Abstract
目的
探讨能够有效预测子宫内膜癌复发患者预后生存的影响因素。
方法
回顾性收集2013年10月−2019年5月共473例Ⅰ~Ⅲ期接受了标准手术治疗的子宫内膜癌患者的临床病理数据,术后随访患者复发情况。整体复发包括局部区域复发和不良预后复发。本研究的终点指标为整体复发、局部区域复发和不良预后复发患者的无复发生存(recurrence-free survival,RFS)和总生存(overall survival,OS)。使用Kaplan-Meier生存曲线评估患者OS和RFS。通过Cox回归分析寻找影响子宫内膜癌复发患者预后生存的因素。
结果
473例患者中,无复发患者406例,总共67例患者复发(14.2%),其中局部区域复发占27例(5.7%),不良预后复发占40例(8.5%),复发患者的中位随访时间为38个月。生存曲线示,无复发组患者RFS和OS不变,而复发组患者,无论是整体复发还是局部区域复发、不良预后复发,RFS和OS均下降(P<0.001);整体复发患者的3年OS率为44.8%,中位生存时间为29个月,中位复发时间为17个月;无复发组患者的3年OS率为98.8%,中位生存时间为40个月;局部区域复发患者3年OS率为59.3%,中位生存时间为27个月,中位复发时间为15个月;不良预后复发患者3年OS率仅为35.0%,中位生存时间为22个月,中位复发时间为10个月。多因素Cox回归分析结果显示, 对于整体复发者而言,FIGO分期为Ⅲ期〔风险比(HR)=3.432,P=0.005〕、Ki-67的表达增高(HR=1.015,P=0.025)和ER的表达降低(HR=0.985,P=0.005)是RFS下降的独立因素,FIGO分期为Ⅲ期(HR=4.918,P=0.005)和PR的表达降低(HR=0.977,P=0.003)是OS下降的独立因素;对于局部区域复发者而言,特殊病理类型(HR=2.545,P=0.049)和Ki-67的表达增高(HR=1.024,P=0.033)是RFS下降的独立影响因素,PR的表达降低(HR=0.973,P=0.009)是OS下降的独立危险因素;对于不良预后复发者而言,FIGO分期Ⅲ期(HR=5.977,P=0.002)和ER表达降低(HR=0.984,P=0.023)是RFS下降的独立危险因素,FIGO分期Ⅲ期(HR=10.098,P=0.001)是OS下降的独立影响因素。
结论
FIGO分期Ⅲ期、Ki-67的表达增高、ER的表达降低可增加患者术后复发的风险;FIGO分期Ⅲ期、PR的表达降低可增加复发患者的死亡风险。
Keywords: 子宫内膜癌, 免疫组化指标, 临床病理因素, 不良复发
Abstract
Objective
To probe for factors that can be used effectively to predict the prognostic survival of patients with endometrial cancer recurrence.
Methods
The clinicopathological data of 473 patients with stage Ⅰ to Ⅲ endometrial cancer who underwent standard surgical treatment from October 2013 to May 2019 were retrospectively collected, and post-operative recurrence of the patients were followed up. Overall recurrence includes local recurrence and poor prognosis recurrence. The endpoint indicators of this study are the recurrence-free survival (RFS) and overall survival (OS) of patients with overall recurrence, local recurrence, and poor prognosis recurrence (PPR). The Kaplan-Meier survival curve was used to evaluate the OS and RFS of patients. Cox proportional-hazards model was used to identify factors affecting the prognostic survival of patients with endometrial cancer recurrence.
Results
Among the 473 patients, 406 did not experience recurrence. A total of 67 patients, accounting for 14.2%, had recurrence. Among them, 27 had local recurrence, accounting for 5.7%, while 40 had poor prognosis recurrence, accounting for 8.5%. The median follow-up time of patients with recurrence was 38 months. The survival curve showed that the RFS and OS of the patients in the recurrence-free group remained unchanged, while the patients in the recurrence group, regardless of whether they had overall recurrence, local recurrence or PPR, experienced a decrease in RFS and OS(P<0.001). The overall 3-year OS rate of patients with recurrence was 44.8%, the median survival time was 29 months, and the median recurrence time was 17 months. The 3-year OS rate of patients in the recurrence-free group was 98.8%, and the median survival time was 40 months; the 3-year OS rate of patients with local recurrence was 59.3%, the median survival time was 27 months, and the median recurrence time was 15 months. The 3-year OS rate of patients with PPR was only 35.0%, the median survival time was 22 months, and the median recurrence time was 10 months. The results of multivariate Cox regression analysis showed that, for overall recurrence patients, FIGO stage Ⅲ (hazard ratio (HR)=3.432, P=0.005), increased expression of K-i67 (HR=1.015, P=0.025), and decreased expression of estrogen receptor (ER) (HR=0.985, P=0.005) are independent factors for the decline in RFS, FIGO stage Ⅲ (HR=4.918, P=0.005) and the decreased expression of progesterone receptor (PR) (HR=0.977, P=0.003) are independent factors for the decrease in OS. For patients with local recurrence, special pathological types (HR=2.545, P=0.049) and increased expression of Ki-67 (HR=1.024, P=0.033) are independent factors influencing the decrease in RFS, while decreased expression of PR (HR=0.973, P=0.009) is an independent risk factor for decreased OS. For patients with PPR, FIGO stage Ⅲ (HR=5.977, P=0.002) and decreased ER expression (HR=0.984, P=0.023) are independent risk factors for the decline in RFS, while FIGO stage Ⅲ (HR=10.098, P=0.001) is an independent factor influencing the decline of OS.
