–2073. doi: 10.3892/etm.2017.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HAN X, LIU C L, ZHANG K, et al Calpain and JNK pathways participate in isoflurane - induced nucleus translocation of apoptosis-inducing factor in the brain of neonatal rats. Toxicol Lett. 2018;285:60–73. doi: 10.1016/j.toxlet.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 8.LIU B, XIA J M, CHEN Y L, et al Sevoflurane-induced endoplasmic reticulum stress contributes to neuroapoptosis and BACE-1 expression in the developing brain: the role of eIF2α. Neurotox Res. 2017;31(2):218–229. doi: 10.1007/s12640-016-9671-z. [DOI] [PubMed] [Google Scholar]
- 9.DENG M, HOFACER R D, JIANG C, et al Brain regional vulnerability to anaesthesia-induced neuroapoptosis shifts with age at exposure and extends into adulthood for some regions. Br J Anaesth. 2014;113(3):443–451. doi: 10.1093/bja/aet469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ISTAPHANOUS G K, HOWARD J, NAN X, et al Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114(3):578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 11.KODAMA M, SATOH Y, OTSUBO Y, et al Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115(5):979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 12.ZHOU X, LI W D, CHEN X H, et al Dose-dependent effects of sevoflurane exposure during early lifetime on apoptosis in hippocampus and neurocognitive outcomes in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol. 2016;8(3):111–119. [PMC free article] [PubMed] [Google Scholar]
- 13.XIAO H Y, LIU B, CHEN Y L, et al Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int J Dev Neurosci. 2016;48:38–49. doi: 10.1016/j.ijdevneu.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 14.CHUNG W, PARK S, HONG J, et al Sevoflurane exposure during the neonatal period induces long-term memory impairment but not autism-like behaviors. Paediatr Anaesth. 2015;25(10):1033–1045. doi: 10.1111/pan.12694. [DOI] [PubMed] [Google Scholar]
- 15.BI C J, CAI Q P, SHAN Y Y, et al Sevoflurane induces neurotoxicity in the developing rat hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1 pathway. Biomed Pharmacother. 2018;108:1469–1476. doi: 10.1016/j.biopha.2018.09.111. [DOI] [PubMed] [Google Scholar]
- 16.LIAO Z X, LI J H, MIAO L P, et al Inhibition of RhoA activity does not rescue synaptic development abnormalities and long-term cognitive impairment after sevoflurane exposure. Neurochem Res. 2021;46(3):468–481. doi: 10.1007/s11064-020-03180-2. [DOI] [PubMed] [Google Scholar]
- 17.XU L L, SHEN J J, DAI S B, et al Tetramethylpyrazine attenuated sevoflurane-induced neurotoxicity by enhancing autophagy through GPR50/CREB pathway in SH-SY5Y cells. Am J Chin Med. 2020;48(4):945–966. doi: 10.1142/S0192415X20500457. [DOI] [PubMed] [Google Scholar]
- 18.LIU B, OU G Y, CHEN Y L, et al Inhibition of protein tyrosine phosphatase 1B protects against sevoflurane-induced neurotoxicity mediated by ER stress in developing brain. Brain Res Bull. 2019;146:28–39. doi: 10.1016/j.brainresbull.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 19.BARTELS D D, MCCANN M E, DAVIDSON A J, et al Estimating pediatric general anesthesia exposure: quantifying duration and risk. Paediatr Anaesth. 2018;28(6):520–527. doi: 10.1111/pan.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MCCANN M E, SORIANO S G. Does general anesthesia affect neurodevelopment in infants and children? BMJ, 2019, 367: 16459[2020-08-05]. https://doi.org/10.1136/bmj.l6459.
- 21.ANDROPOULOS D B, AHMAD H B, HAQ T, et al The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24(3):266–274. doi: 10.1111/pan.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GUERRA G G, ROBERTSON C M T, ALTON G Y, et al Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth. 2011;21(9):932–941. doi: 10.1111/j.1460-9592.2011.03581.x. [DOI] [PubMed] [Google Scholar]
- 23.FARAONE S V, ASHERSON P, BANASCHEWSKI T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers, 2015, 1: 15020[2020-08-05]. https://doi.org/10.1038/nrdp.2015.20.
