Abstract
放射治疗(放疗)作为目前恶性肿瘤的主要治疗手段之一,尤其在胸部肿瘤的多学科综合治疗中发挥着重要作用。随着放疗技术的发展,研究重点已经从提高恶性肿瘤患者整体生存率转为降低放射性相关损伤的发生率。目前,放射性心脏损伤(radiation-induced heart disease, RIHD)已成为胸部肿瘤放疗患者非癌性死亡的主要原因之一,严重影响患者的生存质量和临床预后。近年来,RIHD的发病机制逐渐被阐明,并提出了一些预防和治疗RIHD的潜在手段。本文结合现阶段已报道的RIHD临床表现和病理改变,综述其生物学机制和潜在治疗方案,并指出当前RIHD防治工作中存在的挑战,为预防和治疗RIHD提供参考。
Keywords: 放射治疗, 胸部肿瘤, 放射性心脏损伤
Abstract
Being one of the major therapeutic measures for malignant tumors, radiation therapy, or radiotherapy, plays a particularly crucial role in the multidisciplinary integrated treatment of thoracic tumors. With the development in radiotherapy technology, the research focus has shifted from improving the overall survival of malignant tumor patients to reducing the incidence of radiation-related injuries. Currently, radiation-induced heart disease (RIHD) has become one of the leading non-cancer causes of death in thoracic tumor patients who have undergone radiotherapy, seriously affecting their quality of life and clinical prognosis. In recent years, there has been growing understanding of the pathogenesis of RIHD, and proposals have been made for some potential measures for the prevention and treatment of RIHD. Based on the clinical manifestations and pathological changes of RIHD that have been reported, we herein reviewed the biological mechanism and potential treatment options for RIHD. We also discussed existing challenges in the prevention and treatment of RIHD, intending to provide references for the prevention and treatment of RIHD.
Keywords: Radiation therapy, Thoracic tumor, Radiation-induced heart disease
放射治疗(放疗)是乳腺癌、食管癌和肺癌等胸部实体恶性肿瘤的标准治疗手段。随着调强放疗、图像引导放疗和立体定向放疗等放疗技术的革新,目前放疗精度已得到极大的提高,但仍无法避免相邻的危及器官受照射。尤其是对于乳腺癌、霍奇金淋巴瘤等预后良好的疾病的患者,放疗可能会导致放射性心脏损伤(RIHD)等剂量依赖性的毒副作用[1-2]。例如,霍奇金淋巴瘤放疗患者的心血管疾病发病风险是一般人群的3~5倍[3],发病率高达50%[4]。具体而言,RIHD可表现为器质性心脏病或非器质性心脏病,包括冠状动脉疾病、瓣膜疾病、传导系统疾病、心包疾病以及心肌损伤等[5]。目前关于RIHD的病理机制研究较少,因此深入研究RIHD的发生机制对制定针对性防治措施以及改善放疗患者的远期预后大有裨益。本文首先回顾RIHD已报道的临床表现,并详细阐述相关分子机制。此外,我们还总结了可能适用于预防或治疗RIHD的潜在策略,以期为RIHD的临床防治提供参考。
1. 临床表现
1.1. 冠状动脉疾病