Conclusion
FIGO stage Ⅲ, increased Ki-67 expression, and decreased ER expression can increase patients' risk of postoperative recurrence, and FIGO stage Ⅲ and decreased expression of PR can increase the risk of death in patients with recurrence.
Keywords: Endometrial cancer, Immunohistochemical indicators, Clinicopathological factors, Poor prognosis recurrence
子宫内膜癌是女性生殖系统常见的三大恶性肿瘤之一[1]。近年来,子宫内膜癌的发生率呈上升趋势和年轻化趋势[2-3],此病主要依靠手术治疗,但仍有部分患者出现术后复发。复发的子宫内膜癌治疗相对困难,中位生存期较短[2,4]。OULDAMER等[5]将腹腔转移、腹膜癌和其它器官转移(肺、肝、脑等)定义为不良预后的复发,并发现相较于局部区域复发,不良预后复发总生存率明显降低。因此有关内膜癌复发、转移的因素需要更进一步的探索。
目前,临床上主要根据是否合并高危因素来确定术后是否需要辅助治疗,高危因素包括深肌层浸润、组织低分化(G3)、特殊病理类型及淋巴脉管间隙浸润,美国国立综合癌症网络(NCCN )和国际妇产科联盟(FIGO)指南中将年龄大于60岁也列入高危因素。随着分子生物学研究的发展,免疫组化标志物逐渐纳入恶性肿瘤预后生存评估的研究范围[6-7]。Ki-67蛋白可作为肿瘤增殖、侵袭[8]、预后不良[9-11]的标志物。雌激素受体(ER)、孕激素受体(PR)的异常表达和调节参与了激素相关癌症的发生和发展,并且与癌症治疗的结果有关[12]。p53基因突变和失活可以抑制细胞凋亡促进肿瘤发展[13]。既往研究表明,ER、PR、Ki-67及P53均与子宫内膜癌的预后密切相关,且可以通过免疫组化方法测得并广泛用于常规病理研究[14-18]。
近年来有关子宫内膜癌预后生存的模型研究因主要建立在传统的临床病理参数上,已无法满足准确地预测预后生存情况。另外,目前有关内膜癌复发预后生存的研究报道多集中于整体的复发,而对其具体复发类型和部位的研究较少。因此,本研究拟结合传统临床病理参数和免疫组化指标,探索子宫内膜癌不同类型和部位复发患者预后生存的相关因素。
1. 对象与方法
1.1. 研究对象
回顾性收集2013年10月1日−2019年5月31日于重庆医科大学附属第一医院收治的子宫内膜癌患者的临床资料。纳入标准:①经手术-病理分期确诊为Ⅰ~Ⅲ(FIGO 2009)的子宫内膜癌患者、并接受了标准的全面分期手术治疗,手术方式为至少全子宫+双侧输卵管卵巢切除术,伴或不伴系统性淋巴结切除术。②患者的临床病理资料完整,包括年龄、体质量指数(BMI)、合并症、完整的手术记录、病理结果(组织学类型和分级,肌层浸润深度,宫颈间质浸润与否,免疫组化指标Ki-67、ER、PR、P53)、术后辅助治疗(包括门诊随访、内分泌治疗、局部放疗、全身化疗和联合放化疗)。排除标准:①失访者和未规律随访者;②未按标准手术方式治疗;③非原发性子宫内膜癌、子宫癌肉瘤;④合并其他恶性肿瘤、致命合并症的患者;⑤术前接受了放化疗的患者。术后根据是否合并高危因素,由多学科讨论及结合NCCN指南决定是否补充放疗、化疗或内分泌治疗。本研究获重庆医科大学附属第一医院生物医学伦理审查委员会批准(2020年审469号)。
1.2. 复发与随访
患者经充分手术-术后辅助治疗后,前3年内每3~6个月随访1次,以后每6~12月随访1次。随访的内容包括定期妇科查体、血清肿瘤标志物〔糖类抗原CA125、人附睾蛋白4(HE4)、肿瘤相关表面抗原标志物〕和诊断学性影像学检查(妇科B超、腹部CT、盆腔CT、胸部CT和PET-CT)。记录患者复发转移情况,包括复发转移的时间、部位及生存情况。本研究的终点指标为整体复发、局部区域复发和不良预后复发患者的无复发生存(recurrence-free survival,RFS)和总生存(overall survival,OS)。
整体复发包括局部区域复发和不良预后复发。局部区域复发定义为阴道内、盆腔内和盆腔淋巴结/腹主动脉旁淋巴结复发;不良预后复发定义为腹腔转移、腹膜癌和其它器官转移(肺、肝、脑等)[5]。
RFS时间定义为术后至患者复发或随访截止日期的时间。OS时间定义为术后至患者死亡日期或随访截止日期的时间。
1.3. 组织病理学和免疫组化
所有术后标本均在规定时间内用多聚甲醛液固定后送至重庆医科大学病理实验室进一步处理。将组织制成石蜡包埋的标本。使用HE染色来确认哪些部分是癌性的,然后选择一个具有代表性的癌性部分进行观察。ER、PR、Ki-67和P53的免疫组织化学分析(IHC)在自动免疫染色仪上进行。病理学类型及分级、病变大小、病变浸润范围、免疫组织化学结果由病理实验室的初级病理学医师进行初步判断,再由上级医师进行复核。免疫组织化学参数(Ki-67、ER、PR、P53)结果报告其阳性率。
