Abstract
目的
探索伏立康唑在隐球菌脑膜炎患者中枢神经系统的分布浓度及其关系。
方法
回顾性分析25例非HIV感染的隐球菌脑膜炎成人患者的伏立康唑脑脊液谷浓度与血液谷浓度,并探索影响脑脊液/血液浓度比值的患者因素。
结果
伏立康唑血液谷浓度范围0.38~8.56 mg/L,中位数(P25,P75)为1.81(1.40,3.84) mg/L。伏立康唑脑脊液谷浓度范围0.17~3.92 mg/L,中位数(P25, P75)为1.02 (0.54,1.84) mg/L。伏立康唑脑脊液浓度与脑脊液有核细胞数呈负相关,但无统计学意义(r=−0.377,P=0.063)。伏立康唑脑脊液浓度与血液浓度呈正相关(r=0.736,P<0.001),脑脊液/血液浓度比值中位数(P25,P75)为0.43(0.34,0.68)。脑脊液/血液浓度比值与年龄、体表面积、影像学改变(脑积水)或颅内压无关。
结论
伏立康唑脑脊液浓度与血液浓度呈正相关,且未发现影响其比值的因素。
Keywords: 伏立康唑, 隐球菌, 脑脊液, 脑脊液/血液浓度比值, 影响因素
Abstract
Objective
To investigate the concentrations of voriconazole (VCZ) in the central nervous system (CNS) of patients with cryptococcal meningitis and the relationship thereof.
Methods
We retrospectively analyzed the trough concentration of VCZ in the cerebrospinal fluid (CSF) and the blood of 25 adult patients who had cryptococcal meningitis, and who were not infected with HIV. We also examined patient-level characteristics that could contribute to the differences in CSF/plasma VCZ concentration ratio.
Results
The trough concentration of VCZ in plasma ranged from 0.38 to 8.56 mg/L, and the median (P25, P75) was 1.81 (1.40, 3.84) mg/L. The trough concentration of VCZ in CSF ranged from 0.17 to 3.92 mg/L, and the median (P25, P75) was 1.02 (0.54, 1.84) mg/L. The CSF VCZ trough concentration showed a slight negative correlation with the nucleated cell counts in CSF, but the correlation was not statistically significant (r=−0.377, P=0.063). There was a positive correlation between VCZ concentrations in CSF and that in the plasma (r=0.736, P<0.001), and the median (P25, P75) CSF/plasma ratio was 0.43 (0.34, 0.68). The CSF/plasma ratio did not statistically correlate with age, body surface area (BSA), radiology changes (hydrocephalus), or intracranial pressure.
Conclusion
There is a positive correlation between VCZ concentration in CSF and VCZ concentration in the plasma, and no influencing factors of CSF/plasma ratio were found.
Keywords: Voriconazole, Cryptococcus, Cerebrospinal fluid, Cerebrospinal fluid-to-plasma ratio, Influence factor
