Abstract
Aims
To investigate the relationship between uric acid to high-density lipoprotein cholesterol ratio (UHR) levels and nonalcoholic fatty liver disease (NAFLD) in nonoverweight/obese patients with type 2 diabetes.
Methods
A retrospective study was designed including a total of 343 inpatients with type 2 diabetes whose BMI<24 kg/m2. The population was divided into three groups as the UHR tertiles. Logistic regression analysis was performed to estimate odds ratios (ORs) of UHR for NAFLD. ROC curve analysis was used to estimate the diagnostic value of UHR for NAFLD.
Results
The prevalence rat of NAFLD enhanced progressively from the tertile 1 to tertile 3 of UHR (30.70% vs. 56.52% vs. 73.68%). Logistic regression analysis showed that participants in the higher UHR groups, compared with those in the first tertile group, had higher occurrence risks for NAFLD. The positive association between UHR and NAFLD was independent of age, BMI, blood pressure, hepatic enzymes, and other components of metabolic disorders. ROC curve analysis showed that the area under curve (AUC), sensitivity, and specificity for UHR were 0.697, 0.761, and 0.553, respectively.
Conclusions
In type 2 diabetic patients without overweight or obesity, UHR is significantly associated with NAFLD and can be used as a novel and useful predictor for NAFLD onset.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) has become one of the most frequent liver diseases with increased morbidity which may be up to 25% in general population globally [1]. The pathological mechanism of NAFLD is characterized by abnormal lipid deposition in hepatic cells, excluding excessive drinking, drugs, and other chronic liver disease such as viral hepatitis [2]. NAFLD can cause a large spectrum of hepatic lesions starting with simple liver steatosis [3]. Then, a complex pathological process is triggered involving chronic hepatitis, hepatic fibrosis, and liver cellular injury, eventually developing to liver cirrhosis, even to liver failure and hepatic carcinoma [4]. In addition, the health damages of NAFLD also spread to extra-hepatic organs. NAFLD has been confirmed to be positively associated with many chronic diseases such as atherosclerotic cardiovascular disease (ASCVD) [5], chronic kidney disease [5], and cancers [6]. The association between NAFLD and type 2 diabetes (T2DM) has also been well revealed [7]. Subjects with NAFLD are more likely to suffer from type 2 diabetes than those without NAFLD [8]; meanwhile, NAFLD is fairly common in type 2 diabetic individuals, affecting 28–70% of the population [9]. More importantly, coexistent NAFLD significantly increases the morbidity and mortality of diabetic complications including cardiovascular events [10], diabetes nephropathy, and diabetes retinopathy [11]. In addition, liver fat accumulation has been indicated to be relevant to reduced insulin sensitivity which could aggravate the metabolism disorders of glucose and lipids [12]. The huge influences of NAFLD on health require an early identification of NAFLD in type 2 diabetic patients.
Most of the patients with T2DM are accompanied by overweight or obesity which is widely known as the major risk element for the onset and development of NAFLD [13]. In type 2 diabetics, the presence of obesity was highly suggested to sift for NAFLD according to EASL guideline [14]. However, our previous study showed that NAFLD was also common in nonobese type 2 diabetic patients, and the degree of metabolic disorders and insulin resistance was more serious in patients with both T2DM and NAFLD [15]. Nonetheless, the study on the NAFLD, in type 2 diabetic patients without overweight or obesity, is limited. The screening predictors for NAFLD in those patients have not been well clarified. In some previous research studies, serum uric acid was found to be positively associated with the NAFLD risk independent of components of metabolic syndrome in diabetic populations [16, 17]. HDL cholesterol is an important part of plasma lipid profile. Decreased serum HDL-cholesterol levels have been indicated to be associated with a worse metabolic status [18]. A combination of these two metabolic factors is serum uric acid to HDL-cholesterol ratio (UHR) which has recently attracted increasing attentions as a valuable biomarker for metabolic disorders [19], incident ischemic heart disease [20], and diabetic control [21]. A small amount of studies have found a strong relationship between UHR and nonalcoholic fatty liver disease (NAFLD) [22, 23]; however, no study has concerned the association between UHR and NAFLD in the type 2 diabetic population, especially in nonoverweight/obese diabetic subjects. Thus, we aimed to examine whether UHR was independently correlative with the prevalence of NAFLD in type 2 diabetic patients without overweight or obesity.
