Abstract
目的
探讨外周全血中异常激活的T淋巴细胞亚群与人类免疫缺陷病毒1型(HIV-1)感染者免疫功能恢复的关系,探讨HIV-1 DNA病毒库的大小与T淋巴细胞亚群的关系。
方法
以2019年7月–2020年5月进行常规检测的HIV-1感染者为目标人群,入组时根据抗逆转录病毒疗法(antiretroviral therapy, ART)治疗后CD4+ T细胞是否达到500 cells/ μL,将HIV-1感染者分为两组,其中缺陷组76例,无缺陷组61例。同时选择22例未暴露且HIV-1抗体检测阴性者作为对照组。对以上3组人群的T细胞亚群进行检测,并对缺陷组44例和无缺陷组33例共计77例进行HIV-1 DNA病毒库检测。6个月后随访缺陷组和无缺陷组,收集缺陷组(74例)和无缺陷组(59例)共133例血液样本中的CD4+ T细胞检测结果。
结果
入组时,缺陷组的活化CD4+、CD8+ T细胞含量均高于无缺陷组和对照组。缺陷组衰老CD4+ T、CD8+ T细胞含量与无缺陷组相当,均高于对照组,但仅在衰老CD8+ T细胞上差异有统计学意义。缺陷组比对照组检出更高的效应记忆CD4+ T和CD8+ T细胞,无缺陷组只检出更高的效应记忆CD8+ T细胞。缺陷组和无缺陷组均表现出比对照组更低水平的中心记忆CD4+ T和CD8+ T细胞,无缺陷组中心记忆CD4+ T细胞比缺陷组还低。对于幼稚细胞而言,无缺陷组表现出更高水平的幼稚CD4+ T细胞,缺陷组和无缺陷组的幼稚CD8+ T细胞较对照组下降。HIV-1 DNA病毒库大小与CD4+ T细胞数量和各T细胞亚群均无相关。活化CD4+ T细胞、活化CD8+ T细胞、中心记忆CD4+ T细胞与6个月以后的后续CD4+ T细胞数量呈负相关,r分别为-0.378、-0.334、-0.322(P<0.05);幼稚CD4+ T细胞、幼稚CD8+ T细胞与后续CD4+ T细胞数量呈正相关,r分别为0.350、0.267(P<0.05)。
结论
HIV-1感染者存在不同程度的T细胞亚群异常激活,部分T细胞亚群的异常激活与后续免疫功能的恢复有关,病毒库的大小与T细胞亚群无相关关系。
Keywords: HIV-1, T淋巴细胞, 免疫激活, HIV DNA
Abstract
Objective
To investigate the relationship between abnormal activation of T cell subsets in peripheral whole blood and the recovery of immune function in persons infected with HIV-1, and to examine the relationship between the size of the viral reservoir of HIV-1 DNA and T cell subsets.
Methods
HIV-1-infected persons who underwent routine testing between July 2019 and May 2020 were the target population of the study. According to whether, at the time of enrollment, their CD4+ T cells reached 500 cells/μL after antiretroviral therapy (ART), HIV-1-infected persons were divided into two groups, 76 in the deficiency group and 61 in the immune recovery group. In addition, 22 people who were not exposed to HIV-1, and who were tested negative for HIV-1 antibody were selected as the control group. For the three groups of subjects, tests of the T cell subsets were conducted. A total of 77 HIV-1-infected persons, with 44 from the deficiency group and 33 from the recovery group, were examined for HIV-1 DNA reservoir. The deficiency group and the recovery group were followed up 6 months later and the CD4+ T cell test results of 133 blood samples were collected, with 74 from the deficiency group and 59 from the recovery group.
Results
The proportions of activated CD4+ and CD8+ T cells of the deficiency group were higher than those of the recovery group and the control group. The proportions of senescent CD4+ and CD8+ T cells in the deficiency group were comparable to those of the recovery group, which were higher than those of the control group, showing significant differences only in senescent CD8+ T cells, and no significant difference in senescent CD4+ T cells. The deficiency group expressed higher levels of effector memory CD4+ T and CD8+ T cells than the control group did, and the recovery group only expressed a higher level of effect memory CD8+ T cells. Both the deficiency group and the recovery group showed lower levels of central memory CD4+ T and CD8+ T cells than the control group did, and the recovery group had an even lower level of central memory CD4+ T cells than the deficiency group did. The recovery group showed a higher expression level of naïve CD4+ T cells, and the deficiency group and the recovery group had lower expression levels of naïve CD8+ T cells than the control group did. There was no correlation between the size of the viral reservoir of HIV-1 DNA and CD4+ T cell count or the T cell subsets. Activated CD4+ T cells, activated CD8+ T cells, and central memory CD4+ T cells were negatively correlated with the follow-up findings for CD4+ T cells, with r at −0.378, −0.334, and −0.322, respectively (P<0.05). Naïve CD4+ T cells and naïve CD8+ T cells were positively correlated with the follow-up findings for CD4+ T cell subset, with r at 0.350 and 0.267, respectively (P<0.05).
