Abstract
代谢异常是肿瘤细胞的十大特征之一,肿瘤细胞能够通过代谢重编程满足其快速增殖的物质和能量需求。肿瘤代谢重编程伴随活性氧(reactive oxygen species,ROS)的产生以及抗氧化体系的激活。ROS含量过高会导致氧化损伤甚至细胞死亡,而适量水平的ROS可作为第二信使参与调控多种信号通路。近年来,随着对氧化应激研究的不断深入,发现ROS可直接介导蛋白质发生氧化还原修饰(redox modifications),从而造成蛋白质构象或功能的改变。然而,目前仅报道了3-磷酸甘油醛脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)、M2型丙酮酸激酶(PKM2)等个别代谢酶的氧化还原修饰,其他代谢酶是否受到氧化还原修饰调控并发挥重要功能尚不清楚,靶向代谢酶氧化还原修饰的时空特异性和代偿适应性也是目前的重点和难点。本文将从肿瘤代谢的角度出发,综述近年来报道的有关代谢酶的氧化还原修饰模式、调控机制及其在肿瘤发生发展中的作用,探讨和展望靶向代谢酶氧化还原修饰的肿瘤治疗策略。
Keywords: 肿瘤代谢, 活性氧, 氧化应激, 氧化还原修饰, 肿瘤治疗
Abstract
Metabolic aberrance is one of the hallmarks of cancer. The metabolic patterns in cancer cells are well reprogrammed to provide building blocks and energy for their sustained growth. During tumor metabolic reprogramming, reactive oxygen species (ROS) are generated and the antioxidant systems are activated. High levels of ROS lead to oxidative damage and even cell death, whereas ROS at low levels act as second messenger to regulate many signaling pathways. Recently, with the revisiting of oxidative stress, it has been found that ROS can directly mediate the redox modifications of proteins, resulting in protein conformational and functional alterations. However, only a very small portion of metabolic enzymes, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and PKM2, etc., has been reported to undergo redox modifications. Whether other metabolic enzymes are regulated by redox modifications and thus exhibit critical functions remain largely unknown. Moreover, the specific spatio-temporal targeting of redox modifications of metabolic enzymes, as well as overcoming the existed redox and metabolic adaptation, are key points to be solved. Here, we will review the reported redox modification patterns of metabolic enzymes, the involved regulatory mechanisms and their roles in tumorigenesis and tumor progress. In addition, we will discuss the future therapeutic strategies targeting redox modifications of metabolic enzymes for tumor treatment.