2. Materials and Methods
2.1. Research Population
The objects collected in our research were patients hospitalized in the Endocrine Department, Qilu Hospital of Shandong University Dezhou Hospital, from 2016 to 2022. The participants were selected with these criteria: (1) individuals with type 2 diabetes whose BMI was not more than 24 kg/m2 according to the guidelines for overweight/obesity in China [24] in which the criteria of overweight and obesity were BMI ≥ 24 kg/m2 and BMI ≥ 28 kg/m2, respectively, for Chinese adults, (2) those without large quantities of alcohol intake (alcohol intake<30 g/d for males and<20 g/d for females), and (3) those without a history of other hepatic diseases including viral hepatitis and autoimmune hepatitis. The exclusive criteria were as follows: (1) those with seriously acute or chronic complications such as diabetes ketoacidosis, infection, myocardial infarction, diabetic gangrene, cardiac insufficiency, and kidney failure, (2) those with stringent state, gestation, trauma, and other situations affecting the results, and (3) those using any medicine that can affect the levels of uric acid and HDL cholesterol such as benzbromarone, febrista, statins, and insulin. Ultimately, 343 inpatients were enrolled in the investigation.
We preformed this research in accordance with the regulations of the Helsinki Declaration. Ethical approval was obtained for this cross-sectional study from the Ethics Committee of Qilu Hospital of Shandong University Dezhou Hospital. Written informed consents were provided by all participants.
2.2. Physical Measurements and Biochemical Examinations
The anthropometric indicators such as height, body weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured following the standardized approaches. The body mass index (BMI) was calculated as weight (kg)/height (m2). The general information of the population including age, gender, and medical history was recorded carefully. Fasted blood samples were collected after overnight (for more than 8 hours) and were detected in the department of clinical laboratory, using the automatic biochemical analyzer. The biochemical parameters including serum uric acid (SUA), alanine aminotransferase(ALT), aspartate transferase(AST), γ-glutamyl transpeptidase (γ-GGT), high-density lipoprotein cholesterol (HDL), fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL), blood urea nitrogen (BUN), and serum creatinine (Cr) were measured and recorded by trained technicians. Serum fasting insulin (FIN) was measured with chemiluminescence assay. UHR was obtained dividing serum uric acid levels by HDL-cholesterol levels. Homeostasis model assessment of insulin resistance (HOMA-IR) was used to assess the insulin resistance, which was calculated as fasting insulin (uIU/mL) × fasting glucose (mmol/L)/22.5.
2.3. Examination of Fatty Liver
Liver biopsy was not selected due to its invasive characteristic, and the imaging tool was used to determine the presence of fatty liver based on previous study [22]. An ultrasound scan of the liver was performed for each participant in the department of ultrasound. The diagnosis of NAFLD was based on typical image changes according to the criteria established by the Chinese Association of Liver Disease [25]: (1) the ultrasound beam enhancement in liver antefield, (2) ultrasound beam attenuation in liver far field, and (3) poor visualization of intrahepatic structures, after excluding other forms of hepatic diseases.
2.4. Statistical Analyses
Quantitative variables corresponding to normal distribution were displayed by means ± standard deviations, and those with skewed distribution were presented by medians (intertertile ranges). Qualitative data were expressed as numbers and frequencies. The population was divided into NAFLD group and non-NAFLD group. Independent sample T test (for normal distribution) and Wilcoxon rank sum test (for skewed distribution) were used to assess differences between these two groups. To evaluate the relationship between UHR and NAFLD, all individuals were categorized into three groups according to the tertiles of UHR: UHR tertile 1, <211.21 μmol/mmol; UHR tertile 2, 211.21–295.04 μmol/mmol; and UHR tertile 3, >295.04 μmol/mmol. The comparisons of NAFLD incidences among the tertiles were evaluated by the chi-square test. Logistic regression analysis was conducted to investigate the association of UHR tertiles with the risk of NAFLD by building three test models (model 1: without adjustment; model 2: adjusting for age, gender, BMI, SBP, and DBP; and model 3: adjusting for age, gender, BMI, SBP, DBP, FIN, ALT, AST, γ-GGT, TC, TG, LDL, FBG, BUN, Cr, and HOMA-IR), and the unadjusted and adjusted odds ratios (ORs) as well as 95% confidence intervals were given. Furthermore, the receiver operator characteristic (ROC) curve analysis was applied to estimate the function of UHR for the detection of NAFLD, and the susceptibility and specificity as well as cut-off point were calculated. All analyses were conducted using SPSS 21.0. The P values (two-sided) less than 0.05 were supposed to be statistically significant.