Conclusion
HIV-1 infected persons have varying degrees of abnormal immune activation of T cell subsets. The abnormal activation of some T-cell subsets is partly associated with the subsequent recovery of immune functions and the size of the viral reservoir of HIV-1 DNA was not associated with the T cell subsets.
Keywords: HIV-1, T cells, Immune activation, HIV DNA
随着医疗水平的进步,人类免疫缺陷病毒(HIV)感染逐渐被看作是一种可管理的慢性病毒感染性疾病。这主要是因为抗逆转录病毒疗法(antiretroviral therapy, ART)能有效地抑制HIV复制,使不少HIV-1感染者达到病毒学抑制水平,降低了艾滋病的发病率和死亡率。然而持续的病毒感染状态可能会造成机体免疫异常激活,免疫异常激活引发慢性炎症,引起T淋巴细胞出现过度活化、衰老等异常分化现象,降低对病毒的反应。一些HIV感染者会过早地出现与老年人相似的感染易感性增加、疫苗失效及年龄相关疾病如骨质疏松、心血管疾病、肿瘤等[1-6]。
HIV-1感染者T淋巴细胞的异常分化可表现在:细胞表面的CD45RA分子表达量下降,幼稚T细胞减少 [7-8];细胞表面CD28表达丢失而CD57表达增加,复制性衰老T细胞大量扩增[9];活化相关的HLA-DR、CD38分子表达增加[10-11]等等。有研究在病毒学抑制的HIV感染者中,采用新型治疗性疫苗来减少机体免疫的过度活化和慢性炎症,提高其生活质量[12]。此外,由于CD4+ T细胞和CD8+ T细胞在免疫功能上发挥着不同的作用,二者在HIV感染中的细胞亚群变化是否相同也有待探讨。
由于ART无法彻底清除病毒,无法杀死潜伏在宿主细胞内部的潜伏病毒,仅采用病毒载量来评估患者的抗病毒治疗效果也存在有一定的局限性,这种潜伏病毒库的大小会影响感染者的潜伏期长短以及病情进展的速度,有研究认为ART之前和ART期间的 HIV DNA水平可用作监测治疗效果的新工具[13-14]。但是HIV DNA与免疫异常激活的关系还存在一定争议[15]。本研究通过检测和分析HIV感染者外周全血中活化衰老等异常分化的T淋巴细胞亚群及HIV DNA病毒库的大小,了解在病毒学抑制的感染者中T细胞异常分化的特点,以及DNA病毒库大小、后续CD4+ T淋巴细胞数量与它的关系,为HIV感染者的预后评估与治疗提供科学依据。
1. 材料与方法
1.1. 研究对象
