Abstract
患者12岁,因“阴道流血2+月,盆腔肿物待诊”于2019年9月19日入院。本例患者及家属均明确否认既往己烯雌酚暴露史。入院后予完善相关检查后于全麻下行宫腔镜探查术,术中取宫颈赘生物组织行冰冻切片,病理结果示宫颈恶性肿瘤,遂行经腹广泛全子宫切除术+双侧输卵管切除术+双侧卵巢移位+盆腔淋巴结清扫+腹主动脉旁淋巴结取样。术后切除组织病理示:宫颈透明细胞癌,癌浸润宫颈间质深1/3层,并向下累及阴道壁;癌转移至左闭孔淋巴结、左髂内外淋巴结。切除组织免疫组化染色示:细胞角蛋白7(CK7)(+)、细胞角蛋白20(CK20)(−)、天冬氨酸蛋白酶A(Napsin-A)(+)、细胞黏附分子CD15(+)、肝细胞核因子-1β(HNF-1β)(+)、Sal样蛋白4(SALL-4)(−)、抑癌基因P16蛋白(+)、雌激素受体(ER)(+)、孕激素受体(PR)(−)、抑癌基因P53蛋白灶性+、抑癌基因WT-1蛋白(−)、抗原Ki67阳性率约40%。诊断:宫颈透明细胞癌 ⅢC1p期,术后全身化疗(氟尿嘧啶+顺铂)4周期,25次三维后装放射治疗,随访至今,未见明显复发迹象。此病临床表现与宫颈鳞癌基本相同,但若患者年龄较小,则容易被误诊为功能失调性子宫出血等,需加以鉴别。
Keywords: 宫颈透明细胞癌, 宫颈癌, 透明细胞癌, 宫颈腺癌
Abstract
The 12-year-old patient was admitted to the hospital on September 19, 2019 for “vaginal bleeding for 2+ months and pelvic mass to be diagnosed”. The patient and her family explicitly denied any previous history of diethylstilbestrol exposure. After admission, relevant examinations were conducted and hysteroscopic exploration was performed under general anesthesia. During the procedure, cervical neoplasms were extracted and pathology results indicated cervical cancer. Then, extensive transabdominal hysterectomy+bilateral salpingectomy+bilateral ovarian transposition+pelvic lymph node dissection+para-aortic lymph node sampling were performed. Postoperative pathology analysis of the removed tissue showed that clear cell carcinoma of cervix (CCAC) had infiltrated into 1/3 of the cervical stroma and there was downward involvement of the vaginal wall; the cancer metastasized to the left obturator lymph node and the left internal and external iliac lymph nodes. Immunohistochemical staining of the removed tissue showed the following results: cytokeratin 7 (+), cytokeratin 20 (−), Napsin-A (+), cell adhesion molecule CD15 (+), heatocyte nuclear factor-1 β (+), Sal-like protein 4 (−), tumor suppressor gene P16 protein (+), estrogen receptor (+), progesterone receptor (−), tumor suppressor gene P53 protein (focal positive), tumor suppressor gene WT-1 protein (−) and Ki67 antigen (about 40% positive). The patient was diagnosed with CCAC stage ⅢC1p. Four cycles of postoperative systemic chemotherapy (fluorouracil+cisplatin) and 25 times of three-dimensional afterloading radiotherapy were performed. The patient did follow-up visits and did not show obvious signs of recurrence. The clinical manifestations of this disease are basically the same as those of cervical squamous cell carcinoma, and if the patient is younger, it can be easily misdiagnosed as dysfunctional uterine bleeding, indicating the need for differential diagnosis.