3. Results
3.1. General Characteristics of Research Subjects
There were 209 males and 134 females among these 343 type 2 diabetic participants with average age of 50.96 years and median BMI of 22.68 kg/m2. The total prevalence of NAFLD was 53.6% (184 persons). Physical and biochemical indexes on the basis of the presence of NAFLD are presented in Table 1. Patients with NAFLD were more likely to be heavier and had significantly enhanced levels of SBP, DBP, FIN, ALT, AST, γ-GGT, TC, TG, FBG, LDL-C, SUA, and HOMA-IR and decreased levels of HDL compared to those without NAFLD. Moreover, sensibly higher UHR levels were observed in individuals with NAFLD than in non-NAFLD subjects (294.80 ± 99.90 versus 228.67 ± 88.08, P < 0.001) (Table 1).
Table 1.
Clinical and biochemical characteristics of study subjects and the differences of factors between patients with non-NAFLD and NAFLD.
| General indexes | All patients (n = 343) | NAFLD (n = 184) | Non-NAFLD (n = 159) | T/Z | P |
|---|---|---|---|---|---|
| Age (yr) | 50.96 ± 11.67 | 50.85 ± 11.70 | 51.08 ± 11.66 | 0.18 | >0.05∗ |
| BMI (kg/m2) | 22.68 (21.80–23.30) | 22.94 (22.20–23.45) | 22.21 (20.70–22.96) | −5.72 | <0.001∗ |
| SBP (mmHg) | 131.94 ± 17.68 | 135.19 ± 17.88 | 128.18 ± 16.74 | −3.71 | <0.001∗ |
| DBP (mmHg) | 81.89 ± 11.55 | 84.15 ± 11.14 | 79.27 ± 11.49 | −3.97 | <0.001∗ |
| FINS (pmol/l) | 29.54 (16.76–47.05) | 33.93 (20.24–54.50) | 24.02 (13.23–39.97) | −3.99 | <0.001∗ |
| ALT (IU/L) | 19.4 (15–30.45) | 24.5 (16.8–36.8) | 16.9 (13–21.83) | −5.88 | <0.001∗ |
| AST (IU/L) | 20.07 (16.3–25.7) | 22 (17.4–29.1) | 18.4 (15.08–23.33) | −4.15 | <0.001∗ |
| γ-GGT (IU/L) | 29.25 (19.48–44.08) | 33.6 (23.08–50.63) | 22 (16.28–38.08) | −5.13 | <0.001∗ |
| TC (mmol/L) | 5.14 ± 1.77 | 5.35 ± 2.10 | 4.89 ± 1.25 | −2.42 | <0.05∗ |
| TG (mmol/L) | 1.30 (0.87–1.93) | 1.68 (1.22–2.46) | 0.94 (0.72–1.35) | −8.85 | <0.001∗ |
| HDL (mmol/L) | 1.15 ± 0.27 | 1.09 ± 0.23 | 1.22 ± 0.29 | 4.57 | <0.001∗ |
| LDL (mmol/L) | 3.21 ± 0.89 | 3.32 ± 0.84 | 3.08 ± 0.92 | −2.54 | <0.05∗ |
| FBG (mmol/L) | 7.92 (6.26–10.28) | 8.53 (6.59–10.80) | 7.30 (6.01–9.93) | −2.72 | <0.01∗ |
| BUN (mmol/L) | 5.30 ± 1.47 | 5.21 ± 1.41 | 5.41 ± 1.54 | 1.22 | >0.05∗ |
| Cr (μmol/L) | 63.88 ± 18.23 | 65.42 ± 16.01 | 62.11 ± 20.41 | −1.67 | >0.05∗ |
| SUA (μmol/L) | 287.58 ± 82.82 | 307.48 ± 80.22 | 264.56 ± 80.00 | −4.94 | <0.001∗ |
| HOMA-IR | 10.44 (5.53–18.32) | 12.99 (6.96–20.97) | 7.45 (4.30–13.62) | −4.69 | <0.001∗ |
| UHR (μmol/mmol) | 264.14 ± 100.07 | 294.80 ± 99.90 | 228.67 ± 88.08 | −6.45 | <0.001∗ |