冠状动脉疾病已成为恶性肿瘤患者放疗的主要治疗相关并发症,也是导致患者非肿瘤性死亡的重要原因。一项对2168名乳腺癌患者接受两侧胸壁放疗的回顾性分析显示,放疗毒性与放射线剂量呈线性关系,且没有明显的上限[1]。患者每增加1 Gy心脏受量,其出现冠状动脉事件的风险将升高7.4%[1]。 此外,由于放疗导致的冠状动脉疾病的症状与普通的冠心病类似。因此放疗诱发的冠状动脉疾病与常规冠状动脉疾病之间的鉴别诊断较为困难。
1.2. 瓣膜病
放疗导致的心脏瓣膜病的病理改变主要包括瓣叶回缩、纤维化、增厚及最终钙化形成。与三尖瓣和肺动脉瓣相比,主动脉瓣和二尖瓣受到的影响更大,且一旦辐射剂量超过30 Gy,瓣膜病的发生风险将显著增加[6]。此外,尽管在81%的RIHD患者中发现了心脏瓣膜改变,但超过70%的患者并没有显著症状[7]。研究发现,瓣膜病变的无症状发展时间约为11.5年,而出现功能性瓣膜疾病的时间约为16.5年[8]。
1.3. 传导系统疾病
放疗引起的传导系统疾病通常在放疗后数年至数十年才能被检测,故目前还很难确定与放疗之间的关系[9]。放疗所致的传导系统疾病包括不同程度的房室传导阻滞、房室结节律性心动过缓和病态窦房结综合征等[9]。研究表明放疗导致的心电图异常的患者中,其70%会在放疗结束后半年恢复正常,真正发生完全性传导阻滞病例少见[10]。
1.4. 心包疾病
研究表明70%的RIHD患者存在心包疾病[11]。放射性心包炎的病理特征在于心包囊腔中存在富含蛋白质的渗出物和心包腔中纤维蛋白积累。根据疾病的严重程度和发展情况,心包疾病的临床病变包括从急性心包炎到慢性心包积液,再到心包填塞和缩窄性心包炎等疾病谱[12]。
1.5. 心肌损伤
胸部放疗对微血管的损害会导致心肌慢性缺血,进一步发展可能导致心肌纤维化。进一步研究发现放疗后心内膜心肌层最容易受损[13]。放射性心肌损伤主要表现为限制型心肌病导致的舒张功能受限,部分伴有左心室收缩功能的轻微降低[14]。多数患者没有显著的临床症状,临床诊断率约为10%[15]。而心肌纤维化的出现时间晚,一般在放疗后数年甚至数十年才出现,可能导致患者出现心力衰竭甚至猝死。
2. 发生机制
由于RIHD存在混杂因素多、采样困难及潜伏期长等特点,因此RIHD发病机制尚未完全阐明。下文我们将阐述目前临床前研究对放射性心脏损伤机制的理解。
2.1. 冠状动脉疾病和血管损伤
放射性冠状动脉疾病和血管损伤机制复杂、涉及多个通路,且不同通路之间相互作用,形成正反馈,并最终导致出现持续的不可逆损伤[14]。放疗可通过电离水分子及破坏线粒体呼吸链,产生活性氧(reactive oxygen species, ROS)并使其蓄积。此外,NADPH 氧化酶、环氧合酶等酶类的激活也会加速ROS蓄积。与此同时,抗氧化酶被辐射抑制将导致蓄积的ROS无法被抗氧化剂完全清除,并在体内发生多种化学反应,从而加重氧化应激 [16]。此外,辐射可作用DNA并导致多种类型的损伤,其中DNA双链断裂 (double-strand breaks, DSBs)最为严重。
ROS及DSBs会激活I-κB激酶,介导I-κB降解并释放NF-κB,随后游离的NF-κB将易位至细胞核内。NF-κB与靶基因的启动子区域结合并诱导TNF-α、IL-1、IL-6、IL-8等促炎因子的表达,调控炎症反应[17]。同时NF-κB可通过诱导分泌黏附分子以增加白细胞的黏附能力。渗透的中性粒细胞可导致多种促炎因子的进一步释放,加重内皮细胞破坏;渗透的单核细胞转化为活化的巨噬细胞,而活化的巨噬细胞难以分解ROS氧化产生的低密度脂蛋白,随后逐渐转化为泡沫细胞,此过程与动脉粥样硬化形成密切相关 [18]。此外,NF-κB作用于靶基因,促进NADPH 氧化酶、环氧合酶的表达,从而导致ROS进一步增加,且增加的ROS继续作用于NF-κB,形成正反馈,加速冠状动脉疾病和血管损伤[10](图1)。
图 1.
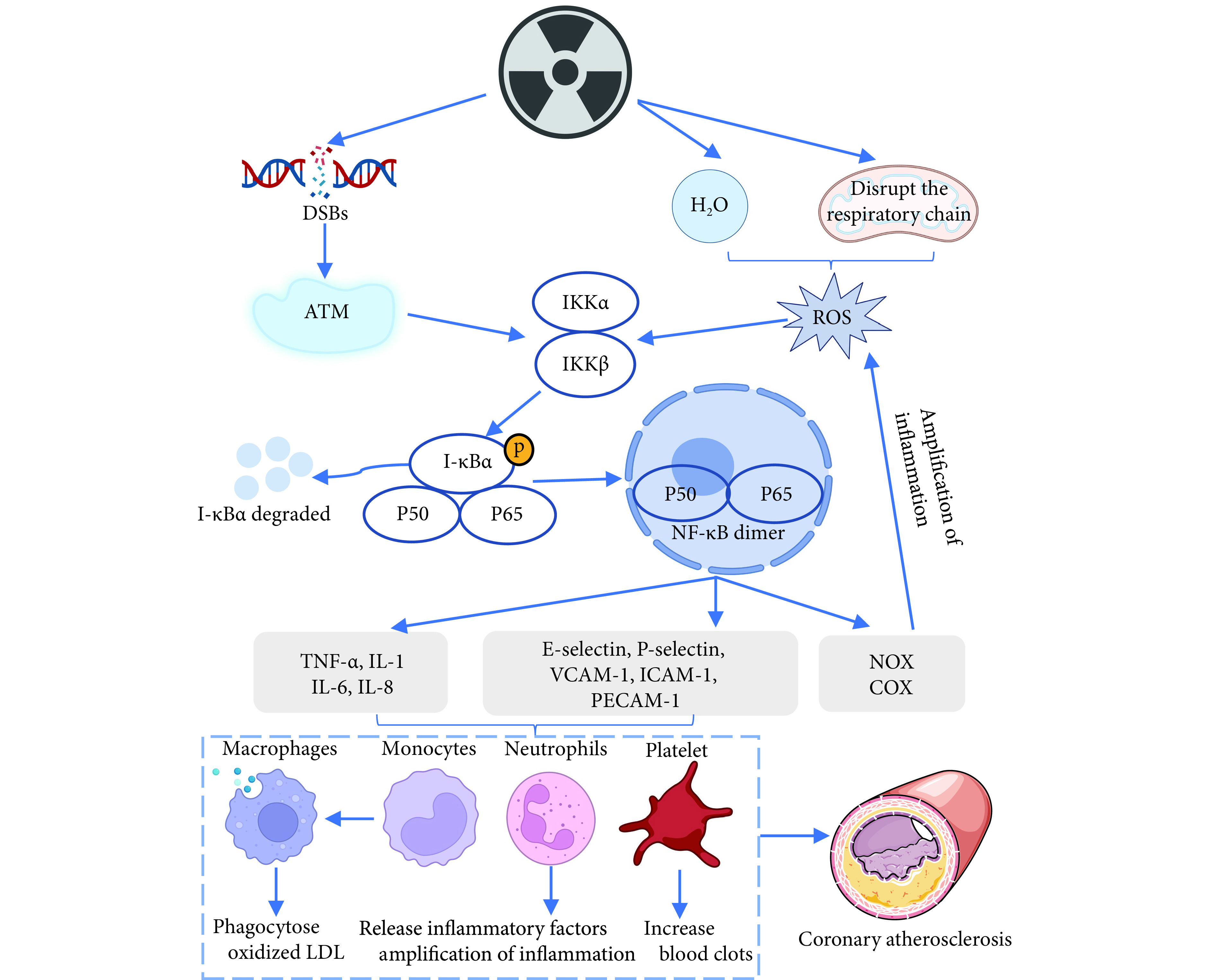
The role of NF-κB in RIHD
NF-κB在RIHD中的作用
DSBs: Double-strand breaks; ATM: Ataxia telangiectasia mutated; ROS: Reactive oxygen species; IKK: I-κB kinase; TNF-α: Tumor necrosis factor alpha; IL-1: Interleukin 1; IL-6: Interleukin 6; IL-8: Interleukin 8; VCAM-1: Vascular cell adhesion molecule-1; ICAM-1: Intercellular adhesion molecule-1; PECAM-1: Platelet endothelial cell adhesion molecule-1; NF-κB: Nuclear factor kappa-B; NOX: NADPH oxidase; COX: Cyclooxygenase; LDL: Low density lipoprotein.