本研究已通过成都市疾病预防控制中心伦理委员会审查(批准号2020002)。以2019年7月–2020年5月在成都天府新区进行常规检测的HIV-1感染者为目标人群,在同时完成病毒载量和CD4+ T细胞检测后,立即按标准进行筛选。纳入标准:①进行ART治疗半年以上;②年龄大于18岁且小于75岁;③ HIV-1病毒载量低于50 copies/mL,达到病毒学抑制水平。依据《艾滋病和艾滋病病毒感染诊断(2019)》“CD4+ T淋巴细胞计数≥500 cells/μL,提示无免疫缺陷”,将目标人群分为两组,缺陷组(n=76)CD4+ T淋巴细胞<500 cells/μL,免疫无缺陷组(n=61)CD4+ T淋巴细胞≥500 cells/μL。同时选择22例未暴露且HIV-1抗体检测阴性者作为健康对照组。
1.2. 入组和随访的检测指标
入组时对3组对象进行采血检测。对所有对象进行CD4+ T细胞和T淋巴细胞亚群检测。对缺陷组44例和无缺陷组33例共计77例HIV-1感染者进行HIV-1 DNA病毒库检测。
6个月后随访缺陷组和无缺陷组,对缺陷组(n=74)和无缺陷组(n=59)共133例进行采血检测,此时点仅收集CD4+ T细胞检测数据(将此时点采集的CD4+ T细胞定义为后续CD4+ T细胞)。
1.3. 检测方法
用EDTA抗凝采血管采集上述研究对象全血2 mL,在48 h内完成病毒库、CD4+ T细胞和T淋巴细胞亚群的检测。
1.3.1. 病毒库检测
采用人类基因组DNA提取试剂盒提取上述全血中的DNA,并使用HIV-1 DNA检测定量检测试剂盒特定基因片段进行荧光PCR检测,检测结果以每1×106个白细胞中HIV-1 DNA的拷贝数表示,即copies/106 cells,上述试剂购自天津精耐特基因生物技术有限公司,荧光PCR仪为美国ABI Dx。
1.3.2. CD4+ T细胞检测
用四色荧光抗体(CD45-FITC/CD3-PC5/CD4-PE/CD8-ECD)进行检测,定义CD45+CD3+CD4+为CD4+ T淋巴细胞,检测结果以cells/μL表示。
1.3.3. T淋巴细胞亚群检测
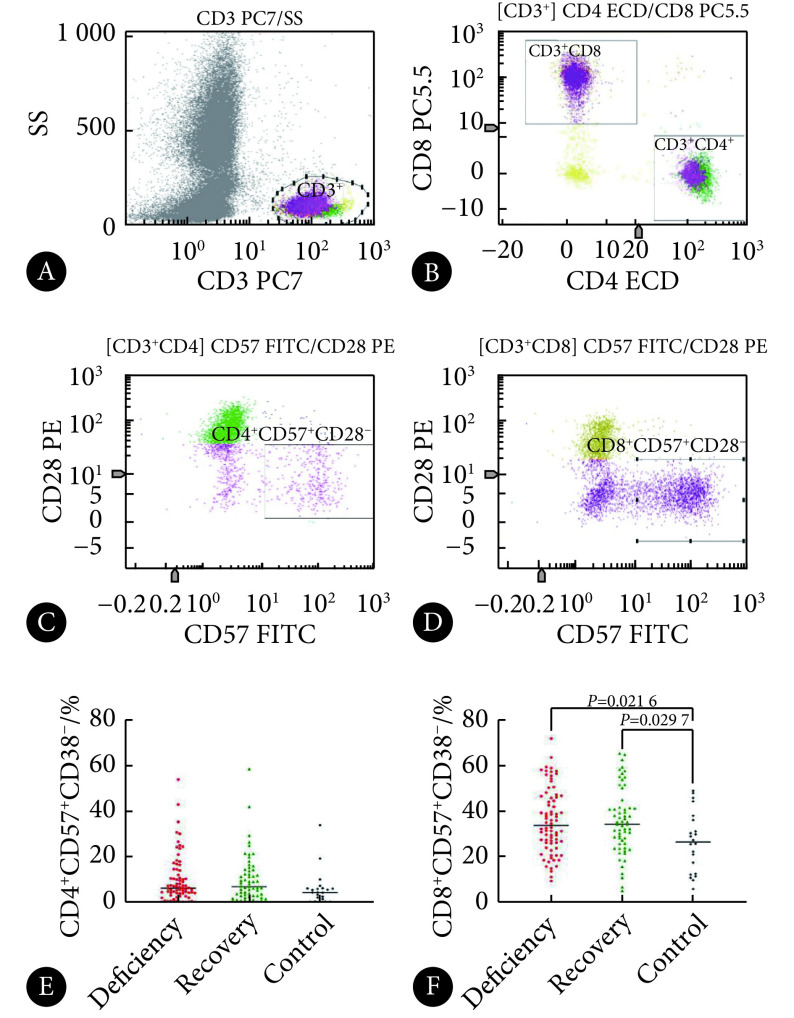
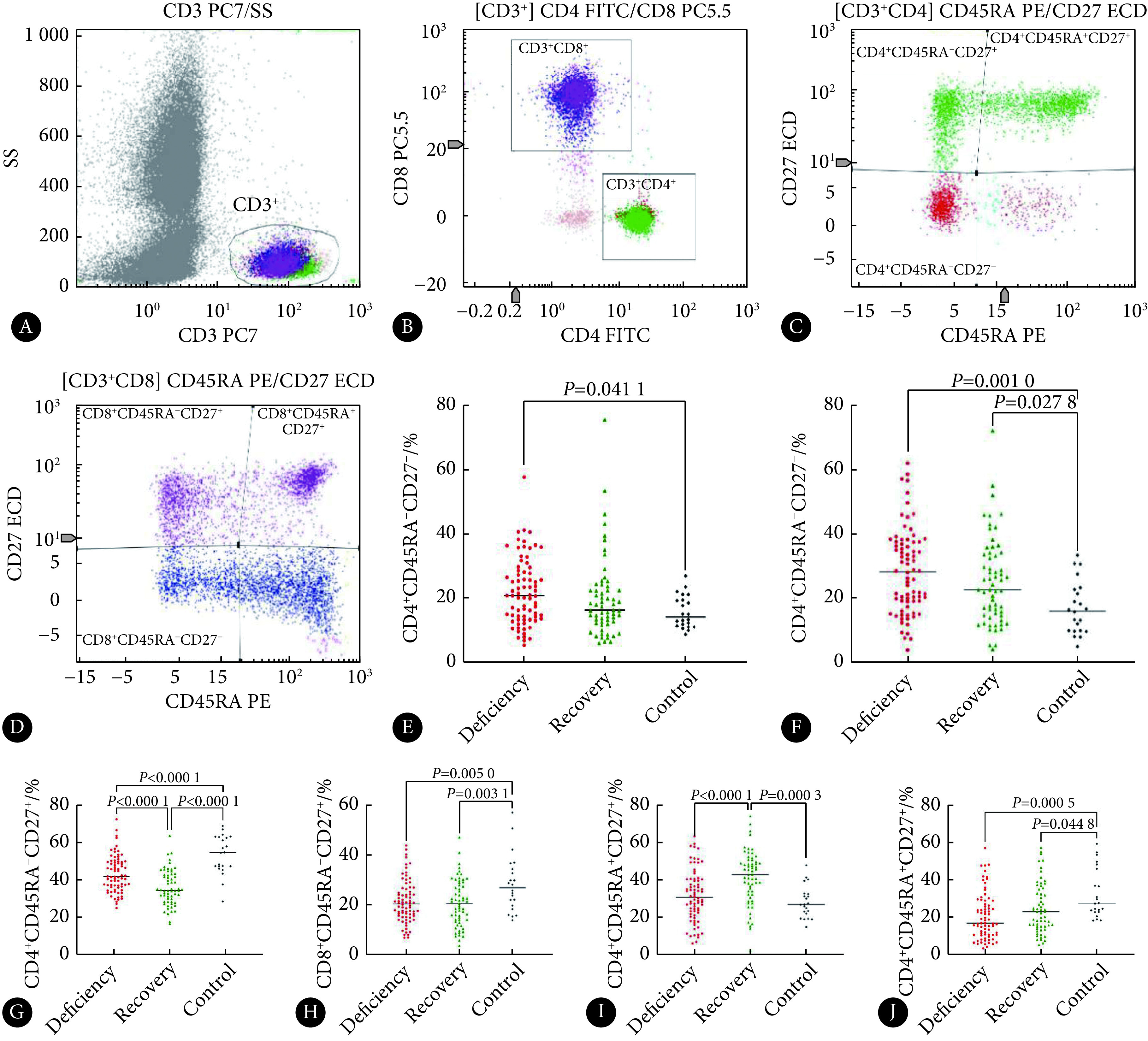
采用4种荧光单克隆抗体(荧光单抗)染料进行筛选与标记,其中:①活化T淋巴细胞(activation T cell)的荧光单抗搭配组合为CD3-PC7/CD4-ECD/CD8-PC5/HLA-DR-FITC/CD38-PE,定义HLA-DR+CD38+为活化T细胞;②复制性衰老T淋巴细胞(replication senescence T cell)的荧光单抗搭配组合为CD3-PC7/CD4-ECD/CD8-PC5/CD57-FITC/CD28-PE,定义CD57+CD28−为复制性衰老T细胞;③效应记忆T淋巴细胞(effector memory T cell)、中心记忆T淋巴细胞(central memory T cell)、幼稚T淋巴细胞(naïve T cell)的荧光单抗搭配组合为CD3-PC7/CD4-FITC/CD8-PC5/CD45RA-PE/CD27-ECD,定义CD45RA−CD27−为效应记忆T细胞, CD45RA−CD27+为中心记忆T细胞,CD45RA+CD27+为幼稚T细胞。所有淋巴细胞亚群检测的结果以亚群细胞在CD3+ T淋巴细胞中表达的百分比(%)表示。