SBP: systolic pressure; DBP: diastolic pressure; FINS: fasting insulin ALT: alanine aminotransferase; AST: aspartate transferase; γ-GGT: γ-glutamyl transpeptidase; TC: total cholesterol; TG: triglycerides; HDL: high-density lipoprotein; LDL: low-density lipoprotein; FBG: fasting blood glucose; BUN: blood urea nitrogen; Cr: creatinine; SUA: serum uric acid; UHR: uric acid to HDL cholesterol ratio; HOMA-IR: insulin resistance index. The t test (for normal distribution) and Wilcoxon rank sum test (for skewed distribution) with different samples were adopted for comparison between groups. ∗P < 0.05 was considered as statistically significant difference.
3.2. Correlation between UHR and the NAFLD Risk
The NAFLD prevalence rat in UHR tertile 1 group was 30.70% and increased to 56.52% and 73.68% in the tertile 2 and tertile 3 groups, respectively (Table 2). The unadjusted ORs for NAFLD in the tertile 2 group and tertile 3 group were 2.93 (95% CI: 1.770–5.04) and 6.32 (95% CI: 3.55–11.24) compared with tertile 1 (Table 3). After adjusting for gender, age, BMI, SBP, and DBP (model 2), the ORs for NAFLD in tertile 2 and tertile 3 were 2.58 (95% CI: 1.42–4.71) and 6.32 (95% CI: 3.24–12.32) (Table 3). With further adjustment for gender, age, BMI, SBP, DBP, FIN, ALT, AST, γ-GGT, TC, TG, LDL, FBG, BUN, Cr, and HOMA-IR (model 3), the ORs of NAFLD remained significantly increased for tertile 2 (OR = 2.17, 95% CI: 1.06–4.48) and tertile 3 (OR = 3.73, 95% CI: 1.53–9.09) (Table 3).
Table 2.
Differences of the prevalence rat of NAFLD among UHR tertiles.
| UHR quartile | Total | NAFLD | Prevalence rat (%) | X 2 | P value |
|---|---|---|---|---|---|
| Quartile 1 | 114 | 35 | 30.70 | ||
| Quartile 2 | 115 | 65 | 56.52 | ||
| Quartile 3 | 114 | 84 | 73.68 | 42.924 | <0.001∗ |
UHR: uric acid to HDL cholesterol ratio. UHR tertile 1, <211.21 μmol/mmol; UHR tertile 2, 211.21–295.04 μmol/mmol; UHR tertile 3, >295.04 μmol/mmol. The chi-square test was used to evaluate the comparisons in NAFLD prevalence among the tertiles. ∗P < 0.05 was considered as statistically significant difference.
Table 3.
Summary of regression analysis of the correlation between UHR quartiles and NAFLD.
| Quartile 1 (n = 114) | Quartile 2 (n = 115) | Quartile 3 (n = 114) | |
|---|---|---|---|
| Model 1 P |
1 | 2.93 (1.770–5.04) <0.001∗ |
6.32 (3.55–11.24) <0.001∗ |
| Model 2 P |
1 | 2.58 (1.42–4.71) <0.01∗ |
6.32 (3.24–12.32) <0.001∗ |
| Model 3 P |
1 | 2.17 (1.06–4.48) <0.05∗ |
3.73 (1.53–9.09) <0.01∗ |
UHR: uric acid to HDL cholesterol ratio. UHR tertile 1, <211.21 μmol/mmol; UHR tertile 2, 211.21–295.04 μmol/mmol; UHR tertile 3, >295.04 μmol/mmol. Model 1: unadjusted analyses. Model 2: adjusted for gender, age, BMI, SBP, and DBP; Model 3: adjusted for gender, age, BMI, SBP, DBP, FIN, ALT, AST, γ-GGT, TC, TG, LDL, FBG, BUN, Cr, and HOMA-IR.
