Abstract
目的
探讨携带三磷酸腺苷结合盒转运子G2(ATP binding cassette transporter G2, ABCG2)胞外囊泡(extracellular vesicle, EVs)调控肺腺癌耐药作用及其分子机制。
方法
取人肺腺癌细胞A549,建立顺铂(cis-Diaminedichloroplatinum, CDDP)耐药的肺腺癌细胞A549/CDDP;利用梯度离心法提取A549及A549/CDDP细胞释放的EVs,分别命名为EVs1及EVs2。EVs1及EVs2干预A549细胞48 h后,细胞分别命名为A549-EVs1及A549-EVs2。利用pCDNA3.1-ABCG2重组质粒转染A549细胞,建立A549/ABCG2细胞;转染空载体的A549细胞命名为A549/pCDNA3.1细胞。以MTT检测计算细胞24 h对CDDP的耐药指数;real-time PCR检测细胞、EVs中ABCG2基因表达;建立荷瘤裸鼠模型,分别接种A549及A549-EVs2细胞至裸鼠皮下,记为对照组及实验组。成瘤后腹腔注射3 mg/kg CDDP,每周1次,共2次。取皮下移植瘤组织,real-time PCR检测ABCG2基因表达,流式细胞术检测皮下移植瘤细胞凋亡率。
结果
以亲代细胞A549为参照,A549/CDDP、A549/ABCG2、A549/pCDNA3.1、A549-EVs1、A549-EVs2细胞24 h对CDDP的耐药指数分别为7.17、10.06、1.02、1.19、5.40。各细胞中、EVs中ABCG2基因的表达水平相比较,A549/CDDP细胞高于A549细胞,A549/ABCG2细胞高于A549/pCDNA3.1和A549细胞,EVs2高于EVs1,A549-EVs2细胞高于A549-EVs1细胞(P<0.01)。实验组移植瘤体积、细胞中ABCG2基因表达大/高于对照组,而细胞凋亡率低于对照组(P<0.05)。
结论
携带ABCG2基因的EVs可以调控肺腺癌细胞耐药。
Keywords: 非小细胞肺癌, 耐药, 胞外囊泡, 裸鼠, 三磷酸腺苷结合转运蛋白G2
Abstract
Objective
To investigate the regulatory role of extracellular vesicles (EVs) carrying ATP binding cassette transporter G2 (ABCG2) on the drug resistance of lung adenocarcinoma cells and the relevant molecular mechanisms.
Methods
A549 cells, human lung adenocarcinoma cells, were used to form cisplatin (or cis-Diaminedichloroplatinum, CDDP)-resistant lung adenocarcinoma cells, i.e., A549/CDDP cells. EVs from A549 and A549/CDDP cells were extracted by gradient centrifugation method and were hence named EVs1 and EVs2, respectively. The A549 cells were treated with EVs1 and EVs2 for 48 hours, and the cells were named A549-EVs1 and A549-EVs2 cells, respectively. A549/ABCG2 cells were established by transfecting A549 cells with pCDNA3.1-ABCG2 recombinant plasmids. On the other hand, A549 cells transfected with empty vectors were named A549/pCDNA3.1 cells. MTT assay was conducted to calculate the 24-hour cell drug resistance index for CDDP. The ABCG2 gene expression in cells and EVs were assessed with real-time PCR. A549 and A549-EVs2 cells were transplanted subcutaneously into nude mice, which were labeled the control group and the experimental group accordingly. After tumor formation, 3 mg/kg CDDP was intraperitoneally injected once a week for two times. The ABCG2 gene expression of subcutaneous transplanted tumor cells was examined by real-time PCR. The cell apoptosis rate of subcutaneous transplanted tumor cells was examined by flow cytometry.
