Abstract
患者,男,42岁,因“发作性晕厥10+个月,胸闷20+ d,胸痛10+ d”入院。诊断为肥厚型心肌病,既往无高血压、糖尿病病史。为了解左室流出道压差及冠脉情况,予以左室心导管检查及冠脉造影术,心导管经右侧桡动脉入路,共使用碘质量浓度为370 mg I/mL的碘普罗胺200 mL,术后患者出现以剧烈头痛、皮质盲、神经精神症状为主要临床表现的造影剂脑病。予以降颅压、镇静及止痛等对症支持治疗,患者自愈。
Keywords: 冠状动脉造影, 碘普罗胺, 造影剂脑病
Abstract
A 42-year-old male was admitted for paroxysmal syncope for 10+ months, chest tightness for 20+ days and chest pain for 10+ days. The patient was diagnosed with hypertrophic cardiomyopathy. The patient did not have a history of hypertension or diabetes. Coronary angiography and left ventricular cardiac catheterization were done in order to examine the coronary artery and the pressure gradient of the left ventricular outflow tract. The cardiac catheterization was performed via a right radial artery approach and a total of 200 mL of 370 mg I/mL iopromide was injected. The patient developed contrast-induced encephalopathy following the cardiac catheterization procedure, displaying severe headache, cortical blindness and neuropsychiatric symptom as the main clinical manifestations. The patient was then given symptomatic and supportive treatment, including decreasing intracranial pressure, analgesics and sedatives, and the patient recovered.
Keywords: Coronary arteriography, Iopromide, Contrast-induced encephalopathy
造影剂脑病是由于造影剂的不良反应引起的一系列神经精神症状,临床上表现主要包括颅内出血、神经毒性脑病、无菌性脑膜炎、精神症状以及皮质盲等[1-2],影像学资料常无明显的变化,大多数患者在注射造影剂后2~12 h内发病,24~72 h内缓解,常常恢复良好。脑血管造影发生造影剂脑病的概率相对较高,约0.3%~2.6%[3],而冠脉造影或心导管检查中发生的可能性较低,其中暂时性的皮质盲更是罕见,在冠脉造影患者的发生率为0.06%[4],椎动脉造影的发生率为0.3%~1%[5],而后循环动脉瘤血管内线圈治疗的发生率为2.9%[6]。四川大学华西医院心内科近期收治了1例因行左心导管检查及冠脉造影术后出现造影剂脑病的肥厚型心肌病患者,本例患者的发病特点为冠脉造影加左室心腔测压术后半小时,出现以皮质盲、精神症状为主的造影剂脑病临床表现,持续时间短暂,呈自限性,予以相应的对症支持治疗后即痊愈,且发病前后影像学检查无明显差异,现报道如下。
1. 病例资料
患者,男,42岁,因“发作性晕厥10+个月,胸闷20+ d,胸痛10+ d”入院。诊断为肥厚型心肌病,既往无高血压、糖尿病病史。为了解左室流出道压差及冠脉情况,则予以左室心导管检查及冠脉造影术,心导管经右侧桡动脉进入左心腔及冠状动脉内,共使用碘质量浓度为370 mg I/mL的碘普罗胺(Ultravist®)200 mL。左室心导管检查示左室流出道总压差为46 mmHg(1 mmHg=0.133 kPa),最大压差位于左心腔的中部至心尖部,无酒精化学消融指针,冠脉造影未见冠脉狭窄。手术顺利,术毕患者未诉不适,转回病房。术后30 min患者诉轻微头疼、手脚和脸麻木感,术后40 min患者感觉头痛难耐、烦躁,恶心,随后伴失明、失聪、意识障碍,即刻查体:脉搏80 min−1,血压159/82 mmHg。手呈僵直状态、双侧瞳孔等大、形圆,光敏,眼底检查(−)。伸舌居中,四肢肌力正常,双侧病理征(−)、颈阻(−)。术后1 h头颅急诊CT(图1)示:颅内未见确切异常密度影。排除急性脑出血后转入CCU,予以甘露醇降颅压,咪达唑仑、地西泮镇静、曲马多止痛等对症支持治疗。术后12 h患者意识、视力等逐渐恢复,伴轻微头痛。术后48 h,患者再次出现剧烈头痛、视物模糊、意识障碍,伴恶心、呕吐,张口呼吸。即刻血气分析显示:低钾血症,静脉补钾后继续加强止痛、降颅压、镇静等对症支持治疗。术后54 h,患者头痛缓解,意识恢复,对答切题。术后3 d,患者病情稳定后予以头颅MRI检查,结果(图2)示:双侧额叶少许斑点稍长T2信号影,枕大池扩张,中线结构无偏移,提示双侧额叶小缺血灶。之后患者未诉头痛、胸闷等不适,术后5 d患者平安出院,并计划定期随访。
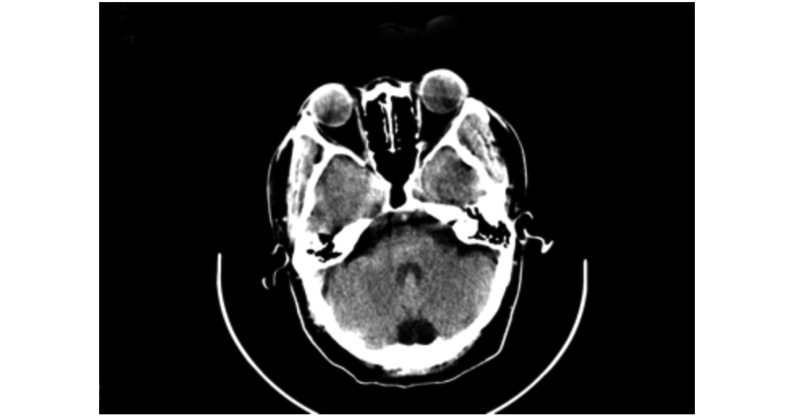
图 1.