研究证实在放疗早期,ROS[19]及DNA修复损伤(DNA damage repair, DDR)[20]可通过磷酸化人静脉内皮细胞中的内皮一氧化氮合酶(endothelial nitric oxide synthase, eNOS)上的第1177位丝氨酸,导致一氧化氮(NO)增加。但ROS与NO反应会产生活性氮物质,导致NO生物利用度下降;同时ROS会促进前列腺素等血管收缩物质生成导致血管舒缩反应受损,并最终导致血管狭窄[21]。此外,放疗还会导致心脏毛细血管减少[22],促进血管内皮细胞内血管性血友病因子表达[23],致使血管中血小板黏附和血栓形成,从而加重缺血缺氧(图2)。
图 2.
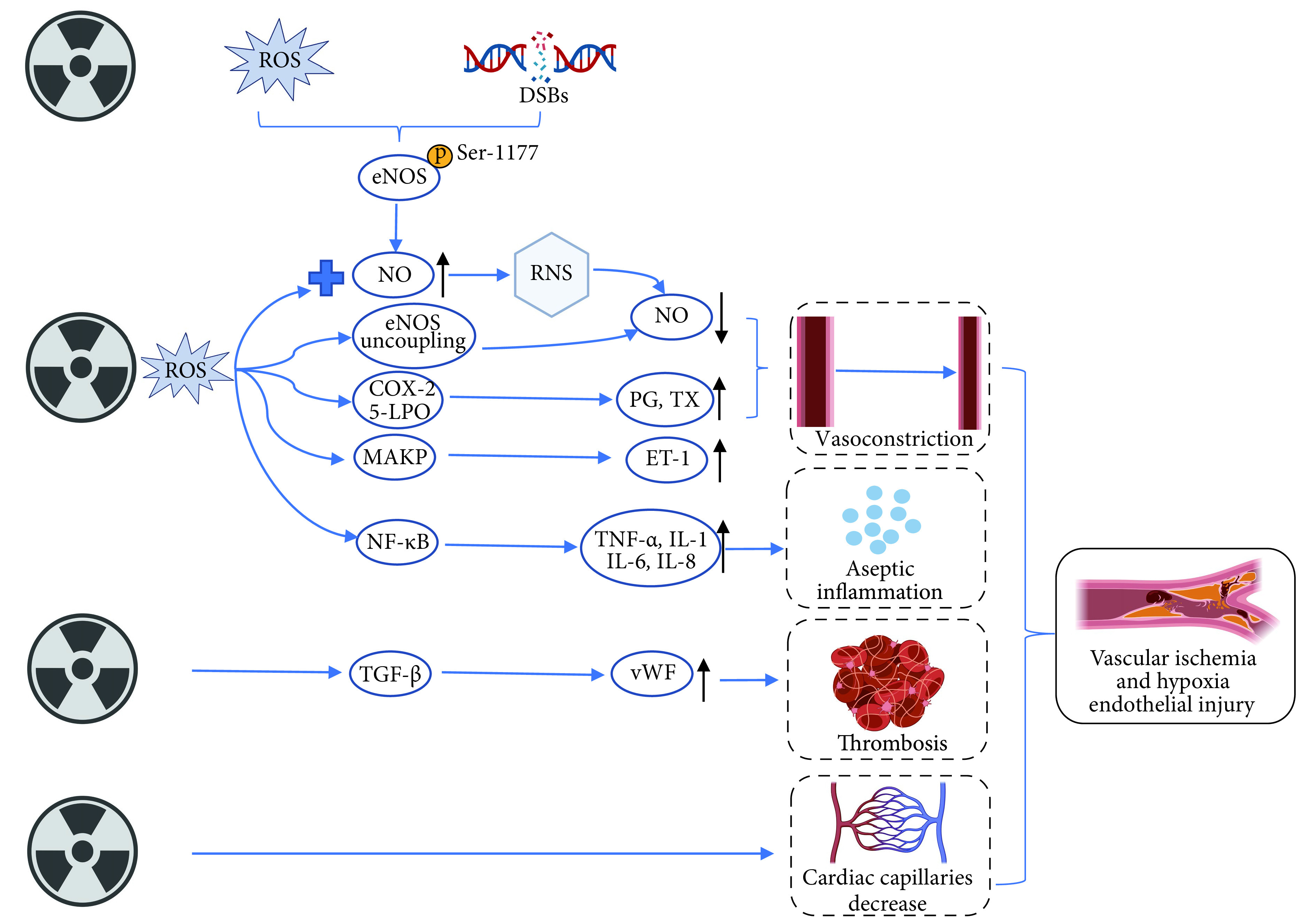
Mechanisms of vascular injury in RIHD
RIHD中血管损伤机制
ROS, DSBs, NF-κB, TNF-α, IL-1, IL-6, and IL-8: The denotations are the same as those in the notes for Fig 1. eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide; RNS: Reactive nitrogen species; COX-2: Cyclooxygenase-2; 5-LPO: 5-Lipoxygenase; PG: Prostaglandin; TX: Thromboxane; MAPK: Mitogen-activated protein kinases; ET-1: Endothelin-1; TGF-β: Transforming growth factor beta; vWF: von Willebrand factor.