1.4. 统计学方法
计量资料用中位数、
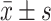 、最小值和最大值表示;计数资料用频率、百分数表示;Kaplan-Meier曲线描绘各组OS、RFS,并进行log-rank检验;利用单因素Cox回归分析筛选与子宫内膜癌复发患者预后生存相关的因素(其中免疫组化指标ER、PR、Ki-67、P53表达的数据形式为百分率,当连续变量处理),P<0.05的因素进一步纳入多因素Cox回归分析;P<0.05为差异有统计学意义。
、最小值和最大值表示;计数资料用频率、百分数表示;Kaplan-Meier曲线描绘各组OS、RFS,并进行log-rank检验;利用单因素Cox回归分析筛选与子宫内膜癌复发患者预后生存相关的因素(其中免疫组化指标ER、PR、Ki-67、P53表达的数据形式为百分率,当连续变量处理),P<0.05的因素进一步纳入多因素Cox回归分析;P<0.05为差异有统计学意义。
2. 结果
2.1. 整体情况
共收集537例患者的临床信息,按照纳入标准筛选后,最终473例纳入回顾性研究中。所有患者均行全子宫+双侧输卵管卵巢切除术。其中52例(10.9%)同时行系统性盆腔淋巴结清扫。大部分为Ⅰ期子宫内膜癌患者(72.7%),术后病理类型为Ⅰ型的子宫内膜癌即子宫内膜样腺癌的患者共411例(86.9%),病理类型为Ⅱ型子宫内膜癌患者共62例(13.1%),包括浆液性癌、黏液性癌、透明细胞癌。免疫组化指标Ki-67阳性百分比的中位数为30%,ER的百分比中位数为80%,PR的百分比中位数为80%,P53的百分比中位数为20%。术后194例(41.0%)患者未接受任何辅助治疗或仅接受内分泌治疗,其余279例(49.0%)患者术后接受了包括盆腔放疗、全身化疗或联合放化疗。所有随访患者的中位随访时间为38个月(范围7~67个月),无复发患者的中位随访时间为40个月。共有67例患者(14.2%)整体复发,其中局部区域复发有27例(5.7%),不良预后复发有40例(8.5%)。
2.2. 不同复发类型患者的生存分析
见图1。无复发组患者和整体复发组患者的OS、RFS差异均有统计学意义(P<0.001)。整体复发患者的3年OS率为44.8%,中位生存时间为29个月,中位复发时间为17个月。进一步分析发现无复发组患者、局部区域复发组患者、不良预后复发组患者的OS差异有统计学意义(P<0.001):无复发患者的3年OS率为98.8%,中位生存时间为40个月;局部区域复发患者3年OS率为59.3%,中位生存时间为27个月,中位复发时间为15个月;不良复发患者3年OS率仅为35.0%,中位生存时间为22个月,中位复发时间为10个月。3组患者的RFS差异有统计学意义(P<0.001)。
图 1.

Kaplan-Meier curves of overall survival (OS) and recurrence-free survival (RFS) of different types of endometrial cancer recurrence
不同复发类型的子宫内膜癌患者总生存和无复发生存的Kaplan-Meier曲线
2.3. 子宫内膜癌复发患者预后生存因素的单因素及多因素分析
由图1可知,无复发组患者RFS和OS不变,而复发组患者,无论是整体复发还是局部区域复发、不良预后复发,RFS和OS均下降。因此,在Cox回归分析中,我们将Y赋值为,0=RFS或OS不变,1=RFS或OS下降,以寻找复发患者和无复发患者共有、但是能引起RFS或OS下降的危险因素。
2.3.1. 影响子宫内膜癌整体复发患者预后生存的因素
纳入整体复发患者(67例)和无复发患者(406例)共473例进行Cox回归分析。单因素分析显示FIGO分期,组织学分级,病理学分型,宫颈间质浸润,肌层浸润深度,辅助治疗方式,免疫组化指标ER、PR、Ki-67、P53对患者的RFS、OS均有影响(P<0.05);年龄≥60岁(P=0.011)、术中未切除淋巴结(P=0.004)对OS也有影响。进一步行多因素分析发现,仅有 FIGO分期Ⅲ期〔风险比(HR)=3.432,P=0.005〕、Ki-67表达增高(HR=1.015,P=0.025)和ER表达降低(HR=0.985,P=0.005)是RFS下降的独立危险因素,FIGO分期Ⅲ期(HR=4.918,P=0.005)和PR表达降低(HR=0.977,P=0.003)是OS下降的独立危险因素。见表1和表2。
表 1. Multivariate Cox regression analysis of factors influencing recurrence-free survival (RFS) of patients with overall recurrence.