Results
Using the parental A549 cells as reference, the 24-h CDDP-resistance indexes of 549/CDDP, A549/ABCG 2, A549/pCDNA3.1, A549-EVs1, A549-EVs2 cells were 7.17, 10.06, 1.02, 1.19 and 5.40, respectively. When comparing the ABCG2 gene expression levels in all cells and EVs, the findings were higher in A549/CDDP cells than those inA549 cells, higher in A549/ABCG2 cells than those in A549/pCDNA3.1 or A549 cells, higher in EVs2 than those in EVs1, and higher in A549-EVs2 than those in A549-EVs1 cells (P<0.01) . The volume of transplanted tumor and theABCG2 gene expression level in the experimental group were higher than those in the control group, while the apoptosis rate was lower than that in the control group (P<0.01).
Conclusion
EVs carrying ABCG2 gene can regulate the drug resistance of lung adenocarcinoma cells.
Keywords: Non-small cell lung cancer, Drug resistance, Extracellular vesicle, Nude mice, ATP binding cassette transporter G2
肺癌是发病及死亡率较高的恶性肿瘤,其中肺腺癌最为常见,其主要治疗方法为化疗,而耐药是影响疗效的主要因素[1-3]。探究肺腺癌耐药机制,对于提高肺腺癌治疗效果具有重大意义。细胞跨膜转运蛋白与耐药密切相关,可通过降低细胞内化疗药物的有效浓度引起耐药[4-5],以三磷酸腺苷结合盒(ATP binding cassette, ABC)转运蛋白为代表[6-11]。本小组发现ABC转运子G2(ABC transporter G2, ABCG2)与食管癌耐药相关[12],但耐药细胞通过何种机制传递耐药信息并未阐明。肿瘤微环境对细胞耐药起着重要的调控作用,其中胞外囊泡(extracellular vesicles, EVs)的作用尤为突出。本小组发现携带linc-VLDLR的EVs可以调控食管癌耐药[13],GIULIA等[14]研究显示,间变性淋巴瘤激酶(anaplastic lymphoma kinase, ALK)参与黑素瘤细胞耐药,携带ALK的EVs可以将耐药信息传递给敏感细胞。然而,EVs调控肺腺癌耐药作用的研究鲜有报道。本文利用A549肺腺癌细胞建立顺铂(cis-Diaminodichloroplatinum, CDDP)耐药的肺腺癌A549/CDDP细胞及过表达ABCG2的A549/ABCG2细胞,研究肺腺癌耐药细胞是否可以通过释放携带ABCG2基因的EVs调控耐药。
1. 材料和方法
1.1. 主要试剂
RPMI1640及胎牛血清(Gibco公司,美国);荧光定量PCR试剂(南京诺唯赞生物科技有限公司,中国);AnnexinⅤ-FITC/PI试剂盒(BD公司,美国);噻唑蓝比色法(MTT)试剂及二甲基亚砜(Sigma公司,美国);注射用顺铂(冻干型)(齐鲁制药有限公司,中国);引物(上海生工生物公司,中国);pCDNA3.1-ABCG2质粒(武汉晶赛公司,中国)。