细胞凋亡、坏死也是导致RIDH的重要原因。ROS和DNA损伤信号分别通过Bcl-2/Bax蛋白家族以及P53蛋白诱导细胞发生凋亡[10, 22]。与此同时,Bcl-2/Bax蛋白家族还能通过改变线粒体通透性诱导细胞凋亡[24]。进一步研究发现RIDH的长期病理变化与蛋白质表达改变和心脏线粒体功能受损有关[25]。此外,放疗还可促进内质网释放 Ca2+,导致线粒体 Ca2+摄取增加,钙超载最终也会导致细胞膜肿胀和释放凋亡因子[26]。
值得注意的是,最近有研究发现与单独的胸腔局部放射相比,10 Gy全身放射导致心脏血管密度显著降低[27]。不同于其他文献报道的放射治疗“远位效应”可能激活免疫从而增强抗肿瘤治疗疗效[28],该研究提示通过体内除心脏以外的结构受到辐射可能加重放射性心脏损伤,但是这种针对心脏的负性 “远位效应”现象尚未得到系统的研究。
2.2. 传导系统疾病和心包疾病
虽然大量研究证实辐射与心脏电生理改变密切相关[29],但目前对于RIHD的传导系统疾病和心包疾病的机制研究较少。放疗可导致心肌缺血,或通过炎症反应导致心肌纤维化并最终损伤传导系统。研究还发现ROS与蛋白质氧化可能会影响受体、酶、转运蛋白等的功能[18]。例如ROS可过度激活Ca2+- 钙调蛋白依赖性蛋白激酶Ⅱ,导致异常的兴奋收缩偶联、心力衰竭以及心律失常[30]。
辐射引起的微血管损伤可导致毛细血管通透性增加和富含蛋白质的渗出液快速出现和发展,最终导致放射性心包炎[9]。而心包增厚的间质和心尖区域的胶原蛋白沉积则会导致心包纤维化。
2.3. 心肌纤维化
心肌纤维化主要表现为胶原蛋白沉积在心脏,并最终置换心肌细胞。 在RIHD的动物模型中,发现在辐射照射后2~6个月会出现心脏功能降低和心肌纤维化,辐射导致心脏的功能和形态改变,可以通过超声心动图和组织学测量[31]。心肌纤维化的机制目前尚不清楚,其发生发展时间长,可能是多因素相互作用的结果。
氧化应激也与心肌纤维化密切相关。TNF-α、IL-1、IL-11等促炎因子及黏附分子的释放,将导致成纤维细胞增加。随后引起微血栓和血管闭塞,从而导致充盈缺损和局灶性缺血,继而进一步加速心肌细胞死亡和纤维化[32]。此外,ROS与脂质氧化产生的脂质过氧化物将导致膜结合受体和酶失活,进而导致组织通透性升高以及细胞蛋白质失活,并最终破坏心肌细胞膜[18]。
此外,放疗引起的心脏纤维化中常出现TGF-β过表达,提示转化生长因子水平升高可能使RIHD恶化。电离辐射损伤可能通过ROS生成、炎症过度活化、微血管损伤、血小板活化以及细胞衰老和凋亡等多个途径使TGF-β发生活化[33]。TGF-β能够通过经典和非经典信号通路触发纤维化,其中经典途径为TGF-β通过Smad转录因子激活Ⅰ型胶原蛋白 、Ⅲ型胶原蛋白、CTGF和α-肌动蛋白等靶基因[34]。 TGF-β还可以通过Rho/ROCK等不依赖Smad的通路发挥作用,进一步促进纤维化[35]。同时,TGF-β也可通过ROS放大上述促纤维化信号,诱导肌成纤维细胞和细胞外基质的形成和累积,加速纤维化的发生发展(图3)。
图 3.
