To the Editor
A 71-year-old man with progressive dysphagia (from solids and subsequently to liquids) was referred to our clinic for orthopedic evaluation. He complained of weight loss due to dysphagia. Currently, he has been diagnosed with gastritis and duodenal diverticulum in other related departments. However, none of these diseases can completely explain his progressive symptoms. He had to be referred to us for further help because he had undergone percutaneous radiofrequency ablation of the cervical intervertebral disc 1 year prior due to cervical disc herniation in another hospital. Considering that new dysphagia may occur after cervical spine surgery [1], we admitted the patient to our department for further evaluation.
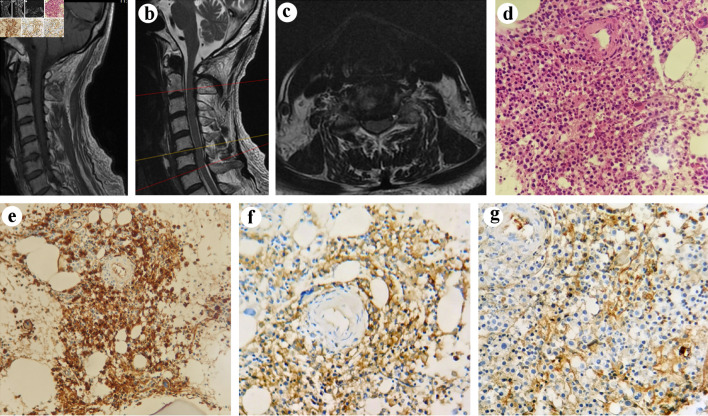
The physical examination on admission was unremarkable. He had normal sensory and manual muscles. Regarding neurological examinations, no abnormal findings were found, including cranial nerve function, deep tendon reflex, or pathologic reflex tests. His laboratory studies of arrival showed (normal reference ranges are given in parenthesis) white blood cell count of 10.9 × 109/L (3.5 - 9.5 × 109/L); neutrophil percentage 81.5% (40-75%); lymphocyte percentage 9.7% (20-50%); monocyte percentage 8.3% (3-10%); hemoglobin 109 g/L (130 - 175 g/L); total serum protein 68.1 g/L (65 - 85 g/L); albumin 29.4 g/L (40 - 55 g/L); and globulin 38.7 g/L (20 - 40 g/L). Magnetic resonance imaging (MRI) (Fig. 1a-c) revealed a mildly herniated cervical disc at C6 - C7 without significant deformation of the spinal cord and large cervical osteophyte, which can preliminarily preclude the reason of cervical spondylosis [2]. However, the possibility of nerve disorders was still kept in mind, and the patient was routinely initiated on methylprednisolone, mannitol and mecobalamin for 5 days. However, he continued to have persistent dysphagia without any relief. Then, we realized that we may have a wrong diagnosis. Therefore, we consulted with neurologists as well as later hematologists and performed additional laboratory tests. Further evaluations showed elevated serum kappa free light chains of 8 (1.7 - 3.7 g/L), elevated serum kappa/lambda ratio of 12.365 (1.35 - 2.65), elevated immunoglobulin (Ig)G of 18 g/L (7 - 16 g/L), IgA 0.732 g/L (0.7 - 4.0 g/L), reduced IgM 0.216 g/L (0.4 - 2.3 g/L), and IgE 19.4 IU/mL (0 - 100 IU/mL). Urine immunoelectrophoresis revealed elevated kappa free light chains of 425 mg/L (0 - 7.1 mg/L). However, he did not have Benee Jones proteinuria. Based on these laboratory studies indicating myeloma, the patient was further transferred to the Hematology Department for definitive diagnosis and management. Bone marrow aspiration and biopsy confirmed multiple myeloma (MM) (27% plasma cells). Histopathologic examination of the specimens obtained from the bone marrow showed hyperplastic plasma cells involved in the trabecular bone (Fig. 1d). Immunohistochemically, plasma cells revealed a positive reaction with CD38, CD138, kappa light chain, and lambda light chain and a negative reaction with CD20 in the bone marrow (Fig. 1e-g). Based on the clinical and pathological information, the patient was finally diagnosed with MM of IgG kappa subtype, and chemotherapy regimen of DCD was then applied (daratumumab 16 mg/kg on days 1, 8, 15, 22, cyclophosphamide 300 mg/m2 on days 1, 8, 15, 22, dexamethasone 20 mg on days 1 - 2, 8 - 9, 15 - 16, 22 - 23). After administration of the chemotherapy regimen, his dysphagia was relieved.
Figure 1.
MRI and pathological images of a 71-year-old male with MM after cervical spine surgery. Tl-weighted scan (a) and T2-weighted gradient echo scan (b) of cervical spine showed the morphology of the bone and spinal cord. Axial T2-weighted MRI of cervical spine showed C5/6 intervertebral disc protrusion (c). (d) The morphology of the tumor cells (H&E × 400). (e-g) Immunohistochemical findings: (e) the tumor cells expressing CD38 (original magnification × 400) and (f) kappa (original magnification × 400), with (g) lambda (original magnification × 400) rarely expressed. MRI: magnetic resonance imaging; MM: multiple myeloma; H&E: hematoxylin and eosin stain.
