Abstract
CB2 cannabinoid receptor activation suppresses pathological pain in animal models. CB2 agonists show promise as therapeutic agents because they lack unwanted side effects commonly associated with direct activation of CB1 receptors. However, the types of pain most responsive to CB2 agonists are incompletely understood and cell types which underlie CB2-mediated anti-allodynic efficacy remain largely unknown. Our laboratory previously reported that the CB2 receptor agonist LY2828360 attenuated the maintenance of neuropathic pain induced by toxic challenge with chemotherapeutic and anti-retroviral agents in mice. Whether these findings generalize to models of inflammatory pain is not known. Here we show that LY2828360 (10 mg/kg i.p.) reversed the maintenance of carrageenan-induced mechanical allodynia in female mice. Anti-allodynic efficacy was fully preserved in global CB1 knock out (KO) mice but absent in CB2 KO mice. The anti-allodynic efficacy of LY2828360 was absent in conditional KO mice lacking CB2 receptors in peripheral sensory neurons (AdvillinCRE/+; CB2f/f). Intraplantar administration of LY2828360 (30 μg i.pl.) reversed carrageenan-induced mechanical allodynia in CB2f/f but not AdvillinCRE/+; CB2f/f mice of both sexes. Thus, CB2 receptors in peripheral sensory neurons underlie the therapeutic effects of LY2828360 injection in the paw. Lastly, qRT-PCR analyses revealed that LY2828360 reduced carrageenan-induced increases in IL-1β and IL-10 mRNA in paw skin. Our results provide evidence that LY2828360 suppresses inflammatory nociception in mice through a neuronal CB2-dependent mechanism that requires peripheral sensory neuron CB2 receptors and suggest that the clinical applications of LY2828360 as an anti-hyperalgesic agent should be re-evaluated.
Keywords: CB2 receptor, cannabinoid, inflammation, pain, dorsal root ganglion
Graphical Abstract
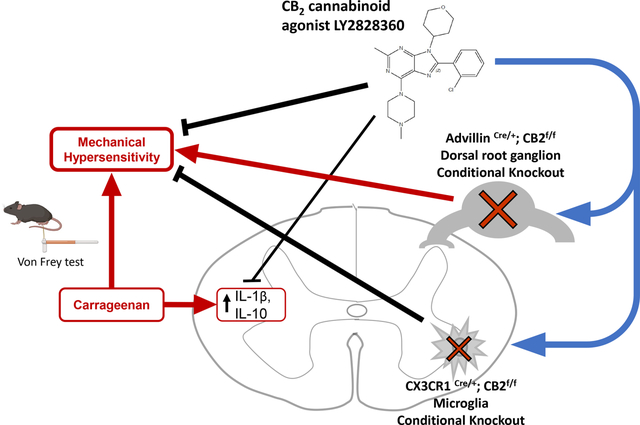
1. Introduction
Engaging targets within the endocannabinoid system can supress pain behavior in preclinical and clinical studies (for review see Lötsch et al., 2018). However, activation of CB1 cannabinoid receptors also produces unwanted side effects (e.g., sedation, altered mental state, memory impairment, and dependence (Howlett et al., 2002; Cooper and Haney, 2009)) that limit clinical utility. By contrast, activation of CB2 cannabinoid receptors suppresses pathological pain in several rodent models without producing adverse CB1-mediated cannabimimetic effects (see Guindon and Hohmann, 2008 for review). We previously reported that CB2 activation reduces neuropathic nociception without producing tolerance or physical dependence (Deng et al., 2015). However, most early studies of CB2 analgesic efficacy employed male rodents exclusively and whether CB2 antinociceptive efficacy is comparable between sexes remains poorly understood. In male rats, CB2 activation reduces mechanical and heat hypersensitivity in the carrageenan model of inflammatory nociception (Nackley, Makriyannis and Hohmann, 2003; Clayton et al., 2002; Elmes et al., 2005) and suppresses transmission of inflammation-evoked neuronal activity measured both immunohistochemically and electrophysiologically (Nackley, Makriyannis and Hohmann, 2003; Nackley, Suplita and Hohmann, 2003; Nackley et al., 2004). CB2 agonism is unlikely to produce deleterious psychoactive effects, possibly due to relative paucity of CB2 receptors in the central nervous system (CNS) and primary localization of these receptors to immune tissues and cells (Munro et al., 1993; Galiègue et al., 1995; Van Sickle et al., 2005). Thus, CB2 agonists may be preferable to analgesic medications with high abuse potential such as opioids (Gutierrez et al., 2011; Xi et al., 2011; Iyer et al., 2020). Both efficacy of CB2 activation in pain models as well as the lack of apparent adverse side effects makes CB2 a favourable target for the development of pharmacotherapies for pain (for review see Soliman et al. 2021). However, a key challenge for clinical translation, given the failure of CB2 agonists in several clinical pain trials (Cabañero, Martín-García and Maldonado, 2021), is determining which pain states are best treated by CB2 agonists.
LY2828360 is a potent CB2 receptor agonist that exhibits similar affinity for human and rat CB2 receptors (Ki = 40.3 nM) and has little affinity for the human CB1 receptor (EC50s = 20.1 and > 100,000 nM for CB2 and CB1 respectively; Hollinshead et al., 2013). We showed that LY2828360 displays strong Gi/o-protein bias, resulting in the inhibition of cAMP and activation of ERK1/2 signaling, without β-arrestin recruitment or CB2 receptor internalization (Lin et al., 2018). LY2828360 attenuated neuropathic nociception produced by the chemotherapeutic agent paclitaxel (Lin et al., 2018; Lin et al., 2022) or the anti-retroviral agent zalcitabine (Carey et al., 2023) and inhibited the development of tolerance to the anti-allodynic effects of morphine in mice (Lin et al., 2018; Carey et al., 2023). Whether this profile of anti-allodynic efficacy generalizes to models of inflammatory pain is unknown. Understanding the range of potential therapeutic indications for this compound is important because LY2828360 failed in a Phase 2 clinical trial for osteoarthritis pain but was demonstrated to be safe for use in humans (Pereira et al., 2013).
The present studies examined the ability of LY2828360 to reduce carrageenan-induced inflammatory nociception and established the mechanism of action underlying these effects. LY2828360 was tested for its ability to reduce carrageenan-induced inflammatory nociception in CB1 knockout (KO) and CB2 KO mice to assess mediation by CB2. To identify specific cell types involved in the anti-allodynic ability of LY2828360, we generated mouse lines with conditional KO (cKO) of CB2 receptors from microglia or macrophages expressing the protein coding gene C-X3-C Motif Chemokine Receptor 1 (CX3CR1CRE/+; CB2f/f mice) or peripheral sensory neurons (AdvillinCRE/+; CB2f/f mice) and asked whether anti-allodynic efficacy of LY2828360 was altered following conditional deletion of CB2 from each cell type. Finally, we asked whether local administration of LY2828360 reduces carrageenan-induced mechanical allodynia in wildtype (WT) mice and in mice lacking CB2 in peripheral sensory neurons. Finally, the ability of LY2828360 to modulate mRNA expression levels of cytokines and chemokines involved in the inflammatory response to carrageenan was examined in lumbar spinal cord, paw skin and spleen of both WT and AdvillinCRE/+; CB2f/f mice.
2. Materials and Methods
2.1. Subjects
Female and male mice between 72 and 274 days of age at the start of the experiment were used, with all mice age matched for each experiment. Mice were housed in a temperature-controlled colony room on a 24-hr light/dark cycle (lights on at 7am; lights off at 7pm), such that behavioral testing occurred during the light phase of the cycle. Mice were maintained on ad libitum food and water. CB2 knockout (KO) mice and wild type (WT) mice on a C57BL/6J background and CB1 KO mice on a CD1 background were used. Mice with conditional KO of CB2 receptors in microglia, excitatory neurons and peripheral sensory afferents were also used. All cKO mouse lines were on a C57BL/6J background. To conditionally delete CB2 from the desired cells, female mice carrying two alleles of the floxed CB2 gene (CB2f/f; López et al., 2018) were bred with male mice carrying two floxed CB2 alleles and one allele of Cre-recombinase in either the CX3CR1 (Yona et al., 2013) or Advillin (Zhou et al., 2010) locus, as appropriate for the experiment. Mice used from this breeding strategy were CX3CR1CRE/+; CB2f/f (deletion of CB2 from microglia), AdvillinCRE/+; CB2f/f (deletion of CB2 from peripheral sensory afferents) and CB2f/f controls (no deletion of CB2, CB2f/f only). CB2f/f mice were generated from the same crosses that produced the conditional KO mice lines used for each experiment and were tested concurrently. KO mice were bred at Indiana University. Wildtype (WT) littermates were included for each experiment as appropriate comparators. All experiments were approved by the Indiana University Animal Care and Use Committee.
2.2. Drugs
LY2828360 (8-(2-chlorophenyl)- 2-methyl-6-(4-methylpiperazin-1-yl)-9-(tetrahydro-2H-pyran-4-yl)-9H- purine) was synthesized by Sai Biotech (Mumbai, India; purity > 98%). LY2828360 was administered intraperitoneally (i.p.) at doses of 1, 3 and 10 mg/kg, and administered in a volume of 10 ml/kg in a vehicle (VEH) consisting of 10% dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO), and the remaining 90% consisted of emulphor (Alkamuls EL-620; Solvay), 95% ethanol (Sigma-Aldrich) and 0.9% saline (Aqualite System; Hospira, Inc., Lake Forest, IL) at a 1:1:18 ratio. LY2828360 was prepared at a concentration of 3 μg/μl in the same vehicle used for i.p. administration and administered at a volume of 10 μl into the paw via direct intraplantar (i.pl.) injection (30 μg/paw). Gabapentin was administered i.p. at a dose of 50 mg/kg, prepared at a concentration of 10 mg/ml in solution with saline. To induce inflammation, λ-Carrageenan (Sigma-Aldrich) was mixed in saline (1% wt/vol solution) and delivered into the right hind paw by intraplantar injection at a volume of 20 μl.
2.3. Assessment of Mechanical Allodynia
Mechanical allodynia was assessed by measuring the paw withdrawal threshold (in grams) to punctate mechanical stimulation, assessed using an electronic von Frey anesthesiometer (IITC Life Sciences Inc., Woodland Hills, CA) as described in our previously published work (Lin et al. 2018; Lin et al., 2022; Carey et al., 2023). First, mice were placed on an elevated testing table with a stainless-steel wire mesh platform (with 0.6 × 0.6 cm gaps in the wire mesh) underneath clear Plexiglass chambers (10.5 × 9 × 7 cm) for 1 hour of habituation prior to testing. Following habituation, baseline measurements of paw withdrawal thresholds were taken using the von Frey anesthesiometer. Immediately following baseline measurements, carrageenan (20 μl) was injected into the right hind paw. Subsequent von Frey measurements were taken at various time points (see specific experiments in procedure section for details). To measure paw withdrawal threshold, a semi-flexible plastic filament with a 0.8 mm diameter was applied vertically to the plantar surface of the hind paws until withdrawal of the paw; a digital readout of the threshold for paw withdrawal was displayed on the anesthesiometer meter and recorded by the experimenter. Each paw was measured twice at every time point, including for baseline measurements (in a right-left-right-left order).
2.4. Tissue collection and quantitative real-time polymerase chain reaction (qRT-PCR)
Mice were perfused with Hank’s Balanced Salt solution (HBSS) 30-min following injection of each pharmacological treatment, and then spleen, lumbar spinal cord, and paw skin (ipsilateral and contralateral paws separated) were flash frozen. Spleen and spinal cord RNA was extracted using TRizol (Invitrogen)/RNeasy (Qiagen) RNA mini Kit according to the manufacturer’s instructions, and paw skin RNA was extracted using a RLT buffer:2-mercapto ethanol mixture in a 100:1 ratio/RNeasy RNA mini Kit. RNA (3000 ng for spleen, 500 ng for spinal cord/paw skin) was used in a Luna Universal One-Step RT-qPCR Kit (New England Biolabs) with the following cycling conditions: 55°C for 10 min, 95°C for 1 min, and 40 cycles of 95°C for 10 sec and 60°C for 1 min. RNA levels in extracts were quantified by cycle threshold (Ct), where Ct levels are inversely proportional to the amount of target nucleic acid in the sample. Relative gene expression for CB2, interleukin 1 beta (IL-1β), interleukin 10 (IL-10), tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein 1 (MCP1) mRNAs were normalized to the housekeeping gene GAPDH and calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). Sequences for qRT-PCR primers were: IL-1β sense 5’-CGT GGA CCT TCC AGG ATG AG-3’; IL-1β anti-sense 5’-CAT CTC GGA GCC TGT AGT GC-3’; IL-10 sense 5’-GGA CTT TAA GGG TTA CTT GGG TTG CC-3’; IL-10 anti-sense 5’-CAT TTT GAT CAT CAT GTA TGC TTC T-3’; TNFα sense 5’-CGT CGT AGC AAA CCA CCA AG-3’; TNFα anti-sense 5’-TAG CAA ATC GGC TGA CGG TG-3’; MCP1 sense 5’-GAA GGA ATG GGT CCA GAC AT-3’; MCP1 anti-sense 5’-ACG GGT CAA CTT CAC ATT CA-3’; CB2 sense 5’-ACG GTG GCT TGG AGT TCA AC-3’; CB2 anti-sense 5’- GCC GGG AGG ACA GGA TAA T-3’; GAPDH sense 5’-AGG TCG GTG TGA ACG GAT TTG-3’; GAPDH anti-sense 5’-TGT AGA CCA TGT AGT TGA GGT CA-3’.
