Abstract
Background
The ACOSOG-Z0011- and the AMAROS-trial obviated the need for axillary surgery in most sentinel node-positive (SLN+) breast cancer patients undergoing breast-conserving surgery (BCS). Data for patients who undergo mastectomy is scarce. The purpose of this study was to investigate patterns of axillary treatment in SLN+ patients treated by mastectomy in the years after the publication of landmark studies regarding axillary treatment in SLN+ breast cancer patients undergoing BCS.
Methods
This was a population-based study in cT1-3N0M0 breast cancer patients treated by mastectomy and staged as SLN+ between 2009 and 2018. The performance of an axillary lymph node dissection (ALND) and/or administration of postmastectomy radiotherapy (PMRT) were primary outcomes and were studied over time.
Results
The study included 10,633 patients. The frequency of ALND performance decreased from 78% in 2009 to 10% in 2018, whereas PMRT increased from 4 to 49% (P < 0.001). In ≥N1a patients, ALND performance decreased from 93 to 20%, whereas PMRT increased to 70% (P < 0.001). In N1mi and N0itc patients, ALND was abandoned during the study period, whereas PMRT increased to 38% and 13% respectively (P < 0.001), respectively. Age, tumor subtype, N-stage, and hospital type affected the likelihood that patients underwent ALND.
Conclusions
In this study in SLN+ breast cancer patients undergoing mastectomy, use of ALND decreased drastically over time. By the end of 2018 most ≥N1a patients received PMRT as the only adjuvant axillary treatment, whereas the majority of N1mi and N0itc patients received no additional treatment.
During the past decade, several randomized trials have cast doubt on the need to perform axillary lymph node dissection (ALND) in patients with sentinel lymph node metastases (SLN+). The Z0011 trial of The American College of Surgeons Oncology Group (ACOSOG), published in 2011, demonstrated that ALND in cT1-2 patients undergoing breast-conserving surgery (BCS) who were found to have one or two positive SLN (SLN+) showed no lower regional recurrence risk or better survival compared with those undergoing sentinel lymph node biopsy (SLNB) only.1,2 The International Breast Cancer Study Group trial (IBCSG 23-01) showed similar results for patients with micrometastases in the SLN.3 The results of the “After Mapping of the Axilla: Radiotherapy or Surgery?” (AMAROS) trial, published in 2014, demonstrated that axillary radiotherapy (RT) could serve as a safe alternative to ALND resulting in equivalent regional control.4
The results of these trials led to a broad discussion about the need of performing ALND in SLN+ patients and about the use of RT as an alternative to ALND in SLN+ patients who would previously had been candidates for ALND. International guidelines suggest to consider foregoing axillary surgery in patients meeting the Z0011 criteria, i.e., patients who were treated by breast-conserving surgery (BCS) followed by routine external beam RT of the breast.5–7 Other guidelines advocate the use of regional RT as an alternative for ALND in SLN+ patients,6 applying the AMAROS results both to patients who undergo BCS as well as to patients treated by mastectomy.
Some years ago, a substantial decrease was reported in ALND frequency among SLN+ patients both in those undergoing BCS and mastectomy.8,9 In a previous Dutch population-based study, describing patients treated from 2011 to 2015, the proportion of SLN+ patients receiving ALND alongside BCS versus mastectomy was 31% versus 52% at the start but had decreased to 11% and 26%, respectively, by the end of the study period.8 These trend lines show a stronger reduction of ALND in the context of BCS versus mastectomy, which may reflect an altered protocol with regard to the anticipated effectivity of ALND in conjunction with BCS. Because for mastectomy patients the Z0011 criteria do not apply, one might expect that postmastectomy radiotherapy (PMRT) would have been applied as a substitute for ALND.
Therefore, the purpose of this study was to investigate patterns of care in axillary treatment for Dutch cT1-3N0 SLN+ breast cancer patients undergoing mastectomy. Furthermore, patient-, tumor-, treatment-, and hospital-related factors that are associated with ALND performance were evaluated.
Methods
Data were obtained from the nationwide population-based Netherlands Cancer Registry (NCR), which is hosted by the Netherlands Comprehensive Cancer Organisation (IKNL). Based on notification through the national pathology database (PALGA) specially trained IKNL data managers register patient-, tumor-, and treatment-related characteristics directly from the patient’s files.
Patients and Hospitals
For the present study, all Dutch adult female patients diagnosed with cT1-3N0M0 invasive breast cancer who underwent mastectomy including SLNB between January 2009 and December 2018 were selected from the NCR. Patients who had SLNs containing metastases were included. Those who received neoadjuvant systemic therapy, underwent mastectomy without SLN biopsy, as well as patients in whom the SLN could not be identified intraoperatively were excluded.