抗真菌药物的血脑屏障透过率和脑脊液(cerebrospinal fluid, CSF)浓度对隐球菌脑膜炎患者预后可能至关重要。两性霉素B、伊曲康唑、泊沙康唑中枢神经系统渗透性极差;氟康唑渗透性良好但为抑菌剂,单药治疗仅适用于在诱导期之后抑制真菌复发[1]。临床研究证实伏立康唑在难治性隐球菌脑膜炎方面具有治疗前景[2]。但伏立康唑脑脊液浓度真实世界数据极少,2003年LUTSAR等[3]首次报道了伏立康唑脑脊液浓度,接下来的10年未见同类研究,直到2014年WIEDERHOLD等[4]报道了173份脑脊液样品的伏立康唑浓度,但这两项研究均未要求在相同时间采集脑脊液与血液浓度标本。收集在相同时间检测了脑脊液和血液浓度的病例十分困难,导致伏立康唑脑脊液/血液浓度比值不精准。本回顾性研究仅纳入相同时间采集伏立康唑脑脊液和血液谷浓度的患者,使脑脊液浓度与血液浓度具有可比性,探索脑脊液/血液浓度比值的影响因素。
1. 资料与方法
1.1. 伦理
本研究为回顾性研究,仅提取电子病历数据和使用有限的数据,患者风险极小。本研究已获四川大学华西医院伦理委员会批准,伦理批准号2020年审(751)号。
1.2. 对象
回顾性审查了四川大学华西医院感染性疾病中心2018年7月–2020年6月收治的隐球菌脑膜炎住院患者资料。纳入所有接受伏立康唑治疗,且相同时间检测脑脊液和血液谷浓度的患者。
1.3. 检测方法
隐球菌脑膜炎是通过脑脊液墨汁染色或真菌培养阳性诊断的,治疗方案为伏立康唑(静脉滴注或口服)单用,或联用两性霉素B或氟胞嘧啶。
伏立康唑给药72 h后采集血液和脑脊液样本,在晨起给药前30 min采集血液谷浓度,采集血液样本后30 min内进行腰椎穿刺以获取脑脊液样本和用压力计测颅内压。实验医学科采用超高效液相色谱-串联质谱法测定血液和脑脊液中伏立康唑浓度:取样本上层清液100 μL置于离心管中,加50 μL内标液(0.2 μg/mL赛庚啶)、50 μL缓冲液(pH9.2)、1 mL甲基叔丁基醚,旋涡混匀,放置1 min,12000 r/min离心5 min,取上层清液800 μL,40 ℃挥干浓缩,加150 μL流动相〔0.02 mol/L醋酸铵缓冲液(pH3.0)-乙腈(60∶40)〕复溶,进样4 μL检测,色谱柱为Waters ACQUITY UPLC BEH C18(2.1×50 mm,1.7 μm),流动相流速0.3 mL/min,采用电喷雾离子源,多离子反应监测模式,正离子扫描进行测定(伏立康唑m/z 350.4→127.2,赛庚啶m/z 288.1→96.2)。
对脑脊液隐球菌培养阳性者,根据美国临床实验室标准学会(Clinical Laboratory Standards Institute, CLSI)标准测定伏立康唑最小抑菌浓度(minimum inhibitory concentration, MIC)[5]。
1.4. 检测指标
通过病历回顾,收集了患者的年龄、性别、体表面积、基础疾病/并发症、伏立康唑代谢酶细胞色素P450(cytochrome P450, CYP)2C19基因(CYP2C19)多态性检测结果、影像学变化(是否有脑积水)、对治疗的短期反应和出院时的结局。根据伏立康唑治疗4周内的疾病预后情况,将患者对治疗的短期反应分为:改善(症状体征改善,脑脊液持续无菌)、稳定(症状体征改善,伴有墨汁染色或真菌培养阳性)和恶化(症状体征持续或恶化,隐球菌复发,或影像学恶化)。从病历提取患者出院时的结局(死亡或存活)。评估伏立康唑脑脊液浓度与各指标的相关性,包括血药浓度、脑脊液有核细胞计数和影像学改变(是否有脑积水)。评估伏立康唑脑脊液/血液浓度比值与各指标的相关性,如年龄、体表面积、颅内压、影像学改变(是否有脑积水)和给药途径(口服或静脉滴注)。
1.5. 统计学方法
计量资料先进行正态性、方差齐性检验,正态分布的计量资料以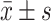 表示,两组间比较采用t检验,不符合正态分布者以中位数(P25,P75)表示,两组间比较采用Mann-Whitney U检验;两个连续变量的相关性分析采用Pearson相关系数。P≤0.05为差异有统计学意义。
表示,两组间比较采用t检验,不符合正态分布者以中位数(P25,P75)表示,两组间比较采用Mann-Whitney U检验;两个连续变量的相关性分析采用Pearson相关系数。P≤0.05为差异有统计学意义。
2. 结果
2.1. 接受伏立康唑治疗的隐球菌脑膜炎患者的人口学及临床特征
25例符合纳入标准的患者全部纳入研究(表1)。患者样本在性别上大致平衡(男12例、女13例),中位年龄41岁(范围27~67岁)。患者均未患有艾滋病,1例合并肾移植(No.1),1例合并系统性红斑狼疮和狼疮肾炎(No.25),其他患者在隐球菌脑膜炎发病前相对健康。
表 1. Demographic and clinical data characteristics of patients who received voriconazole for cryptococcal meningitis.