3.3. The Detective Ability of UHR for NAFLD
ROC curve analysis was used to assess the detective ability of UHR for NAFLD occurrence. The area under curve (AUC) of UHR was 0.697 which was higher than that of SUA (0.661) and HDL (0.637) (Figure 1). The sensitivity and specificity of UHR were 0.761 and 0.553, respectively (Table 4). The Youden index and cut-off point of UHR were 0.314 and 222.26, respectively (Table 4).
Figure 1.
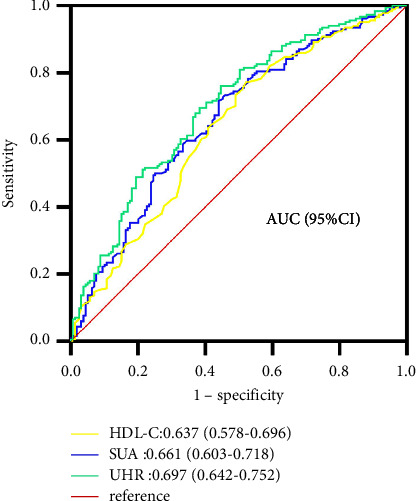
ROC curves for UHR, compared to SUA and HDL-C alone. The indicative ability of UHR is greater compared to that of SUA or HDL-C alone according to its AUC.
Table 4.
ROC curve analysis for UHR, SUA, and HDL-C.
| Factor | AUC | 95% CI | Sensitivity | Specificity | Youden index | Cut-off point | P value |
|---|---|---|---|---|---|---|---|
| UHR | 0.697 | 0.642–0.752 | 0.761 | 0.553 | 0.314 | 222.26 | <0.001∗ |
| SUA | 0.661 | 0.603–0.718 | 0.717 | 0.559 | 0.277 | 259.35 | <0.001∗ |
| HDL-C | 0.637 | 0.578–0.696 | 0.478 | 0.772 | 0.249 | 1.22 | <0.001∗ |
UHR: uric acid to HDL cholesterol ratio; SUA: serum uric acid; HDL: high-density lipoprotein; AUC: area under curve.
4. Discussion and Conclusions
Our research confirmed that increased UHR levels were independently relevant to higher risks of NAFLD in nonoverweight/obese type 2 diabetics. UHR can be considered as a useful biomarker for NAFLD in nonoverweight/obese patients with type 2 diabetes.
Nonalcoholic fatty liver disease and type 2 diabetes are both the most common chronic conditions worldwide driving huge economic and health burdens [26, 27]. The relationship between NAFLD and type 2 diabetes has been widely clarified. The occurrence of NAFLD is quite universal in population with T2DM, with the maximal morbidity at 70% for NAFLD in Europe and the minimum morbidity at 30% in Africa [28]. Moreover, the coexistence of T2DM and NAFLD significantly increases the risks of diabetic complications. Targher et al. [10] showed that NAFLD independently enhanced the risk of the development of cardiovascular diseases by 1.87-fold in type 2 diabetic individuals. In other studies, hypertension and metabolic syndrome were indicated to be more prevalent in subjects with both T2DM and NAFLD than those having T2DM only [29]. Nevertheless, most of previous studies were conducted in patients with T2DM without excluding the impact of obesity. In fact, the beginning of type 2 diabetes appears mostly in obese population, and the incidence of overweight and obesity in people with T2DM is up to 50.9%–98.6% [30]. Overweight and obesity are well known to be interlinked risk factors for both NAFLD prevalence and development [31]. Therefore, it is easy to identify NAFLD in overweight and obese patients. However, the exploration for NAFLD among type 2 diabetics without overweight or obesity is very scarce. Our study showed that, among population with T2DM whose BMI < 24 kg/m2, NAFLD covered approximately half of the participants and was associated with more serious metabolic disorders and insulin resistance, highlighting the importance of early identification of NAFLD in these patients.