Head CT image showed no definite abnormality 1 hour after the procedure
术后1 h头颅CT显示未见确切异常密度影
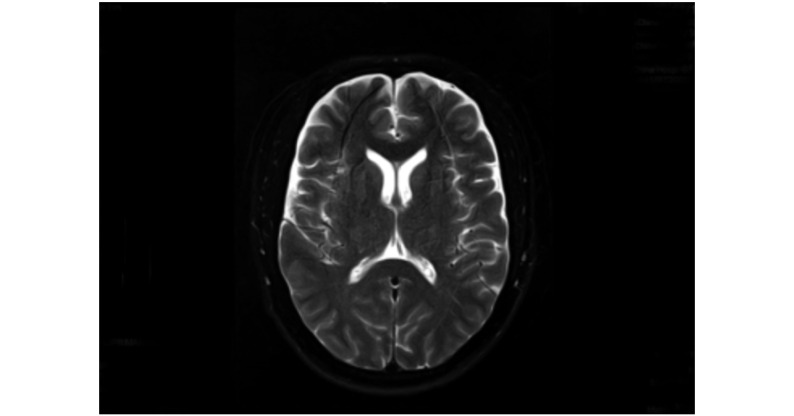
图 2.
Head MRI demonstrated a little hyperintense areas in the bilateral frontal lobe of the brain on T2-weighted images 3 days after the procedure
术后3 d头颅MRI双侧额叶少许斑点稍长T2信号影
2. 讨论
造影剂对血脑屏障局限性地破坏被认为是造影剂脑病的主要发病机制[7-8],造影剂对脑或脊髓有直接的毒性作用,破坏血脑屏障,从而允许更多的造影剂进入,形成恶性循环。而且神经毒性往往会导致选择性的皮质损伤[9],特别是薄弱的视皮质区,易损伤,形成皮质盲。其毒性作用可能是由碘造影剂的特性所决定,目前临床上常使用的是第二代非离子型造影剂。虽然具有低渗透压、良好的耐受性,性能较稳定等特性,本例则是碘普罗胺(Ultravist®)诱发造影剂脑病。同时造影剂可能直接导致脑组织短暂性损伤[10],引起脑组织水肿,出现颅内压增高的表现及精神症状,精神障碍常继发于额、颞叶损伤。本例患者出现了烦躁、谵妄等意识障碍且伴有颅内压增高的临床表现,结合头颅MRI显示双侧额叶小缺血灶,推测可能是由造影剂导致额叶脑组织短暂性损伤。微血栓进入大脑也是造影剂脑病发病机制之一,当导丝穿过右锁骨动脉及头臂干时,微血栓可能进入大脑,特别是经上肢桡动脉操作时,发生可能性相对较大,在冲洗导管时发现许多微血栓的形成,可在脑血管床形成栓塞,导致局灶性神经功能缺失。且研究表明有92.1%的微栓塞为气栓,仅7.9%为固体栓塞[11]。本例患者头颅MRI显示双侧额叶小缺血灶,也不能完全排除微小血栓引起的相应症状,遗憾的是由于缺乏经验未及时给患者做头颅弥散MRI,不能确定否为新鲜缺血灶。
有别于以往的报道,本例患者为肥厚型心肌病,且曾出现一过性失聪,待意识恢复后,听力恢复正常。令人困惑的是皮层听觉区位于颞上回,但该患者未有任何影像学检查提示颞上回受损。同时患者在术后12 h,临床症状一度好转,术后48 h再次出现神经系统症状,推测可能是患者症状缓解后,用药强度变化导致症状反复,也可能是造影剂引发的脑组迟发性变态反应,有文献报道免疫机制参与造影剂脑病[12],特别是造影剂过敏的患者,但其具体原因有待进一步探究。
诊断造影剂脑病应排除其他原因引起的脑出血、脑梗塞、癔症、视网膜中动脉栓塞、球后视神经炎等疾病,结合临床表现及相关检查后做出诊断。神经系统查体、眼科专科检查、头颅CT、头颅MRI、脑电图、血气分析等有助于诊断,特别强调弥散加权MRI对检测大脑能量耗竭及细胞毒性水肿非常敏感[13],有助于发现新鲜的小梗塞灶,必要时可做延迟磁共振显像,可发现3 d内信号的异常[14]。
造影剂脑病的治疗没有特异性,其原则主要是对症支持治疗。在吸氧基础上,若伴随明显的神经精神症状,如谵妄、癫痫,予以苯二氮类药物等镇静;若颅内压增高,通过甘露醇、利尿等降颅压;若出现皮质盲,可行高压氧治疗;必要时可适当使用激素,减少细胞毒性。同时积极治疗原发疾病。本例患者于术后48 h内病情反复,提示用药剂量在患者病情暂时缓解后仍需维持,直至病情稳定。
综上,造影剂脑病是一种较罕见的病症,少数患者病情较危重,不可逆,导致永久性的大脑功能障碍,甚至死亡,但大多数患者的发病常有自限性,短时间内恢复良好。随着介入技术的发展,含碘造影剂在临床诊治上应用范围越来约广,所以我们要警惕造影剂脑病的发生,避免不必要的死亡。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
四川省科技厅重点研发项目(No. 2020YFS0244、No. 2020YFS0242)和四川大学华西第二医院新芽基金(No. kx128)资助
Contributor Information
文 张 (Wen ZHANG), Email: 1055130496@qq.com.