Keywords: Tumor metabolism, Reactive oxygen species, Oxidative stress, Redox modifications, Tumor therapy
上世纪20年代,德国生物化学家WARBURG发现,即使在有氧条件下,肿瘤细胞仍然能够大量摄取葡萄糖进行糖酵解,这种现象被称为瓦博格效应(Warburg effect,有氧糖酵解)[1]。肿瘤细胞通过有氧糖酵解分解葡萄糖,一方面合成ATP提供能量,另一方面为生物大分子合成提供前体,从而满足肿瘤细胞快速生长所需的能量和物质需求。数十年来,国内外大量研究已证实肿瘤代谢在肿瘤发生发展过程中发挥重要作用,针对糖酵解[2-3]、三羧酸循环[4-5]、氨基酸代谢[6-8]、脂代谢[9-10]等代谢途径的多种关键代谢酶的小分子抑制剂正不断被开发,部分已进入临床试验,具有很好的临床应用前景。
氧化应激是肿瘤细胞的一个重要生物学特征。与正常细胞相比,肿瘤细胞往往具有较高的活性氧(reactive oxygen species,ROS)水平,从而造成氧化应激。一定阈值的氧化应激(oxidative eustress)可通过调控细胞内的多种信号通路以促进肿瘤发生发展,而过度的氧化应激(oxidative distress)则会导致氧化损伤甚至肿瘤细胞死亡[11- 12]。因此,肿瘤细胞在具有较高ROS水平的同时,也会激活细胞内抗氧化体系缓解氧化应激压力,维持细胞内氧化还原稳态,促进肿瘤细胞存活。已有大量研究表明,肿瘤细胞代谢重编程在ROS产生、抗氧化系统激活以及维持氧化还原稳态的过程中发挥重要作用[13- 14]。
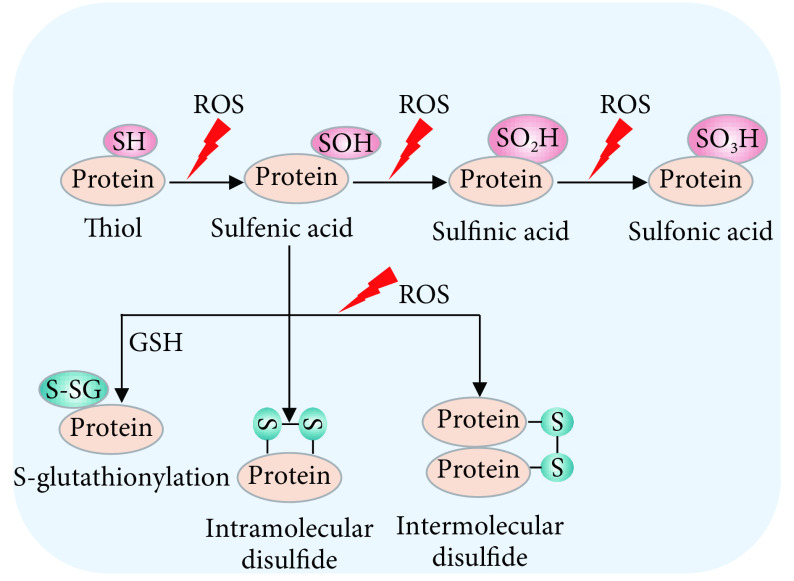
近年来,随着对氧化应激和氧化还原信号调控的再认识,研究发现ROS可以直接介导蛋白质的氧化还原修饰(redox modifications,一种翻译后修饰模式),从而改变蛋白质的构象和功能[15-16]。这种氧化还原修饰主要发生在蛋白质的活泼半胱氨酸上的巯基(RSH)侧链[17]。在ROS刺激下,半胱氨酸上的还原型巯基发生去质子化,被氧化形成次磺酸(sulfenic acids,RSOH)。随后,次磺酸可与邻位巯基反应形成分子内或分子间二硫键(disulfide bonds,RS-SR或RS-SR'),或与谷胱甘肽(GSH)结合发生谷胱甘肽化(S-glutathionylation,RS-SG)修饰。次磺酸还可被进一步氧化形成亚磺酸(sulfinic acids,RSO2H)或磺酸(sulfonic acids,RSO3H)(图1)。除磺酸化修饰外,蛋白质的其它氧化还原修饰是可逆性的,硫氧还蛋白(thioredoxins,Trxs)、谷氧还蛋白(glutaredoxins,Grxs)和过氧化物氧还蛋白(peroxiredoxins,Prdxs)等抗氧化酶在逆转蛋白质的氧化还原修饰中发挥重要作用[18-20]。通过以上形式的氧化还原修饰,蛋白质的构象或蛋白质间的相互作用发生变化,最终导致蛋白质的功能改变。
图 1.
The redox modification patterns of protein cysteines
蛋白质的氧化还原修饰翻译模式
ROS can induce the redox modifications of thiols on the cysteines of proteins. The redox modification patterns include sulfenylation (RSOH), disulfide (RS-SR or RS-SR') formation, S-glutathionylation (RS-SG), sulfinylation (RSO2H) and sulfonylation (RSO3H).
已有研究表明,糖酵解、三羧酸循环、脂代谢、能量代谢和氨基酸代谢等代谢过程中的一些代谢酶可受到氧化还原修饰的调控。本文将论述代谢酶的氧化还原修饰调控机制及其在肿瘤中的作用,以期为肿瘤代谢和氧化应激的研究提供新的切入点,为抗肿瘤药物研发提供新的思路。
1. 代谢酶的氧化还原修饰调控
以往研究表明,ROS可通过翻译后修饰调节代谢酶的功能。例如,在氧化应激条件下,糖酵解途径的M2型丙酮酸激酶(pyruvate kinase M2,PKM2)的乙酰化水平降低,从而抑制其被溶酶体降解,导致肾细胞癌耐药[21]。此外,氧化应激可抑制苹果酸脱氢酶(malate dehydrogenase 1,MDH1)的精氨酸甲基化修饰,导致MDH1激活并产生还原型烟酰胺腺嘌呤二核苷酸磷酸(reduced nicotinamide adenine dinucleotide phosphate,NADPH)缓冲氧化应激,促进胰腺导管癌细胞存活[22]。然而,ROS介导这些代谢酶的翻译后修饰并非直接调控,而是需要相关翻译后修饰酶的参与,至于代谢酶如何直接感知氧化应激信号尚不清楚。