影响整体复发患者RFS的多因素Cox回归分析
| Variables | RFS | |||
| Hazard ratio | 95%CI | P | ||
| ER: Estrogen receptor; PR: Progesterone receptor; Follow-up: No adjuvant therapy or only endocrine therapy after surgery; CT: Chemotherapy; RT: Radiotherapy; CI: Confidence interval. | ||||
| FIGO stage | ||||
| Ⅰ | 1.000 | Ref | ||
| Ⅱ | 2.624 | 0.898-7.662 | 0.078 | |
| Ⅲ | 3.432 | 1.451-8.116 | 0.005 | |
| Grade | ||||
| 1 | 1.000 | Ref | ||
| 2 | 1.519 | 0.343-6.723 | 0.582 | |
| 3 | 1.547 | 0.329-7.273 | 0.580 | |
| Pathological type | ||||
| Endometrioid carcinoma | 1.000 | Ref | ||
| Special types | 1.614 | 0.894-2.915 | 0.112 | |
| Myometrial invasion | ||||
| <1/2 | 1.000 | Ref | ||
| ≥1/2 | 1.601 | 0.885-2.896 | 0.120 | |
| Cervical stromal invasion | ||||
| No | 1.000 | Ref | ||
| Yes | 1.464 | 0.663-3.233 | 0.345 | |
| Ki-67 positive percentage | 1.015 | 1.002-1.028 | 0.025 | |
| ER positive percentage | 0.985 | 0.975-0.995 | 0.005 | |
| PR positive percentage | 0.996 | 0.985-1.007 | 0.465 | |
| P53 positive percentage | 1.008 | 1.000-1.015 | 0.054 | |
| Adjuvant therapy | ||||
| Follow-up | 1.000 | Ref | ||
| Only CT | 0.624 | 0.286-1.358 | 0.234 | |
| Only RT | 0.284 | 0.035-2.345 | 0.243 | |
| CT and RT | 0.686 | 0.300-1.570 | 0.372 | |
表 2. Multivariate analysis of factors influencing overall survival (OS) of patients with overall recurrence.
影响整体复发患者OS的多因素Cox回归分析
| Variables | OS | ||
| Hazard ratio | 95%CI | P | |
| ER, PR, follow-up, CT, RT,CI: For details see the notes of table 1. | |||
| Age | |||
| <60 yr. | 1.000 | Ref | |
| ≥60 yr. | 1.107 | 0.531-2.306 | 0.786 |
| FIGO stage | |||
| Ⅰ | 1.000 | Ref | |
| Ⅱ | 4.583 | 1.041-20.174 | 0.044 |
| Ⅲ | 4.918 | 1.638-14.764 | 0.005 |
| Grade | |||
| 1 | 1.000 | Ref | |
| 2 | 1.102 | 0.132-9.180 | 0.929 |
| 3 | 1.733 | 0.198-15.187 | 0.619 |
| Pathological type | |||
| Endometrioid carcinoma | 1.000 | Ref | |
| Special types | 1.064 | 0.502-2.252 | 0.872 |
| Myometrial invasion | |||
| <1/2 | 1.000 | Ref | |
| ≥1/2 | 1.885 | 0.858-4.141 | 0.114 |
| Cervical stromal invasion | |||
| No | 1.000 | Ref | |
| Yes | 0.868 | 0.325-2.317 | 0.777 |
| Lymphadenectomy | |||
| Yes | 1.000 | Ref | |
| No | 1.553 | 0.701-3.439 | 0.278 |
| Ki-67 positive percentage | 1.005 | 0.988-1.022 | 0.589 |
| ER positive percentage | 0.992 | 0.978-1.005 | 0.228 |
| PR positive percentage | 0.977 | 0.962-0.992 | 0.003 |
| P53 positive percentage | 1.008 | 0.998-1.018 | 0.111 |
| Adjuvant therapy | |||
| Follow-up | 1.000 | Ref | |
| Only CT | 2.421 | 0.838-6.994 | 0.102 |
| Only RT | 0.691 | 0.314-1.521 | 0.358 |
| CT and RT | 1.625 | 0.306-8.630 | 0.569 |
2.3.2. 影响子宫内膜癌局部区域复发患者预后生存的因素
纳入443例局部区域复发患者(27例)和无复发患者(406例)进行 Cox回归分析,结果见表3、表4。影响局部区域复发患者RFS的单因素分析的结果与整体复发患者的分析结果类似,FIGO分期,组织学分级,病理学类型,宫颈间质浸润,肌层浸润深度,辅助治疗,免疫组化指标ER、PR、Ki-67、P53对患者的局部区域复发均有显著影响(P<0.05)。进一步的多因素分析提示仅有特殊病理类型(HR=2.545,P=0.049)和Ki-67表达增高(HR=1.024,P=0.033)是患者RFS下降的独立影响因素。单因素分析示年龄≥60岁,FIGO分期,组织学分级,病理学类型,宫颈间质浸润,肌层浸润深度,术中未切除淋巴结,免疫组化指标ER、PR、Ki-67对患者的OS有影响(P<0.05),将上述因素纳入多因素分析,结果提示仅PR的表达降低(HR=0.973,P=0.009)是患者OS下降的独立危险因素。
表 3. Multivariate analysis of factors influencing recurrence-free survival (RFS) of patients with local recurrence.