接受伏立康唑治疗的隐球菌脑膜炎患者的人口学及临床特征
| Patient No. | Age/yr. | Sex# | BSA/m2 | Radiology changes | Nucleated cell count in CSF/uL | Intracranial pressure/mmH2O | Underling diseases or comorbitity | Response to therapy | VCZ dose | MIC of isolates if culture positive/(mg/L) | VCZ concentration | Treatment▲ | Hospital discharge status△ | ||
| CSF/(mg/L) | Plasma/(mg/L) | CSF/plasma ratio | |||||||||||||
| BSA: body surface area; CSF: cerebrospinal fluid; SLE: systemic lupus erythematosus; LN: lupus nephritis; VCZ: voriconazole.†The CSF sample of patient No. 4 was obtained from lumbar drainage (the CSF samples of all other patients were obtained via lumbar puncture); *Patients with CYP2C19 gene polymorphism test results showed extensive metabolizer (EM); #M: male; F: female. Radiology changes: hydrocephalus. §The dosage was adjusted because the plasma concentration test results were not in the recommended range (1.5-5.5 mg/L). ▲Dex: dexamethasone; 5-Flu: 5-flucytosine; Pred: prednisone; MP: methylprednisolone; HCQ: hydroxychloroquine. △T: therapy continued but stable; L: leave hospital voluntarily but stable. 1 mmH2O=0.0098 kPa. | |||||||||||||||
| 1 | 42 | M | 1.66 | None | 15 | 110 | Kidney transplantation | Improved | 0.2 g bid po | 0.03 | 0.56 | 1.56 | 0.36 | VCZ+5-Flu+Pred | Cured |
| 2 | 44 | F | 1.43 | None | 6 | 230 | Anemia | Improved | 0.2 g bid po | None | 3.24 | 3.97 | 0.82 | VCZ+Amphotericin B | T |
| 3 | 45 | F | 1.43 | None | 10 | 230 | None | Improved | 0.2 g bid po | None | 0.72 | 2.05 | 0.35 | VCZ+Amphotericin B | T |
| 4† | 56 | M | 1.78 | None | 10 | 200 | Diabetes mellitus | Improved | 0.2 g bid po | 0.25 | 1.9 | 3.72 | 0.51 | VCZ | T |
| 5 | 41 | M | 1.73 | None | 11 | 230 | HBV infection | Improved | 0.2 g q12h iv | None | 3.92 | 4.8 | 0.82 | VCZ+Amphotericin B | T |
| 6 | 38 | F | 1.32 | None | 15 | 150 | Subacute thyroiditis | Improved | 0.2 g q12h iv | None | 0.6 | 1.4 | 0.43 | VCZ+5-Flu | T |
| 7 | 44 | F | 1.51 | None | 19 | 230 | Cirrhosis | Stable | 0.2 g q12h iv | 0.12 | 1.79 | 5.1 | 0.35 | VCZ | T |
| 8 | 34 | F | 1.62 | None | 60 | 200 | None | Improved | 0.2 g bid po | 0.06 | 0.4 | 1.4 | 0.29 | VCZ+Amphotericin B+Pred | T |
| 9 | 35 | F | 1.63 | None | 330 | 230 | None | Improved | 0.2 g bid po | 0.12 | 0.66 | 1.75 | 0.38 | VCZ+Amphotericin B+Pred | T |
| 10 | 39 | F | 1.30 | None | 50 | 160 | Depression | Improved | 0.2 g q12h iv | 0.25 | 1.34 | 1.81 | 0.74 | VCZ+Amphotericin B+Dex | T |