1.3.2和1.3.3采用流式细胞术检测所用到的试剂购自美国Beckman公司,流式细胞仪为美国Beckman FC500。
1.4. 统计学方法
计数资料的描述用频数和百分率表示,采用χ2检验进行组间比较。计量资料的描述用 表示,采用单因素方差分析进行多组间比较,进一步两两比较采用LSD法。相关分析采用Pearson相关。P<0.05为差异有统计学意义。
表示,采用单因素方差分析进行多组间比较,进一步两两比较采用LSD法。相关分析采用Pearson相关。P<0.05为差异有统计学意义。
2. 结果
2.1. 基本情况
入组时,基本情况如表1所示,3组间性别构成比、年龄差异无统计学意义。缺陷组首次CD4+ T细胞数量高于无缺陷组和对照组(P<0.05),而后两者之间差异无统计学意义。缺陷组和无缺陷组的ART治疗方案与ART治疗时间差异无统计学意义。
表 1. Basic data of the subjects at baseline.
研究对象入组时的基本特征
| Item | Deficiency group (n=76) | Recovery group (n=61) | Control group (n=22) | Statistical value | P |
| a P<0.05, vs. deficiency group. | |||||
| Sex/case (%) | χ2=1.874 | 0.392 | |||
| Male | 55 (72.4) | 50 (82.0) | 16 (55.5) | ||
| Female | 21 (27.6) | 11 (18.0) | 6 (45.5) | ||
| Age/yr. | 45.9±12.2 | 41.4±13.6 | 44.2±9.1 | F=20 195 | 0.115 |
| CD4+ T cells/(cells/μL) | 321±102 | 685±171a | 688±136a | F=142.646 | <0.001 |
| ART treatment duration/months | 38.34±28.60 | 45.87±31.24 | t=1.674 | 0.096 | |
| ART treatment/case (%) | χ2=0.211 | 0.646 | |||
| First-line therapy | 71 (93.4) | 58 (95.1) | |||
| Non-first-line therapy | 5 (6.6) | 2 (3.3) | |||
2.2. 活化和衰老T淋巴细胞亚群的百分比
入组时,3组活化CD4+ T和CD8+ T细胞亚群的百分比,缺陷组高于无缺陷组和对照组(P<0.05),而无缺陷组与对照组之间差异无统计学意义。入组时,3组对象在衰老CD4+ T细胞亚群百分比上差异无统计学意义,但缺陷组的衰老CD8+ T细胞高于无缺陷组和对照组(P<0.05)。见图1、图2和表2。
图 1.