2.5. Experimental Procedure
2.5.1. General experimental procedures
Paw withdrawal thresholds were measured in duplicate in each paw prior to carrageenan administration (baseline), after allowing for 1 hour of habituation. Carrageenan was injected i.pl. immediately following completion of baseline measurements. Paw withdrawal thresholds were subsequently measured either 3- or 4-hours post-carrageenan to document the presence of inflammatory nociception prior to pharmacological treatments, based upon the protocol described below. Timing of pharmacological manipulations was optimized for specific experiments as described below.
2.5.2. Experiment 1: Dose response of LY2828360 effect in carrageenan-induced inflammatory pain
We evaluated the dose response of LY2828360 in suppressing the maintenance of carrageenan-induced inflammation in C57BL/6J female mice. Mice (n = 6 per group) were injected intraperitoneally (i.p.) with either 1, 3 or 10 mg/kg LY2828360 or VEH 30 mins prior to drug testing. Testing of drug effects began 4-hours after carrageenan administration.
2.5.3. Experiment 2: Are the anti-allodynic effects of LY2828360 preserved in CB1 KO mice?
To rule out the possibility that LY2828360 is producing anti-allodynic effects through CB1 receptors, we examined the ability of LY2828360 (10 mg/kg i.p.) to suppress inflammatory nociception in female CB1 receptor KO mice (n = 7–8 per group). LY2828360 (10 mg/kg i.p.) or vehicle was injected 30-mins prior to assessment of pharmacological effects 4 and 6 hours after carrageenan injection.
2.5.4. Experiment 3: Is anti-allodynic efficacy of a reference agent preserved in CB2 KO mice?
The ability of gabapentin to reduce carrageenan-induced mechanical allodynia in female WT (C57 strain) mice and CB2 KO mice (n = 8 per group) was determined. Gabapentin (50 mg/kg i.p.) or vehicle was injected 30-mins prior to the 6-hour post-carrageenan measurement. Subsequent measurements occurred at 7 and 24-hours post-carrageenan.
2.5.5. Experiment 4: Role of CB2 receptors in the anti-allodynic effect of LY2828360
To ascertain whether the anti-allodynic effects of LY2828360 were CB2-mediated, female CB2 KO mice were treated with either LY2828360 (10 mg/kg i.p.) or its vehicle (n = 6 per group) during the maintenance phase of carrageenan-induced inflammatory nociception. Experimental procedures were conducted identically as those in Experiment 2.
2.5.6. Experiment 5: Are the anti-allodynic effects of LY2828360 preserved in CB2f/f mice receiving intraplantar injection of carrageenan?
The ability of LY2828360 to reduce carrageenan-induced mechanical allodynia relative to vehicle treatment was assessed in female CB2f/f control mice (n = 10 per group). Experimental procedures were the same as in Experiment 3.
2.5.7. Experiment 6: Impact of cKO of CB2 receptors in microglia and macrophages on the anti-allodynic effect of LY2828360
To ascertain whether LY2828360 is exerting its anti-allodynic effect by engaging CB2 receptors on microglia or macrophages, we administered LY2828360 or its vehicle to female CX3CR1CRE/+; CB2f/f mice (n = 4–7 per group). Experimental procedures were the same as in Experiment 3. LY2828360 (10 mg/kg i.p.) or vehicle was administered 30-mins prior to the 6-hour post-carrageenan measurement.
2.5.8. Experiment 7: Effect of carrageenan in mice with cKO of CB2 from peripheral sensory neurons
To ensure that carrageenan-induced inflammatory nociception develops normally following conditional deletion of CB2 receptors from peripheral sensory neurons, carrageenan-induced mechanical allodynia was measured in female CB2f/f and AdvillinCRE/+; CB2f/f mice (n = 7 per group). Paw withdrawal thresholds were reassessed 2, 4, 6 and 24-hours post carrageenan.
2.5.9. Experiment 8: Impact of cKO of CB2 from peripheral sensory neurons on anti-allodynic effect of LY2828360
To ascertain the possible contribution of CB2 receptors in peripheral sensory neurons to the anti-allodynic effects of LY2828360, we asked whether antiallodynic effects of LY2828360 would be attenuated in female AdvillinCRE/+; CB2f/f mice (n = 7 per group). Experimental procedures were conducted the same as in Experiment 3.
2.5.10. Experiment 9 and 10: Local paw injection of LY2828360 into the paw of CB2f/f and AdvillinCRE/+; CB2f/f male and female mice
To ascertain whether LY2828360 suppresses carrageenan-induced inflammatory nociception through a peripheral mechanism, vehicle or LY2828360 (30 μg i.pl.) was injected locally into the paw ipsilateral or contralateral to the paw injected with carrageenan. Mechanical paw withdrawal thresholds were assessed before and after intraplantar injections of carrageenan, drug or vehicle in four separate groups of mice: female CB2f/f mice (n = 5 per group), female AdvillinCRE/+; CB2f/f mice (n = 5 per group), male CB2f/f mice, (n = 5 per group) and male AdvillinCRE/+; CB2f/f mice (n = 4–5 per group). Mice were injected with either LY2828360 or VEH unilaterally into the plantar hindpaw surface either ipsi- or contra-lateral to the carrageenan-injected paw. Intraplantar injections were performed 30 min prior to a 6-hour post-carrageenan measurement. Additional measurements were taken at 7- and 24-hours post-carrageenan.
2.5.11. Experiment 11: Impact of intraplantar injection of LY2828360 in the absence of carrageenan
To ascertain whether LY2828360 was antinociceptive in the absence of carrageenan, LY2828360 (30 μg i.pl.) or vehicle was administered locally into the paw of naïve male C57 WT mice (n = 5 per group). Experimental procedures were conducted as in Experiment 7, with the exception that an equivalent volume of saline was injected into the paw in lieu of carrageenan.
2.5.12. Experiment 12: Impact of LY2828360 on mRNA expression levels of inflammatory-related markers following intraplantar injection of carrageenan
To ascertain whether LY2828360 reduces mRNA levels of inflammatory markers in the spleen, spinal cord, or paw skin, LY2828360 (10 mg/kg i.p.) or VEH was administered to female C57 WT mice (n = 8 per group) 3.5 hrs after intraplantar carrageenan injection. Tissue was also collected from a group of otherwise naïve animals (n=8) as a comparator.
2.5.13. Experiment 13: qRT-PCR analysis to assess the impact of intraplantar carrageenan in the spinal cord of mice lacking CB2 in AdvillinCRE expressing cells
To ascertain whether carrageenan increases inflammatory markers in the lumbar spinal cord of male and female CB2f/f and AdvillinCRE/+; CB2f/f mice, equivalent volumes (20 μl) of carrageenan or saline were administered into a single hind paw (n = 5–6 per group, mixed sex). Then, 6 hrs post-injection mice were euthanized using live decapitation and their spinal cord was extracted and flash frozen.
2.5.14. Experiment 14: Impact of LY2828360 on mRNA expression levels of inflammatory markers following intraplantar injection of carrageenan in mice lacking CB2 in AdvillinCRE expressing cells
To ascertain whether LY2828360 reduces inflammatory markers in the spleen, spinal cord, or paw skin of female CB2f/f and AdvillinCRE/+; CB2f/f mice, LY2828360 (10 mg/kg i.p.) or VEH was administered (n = 5 per group) 3.5 hrs after intraplantar carrageenan injection.
3. Results
3.1. General Behavioral Results
In general, prior to pharmacological treatments, carrageenan lowered paw withdrawal thresholds in the ipsilateral (i.e. carrageenan-injected) paw relative to baseline (pre-injection) levels (Table 1), but no difference was observed between group or time points in the paw contralateral to carrageenan injection in any study (data not shown). Following pharmacological treatments, differences in contralateral paw withdrawal thresholds were only detected in a subset of studies (Table 2).
Table 1.
Summary of statistical analyses evaluating development of carrageenan-induced hypersensitivity in the carrageenan-injected ipsilateral paw prior to pharmacological treatments (mechanical withdrawal thresholds).
| Figure | Group | Time | Interaction |
|---|---|---|---|
|
| |||
| 1A | F3,19= 0.06245, p = 0.9790 | - | - |
|
| |||
| 2A | F1,13= 0.1325, p = 0.7218 | F1,13= 0.02251, p = 0.8830 | F1,13= 0.2915, p = 0.5984 |
|
| |||
| 3A | F1,14= 2.444, p = 0.1403 | F1,14 = 42.59, p < 0.0001**** | F1,14 = 0.8586, p = 0.3698 |
| 3C | F1,14= 2.2588, p = 0.6189 | F1,14 = 30.25, p < 0.0001**** | F1,14 = 2.352, p = 0.1474 |
| 3E | F1,10= 3.428, p = 0.0938 | F1,10= 7.06, p = 0.0240* | F1,10= 0.004534, p = 0.9476 |
|
| |||
| 4A | F1,17= 0.9811, p = 0.3358 | F1,17= 11.42, p = 0.0036** | F1,17= 0.01227, p = 0.9131 |
| 4C | F1,12= 0.6703, p = 0.4289 | F1,12= 28.54, p = 0.0002*** | F1,12= 0.3348, p = 0.5736 |
| 4E | F1,12 = 0.0001301, p = 0.9911 | F1,12 = 29.78, p = 0.0001*** | F1,12 = 0.1282, p = 0.7265 |
|
| |||
| 5A | F1,11 = 0.3124, p = 0.5874 | F1,11 = 10.93, p = 0.0070* | F1,11 = 4.24, p = 0.0640 |
| 5C | F1,10= 3.217, p = 0.1031 | F1,10= 4.543, p = 0.0589 | F1,10= 1.141, p = 0.3105 |
| 5E | F1,8= 1.797, p = 0.2169 | F1,8= 70.26, p < 0.0001**** | F1,8= 2.068, p = 0.1883 |
| 5G | F1,8= 1.76, p = 0.2213 | F1,8= 3.733, p = 0.0894 | F1,8= 0.1367, p = 0.7212 |
|
| |||
| 6A | F1,11 = 0.02834, p = 0.8694 | F1,11 = 22.65, p = 0.0006*** | F1,11 = 3.876, p = 0.0747 |
| 6C | F1,10= 3.439, p = 0.0934 | F1,10= 10.49, p = 0.0089** | F1,10= 0.001533, p = 0.9695 |
| 6E | F1,8= 0.4482, p = 0.5220 | F1,8= 26.76, p = 0.0009*** | F1,8= 1.888, p = 0.2067 |
| 6G | F1,6= 0.6613, p = 0.4472 | F1,6= 10.22, p = 0.0187* | F1,6= 0.07212, p = 0.7973 |
Table 2.
Summary of statistical analyses of mechanical withdrawal thresholds in the contralateral paw following pharmacological treatments
| Figure | Group | Time | Interaction |
|---|---|---|---|
|
| |||
| 1B | F3,19= 2.046, p = 0.1415 | - | - |
|
| |||
| 2B | F1,13= 0.1908, p = 0.6694 | F1,13= 2.648, p = 0.1277 | F1,13= 0.00134, p = 0.9714 |
|
| |||
| 3B | F1,14 = 5.834, p = 0.0300* | F2,28= 0.4645, p = 0.6332 | F2,28= 0.6038, p = 0.5537 |
| 3D | F1,14 = 1.958, p = 0.1835 | F2,28= 4.114, p = 0.0271* | F2,28= 0.04266, p = 0.9583 |
| 3F | F1,10= 0.8129, p = 0.3885 | F1,10= 0.04466, p = 0.8369 | F1,10= 0.06597, p = 0.8025 |
|
| |||
| 4B | F1,17= 0.3021, p = 0.5897 | F2,34 = 0.1654, p = 0.8482 | F2,34 = 0.551, p = 0.5815 |
| 4D | F1,12 = 0.0002904, p = 0.9867 | F2,24 = 0.7491, p = 0.4835 | F2,24 = 0.07709, p = 0.9260 |
| 4F | F1,12 = 1.903, p = 0.1929 | F2,24= 0.5071, p = 0.6086 | F2,24= 1.671, p = 0.2093 |
|
| |||
| 5B | F1,12= 1.845, p = 0.1993 | F2,24= 0.127, p = 0.8813 | F2,24= 0.7871, p = 0.4666 |
| 5D | F1,10 = 23.31, p = 0.0007*** | F2,20= 1.737, p = 0.2016 | F2,20= 1.921, p = 0.1726 |
| 5F | F1,8= 0.02062, p = 0.8894 | F2,16 = 2.692, p = 0.0982 | F2,16= 3.721, p = 0.0471* |
| 5H | F1,8= 0.3147, p = 0.5902 | F2,16 = 0.2489, p = 0.7826 | F2,16= 2.94, p = 0.0818 |
|
| |||
| 6B | F1,12= 0.7557, p = 0.4017 | F2,24 = 3.07, p = 0.0744 | F2,24= 0.5455, p = 0.5900 |
| 6D | F1,10= 2.181, p = 0.1705 | F2,20 = 0.08355, p = 0.9202 | F2,20= 1.112, p = 0.3529 |
| 6B | F1,8= 6.008, p = 0.0399* | F2,16= 0.5274, p = 0.6001 | F2,16= 0.2469, p = 0.7841 |
| 6D | F1,6= 0.1611, p = 0.7020 | F2,12 = 0.5367, p = 0.5981 | F2,12 = 2.368, p = 0.1358 |
3.2. Experiment 1: Dose response of LY2828360 effect in carrageenan-induced inflammatory pain
LY2828360 increased paw withdrawal thresholds (F3,19 = 3.904, p = 0.0250) in the carrageenan-injected (ipsilateral) paw. Dunnett’s multiple comparisons test revealed that 10 mg/kg i.p. LY2828360 increased paw withdrawal thresholds relative to VEH treatment (p = 0.0150), with no difference between VEH and either 1 mg/kg (p = 0.9253) or 3 mg/kg (p = 0.8904) LY2828360 doses detected (Fig. 1A).