| 11* | 40 | F | 1.33 | None | 140 | 150 | None | Improved | 0.2 g q12h iv | None | 0.42 | 2.59 | 0.16 | VCZ+Amphotericin B+Dex | T |
| 12 | 30 | F | 1.25 | None | 10 | 93 | None | Improved | 0.2 g q12h iv | 0.12 | 2.07 | 5.71 | 0.36 | VCZ+Amphotericin B+5-Flu+MP | T |
| 13 | 47 | M | 1.74 | None | 140 | 150 | Pheumonia | Stable | 0.2 g q12h iv | None | 0.42 | 2.59 | 0.16 | VCZ+Amphotericin B+Dex | L |
| 14 | 47 | M | 1.73 | None | 20 | 120 | HBV infection | Improved | 0.2 g q12h iv | None | 1.64 | 2.73 | 0.60 | VCZ+Amphotericin B+Dex | T |
| 15 | 45 | F | 1.69 | None | 18 | 170 | Pheumonia | Improved | 0.2 g q12h iv | 0.12 | 1.16 | 1.73 | 0.67 | VCZ+Amphotericin B+Pred | T |
| 16 | 39 | M | 1.69 | None | 2 | 230 | Hypertention | Improved | 0.2 g q12h iv | None | 1.1 | 1.4 | 0.79 | VCZ+Dex | T |
| 17* | 27 | M | 1.74 | Yes | 80 | 120 | HBV infection | Improved | 0.2 g bid po | 0.12 | 0.17 | 0.38§ | 0.45 | VCZ+5-Flu | T |
| 18* | 32 | M | 1.77 | Yes | 0 | 170 | None | Improved | 0.2 g bid po | 0.008 | 2.19 | 5.82 | 0.38 | VCZ+5-Flu | T |
| 19 | 38 | F | 1.32 | Yes | 40 | 120 | None | Improved | 0.2 g q12h iv | None | 0.59 | 1.17 | 0.50 | VCZ+5-Flu | T |
| 20 | 67 | M | 1.69 | Yes | 80 | 100 | None | Improved | 0.15g bid po | None | 0.41 | 1.29 | 0.32 | VCZ+Amphotericin B | T |
| 21 | 44 | F | 1.41 | Yes | 0 | 230 | None | Improved | 0.2 g bid po | 0.15 | 0.51 | 1.65 | 0.31 | VCZ+Amphotericin B | T |
| 22 | 43 | F | 1.41 | Yes | 21 | 190 | None | Improved | 0.2 g bid po | None | 1.02 | 1.5 | 0.68 | VCZ+Amphotericin B | T |
| 23 | 33 | M | 1.86 | Yes | 40 | 230 | None | Improved | 0.3g q12h iv | None | 1.02 | 2.24 | 0.46 | VCZ+Amphotericin B liposome | Cured |
| 24* | 31 | M | 1.78 | Yes | 0 | 170 | T cell dysfunction | Improved | 0.2 g q12h iv | None | 2.29 | 8.56§ | 0.27 | VCZ+5-Flu | T |
| 25 | 41 | M | 1.41 | Yes | 20 | 170 | SLE, LN | Stable | 0.15 g bid po | None | 0.6 | 0.79§ | 0.76 | VCZ+5-Flu+MP+HCQ | Death |
11例患者隐球菌培养阳性,均对伏立康唑敏感,MIC范围0.008~0.25 μg/mL。4例患者进行了CYP2C19基因多态性检测,均为正常代谢型。大多数患者在伏立康唑治疗4周内的反应为改善(22/25)或稳定(3/25)。1例长期用药患者(No.25)在出院前因病情恶化而死亡。
2.2. 伏立康唑血液及脑脊液谷浓度
伏立康唑血液谷浓度范围0.38~8.56 mg/L,中位数(P25, P75)为1.81(1.40,3.84) mg/L。我院实验医学科推荐的参考范围为1.5~5.5 mg/L。7例患者的样本低于该推荐浓度范围下限,3例患者的样本高于该推荐浓度范围上限,但这10例患者中只有3例(No.17、No.24、No.25)随后进行了剂量调整。
伏立康唑脑脊液谷浓度范围0.17~3.92 mg/L,中位数(P25, P75)为1.02(0.54,1.84) mg/L,脑脊液/血液浓度比值范围0.16~0.82,中位数(P25, P75)为0.43(0.34,0.68)。
伏立康唑脑脊液谷浓度与血液谷浓度呈中度正相关(r=0.736,P<0.001)(图1);与脑脊液有核细胞数呈负相关,但无统计学意义(r=−0.377,P=0.063)(图2)。在有无影像学改变的两组患者间(有脑积水组9例、无脑积水组16例),伏立康唑脑脊液浓度差异无统计学意义(Mann-Whitney U检验,U=54.500,Z=−0.991,P=0.330)(图3)。
图 1.