Construction of Variables
Patients were subdivided in groups according to axillary treatment following SLNB: ALND, PMRT, a combination of the two (ALND + PMRT), or no subsequent axillary treatment. Detailed information regarding radiation fields was not available. In the Netherlands, the indication for RT of the chest wall in the primary setting is dependent on the estimated risk of recurrence and the absence or presence of risk factors. In case regional RT is indicated in postmastectomy patients (dependent on the extent of nodal disease and the absence or presence of risk factors), the chest wall is generally included in the radiotherapy field. Metastatic lymph node involvement was categorized into isolated tumor cells (N0itc), micrometastases (N1mi) or macrometastases (≥N1a) based on the pathology examination of the retrieved SLNs. Hospitals were categorized based on surgical hospital volume. They were divided into low volume (<150 breast cancer operations for primary breast cancer), middle volume (150-300 operations), and high volume (>300 operations) on average per year. Cutoff points were based on those reported by EUSOMA, the European Society of Breast Cancer Specialists,10 and those reported in an article from Greenup et al.11 Hospitals also were categorized by their teaching status as general nonteaching, teaching, or academic centers.
Statistical Analysis
Patient-, tumor-, treatment-, and hospital-related characteristics are presented as baseline characteristics according to the different treatment groups and compared by using chi-squared tests. Descriptive analyses were used to report on the annual proportions of axillary treatments. Univariable and multivariable logistic regression analyses were used to identify patient-, tumor-, treatment-, and hospital-related factors that are associated with ALND performance. P value < 0.05 was considered statistically significant. Data analyses were performed using Stata version 17.0 (StataCorp, TX).
Results
Patients
In total 10,633 patients were included in the analysis. Most of the SLN+ patients were diagnosed with a cT1-2 tumor (93%, n = 9864). The remaining 7% of the patients were diagnosed with a cT3 tumor (n = 769; Table 1). In most of the patients receiving SLNB alone and no ALND (n = 6457), one to three lymph nodes were removed and examined (83%, n = 5355; median 2; IQR 1-3).
Table 1.
Baseline characteristics of all SLN+ patients treated with ALND, ALND + PMRT, PMRT, or no adjuvant axillary treatment (n = 10,633)
| Characteristics | N overall | ALND | ALND + PMRT | PMRT | No adjuvant axillary treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | N | % | N | % | N | % | N | % | |
| Year of diagnosis | |||||||||
| 2009 | 854 | 462 | 54 | 206 | 24 | 33 | 4 | 153 | 18 |
| 2010 | 1041 | 561 | 54 | 198 | 19 | 52 | 5 | 230 | 22 |
| 2011 | 1205 | 537 | 45 | 216 | 18 | 121 | 10 | 331 | 28 |
| 2012 | 1197 | 431 | 36 | 187 | 16 | 180 | 15 | 399 | 33 |
| 2013 | 1157 | 342 | 30 | 134 | 12 | 258 | 22 | 423 | 37 |
| 2014 | 1142 | 205 | 18 | 132 | 12 | 362 | 32 | 443 | 39 |
| 2015 | 1098 | 137 | 13 | 75 | 7 | 477 | 43 | 409 | 37 |
| 2016 | 1008 | 86 | 9 | 56 | 6 | 458 | 45 | 408 | 41 |
| 2017 | 982 | 67 | 7 | 49 | 5 | 451 | 46 | 415 | 42 |
| 2018 | 949 | 64 | 7 | 31 | 3 | 463 | 49 | 391 | 41 |
| Age group (year) | |||||||||