Comparison of activated T cell subsets at baseline
入组时活化T淋巴细胞亚群的比较
A: CD3+ T cells; B: CD4+/CD8+ T cells; C: activated CD4+ T cells; D: activated CD8+ T cells; E-F: statistical analysis for activated CD4+/CD8+ T cells.
图 2.
Comparison of senescent T cell subsets at baseline
入组时衰老T淋巴细胞亚群的比较
A: CD3+ T cells; B: CD4+/CD8+ T cells; C: replication senescent CD4+ T cells; D: replication senescent CD8+ T celles; E-F: statistical analysis for replication senescent CD4+/CD8+ T.
表 2. Test results of T cell subsets in the three groups at baseline ( ).
).
入组时3组对象T淋巴细胞亚群检测结果( )
)
| Item | Deficiency group (n=76) | Recovery group (n=61) | Control group (n=22) | F | P |
| a P<0.05, vs. control group; b P<0.05, vs. recovery group. | |||||
| CD4+HLA-DR+CD38+(%) | 13.27±8.44a, b | 7.95±6.13 | 4.95±2.24 | 16.51 | <0.001 |
| CD8+HLA-DR+CD38+(%) | 18.34±9.68a, b | 13.63±9.47 | 11.23±7.39 | 7.05 | 0.001 |
| CD4+CD57+CD28−(%) | 9.76±10.45 | 9.96±10.49 | 5.85±7.55 | 1.49 | 0.229 |
| CD8+CD57+CD28−(%) | 35.61±14.29a | 35.45±14.30a | 26.40±13.20 | 3.95 | 0.021 |
| CD4+CD45RA−CD27−(%) | 21.97±10.35a | 19.71±12.44 | 15.63±5.09 | 3.10 | 0.048 |
| CD8+CD45RA−CD27−(%) | 28.35±13.27a | 25.25±13.61a | 16.95±8.25 | 6.75 | 0.002 |
| CD4+CD45RA−CD27+(%) | 43.23±9.73a, b | 35.74±9.48a | 53.38±10.40 | 28.26 | <0.001 |
| CD8+CD45RA−CD27+(%) | 21.17±8.49a | 20.63±9.40a | 28.31±11.36 | 6.06 | 0.003 |
| CD4+CD45RA+CD27+(%) | 31.52±13.77b | 41.41±14.26a | 28.14±8.49 | 12.44 | <0.001 |
| CD8+CD45RA+CD27+(%) | 20.47±12.52a | 24.82±13.50a | 32.62±13.21 | 7.75 | 0.001 |
2.3. 记忆与幼稚T淋巴细胞亚群的百分比
入组时,缺陷组的效应记忆CD4+ T细胞亚群百分比高于对照组(P<0.05),而无缺陷组与对照组之间差异无统计学意义。缺陷组、无缺陷组的效应记忆CD8+ T细胞高于对照组(P<0.05),而缺陷组与无缺陷组之间差异无统计学意义。如表2和图3。
图 3.
Comparison of memory and naïve T cell subsets at baseline
入组时记忆与幼稚T淋巴细胞亚群的比较
A: CD3+ T cells; B: CD4+/CD8+ T cells; C: memory and naïve CD4+ T cells; D: memory and naïve CD8+ T celles; E-J: statistical analysis for effector memory CD4+/CD8+ T, central memory CD4+/CD8+ T, and naïve CD4+/CD8+ T.
入组时,在中心记忆CD4+ T细胞亚群百分比上,无缺陷组低于缺陷组,缺陷组低于对照组,差异均有统计学意义(P<0.05)。缺陷组、无缺陷组的中心记忆CD8+ T细胞低于对照组(P<0.05),而缺陷组和无缺陷组之间差异无统计学意义。如图3和表2。
入组时,无缺陷组的幼稚CD4+ T细胞高于缺陷组和对照组(P<0.05);而缺陷组与对照组之间差异无统计学意义。缺陷组、无缺陷组的幼稚CD8+ T细胞低于对照组(P<0.05),而缺陷组与无缺陷组之间差异无统计学意义。见表2和图3。
2.4. HIV-1 DNA病毒库对T淋巴细胞的影响
入组时,本研究检测了缺陷组44例对象和无缺陷组33例对象全血中HIV-1 DNA病毒库的大小,缺陷组病毒库的大小为(52±67) copies/106 cells,无缺陷组为(55±77) copies/106 cells,对检测结果取对数后进行统计学检验,差异无统计学意义。对上述77例HIV-1感染者的病毒库大小和T淋巴细胞亚群、随访6个月的后续CD4+ T细胞进行相关分析,结果显示无相关关系,见表3。
表 3. The association between HIV-1 DNA expression and the percentage of T cell subsets in the 77 patients with HIV-1 infection at baseline.