Keywords: Clear cell carcinoma of cervix, Carcinoma of cervix, Clear cell cancer, Adenocarcinoma of cervix
在女性癌症中宫颈癌的发病率和死亡率都居于第四位[1],而宫颈透明细胞癌是一类较特殊的宫颈腺癌,发病率约为宫颈腺癌的4%[2],国内外关于宫颈透明细胞癌的报道较少,近期我院收治一名年仅12岁的 ⅢC[3]期宫颈透明细胞癌,患儿术后一般情况可。此病例报道如下。
1. 病例资料
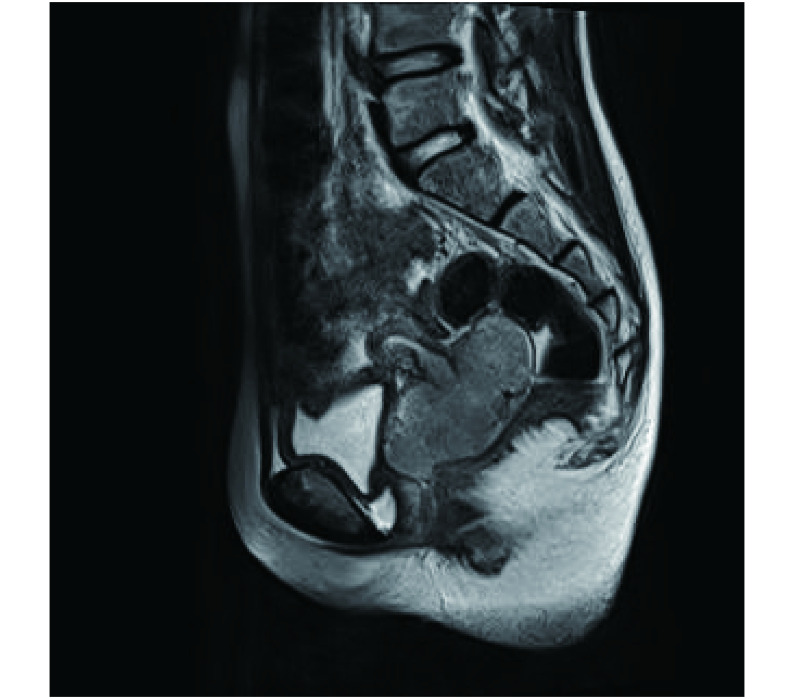
患儿12岁,因“阴道流血2+月,盆腔肿物待诊”于2019年9月19日入我院。2个月前(2019年7月)患者出现持续少量阴道流血,2019年8月22日查甲胎蛋白:1.6 μg/L,癌胚抗原:0.8 ng/mL,糖类抗原CA125:22.9 U/mL,糖类抗原CA19-9:27.1 U/mL,人绒毛膜促性腺激素:2.0 mIU/mL;彩超提示(图1):盆腔内查见大小约6.7 cm×3.4 cm×6.8 cm不均质弱回声,边界较清,形态欠规则,内可见片状强回声,该团块与子宫下段及宫颈分界不清,似凸向宫腔,其内探及血流信号,血流阻力指数=0.58。2019年9月6日MRI示(图2、图3):阴道穹窿及宫颈管内占位,该占位与宫颈前唇关系密切,上缘近颈体交界,宫颈前唇间质环部分缺如,考虑宫颈或内膜来源肿瘤性病变,并凸入阴道穹窿,左侧闭孔淋巴结长大,肿瘤转移不能除外;双侧附件、膀胱和直肠未见异常,盆腔少量积液。自述便秘,余无特殊不适。入院后肛查:子宫颈处扪及一肿物。全麻下行“宫腔镜检查术+宫颈活检术”,术中见:阴道内扪及大小约7 cm肿物,肿物表面粗糙,质朽。宫腔镜(图4)下见肿物呈鱼肉样,表面血管丰富,予切除并钳夹肿物后仍未见明确宫颈组织。术中取宫颈赘生物组织行冰冻切片,结果示宫颈赘生物为恶性肿瘤,家属签字同意后,行经腹广泛全子宫切除术+双侧输卵管切除术+双侧卵巢移位+盆腔淋巴结清扫+腹主动脉旁淋巴结取样术。术中发现左侧阴道前壁肿瘤穿透至膀胱后壁,左侧髂内外淋巴结、左侧闭孔淋巴结、右侧髂内外淋巴结增大变硬,余淋巴结未见明显异常。术毕剖视子宫:宫颈呈鱼肉样肿物,质朽,肿物侵及宫颈间质近全层,左侧上似达颈体交界处,肿瘤侵及左侧阴道及阴道穹窿,穿至阴道膀胱面。术后组织病理示(图5):宫颈透明细胞癌,癌浸润宫颈间质深1/3层,并向下累及阴道壁;癌未累及送检部分阴道组织、子宫颈体交界、双侧输卵管、宫旁组织(双侧)及盆侧壁(双侧)手术切缘。癌转移至左闭孔淋巴结3/7枚、左髂内外1/2枚;未转移至其他各组淋巴结。免疫组织化学(IHC)染色:细胞角蛋白7(CK7)(+)、细胞角蛋白20(CK20)(−)、天冬氨酸蛋白酶A(Napsin-A)(+)、细胞黏附分子CD15+、肝细胞核因子-1β(HNF-1β)(+)、Sal样蛋白4(SALL-4)(−)、抑癌基因P16蛋白(+)、雌激素受体(ER)(+)、孕激素受体(PR)(−)、抑癌基因P53蛋白灶性+、抑癌基因WT-1蛋白(−)、抗原Ki67阳性率约40%。术后宫颈组织行HPV检测(PCR-反向点杂交法)提示HPV23种亚型均为阴性。据FIGO宫颈癌分期(2018)诊断为宫颈透明细胞癌ⅢC1p期,截至2020年1月13日,患者已行全身化疗(氟尿嘧啶+顺铂)4周期,25次三维后装放射治疗,目前患者随访6个月,2020年3月30日 复查糖类抗原CA125:7.0 U/mL,糖类抗原CA19-9:34.6 U/mL,未见明显肿瘤复发迹象。
图 1.