- 24.CASTELLANOS F X, TANNOCK R Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 25.ING C, SUN M, OLFSON M, et al Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis. Anesth Analg. 2017;125(6):1988–1998. doi: 10.1213/ANE.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HU D, FLICK R P, ZACCARIELLO M J, et al Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127(2):227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TSAI C J, LEE C T C, LIANG S H Y, et al Risk of ADHD after multiple exposures to general anesthesia: a nationwide retrospective cohort study. J Atten Disord. 2018;22(3):229–239. doi: 10.1177/1087054715587094. [DOI] [PubMed] [Google Scholar]
- 28.SPRUNG J, FLICK R P, KATUSIC S K, et al Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87(2):120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EFRON D, VUTSKITS L, DAVIDSON A J Can we really suggest that anesthesia might cause attention-deficit/hyperactivity disorder? Anesthesiology. 2017;127(2):209–211. doi: 10.1097/ALN.0000000000001736. [DOI] [PubMed] [Google Scholar]
- 30.SPRUNG J, SCHROEDER D R, HANSEN T G, et al Is anesthetic exposure in early life associated with ADHD? Paediatr Anaesth. 2014;24(12):1305–1306. doi: 10.1111/pan.12536. [DOI] [PubMed] [Google Scholar]
- 31.KO W R, LIAW Y P, HUANG J Y, et al Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: a nationwide, retrospective matched-cohort study. Paediatr Anaesth. 2014;24(7):741–748. doi: 10.1111/pan.12371. [DOI] [PubMed] [Google Scholar]
- 32.JI M H, WANG X M, SUN X R, et al Environmental enrichment ameliorates neonatal sevoflurane exposure-induced cognitive and synaptic plasticity impairments. J Mol Neurosci. 2015;57(3):358–365. doi: 10.1007/s12031-015-0627-1. [DOI] [PubMed] [Google Scholar]
- 33.LIU G H, ZHU T G, ZHANG A H, et al Heightened stress response and cognitive impairment after repeated neonatal sevoflurane exposures might be linked to excessive GABAAR-mediated depolarization. J Anesth. 2016;30(5):834–841. doi: 10.1007/s00540-016-2215-0. [DOI] [PubMed] [Google Scholar]
- 34.XIA Y M, XU H, JIA C F, et al Tanshinone ⅡA attenuates sevoflurane neurotoxicity in neonatal mice. Anesth Analg. 2017;124(4):1244–1252. doi: 10.1213/ANE.0000000000001942. [DOI] [PubMed] [Google Scholar]
- 35.MURPHY K L, MCGAUGHY J, CROXSON P L, et al Exposure to sevoflurane anesthesia during development does not impair aspects of attention during adulthood in rats. Neurotoxicol Teratol. 2017;60:87–94. doi: 10.1016/j.ntt.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 36.LI Y, YIN A Q, SUN X, et al Deficiency of tumor suppressor NDRG2 leads to attention deficit and hyperactive behavior. J Clin Invest. 2017;127(12):4270–4284. doi: 10.1172/JCI94455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.JEVTOVIC-TODOROVIC V Developmental synaptogenesis and general anesthesia: a kiss of death? Curr Pharm Des. 2012;18(38):6225–6231. doi: 10.2174/138161212803832380. [DOI] [PubMed] [Google Scholar]
- 38.VLISIDES P, XIE Z Neurotoxicity of general anesthetics: an update. Curr Pharm Des. 2012;18(38):6232–6240. doi: 10.2174/138161212803832344. [DOI] [PubMed] [Google Scholar]
- 39.LIN E P, LEE J R, LEE C S, et al Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol Teratol. 2017;60:117–128. doi: 10.1016/j.ntt.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 40.VUTSKITS L, XIE Z Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 41.JEVTOVIC-TODOROVIC V General anesthetics and neurotoxicity: how much do we know? Anesthesiol Clin. 2016;34(3):439–451. doi: 10.1016/j.anclin.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.BAHI A, SCHWED J S, WALTER M, et al Anxiolytic and antidepressant-like activities of the novel and potent non-imidazole histamine H₃ receptor antagonist ST-1283. Drug Des Devel Ther. 2014;8:627–637. doi: 10.2147/DDDT.S63088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.HEDAYATI MOGHADAM M, REZAEE S A R, HOSSEINI M, et al HTLV-1 infection-induced motor dysfunction, memory impairment, depression, and brain tissues oxidative damage in female BALB/c mice. Life Sci. 2018;212:9–19. doi: 10.1016/j.lfs.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 44.VISHNOI S, RAISUDDIN S, PARVEZ S Modulatory effects of an NMDAR partial agonist in MK-801-induced memory impairment. Neuroscience. 2015;311:22–33. doi: 10.1016/j.neuroscience.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 45.JI M H, WANG Z Y, SUN X R, et al Repeated neonatal sevoflurane exposure-induced developmental delays of parvalbumin interneurons and cognitive impairments are reversed by environmental enrichment. Mol Neurobiol. 2017;54(5):3759–3770. doi: 10.1007/s12035-016-9943-x. [DOI] [PubMed] [Google Scholar]
- 46.SANDERS M J, WILTGEN B J, FANSELOW M S The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463(1/2/3):217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- 47.TERRANDO N, MONACO C, MA D, et al Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.GUI L L, LEI X, ZUO Z Y Decrease of glial cell-derived neurotrophic factor contributes to anesthesia- and surgery-induced learning and memory dysfunction in neonatal rats. J Mol Med (Berl) 2017;95(4):369–379. doi: 10.1007/s00109-017-1521-9. [DOI] [PubMed] [Google Scholar]
- 49.VORHEES C V, WILLIAMS M T Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NORRIS D Short-term memory and long-term memory are still different. Psychol Bull. 2017;143(9):992–1009. doi: 10.1037/bul0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GUO S B, LIU L D, WANG C, et al Repeated exposure to sevoflurane impairs the learning and memory of older male rats. Life Sci. 2018;192:75–83. doi: 10.1016/j.lfs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 52.JU X S, CUI J C, LEE Y L, et al Increasing the interval between repeated anesthetic exposures reduces long-lasting synaptic changes in late post-natal mice. J Neurochem. 2021;156(1):76–87. doi: 10.1111/jnc.15121. [DOI] [PubMed] [Google Scholar]