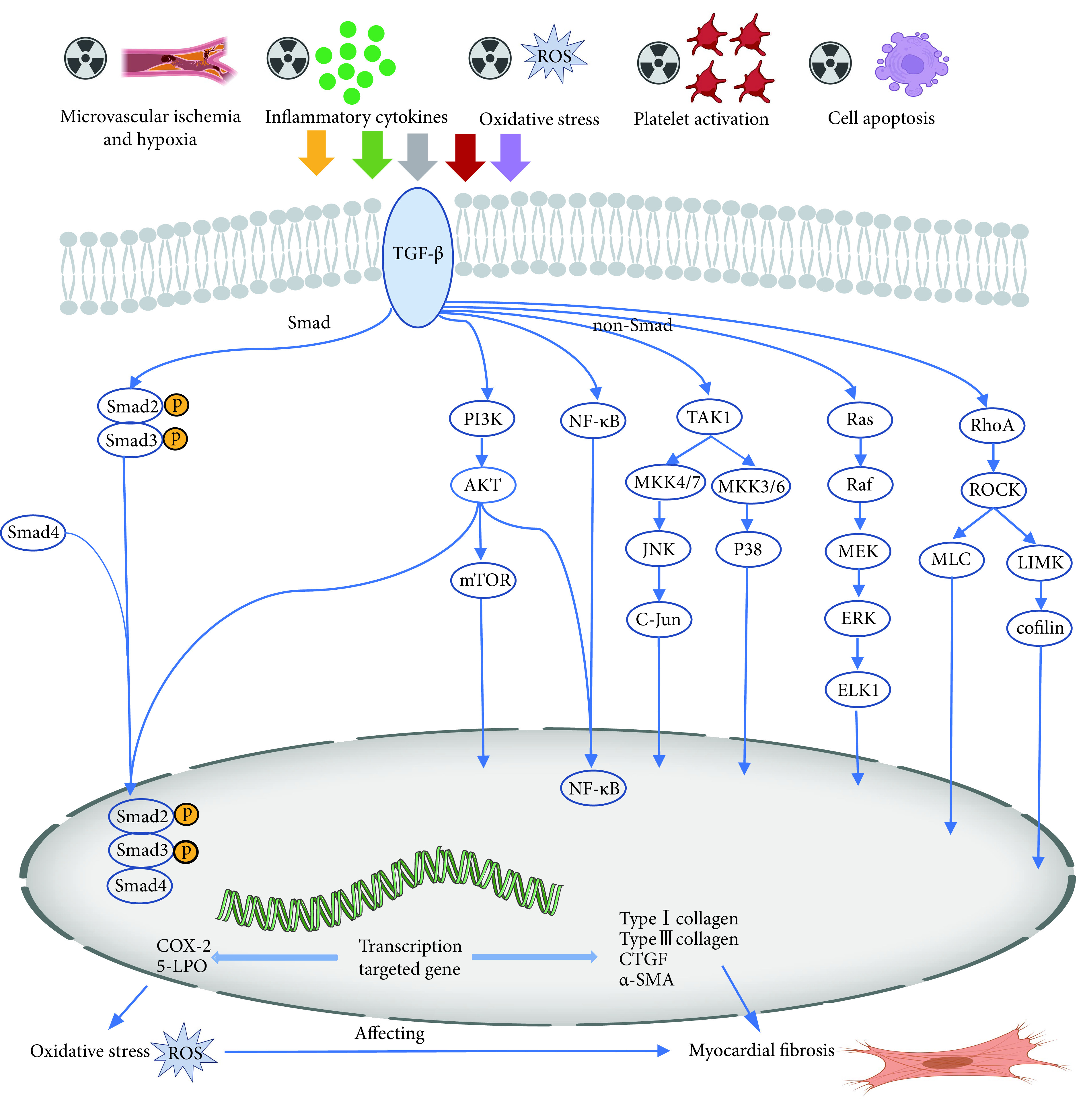
The role of TGF-β in myocardial fibrosis induced by radiotherapy
TGF-β在放疗导致心肌纤维化中的作用
ROS, TGF-β, NF-κB, COX-2, and 5-LPO: The denotations are the same as those in the notes for Fig 1 and Fig 2. PI3K: Phosphoinositide 3-kinase; AKT/PKB: Protein kinase B; mTOR: Mammalian target of rapamycin; TAK1: TGF-β-activated kinase 1; MKK: Mitogen-activated kinase kinase; JNK: c-Jun amino terminal kinase; Ras: Rat sarcoma; Raf: Rapidly accelerated fibrosarcoma; MEK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; ELK1: Ets-like protein 1; RhoA: Ras homolog family member A; ROCK: Rho Kinase; MLC: Myosin light chain; LIMK: LIM kinase; CTGF: Connective tissue growth factor; α-SMA: α-Smooth muscle actin.
心肌纤维化的另一个重要介质是血小板衍生生长因子(PDGF)家族的因子。研究发现通过转基因技术过度表达心脏PDGF-C和PDGF-D会导致广泛的心脏纤维化[36-37]。一项有趣的研究将循环microRNA鉴定为辐射诱发的心脏毒性的生物标志物,提示microRNA可能在RIHD的进程中发挥重要作用[38]。还有部分研究者发现放疗后肾素-血管紧张素-醛固酮系统激活可启动心肌细胞重塑[39]。另外,心脏照射可以增加肥大细胞的数量,可能也与RIHD的进展有关[40]。然而,在其他研究中发现肥大细胞缺乏的大鼠表现出比对照组更严重的改变[41],这表明肥大细胞在RIHD中可能起保护作用。总之,肥大细胞在RIHD中的具体作用仍存在一定争议。
3. 潜在治疗策略
缩小照射范围、降低照射剂量是预防RIHD最重要、最有效的措施。但由于胸腔放疗时心脏无法完全避免接受照射剂量,因此研发减轻RIHD的药物具有重要临床意义。
大量研究证实氧化应激在RIHD的进程中扮演重要角色[18],因此抗氧化可能是一种潜在治疗策略。在胸部放疗前使用己酮可可碱和α-生育酚等抗氧化剂可显著改善由辐射引起的左心室舒张压升高及心肌纤维化[42]。还有研究发现在单次高剂量心脏照射前应用氨磷汀等细胞保护剂可通过清除自由基以避免心肌出现纤维化和功能丧失[43]。此外,在动物模型中也观察到其他抗氧化剂有助于改善RIHD[44-48]。
缺血性心脏病和慢性心力衰竭的心脏保护药物也被用于减轻RIHD。在既往动物实验研究中,他汀类药物已显示出对大鼠的放射性心脏纤维化的治疗效果[49],但在其他研究中,发现给予阿托伐他汀后并没有改善放疗导致的小鼠的动脉粥样硬化[50]。RABENDER等[51]发现连续给予20周TGF-β受体1抑制剂IPW-5371可以保留接受胸腔照射的小鼠的心脏收缩功能,并改善心脏纤维化。在RIHD的大鼠模型上观察到骨髓间充质干细胞尾静脉注射可增强大鼠心脏功能,减轻心肌纤维化,可能是放射性心肌损伤患者治疗的新选择[52]。 其他一些药物,例如卡托普利、右雷佐生、黄芪生脉饮等在临床前实验中都显示出RIDH的治疗作用,但临床实用性尚未得到证实[53-56]。表1总结了能够缓解RIHD的潜在治疗策略。
表 1. Potential therapeutic strategies for alleviating RIHD.