| < 40 | 556 | 165 | 30 | 111 | 20 | 150 | 27 | 130 | 23 |
| 40–49 | 1979 | 624 | 32 | 317 | 16 | 492 | 25 | 546 | 28 |
| 50–59 | 2521 | 774 | 31 | 328 | 13 | 681 | 27 | 738 | 29 |
| 60–69 | 2498 | 675 | 27 | 311 | 13 | 681 | 27 | 831 | 33 |
| 70–79 | 1754 | 426 | 24 | 150 | 9 | 547 | 31 | 631 | 36 |
| > 79 | 1325 | 228 | 17 | 67 | 5 | 304 | 23 | 726 | 55 |
| Histological tumour type | |||||||||
| Ductal | 7372 | 2193 | 30 | 836 | 11 | 1850 | 25 | 2493 | 34 |
| Lobular | 2419 | 493 | 20 | 361 | 15 | 750 | 31 | 815 | 34 |
| Mixed | 614 | 152 | 25 | 65 | 11 | 208 | 34 | 189 | 31 |
| Other | 228 | 54 | 24 | 22 | 10 | 47 | 21 | 105 | 46 |
| Differentiation grade | |||||||||
| I | 1806 | 544 | 30 | 159 | 9 | 419 | 23 | 684 | 38 |
| II | 5808 | 1502 | 26 | 684 | 12 | 1557 | 27 | 2065 | 36 |
| III | 2759 | 755 | 27 | 413 | 15 | 833 | 30 | 758 | 28 |
| Unknown | 260 | 91 | 35 | 28 | 11 | 46 | 18 | 95 | 37 |
| Clinical tumour stage | |||||||||
| cT1 | 4730 | 1466 | 31 | 444 | 9 | 1054 | 22 | 1766 | 37 |
| cT2 | 5134 | 1330 | 26 | 694 | 14 | 1453 | 28 | 1657 | 32 |
| cT3 | 769 | 96 | 13 | 146 | 19 | 348 | 45 | 179 | 23 |
| Multifocality | |||||||||
| No | 7165 | 2002 | 28 | 867 | 12 | 1739 | 24 | 2557 | 36 |
| Yes | 3424 | 877 | 26 | 408 | 12 | 1107 | 32 | 1032 | 30 |
| Unknown | 35 | 7 | 20 | 9 | 26 | 7 | 20 | 12 | 34 |
| Breast cancer subtype | |||||||||
| HR+/HER2− | 8535 | 2290 | 27 | 999 | 12 | 2345 | 28 | 2901 | 34 |
| HR+/HER2+ | 899 | 260 | 29 | 108 | 12 | 218 | 24 | 313 | 35 |
| HR−/HER2+ | 359 | 108 | 30 | 57 | 16 | 82 | 23 | 112 | 31 |
| HR−/HER2− | 625 | 193 | 31 | 104 | 17 | 156 | 25 | 172 | 28 |
| Other/unknown | 215 | 41 | 19 | 16 | 7 | 54 | 25 | 104 | 48 |
| Pathological N-stage | |||||||||
| Isolated tumour cells | 2170 | 70 | 4 | 17 | 1 | 224 | 10 | 1850 | 85 |
| Micrometastasis | 2847 | 743 | 26 | 104 | 4 | 739 | 26 | 1261 | 44 |
| Macrometastasis | 5616 | 2070 | 37 | 1163 | 21 | 1892 | 34 | 491 | 9 |
| Hormonal therapy | |||||||||
| No | 2106 | 509 | 24 | 234 | 11 | 461 | 22 | 902 | 43 |
| Yes | 8527 | 2383 | 28 | 1050 | 12 | 2394 | 28 | 2700 | 32 |
| Chemotherapy | |||||||||
| No | 5199 | 1101 | 21 | 243 | 5 | 1360 | 26 | 2495 | 48 |
| Yes | 5434 | 1791 | 33 | 1041 | 19 | 1495 | 28 | 1107 | 20 |
| Hospital volume | |||||||||
| < 150 resections per year | 4277 | 1277 | 30 | 512 | 12 | 1021 | 24 | 1467 | 34 |
| 150–300 resections per year | 5951 | 1515 | 26 | 733 | 12 | 1685 | 28 | 2018 | 34 |
| > 300 resections per year | 400 | 99 | 25 | 37 | 9 | 148 | 37 | 116 | 29 |
| Hospital type | |||||||||
| General nonteaching | 4604 | 1288 | 23 | 498 | 11 | 1247 | 27 | 1571 | 34 |
| Teaching hospital | 5130 | 1402 | 27 | 707 | 14 | 1334 | 26 | 1687 | 33 |
| Academic hospital | 894 | 201 | 23 | 77 | 9 | 273 | 31 | 343 | 38 |
ALND axillary lymph node dissection, PMRT postmastectomy radiotherapy, HR hormone receptor, HER2+ human epidermal growth factor receptor 2, SLNB sentinel lymph node biopsy
Trends in Axillary Treatment in cT1-3 SLN+ Breast Cancer Patients Undergoing Mastectomy
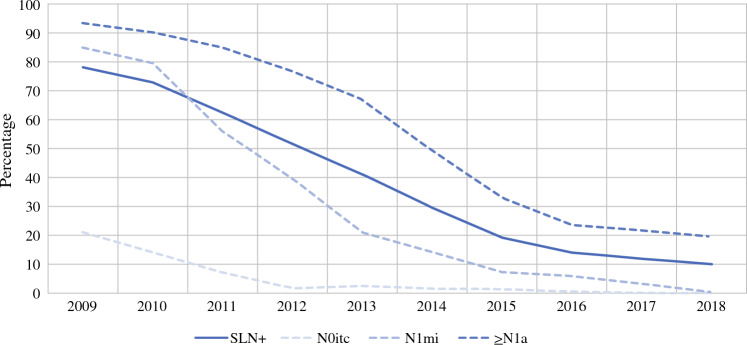
The proportion of SLN+ patients who underwent ALND following mastectomy (n = 10,633) decreased from 78% in 2009 to 10% in 2018 (Fig. 1). The frequency of ALND decreased from 93 to 20% in ≥N1a patients, from 85% to 0.4% in N1mi patients, and from 21% to 0% in N0itc patients, respectively (Fig. 1).