Our research confirmed that UHR had significant ability for detecting NAFLD in nonoverweight/obese type 2 diabetics. We observed that the UHR levels were significantly increased in patients with NAFLD compared to those without NAFLD. In addition, the prevalence of NAFLD increased progressively from the lowest tertile to the highest tertile of UHR. Logistic regression analysis showed that participants in the higher UHR tertiles, compared with those in the first tertile, had higher risks for NAFLD, suggesting that individuals with increased UHR are more possible to have NAFLD compared with those with reduced UHR. This association between UHR and NAFLD risks was independent of multiple confounding factors including age, gender, BMI, blood pressure, hepatic enzymes, and other components of metabolic syndrome. Furthermore, the ROC analysis showed that UHR had a significant predictive power for the onset of NAFLD which was better than SUA and HDL alone. These findings suggest that UHR can serve as a potential biomarker for NAFLD in nonoverweight/obese type 2 diabetic populations.
Uric acid is the end product of purine metabolism. Various chronic diseases, such as cardiovascular disease (CVD) [32], insulin resistance, type 2 diabetes, and high blood pressure [33], are frequently found to be related to uric acid increase. Previous studies have confirmed that SUA was positively associated with NAFLD after adjusting for multiple factors [34]. Hyperuricemia can induce the development of insulin resistance, mitochondrial oxidative stress, and inflammation response, which are all risk factors for liver fibrosis [35, 36]. HDL cholesterol plays important roles in metabolic syndrome and is also closely associated with NAFLD [37]. One of the lipid profiles in subjects with NAFLD is characterized by reduced HDL-cholesterol [38]. Low HDL cholesterol was found to be associated with a worse metabolic status, which significantly increased the risk of NAFLD [39]. The UHR, which is calculated dividing serum uric acid levels by HDL-cholesterol, has recently attracted increasing attentions. UHR has been showed to be increased in metabolic syndrome and suggested as a more sensitive predictor of metabolic syndrome than every other markers of this syndrome [40]. Aktas et al. [21] suggested that UHR could serve as a useful predictor of diabetic control in type 2 diabetic males, owning to its positive association with HbA1c and FPG levels. A small number of previous studies have shown that UHR was positively correlated with NAFLD [22]. Zhang et al. [23] suggested that UHR was independently related to an enhanced risk of NAFLD, in which when the levels of UHR increased by 1%, the risk for NAFLD occurrence increased by 10.5%. Our research presented for the first time that, in nonoverweight/obese type 2 diabetic subjects, higher UHR was strongly and independently associated with an increased risk of NAFLD. UHR can be measured as a useful and economical predictor for the onset of NAFLD.
There are several shortcomings in this study. First, this was a single-center study and the sample size was relative small. Thus, the conclusion may not be universal. Second, patients with NAFLD in the present study were diagnosed by ultrasound, which is relatively insensitive for the degree of liver fibrosis. Hence, association between UHR and the severity of hepatic steatosis could not be determined. Third, there was no consideration for other obesity-related factors including waist circumstance and body fat content. Therefore, some obese patients with normal BMI were not been identified. Therefore, well-designed cohort studies with a larger sample should be conducted to further explore the screening capacity of UHR for NAFLD in nonoverweight/obese patients with T2DM.
5. Conclusion
Our study demonstrated that, in nonoverweight/obese patients with type 2 diabetes, UHR levels were positively associated with NAFLD occurrence, independent of hepatic enzymes and multiple metabolic risk factors. UHR is a reliable predictor to stratify the higher risks of NAFLD in type 2 diabetes patients without overweight or obesity.
Acknowledgments
The authors would be grateful for language services by Duoease Scientific Service Center. This article was founded by the Natural Science Foundation of Shandong Province (fund code: ZR2021QH181).