近年来研究发现,ROS可通过对代谢酶的氧化还原修饰直接调控相关代谢酶的功能,为代谢酶如何感知氧化应激信号提供了新思路。目前,已有大量研究证实包括糖酵解、三羧酸循环、脂代谢、能量代谢和氨基酸代谢等在内的多个代谢过程中的代谢酶可受到氧化还原修饰调控。
1.1. 糖酵解代谢酶的氧化还原修饰调控
许多糖酵解酶含有高度保守的半胱氨酸,这些活泼半胱氨酸在ROS刺激下易发生氧化还原修饰。例如,介导糖酵解第一步酶促反应的己糖激酶(hexokinases,HKs)可将葡萄糖磷酸化形成6-磷酸葡萄糖(glucose-6-phosphate,G6P)。研究发现,脱氢抗坏血酸(dehydroascorbic acid,DHA)可与己糖激酶1(HK1)的活性半胱氨酸共价结合,使其不可逆地失去酶活性[23-24]。
磷酸丙糖异构酶(triosephosphate isomerase,TPI)可催化磷酸二羟基丙酮(dihydroxyacetone phosphate,DHAP)的异构化,形成3-磷酸甘油醛(glyceraldehyde-3-phosphate,G3P)。ROS可导致TPI发生氧化(可能通过分子内二硫键形成同源二聚体),氧化型TPI随后发生降解,从而导致糖酵解转向磷酸戊糖途径(pentose phosphate pathway,PPP)产生NADPH以缓冲氧化应激压力[25]。
G3P是3-磷酸甘油醛脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)的底物。GAPDH的第152位半胱氨酸(Cys152)是一个保守的活泼半胱氨酸位点,在氧化应激条件下可被氧化发生次磺酸化或谷胱甘肽化修饰。Cys152的氧化还原修饰可抑制GAPDH的活性,使得代谢通量从糖酵解转向PPP途径,促进肿瘤细胞在氧化应激压力下的存活[26-27]。然而有趣的是,研究发现Cys152的氧化并不是由ROS直接介导的,而是由非活性位点的第156位半胱氨酸(Cys156)先发生氧化,随后通过质子接力(proton relay)将其转移至Cys152[28-29]。此外,在氧化应激下,GAPDH的Cys152也可被氧化形成分子间二硫键,导致GAPDH的聚集和细胞死亡[30-31]。
GAPDH的产物1,3-二磷酸甘油酸(1, 3-bisphosphoglyceric acid,1,3-BPG)通过磷酸甘油酸激酶1(phosphoglycerate kinase 1,PGK1)转化为3-磷酸甘油酸(3-phosphoglycerate,3PG),然后在磷酸甘油酸变位酶1(phosphoglycerate mutase 1,PGAM1)的作用下生成2-磷酸甘油酸(2-phosphoglycerate,2PG)[32]。2PG可通过α-烯醇酶(α-enolase,ENO1)转化为磷酸烯醇丙酮酸(phosphoenolpyruvate,PEP)。研究发现,氧化应激可导致ENO1发生谷胱甘肽化或其他氧化还原修饰而失活[33],但具体修饰位点尚不清楚。
PEP作为PKM2的底物,可被PKM2磷酸化生成丙酮酸。在氧化应激条件下,PKM2被报道可在第358位半胱氨酸(Cys358)发生氧化并失活,导致糖酵解途径受阻,细胞代谢途径转向PPP旁路合成NADPH进行ROS解毒,促进肺癌细胞在氧化应激压力下的存活[34]。尽管有些糖酵解代谢酶的氧化还原修饰模式尚未完全阐明,但这些研究表明代谢酶可通过氧化还原修饰以缓冲氧化应激压力,进而维持细胞内氧化还原稳态,促进细胞存活。
1.2. 三羧酸循环代谢酶的氧化还原修饰调控
糖酵解产生的丙酮酸进入线粒体后,被丙酮酸脱氢酶(pyruvate dehydrogenase,PDH)脱氢生成乙酰辅酶A(acetyl-CoA,AcCoA),从而参与三羧酸循环过程。PDH的酶活受到丙酮酸脱氢酶激酶2(pyruvate dehydrogenase kinase 2,PDHK2)的调控,PDHK2可使PDH发生磷酸化而失活。研究表明,线粒体呼吸链产生的ROS可将PDHK2的第45和392位半胱氨酸(Cys45和Cys392)氧化,从而抑制PDHK2活性,导致PDH去磷酸化激活,最终促进三羧酸循环[35]。在三羧酸循环过程中,AcCoA可与草酰乙酸(oxaloacetate,OAA)结合生成柠檬酸。柠檬酸随后被顺乌头酸酶(aconitase)催化生成异柠檬酸。有报道表明,在H2O2处理下,aconitase可发生氧化聚集、失活和降解[36],但氧化还原修饰在其中是否发挥调控作用尚不清楚。