Fig. 1.
Frequency of ALND in sentinel node-positive patients undergoing amputation. SLN+ sentinel lymph node positive, N0itc isolated tumor cells, N1mi micrometastases, ≥N1a macrometastases
Figure 2 shows the trend of adjuvant axillary treatment. Both ALND and ALND combined with PMRT decreased from 54% in 2009 to 7% in 2018 and from 24 to 3%, respectively. The use of PMRT as the only type of adjuvant treatment increased from 4 to 49% (P < 0.001 for all). For patients with a cT3 tumor, ALND (ALND alone or combined with PMRT) decreased from 72 to 13%. Excluding patients with T3 tumors had no significant impact on the results for the whole group. In the selection of patients with cT1-2 tumors, the proportion of ALND decreased from 55 to 7% and treatment with PMRT increased from 4 to 48%.
Fig. 2.
Frequency of axillary treatment in sentinel node-positive patients. PMRT postmastectomy radiotherapy, ALND axillary lymph node dissection
The trends of adjuvant axillary treatment varied for the different N+ categories groups. In ≥N1a patients, the increase of PMRT from 2% in 2009 to 70% in 2018 was accompanied by a decrease in ALND from 57 to 13% (P < 0.001 for all; Fig. 3a). In the N1mi group, the decrease of ALND appeared most prominent from 75 to 0.4% (P < 0.001; Fig. 3b). This decrease in ALND performance was only in part accompanied by an increase of PMRT from 4 to 38% (P < 0.001). In the latter years, a substantial number of patients did not receive axillary treatment at all. In N0itc patients, ALND was abandoned rapidly from 17% to approximately 0% since 2012 (P < 0.001; Fig. 3c). The use of PMRT being approximately 10% throughout the study period.
Fig. 3.
A Frequency of axillary treatment in ≥N1a patients. B Frequency of axillary treatment in N1mi patients. C Frequency of axillary treatment in N0itc patients. PMRT postmastectomy radiotherapy, ALND axillary lymph node dissection
Patients-, Tumor-, and Hospital Characteristics which Influence the Choice of Omitting ALND
In addition to the effect of time, factors that were associated with a decreased chance of undergoing ALND were patients > 79 years (odds ratio [OR] 0.27; 95% confidence interval [CI] 0.21-0.35) compared with age 50-59 years, treatment with PMRT (OR 0.14; 95% CI 0.12–0.17), patients with tumor’s differentiation grade II (OR 0.83; 95% CI 0.70–0.98) compared with grade I, and patients with sentinel nodes containing isolated tumor cells (OR 0.00; 95% CI 0.00–0.01) or micrometastases (OR 0.10; 95% CI 0.08–0.11) compared with macrometastases.
Factors that were associated with a higher chance of ALND performance were age < 40 years (OR 1.28; 95% CI 0.96–1.70) compared with age 50-59 years, lobular (OR 1.23; 95% CI 1.05–1.43) compared with ductal tumor type, basal-like (OR 1.83; 95% CI 1.33–2.53) compared with hormone receptor-positive (HR+)/HER2 receptor-negative tumor subtype, receiving chemotherapy (OR 2.34; 95% CI 1.98–2.77) compared with not receiving adjuvant chemotherapy, as well as treatment outside an academic institution (teaching hospital: OR 2.19; 95% CI 1.71–2.81, general hospital: OR 1.58; 95% CI 1.25–2.00) (Table 2).
Table 2.