Correlation between VCZ concentration in CSF and plasma VCZ concentration
伏立康唑脑脊液与血液浓度相关性
图 2.
Correlation between VCZ concentration in CSF and CSF nucleated cell count
伏立康唑脑脊液浓度与脑脊液有核细胞数相关性
图 3.
Distribution of VCZ concentration in the CSF of patients without hydrocephalus and those with hydrocephalus
脑积水患者与无脑积水患者伏立康唑脑脊液浓度的分布
2.3. 伏立康唑脑脊液/血液浓度比值的影响因素
伏立康唑脑脊液/血液浓度比值与年龄(图4)、体表面积(图5)、颅内压(图6)、影像学改变(图7)、给药途径(图8)均无相关性。
图 4.
Correlation between CSF/plasma concentration ratio of VCZ and age
伏立康唑脑脊液/血液浓度比值与年龄的相关性
图 5.
Correlation between CSF/plasma ratio of VCZ and body surface area (BSA)
伏立康唑脑脊液/血液浓度比值与体表面积的相关性
图 6.
Correlation between CSF/plasma ratio of VCZ and intracranial pressure
伏立康唑脑脊液/血液浓度比值与颅内压的相关性
1 mmH2O=0.0098 kPa.
图 7.
Distribution of CSF/plasma ratio of VCZ in patients with or without radiology changes ( i. e., hydrocephalus)
脑积水患者与无脑积水患者伏立康唑脑脊液/血液浓度比值的分布
图 8.
Distribution of VCZ CSF/plasma ratio in patients who received VCZ through oral administration and those who received VCZ through injection
口服患者与注射患者伏立康唑脑脊液/血液浓度比值的分布
3. 讨论
隐球菌感染易发于但不限于免疫功能低下者,尤其是艾滋病或器官移植患者,最常见的感染部位是中枢神经系统,隐球菌脑膜炎预后不良。抗真菌治疗是隐球菌脑膜炎的主要治疗方法,两性霉素B联合氟胞嘧啶是主要方案,尽管两性霉素B中枢神经系统透过率低且存在严重不良反应风险,但临床医生仍认可两性霉素B在诱导期的强效杀菌作用。近年来,伏立康唑因其中枢神经系统渗透性好和抗真菌作用强,在隐球菌脑膜炎治疗中发挥着越来越重要的作用。伏立康唑是小分子亲脂性抗真菌药物(相对分子质量349,脂水分配系数1.8),血浆蛋白结合能力中等,这些特征使其具有良好的生物膜穿透性[6]。我们在隐球菌脑膜炎患者样本(大多数无免疫受限)中,测定了伏立康唑脑脊液/血液浓度比值的中位数为0.43(范围0.16~0.82)。本研究的发现与文献报道相似,LUTSAR等[3]发现免疫缺陷患者脑脊液/血液比值中位数为0.46(范围0.22~1.00),WIEDERHOLD等[4]报道的比值中位数为0.52(范围0.00~1.22)。
本研究的药敏试验数据也证实伏立康唑具有良好的抗真菌作用。根据CLSI标准,若伏立康唑对隐球菌MIC≥1 μg/mL则为耐药[5,7]。11例患者分离出的隐球菌对伏立康唑均敏感,其MIC范围0.008~0.25 μg/mL。本研究中,伏立康唑脑脊液谷浓度相对较低,但远高于分离株的MIC值。笔者推断,除No.25患者(合并系统性红斑狼疮和狼疮肾炎)外,大多数患者伏立康唑脑脊液浓度较低但已足够,均可获得较好的治疗效果。