Fig. 1.
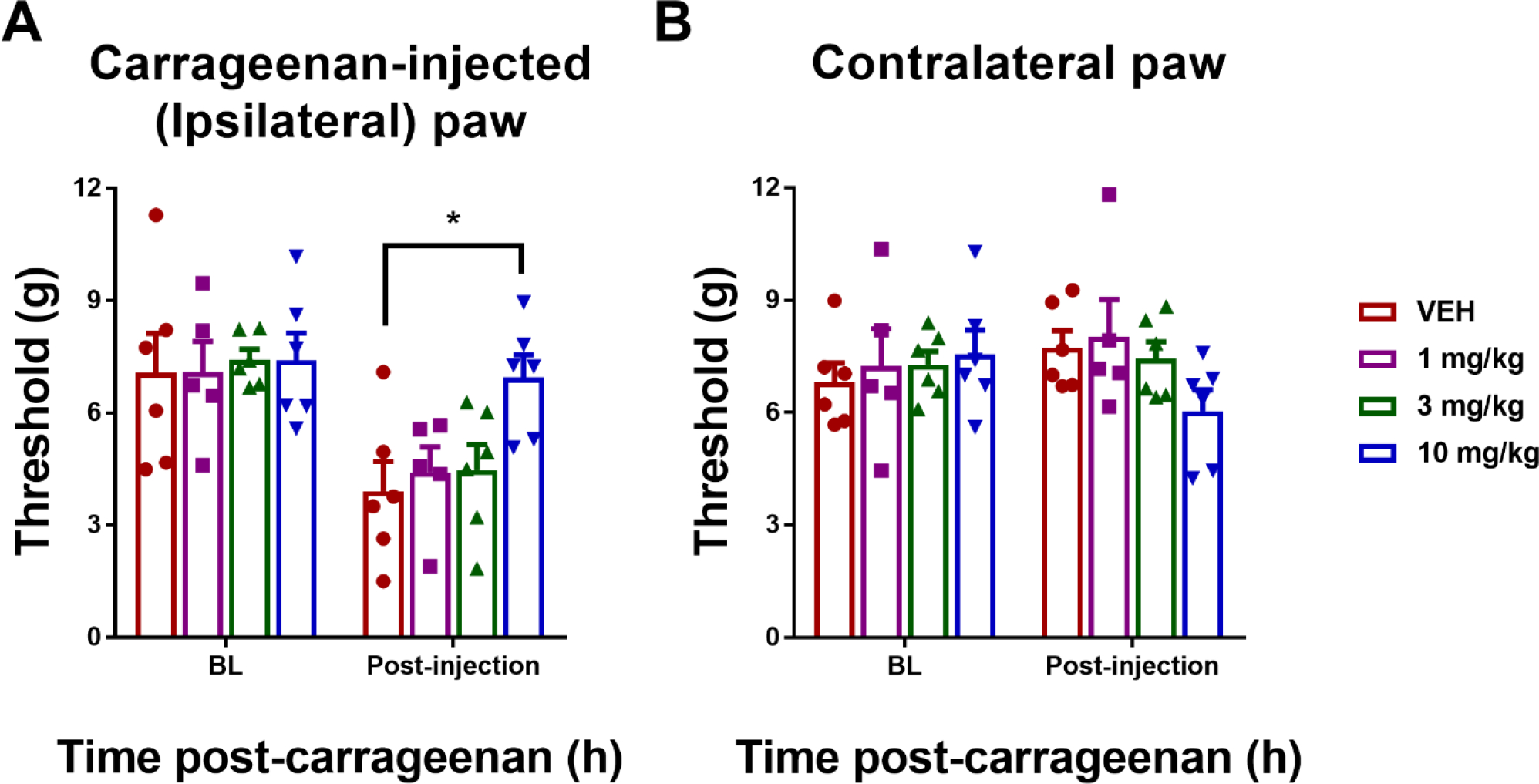
Dose response of LY2828360 in suppressing carrageenan-induced mechanical allodynia in female C57 WT mice (n = 6 per group). LY2828360 reduced carrageenan-induced mechanical allodynia at a dose of 10 mg/kg i.p. but not at lower doses (1 or 3 mg/kg i.p.). LY2828360 (10 mg/kg) increased paw withdrawal thresholds in the ipsilateral paw (A) but not the contralateral paw (B) in a mouse model of carrageenan-induced inflammatory pain. Data show mean (± SEM) threshold for the ipsilateral and contralateral paw in Experiment 1. *p < 0.05 vs. VEH; ###p < 0.001 vs. baseline (BL).
3.3. Experiment 2: Are the anti-allodynic effects of LY2828360 preserved in CB1 KO mice?
In CB1 KO mice, LY2828360 (10 mg/kg i.p.) increased paw withdrawal thresholds in the carrageenan-injected (ipsilateral) paw across the observation interval (Group: F1,13 = 34.82, p < 0.0001; Time: F1,13 = 34.82, p = 0.2142; Interaction: F1,13 = 0.6893, p = 0.4214); Sidak’s multiple comparisons test revealed that LY2828360 increased paw withdrawal thresholds at the 4-hr (p = 0.0009) and 6-hr post carrageenan time-point (p = 0.0279) relative to VEH (Fig. 2A).
Fig. 2.
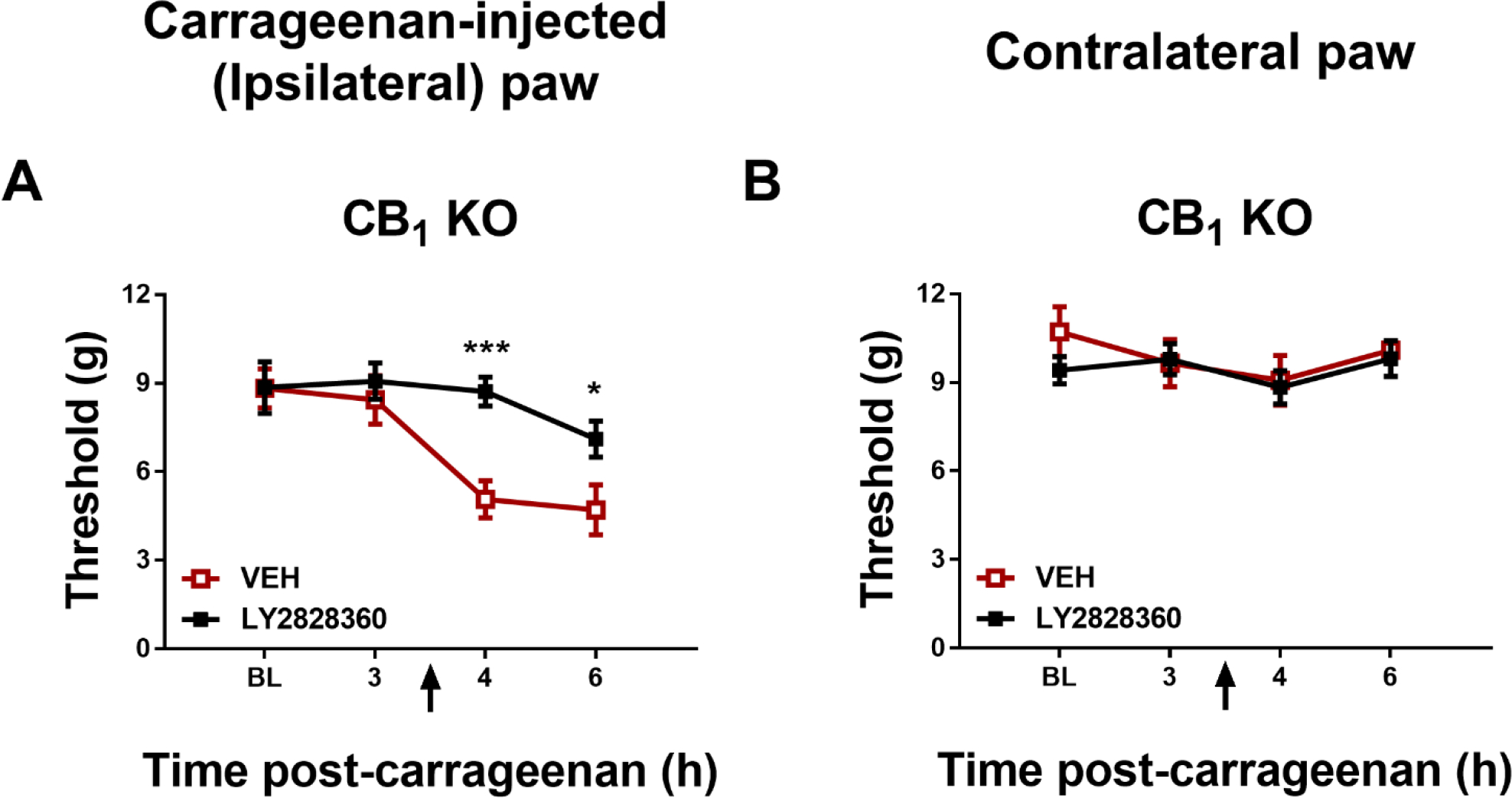
LY2828360 (10 mg/kg i.p.) increased mechanical paw withdrawal thresholds in the ipsilateral (A) but not contralateral (B) paw of female CB1 KO mice in a mouse model of carrageenan-induced inflammatory pain (n = 7–8 per group). Data show mean (± SEM) threshold for the ipsilateral and contralateral paw in Experiment 2. *p < 0.05, ***p < 0.001 vs. VEH.
3.4. Experiment 3: Is anti-allodynic efficacy of a reference agent preserved in CB2 KO mice?
In C57 WT control mice, gabapentin (50 mg/kg i.p.) increased paw withdrawal thresholds in the carrageenan-injected paw, paw withdrawal thresholds changed over time and the interaction was significant (Group: F1,14 = 20, p = 0.0005; Time: F2,28 = 7.929, p = 0.0019; Interaction: F2,28 = 7.184, p = 0.0030); Sidak’s multiple comparisons test revealed that gabapentin increased mechanical paw withdrawal thresholds compared to VEH at the 6-hr (p < 0.0001) and 7-hr post carrageenan timepoints (p = 0.0010) (Fig. 3A). In CB2 KO mice, gabapentin (50 mg/kg i.p.) increased paw withdrawal thresholds in the carrageenan-injected paw in a time-dependent manner (Group: F1,14 = 16.36, p = 0.0012; Time: F2,28 = 3.266, p = 0.0531; Interaction: F2,28 = 4.682, p = 0.0176) compared to vehicle at both the 6- (p = 0.0001; Sidak’s multiple comparison test) and 7-h post carrageenan timepoints (p = 0.0021) (Fig. 3C).
Fig. 3.