Anterior cervical decompression and fusion are the most commonly used approaches for cervical disc herniation to achieve decompression and fusion by means of autografts or cages [3, 4]. Dysphagia after cervical spine surgery, regardless of whether the anterior or posterior approach is used, is a common complication, and its risk factors include female sex, advanced age, multilevel surgery, longer operating time and severe preoperative neck pain [1, 5]. Normally, dysphagia following cervical spine surgery is transient; however, some new dysphagia can still occur at 1 year post surgery, especially in patients who undergo an anterior approach [1]. Percutaneous radiofrequency ablation of herniation is another minimally invasive approach for the treatment of disc herniation. In contrast to fusion surgery, the procedure only requires a needle to puncture into the disc space under the guidance of X-ray [6]. However, most orthopedic surgeons are unfamiliar with this procedure and remain confused with its safety, which might lead to misdiagnosis in the current setting. Furthermore, a few cases of MM with the presentation of dysphagia have been reported [7, 8]. However, MM presenting as dysphagia after cervical spine surgery is rarely reported.
MM, a neoplasm of clonal plasma cells originating from the postgerminal lymphoid B-cell lineage, is a common hematologic malignancy and tends to be present in the patients approximately at the age of 66 - 70 years [9]. Accordingly, neoplastic cells in bone marrow tend to express plasma cell antigens, such as CD19, CD38, and CD138, but not CD20 [10]. Immunotherapy, such as targeting CD38, has emerged as a powerful tool for the treatment of MM [10, 11]. The neoplastic cells in our case showed positivity for CD38 and CD138 and a negative reaction with CD20, which is in accordance with its pathogenesis, and showed improvement after administration of daratumumab, a monoclonal antibody against the cell surface antigen CD38. Our case was unique in that the tumor complicated dysphagia after cervical spine surgery. Therefore, MM may become an unexpected cause of dysphagia after cervical spine surgery. It is particularly important to recognize the possibility of MM after cervical spine surgery in old patients presenting with dysphagia, as it may mimic a common complication of cervical spine surgery.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the Natural Science Foundation of Shandong Province (grant no.ZR2023QH517).
Conflict of Interest
The authors declared no conflict of interest.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
CJC and LFC participated in the drafting, writing, and revising of the manuscript. YW, GHZ, YJR, YBQ, and YGW participated in the data selection and analysis. CJC, KNZ, and YY contributed to the study concept and acquired and analyzed the data. All authors contributed to the drafting of the manuscript and figure preparation.
Data Availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Sakai K, Yoshii T, Arai Y, Hirai T, Torigoe I, Inose H, Tomori M. et al. A prospective cohort study of dysphagia after subaxial cervical spine surgery. Spine (Phila Pa 1976) 2021;46(8):492–498. doi: 10.1097/BRS.0000000000003842. [DOI] [PubMed] [Google Scholar]
- 2.Kapetanakis S, Vasileiadis I, Papanas N, Goulimari R, Maltezos E. Can a giant cervical osteophyte cause dysphagia and airway obstruction? A case report. Wien Klin Wochenschr. 2011;123(9-10):291–293. doi: 10.1007/s00508-011-1564-9. [DOI] [PubMed] [Google Scholar]
- 3.Mazas S, Benzakour A, Castelain JE, Damade C, Ghailane S, Gille O. Cervical disc herniation: which surgery? Int Orthop. 2019;43(4):761–766. doi: 10.1007/s00264-018-4221-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Gao X, Li H, Pan X, Wang S. Intravertebral insertion of interbody fusion cage via transpedicular approach for the treatment of stage III Kummell disease: a technical note and case presentation. Br J Neurosurg. 2021:1–6. doi: 10.1080/02688697.2021.1892590. [DOI] [PubMed] [Google Scholar]
- 5.Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery: a systematic review. Bone Joint J. 2013;95-B(7):868–873. doi: 10.1302/0301-620X.95B7.31029. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Yan DL, Zhang ZH. Percutaneous cervical nucleoplasty in the treatment of cervical disc herniation. Eur Spine J. 2008;17(12):1664–1669. doi: 10.1007/s00586-008-0786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez J, Wahab A, Kesari K. Dysphagia unveiling systemic immunoglobulin light-chain amyloidosis with multiple myeloma. BMJ Case Rep. 2018;2018:bcr-2018-226331. doi: 10.1136/bcr-2018-226331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortson JK, Nuriddin A, McCarter F, Henderson VJ, Patel V. Multiple myeloma in a patient with hoarseness, dysphagia, aspiration, and cervical lymphadenopathy. Ear Nose Throat J. 2004;83(4):274. [PubMed] [Google Scholar]
- 9.Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016;43(6):676–681. doi: 10.1053/j.seminoncol.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Donk N, Usmani SZ, Yong K. CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. 2021;8(6):e446–e461. doi: 10.1016/S2352-3026(21)00057-0. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith SR, Foley N, Schroeder MA. Daratumumab for the treatment of multiple myeloma. Drugs Today (Barc) 2021;57(10):591–605. doi: 10.1358/dot.2021.57.10.3313853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.