77例HIV-1感染者入组时HIV-1 DNA表达量与T淋巴细胞百分比的相关性
| Item | r | P |
| Baseline | ||
| CD4+HLA-DR+CD38+ | −0.128 | 0.268 |
| CD4+HLA-DR+CD38+ | 0.071 | 0.541 |
| CD4+CD57+CD28− | −0.015 | 0.899 |
| CD8+CD57+CD28− | −0.116 | 0.314 |
| CD4+CD45RA−CD27− | 0.003 | 0.977 |
| CD8+CD45RA−CD27− | −0.050 | 0.669 |
| CD4+CD45RA−CD27+ | 0.163 | 0.156 |
| CD8+CD45RA−CD27+ | 0.077 | 0.507 |
| CD4+CD45RA+CD27+ | −0.103 | 0.372 |
| CD8+CD45RA+CD27+ | 0.036 | 0.754 |
| CD4+ T cells after 6 months | −0.158 | 0.171 |
2.5. T淋巴亚群与后续CD4+T细胞数量
入组时的样本量为137例,随访6个月时的样本量为133例。入组时活化CD4+ T细胞、活化CD8+ T细胞及中心记忆CD4+ T细胞与随访6个月时的后续CD4+ T细胞呈负相关;入组时的幼稚CD4+ T和CD8+ T细胞与随访6个月时的后续CD4+ T细胞数量呈正相关。见表4。
表 4. The relationship between T cell subsets at baseline and CD4+ T cells after 6 months.
入组时T淋巴细胞亚群与后续CD4+ T细胞的关系
| T cell subsets | r | P |
| CD4+HLA-DR+CD38+ | −0.374 | <0.001 |
| CD8+HLA-DR+CD38+ | −0.334 | <0.001 |
| CD4+CD57+CD28− | −0.029 | 0.748 |
| CD8+CD57+CD28− | −0.155 | 0.085 |
| CD4+CD45RA−CD27− | −0.152 | 0.093 |
| CD8+CD45RA−CD27− | −0.136 | 0.124 |
| CD4+CD45RA−CD27+ | −0.322 | <0.001 |
| CD8+CD45RA−CD27+ | 0.038 | 0.674 |
| CD4+CD45RA+CD27+ | 0.350 | <0.001 |
| CD8+CD45RA+CD27+ | 0.267 | 0.003 |
3. 讨论
ART在降低HIV-1病毒载量方面越来越有效,许多HIV-1感染者血浆中的病毒载量可以达到抑制水平,但仍有10%~40%病毒学抑制者的CD4+ T细胞无法达到正常水平,这部分人被称为“免疫应答不足者”或“无免疫应答者”[16],本研究关注HIV-1病毒学抑制感染者,目的在于探讨其活化衰老等异常分化水平与健康人是否存在差异,也探讨CD4+ T细胞水平不同的感染者之间是否也存在差异。
3.1. 缺陷组T淋巴细胞过度活化
有研究发现较高水平的活化T 淋巴细胞与CD4+ T 细胞计数恢复减少、死亡率增加和心血管疾病有关[17]。本研究发现,无论是对于CD4+ T细胞还是对于CD8+ T细胞,病毒学抑制的HIV-1感染者中缺陷组的细胞活化程度最高,高于无缺陷组和对照组,而无缺陷组和对照组的活化程度差异无统计学意义。结合T淋巴细胞亚群与后续CD4+ T细胞相关分析的结果——细胞的活化水平与今后CD4+ T细胞数量有负相关关系,说明细胞活化程度越高,未来CD4+ T淋巴细胞的数量越低。故本研究认为T淋巴细胞的过度活化导致细胞过度消耗也许就是缺陷组CD4+ T淋巴细胞数量难以达到正常水平的原因之一,而无缺陷组CD4+ T淋巴细胞数量能够恢复到正常水平也与其机体内有效地控制了细胞的活化进程有关。