1.2. 细胞株
1.2.1. 人肺腺癌细胞株A549
A549细胞由河北省肿瘤研究所提供,使用含10%胎牛血清的RPMI1640培养基(青链霉素各100 U/L)进行培养,置37 ℃、体积分数5%CO2的温箱中。
1.2.2. A549/CDDP细胞
逐渐增加A549细胞培养液中CDDP质量浓度结合CDDP大剂量冲击,CDDP质量浓度从0.1 μg/mL逐渐增至3 μg/mL,历时6个月的细胞培养,最终使用1 μg/mL CDDP为维持质量浓度,建立顺铂耐药的肺腺癌细胞,命名为A549/CDDP细胞。
1.2.3. A549-EVs1及A549-EVs2细胞
采用梯度离心法提取细胞释放的EVs。将处于对数生长期的A549及A549/CDDP细胞分别接种至细胞培养瓶中,培养48 h后利用梯度离心法收集细胞培养液中A549及A549/CDDP细胞释放的EVs,分别标记为EVs1及EVs2。梯度离心法的主要步骤包括[15]:2000 r/min,10 min;3000 r/min,30 min;11000 r/min,1 h。收集离心后的上清,将上清以超高速110000×g离心16 h,弃上清,用适量PBS溶解EVs沉淀,Nanodrop法定量,调整质量浓度为500 μg/mL,−80 ℃分装保存。将1×105mL−1的A549细胞接种至6孔板中,细胞贴壁后,分别加入EVs1及EVs2,使终质量浓度达到50 μg/mL,每组设置3个复孔,培养48 h后收集细胞,分别标记为A549-EVs1及A549-EVs2细胞。
1.2.4. A549/ABCG2细胞和A549/pCDNA3.1细胞
利用基因转染方法建立过表达ABCG2的肺腺癌细胞。将处于对数生长期的肺腺癌A549细胞4×105/孔接种于6孔板中,细胞贴壁后,进行细胞转染。用无牛清的RPMI1640培养液冲洗细胞2次后弃去上清液,每孔加入2 mL无牛清及抗生素的RPMI1640培养液,试剂1(5 μL LipofectamineTM2000脂质体与245 μL无牛清及抗生素的RPMI1640培养液混合,室温放置5 min)与试剂2(2 μL pCDNA3.1-ABCG2重组质粒与248 μL无牛清及抗生素的RPMI1640培养液混合,室温放置5 min)轻柔混合,室温放置20 min后加入6孔板每孔中,转染72 h后使用G418进行转染细胞筛选,细胞命名为A549/ABCG2细胞;转染空载体pCDNA3.1的A549细胞命名为A549/pCDNA3.1细胞。
1.3. 实验方法
1.3.1. MTT法检测细胞对CDDP的耐药
将1×104mL−1 A549、A549/CDDP、A549-EVs1、A549-EVs2、A549/ABCG2及A549/pCDNA3.1分别接种至96孔板,待细胞贴壁后小心倒去培养液,各设置9个质量浓度,分别加入不同质量浓度的CDDP(0、0.01、0.05、0.1、0.5、1、5、10、50 μg/mL),并以0 μg/mL CDDP组为对照组(使用生理盐水代替CDDP);每个质量浓度重复3个复孔,另设调零孔。CO2温箱中孵育24 h,而后每孔加入MTT溶液(5 mg/mL) 20 μL,继续培养4 h,弃去培养基,每孔加入180 μL二甲基亚砜,振荡10 min。酶标仪测定各孔吸光度A490值,计算细胞生长抑制率〔 (1−用药组A490÷对照组A490 )×100 % 〕,以绘制细胞生长曲线,对应得出24 h的半数抑制浓度(half inhibition concentration, IC50 ),计算细胞24 h对CDDP的耐药指数。耐药指数=耐药细胞IC50÷亲代细胞IC50,亲代细胞均为A549。
1.3.2. 荧光定量real-time PCR方法检测细胞、EVs中ABCG2基因表达