影响局部复发患者RFS的多因素Cox回归分析
| Variables | RFS | ||
| Hazard ratio | 95%CI | P | |
| ER, PR, follow-up, CT, RT, CI: For details see the notes of table 1. | |||
| FIGO stage | |||
| Ⅰ | 1.000 | Ref | |
| Ⅱ | 1.488 | 0.188-11.784 | 0.707 |
| Ⅲ | 1.150 | 0.208-6.359 | 0.873 |
| Grade | |||
| 1 | 1.000 | Ref | |
| 2 | 1.169 | 0.137-9.937 | 0.887 |
| 3 | 2.177 | 0.240-19.754 | 0.489 |
| Pathological type | |||
| Endometrioid carcinoma | 1.000 | Ref | |
| Special types | 2.545 | 1.004-6.446 | 0.049 |
| Myometrial invasion | |||
| <1/2 | 1.000 | Ref | |
| ≥1/2 | 1.516 | 0.563-4.083 | 0.410 |
| Cervical stromal invasion | |||
| No | 1.000 | Ref | |
| Yes | 3.698 | 0.578-23.647 | 0.167 |
| Ki-67 positive percentage | 1.024 | 1.002-1.047 | 0.033 |
| ER positive percentage | 0.987 | 0.972-1.002 | 0.092 |
| PR positive percentage | 0.995 | 0.979-1.012 | 0.567 |
| P53 positive percentage | 1.005 | 0.992-1.018 | 0.497 |
| Adjuvant therapy | |||
| Follow-up | 1.000 | Ref | |
| Only CT | 0.517 | 0.163-1.640 | 0.263 |
| Only RT | 0.000 | 0.000 | 0.978 |
| CT and RT | 0.433 | 0.114-1.647 | 0.219 |
表 4. Multivariate analysis of factors influencing overall survival (OS) of patients with local recurrence.
影响局部复发患者OS的多因素Cox回归分析
| Variables | OS | ||
| Hazard ratio | 95%CI | P | |
| ER: Estrogen receptor; PR: Progesterone receptor; CI: Confidence interval. | |||
| Age | |||
| <60 yr. | 1.000 | Ref | |
| ≥60 yr. | 1.879 | 0.589-5.996 | 0.287 |
| FIGO stage | |||
| Ⅰ | 1.000 | Ref | |
| Ⅱ | 8.157 | 0.567-117.26 | 0.123 |
| Ⅲ | 2.277 | 0.364-14.232 | 0.379 |
| Grade | |||
| 1 | 1.000 | Ref | |
| 2 | 1697 | 0.000 | 0.922 |
| 3 | 6520 | 0.000 | 0.908 |
| Pathological type | |||
| Endometrioid carcinoma | 1.000 | Ref | |
| Special types | 0.850 | 0.246-2.940 | 0.797 |
| Myometrial invasion | |||
| <1/2 | 1.000 | Ref | |
| ≥1/2 | 1.292 | 0.416-4.017 | 0.658 |
| Cervical stromal invasion | |||
| No | 1.000 | Ref | |
| Yes | 1.178 | 0.124-11.165 | 0.886 |
| Lymphadenectomy | |||
| Yes | 1.000 | Ref | |
| No | 2.646 | 0.719-9.744 | 0.143 |
| Ki-67 positive percentage | 1.010 | 0.983-1.038 | 0.462 |
| ER positive percentage | 1.009 | 0.992-1.027 | 0.309 |
| PR positive percentage | 0.973 | 0.952-0.993 | 0.009 |
2.3.3. 影响子宫内膜癌不良复发患者预后生存的因素
见表5和表6。将不良预后复发患者(40例)和无复发患者(406例)共446例患者进行Cox回归分析。单因素分析提示分期、组织学分级、病理学类型、宫颈间质浸润、肌层浸润深度、辅助治疗,免疫组化指标ER、PR、Ki-67、P53对RFS和OS均有影响(P<0.05),另外年龄≥60岁(HR=2.070,P=0.044)、术中未切除淋巴结(HR=2.767,P=0.013)也是OS的影响因素。而多因素分析提示,FIGO分期Ⅲ期(HR=5.977,P=0.002)和ER表达降低(HR=0.984,P=0.023)是RFS降低的独立危险因素;仅有FIGO分期(HR=10.098,P=0.001)是OS降低的独立危险因素。
表 5. Multivariate Cox regression analysis of factors influencing recurrence-free survival (RFS) of patients with poor prognosis recurrence.