缓解放射性心脏损伤的潜在治疗对策
| Strategy | Mechanism | Result |
| MSCs: Mesenchymal stem cells; MMPs: Matrix metalloproteinases; RAAS: Renin-angiotensin-aldosterone system; +: Positive result; −: Negative result. | ||
|
Pentoxifylline and α-tocopherol[42], Amifostine[43], black grape juice[44], Hydrogen-rich water[45], melatonin[46], hesperidin[47], lycopene[48] |
Antioxidant | + |
| Statins [49- 50] | Lower cholesterol | +, − |
| IPW-5371[51] | Inhibit TGF-β receptor 1 | + |
| MSCs[52] | Promote DNA repair | + |
| Captopril[53] | Inhibit the RAAS system | + |
| Dexrazoxane[54] | Anti-apoptotic | + |
| Colchicine[55] |
Anti-inflammatory and anti-coagulant |
+ |
| Huangqi Shengmai Yin[56] |
Regulate TGF-β1/Smads and MMPs |
+ |
4. 临床防治策略
由于RIHD多为晚期并发症,因此推荐有纵隔胸部放疗史的患者,即使无症状,在放疗后5~10年也应通过影像学及心肌标志物进行心脏风险评估。尤其是对于存在照射剂量>30 Gy、照射剂量<30 Gy但同时接受蒽环类药物治疗、年龄<50岁、每天单次照射剂量>2 Gy、心脏在照射野内、存在心血管危险因素和既往有心血管疾病等高危因素的放疗患者,应更早进行筛查以便评估是否进行早期干预[57]。
目前尚无预防或治疗 RIHD 的特定药物,因此RIHD的治疗方案与普通心脏病患者相同。对于放疗引起的冠状动脉疾病患者,治疗方式包括药物治疗、改变生活行为方式、经皮冠状动脉介入治疗和冠状动脉搭桥术等[58]。对于由放疗引起的瓣膜疾病的患者,通常建议其更换心脏瓣膜,且经导管主动脉瓣植入术可能更为合适[59]。此外,对于放疗引起的急性心包炎的患者可用利尿药及非甾体类抗炎药治疗以控制症状,而对于慢性心包炎患者,可考虑手术治疗但通常预后较差[60]。放射性心肌病的治疗手段与其他类型的心肌病相似,通常是针对相应症状进行处理;而在疾病末期心力衰竭阶段,心脏移植可能是一种可选择的治疗手段[61]。
5. 结论
尽管近些年胸腔放疗在技术和物理方面都有显著发展,但RIHD仍然是放疗毒副反应中需要关注的重要问题。目前对RIHD的分子机制的认识还不够清楚,相关临床研究报告也较少,阻碍了针对RIHD的精准及有效防治措施的发现。RIHD 进程中的各种信号通路可能是的潜在治疗靶点,现已证明了几种化合物的有效作用,但有关这些药物在临床中的研究数据有限,还需进一步研究来改善这一现状。
* * *
利益冲突 所有作者均声明不存在利益冲突
Contributor Information
华菊 杨 (Hua-ju YANG), Email: yhjyanghuaju@163.com.
炳文 邹 (Bing-wen ZOU), Email: zoubingwen81@163.com.
References
- 1.DARBY S C, EWERTZ M, MCGALE P, et al Risk of ischemic heart disease in women after radiotherapy for breast cancer. NEJM. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 2.HADDY N, DIALLO S, EL-FAYECH C, et al Cardiac diseases following childhood cancer treatment: Cohort study. Circulation. 2016;133(1):31–38. doi: 10.1161/CIRCULATIONAHA.115.016686. [DOI] [PubMed] [Google Scholar]
- 3.ALEMAN B M, VAN DEN BELT-DUSEBOUT A W, DE BRUIN M L, et al Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 4.VAN NIMWEGEN F A, SCHAAPVELD M, JANUS C P, et al Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Internal Medi. 2015;175(6):1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 5.LESTUZZI C, MASCARIN M, COASSIN E, et al. Cardiologic long-term follow-up of patients treated with chest radiotherapy: When and how? Front Cardiovasc Med, 2021, 8: 671001[2022-05-31]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8572927/.
- 6.CUTTER D J, SCHAAPVELD M, DARBY S C, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst, 2015, 107(4): djv008[2022-05-31].https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4394894/.