使用RNA isolater提取细胞(A549、A549/CDDP、A549/ABCG2、A549/pCDNA3.1、A549-EVs1及A549-EVs2细胞)、EVs(EVs1及EVs2)中的RNA,反转录为cDNA。常规real-time PCR方法进行实验,采用SYBR-Green Ⅰ作为荧光染料,实验重复3次。ABCG2上游引物3′-ACG ATG TCT TTG GTG TGA GAC TGG-5′,下游引物3′-ATT TCG TCC CGT AGC TAG AGA GTG-5′,扩增产物长度280 bp。内参GAPDH上游引物3′-CACTACCGTACCTGACACCA-5′,下游引物3′-ATGTCGTTGTCCCACCACCT-5′,扩增产物长度452 bp。扩增条件:预变性,95 ℃ 5 min(1循环);扩增,95 ℃ 15 s、58 ℃ 30 s、72 ℃ 25 s(40循环);熔解曲线,95 ℃ 15 s、58 ℃ 1 min、95 ℃ 30 s(1循环)。应用2−ΔCt值法计算ABCG2 mRNA的相对表达量。
1.3.3. 建立肺腺癌细胞荷瘤裸鼠
为了探讨来源于肺腺癌耐药细胞释放的EVs2调控耐药的作用,接种A549-EVs2细胞至小鼠皮下,进行实验。4周龄BALB/c nu/nu裸小鼠,12只,雌雄各半,购自北京维通利华实验动物技术有限公司,动物许可证号SCXK(京)2012-0001。动物实验分2组,每组小鼠6只,雌雄各半。实验组,皮下接种A549-EVs2细胞;对照组,皮下接种A549细胞。裸鼠皮下接种肺腺癌细胞成瘤后(瘤直径约0.5 cm)开始腹腔注射药物(3 mg/kg CDDP),1次/周,共2次,实验周期为2周。每隔1 d,测量裸鼠体质量和观察裸鼠饮食水及活动情况,每周测皮下移植瘤长径(a,单位为mm)、短径(b,单位为mm)和体积(V,单位为mm3)1次。V=a×(b)2/2。皮下移植瘤成长至瘤直径约5 mm,判定裸鼠皮下移植瘤模型建立成功。
1.3.4. 荧光定量real-time PCR方法检测皮下移植瘤中ABCG2基因表达
使用RNA isolater提取皮下移植瘤组织(将组织进行研磨后提取RNA)中的RNA,反转录为cDNA,后续步骤与1.3.2相同。检测皮下移植瘤中ABCG2基因表达。
1.3.5. 流式细胞术方法检测裸鼠皮下移植瘤细胞凋亡
裸鼠皮下移植瘤离体后立即使用网搓法制备单细胞悬液,调整细胞浓度为1×107mL−1。取100 μL制备的细胞悬液,加入5 mL PBS混匀后,离心弃去上清,加入100 μL1×Binding buffer混匀细胞,加入5 μL AnnexinⅤ-FITC,避光放置15 min,而后加入10 μL PI染液避光放置10 min,加入385 μL 1×Binding buffer后流式细胞仪检测。采用Expo32 ADC v1.2软件分析荧光数据,计算细胞凋亡率。
1.4. 统计学方法
实验数据以
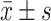 表示。采用单因素方差分析及独立样本t检验进行组间比较,P<0.05为差异有统计学意义。
表示。采用单因素方差分析及独立样本t检验进行组间比较,P<0.05为差异有统计学意义。
2. 结果
2.1. 肺腺癌顺铂耐药细胞建立、耐药特征及ABCG2表达
细胞生长抑制率曲线见图1A。CDDP作用A549/CDDP细胞24 h的IC50高于其亲代细胞A549〔(26.32±2.93) μg/mL vs. (3.67±0.20) μg/mL,P<0.01〕。A549/CDDP细胞对CDDP的耐药指数为7.17。ABCG2 mRNA在A549/CDDP细胞中的表达水平(0.46±0.05)高于A549细胞(0.20±0.01),差异有统计学意义(P<0.01)。
图 1.

Growth inhibition rate curves of different lung adenocarcinoma cells treated with CDDP for 24 h (n=3)
顺铂作用不同肺腺癌细胞24 h的生长抑制率曲线图(n=3)
A: A549 and A549/CDDP; B: A549, A549/PCDNA3.1, and A549/ABCG2; C: A549-EVs1and A549-EVs2.