Univariable and multivariable analysis patient, tumor, and hospital characteristics associated with the performance of ALND
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| N | % ALND | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Year of incidence | ||||||
| 2009 | 854 | 78 | 1.334 | 1.079–1.650 | 1.65 | 1.20–2.27 |
| 2010 | 1041 | 73 | Ref | Ref | ||
| 2011 | 1205 | 62 | 0.619 | 0.517–0.741 | 0.45 | 0.34–0.59 |
| 2012 | 1197 | 52 | 0.397 | 0.332–0.474 | 0.21 | 0.17–0.28 |
| 2013 | 1157 | 41 | 0.260 | 0.217–0.311 | 0.12 | 0.09–0.16 |
| 2014 | 1142 | 30 | 0.156 | 0.129–0.187 | 0.08 | 0.06–0.10 |
| 2015 | 1098 | 19 | 0.089 | 0.073–0.109 | 0.04 | 0.03–0.05 |
| 2016 | 1008 | 14 | 0.061 | 0.049–0.076 | 0.03 | 0.02–0.04 |
| 2017 | 982 | 12 | 0.050 | 0.039–0.063 | 0.02 | 0.02–0.03 |
| 2018 | 949 | 10 | 0.041 | 0.032–0.053 | 0.02 | 0.01–0.03 |
| Age (year) | ||||||
| < 40 | 556 | 50 | 1.27 | 1.06–1.53 | 1.32 | 1.00–1.76 |
| 40–49 | 1.979 | 48 | 1.17 | 1.04–1.31 | 1.08 | 0.86–1.25 |
| 50–59 | 2521 | 44 | Ref | Ref | ||
| 60–69 | 2498 | 39 | 0.84 | 0.75–0.94 | 1.04 | 0.88–1.24 |
| 70–79 | 1754 | 33 | 0.63 | 0.55–0.71 | 0.93 | 0.74–1.16 |
| > 79 | 1325 | 22 | 0.37 | 0.32–0.43 | 0.27 | 0.21–0.35 |
| Histological tumor type | ||||||
| Ductal | 7372 | 41 | Ref | Ref | ||
| Lobular | 2419 | 35 | 0.78 | 0.71–0.86 | 1.23 | 1.05–1.43 |
| Mixed | 614 | 35 | 0.78 | 0.66–0.93 | 0.86 | 0.66–1.12 |
| Other | 228 | 33 | 0.72 | 0.54–0.95 | 0.91 | 0.59–1.42 |
| Differentiation grade | ||||||
| I | 1806 | 39 | Ref | Ref | ||
| II | 5808 | 38 | 0.95 | 0.85–1.06 | 0.83 | 0.70–0.98 |
| III | 2759 | 42 | 1.15 | 1.02–1.30 | 1.00 | 0.82–1.22 |
| Unknown | 260 | 46 | ||||
| Clinical tumour stage | ||||||
| cT1 | 4730 | 40 | Ref | Ref | ||
| cT2 | 5134 | 39 | 0.96 | 0.89–1.04 | 1.08 | 0.95–1.23 |
| cT3 | 769 | 31 | 0.68 | 0.58–0.80 | 1.11 | 0.87–1.41 |
| Multifocality | ||||||
| No | 7165 | 40 | Ref | Ref | ||
| Yes | 3424 | 38 | 0.90 | 0.83–0.98 | 0.98 | 0.86–1.11 |
| Unknown | 35 | 46 | ||||
| Pathological N-stage | ||||||
| Isolated tumour cells | 2170 | 4 | 0.034 | 0.028–0.042 | 0.00 | 0.00–0.01 |
| Micrometastasis | 2847 | 30 | 0.312 | 0.284–0.344 | 0.10 | 0.08–0.11 |
| Macrometastasis | 5616 | 58 | Ref | Ref | ||
| Breast cancer subtype | ||||||
| HR+/HER2− | 8535 | 39 | Ref | Ref | ||
| HR+/HER2+ | 899 | 41 | 1.11 | 0.96–1.27 | 0.75 | 0.60–0.93 |
| HR−/HER2+ | 359 | 46 | 1.36 | 1.10–1.68 | 1.15 | 0.78–1.69 |
| HR−/HER2− | 625 | 48 | 1.44 | 1.23–1.70 | 1.83 | 1.33–2.53 |
| Unknown | 215 | 27 | 0.58 | 0.42–0.78 | 0.72 | 0.45–1.16 |
| Adjuvant hormonal therapy | ||||||
| No | 2106 | 35 | Ref | Ref | ||
| Yes | 8527 | 40 | 1.24 | 1.12.–1.37 | 1.26 | 1.02–1.55 |
| Adjuvant chemotherapy | ||||||
| No | 5199 | 26 | Ref | Ref | ||
| Yes | 5434 | 52 | 3.12 | 2.88–3.39 | 2.34 | 1.98–2.77 |
| Radiotherapy | ||||||
| No | 6494 | 45 | Ref | Ref | ||
| Yes | 4139 | 31 | 0.56 | 0.52–0.61 | 0.14 | 0.12–0.17 |
| Hospital volume | ||||||
| Low (< 150) | 4277 | 42 | Ref | Ref | ||
| Medium (150–300) | 5951 | 38 | 0.84 | 0.78–0.91 | 0.75 | 0.65–0.86 |
| High (> 300) | 400 | 34 | 0.72 | 0.58–0.89 | 0.91 | 0.65–1.26 |
| Hospital type | ||||||
| Academic | 894 | 31 | Ref | Ref | ||
| Teaching | 5130 | 41 | 1.55 | 1.33–1.80 | 2.19 | 1.71–2.81 |
| General | 4604 | 39 | 1.40 | 1.20–1.64 | 1.58 | 1.25–2.00 |
ALND axillary lymph node dissection (with or without postmastectomy radiotherapy); HR hormone receptor; HER2+ human epidermal growth factor receptor 2; SLNB sentinel lymph node biopsy
Discussion
In this population-based study in Dutch cT1-3N0M0 breast cancer patients who underwent mastectomy and were SLN+, a substantial decrease in the proportion of patients undergoing ALND was observed. In patients diagnosed with ≥N1a disease, ALND performance decreased and PMRT increased substantially over the years, whereas in patients with isolated tumor cells and micrometastasis, a substantial proportion had no adjuvant regional treatment at the end of the study period.