- 7.ELLAHHAM S, KHALOUF A, ELKHAZENDAR M, et al An overview of radiation-induced heart disease. Radiat Oncol J. 2022;40(2):89–102. doi: 10.3857/roj.2021.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CARLSON R G, MAYFIELD W R, NORMANN S, et al Radiation-associated valvular disease. Chest. 1991;99(3):538–545. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- 9.DONNELLAN E, PHELAN D, MCCARTHY C P, et al Radiation-induced heart disease: A practical guide to diagnosis and management. Cleve Clin J Med. 2016;83(12):914–922. doi: 10.3949/ccjm.83a.15104. [DOI] [PubMed] [Google Scholar]
- 10.WANG H, WEI J, ZHENG Q, et al Radiation-induced heart disease: A review of classification, mechanism and prevention. Int J Biol Sci. 2019;15(10):2128–2138. doi: 10.7150/ijbs.35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VEINOT J P, EDWARDS W D J H P Pathology of radiation-induced heart disease: A surgical and autopsy study of 27 cases. Hum Pathol. 1996;27(8):766–773. doi: 10.1016/S0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 12.ALA C K, KLEIN A L, MOSLEHI J J Cancer treatment-associated pericardial disease: Epidemiology, clinical presentation, diagnosis, and management. Curr Cardiol Rep. 2019;21(12):156. doi: 10.1007/s11886-019-1225-6. [DOI] [PubMed] [Google Scholar]
- 13.WALKER V, LAIREZ O, FONDARD O, et al Myocardial deformation after radiotherapy: A layer-specific and territorial longitudinal strain analysis in a cohort of left-sided breast cancer patients (BACCARAT study) Radiat Oncol. 2020;15(1):201. doi: 10.1186/s13014-020-01635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SáRKöZY M, VARGA Z, GÁSPÁR R, et al Pathomechanisms and therapeutic opportunities in radiation-induced heart disease: From bench to bedside. Clin Res Cardiol. 2021;110(4):507–531. doi: 10.1007/s00392-021-01809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CHANG H M, OKWUOSA T M, SCARABELLI T, et al Cardiovascular complications of cancer therapy: Best practices in diagnosis, prevention, and management: Part 2. J Am Coll Cardiol. 2017;70(20):2552–2565. doi: 10.1016/j.jacc.2017.09.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AZZAM E I, JAY GERIN J P, PAIN D Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1/2):48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MORGAN M J, LIU Z G Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PING Z, PENG Y, LANG H, et al. Oxidative stress in radiation-induced cardiotoxicity. Oxid Med Cell Longev, 2020, 2020: 3579143[2022-5-31]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7071808/.
- 19.SAKATA K, KONDO T, MIZUNO N, et al Roles of ROS and PKC-βII in ionizing radiation-induced eNOS activation in human vascular endothelial cells. Vascul Pharmacol. 2015;70:55–65. doi: 10.1016/j.vph.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 20.NAGANE M, KUPPUSAMY M L, AN J, et al Ataxia-telangiectasia mutated (ATM) kinase regulates eNOS expression and modulates radiosensitivity in endothelial cells exposed to ionizing radiation. Radiat Res. 2018;189(5):519–528. doi: 10.1667/RR14781.1. [DOI] [PubMed] [Google Scholar]
- 21.BASELET B, SONVEAUX P, BAATOUT S, et al Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell Mol Life Sci. 2019;76(4):699–728. doi: 10.1007/s00018-018-2956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LEE C L, MODING E J, CUNEO K C, et al p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal. 2012;5(234):ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BOERMA M, KRUSE J J, VAN LOENEN M, et al Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Strahlenther Onkol. 2004;180(2):109–116. doi: 10.1007/s00066-004-1138-0. [DOI] [PubMed] [Google Scholar]
- 24.SRIDHARAN V, AYKIN-BURNS N, TRIPATHI P, et al Radiation-induced alterations in mitochondria of the rat heart. Radiat Res. 2014;181(3):324–334. doi: 10.1667/RR13452.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BARJAKTAROVIC Z, SHYLA A, AZIMZADEH O, et al Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother Oncol. 2013;106(3):404–410. doi: 10.1016/j.radonc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 26.LIVINGSTON K, SCHLAAK R A, PUCKETT L L, et al. The role of mitochondrial dysfunction in radiation-induced heart disease: From bench to bedside. Front Cardiovasc Med, 2020, 7: 20[2022-05-31]. https://www.frontiersin.org/articles/10.3389/fcvm.2020.00020/full.
- 27.BAKER J E, FISH B L, SU J, et al 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol. 2009;85(12):1089–1100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.POSTOW M A, CALLAHAN M K, BARKER C A, et al Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.BECKER B V, SEEGER T, BEIERT T, et al Impact of ionizing radiation on electrophysiological behavior of human-induced iPSC-derived cardiomyocytes on multielectrode arrays. Health Phys. 2018;115(1):21–28. doi: 10.1097/HP.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 30.LUCZAK E D, ANDERSON M E CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol. 2014;73:112–116. doi: 10.1016/j.yjmcc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DREYFUSS A D, GOIA D, SHONIYOZOV K, et al A novel mouse model of radiation-induced cardiac injury reveals biological and radiological biomarkers of cardiac dysfunction with potential clinical relevance. Clin Cancer Res. 2021;27(8):2266–2276. doi: 10.1158/1078-0432.CCR-20-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WANG B, WEI J, MENG L, et al. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed Pharmacother, 2020, 121: 109560[2022-05-31]. https://www.sciencedirect.com/science/article/pii/S0753332219351832?via%3Dihub.
- 33.AHAMED J, LAURENCE J Role of platelet-derived transforming growth factor-β1 and reactive oxygen species in radiation-induced organ fibrosis. Antioxid Redox Signal. 2017;27(13):977–988. doi: 10.1089/ars.2017.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FARHOOD B, KHODAMORADI E, HOSEINI-GHAHFAROKHI M, et al. TGF-β in radiotherapy: Mechanisms of tumor resistance and normal tissues injury. Pharmacol Res, 2020, 155: 104745[2022-05-31]. https://www.sciencedirect.com/science/article/abs/pii/S104366181933049X?via%3Dihub.
- 35.ZHANG Y E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb Perspect Biol, 2017, 9(2): a022129[2022-05-31]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5287080/.