Data Availability
The data are unavailable because the data sharing should be agreed by the authors' ethics committee. In addition, the authors will conduct further studies based on these data.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology . 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.LaBrecque D. R., Abbas Z., Anania F., et al. World Gastroenterology Organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Journal of Clinical Gastroenterology . 2014;48(6):467–473. doi: 10.1097/mcg.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N., Younossi Z., Lavine J. E., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology . 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.McPherson S., Hardy T., Henderson E., Burt A. D., Day C. P., Anstee Q. M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. Journal of Hepatology . 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Han E., Lee Y. H. Non-alcoholic fatty liver disease: the emerging burden in cardiometabolic and renal diseases. Diabetes & Metabolism J . 2017;41(6):430–437. doi: 10.4093/dmj.2017.41.6.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., Zhao X., Chen S., et al. Associations between nonalcoholic fatty liver disease and cancers in a large cohort in China. Clinical Gastroenterology and Hepatology . 2021;19(4):788–796. doi: 10.1016/j.cgh.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Hamed A. E., Elwan N., Naguib M., et al. Diabetes association with liver diseases: an overview for clinicians. Endocrine, Metabolic and Immune Disorders-Drug Targets . 2019;19(3):274–280. doi: 10.2174/1871530318666181116111945. [DOI] [PubMed] [Google Scholar]
- 8.Lallukka S., Yki-Järvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Practice & Research Clinical Endocrinology & Metabolism . 2016;30(3):385–395. doi: 10.1016/j.beem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Williamson R. M., Price J. F., Glancy S., et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care . 2011;34(5):1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targher G., Bertolini L., Rodella S., et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care . 2007;30(8):2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 11.Targher G., Bertolini L., Rodella S., et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia . 2008;51(3):444–450. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 12.Ryysy L., Häkkinen A. M., Goto T., et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes . 2000;49(5):749–758. doi: 10.2337/diabetes.49.5.749. [DOI] [PubMed] [Google Scholar]
- 13.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology . 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver EASL. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of Hepatology . 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y., Liu J., Shi H., Hu W., Song L., Zhao Q. Serum uric acid is positively associated with the prevalence of nonalcoholic fatty liver in non-obese type 2 diabetes patients in a Chinese population. Journal of Diabetes and Its Complications . 2021;35(5) doi: 10.1016/j.jdiacomp.2021.107874.107874 [DOI] [PubMed] [Google Scholar]
- 16.Zelber-Sagi S., Ben-Assuli O., Rabinowich L., Shalev V., Shibolet O., Chodick G. Response to the relationship between serum uric acid levels and NAFLD. Liver International . 2016;36(5):769–770. doi: 10.1111/liv.13101. [DOI] [PubMed] [Google Scholar]
- 17.Abbasi S., Haleem N., Jadoon S., Farooq A. Association of non-alcoholic fatty liver disease with serum uric acid. Journal of Ayub Medical College, Abbottabad . 2019;31(1):64–66. [PubMed] [Google Scholar]
- 18.Eckel R. H., Alberti K. G., Grundy S. M., Zimmet P. Z. The metabolic syndrome. The Lancet . 2010;375(9710):181–183. doi: 10.1016/s0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 19.Yazdi F., Baghaei M. H., Baniasad A., Naghibzadeh-Tahami A., Najafipour H., Gozashti M. H. Investigating the relationship between serum uric acid to high-density lipoprotein ratio and metabolic syndrome. Endocrinology, Diabetes & Metabolism . 2022;5(1) doi: 10.1002/edm2.311.e00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park B., Jung D. H., Lee Y. J. Predictive value of serum uric acid to HDL cholesterol ratio for incident ischemic heart disease in non-diabetic Koreans. Biomedicines . 2022;10(6):p. 1422. doi: 10.3390/biomedicines10061422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aktas G., Kocak M. Z., Bilgin S., Atak B. M., Duman T. T., Kurtkulagi O. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. The Aging Male . 2020;23(5):1098–1102. doi: 10.1080/13685538.2019.1678126. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W., Liang A., Shi P., et al. Higher serum uric acid to HDL-cholesterol ratio is associated with onset of non-alcoholic fatty liver disease in a non-obese Chinese population with normal blood lipid levels. BMC Gastroenterology . 2022;22(1):p. 196. doi: 10.1186/s12876-022-02263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y. N., Wang Q. Q., Chen Y. S., Shen C., Xu C. F. Association between serum uric acid to HDL-cholesterol ratio and nonalcoholic fatty liver disease in lean Chinese adults. International Journal of Endocrinology . 2020;2020:6. doi: 10.1155/2020/5953461.5953461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C., Lu F. C., Department of Disease Control Ministry of Health PR China The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomedical and Environmental Sciences . 2004;17(l):1–36. [PubMed] [Google Scholar]
- 25.Fan J. G., Wei L., Zhuang H., et al. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China) Journal of Digestive Diseases . 2019;20(4):163–173. doi: 10.1111/1751-2980.12685. [DOI] [PubMed] [Google Scholar]
- 26.Younossi Z. M., Tampi R., Priyadarshini M., Nader F., Younossi I. M., Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology . 2019;69(2):564–572. doi: 10.1002/hep.30254. [DOI] [PubMed] [Google Scholar]
- 27.Pearson E. R. Type 2 diabetes: a multifaceted disease. Diabetologia . 2019;62(7):1107–1112. doi: 10.1007/s00125-019-4909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younossi Z. M., Golabi P., de Avila L., et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. Journal of Hepatology . 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Hazlehurst J. M., Woods C., Marjot T., Cobbold J. F., Tomlinson J. W. Non-alcoholic fatty liver disease and diabetes. Metabolism . 2016;65(8):1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chobot A., Górowska-Kowolik K., Sokołowska M., Jarosz-Chobot P. Obesity and diabetes-Not only a simple link between two epidemics. Diabetes Metab Res Rev . 2018;34(7) doi: 10.1002/dmrr.3042.e3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillenbrand A., Kiebler B., Schwab C., et al. Prevalence of non-alcoholic fatty liver disease in four different weight related patient groups: association with small bowel length and risk factors. BMC Research Notes . 2015;8(1):p. 290. doi: 10.1186/s13104-015-1224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndrepepa G. Uric acid and cardiovascular disease. Clinica Chimica Acta . 2018;484:150–163. doi: 10.1016/j.cca.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 33.Mortada I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: an emerging association. Current Hypertension Reports . 2017;19(9):p. 69. doi: 10.1007/s11906-017-0770-x. [DOI] [PubMed] [Google Scholar]
- 34.Sirota J. C., McFann K., Targher G., Johnson R. J., Chonchol M., Jalal D. I. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism . 2013;62(3):392–399. doi: 10.1016/j.metabol.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein L. L., Dong M. H., Loomba R. Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis: current status. Advances in Therapy . 2009;26(10):893–907. doi: 10.1007/s12325-009-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanaspa M. A., Sanchez-Lozada L. G., Choi Y. J., et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress:potential role in fructose-dependent and-independent fatty liver. Journal of Biological Chemistry . 2012;287(48):40732–40744. doi: 10.1074/jbc.m112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemes K., Åberg F. Interpreting lipoproteins in nonalcoholic fatty liver disease. Current Opinion in Lipidology . 2017;28(4):355–360. doi: 10.1097/mol.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 38.Verwer B. J., Scheffer P. G., Vermue R. P., Pouwels P. J., Diamant M., Tushuizen M. E. NAFLD is related to post-prandial triglyceride-enrichment of HDL particles in association with endothelial and HDL dysfunction. Liver International . 2020;40(10):2439–2444. doi: 10.1111/liv.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumner A. E., Zhou J., Doumatey A., et al. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in west africans and african Americans with metabolic syndrome: implications for cardiovascular disease prevention. Global Heart . 2010;5(3):75–80. doi: 10.1016/j.cvdpc.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocak M. Z., Aktas G., Erkus E., Sincer I., Atak B., Duman T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Revista da Associação Médica Brasileira . 1992;65(1):9–15. doi: 10.1590/1806-9282.65.1.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are unavailable because the data sharing should be agreed by the authors' ethics committee. In addition, the authors will conduct further studies based on these data.