Gynecological ultrasound of the patient
患儿妇科B超
图 2.
T2 axial MRI of the patient
患儿MRI T2轴位
图 3.
T2 sagittal position MRI of the patient
患儿MRI T2矢状位
图 4.
Picture taken during hysteroscopy
宫腔镜术中照片
图 5.
Pathological section of cervix under microscope. ×200
患儿宫颈组织镜下病理切片。×200
Left: HE staining; Right: IHC staining of P53.
2. 讨论
2.1. 一般情况
宫颈透明细胞腺癌的发病年龄大多集中于15~29岁及40~54岁[4],国外报道的年龄最小病例仅1岁[5],国内报道的年龄最小病例仅6岁[6- 7],但总的来看,国内外年龄小于15岁的宫颈透明细胞癌病例均鲜有报道。
2.2. 病因
目前认为遗传,bcl-2、p53 基因过度表达,微卫星重复序列的不稳定性等因素可能都是与宫颈透明细胞癌发病相关的因素[8-10]。也有报告指出宫颈透明细胞癌和子宫内膜异位症之间可能有联系[11]。既往有研究明确指出宫颈透明细胞癌与宫内己烯雌酚(diethylstilbestrol,DES)暴露史相关,但HUO等[12]的研究提示原发性宫颈透明细胞癌与有宫内己烯雌酚暴露史的宫颈透明细胞癌只有第一个5年生存率有较大差别;且自1971年,己烯雌酚不再用于治疗早期先兆流产,近年来报道的宫颈透明细胞癌病例绝大多数无“DES暴露史”[13],可基本排除宫颈透明细胞癌与DES的相关性 [14],本例患者及家属均明确否认既往DES暴露史。此外,有研究认为宫颈透明细胞癌与宫颈鳞癌的“高危因素”〔年龄大、流行病学的风险、多个性伴侣、吸烟史、社会经济地位的不同、使用避孕药和人乳头瘤病毒(HPV)感染等〕无明显相关性[15-17]。目前尚无关于孕产次与宫颈透明细胞癌发病相关性的分析,本次查阅的相关报告暂未体现明显关联。因宫颈透明细胞癌较为少见,可研究的病例数目较少,其发病机制尚无定论,仍需要更进一步的探究。
2.3. 诊断
目前宫颈透明细胞癌的诊断主要依靠组织病理学诊断,液基细胞检测(liquid-based cytologic test,LCT)、液基薄层细胞检测(thinprep cytologic test,TCT)及HPV-DNA检测是有效的宫颈癌筛查措施,但宫颈腺癌与HPV感染有较低的相关性[15- 16, 18],国际子宫颈腺癌标准和分类(the International Endocervical Adenocarcinoma Criteria and Classification,IECC)将宫颈透明细胞癌列为非人乳头瘤病毒相关性宫颈癌(non-human papillomavirus-related adenocarcinomas,NHPVA)[18],PARK等[19]的研究表明在NHPVA中,仅仅有3%的HPV核糖核酸原位杂交(ribonucleic acid in-situ hybridisation,RISH)结果阳性,本例患者HPV结果为阴性,因此宫颈透明细胞癌难以通过筛查的方式被早期发现。
2.4. 临床表现
宫颈透明细胞癌临床表现与宫颈鳞癌基本相同,大多表现为阴道持续流血、流液,不规则阴道出血等,但若患者年龄较小,则容易被误诊为功能失调性子宫出血等,从而延误治疗[9-10, 20-21]。国内外均有过类似报道,这足以提醒我们,任何阶段的女性,一旦出现类似症状,都应该积极将宫颈透明细胞癌纳入可疑诊断之中。
2.5. 治疗
目前普遍认为宫颈透明细胞癌治疗方式与宫颈鳞癌及其他病理类型宫颈癌相似,早期宫颈透明细胞癌首选治疗方式仍为宫颈癌根治术,即根治性子宫切除+盆腔淋巴结切除术,必要时辅助治疗。因宫颈透明细胞癌患者中年轻患者较多,其保留卵巢或保留生育能力的治疗方案成为目前值得探讨的问题,国内外多位专家研究指出:对于早期宫颈透明细胞癌患者,保留生育能力或保留卵巢的治疗方案是可行的[22-23]。当然,宫颈透明细胞癌发病率较低,且大部分保留卵巢或生育能力患者依然较年幼,需随访更长时间,保留生育能力手术方式对患者的预后还有待进一步探究。
2.6. 预后