Ten years after the publication of the Z0011 and AMAROS trials, the proportion of Dutch patients undergoing mastectomy who were SLN+ and underwent ALND decreased to 10%. This seems to reflect the clinicians’ confidence in a restrained surgical policy in this category of patients, albeit that the aforementioned trials included patients undergoing BCS exclusively (Z0011) or mostly (82% in the AMAROS trial).2,4,12 A recent population-based study from the United States in a similar cohort of 12,190 patients also showed a decrease in the proportion patients undergoing ALND from 58% in 2005 to 36% in 2014,13 whereas another large, population-based study in Germany showed a decrease from 90% in 2008 to 56% in 2015.14
The present study shows replacement of ALND with PMRT as axillary treatment after mastectomy in patients staged as ≥N1a. While only 20% of ≥N1a patients underwent ALND at the end of the study period, 70% received PMRT. This trend to omit ALND and increasingly use PMRT has been reported by others,13,15 arguing in favor of this treatment switch citing the evidence from the AMAROS trial results. In addition, a remarkable decrease in both performing ALND and administering PMRT as adjuvant axillary treatment is observed. Others also reported this decrease.16 Proceeding with PMRT instead of ALND in SLN+ patients precludes the identification of patients with N2 or N3 disease. Long-term outcome remains to be awaited, but the short-term advantage in terms of less arm morbidity when fewer patients undergo both local treatment modalities goes without saying.
In N0itc and N1mi patients, the decreasing trend in axillary surgery was observed earlier during the study period and the decrease was to a lesser extent accompanied by an increase in PMRT compared with ≥N1a patients. This may partly be clarified by the Dutch breast cancer treatment guideline from 2012,17 which recommended that adjuvant axillary treatment was unnecessary in N0itc patients and questioned the need of axillary treatment in a selection of N1mi patients, e.g., depending on the number of lymph nodes that contained micrometastasis or the presence of other risk factors, such as young age (<40 years), grade 3 disease, lymphovascular invasion, or triple-negative disease. The conceivable association between the degree of metastatic lymph node involvement and the proportion of patients who undergo axillary surgery also was observed by others.13–15,18 Apart from the observed decreased performance of ALND, the association between the extent of metastatic involvement of the SLN and the subsequent administration of PMRT suggests that in SLN+ patients who undergo mastectomy and are diagnosed with ≥N1a disease, the AMAROS trials results are adhered to, whereas in patients with N1mi and N0itc, adjuvant treatment is considered unnecessary by many clinicians in the majority of patients.3,16,19
In addition to N-stage and histologic subtype of the tumor, several other factors were associated with the decision of whether to perform ALND. Women older than age 79 years had a lower chance of undergoing ALND, whereas women younger than age 40 years and women with basal-like tumor subtype had a higher chance of undergoing ALND.14,15 It seems that surgeons are more reserved in omitting ALND in young patients with an aggressive tumor subtype, albeit that a recent study suggests that clinicians may forego ALND in young patients when PMRT will be administered.20 Furthermore, the results of our study showed that patients who undergo adjuvant chemotherapy also were more likely to receive ALND. The higher likelihood of macrometastatic disease or high-grade disease in patients undergoing adjuvant chemotherapy probably contributes to this correlation, albeit that hospital type and the innovative characteric within a hospital also influences the use of systemic therapies and axillary treatment.