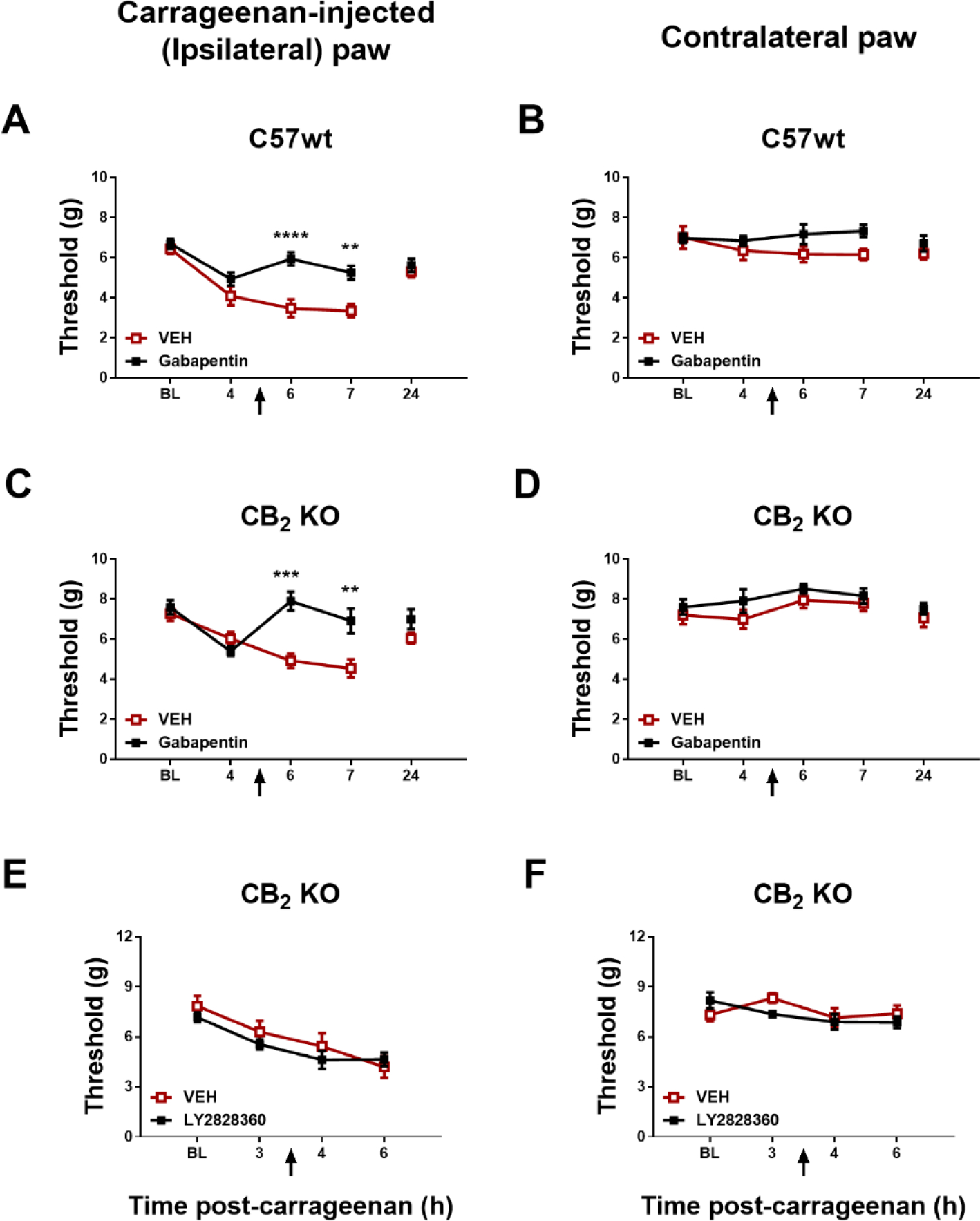
Gabapentin (50 mg/kg i.p.) increased mechanical paw withdrawal thresholds in the paw ipsilateral (A) or contralateral (B) to carrageenan injection in female C57 WT and CB2 KO mice (n = 8 per group). LY2828360 (10 mg/kg i.p.) did not alter paw withdrawal thresholds in the paw ipsilateral (A) or contralateral (B) to carrageenan injection in female CB2 KO mice (n = 6 per group). Mean (± SEM) threshold for the ipsilateral and contralateral paw in Experiment 3 and 4. **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. VEH.
3.5. Experiment 4: Role of CB2 in the anti-allodynic effect of LY2828360
In CB2 KO mice, LY2828360 (10 mg/kg i.p.) did not alter paw withdrawal thresholds in the carrageenan-injected paw relative to vehicle treatment (Group: F1,10 = 0.05587, p = 0.8179; Time: F1,10 = 1.919, p = 0.1961; Interaction: F1,10 = 2.115, p = 0.1765) (Fig. 3E).
3.6. Experiment 5: Are the anti-allodynic effects of LY2828360 preserved in CB2f/f mice receiving intraplantar injection of carrageenan?
In CB2f/f control mice, LY2828360 (10 mg/kg i.p.) increased paw withdrawal thresholds in the carrageenan-injected paw, paw withdrawal thresholds changed over time and the interaction approached significance (Group: F1,17 = 7.832, p = 0.0123; Time: F2,34 = 10.93, p = 0.0002; Interaction: F2,34 = 3.145, p = 0.0558); Sidak’s multiple comparisons test revealed that LY2828360 increased mechanical paw withdrawal thresholds compared to VEH at the 6-hr timepoint (p = 0.0029) (Fig. 4A).
Fig. 4.
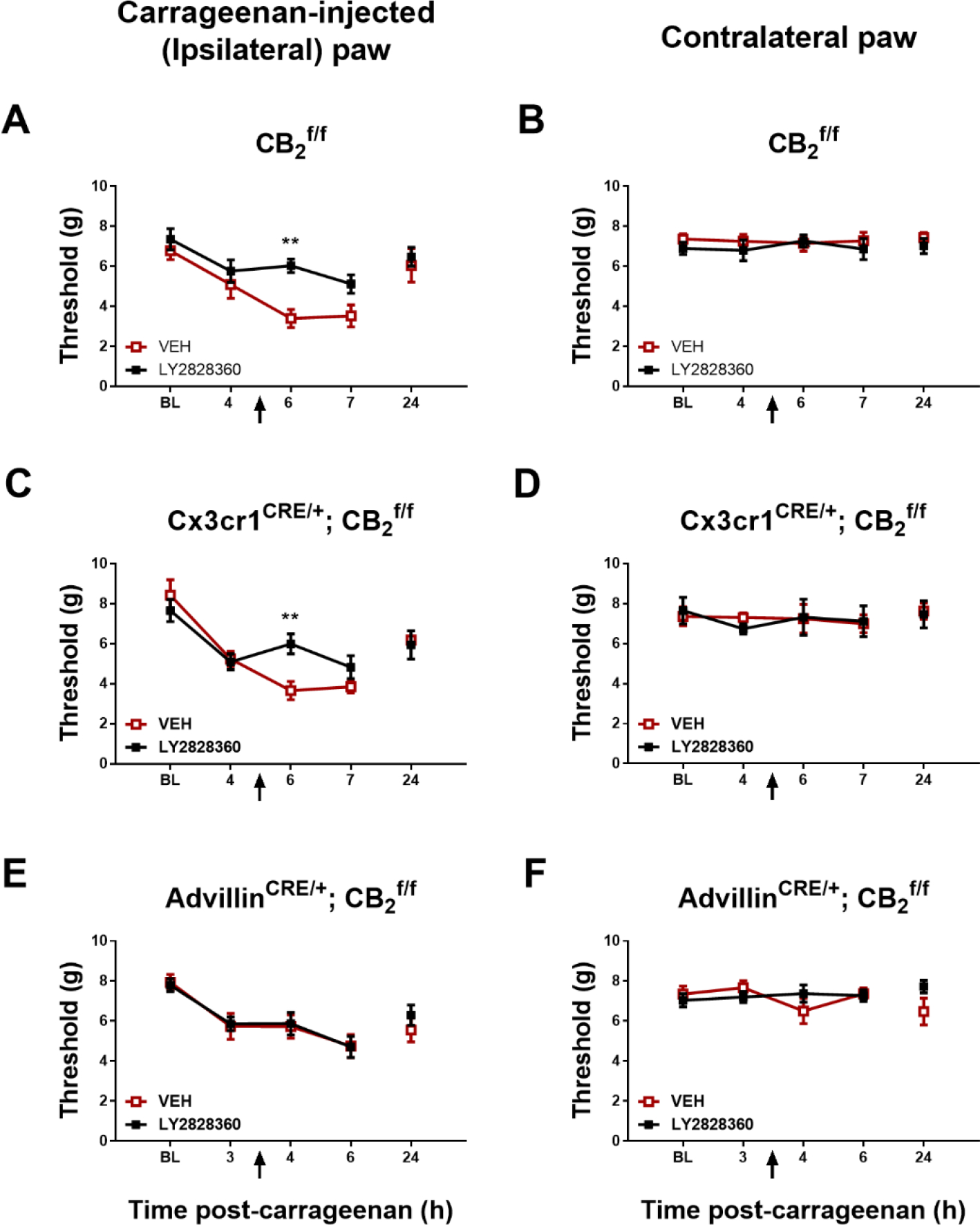
LY2828360 (10 mg/kg i.p.) increased mechanical paw withdrawal thresholds in paw ipsilateral but not contralateral to carrageenan injection in female CB2f/f and CX3CR1CRE/+; CB2f/f mice (n = 4–10 per group). LY2828360 (10 mg/kg i.p.) did not alter paw withdrawal thresholds in paw ipsilateral or contralateral to carrageenan injection in female AdvillinCRE/+; CB2f/f mice (n = 7 per group). Mean (± SEM) threshold for the ipsilateral and contralateral paw in Experiment 5, 6, 7 and 9. **p < 0.01 vs. VEH.
3.7. Experiment 6: Impact of cKO of CB2 receptors in microglia and macrophages on the antiallodynic effect of LY2828360
In CX3CR1CRE/+; CB2f/f mice, paw withdrawal thresholds in the carrageenan-injected paw differed across time (Time: F2,24 = 8.956, p = 0.0012). LY2828360 (10 mg/kg i.p.) trended to alter paw withdrawal thresholds as a function of group and the interaction was significant (Group: F1,12 = 4.562, p = 0.0540; Interaction: F2,24 = 4.729, p = 0.0186); ); Sidak’s multiple comparisons test revealed that LY2828360 increased mechanical paw withdrawal thresholds compared to VEH at the 6-hr timepoint (p = 0.0046) indicating that anti-allodynic efficacy was preserved in this cKO mouse line (Fig. 4C).
3.8. Experiment 7: Effect of carrageenan in mice with cKO of CB2 from peripheral sensory neuron
In the absence of pharmacological manipulations, carrageenan lowered paw withdrawal thresholds in the ipsilateral (carrageenan-injected) paw of Advillin+/+; CB2f/f and AdvillinCRE/+; CB2f/f relative to baseline (Time: F5,60 = 22.11, p < 0.0001), consistent with the development of mechanical allodynia. This effect occurred irrespective of genotype (group) and the interaction was not significant (Genotype: F1,12 = 1.794, p = 0.2052); Interaction: F5,60 = 0.5866, p = 0.7101). Paw withdrawal thresholds did not differ as a function of genotype, time, or interaction in the non-inflamed contralateral paw (Group: F1,12 = 0.1758, p = 0.6825; Time: F5,60 = 1.272, p = 0.2876; Interaction: F5,60 = 1.361, p = 0.2519) (Data not shown).
3.9. Experiment 8: Impact of cKO of CB2 receptors from peripheral sensory neurons on the anti-allodynic effect of LY2828360
In AdvillinCRE/+; CB2f/f mice, paw withdrawal threshold in the carrageenan-injected paw differed across time (Time: F2,24 = 4.916, p = 0.0162), but LY2828360 (10 mg/kg i.p.) did not alter paw withdrawal thresholds as a function of group and the interaction was not significant (Group: F1,12 = 0.2013, p = 0.6617; Interaction: F2,24 = 0.4772, p = 0.6263) (Fig. 4E). Thus, anti-allodynic efficacy of LY2828360 was absent in cKO mice following deletion of CB2 from peripheral sensory neurons.
3.10. Experiment 9: Local paw injection of LY2828360 into the ipsilateral paw of CB2f/f and AdvillinCRE/+; CB2f/f male and female mice
In female CB2f/f mice receiving local injections in ipsilateral paw, LY2828360 (30 μg i.pl.) increased paw withdrawal thresholds in the carrageenan-injected paw, and paw withdrawal thresholds changed over time (Group: F1,11 = 16.23, p = 0.0020; Time: F2,22 = 12.83, p = 0.0002; Interaction: F2,22 = 1.629, p = 0.2190); Sidak’s multiple comparisons test revealed that LY2828360 increased mechanical paw withdrawal thresholds compared to VEH at the 6-hr (p = 0.0107) and 7-hr (p = 0.0051) timepoints (Fig. 5A). In female AdvillinCRE/+; CB2f/f mice receiving injections in ipsilateral paw, paw withdrawal threshold in the carrageenan-injected paw differed across time (Time: F2,20 = 17.93, p < 0.0001), but LY2828360 (30 μg i.pl.) did not alter paw withdrawal thresholds as a function of group or interaction (Group: F1,10 = 0.682, p = 0.4282; Interaction: F2,20 = 0.2997, p = 0.7443) (Fig. 5C).
Fig. 5.