迄今为止,隐球菌脑膜炎治疗用药伏立康唑的治疗药物监测(therapeutic drug monitoring, TDM)仍采用血液标本,尽管脑脊液药物浓度监测在剂量调整中更为直接和准确。伏立康唑血液浓度与曲霉病等侵袭性真菌病的临床结局有明显的相关性[4,8-10]。但在隐球菌脑膜炎治疗中,血液或脑脊液浓度推荐范围尚无共识。本研究为隐球菌脑膜炎患者的伏立康唑脑脊液浓度提供了初步数据,如预期的那样,伏立康唑脑脊液浓度与血液浓度呈正相关,相关性为中等强度(r=0.736)。脑脊液/血液浓度比值离散程度大(范围0.16~0.82),提示伏立康唑脑脊液浓度与血液浓度可能存在偏差,为后续脑脊液TDM研究奠定了基础。研究还发现伏立康唑脑脊液浓度与脑脊液有核细胞数负相关,但无统计学意义。笔者认为,较高的脑脊液药物浓度具有更好的抗菌作用,这将减轻炎症反应,表现为较低的有核细胞数。
研究不同个体伏立康唑脑脊液/血液浓度比值差异的其他影响因素也有价值。之前的研究分析了颅内压和给药途径[11],颅内压的升高可能是有效给药的障碍[12],然而本研究并没有发现伏立康唑脑脊液/血液浓度比值与颅内压之间的显著相关性,另一研究显示颅内压变化不影响脑脊液两性霉素B、氟康唑的浓度[13]。有研究显示脑脊液/血液浓度比值与年龄呈负相关[14],本研究未发现这一规律。本研究发现脑脊液/血液浓度比值与体表面积、影像学改变或给药途径无相关性。CYP2C19等代谢酶参与肝微粒体伏立康唑的代谢,CYP2C19具有基因多态性,个体基因突变会改变伏立康唑代谢速率。本研究仅4例患者检测了CYP2C19基因多态性,均为正常代谢型。
隐球菌脑膜炎最佳治疗效果取决于脑膜组织中有生物活性的抗真菌药物的浓度,但组织药物浓度难以测得,备选检测脑脊液浓度。用脑脊液浓度替代组织浓度,可能会低估脑膜或脑实质浓度[1]。动物实验证实伏立康唑有很好的脑组织分布,豚鼠伏立康唑脑间质浓度为血药浓度的200%,脑脊液浓度为血药浓度的50%[3]。2003年LUTSAR等[3]对14名患者36份脑脊液样本进行了伏立康唑浓度检测,对2例治疗后死亡的患者取脑组织标本,结果显示伏立康唑脑组织浓度高于脑脊液浓度。HENRY等[15]通过磁共振波谱法测得健康成人伏立康唑脑组织/血液浓度比值在给药前(稳态谷浓度)为3.0,给药后2 h为1.9。基于脑脊液浓度制定伏立康唑TDM策略时,必须考虑到药物在血液、脑脊液、脑膜和脑实质之间的分布差异。
本研究是一项回顾性的基于单一机构的小样本研究,因为收集在相同时间检测了脑脊液和血液浓度的病例十分困难。据笔者所知,这是我国医疗机构首次对伏立康唑脑脊液浓度进行研究,填补了伏立康唑TDM真实世界数据研究的空白。本研究得出的分析结果需要在更大的样本中进行验证。其次,研究样本仅代表成人人群,而文献提示儿童患者的血液、脑脊液伏立康唑浓度变异更大[4,14],对儿科患者,更严格地基于TDM使用伏立康唑是必要的。
总之,本研究是独特的,纳入了一组隐球菌脑膜炎成人患者,定量研究了脑脊液药物浓度与血药浓度之间的关系,为未来伏立康唑治疗隐球菌脑膜炎开发更精准的TDM管理策略提供了重要数据。未来的研究需要纳入更大数量、更具代表性、多样性的患者样本,以便更好地收集患者特征,包括可能致伏立康唑代谢变异的遗传因素。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
四川省科技计划(No. 2020YFS0138、No. 2020YFQ0010)资助
Contributor Information
桂荣 肖 (Gui-rong XIAO), Email: 407903196@qq.com.