Albeit that patients with a cT3 tumor were not included in the Z0011 and AMAROS trial, we decided to include these patients in our dataset to evaluate patterns of care for this particular subgroup. Despite the lack of evidence to deescalate axillary treatment within this category of patients, the results of our study illustrated a similar decreasing trend in the performance of ALND in patients with cT3 tumors compared with those with T1-2 tumors.
The finding that patients treated outside an academic hospital were more likely to undergo axillary surgery is in line with the findings of another study from the Netherlands8 but contrasts with the opposite finding of three cohort studies from the United States and Germany.13–15 In the German study, patients who were treated in community cancer centers, in comparison to academic cancer centers, were more likely to undergo treatment with SLN dissection without ALND or PMRT (37.4% and 32.1%, respectively).14 Weiss et al. showed similar results; 37-38% of the patients treated in community centers only underwent SLN biopsy versus 32% in academic centers. The latter authors also observed that patients with public insurance were more likely to receive SLN biopsy only.13 Then again, in another American study, it was observed that patients undergoing an upfront ALND were more likely to be treated in a community center than those undergoing SLN biopsy alone.15 This all implies that opinions regarding axillary treatment differ between institutions, clinicians, and surgical societies.
The main strengths of the present study are the size of the study population, the quality of the items that were uniformly registered by personnel of the NCR, and the study period of 10 years. As a result, robust data regarding treatment trends are presented. Some limitations of the study are the absence of the number of removed and examined sentinel nodes in patients who underwent ALND following SLN biopsy, the timing of axillary surgery (SLN biopsy with the ALND versus delayed ALND), and the absence of information regarding the radiation fields. In the Netherlands, the indication for RT of the chest wall in the primary setting is dependent on the estimated risk of recurrence and the absence or presence of risk factors. If case regional RT is indicated in postmastectomy patients (dependent on the extent of nodal disease and the absence or presence of risk factors), the chest wall is generally included in the radiotherapy field. Another important limitation of the study design is the absence of follow-up information, because this is not routinely collected for all patients in the NCR. While evidence from clinical trials support the interchangeability of ALND and regional RT in patients treated with BCS, we are still awaiting the results of several clinical trials exploring the impact of omitting adjuvant local treatment in SLN+ patients who undergo mastectomy.21–23 These trials mostly included patients treated with BCS, whereas data specifically for patients undergoing mastectomy is scarce. The Dutch BOOG 2013-07 registry study assessed the oncologic safety of different extents of additional axillary treatment following a positive SLN, specifically in patients who underwent mastectomy.24 Follow-up of this trial was recently completed. While awaiting the results of these trials to determine optimal axillary treatment strategies in postmastectomy patients with a positive SLN, it seems sensible to avoid treating patients with both ALND and regional RT, because this combination is associated with the worst patient-reported outcomes compared with less invasive axillary treatments (SLNB or regional RT only).25 Based on the results of our study, specialists seem to already actively avoid this combination in daily practice, because these rates decreased further each year.
Conclusions
This study shows a descending trend in the execution of ALND in SLN+ Dutch cT1-3N0M0 breast cancer patients undergoing mastectomy within the 10 years following the AMAROS and Z0011 trial results. ALND was omitted in the vast majority of SLN+ patients. In ≥N1a patients, PMRT increased drastically, whereas less than half of N1mi and only a tenth of N0itc patients received PMRT as the only adjuvant axillary treatment by the end of 2018.
Funding
None.
Data Availability
The data that support the findings of this study are available upon reasonable request.
DISCLOSURES
The authors report no conflicts of interest.
Ethics Approval
This article does not contain any studies involving animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised con- trolled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nationaal Borstkanker Overleg Nederland (NABON). Richtlijn behandeling van het mammacarcinoom. https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html. Accessed 10 Apr 2021.
- 6.National Comprehensive Cancer Network clinical practice guidelines in oncology. Breast cancer (ver 5.2020). https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf. Accessed 7 Apr 2021.