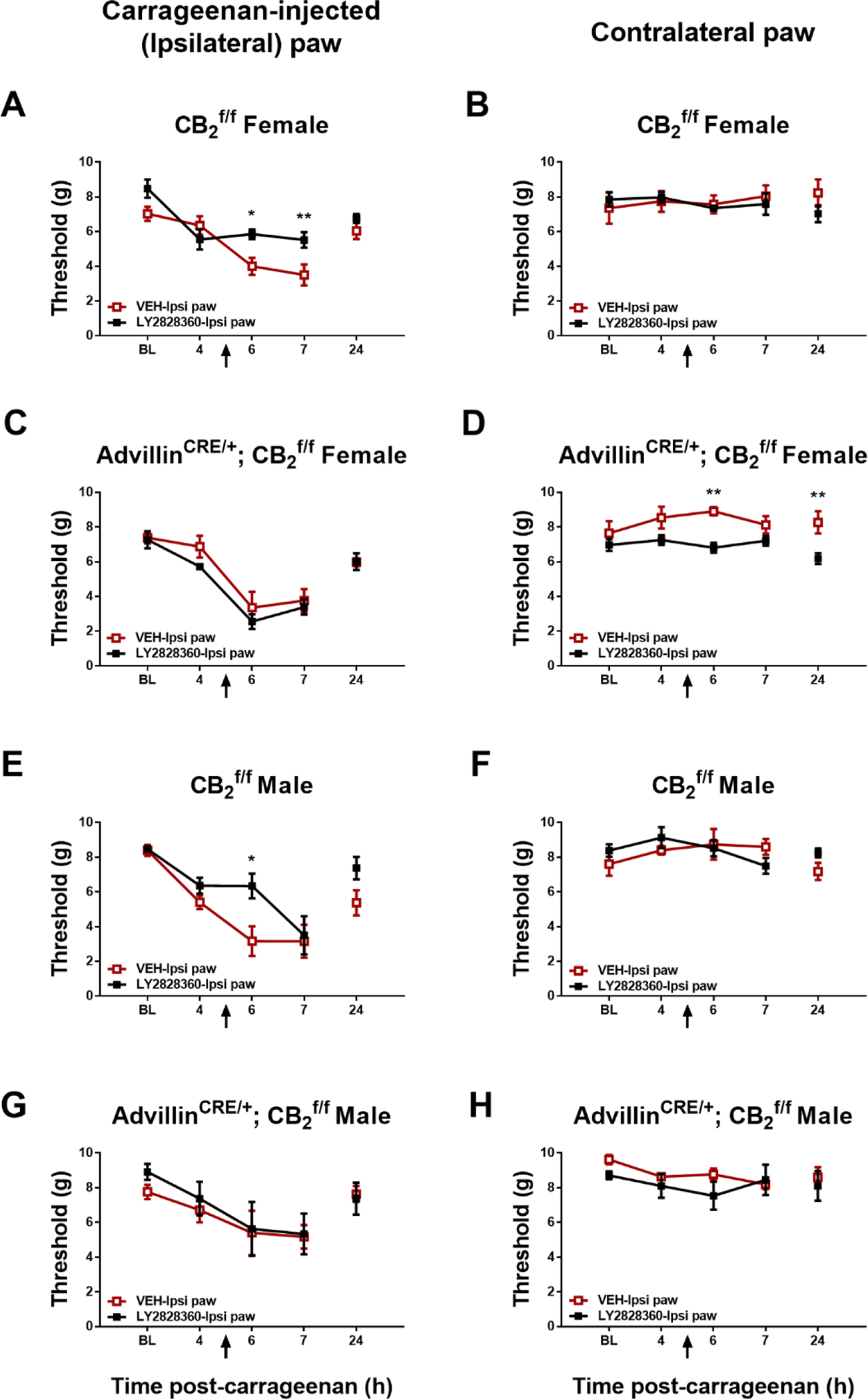
Impact of ipsilateral intraplantar LY2828360 (30 μg i.pl.) injection on paw withdrawal thresholds in the paw ipsilateral or contralateral to carrageenan injection in male and female CB2f/f mice and AdvillinCRE/+; CB2f/f mice (n = 4–5 per group). Mean (± SEM) threshold for the ipsilateral and contralateral paw in Experiment 9. *p < 0.05, **p < 0.01 vs. VEH-ipsi paw.
In male CB2f/f mice receiving injections in ipsilateral paw, paw withdrawal thresholds in the carrageenan-injected paw differed across time and the interaction was significant (Time: F2,16 = 29.16, p < 0.0001; Interaction: F2,16 = 6.338, p = 0.0094), but LY2828360 (30 μg i.pl.) did not alter paw withdrawal thresholds as a function of group (Group: F1,8 = 2.781, p = 0.1339); Sidak’s multiple comparisons test revealed that paw withdrawal thresholds were higher in the ipsilateral-LY2828360 paw injection groups relative to ipsilateral-VEH and at the 6-hr (p = 0.0410) timepoint (Fig. 5E). In male AdvillinCRE/+; CB2f/f mice receiving injections in ipsilateral paw, paw withdrawal threshold in the carrageenan-injected paw differed across time (Time: F2,16 = 7.42, p = 0.0052), but LY2828360 (30 μg i.pl.) did not alter paw withdrawal thresholds as a function of group or interaction (Group: F1,8 = 0.0011, p = 0.9744; Interaction: F2,16 = 0.0845, p = 0.9194) (Fig. 5G). Thus, local injection of LY2828360 in the carrageenan-injected paw failed to suppress mechanical allodynia in either female or male AdvillinCRE/+; CB2f/f mice.
3.11. Experiment 10: Local paw injection of LY2828360 in contralateral paw of CB2f/f and AdvillinCRE/+; CB2f/f male and female mice
In female CB2f/f mice receiving injections in contralateral paw, paw withdrawal thresholds in the carrageenan-injected paw differed across time (Time: F2,22 = 16.68, p < 0.0001), but LY2828360 (30 μg i.pl.) did not alter paw withdrawal thresholds as a function of group and the interaction was not significant (Group: F1,11 = 4.074, p = 0.0686; Interaction: F2,22 = 0.739, p = 0.4891 (Fig. 6A). In female AdvillinCRE/+; CB2f/f mice receiving injections in contralateral paw, withdrawal thresholds in the carrageenan-injected paw did not differ as a function of group, time or interaction (Group: F1,10 = 2.198, p = 0.1690; Time: F2,20 = 0.3946, p = 0.6791; Interaction: F2,20 = 0.05654, p = 0.9452) (Fig. 6C).
Fig. 6.
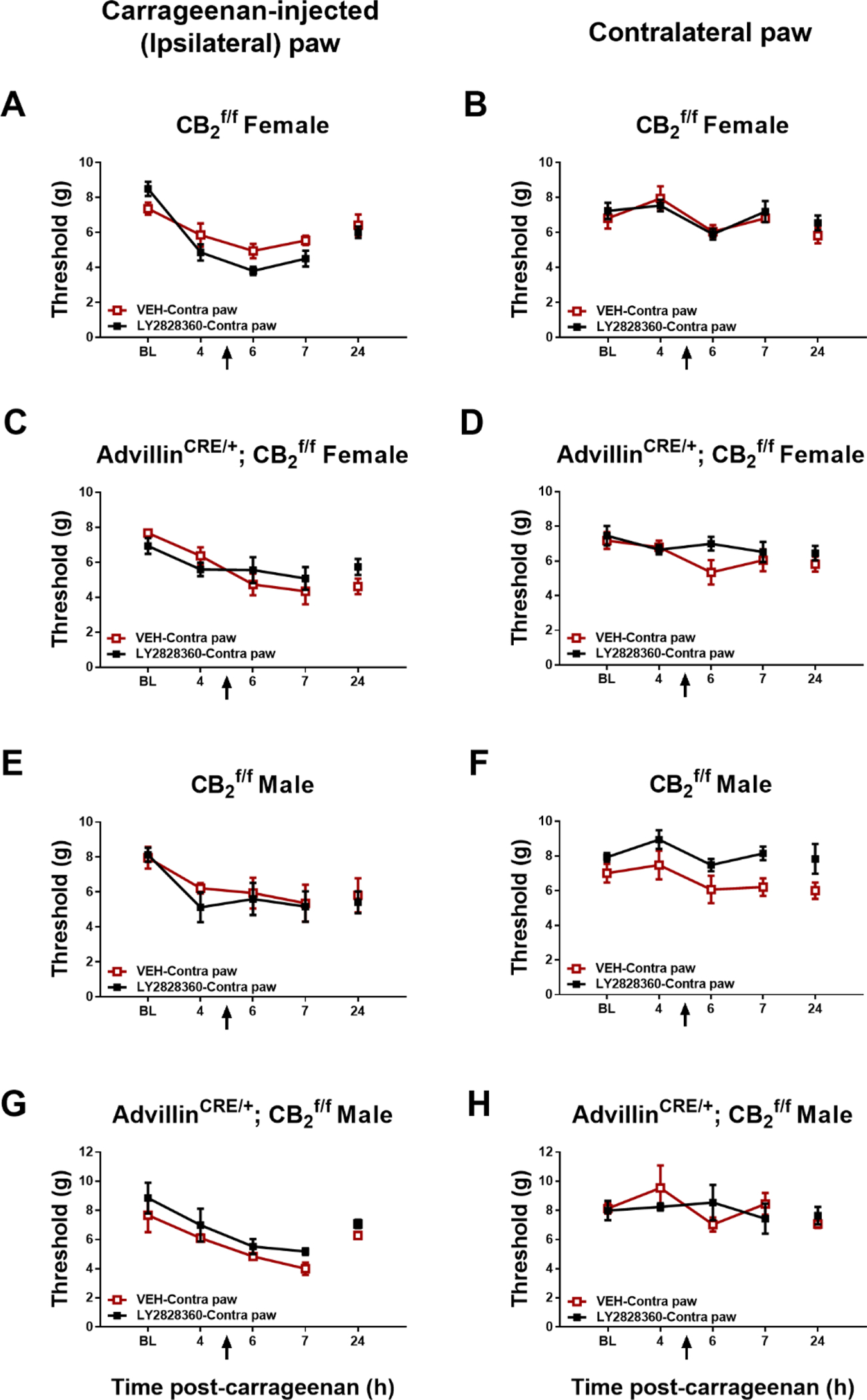
Impact of contralateral intraplantar LY2828360 (30 μg i.pl.) injection on paw withdrawal thresholds in the paw ipsilateral or contralateral to carrageenan injection in female and male CB2f/f and AdvillinCRE/+; CB2f/f mice (n = 4–5 per group). Mean (± SEM) threshold for the ipsilateral and contralateral paw in Experiment 10.
In male CB2f/f mice receiving injections in contralateral paw, withdrawal thresholds in the carrageenan-injected paw did not differ as a function of group, time or interaction (Group: F1,8 = 0.09111, p = 0.7705; Time: F2,16 = 0.3005, p = 0.7446; Interaction: F2,16 = 0.01146, p = 0.9886) (Fig. 6E). In male AdvillinCRE/+; CB2f/f mice receiving injections in contralateral paw, LY2828360 (30 μg i.pl.) increased paw withdrawal thresholds in the carrageenan-injected paw, and paw withdrawal thresholds changed over time but the interaction was not significant (Group: F1,6 = 14.01, p = 0.0096; Time: F2,12 = 19.76, p = 0.0002; Interaction: F2,12 = 0.2941, p = 0.7504) (Fig. 6G).
3.12. Experiment 11: Impact of intraplantar injection of LY2828360 in the absence of carrageenan
In the absence of carrageenan injection, paw withdrawal thresholds did not differ as a function of group, time, or interaction in the saline-injected paw of C57 WT mice before (Group: F1,8 = 0.2198, p = 0.6517 ; Time: F1,8 = 1.216, p = 0.2532 ; Interaction: F1,8 = 1.526, p = 0.2518) or after (Group: F1,8 = 0.07089, p = 0.7968 ; Time: F2,16 = 1.431, p = 0.2681; Interaction: F2,16 = 0.5819, p = 0.5702) pharmacological treatments. Paw withdrawal thresholds did not differ as a function of group, time, or interaction in the non-injected paw of C57 WT mice that received local injections of saline either before (Group: F1,8 = 0.9938, p = 0.3480 ; Time: F1,8 = 4.295, p = 0.0719 ; Interaction: F1,8 = 0.2152, p = 0.6551) or after (Group: F1,8 = 0.02304, p = 0.8831 ; Time: F2,16 = 0.08789, p = 0.9163 ; Interaction: F2,16 = 0.5543, p = 0.5851) pharmacological treatments (Data not shown).
3.13. Experiment 12: Impact of LY2828360 on mRNA expression levels of inflammatory-related markers following intraplantar injection of carrageenan
Carrageenan injection in the paw increased mRNA expression levels of cytokines IL-1β (F2,21 = 28.59, p < 0.0001), IL-10 (F2,21 = 46.16, p < 0.0001), TNFα (F2,21 = 25.76, p < 0.0001) and the chemokine MCP1 (F2,21 = 22.65, p < 0.0001) in the injected (ipsilateral) paw without changing levels of CB2 mRNA (F2,21 = 2.219, p = 0.1336) (Fig. 7A). Tukey’s multiple comparison test revealed that carrageenan increased mRNA expression levels of IL-1β, IL-10, TNFα and MCP1 in the VEH (i.p.) injected group vs. the naïve (no carrageenan) group (p < 0.0001 for all comparisons). LY2828360 also decreased mRNA expression levels of IL-1β and IL-10 in the carrageenan-treated groups that received LY2828360 (i.p) relative to VEH (i.p.) injections (p < 0.04 for all comparisons). No changes were observed in mRNA expression levels of any of these markers in paw skin derived from the non-injected contralateral paw (p > 0.2 for all comparisons; Fig. 7B). Carrageenan injection increased mRNA expression levels of IL-1β (F2,21 = 8.944, p = 0.0015) and MCP1 (F2,21 = 4.435, p = 0.0247) in the lumbar spinal cord without changing levels of IL-10 (F2,21 = 2.752, p = 0.0868), TNFα (F2,21 = 0.2378, p = 0.7905) or CB2 (F2,21 = 1.024, p = 0.3764) mRNA (Fig. 7C). Tukey’s multiple comparison test revealed that carrageenan increased mRNA expression of IL-1β in the VEH (i.p.) injected group relative to the naïve group (p = 0.0173). Carrageenan injection increased mRNA expression levels of IL-1β (F2,19 = 18.74, p < 0.0001) in the spleen without changing levels of IL-10 (F2,20 = 2.567, p = 0.1018), TNFα (F2,20 = 0.7415, p = 0.4890), MCP1 (F2,20 = 1.116, p = 0.3472) or CB2 (F2,20 = 0.2507, p = 0.7807) mRNA (Fig. 7D). Tukey’s multiple comparison test revealed that carrageenan increased mRNA expression of IL-1β in the VEH injected group vs naïve group (p = 0.0006).