1.3. 脂代谢酶的氧化还原修饰调控
三羧酸循环中生成的柠檬酸也可转化成AcCoA,进而参与脂代谢。研究发现,脂代谢中的一些代谢酶也受到氧化还原修饰的调控。例如,酰基辅酶A:胆固醇酰基转移酶2(acyl-CoA:cholesterol acyltransferase 2,ACAT2)可催化游离的胆固醇和脂肪酸转化为胆固醇酯(cholesterolester,CE),以便将其储存在脂滴中[37]。细胞内游离胆固醇和脂肪酸含量过高会造成线粒体功能障碍和ROS积累,导致ACAT2的第277位半胱氨酸(Cys277)发生次磺酸化修饰。ACAT2的次磺酸化修饰可抑制Cys277的泛素化,从而抑制ACAT2的降解并促进胆固醇酯的生成,降低细胞的脂毒性[38]。
脂代谢中的另一种氧化还原敏感蛋白是线粒体三功能蛋白β亚基(β subunit of mitochondrial trifunctional protein,TPβ),它是脂肪酸氧化(fatty acid oxidation,FAO)过程的一个关键限速酶。研究发现,在ROS刺激下,TPβ第458位半胱氨酸(Cys458)发生氧化而失活。在葡萄糖匮乏引起的氧化应激下,核受体Nur77进入线粒体被ROS氧化,以此保护TPβ的Cys458免受氧化作用,从而导致FAO介导的NADPH产生,缓冲细胞内氧化应激,促进黑色素瘤细胞的存活和转移[39]。脂代谢中的其它代谢酶是否受到氧化还原修饰调控还有待进一步研究。
1.4. 能量代谢酶的氧化还原修饰调控
此外,能量代谢过程中的一些代谢酶在氧化应激下也可发生氧化还原修饰。腺苷酸活化蛋白激酶(5′-AMP-activated protein kinase,AMPK)是维持细胞内能量稳态的关键能量传感器,当细胞内ATP含量降低或AMP/ATP比值增加时,AMPK被活化并促进ATP生成,使细胞能量得以恢复。在H2O2处理条件下,AMPK第299和304位半胱氨酸(Cys299和Cys304)可发生谷胱甘肽化修饰,导致AMPK激活[40]。值得注意的是,即使在细胞内ATP水平不降低的情况下,H2O2诱导的AMPK谷胱甘肽化修饰和活化仍可发生,这可能是由于氧化还原修饰直接改变了AMPK构象[41]。有趣的是,ROS也可促使AMPK第130和174位半胱氨酸(Cys130与Cys174)氧化形成分子间二硫键,导致AMPK的活性受到抑制。在氧化应激或能量匮乏等应激条件下,Trx1可与AMPK结合并将分子间二硫键还原,从而抑制AMPK的氧化和聚集[42-43]。从以上研究结果可以推测,在氧化应激条件下,Trx1是决定AMPK功能的关键分子,这一过程与AMP/ATP的比例无关。
此外,肌酸激酶(creatine kinase,CK)是能量代谢的另一个关键代谢酶。在氧化应激条件下,CK位于活性中心的第283位半胱氨酸(Cys283)可发生谷胱甘肽化修饰,导致其酶活减弱[44]。CK的这一氧化还原修饰是否在肿瘤发生发展中发挥作用亦有待于进一步研究。
1.5. 氨基酸代谢酶的氧化还原修饰调控
半胱氨酸是合成还原型GSH的重要原料,在维持细胞内氧化还原平衡过程中发挥重要作用。一碳单位代谢中的S-腺苷甲硫氨酸(S-adenosyl methionine,SAM)循环可产生同型半胱氨酸,其可通过转硫化途径合成胱硫醚这一半胱氨酸前体。胱硫醚β合成酶(cystathionine beta-synthase,CBS)是同型半胱氨酸转硫代谢的限速酶。CBS包含一个CXXC基序,在ROS刺激下,该基序上的第272和275位半胱氨酸(Cys272和Cys275)可氧化形成分子内二硫键,导致酶活降低[45]。另有研究表明,在H2O2处理下,CBS第346位半胱氨酸(Cys346)可发生谷胱甘肽化修饰,导致CBS酶活增强,从而促进同型半胱氨酸的转硫代谢[46]。除代谢酶外,胱氨酸/谷氨酸反向转运系统xCT(cystine/glutamate antiporter xCT)在平衡细胞内的谷氨酸和半胱氨酸水平中也发挥着重要作用。在氧化应激条件下,xCT轻链上的第158位半胱氨酸(Cys158)与其4F2重链上的第10位半胱氨酸(4F2hc,Cys109)可通过二硫键形成二聚体,从而使其具有转运谷氨酸和胱氨酸的功能[47-48]。
综上,ROS介导的代谢酶的直接氧化还原修饰可调控代谢酶的功能,从而调控肿瘤代谢重编程,在肿瘤发生发展过程中发挥重要作用(图2)。将来有望发现更多可被氧化还原修饰调控的关键代谢酶。
图 2.