- 36.PONTÉN A, LI X, THORÉN P, et al Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol. 2003;163(2):673–682. doi: 10.1016/S0002-9440(10)63694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.PONTÉN A, BERGSTEN FOLESTAD E, PIETRAS K, et al Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res. 2005;97(10):1036–1045. doi: 10.1161/01.RES.0000190590.31545.d4. [DOI] [PubMed] [Google Scholar]
- 38.HAWKINS P G, SUN Y, DESS R T, et al Circulating microRNAs as biomarkers of radiation-induced cardiac toxicity in non-small-cell lung cancer. J Cancer Res Clin Oncol. 2019;145(6):1635–1643. doi: 10.1007/s00432-019-02903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WANG B, WANG H, ZHANG M, et al Radiation-induced myocardial fibrosis: Mechanisms underlying its pathogenesis and therapeutic strategies. J Cell Mol Med. 2020;24(14):7717–7729. doi: 10.1111/jcmm.15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.BOERMA M, ZURCHER C, ESVELDT I, et al Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncol Rep. 2004;12(2):213–219. doi: 10.3892/or.12.2.213. [DOI] [PubMed] [Google Scholar]
- 41.BOERMA M, WANG J, WONDERGEM J, et al Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65(8):3100–3107. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 42.BOERMA M, ROBERTO K A, HAUER-JENSEN M Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72(1):170–177. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.KRUSE J J, STROOTMAN E G, WONDERGEM J Effects of amifostine on radiation-induced cardiac damage. Acta Oncol. 2003;42(1):4–9. doi: 10.1080/0891060310002168. [DOI] [PubMed] [Google Scholar]
- 44.DE FREITAS R B, BOLIGON A A, ROVANI B T, et al Effect of black grape juice against heart damage from acute gamma TBI in rats. Molecules. 2013;18(10):12154–12167. doi: 10.3390/molecules181012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.QIAN L, CAO F, CUI J, et al The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res. 2010;51(6):741–747. doi: 10.1269/jrr.10093. [DOI] [PubMed] [Google Scholar]
- 46.GÜRSES I, ÖZEREN M, SERIN M, et al Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol Res Pract. 2014;210(12):863–871. doi: 10.1016/j.prp.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 47.REZAEYAN A, HADDADI G H, HOSSEINZADEH M, et al Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J Med Phys. 2016;41(3):182–191. doi: 10.4103/0971-6203.189482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.GAJOWIK A, DOBRZYŃSKA M M The evaluation of protective effect of lycopene against genotoxic influence of X-irradiation in human blood lymphocytes. Radiat Environ Biophys. 2017;56(4):413–422. doi: 10.1007/s00411-017-0713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ZHANG K, HE X, ZHOU Y, et al Atorvastatin ameliorates radiation-induced cardiac fibrosis in rats. Radiat Res. 2015;184(6):611–620. doi: 10.1667/RR14075.1. [DOI] [PubMed] [Google Scholar]
- 50.HOVING S, HEENEMAN S, GIJBELS M J, et al Anti-inflammatory and anti-thrombotic intervention strategies using atorvastatin, clopidogrel and knock-down of CD40L do not modify radiation-induced atherosclerosis in ApoE null mice. Radiother Oncol. 2011;101(1):100–108. doi: 10.1016/j.radonc.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 51.RABENDER C, MEZZAROMA E, MAURO A G, et al IPW-5371 proves effective as a radiation countermeasure by mitigating radiation-induced late effects. Radiat Res. 2016;186(5):478–488. doi: 10.1667/RR14403.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GAO S, ZHAO Z, WU R, et al Bone marrow mesenchymal stem cell transplantation improves radiation-induced heart injury through DNA damage repair in rat model. Radiat Environ Biophys. 2017;56(1):63–77. doi: 10.1007/s00411-016-0675-0. [DOI] [PubMed] [Google Scholar]
- 53.VAN DER VEEN S J, GHOBADI G, DE BOER R A, et al ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114(1):96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 54.LI L, NIE X, ZHANG P, et al Dexrazoxane ameliorates radiation-induced heart disease in a rat model. Aging (Albany NY) 2021;13(3):3699–3711. doi: 10.18632/aging.202332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'HERRON T, LAFFERTY J Prophylactic use of colchicine in preventing radiation induced coronary artery disease. Med Hypotheses. 2018;111:58–60. doi: 10.1016/j.mehy.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 56.GU J, LIU Y, WU H, et al. Huangqi Shengmai Yin protects against radiation-induced cardiac fibrosis injury by regulating the TGF-beta1/Smads and MMPs. Evid Based Complement Alternat Med, 2019, 2019: 1358469[2022-05-31]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6535819/.
- 57.MITCHELL J D, CEHIC D A, MORGIA M, et al Cardiovascular manifestations from therapeutic radiation: A multidisciplinary expert consensus statement from the international cardio-oncology society. JACC Card Oncol. 2021;3(3):360–380. doi: 10.1016/j.jaccao.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DESAI M Y, WINDECKER S, LANCELLOTTI P, et al Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC scientific expert panel. J Am Coll Cardiol. 2019;74(7):905–927. doi: 10.1016/j.jacc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 59.ZHANG D, GUO W, AL-HIJJI M A, et al. Outcomes of patients with severe symptomatic aortic valve stenosis after chest radiation: Transcatheter versus surgical aortic valve replacement. J Am Heart Assoc, 2019, 8(10): e012110[2022-05-31]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6585322/.
- 60.SZPAKOWSKI N, DESAI M Y Radiation-associated pericardial disease. Curr Cardiol Rep. 2019;21(9):97. doi: 10.1007/s11886-019-1192-y. [DOI] [PubMed] [Google Scholar]
- 61.SAXENA P, JOYCE L D, DALY R C, et al Cardiac transplantation for radiation-induced cardiomyopathy: The Mayo clinic experience. Ann Thorac Surg. 2014;98(6):2115–2121. doi: 10.1016/j.athoracsur.2014.06.056. [DOI] [PubMed] [Google Scholar]