Fig. 7.
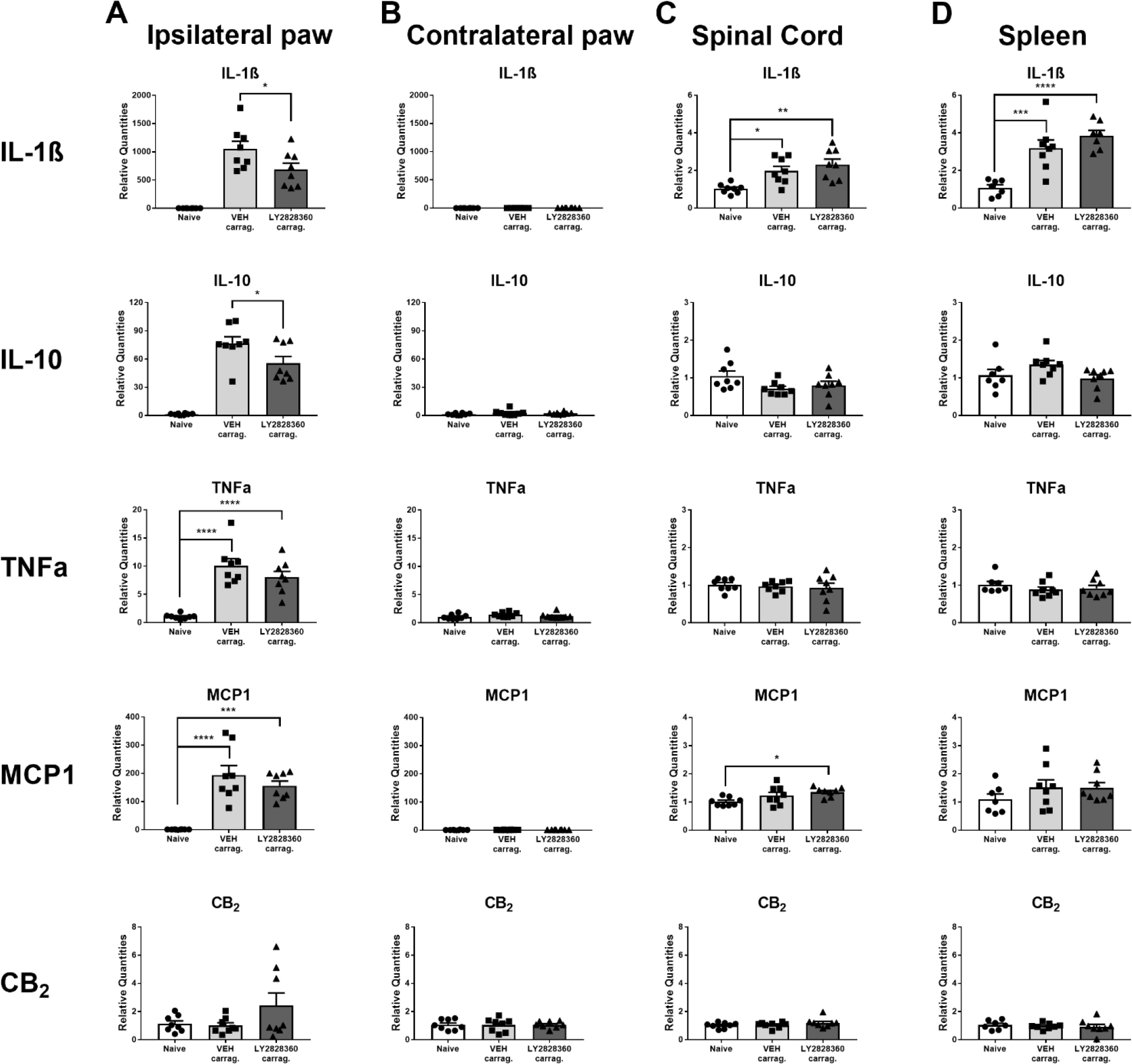
Intraplantar carrageenan increases levels of IL-1β, IL-10, TNFα and MCP1 mRNAs in the ipsilateral paw. Intraplantar carrageenan increased IL-1β and MCP1 mRNAs in the lumbar spinal cord and increased IL-1β mRNA in the spleen of female WT mice. LY2828360 (10 mg/kg i.p.) reduced IL-1β and IL-10 mRNA expression compared to VEH in the ipsilateral paw but did not affect mRNA expression levels of any marker in the contralateral paw, spinal cord or the spleen compared to VEH (n = 8 per group). Mean (± SEM) 2−ΔΔCT values for Experiment 12. Asterisk indicates a group difference; *p < 0.05, ***p < 0.001, ****p < 0.0001.
3.14. Experiment 13: qRT-PCR analysis to assess the impact of intraplantar carrageenan in the lumbar spinal cord of mice lacking CB2 in AdvillinCRE expressing cells
Intraplantar carrageenan increased MCP1 mRNA levels relative to intraplantar saline in the lumbar spinal cord of AdvillinCRE/+; CBf/f mice (F1,20 = 5.541, p = 0.0289), but no difference in genotype or interaction was observed (Genotype: F1,20 = 3.03, p = 0.0971; Interaction: F1,20 = 2.259, p = 0.1485) (Fig. 8C). Sidak’s multiple comparison test revealed that carrageenan increased MCP1 mRNA levels increased in the lumbar spinal cord of carrageenan-injected AdvillinCRE/+; CB2f/f mice compared to saline-injected AdvillinCRE/+; CB2f/f mice (p = 0.0197). Levels of IL-1β (Genotype: F1,19 = 0.4429, p = 0.5137; Treatment: F1,19 = 0.4526, p = 0.5092; Interaction: F1,19 = 0.6662, p = 0.4245) and TNFα (Genotype: F1,20 = 0.846, p = 0.3686; Treatment: F1,20 = 4.237, p = 0.0528; Interaction: F1,20 = 0.6048, p = 0.4459) mRNA expression levels did not differ in lumbar spinal cord as a function of carrageenan injection or genotype and the interaction was not significant (Fig. 8A–B).
Fig. 8.
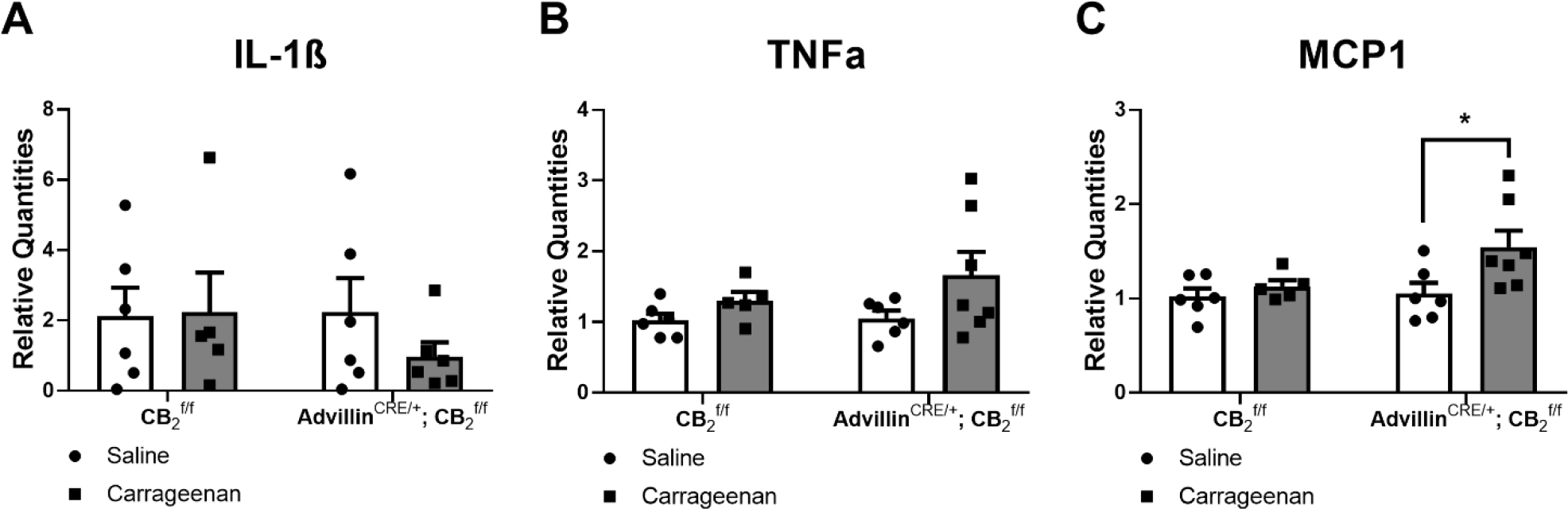
Carrageenan increased MCP1 mRNA expression levels in the lumbar spinal cord of male and female AdvillinCRE/+; CB2f/f mice. Mean (± SEM) 2−ΔΔCT values for Experiment 13 (n = 5–6 per group). Asterisk indicates a group difference; *p < 0.05, *p < 0.05 vs. saline.
3.15. Experiment 14: Impact of LY2828360 on mRNA expression levels of inflammatory markers following intraplantar injection of carrageenan in mice lacking CB2 in AdvillinCRE expressing cells
mRNA expression levels of IL-1β, IL-10, TNFα and MCP1 did not differ as a function of genotype, drug treatment or interaction in carrageenan-injected CB2f/f and AdvillinCRE/+; CB2f/f mice receiving LY2828360 (10 mg/kg i.p.) or vehicle in any tissue evaluated (i.e. ipsilateral paw skin, contralateral paw skin, lumbar spinal cord or spleen). However, mRNA expression levels of TNFα trended to increase in the spleen of AdvillinCRE/+; CB2f/f mice relative to CB2f/f mice similarly receiving intraplantar carrageenan injections (Genotype: F1,15 = 3.732, p = 0.0725) (Table 3).
Table 3.