涂云霞等[23]的分析认为早期诊断的宫颈透明细胞癌患者有较好的预后,且中晚期患者中经手术治疗后再行放化疗比单纯放化疗的预后好,REICH等[9]的研究指出ⅠA期宫颈透明细胞癌患者与宫颈腺癌及宫颈鳞癌相比,其生存率较低(约为67%),但三者差异无统计学意义。胡瑞等[6]的研究证明宫颈透明细胞癌患者中伴有淋巴结转移的预后极差,针对不同的患者采取手术加放化疗结合的方式有助于改善患者的预后。与宫颈鳞癌相似,传统的危险因素(淋巴结阳性、手术切缘阳性、肿瘤直径大于4 cm、淋巴管间隙受累、深1/3宫颈间质受累等)应作为早期宫颈透明细胞癌放化疗的适应证,但诊断为宫颈透明细胞癌并不代表生存率低[15]。总体上,大部分宫颈透明细胞癌患者能在Ⅰ~ⅡA期得到确诊并积极治疗,预后情况较乐观,本例患者“阴道流血2+月”,确诊时即ⅢC期亦是十分少见的。
综上所述,宫颈透明细胞癌是目前较罕见的妇科恶性肿瘤,其发病年龄呈“两个高峰”,此病发病率低,该疾病的病因尚不明确,国内外都没有较成熟、系统的诊治方案,需根据患者制定个体化的治疗方案。但总体来说,早期宫颈透明细胞癌患者预后较好,晚期患者预后不甚理想,因此,早发现、早诊断、早治疗依然是最有效的应对方法。此外,本例患者术后持续规范治疗,截止2020年5月尚未见明显复发迹象,但由于本病例随访时间较短,且与本病例同龄患者数据较少,无可靠数据作为参考,远期预后需随访更长时间。
* * *
利益冲突 所有作者均声明不存在利益冲突
References
- 1.BRAY F, FERLAY J, SOERJOMATARAM I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.PAL S, JANA S, BOSE K Clear cell carcinoma of cervix in a postmenopausal woman: a case report. J Midlife Health. 2015;6(2):85–87. doi: 10.4103/0976-7800.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.李静, 索红燕, 孔为民 《国际妇产科联盟(FIGO)2018癌症报告: 宫颈癌新分期及诊治指南》解读. 中国临床医生杂志. 2019;47(6):646–649. doi: 10.3969/j.issn.2095-8552.2019.06.008. [DOI] [Google Scholar]
- 4.HUO D, ANDERSON D, PALMER J R, et al Incidence rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix: update after 40-year follow-up. Gynecol Oncol. 2017;146(3):566–571. doi: 10.1016/j.ygyno.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 5.ARORA A, RASTOGI A, NEYAZ A, et al. Clear cell adenocarcinoma of cervix in 1-year-old girl without in utero exposure to diethylstilbestrol: an uncommon tumour at an uncommon age and site. BMJ Case Rep, 2017[2020-04-28]. https://www.ncbi.nlm.nih.gov/pubmed/28302659. doi: 10.1136/bcr-2016-218730.
- 6.胡瑞, 侯文静, 张梦真 宫颈透明细胞癌13例临床分析. 国际妇产科学杂志. 2019;46(2):146–149. doi: 10.3969/j.issn.1674-1870.2019.02.006. [DOI] [Google Scholar]
- 7.SU Y, ZHANG C, HOU W, et al. Fertility-preserving local excision under a hysteroscope with combined chemotherapy in a 6-year-old child with clear cell adenocarcinoma of the cervix: a case report and review of the literature. Medicine (Baltimore), 2020, 99(5): e18646[2020-05-01]. https://www.ncbi.nlm.nih.gov/pubmed/32000369.doi: 10.1097/MD.0000000000018646.