- 7.Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(5):561–564. doi: 10.1200/JCO.2016.71.0947. [DOI] [PubMed] [Google Scholar]
- 8.Poodt IGM, Spronk PER, Vugts G, et al. Trends on axillary surgery in nondistant metastatic breast cancer patients treated between 2011 and 2015: a Dutch population-based study in the ACOSOG-Z0011 and AMAROS Era. Ann Surg. 2018;268(6):1084–1090. doi: 10.1097/SLA.0000000000002440. [DOI] [PubMed] [Google Scholar]
- 9.Gondos A, Jansen L, Heil J, et al. Time trends in axilla management among early breast cancer patients: persisting major variation in clinical practice across European centers. Acta Oncol. 2016;55(6):712e9. doi: 10.3109/0284186X.2015.1136751. [DOI] [PubMed] [Google Scholar]
- 10.Wilson ARM, Marotti L, Bianchi S, et al. The requirements of a specialist Breast Centre. Eur J Cancer. 2013;49(17):3579–3587. doi: 10.1016/j.ejca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Greenup RA, Obeng-Gyasi S, Thomas S, et al. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018;267(2):375–381. doi: 10.1097/SLA.0000000000002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels SA, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year result of the randomized controlled EORTC 10981–22023 AMAROS trial. J Clin Oncol. 2022;2022:JCO2201565. doi: 10.1200/JCO.22.01565. [DOI] [PubMed] [Google Scholar]
- 13.Weiss A, Lin H, Babiera GV, et al. Evolution in practice patterns of axillary management following mastectomy in patients with 1–2 positive sentinel nodes. Breast Cancer Res Treat. 2019;176(2):435–444. doi: 10.1007/s10549-019-05243-7. [DOI] [PubMed] [Google Scholar]
- 14.Hennigs A, Riedel F, Feißt M, et al. Evolution of the use of completion axillary limph node dissection in patients with T1/2N0M0 breast cancer and tumour-involved sentinel lymph nodes undergoing mastectomy: a cohort study. Ann Surg Oncol. 2019;26(8):2435–2443. doi: 10.1245/s10434-019-07388-7. [DOI] [PubMed] [Google Scholar]
- 15.Gaines S, Suss N, Barrera E, et al. Axillary surgery for early-stage, node-positive mastectomy patients and the use of postmastectomy chest wall radiation therapy. Ann Surg Oncol. 2018;25(8):2220–2228. doi: 10.1245/s10434-018-6409-6. [DOI] [PubMed] [Google Scholar]
- 16.Kantor O, Means J, Grossmith S, et al. Optimizing axillary management in clinical T1–2N0 mastectomy patients with positive sentinel lymph nodes. Ann Surg Oncol. 2021 doi: 10.1245/s10434-021-10726-3. [DOI] [PubMed] [Google Scholar]
- 17.Nationaal Borstkanker Overleg Nederland (NABON). Richtlijn Behandeling van het Mammacarcinoom 2012. https://www.nabon.nl/wp-content/uploads/2022/11/Richtlijn-mammacarcinoom-2012.pdf. Accessed 8 Mar 2023.
- 18.Moossdorff M, Nakhlis F, Hu J, et al. The potential impact of AMAROS on the management of the axilla in patients with clinical T1–2N0 breast cancer undergoing primary total mastectomy. Ann Surg. 2018;25(9):2612–2619. doi: 10.1245/s10434-018-6519-1. [DOI] [PubMed] [Google Scholar]
- 19.Solá M, Alberro JA, Fraile M, et al. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20(1):120–127. doi: 10.1245/s10434-012-2569-y. [DOI] [PubMed] [Google Scholar]
- 20.Tadros AB, Moo TA, Stempel M, Zabor EC, Khan AJ, Morrow M. Axillary management for young women with breast cancer varies between patients electing breast-conservation therapy or mastectomy. Breast Cancer Res Treat. 2020;180:197–205. doi: 10.1007/s10549-019-05520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tinterri C, Canavese G, Bruzzi P, Dozin B. SINODAR ONE, an ongoing randomized clinical trial to assess the role of axillary surgery in breast cancer patients with one or two macrometastatic sentinel nodes. Breast. 2016;30:197–200. doi: 10.1016/j.breast.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 22.de Boniface J, Frisell J, Andersson Y, et al. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017;17(1):379. doi: 10.1186/s12885-017-3361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal A, Mann GB, Fallowfield L, et al. POSNOC-POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early-stage breast cancer who have metastases in one or two sentinel nodes. BMJ Open. 2021;11(12):e054365. doi: 10.1136/bmjopen-2021-054365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Roozendaal LM, de Wilt JHW, van Dalen T, et al. The value of completion axillary treatment in sentinel node positive breast cancer patients undergoing a mastectomy: a Dutch randomized controlled multicentre trial (BOOG 2013–07) BMC Cancer. 2015;15:610. doi: 10.1186/s12885-015-1613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregorowitsch ML, Verkooijen HM, Houweling A, et al. Impact of modern-day axillary treatment on patients reported arm morbidity and physical functioning in breast cancer patients. Radiother Oncol. 2019;131:221–228. doi: 10.1016/j.radonc.2018.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.