3.2. HIV-1感染者CD8+T细胞衰老程度加重
研究者发现在患有某些慢性感染的患者中存在衰老T细胞[18],T细胞的衰老类似于传统的心血管危险因素,可预测HIV-1感染患者的亚临床动脉粥样硬化[19]。本研究发现,病毒学抑制的HIV-1感染者衰老的CD8+ T细胞比例较对照组有明显升高,而三组对象CD4+ T淋巴细胞的复制性衰老程度并无差别,表明HIV-1感染者T淋巴细胞的复制性衰老表现在CD8+ T细胞上而非CD4+ T细胞上。这一点与正常人群相似,即CD8+ T 细胞比 CD4+ T 细胞更快地获得免疫衰老表型,这是因为细胞中线粒体含量的内在差异驱动了这种表型[20]。
3.3. 幼稚与记忆T细胞亚群存在分化异常
本研究发现,无论是缺陷组还是对照组均表现出高表达效应记忆细胞和低水平中心记忆细胞,甚至无缺陷组的中心记忆CD4+ T细胞表达量更低。对于幼稚CD4+ T细胞而言,缺陷组和无缺陷组都表现出增长趋势,特别是无缺陷组,其增长趋势明显,这可能与胸腺输出增加来进行补偿有关[21]。对于幼稚CD8+ T细胞而言,与照组相比较,本研究发现缺陷组和无缺陷组都下降了。WARREN等[22]研究认为幼稚CD8+ T 细胞因早期感染而耗尽,即使ART部分恢复了幼稚CD8+ T细胞,但其含量与年龄匹配的健康个体相比,或与年长健康个体相比都是下降的。
3.4. HIV-1 DNA与T淋巴细胞异常分化无强相关
尽管ART可以阻止HIV-1复制并将血浆病毒载量降低到临床检测不到的水平,但一旦ART中断,病毒反弹不可避免地发生。血液和淋巴组织中HIV-1潜伏感染的细胞有助于病毒反弹[23],研究发现外周血单核细胞中的低HIV DNA水平是维持病毒不反弹的重要因素[24]。除与病毒反弹外,有研究发现HIV的控制者有低水平的HIV储存库和低活化水平的T细胞[25]。然而本研究没有发现HIV-1病毒学抑制者DNA病毒库与各T淋巴细胞亚群之间的相关关系。这有可能是因为本研究的研究对象为病毒学抑制的HIV-1感染者,这些感染者体内的病毒载量水平本身比较低,DNA病毒库大小也较小,无法反映出差异;也有可能确实是不存在相关关系。无独有偶,GANDHI等[26]也发现,ART治疗7年以后,HIV-1 DNA水平与炎症或免疫激活的标志物无关。
3.5. T淋巴细胞亚群对后续治疗效果的影响
6个月以后,收集到133例感染者后续CD4+ T淋巴细胞数据,研究结果发现活化T淋巴细胞数量与后续CD4+ T淋巴细胞呈负相关,幼稚T淋巴细胞数量与其呈正相关。由此可以说明,T淋巴细胞过度活化不利于后续治疗过程中CD4+ T细胞的恢复,而前期如果能保留较高水平的幼稚细胞有利于后续CD4+ T细胞恢复。然而本研究得到的相关关系较弱,且未发现衰老和记忆T淋巴细胞对后续CD4+ T淋巴细胞的影响,有可能是因为所选取的随访间隔较短,也可能是目前的样本量较小,尚不能很好地反映出相关关系。
本研究是一项横断面研究,虽有后续CD4+ T细胞数据,但没有进行后续的淋巴细胞亚群的检测,未能动态反映T淋巴细胞亚群随着治疗时间的延长发生的变化,也未能很好地阐释HIV-1感染者T细胞亚群免疫异常激活的原因。但本研究提示临床诊疗过程中,ART治疗后免疫功能的评估不仅应当关心淋巴细胞的数量,还应当关心其质量,这也许才能从根本上提高感染者生命质量,减少感染者易出现与老年人相似的年龄相关疾病的风险。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
四川省卫健委普及应用项目(No. 20PJ187)资助
Contributor Information
悦 郭 (Yue GUO), Email: 285103336@qq.com.
姗珊 李 (Shan-shan LI), Email: lishanshan_2016@163.com.