勇 贺 (Yong HE), Email: zznnyeah@163.com.
References
- 1.LEONG S, FANNING N F Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. 2012;18(1):33–41. doi: 10.1177/159101991201800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DATTANI A, AU L, TAY K H, et al Contrast-induced encephalopathy following coronary angiography with no radiological features: A case report and literature review. Cardiology. 2018;139(3):197–201. doi: 10.1159/000486636. [DOI] [PubMed] [Google Scholar]
- 3.HAMRA M, BAKHIT Y, KHAN M, et al Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331–335. doi: 10.2217/fca-2016-0075. [DOI] [PubMed] [Google Scholar]
- 4.ZWICKER J C, SILA C A MRI findings in a case of transient cortical blindness after cardiac catheterization. Catheter Cardiovasc Interv. 2002;57(1):47–49. doi: 10.1002/ccd.10246. [DOI] [PubMed] [Google Scholar]
- 5.DE BONO D Complications of diagnostic cardiac catheterisation: Results from 34, 041 patients in the United Kingdom confidential enquiry into cardiac catheter complications.The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. Br Heart J. 1993;70(3):297–300. doi: 10.1136/hrt.70.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TATLI E, BUYUKLU M, ALTUN A An unusual but dramatic complication of coronary angiography: transient cortical blindness. Int J Cardiol. 2007;121(1):e4–6. doi: 10.1016/j.ijcard.2007.04.129. [DOI] [PubMed] [Google Scholar]
- 7.RIAHI L, MEDIOUNI M, MESSELMANI M, et al A singular manifestation of contrast-induced encephalopathy following coronary angiography. Neurol India. 2019;67(6):1525–1527. doi: 10.4103/0028-3886.273634. [DOI] [PubMed] [Google Scholar]
- 8.SPINA R, SIMON N, MARKUS S R, et al Contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv. 2017;90(2):257–268. doi: 10.1002/ccd.26871. [DOI] [PubMed] [Google Scholar]
- 9.FRONTERA J A, PILE-SPELLMAN J, MOHR J P Contrast-induced neurotoxicity and selective cortical injury. Cerebrovasc Dis. 2007;24(1):148–151. doi: 10.1159/000103621. [DOI] [PubMed] [Google Scholar]
- 10.CHISCI E, SETACCI F, DE DONATO G, et al A case of contrast-induced encephalopathy using iodixanol. J Endovasc Ther. 2011;18(4):540–544. doi: 10.1583/11-3476.1. [DOI] [PubMed] [Google Scholar]
- 11.LUND C, NES R B, UGELSTAD T P, et al Cerebral emboli during left heart catheterization may cause acute brain injury. Eur Heart J. 2005;26(13):1269–1275. doi: 10.1093/eurheartj/ehi148. [DOI] [PubMed] [Google Scholar]
- 12.DEMIRTAS M, BIRAND A, USAL A Transient cortical blindness after second coronary angiography: Is immunological mechanism possible? Cathet Cardiovasc Diagn. 1994;31(2):161. doi: 10.1002/ccd.1810310220. [DOI] [PubMed] [Google Scholar]
- 13.YONEDA Y, YAMAMOTO S Cerebral cortical laminar necrosis on diffusion-weighted MRI in hypoglycaemic encephalopathy. Diabet Med. 2005;22(8):1098–1100. doi: 10.1111/j.1464-5491.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 14.KAWAHARA H, TAKEDA Y, TANAKA A, et al Does diffusion-weighted magnetic resonance imaging enable detection of early ischemic change following transient cerebral ischemia? J Neurol Sci. 2000;181(1/2):73–81. doi: 10.1016/S0022-510X(00)00433-0. [DOI] [PubMed] [Google Scholar]