2.2. A549/ABCG2细胞耐药性和ABCG2表达
细胞生长抑制率曲线见图1B。CDDP对A549/ABCG2细胞24 h的IC50值〔(36.92±1.72) μg/mL〕高于对A549 〔(3.67±0.20) μg/mL〕及对A549/pCDNA3.1细胞〔(3.73±0.07) μg/mL〕(P<0.01),而CDDP对A549细胞与对A549/pCDNA3.1细胞24 h的IC50值差异无统计学意义。A549/pCDNA3.1及A549/ABCG2细胞对CDDP耐药指数分别为1.02和10.06。A549/ABCG2细胞中ABCG2 mRNA表达水平(0.68±0.04)高于其亲代细胞A549 (0.20±0.01)及空载体细胞A549/pCDNA3.1 (0.21±0.02)(P<0.01),而后两者中ABCG2 mRNA的表达水平差异无统计学意义。
2.3. EVs干预肺腺癌A549细胞后耐药性和ABCG2表达
细胞生长抑制率曲线见图1C。CDDP干预A549-EVs1 24 h的IC50值低于A549-EVs2细胞〔(4.38±0.11) μg/mL vs. (19.82±0.64) μg/mL,P<0.01〕。A549-EVs1及 A549-EVs224 h对CDDP的耐药指数分别为1.19和5.40。ABCG2 mRNA在EVs2中的表达水平(0.57±0.04)高于EVs1(0.25±0.01)(P<0.01),在A549-EVs2细胞中的表达水平(0.41±0.03)高于A549-EVs1(0.23±0.02)(P<0.01)。后续实验选择A549-EVs2细胞进行。
2.4. 荷瘤裸鼠实验检测EVs调控肺腺癌细胞耐药
肺腺癌细胞(A549及A549-EVs2)接种裸鼠皮下形成皮下移植瘤, 1周时肉眼已可见实验组的裸鼠皮下移植瘤体积大于对照组,见图2A。与对照组相比,第1周、第2周,实验组皮下移植瘤体积均增大(P<0.05),见图2B。实验组皮下移植瘤细胞中ABCG2 mRNA表达水平(0.43±0.04)高于对照组(0.22±0.03)(P<0.01)。实验组皮下移植瘤细胞凋亡率(9.50%±1.41%)低于对照组(19.03%±2.43%)(P<0.01),见图2C。
图 2.

EVs’ regulation of multidrug resistance of lung adenocarcinoma and its mechanism were investigated in tumor-bearing nude mice
荷瘤裸鼠实验研究EVs调控肺腺癌多药耐药作用及机制
A: Nude mouse model of lung adenocarcinoma subcutaneously transplanted tumor (1 week); B: The growth rate of subcutaneously transplanted tumor in the experimental group was significantly faster than that in the control group, *P<0.05,n=6; C: The apoptosis rate of subcutaneous transplanted tumor cells was detected by flow cytometry.
3. 讨论