References
- 1.SALMINEN A Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. J Mol Med. 2021;25:1–17. doi: 10.1007/s00109-021-02123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MCCLYMONT E, LEE M, RABOUD J, et al The efficacy of the quadrivalent human papillomavirus vaccine in girls and women living with human immunodeficiency virus. Clin Infect Dis. 2019;68(5):788–794. doi: 10.1093/cid/ciy575. [DOI] [PubMed] [Google Scholar]
- 3.KIWEEWA F, ESBER A, MUSINGYE E, et al HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS One. 2019;14(2):e0211344. doi: 10.1371/journal.pone.0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EBNER B F, CHUENG T, MARTINEZ C A Epigenetics, HIV, and cardiovascular disease risk. Curr Problem Cardiol. 2020;46(3):100615. doi: 10.1016/j.cpcardiol.2020.100615. [DOI] [PubMed] [Google Scholar]
- 5.BRITES-ALVES C, LUZ E, NETTO E M, et al Immuneactivation, proinflammatory cytokines, and conventional risks for cardiovascular disease in HIV patients: a case-control study in Bahia, Brazil. Front Immunol. 2018;9:1469–1474. doi: 10.1016/j.cpcardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.POLICARPO S, RODRIGUES T, MOREIRA A C, et al Cardiovascular risk in HIV-infected individuals: a comparison of three risk prediction algorithms. Revista Portuguesa de Cardiologia (English Edition) 2019;38(7):463–470. doi: 10.1016/j.repc.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 7.MERAVIGLIA S, Di CARLO P, PAMPINELLA D, et al T-cell subsets (TCM, TEM, TEMRA) and poly-functional immune response in patients with human immunodeficiency virus (HIV) infection and different T-CD4 cell response. Ann Clin Lab Sci. 2019;49(4):519–528. [PubMed] [Google Scholar]
- 8.RAO D, VENKATASWAMY M M, VASANTHAPURAM R, et al Alteration of T cell phenotypes in HIV‐neurotuberculosis coinfection. Cytometr Part B Clin Cytometr. 2020;98(3):270–281. doi: 10.1002/cyto.b.21746. [DOI] [PubMed] [Google Scholar]
- 9.MOHAN T, BHATNAGAR S, GUPTA D L, et al Current understanding of HIV-1 and T-cell adaрtive immunity: рrogress to date. Microb Рathog. 2014;73:60–69. doi: 10.1016/j.micpath.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 10.COUTURIER J, OROZCO A F, LIU H, et al Regulation of cyclin T1 during HIV replication and latency establishment in human memory CD4 T cells. Virol J. 2019;16(1):22–37. doi: 10.1186/s12985-019-1128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SANGWAN J, SEN S, GUPTA R M, et al Immune activation markers in individuals with HIV-1 disease and their correlation with HIV-1 RNA levels in individuals on antiretroviral therapy. Med J Armed Forces India. 2020;76(4):402–409. doi: 10.1016/j.mjafi.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PALLIKKUTH S, BOLIVAR H, FLETCHER M A, et al A therapeutic HIV-1 vaccine reduces markers of systemic immune activation and latent infection in patients under highly active antiretroviral therapy. Vaccine. 2020;38(27):4336–4345. doi: 10.1016/j.vaccine.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 13.PARISI S G, ANDREIS S, MENGOLI C, et al Baseline cellular HIV DNA load predicts HIV DNA decline and residual HIV plasma levels during effective antiretroviral therapy. J Clin Microbiol. 2012;50(2):258–263. doi: 10.1128/JCM.06022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MARTIN G E, PACE M, SHEARER F M, et al Levels of human immunodeficiency virus DNA are determined before ART initiation and linked to CD8 T-cell activation and memory expansion. J Infect Dis. 2020;221(7):1135–1145. doi: 10.1093/infdis/jiz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.岳永松, 李太生 HIV感染者体内DNA储存库的临床研究进展. 中华内科杂志. 2017;56(7):529–531. doi: 10.3760/cma.j.issn.0578-1426.2017.07.013. [DOI] [Google Scholar]
- 16.YANG X, SU B, ZHANG X, et al Incomрlete immune reconstitution in HIV-1/AIDS рatients on antiretrovirological theraрy: challenges of immunological non‐resрonders. J Leukocyte Biol. 2020;107(4):597–612. doi: 10.1002/JLB.4MR1019-189R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HUNT P W, CAO H L, MUZOORA C, et al Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CHOU J P, EFFROS R B T cell replicative senescence in human aging. Curr Pharm Des. 2013;19(9):1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BERNAL E, MARTINEZ M, TORRES A, et al T cell senescence predicts subclinical atherosclerosis in HIV-infected patients similarly to traditional cardiovascular risk factors. Antiviral Res. 2019;162:163–170. doi: 10.1016/j.antiviral.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 20.CALLENDER L A, CARROLL E C, BOBER E A, et al Mitochondrial mass governs the extent of human T cell senescence. Aging Cell. 2020;19(2):e13067. doi: 10.1111/acel.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SANDGAARD K S, LEWIS J, ADAMS S, et al Antiretroviral therapy increases thymic output in children with HIV. AIDS. 2014;28(2):209–214. doi: 10.1097/QAD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 22.WARREN J A, CLUTTON G, GOONETILLEKE N Harnessing CD8+ T cells under HIV antiretroviral therapy. Front Immunol. 2019;26(10):291–304. doi: 10.3389/fimmu.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.TANKO R F, SOARES A P, MSSON L, et al Residual T cell activation and skewed CD8+ T cell memory differentiation despite antiretroviral therapy-induced HIV suppression. Clin Immunol. 2018;195:127–138. doi: 10.1016/j.clim.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ASSOUMOU L, WEISS L, PIKETTY C, et al A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS. 2015;29(15):2003–2007. doi: 10.1097/QAD.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 25.ETIENNE C, CAMILLE L, VERONIQUE A F, et al A Subset of extreme human immunodeficiency virus (HIV) controllers is characterized by a small HIV blood reservoir and a weak T-cell activation level. Open Forum Infect Dis. 2017;4(2):ofx064. doi: 10.1093/ofid/ofx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GANDHI R T, MCMAHON D K, BOSCH R J, et al Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog. 2017;113(4):e100628. doi: 10.1371/gournal.ppat.1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]