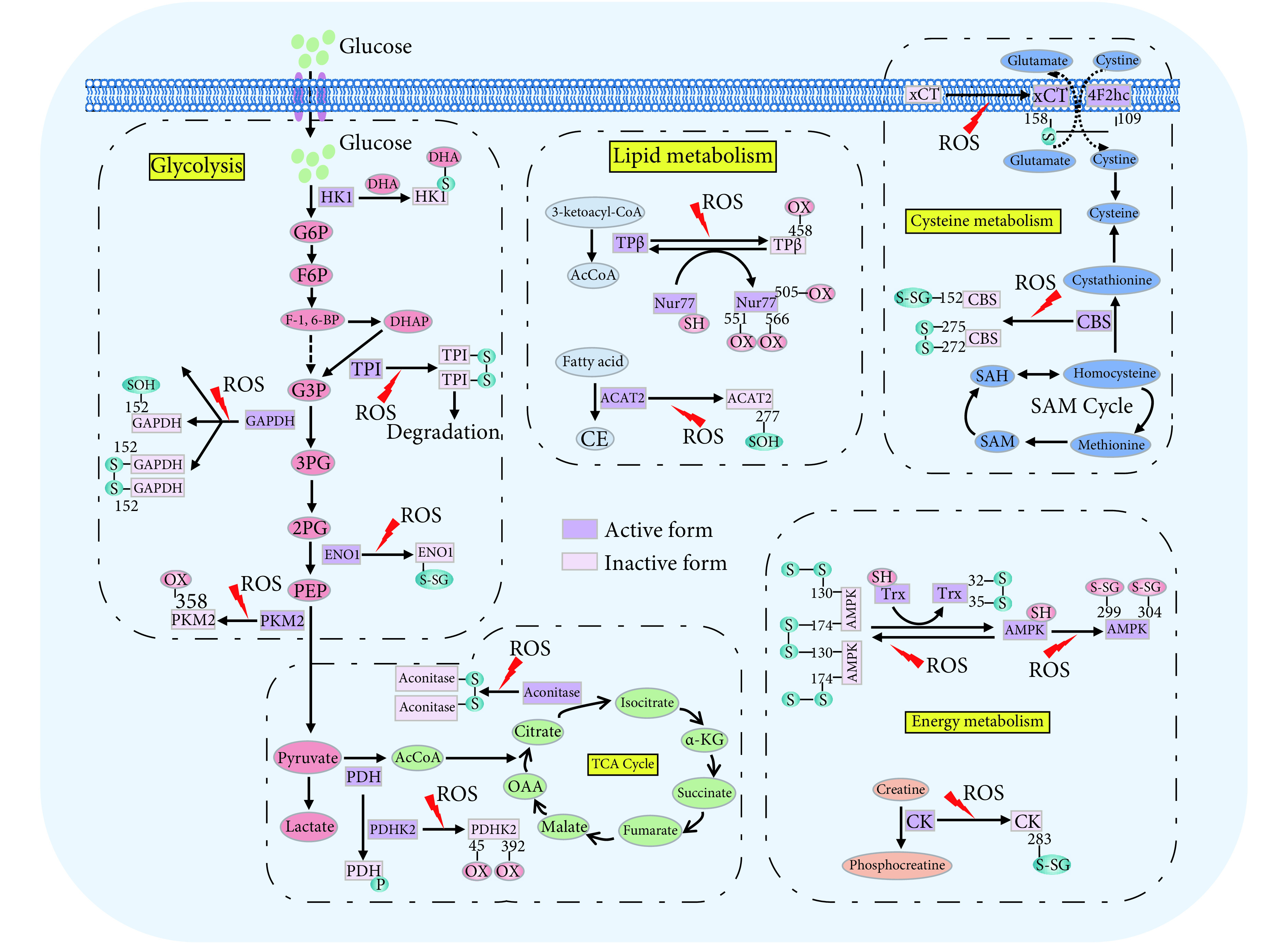
Oxidative modifications of metabolic enzymes
代谢酶的氧化还原修饰模式
In response to ROS-mediated oxidative stress, many metabolic enzymes can undergo redox modifications, including glycolytic enzymes (HK1, TPI, GAPDH, ENO, PKM2, and PDHK2, etc.), TCA cycle enzymes (Aconitase, PDHK2, etc.), lipid metabolism enzymes (ACAT2, TPβ, etc.), energy metabolism enzymes (AMPK, CK, etc.), and amino acid metabolism enzymes (CBS, etc.), leading to the alteration of metabolic patterns.