ABC转运蛋白与肿瘤细胞耐药产生密切相关[4-11],其中以ABCB1及ABCG2研究较多[10-11, 16-20]。本文首先研究ABCG2是否参与肺腺癌耐药,肺腺癌耐药细胞A549/CDDP及A549/ABCG2的耐药指数和ABCG2表达结果显示,耐药性增加的同时,ABCG2表达增加。本小组在以往的研究中发现ABCG2基因与食管癌耐药密切相关[12],ABCG2蛋白可以外排进入食管癌细胞中的化疗药物,引起细胞内有效药物浓度的降低,从而导致耐药;EMERY等[16]研究显示,ABCG2及XIAP基因高表达的脑胶质瘤患者预后较差,参与了脑胶质瘤耐药的形成;JI等[17]研究显示,Selonsertib(GS-4997)通过抑制ABCB1及ABCG2外排药物作用而逆转细胞耐药。本研究结果支持研究假设,且与以上文献报道结果一致,显示了ABCG2参与肿瘤细胞的耐药。接下来,本研究欲揭示肺腺癌耐药细胞通过何种机制传递耐药信息。
肿瘤微环境中EVs对于肿瘤侵袭、转移及耐药具有重要的调控作用[21-24]。EVs是细胞遭受刺激时产生并释放的超微囊性结构,是细胞旁分泌的生物活性物质,包括外泌体、微泡和凋亡小体等。来源于肿瘤细胞的EVs,在其形成过程中会功能性选择与其亲代细胞相关的核酸、蛋白质等信号分子,这些信号分子在EVs与靶细胞相互作用后被释放到靶细胞中,从而改变靶细胞的特性。我们设想,肺腺癌耐药细胞是否通过EVs传递耐药信息?本研究利用差速离心法提取肺腺癌耐药细胞A549/CDDP释放的EVs2,检测到A549/CDDP释放的EVs2中ABCG2表达水平显著高于其亲代细胞(A549)释放的EVs1中的表达。我们推测,EVs2携带参与耐药的ABCG2基因信息,当EVs2作用肺腺癌A549细胞后,A549细胞产生耐药,并同时表现出ABCG2基因信息的表达升高。较多的文献显示,EVs参与肿瘤耐药的产生:OZAWA等[25]研究显示,来源于三阴性乳腺癌HCC1806细胞的EVs促进了正常乳腺细胞MCF10A的增殖和耐药产生;GOLER-BARON等[26]研究发现,富集ABCG2的EVs可以引起乳腺癌细胞耐药;HE等[27]亦发现,对索拉非尼耐药的肾癌细胞释放的EVs中高表达miR-31-5p,高表达miR-31-5p的EVs可以下调靶细胞中MutL homolog 1 (MLH1)基因表达,将索拉非尼耐药细胞的耐药信息传递给敏感细胞,从而将耐药信息放大到整个肿瘤;同时,本课题组研究显示,携带linc-VLDLR的EVs可以通过调控靶细胞中ABCG2的表达,从而引起食管癌细胞耐药[13]。本研究结果支持我们的推测,且同上述文献报道的结果相一致,这提示,携带ABCG2的EVs可以将耐药信息传递给靶细胞,引起细胞耐药。
综上所述,本研究体内外实验显示,携带 ABCG2的EVs通过调控靶细胞中ABCG2表达从而引起肺腺癌细胞耐药,为肺腺癌耐药研究提供新思路,为研究EVs调控肿瘤耐药作用提供基础数据。但是,肿瘤耐药产生是由多基因、多途径调控,本文仅研究了携带ABCG2的EVs对肺腺癌细胞耐药的调控,在今后的实验中会进一步研究EVs调控肿瘤耐药的分子机制、EVs中非编码RNA对肿瘤耐药的调控机制,以及EVs作为纳米颗粒在肿瘤治疗及诊断中的作用。
* * *
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
河北省政府资助专科能力建设和专科带头人培养专项基金[冀财预复(2018)674]和河北省医学科学研究重点课题(No. 20210765、No. 20210987)资助
Contributor Information
雷 王 (Lei WANG), Email: yuankundu@163.com.
亮 刘 (Liang LIU), Email: aliangdaziran@163.com.