- 8.BOYD J, TAKAHASHI H, WAGGONER S E, et al Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in utero. Cancer. 1996;77(3):507–513. doi: 10.1002/(SICI)1097-0142(19960201)77:3<507::AID-CNCR12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.REICH O, TAMUSSINO K, LAHOUSEN M, et al Clear cell carcinoma of the uterine cervix: pathology and prognosis in surgically treated stage ⅠB-ⅡB disease in women not exposed in utero to diethylstilbestrol. Gynecol Oncol. 2000;76(3):331–335. doi: 10.1006/gyno.1999.5700. [DOI] [PubMed] [Google Scholar]
- 10.黄燕明, 吴绪峰, 金晶 青少年宫颈透明细胞癌1例并文献复习. 中国实用妇科与产科杂志. 2010;26(11):883–885. [Google Scholar]
- 11.HIROMURA T, TANAKA Y O, NISHIOKA T, et al. Clear cell adenocarcinoma of the uterine cervix arising from a background of cervical endometriosis.Br J Radiol, 2009, 82(973): e20-e22[2020-05-05]. https://www.ncbi.nlm.nih.gov/pubmed/19095810.
- 12.HUO D, ANDERSON D, HERBST A L Follow-up of patients with clear-cell adenocarcinoma of the vagina and cervix. N Engl J Med. 2018;378(18):1746–1748. doi: 10.1056/NEJMc1800097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WANG D, ZHAO C, FU L, et al. Primary clear cell adenocarcinoma of the cervix: a clinical analysis of 18 cases without exposure to diethylstilbestrol. Obstet Gynecol Int, 2019, 2019: 9465375[2020-05-05]. https://www.ncbi.nlm.nih.gov/pubmed/31049066. doi: 10.1155/2019/9465375.
- 14.HASANZADEH M, JAFARIAN A H, SERESHT L M Primary clear cell carcinoma with no diethylstilbestrol exposure; Case series. Iran J Med Sci. 2019;44(2):163–167. [PMC free article] [PubMed] [Google Scholar]
- 15.THOMAS M B, WRIGHT J D, LEISER A L, et al Clear cell carcinoma of the cervix: A multi-institutional review in the post-DES era. Gynecol Oncol. 2008;109(3):335–339. doi: 10.1016/j.ygyno.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PIROG E C, LLOVERAS B, MOLIJN A, et al HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27(12):1559–1567. doi: 10.1038/modpathol.2014.55. [DOI] [PubMed] [Google Scholar]
- 17.TANTITAMIT T, HAMONTRI S, RANGSIRATANAKUL L. Clear cell adenocarcinoma of the cervix in second generation young women who are without maternal exposure to diethylstilbestrol: A case report. Gynecol Oncol Rep, 2017, 20: 34-36[2020-04-14]. https://www.ncbi.nlm.nih.gov/pubmed/28275694. doi: 10.1016/j.gore.2017.02.008.
- 18.STOLNICU S, HOANG L, SOSLOW R A Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019;475(5):537–549. doi: 10.1007/s00428-019-02601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PARK K J Cervical adenocarcinoma: integration of HPV status, pattern of invasion, morphology and molecular markers into classification. Histopathology. 2020;76(1):112–127. doi: 10.1111/his.13995. [DOI] [PubMed] [Google Scholar]
- 20.STEWART J, 3rd, BEVANS-WILKINS K, YE C, et al Clear-cell endocervical adenocarcinoma in a 19-year-old woman. Diagn Cytopathol. 2006;34(12):839–842. doi: 10.1002/dc.20569. [DOI] [PubMed] [Google Scholar]
- 21.GOTTWALD L, KORCZYNSKI J, GORA E, et al Clear cell adenocarcinoma of the uterine cervix in a 24-year-old woman. Case report and review of the literature. Arch Med Sci. 2012;8(3):578–581. doi: 10.5114/aoms.2012.29414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LESTER F C, FARMER D L, RABBAN J T, et al. Radical trachelectomy for clear cell carcinoma of the cervix in a 6-year old: A case report, review, and description of the surgical technique. J Pediatr Surg, 2010, 45(8): E1-E5[2020-05-28]. https://www.ncbi.nlm.nih.gov/pubmed/20713196.doi: 10.1016/j.jpedsurg.2010.05.032.
- 23.涂云霞, 华金仁, 余期龙, 等 宫颈透明细胞癌50例临床病例分析. 现代肿瘤医学. 2017;25(10):1609–1612. doi: 10.3969/j.issn.1672-4992.2017.10.025. [DOI] [Google Scholar]