影响不良预后复发患者RFS的多因素Cox回归分析
| Variables | RFS | ||
| Hazard ratio | 95%CI | P | |
| ER, PR, follow-up, CT, RT, CI: For details see the notes of table 1. | |||
| FIGO stage | |||
| Ⅰ | 1.000 | Ref | |
| Ⅱ | 3.495 | 0.837-14.601 | 0.086 |
| Ⅲ | 5.977 | 1.895-18.855 | 0.002 |
| Grade | |||
| 1 | 1.000 | Ref | |
| 2 | 1.940 | 0.238-15.805 | 0.536 |
| 3 | 1.485 | 0.168-13.088 | 0.722 |
| Pathological type | |||
| Endometrioid carcinoma | 1.000 | Ref | |
| Special types | 1.693 | 0.778-3.684 | 0.185 |
| Myometrial invasion | |||
| <1/2 | 1.000 | Ref | |
| ≥1/2 | 1.928 | 0.873-4.256 | 0.104 |
| Cervical stromal invasion | |||
| No | 1.000 | Ref | |
| Yes | 1.273 | 0.515-3.144 | 0.601 |
| Ki-67 positive percentage | 1.007 | 0.991-1.024 | 0.379 |
| ER positive percentage | 0.984 | 0.970-0.998 | 0.023 |
| PR positive percentage | 0.996 | 0.981-1.010 | 0.561 |
| P53 positivepercentage | 1.008 | 0.998-1.018 | 0.116 |
| Adjuvant therapy | |||
| Follow-up | 1.000 | Ref | |
| Only CT | 0.477 | 0.151-1.509 | 0.208 |
| Only RT | 0.405 | 0.042-3.860 | 0.432 |
| CT and RT | 0.604 | 0.184-1.984 | 0.406 |
表 6. Multivariate Cox regression analysis of factors influencing overall survival (OS) of patients with poor prognosis recurrence.
影响不良预后复发患者OS的多因素Cox回归分析
| Variables | OS | ||
| Hazard ratio | 95%CI | P | |
| ER, PR, follow-up, CT, RT, CI: For details see the notes of table 1. | |||
| Age | |||
| <60 yr. | 1.000 | Ref | |
| ≥60 yr. | 0.905 | 0.377-2.177 | 0.824 |
| FIGO stage | |||
| Ⅰ | 1.000 | Ref | |
| Ⅱ | 7.906 | 1.328-47.059 | 0.023 |
| Ⅲ | 10.098 | 2.507-40.685 | 0.001 |
| Grade | |||
| 1 | 1.000 | Ref | |
| 2 | 1.249 | 0.142-10.978 | 0.841 |
| 3 | 1.240 | 0.129-11.926 | 0.852 |
| Pathological type | |||
| Endometrioid carcinoma | 1.000 | Ref | |
| Special types | 1.588 | 0.631-4.000 | 0.326 |
| Myometrial invasion | |||
| <1/2 | 1.000 | Ref | |
| ≥1/2 | 1.994 | 0.756-5.258 | 0.163 |
| Cervical stromal invasion | |||
| No | 1.000 | Ref | |
| Yes | 0.738 | 0.242-2.251 | 0.593 |
| Lymphadenectomy | |||
| Yes | 1.000 | Ref | |
| No | 2.252 | 0.868-5.843 | 0.095 |
| Ki-67 positive percentage | 0.993 | 0.972-1.014 | 0.497 |
| ER positive percentage | 0.983 | 0.965-1.001 | 0.063 |
| PR positive percentage | 0.982 | 0.964-1.000 | 0.056 |
| P53 positive percentage | 1.008 | 0.996-1.019 | 0.203 |
| Adjuvant therapy | |||
| Follow-up | 1.000 | Ref | |
| Only CT | 4.636 | 1.138-18.891 | 0.052 |
| Only RT | 1.042 | 0.406-2.669 | 0.932 |
| CT and RT | 3.128 | 0.565-17.312 | 0.191 |
3. 讨论
寻找子宫内膜癌预后生存的危险因素一直是研究的热点,目前有关子宫内膜癌预后生存的预测模型研究多集中于组织病理学类型、组织学分级、肌层浸润深度、淋巴脉管间隙浸润等传统的临床病理因素,但仅靠传统的临床病理因素无法完全准确地预测子宫内膜癌的预后生存[19]。近年来免疫组化指标逐渐纳入各种肿瘤复发风险评估的范围。对于子宫内膜癌,有研究表示Ki-67、ER、PR和P53的表达与子宫内膜癌的预后生存密切相关,目前尚无免疫组化指标作为补充术后辅助治疗的统一评估标准。随着分子生物学的发展,应用有效的免疫组化标志物结合临床参数预测预后生存风险是临床研究发展趋势,可以提高对子宫内膜癌患者预后判断的准确性,有助于指导我们对合并高危因素的患者采取积极有效的治疗方案,避免对低危患者过度治疗。