2. 靶向代谢酶氧化还原修饰的肿瘤治疗新策略
肿瘤代谢酶氧化还原修饰的研究为靶向肿瘤代谢和氧化应激的肿瘤治疗策略提供了新的契机。鉴于此,抗氧化剂理论上可抑制氧化应激和氧化还原修饰调控,从而发挥良好的抗肿瘤作用。然而,多个临床试验表明,膳食补充常用的抗氧化剂,如N-乙酰半胱氨酸(N-acetylcysteine,NAC)、维生素E、β-胡萝卜素等,在某些情况下可抑制肝癌、胃癌等肿瘤的发生,但对肺癌、头颈肿瘤等又无显著抑制作用,甚至可增加前列腺癌和肺癌等肿瘤的发病风险。造成这些矛盾结果的原因可能是由于这些抗氧化剂的抗氧化作用是非靶向的,不能在特定的器官、组织和细胞水平特异地抑制某一蛋白质的氧化还原修饰[13, 49]。近年来的一项研究发现,高剂量的维生素C处理可导致GSH大量消耗和ROS积累,进而促进GAPDH第152位半胱氨酸(Cys152)谷胱甘肽化修饰,导致GAPDH失活,最终通过抑制糖酵解和消耗ATP杀死KRAS和BRAF突变的结肠癌细胞[26]。然而,维生素C诱导的ROS积累是一种普遍性效应,不可避免地会导致除GAPDH以外其他蛋白质的氧化,从而可能对肿瘤抑制效果产生协同作用、中和作用甚至造成对正常细胞的毒副作用。
为了解决靶向蛋白质氧化还原修饰的特异性问题,CRAVATT教授带领的研究小组开发了竞争性的同位素串联正交蛋白水解-基于活性的蛋白质组学(isotopic tandem orthogonal proteolysis-activity-based protein profiling,isoTOP-ABPP)筛选技术。通过竞争性isoTOP-ABPP筛选结合基于片段的配体发现策略(fragment-based ligand discovery,FBLD),BACKUS等[50]建立了可与半胱氨酸共价反应的亲电小分子化合物文库。随后,这一研究小组以经典的抗氧化核转录因子NF-E2相关因子(nuclear factor erythroid-2-related factor 2,Nrf2)为研究对象,通过对这一亲电性小分子化合物文库进行筛选,发现BPK-29能与Nrf2的调节蛋白NR0B1第274位半胱氨酸(Cys274)特异性共价结合,从而破坏NR0B1蛋白复合物,抑制KEAP1突变型非小细胞肺癌细胞的生长[51]。这一研究证实了特异性靶向蛋白质氧化还原修饰的可行性。此外,亲电小分子也可被设计用于将活泼游离的巯基烷基化从而抑制巯基的氧化修饰,或与巯基形成二硫键从而抑制邻位二硫键的还原转位。除亲电小分子外,也可设计含有巯基的亲核小分子与靶蛋白的二硫键竞争性结合,从而导致二硫键断裂,抑制蛋白质的氧化还原修饰[52]。相信随着该领域的发展,具有潜在抗肿瘤活性且能特异靶向代谢酶氧化还原修饰的小分子化合物将会被陆续发现和开发。
3. 总结和展望
鉴于代谢重编程和氧化应激在肿瘤发生发展中的重要作用,近年来靶向代谢和氧化应激的肿瘤治疗策略已被广泛研究。针对糖酵解、三羧酸循环、氨基酸代谢、脂代谢等代谢过程的小分子抑制剂的研发如火如荼,促氧化剂和抗氧化剂的抗肿瘤研究也被广泛开展,其中部分已进入临床试验。但目前已被批准用于临床的小分子药物很少,一方面是由于靶向代谢或氧化应激会造成肿瘤细胞代谢或氧化还原的适应性调节,导致肿瘤耐药或治疗效果欠佳;另一方面是由于患者个体差异以及肿瘤微环境导致的代谢和氧化还原状态的时空性和异质性,因此亟需开发有效的手段在治疗前和治疗过程中对患者体内真实的代谢和氧化还原状态进行动态监测,从而指导和优化治疗措施。此外,需要更加深入地理解肿瘤代谢和氧化应激的调控机制,从而开发特异性更强的小分子抑制剂。
近年来,代谢酶氧化还原修饰的发现为开发抗肿瘤药物提供了新的切入点。然而,目前仅报道了个别代谢酶的氧化还原修饰调控,其他代谢酶是否受到氧化还原修饰尚不清楚。VAN DER REEST等[53]采用基于稳定同位素碘乙酰胺的半胱氨酸标记技术(stable isotope cysteine labelling with iodoacetamide,SICyLIA)结合质谱分析,针对代谢酶和代谢相关蛋白的氧化还原修饰进行了高通量筛选,为代谢酶的氧化还原修饰研究提供了有力的证据及参考。后续研究将针对潜在的可受氧化还原修饰的代谢酶进行逐一功能阐释,并明确代谢酶氧化还原修饰的重要生物学功能和作为药物靶标的潜在应用价值。此外,目前尽管传统的抗氧化剂可通过缓冲ROS水平而抑制氧化还原修饰,但这种抑制效果不能特异性地针对某一特定代谢酶,可能会导致治疗效果不佳或造成严重的副反应。开发可特异性靶向某一特定代谢酶的氧化还原修饰的小分子化合物是该领域的重点和难点,其有望为抗肿瘤药物的开发带来新的机遇。
Funding Statement
国家自然科学基金(No. 81821002、No. 81790251、No. 81872277、No. 82073081),国家重点研发计划(No. 2020YFA0509400、No. 2020YFC2002705),广东省基础与应用基础研究重大项目(No. 2019B030302012)和四川省科技计划项目(No. 2020YJ0107)资助
References
- 1.WARBURG O, WIND F, NEGELEIN E The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VERNIERI C, CASOLA S, FOIANI M, et al Targeting cancer metabolism: dietary and pharmacologic interventions. Cancer Discov. 2016;6(12):1315–1333. doi: 10.1158/2159-8290.CD-16-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WANG C, HE C, LU S, et al Autophagy activated by silibinin contributes to glioma cell death via induction of oxidative stress-mediated BNIP3-dependent nuclear translocation of AIF. Cell Death Dis. 2020;11(8):1–16. doi: 10.1038/s41419-020-02866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HAUGRUD A B, ZHUANG Y, COPPOCK J D, et al Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast Cancer Res Treat. 2014;147(3):539–550. doi: 10.1007/s10549-014-3128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BERGAGGIO E, RIGANTI C, GARAFFO G, et al IDH2 inhibition enhances proteasome inhibitor responsiveness in hematological malignancies. Blood. 2019;133(2):156–167. doi: 10.1182/blood-2018-05-850826. [DOI] [PubMed] [Google Scholar]
- 6.XIANG Y, STINE Z E, XIA J, et al Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125(6):2293–2306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.YUAN L, SHENG X, CLARK L H, et al Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer. Am J Transl Res. 2016;8(10):4265–4277. [PMC free article] [PubMed] [Google Scholar]