References
- 1.ZAPPA C, MOUSA S A Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.陈万青, 郑荣寿, 张思维, 等 2013年中国恶性肿瘤发病和死亡分析. 中国肿瘤. 2017;26(1):1–7. [Google Scholar]
- 3.曹毛毛, 陈万青 中国恶性肿瘤流行情况及防控现状. 中国肿瘤临床. 2019;46(3):145–149. doi: 10.3969/j.issn.1000-8179.2019.03.283. [DOI] [Google Scholar]
- 4.CHOI Y, YU A ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BOESCH M, ZEIMET A, RUMPOLD H, et al Drug transporter-mediated protection of cancer stem cells from ionophore antibiotics. Stem Cells Transl Med. 2015;4(9):1028–1032. doi: 10.5966/sctm.2015-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BRUHN O, CASCORBI I Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin Drug Metab Toxicol. 2014;10(10):1337–1354. doi: 10.1517/17425255.2014.952630. [DOI] [PubMed] [Google Scholar]
- 7.XU J, PENG H, ZHANG J Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14(6):689–701. doi: 10.2174/092986707780059580. [DOI] [PubMed] [Google Scholar]
- 8.CHEN Y, BIEBER M, TENG N Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog. 2014;53(8):625–634. doi: 10.1002/mc.22015. [DOI] [PubMed] [Google Scholar]
- 9.HAN J, LIM H, YOO Y, et al Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110(1):138–147. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 10.HASANABADY M, KALALINIA F ABCG2 inhibition as a therapeutic approach for overcoming multidrug resistance in cancer. J Biosci. 2016;41(2):313–324. doi: 10.1007/s12038-016-9601-5. [DOI] [PubMed] [Google Scholar]
- 11.LEPPER E, NOOTER K, VERWEIJ J, et al Mechanisms of resistance to anticancer drugs: The role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6(2):115–138. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 12.WANG L, LIU L, CHEN Y, et al Correlation between adenosine triphosphate (ATP)-binding cassette transporter G2 (ABCG2) and drug resistance of esophageal cancer and reversal of drug resistance by artesunate. Pathol Res Pract. 2018;214(9):1467–1473. doi: 10.1016/j.prp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 13.CHEN Y, LIU L, LI J, et al Effects of long noncoding RNA (linc-VLDLR) existing in extracellular vesicles on the occurrence and multidrug resistance of esophageal cancer cells. Pathol Res Pract. 2019;215(3):470–477. doi: 10.1016/j.prp.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 14.GIULIA C, DEMETRA P, INES K, et al. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol Cancer, 2018, 17(1): 145[2021-05-04]. https://doi.org/10.1186/s12943-018-0886-x.
- 15.KENJI T, IRENE K, JOSEPH W, et al Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EMERY I, GOPALAN A, WOOD S, et al Expression and function of ABCG2 and XIAP in glioblastomas. J Neurooncol. 2017;133(1):47–57. doi: 10.1007/s11060-017-2422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.JI N, YANG Y, CAI C, et al. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1- and ABCG2-overexpressing cancer cells. Cancer Lett, 2019, 440-441: 82-93[2021-05-04]. https://doi.org/10.1016/j.canlet.2018.10.007.
- 18.XIE Z, LV K, XIONG Y, et al ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from colon cancer. Oncol Res Treat. 2014;37(11):666–668. doi: 10.1159/000368842. [DOI] [PubMed] [Google Scholar]
- 19.HUANG L, LU Q, HAN Y, et al. ABCG2/V-ATPase was associated with the drug resistance and tumor metastasis of esophageal squamous cancer cells. Diagn Pathol, 2012, 7: 180[2021-05-04]. https://doi.org/10.1186/1746-1596-7-180.
- 20.DAS S, MUKHERJEE P, CHATTERJEE R, et al Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol Cancer Ther. 2019;18(3):680–692. doi: 10.1158/1535-7163.MCT-18-0409. [DOI] [PubMed] [Google Scholar]
- 21.SOYSAL S, TZANKOV A, MUENST S Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82(3/4):142–152. doi: 10.1159/000430499. [DOI] [PubMed] [Google Scholar]
- 22.ALTORKI N, MARKOWITZ G, GAO D, et al The lung microenvironment: An important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19(1):9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MAACHA S, BHAT A, JIMENEZ L, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer, 2019, 18(1): 55[2021-05-04]. https://doi.org/10.1186/s12943-019-0965-7.
- 24.SUN Y Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016;380(1):205–215. doi: 10.1016/j.canlet.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 25.OZAWA P, ALKHILAIWI F, CAVALLI I, et al Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res Treat. 2018;172(3):713–723. doi: 10.1007/s10549-018-4925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GOLER V, ASSARAF Y. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS One, 2011, 6(1): e16007[2021-05-04]. https://doi.org/10.1371/journal.pone.0016007.
- 27.HE J, MIN L, HE Y, et al Extracellular vesicles transmitted miR-31-5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma. Int J Cancer. 2020;146(4):1052–1063. doi: 10.1002/ijc.32543. [DOI] [PubMed] [Google Scholar]