在本研究中,我们纳入了4项临床常用免疫组化标记物(ER、PR、Ki-67及P53),并结合经典临床病理参数,以预测子宫内膜癌不同类型及部位的复发。结果显示,对于整体复发者而言,FIGO分期为Ⅲ期、Ki-67的表达增高和ER的表达降低可促进RFS下降(即整体复发风险增加),FIGO分期为Ⅲ期和PR的表达降低可促进OS下降(即死亡风险增加)。这提示我们,对于分期晚、ER和PR低表达、Ki-67高表达的患者,我们应该在术后采取更积极有效的术后管理。而进一步分析发现,特殊病理类型和Ki-67表达增高的局部复发的风险增加,PR表达降低的局部复发患者死亡风险增加;FIGO分期Ⅲ期和ER表达降低的患者不良预后复发风险增加,FIGO分期Ⅲ期的不良预后复发患者死亡风险增加。这提示我们在术后更需要重视不良预后复发的患者,例如补充全身化疗、激素治疗、延长辅助治疗周期等,从而减少不良预后复发的风险,而在治疗结束后临床医师应该适当缩短术后随访的间隔时间,并在随访时关注患者的腹腔、胸部等全身其他器官的影像学检查。对于高Ki-67、低PR表达和特殊病理类型患者,其相对发生局部区域复发的风险较高,尽管该类患者的OS优于不良复发预后患者,我们也应该重视该类患者的术后管理,例如更积极的补充近距离放疗、盆腔外照射等,从而降低其局部区域复发的风险,警惕高Ki-67、低PR表达患者发展成不良预后复发;同时在术后应该更加密切的随访,在随访时重视盆腔影像学检查结果。预测可能出现的不同复发类型,有助于临床医师制定针对性的和个性化的诊疗方案,一方面达到精准治疗的目的,减少术后辅助治疗所带来的并发症给患者造成的痛苦,另一方面,可以合理调度医疗资源,对不同预后类型患者进行不同的医疗资源分配。
有相关研究报道,病理学分级高、深肌层浸润、宫颈间质浸润受累、P53高表达的患者复发率增高、预后更差[20-23]。有研究报道术后辅助治疗能够有效降低患者复发和改善OS[24]。本研究不能否定其他因素预测复发的重要性,但上述因素在本研究未得到证实。
本研究存在一定局限性,首先本研究采用了单中心回顾性研究,需多中心前瞻性实验进一步验证。其次,本研究仅证明Ki-67和ER的表达影响患者预后,并未进一步明确这两个因素影响预后的具体截断值。另外,本研究中免疫组化指标是以百分率表示并做连续变量处理,而不是以二分类变量表示,可能导致HR绝对值过小。
综上,FIGO分期Ⅲ期、Ki-67的表达增高、ER的表达降低可增加患者术后复发的风险;FIGO分期Ⅲ期、PR的表达降低可增加复发患者的死亡风险。我们认为,应该在术后关注合并高危因素的子宫内膜癌患者,并对她们进行分层分类、精细化、个体化管理,从而帮助改善患者预后生存。
* * *
利益冲突 所有作者均声明不存在利益冲突
References
- 1.BRAY F, FERLAY J, SOERJOMATARAM I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.MCALPINE J N, TEMKIN S M, MACKAY H J Endometrial cancer: not your grandmother's cancer. Cancer. 2016;122(18):2787–2798. doi: 10.1002/cncr.30094. [DOI] [PubMed] [Google Scholar]
- 3.MOORE K, BREWER M A Endometrial cancer: is this a new disease? Am Soc Clin Oncol Educ Book. 2017;37:435–442. doi: 10.1200/edbk_175666. [DOI] [PubMed] [Google Scholar]
- 4.CONNOR E V, ROSE P G Management strategies for recurrent endometrial cancer. Expert Rev Anticancer Ther. 2018;18(9):873–885. doi: 10.1080/14737140.2018.1491311. [DOI] [PubMed] [Google Scholar]
- 5.OULDAMER L, BENDIFALLAH S, BODY G, et al Predicting poor prognosis recurrence in women with endometrial cancer: a nomogram developed by the FRANCOGYN study group. Br J Cancer. 2016;115(11):1296–1303. doi: 10.1038/bjc.2016.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WANG C, WANG J, CHEN Z, et al Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: a systematic review. Chin J Cancer. 2017;36:65[2020-08-12]. https://doi.org/10.1186/s40880-017-0232-5. doi: 10.1186/s40880-017-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.INOUE C, TAMATSUKI D, MIKI Y, et al Prognostic significance of combining immunohistochemical markers for cancer-associated fibroblasts in lung adenocarcinoma tissue. Virchows Arch. 2019;475(2):181–189. doi: 10.1007/s00428-019-02587-9. [DOI] [PubMed] [Google Scholar]
- 8.MENON S S, GURUVAYOORAPPAN C, SAKTHIVEL K M, et al Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45. doi: 10.1016/j.cca.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 9.JURÍKOVÁ M, DANIHEL Ĺ, POLÁK Š, et al Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016;118(5):544–552. doi: 10.1016/j.acthis.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 10.SORBYE S W, KILVAER T K, VALKOV A, et al Prognostic impact of Jab1, p16, p21, p62, Ki67 and Skp2 in soft tissue sarcomas. PLoS One. 2012;7(10):e470685[2020-08-07]. https://doi.org/10.1371/journal.pone.0047068. doi: 10.1371/journal.pone.0047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GRANT L, BANERJI S, MURPHY L, et al Androgen receptor and Ki67 expression and survival outcomes in non-small cell lung cancer. Horm Cancer. 