光敏 唐 (Guang-min TANG), Email: 52930382@qq.com.
References
- 1.FELTON T, TROKE P F, HOPE W W Tissue penetration of antifungal agents. Clin Microbiol Rev. 2014;27(1):68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PERFECT J R, MARR K A, WALSH T J, et al Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36(9):1122–1131. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- 3.LUTSAR I, ROFFEY S, TROKE P Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis. 2003;37(5):728–732. doi: 10.1086/377131. [DOI] [PubMed] [Google Scholar]
- 4.WIEDERHOLD N P, PENNICK G J, DORSEY S A, et al A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother. 2014;58(1):424–431. doi: 10.1128/AAC.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FOTHERGILL A W. Antifungal susceptibility testing: Clinical Laboratory and Standards Institute (CLSI) methods// Hall G S. Interactions of yeasts, moulds, and antifungal agents. New Jersey: Humana Press, 2012: 65−74.
- 6.KETHIREDDY S, ANDES D CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol. 2007;3(4):573–581. doi: 10.1517/17425255.3.4.573. [DOI] [PubMed] [Google Scholar]
- 7.PFALLER M A, ANDES D, ARENDRUP M C, et al Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis. 2011;70(3):330–343. doi: 10.1016/j.diagmicrobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 8.KUO I F, ENSOM M H Role of therapeutic drug monitoring of voriconazole in the treatment of invasive fungal infections. Can J Hosp Pharm. 2009;62(6):469–482. doi: 10.4212/cjhp.v62i6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PASCUAL A, CALANDRA T, BOLAY S, et al Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 10.TROKE P F, HOCKEY H P, HOPE W W Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother. 2011;55(10):4782–4788. doi: 10.1128/AAC.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CHANG T, OLSON J A, PROFFITT R T, et al Differences in tissue drug concentrations following intravenous versus intraperitoneal treatment with amphotericin B deoxycholate or liposomal amphotericin B. Med Mycol. 2010;48(2):430–435. doi: 10.3109/13693780903208249. [DOI] [PubMed] [Google Scholar]
- 12.LO E H, SINGHAL A B, TORCHILIN V P, et al Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38(1-2):140–148. doi: 10.1016/S0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.WIRTH F, De AZEVEDO M I, PILLA C, et al Relationship between intracranial pressure and antifungal agents levels in the CSF of patients with cryptococcal meningitis. Med Mycol. 2018;56(3):257–262. doi: 10.1093/mmy/myx054. [DOI] [PubMed] [Google Scholar]
- 14.KOBAYASHI R, SANO H, KISHIMOTO K, et al Voriconazole concentrations in cerebrospinal fluid during prophylactic use in children with acute myelogenous leukemia. Pediatr Infect Dis J. 2016;35(3):297–298. doi: 10.1097/INF.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 15.HENRY M E, BOLO N R, ZUO C S, et al Quantification of brain voriconazole levels in healthy adults using fluorine magnetic resonance spectroscopy. Antimicrob Agents Chemother. 2013;57(11):5271–5276. doi: 10.1128/AAC.00394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]