Summary of statistical analyses of qRT-PCR data used to assess the impact of LY2828360 on inflammatory markers following intraplantar injection of carrageenan in mice lacking CB2 in AdvillinCRE expressing cells (Experiment 15)
| Marker | Genotype | Drug | Interaction |
|---|---|---|---|
|
| |||
| Ipsilateral paw | |||
|
| |||
| IL-1β | F1,16 = 0.07951, p = 0.7816 | F1,16 = 0.02824, p = 0.8687 | F1,16 = 0.6682, p = 0.4257 |
| IL-10 | F1,16 = 0.3808, p = 0.5458 | F1,16 = 0.5689, p = 0.4616 | F1,16 = 0.09311, p = 0.7642 |
| TNFa | F1,16 = 1.317, p = 0.2680 | F1,16 = 0.09746, p = 0.7589 | F1,16 = 0.1154, p = 0.7385 |
| MCP1 | F1,16 = 0.0001717, p = 0.9897 | F1,16 = 0.7937, p = 0.3862 | F1,16 = 0.3792, p = 0.5467 |
|
| |||
| Contralateral paw | |||
|
| |||
| IL-1β | F1,15= 1.197, p = 0.2911 | F1,15= 0.1709, p = 0.6852 | F1,15= 2.981, p = 0.1048 |
| IL-10 | F1,16= 0.4288, p = 0.5219 | F1,16= 0.1886, p = 0.6699 | F1,16= 1.99, p = 0.1775 |
| TNFa | F1,16 = 3.017, p = 0.1016 | F1,16 = 0.3696, p = 0.5518 | F1,16 = 0.05098, p = 0.8242 |
| MCP1 | F1,16 = 0.2327, p = 0.6361 | F1,16 = 1.07, p = 0.3163 | F1,16 = 0.1399, p = 0.7133 |
|
| |||
| Lumbar spinal cord | |||
|
| |||
| IL-1β | F1,15= 0.1067, p = 0.7485 | F1,15= 0.538, p = 0.4746 | F1,15= 1.079, p = 0.3155 |
| IL-10 | F1,15= 1.904, p = 0.1878 | F1,15= 0.3604, p = 0.5572 | F1,15= 0.4234, p = 0.5251 |
| TNFa | F1,15= 1.736, p = 0.2075 | F1,15= 0.3809, p = 0.5464 | F1,15= 2.095, p = 0.1684 |
| MCP1 | F1,16 = 0.003402, p = 0.9542 | F1,16 = 0.7486, p = 0.3997 | F1,16 = 0.7826, p = 0.3894 |
|
| |||
| Spleen | |||
|
| |||
| IL-1β | F1,15= 1.535, p = 0.2343 | F1,15= 2.46, p = 0.1376 | F1,15= 1.695, p = 0.2126 |
| IL-10 | F1,15= 0.88, p = 0.3631 | F1,15= 0.1186, p = 0.7353 | F1,15= 1.236, p = 0.2838 |
| TNFa | F1,15= 3.732, p = 0.0725 | F1,15= 0.3165, p = 0.5820 | F1,15= 0.6876, p = 0.4200 |
| MCP1 | F1,15= 0.05494, p = 0.8178 | F1,15= 0.1425, p = 0.7111 | F1,15= 0.4086, p = 0.5323 |
4. Discussion
Activation of the cannabinoid CB2 receptor suppresses pathological pain in a variety of animal models (e.g. Guindon and Hohmann, 2008; Murineddu et al., 2013; Cabañero et al., 2021; Soliman et al. 2021). However, lack of knowledge about the types of pain most responsive to CB2 agonists and cell types that underlie the therapeutic effects of CB2 agonists, amongst other factors (e.g. biased signaling of CB2 agonists, species differences in agonist efficacy), have hindered prospects for successful clinical translation. We previously reported that the slowly signaling G protein-biased CB2 receptor agonist LY2828360 effectively reduced paclitaxel- (Lin et al., 2018; Lin et al., 2022) and anti-retroviral- (Carey et al. 2023) induced neuropathic nociception. However, it was not known whether LY2828360 attenuated inflammatory nociception and if its efficacy varied by sex. Here we show that the CB2 receptor agonist LY2828360 effectively reduces mechanical allodynia in a carrageenan-induced inflammatory pain model at a dose of 10 mg/kg i.p., but not at lower doses, suggesting that it is less potent in reversing carrageenan-induced inflammatory nociception compared to paclitaxel- or 2,3-dideoxycytidine (ddC)-induced neuropathic nociception (Lin et al., 2018; Lin et al., 2022; Carey et al., 2023). This effect was CB2 receptor dependent as anti-allodynic efficacy was absent in CB2 receptor KO mice and preserved in CB1 receptor KO mice. Thus, the antiallodynic effects of LY2828360 are mediated by CB2 receptors and independent of CB1 receptors. By contrast, the anticonvulsant drug gabapentin which is also prescribed as a treatment for neuropathic pain (Bennett and Simpson, 2004) was equally efficacious in suppressing carrageenan-induced allodynia in both wild type and CB2 receptor KO mice. This observation suggests that, unlike LY2828360, the anti-allodynic effect of gabapentin in suppressing the maintenance of carrageenan-induced inflammatory nociception was independent of the CB2 receptor.
To further investigate the cell types responsible for the anti-allodynic effect of LY2828360, we tested its efficacy in mice lacking CB2 receptors in specific populations of cells after establishment of carrageenan-induced inflammatory nociception. LY2828360 (10 mg/kg i.p.) failed to suppress carrageenan-induced mechanical allodynia in mice with conditional deletion of the CB2 receptor from peripheral sensory neurons (AdvillinCRE/+; CB2f/f mice) at the dose tested but retained efficacy in mice with conditional deletion of CB2 from microglia and macrophages expressing CX3CR1 (CX3CR1CRE/+; CB2f/f mice) and control mice (CB2f/f). Thus, activation of CB2 receptors in peripheral sensory neurons is necessary for the anti-allodynic effects of LY2828360 in a mouse model of carrageenan-induced inflammatory pain, whereas CB2 receptors in CX3CR1-expressing microglia/macrophages are not required for anti-allodynic efficacy under our experimental conditions. Our results do not preclude the possibility that higher doses of LY2828360 or different CB2 agonists might be effective in AdvillinCRE/+; CB2f/f mice or implicated in other pain models. Additionally, given our CX3CR1CRE/+; CB2f/f mouse model is specific to microglia or macrophages expressing the protein coding gene C-X3-C Motif Chemokine Receptor 1 our results do not preclude a role for other cell types/classes of microglia in contributing to the anti-allodynic effect in our studies. The retained efficacy of LY2828360 in CX3CR1CRE/+; CB2f/f mice is likely due to lack of involvement of CB2 receptors in CX3CR1-expressing microglial/macrophages in the ability of this agonist to reduce carrageenan-induced inflammatory pain and not because of a failure of the CB2 receptor conditional KO mouse model used; this mouse model has been used successfully to unmask behavioral phenotypes mediated by CB2 agonists in other studies in our labs (Behlke et al., 2022 and unpublished data). Moreover, because CB2f/f and AdvillinCRE/+; CB2f/f mice do not differ in their development of carrageenan-induced inflammatory pain, we conclude that the results seen in mice lacking CB2 receptors in peripheral sensory neurons is specifically due to the loss of efficacy of LY2828360 in the cKO and not due to loss of CB2 receptors in sensory neurons affecting the development of inflammatory nociception. We previously reported that otherwise naïve AdvillinCRE/+; CB2f/f mice show lower levels of mRNA for CB2 and GFP in DRG, but not paw skin, lumbar spinal cord or spleen, indicating that the cKO is selective for CB2 in DRG (Carey et al., 2023).
Local administration of CB2 receptor agonists in the paw reduces pain behavior in naïve rats in the plantar test as well as in diverse models of inflammatory pain (Malan et al., 2001, 2002; Quartilho et al., 2003; Gutierrez et al., 2007). In the present studies, peripheral mechanisms of anti-allodynic efficacy were also evaluated by using local administration of LY2828360 into the carrageenan-treated hind paw of CB2f/f mice and AdvillinCRE/+; CB2f/f mice. LY2828360 injected into the carrageenan-inflamed paw reduced mechanical allodynia compared to VEH (i.pl.) treatment in CB2f/f but not AdvillinCRE/+; CB2f/f mice. This observation provides further evidence that anti-allodynic efficacy of LY2828360 is dependent on CB2 receptors arising from DRG sensory neurons and provides additional evidence that LY2828360 can exert its effect at the local site of injury. Conversely, when LY2828360 was injected into the paw opposite to the site of injury (contralateral paw) it did not alter responsiveness relative to VEH groups in either AdvillinCRE/+; CB2f/f or Advillin+/+; CB2f/f mice. These observations suggest that LY2828360 was unlikely to reach the systemic circulation when injected locally into the paw and confirm our results suggesting that intraplantar injection of LY2828360 in the carrageenan-injected paw is suppressing allodynia via a local site of action. Our data also suggest that an intraplantar LY2828360 (30 μg i.pl.) injection does not produce an antinociceptive effect (i.e. in our assessments of mechanical paw withdrawal thresholds) in the absence of carrageenan, an effect that we confirmed by injecting LY2828360 into the paw of WT mice in the absence of carrageenan-induced inflammation.
Carrageenan injected locally in the paw increased mRNA expression levels of pro-inflammatory cytokines IL-1β and TNFα, the anti-inflammatory cytokine IL-10 and the chemokine MCP1 selectively in the paw skin ipsilateral to carrageenan injection. Activation of the CB2 receptor by LY2828360 suppressed mRNA expression levels of cytokines that are involved in carrageenan’s response, specifically the pro-inflammatory cytokine IL-1β and the anti-inflammatory cytokine IL-10 in the carrageenan-injected paw skin compared to VEH treatment. Moreover, unlike in the WT mice, LY2828360 did not modulate these cytokines in either CB2f/f or AdvillinCRE/+; CB2f/f mice. Differing results between these two studies may be because of different time points of tissue collection of these two experiments or a genotype difference between the WT and the CB2f/f mice. Additionally, our results were not powered to detect sex differences in studies in which mixed sexes were combined.
Few studies examining the effect of carrageenan on IL-10 in the paw skin have been published and none have examined the impact of CB2 receptor agonists on IL-10 modulation in this tissue. qRT-PCR studies have shown both a decrease or no change in mRNA levels of the anti-inflammatory cytokine IL-10 in the paw skin following carrageenan injection (Zhang et al., 2020; Gong et al., 2009) and increase in response to CB2 receptor activation (eg. Robinson et al., 2015; Saroz et al., 2019). The carrageenan-induced increase in IL-10 mRNA observed in our study may be due to dose of carrageenan employed, species used or reflect the timepoint at which we collected tissue for our qRT-PCR studies. Modulation of these cytokines is a dynamic process a single timepoint is not representative of the entire process. Future studies using qRT-PCR should use tissues collected at several timepoints to get a more comprehensive understanding of how these cytokines are modulated by carrageenan and CB2 receptor activation in paw skin tissue.
In the present study, we used Advillin-CRE; CB2f/f mice, in which the Advillin promoter drives Cre-recombinase expression within sensory neurons, and crossed these mice with CB2f/f mice, to generate putative sensory neuron-specific CB2 knockout mice. This approach leverages the fact that the actin binding protein Advillin is expressed in virtually all somatic sensory neurons (Hunter et al., 2018; Zhou et al., 2010). However, several caveats in interpretation of our results warrant discussion. Selectivity of our AdvillinCRE/+; CB2f/f mouse line to delete CB2 receptors from primary sensory neurons is based upon promoter-based genetic recombination using the Cre-lox approach. It is, thus, important to keep in mind that developmental changes may impact constitutive Advillin-driven genomic editing, and recent reports suggest that Advillin expression is not restricted to primary sensory neurons (Hunter et al., 2018). Advillin expression and Advillin promoter driven EGFP and Cre-recombinase expression have recently been reported in the dorsal habenula, endocrine cells in the gut, Merkel cells in the skin and some neurons of the autonomic nervous system (Hunter et al., 2018). However, Zappia and colleagues did not find evidence of Advillin-Cre expression in keratinocytes of the skin or in spinal cord sections of adult mice when Cas9-GFP reporter was used to visualize Cre+ neurons (Zappia et al., 2017). These latter observations suggest that the loss of CB2 agonist-mediated anti-allodynic efficacy in our AdvillinCRE/+; CB2f/f cKO mice likely reflect deletion of CB2 from primary sensory neurons, rather than from skin or spinal cord. This interpretation is consistent with results from our qPCR validation studies where mRNA expression levels of both CB2 and EGFP were decreased in DRG derived from AdvillinCRE/+; CB2f/f mice, relative to CB2f/f control mice, but no such decreases were observed in paw skin, lumbar spinal cord, or spleen (Carey et al., 2023). Nonetheless, our studies did not specifically evaluate localization of CB2 to primary sensory neurons (as opposed to satellite glial cells) that would be present in DRG tissues. Challenges surrounding the use of CB2 as well as anti-GFP antibodies for localization studies have been previously described by our groups and others (Lin et al., 2022; Carey et al., 2023) and motivated the present cKO approach. We recently reported localization of EGFP reporter to cells of the epidermis (including keratinocytes, Langerhans and dendritic cells) of our CB2f/f mice that were treated with paclitaxel, and also detected sparse anti-GFP immunolabeling in A-Beta fibers and Merkel cell endings but not in intraepidermal nerve fibers (Lin et al., 2022). These latter observations are consistent with putative localization of CB2 to medium and large diameter DRG cells (Hunter et al., 2018). Nonetheless, expression of CB2 mRNA or protein in DRG is far from robust and a number of early reports found expression levels to be near or below detection threshold in the absence of inflammation or injury. Using in situ hybridization, CB2 mRNA expression levels were below the threshold for detection in normal DRG (Hohmann and Herkenham, 1999) and trigeminal ganglia (Price et al., 2003) derived from naïve rats under conditions in which CB1 mRNA was detected in the same sensory ganglia and robust CB2 mRNA was measured in spleen. CB2 protein was similarly reported to be below threshold for detection in DRG of otherwise naïve rats but markedly upregulated following peripheral nerve injury (Wotherspoon et al., 2005); this signal was also absent in CB2 KO mice. Using more sensitive RNAscope in situ hybridization techniques, Ghosh and colleagues recently documented an increase in CB2 mRNA specifically in medium- and large-sized DRG neurons (cells that co-expressed the neuronal marker NeuN) at 10 days following spinal nerve ligation (SNL) (Ghosh et al., 2022). Epigenetic mechanisms are also implicated in both development of DRG hyperexcitability following injury and development of pathological pain. Histone modifications at the Cnr2 promoter were shown to be highly correlated with nerve injury induced CB2 upregulation in rat DRG. Thus, peripheral nerve injury promoted a delayed and sustained increase in CB2 protein expression in primary sensory neurons of rats via epigenetic bivalent histone modification (Ghosh et al., 2022).