- 8.JONES C L, STEVENS B M, D'ALESSANDRO A, et al Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell. 2018;34(5):724–740. doi: 10.1016/j.ccell.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CHENG S, WANG G, WANG Y, et al Fatty acid oxidation inhibitor etomoxir suppresses tumor progression and induces cell cycle arrest via PPARγ-mediated pathway in bladder cancer. Clin Sci. 2019;133(15):1745–1758. doi: 10.1042/CS20190587. [DOI] [PubMed] [Google Scholar]
- 10.LI L, JIANG Z, YAO Y, et al. (−)-Hydroxycitric acid regulates energy metabolism by activation of AMPK-PGC1α-NRF1 signal pathway in primary chicken hepatocytes. Life Sci, 2020, 254: 117785 [2020-04-20]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5648812/. doi: 10.1038/s41467-017-01106-1.
- 11.SOSA V, MOLIN T, SOMOZA R, et al Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 12.SIES H, JONES D P Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 13.WANG K, JIANG J, LEI Y, et al Targeting metabolic–redox circuits for cancer therapy. Trends Biochem Sci. 2019;44(5):401–414. doi: 10.1016/j.tibs.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 14.CLEMENTINO M, SHI X, ZHANG Z Oxidative stress and metabolic reprogramming in Cr (Ⅵ) carcinogenesis. Curr Opin Toxicol. 2018;8(1):20–27. doi: 10.1016/j.cotox.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SONG I K, LEE J J, CHO J H, et al Degradation of redox-sensitive proteins including peroxiredoxins and DJ-1 is promoted by oxidation-induced conformational changes and ubiquitination. Sci Rep. 2016;6(1):1–15. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SMITH K A, WAYPA G B, SCHUMACKER P T Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017;13(1):228–234. doi: 10.1016/j.redox.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MOLDOGAZIEVA N T, LUTSENKO S V, TERENTIEV A A Reactive oxygen and nitrogen species–induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Res. 2018;78(21):6040–6047. doi: 10.1158/0008-5472.CAN-18-0980. [DOI] [PubMed] [Google Scholar]
- 18.REN X, ZOU L, ZHANG X, et al Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid Redox Signal. 2017;27(13):989–1010. doi: 10.1089/ars.2016.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BEGAS P, LIEDGENS L, MOSELER A, et al Glutaredoxin catalysis requires two distinct glutathione interaction sites. Nat Commun. 2017;8(1):1–13. doi: 10.1038/s41467-016-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ELKO E A, CUNNIFF B, SEWARD D J, et al Peroxiredoxins and beyond; redox systems regulating lung physiology and disease. Antioxid Redox Signal. 2019;31(14):1070–1091. doi: 10.1089/ars.2019.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SHANMUGASUNDARAM K, NAYAK B, FRIEDRICHS W, et al. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun, 2017, 8(1): 997[2020-04-20]. https://www.nature.com/articles/s41467-017-01106-1. doi: 10.1038/s41467-017-01106-1.
- 22.WANG Y, ZHOU W, WANG J, et al Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol Cell. 2016;64(4):673–687. doi: 10.1016/j.molcel.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 23.FIORANI M, DE SANCTIS R, SCARLATTI F, et al Dehydroascorbic acid irreversibly inhibits hexokinase activity. Mol Cell Biochem. 2000;209(1):145–153. doi: 10.1023/a:1007168032289. [DOI] [PubMed] [Google Scholar]
- 24.HENEBERG P Redox regulation of hexokinases. Antioxid Redox Signal. 2019;30(3):415–442. doi: 10.1089/ars.2017.7255. [DOI] [PubMed] [Google Scholar]
- 25.DUMONT S, BYKOVA N, PELLETIER G, et al. Arabidopsis thaliana cytosolic triosephosphate isomerase from is reversibly modified by glutathione on cysteines 127 and 218. Front Plant Sci, 2016, 7: 1942[2020-04-20]. https://www.frontiersin.org/articles/10.3389/fpls.2016.01942/full. doi: 10.3389/fpls.2016.01942.