2018;9(4):288–294. doi: 10.1007/s12672-018-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RONG C, MEINERT É F R C, HESS J Estrogen receptor signaling in radiotherapy: from molecular mechanisms to clinical studies. Int J Mol Sci. 2018;19(3):713. doi: 10.3390/ijms19030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BYKOV V J N, ERIKSSON S E, BIANCHI J, et al Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 14.DI DONATO V, IACOBELLI V, SCHIAVI M C, et al Impact of hormone receptor status and Ki-67 expression on disease-free survival in patients affected by high-risk endometrial cancer. Int J Gynecol Cancer. 2018;28(3):505–513. doi: 10.1097/igc.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 15.SHEN F, GAO Y, DING J, et al Is the positivity of estrogen receptor or progesterone receptor different between type 1 and type 2 endometrial cancer? Oncotarget. 2017;8(1):506–511. doi: 10.18632/oncotarget.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.YANG B, SHAN B, XUE X, et al Predicting lymph node metastasis in endometrial cancer using serum CA125 combined with immunohistochemical markers PR and Ki67, and a comparison with other prediction models. PLoS One. 2016;11(5):e0155145[2020-08-07]. doi: https://doi.org/10.1371/journal.pone.0155145. doi: 10.1371/journal.pone.0155145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LI M, ZHAO L, SHEN D, et al Clinical implications and prognostic value of single and combined biomarkers in endometrial carcinoma. Chin Med J (Engl) 2014;127(8):1459–1463. [PubMed] [Google Scholar]
- 18.MARKOVA I, DUSKOVA M, LUBUSKY M, et al Selected immunohistochemical prognostic factors in endometrial cancer. Int J Gynecol Cancer. 2010;20(4):576–582. doi: 10.1111/IGC.0b013e3181d80ac4. [DOI] [PubMed] [Google Scholar]
- 19.BENDIFALLAH S, CANLORBE G, COLLINET P, et al Just how accurate are the major risk stratification systems for early-stage endometrial cancer? Br J Cancer. 2015;112(5):793–801. doi: 10.1038/bjc.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SADOZYE A H, HARRAND R L, REED N S Lymphovascular space invasion as a risk factor in early endometrial cancer. Curr Oncol Rep. 2016;18(4):24. doi: 10.1007/s11912-016-0505-1. [DOI] [PubMed] [Google Scholar]
- 21.WEINBERG L E, KUNOS C A, ZANOTTI K M Lymphovascular space invasion (LVSI) is an isolated poor prognostic factor for recurrence and survival among women with intermediate- to high-risk early-stage endometrioid endometrial cancer. Int J Gynecol Cancer. 2013;23(8):1438–1445. doi: 10.1097/IGC.0b013e3182a16c93. [DOI] [PubMed] [Google Scholar]
- 22.TAKAHASHI K, YUNOKAWA M, SASADA S, et al A novel prediction score for predicting the baseline risk of recurrence of stage I-Ⅱ endometrial carcinoma. J Gynecol Oncol. 2019;30(1):e85[2020-08-07]. https://doi.org/10.3802/jgo.2019.30.e8. doi: 10.3802/jgo.2019.30.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KÖBEL M, RONNETT B M, SINGH N, et al Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019;38(Suppl 1):S123–S131. doi: 10.1097/pgp.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EMONS G, VORDERMARK D Adjuvant treatment for endometrial cancer. Curr Opin Oncol. 2019;31(5):404–410. doi: 10.1097/cco.0000000000000558. [DOI] [PubMed] [Google Scholar]