Less is known about how expression levels of CB2 may change in inflammatory pain models, where CB2 mRNA expression levels may also be dynamically regulated. In support of this hypothesis, CB2 mRNA is upregulated in DRG ipsilateral to injury, but not in spinal cord, using qPCR approaches in a rat model of inflammatory pain induced by Complete Freund’s adjuvant (CFA) inflammation of the hindpaw (Hsieh et al., 2011). Further, using qPCR approaches, CB2 mRNA expression in both DRG and spinal cord was increased following spinal nerve ligation (SNL) under conditions in which no changes in CB2 mRNA expression levels were observed in paw skin, hippocampus, thalamus, sensory cortex and brainstem (Hsieh et al., 2011). These observations are consistent with a role for the DRG in contributing to peripheral CB2 antinociceptive mechanisms. Furthermore, intra-DRG application of structurally distinct CB2 agonists (AM1241, A-836339) produced analgesic effects in both CFA and SNL models in rats, specifically demonstrating the DRG as a site of CB2 analgesic action in both inflammatory and neuropathic pain models (Hsieh et al., 2011). The CB2 agonist JWH133 also inhibited the amplitude of dorsal root-evoked glutamatergic excitatory postsynaptic currents in spinal dorsal horn of rats with SNL, but not in sham-operated rats. Finally, localization of CB2 to human primary sensory neurons has been reported using multiple CB2 antibodies (Anand et al., 2008) and CB2 agonists also suppressed capsaicin-evoked Ca++ responses in human primary DRG cultures under conditions in which pharmacological specificity was established (Anand et al., 2008). These studies collectively suggest that CB2 receptors on DRG are functional in humans (Anand et al., 2008). Thus, spatial transcriptomics of donor-derived human DRG (Tavares-Ferreira et al., 2022) would not necessarily reflect expression patterns observed in pathological pain states. More work is necessary to assess the contributions of neuronal and non-neuronal CB2 receptors, that may be differentially regulated in response to inflammation and injury, in the peripheral antinociceptive effects of CB2 agonists, and determine whether differences in CB2 localization exist across species.
Our results provide the first evidence that LY2828360 suppresses inflammatory nociception through a CB2-dependent mechanism in mice. Our findings align with previous research from our lab and others suggesting that CB2 receptor agonists can reduce inflammatory pain behaviours and neurochemical markers of inflammation-evoked neuronal activation in lumbar spinal cord (Nackley, Makriyannis and Hohmann, 2003; Clayton et al., 2002; Elmes et al., 2005), extending the results to both sexes. LY2828360 is of particular interest as a CB2 receptor agonist because it has recently been shown to reduce neuropathic nociception through a CB2-mediated mechanism without producing tolerance and can also prevent tolerance to morphine (Lin et al., 2018; Lin et al., 2022; Carey et al., 2023). LY2828360 exhibits functional selectivity as a G protein-biased agonist that does not cause internalization of the CB2 receptor or arrestin recruitment (Lin et al., 2018). More work is necessary to determine if the biased nature of this agonist specifically engages therapeutically relevant pathways. It is possible that the choice of therapeutic indication (i.e. osteoarthritis) rather than the small molecule or receptor target accounts for why LY2828360 failed for efficacy in a phase 2 clinical trial for knee pain due to osteoarthritis (Pereira et al., 2013). Lastly, more work is necessary to examine the impact of LY2828360 on hindpaw edema. Our results contribute to an emerging body of literature (Lin et al., 2018; Lin et al., 2022; Carey et al., 2023) which collectively suggest that the clinical application of this small molecule CB2 agonist should be re-evaluated in neuropathic or inflammatory pain conditions.
Highlights.
CB2 receptor agonist LY2828360 reduced carrageenan-induced mechanical allodynia
Anti-allodynic effects of LY2828360 are CB2 receptor dependent
Peripheral sensory neuron CB2 receptors are required for efficacy of LY2828360
LY2828360 can exert anti-allodynic effect locally at the site of injury
LY2828360 suppresses cytokines involved in carrageenan’s inflammatory response
Funding:
This work was supported by the National Institute on Drug Abuse [DA047858, DA041229, DA042584, DA009158], an Indiana Addiction Grand Challenge Grant, the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin and the Ministerio de Economía y Competitividad [SAF 2016-75959-R, SAF PID2019-108992RB-I00]. K.G. was supported by the Harlan Scholars Research Program.
Funding was provided by Agencia Estatal de investigación - Ministerio de Ciencia e Innovación (PID2019-108992RB-I00).
Footnotes
Declarations of interest: none
Credit author statement
Kelsey G. Guenther: Investigation, Formal Analysis, Writing – Original draft, Writing – Review & Editing, Visualization
Zhili Xu: Investigation, Formal Analysis
Julian Romero: Resources
Cecilia J. Hillard: Resources
Ken Mackie: Resources, Writing – Review & Editing, Funding acquisition
Andrea G. Hohmann: Conceptualization, Methodology, Resources, Writing – Original draft, Writing – Review & Editing, Visualization, Supervision, Funding acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP, Anand P 2008. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 138, 667–680. [DOI] [PubMed] [Google Scholar]
- Behlke CA, Krause L, Sterrett J, Moe A, Hillard CJ Cell specific roles of the CB2R in the acute locomotor response to cocaine [abstract]. In: 32nd Annual Symposium of the International Cannabinoid research society; 2022 June 25–30; Galway, Ireland: ICRS; 2022. Abstract nr 34. [Google Scholar]
- Beltramo M, Bernardini N, Bertorello R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A 2006. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 23, 1530–1538. [DOI] [PubMed] [Google Scholar]
- Bennett MI, Simpson KH 2004. Gabapentin in the treatment of neuropathic pain. Palliat Med. 18, 5–11. [DOI] [PubMed] [Google Scholar]
- Cabañero D, Martín-García E, Maldonado R 2021. The CB2 cannabinoid receptor as a therapeutic target in the central nervous system. Expert Opinion on Therapeutic Targets. 25, 659–676. [DOI] [PubMed] [Google Scholar]
- Carey LM, Xu Z, Rajic G, Makriyannis A, Romero J, Hillard C, Mackie K, Hohmann AG 2023. Peripheral sensory neuron CB2 cannabinoid receptors are necessary for both CB2-mediated antinociceptive efficacy and sparing of morphine tolerance in a mouse model of anti-reroviral toxic neuropathy. Pharmacol Research. 187, 106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O’Shaughnessy CT 2002. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 96, 253–260. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M 2009. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatr. 21, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL., Makriyannis A, Mackie K, Hohmann AG. 2015. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiat. 77, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M, Giroud JP, Willoughby DA 1971. Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 104, 15–29. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, Chapman V 2005. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 118, 327–335. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Marchand MA, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Cassellas P 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 232, 54–61. [DOI] [PubMed] [Google Scholar]
- Gong D, Geng C, Jiang L, Cao J, Yoshimura H, Zhong L 2008. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother Res. 23, 646–650. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Zhang GF, Chen H, Chen SR, Pan HL 2022. Cannabinoid CB2 receptors are upregulated via bivalent histone modifications and control primary afferent input to the spinal cord in neuropathic pain. J Biol Chem. 298, 101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidon J, Hohmann AG 2008. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 153, 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T, Crystal JD, Zvonok AM, Makriyannis A, Hohmann AG 2011. Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain. 152, 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG 2007. Activation of peripheral cannabinoid CB1 and CB2 receptors supresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 150, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A 2004. Selective activation of cannabinoid CB2 receptors supresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 308, 446–453. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M 1999. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 92, 1171–1175. [DOI] [PubMed] [Google Scholar]
- Hollinshead SP, Tidwell MW, Palmer J, Guidetti R, Sanderson A, Johnson MP, Chambers MG, Oskins J, Stratford R, Astles PC 2013. Selective cannabinoid receptor type 2 (CB2) agonists: Optimization of a series of purines leading to the identification of a clinical candidate for the treatment of osteoarthritic pain. J Med Chem. 56, 5722–5733. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG 2002. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 54, 161–202. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD 2011. Central and peripheral sites of action for CB₂ receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 162, 428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DV, Smaila BD, Lopes DM, Takatoh J, Denk F, Ramer MS 2018. Advillin is expressed in all adult neural crest-derived neurons. eNeuro. 5, e0077–18.2018 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG 2020. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur J Pharmacol. 886, 173544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Dhopeshwarkar AS, Huibregtse M, Mackie K, Hohmann AG 2018. Slowly signaling G-protein biased CB2 cannabinoid receptor agonist LY2828360 supresses neuropathic pain with sustained efficacy and attenuates morphine tolerance and dependence. Mol Pharmacol. 93, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Xu Z, Carey L, Romero J, Makriyannis A, Hillard CJ, Ruggiero E, Dockum M, Houk G, Mackie K, Albrecht PJ, Rice FL, Hohmann AG. 2022. A peripheral CB2 cannabinoid receptor mechanism suppresses chemotherapy-induced peripheral neuropathy: evidence from a CB2 reporter mouse. Pain. 163, 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25, 402–408. [DOI] [PubMed] [Google Scholar]
- López A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vásquez C, Amores M, Ruiz-Pérez G, García-García E, Beatka M, Tolón RM, Dittel BN, Hilliard CJ, Romero J 2018. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation. 15, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Weyer-Menkhoff I, Tegeder I 2018. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain. 22, 471–484. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Vanderah T, Porreca F, Makriyannis A 2001. CB2 cannabinoid receptor-mediated peripheral anti-nociception. Pain. 93, 239–245. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F 2002. Inhibition of pain responses by activation of CB2 cannabinoid receptors. Chem Phys Lipids. 121, 191–200. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M 1993, Molecular characterization of a peripheral receptor for cannabinoids. Nature. 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Murineddu G, Deligia F, Dore A, Pinna G, Asproni B, Pinna GA 2013. Different classes of CB2 ligands potentially useful in the treatment of pain. Recent Pat CNS Drug Discov. 8, 42–69. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG 2003. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 119, 747–757. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Suplita II RL, Hohmann AG 2003. A peripheral cannabinoid mechanism supresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 117, 659–670. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG 2004. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 92, 3562–3574. [DOI] [PubMed] [Google Scholar]
- Pereira A, Chappell A, Dethy J, Hoeck H, Arendt-Nielsen L, Verfaille S, Boulanger B, Jullion A, Johnson M, McNearney T 2013. A proof-of concept (poc) study including experimental pain models (epms) to assess the effecrs of a CB2 agonist (LY2828360) in the treatment of patients with osteoarthritic (oa) knee pain. Clin Pharmacol Ther. 93, S56–S57. [Google Scholar]
- Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM 2003. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 120, 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP. 2003. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 99, 955–960. [DOI] [PubMed] [Google Scholar]
- Robinson RH, Meissler JJ, Fan X, Yu D, Adler MW, Eisenstien TK 2015. A CB2-selective cannabinoid supresses T-cell activities and increases Tregs and IL-10. J Neuroimmune Pharmacol. 10, 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Wang Z, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG 1996. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 118, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL 2019. Cannabinoid receptor 2 (CB2) signals via G-alpha-s and induces IL-6 and IL-10 cytokine secretion in human primary leukocytes. ACS Pharmacol Transl Sci. 2, 414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman N, Haroutounian S, Hohmann AG, Krane E, Liao J, Macleod M, Segelcke D, Sena C, Thomas J, Vollert J, Wever K, Alaverdyan H, Barakat A, Bathlow T, Harris Bozer AL, Davidson A, Diaz-delCastillo M, Dolgorukova A, Ferdousi MI, Healy C, Hong S, Hopkins M, James A, Leake HB, Malewicz NM, Mansfield M, Mardon AK, Mattimoe D, McLoone AP, Noes-Holt G, Pogatzi-Zahn EM, Power E, Pradier B, Romanos-Sirakis E, Segelcke A, Vinagre R, Yanes JA, Zhang J, Zhang XY, Finn DP, Rice ASC 2021. Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain. 162, S26–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares-Ferreira D, Shiers S, Ray PR, Wangzhou A, Jeevakumar V, Sankaranarayanan I, Cervantes AM, Reese JC, Chamessian A, Copits BA, Dougherty PM, Gereau RW 4th, Burton MD, Dussor G, Price TJ 2022. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci Transl Med. 14, eabj8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, Mark L, Pearson MS, Miller W, Shan S, Rabadi L, Rotshteyn Y, Chaffer SM, Turchin PI, Elsemore DA, Toth M, Koetzner L, Whiteside GT 2005. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, ataxia and catalepsy. Neuropharmacology. 48, 658–672. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA 2005, Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 310, 329–332. [DOI] [PubMed] [Google Scholar]
- Winters CA, Risley EA, Nuss GW 1962. Carrageenin-induces edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med. 111, 544–547. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J 2005. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 135, 235–245. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL 2011. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 14, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim K, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 38, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia KJ, O’Hara CL, Moehring F, Kwan KY, Stucky CL 2017. Sensory Neuron-Specific Deletion of TRPA1 Results in Mechanical Cutaneous Sensory Deficits. eNeuro. 4, e0069–16 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Shang C, Tian Z, Amin HK, Kassab RB, Abdel Moneim AE, Zhang Y 2020. Diallyl disulfide supresses inflammatory and oxidative machineries following carrageenan injection-induced paw edema in mice. Mediators Inflamm. 2020, 8508906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang L, Hasegawa H, Amin P, Han B, Kaneko S, He Y, Wang F 2010. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Netl Acad Sci. USA 17, 9424–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]