- 26.YUN J, MULLARKY E, LU C, et al Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MALLER C, SCHR DER E, EATON P Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxid Redox Signal. 2011;14(1):49–60. doi: 10.1089/ars.2010.3149. [DOI] [PubMed] [Google Scholar]
- 28.PERALTA D, BRONOWSKA A, MORGAN B, et al A proton relay enhances H2O2sensitivity of GAPDH to facilitate metabolic adaptation . Nat Chem Biol. 2015;11(2):156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 29.YANG S, ZHAI Q Cytosolic GAPDH: a key mediator in redox signal transduction in plants. Biologia Plantarum. 2017;61(3):417–426. doi: 10.1007/s10535-017-0706-y. [DOI] [Google Scholar]
- 30.REISZ J A, WITHER M J, DZIECIATKOWSKA M, et al Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 31.GERSZON J, RODACKA A Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase in neurodegenerative processes and the role of low molecular weight compounds in counteracting its aggregation and nuclear translocation. Ageing Res Rev. 2018;48(1):21–31. doi: 10.1016/j.arr.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 32.HAY N Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16(10):635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.KIM H J, LEE H-R, KIM C S, et al Investigation of protein expression profiles of erythritol-producing Candida magnoliae in response to glucose perturbation. Enzyme Microb Technol. 2013;53(3):174–180. doi: 10.1016/j.enzmictec.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 34.ANASTASIOU D, POULOGIANNIS G, ASARA J M, et al Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HURD T, COLLINS Y, ABAKUMOVA I, et al Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. J Biol Chem. 2012;287(42):35153–35160. doi: 10.1074/jbc.M112.400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LARSEN F J, SCHIFFER T A, ØRTENBLAD N, et al High‐intensity sprint training inhibits mitochondrial respiration through aconitase inactivation. FASEB J. 2016;30(1):417–427. doi: 10.1096/fj.15-276857. [DOI] [PubMed] [Google Scholar]
- 37.RÖHRIG F, SCHULZE A The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 38.WANG Y J, BIAN Y, LUO J, et al Cholesterol and fatty acids regulate cysteine ubiquitylation of ACAT2 through competitive oxidation. Nat Cell Biol. 2017;19(7):808–819. doi: 10.1038/ncb3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LI X, WANG Z, ZHENG Y, et al Nuclear receptor Nur77 facilitates melanoma cell survival under metabolic stress by protecting fatty acid oxidation. Mol Cell. 2018;69(3):480–492. doi: 10.1016/j.molcel.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 40.ZMIJEWSKI J W, BANERJEE S, BAE H, et al Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285(43):33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ZHANG C, HAWLEY S, ZONG Y, et al Fructose-1, 6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548(7665):112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SHAO D, OKA S, LIU T, et al A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metab. 2014;19(2):232–245. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.XIE N, YUAN K, ZHOU L, et al PRKAA/AMPK restricts HBV replication through promotion of autophagic degradation. Autophagy. 2016;12(9):1507–1520. doi: 10.1080/15548627.2016.1191857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.REDDY S, JONES A, CROSS C, et al Inactivation of creatine kinase by S-glutathionylation of the active-site cysteine residue. Biochem J. 2000;347(3):821–827. doi: 10.1042/bj3470821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NIU W, WANG J, QIAN J, et al Allosteric control of human cystathionine beta-synthase activity by a redox active disulfide bond. J Biol Chem. 2018;293(7):2523–2533. doi: 10.1074/jbc.RA117.000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NIU W N, YADAV P K, ADAMEC J, et al S-glutathionylation enhances human cystathionine beta-synthase activity under oxidative stress conditions. Antioxid Redox Signal. 2015;22(5):350–361. doi: 10.1089/ars.2014.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SATO H, TAMBA M, ISHII T, et al Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274(17):11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 48.PAUL B, SBODIO J, SNYDER S Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci. 2018;39(5):513–524. doi: 10.1016/j.tips.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ALDINI G, ALTOMARE A, BARON G, et al N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52(7):751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 50.BACKUS K, CORREIA B, LUM K, et al Proteome-wide covalent ligand discovery in native biological systems. Nature. 2016;534(7608):570–574. doi: 10.1038/nature18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.BAR-PELED L, KEMPER E, SUCIU R, et al Chemical proteomics identifies druggable vulnerabilities in a genetically defined cancer. Cell. 2017;171(3):696–709. doi: 10.1016/j.cell.2017.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.HOGG P J Targering allosteric disulfide bonds in cancer. Nat Rev Cancer. 2013;13(6):425–431. doi: 10.1038/nrc3519. [DOI] [PubMed] [Google Scholar]
- 53.VAN DER REEST J, LILLA S, ZHENG L, et al. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat Commun, 2018, 9(1): 1581[2020-04-20]. https://www.nature.com/articles/s41467-018-04003-3. doi: 10.1038/s41467-018-04003-3.