Abstract
Cancer stem cells (CSCs) cause drug resistance in cancer due to its extensive drug efflux, DNA repair and self-renewal capability. ATP binding cassette subfamily G member 2 (ABCG2) efflux pump afford protection to CSCs in tumors, shielding them from the adverse effects of chemotherapy. Although the role of ABCG2 in cancer progression, invasiveness, recurrence are known but its role in metastasis and angiogenesis are not clear. Here, using in vitro (CSCs enriched side population [SP] cells), ex vivo (patient derived primary cells), in ovo (fertilized egg embryo) and in vivo (patient derived primary tissue mediated xenograft (PDX)) system, we have systematically studied the role of ABCG2 in angiogenesis and the regulation of the process by Curcumin (Cur) and Quinacrine (QC). Cur + QC inhibited the proliferation, invasion, migration and expression of representative markers of metastasis and angiogenesis. Following hypoxia, ABCG2 enriched cells released angiogenic factor vascular endothelial growth factor A (VEGF A) and induced the angiogenesis via PI3K-Akt-eNOS cascade. Cur + QC inhibited the ABCG2 expression and thus reduced the angiogenesis. Interestingly, overexpression of ABCG2 in SP cells and incubation of purified ABCG2 protein in media induced the angiogenesis but knockdown of ABCG2 decreased the vascularization. In agreement with in vitro results, ex vivo data showed similar phenomena. An induction of vascularization was noticed in PDX mice but reduction of vascularization was also observed after treatment of Cur + QC. Thus, data suggested that in hypoxia, ABCG2 enhances the production of angiogenesis factor VEGF A which in turn induced angiogenesis and Cur + QC inhibited the process by inhibiting ABCG2 in breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-022-00692-0.
Keywords: Curcumin, Quinacrine, ABCG2, Cancer stem cells, HUVECs, Angiogenesis
Introduction
Undoubtedly, breast cancer is the most frequently occurring cancer among women worldwide (Hashemitabar et al. 2019). However, most women with primary breast cancer experience subclinical metastases that eventually develop to distant metastasis that makes more perplex for curability of cancer (Taheri et al. 2017).
The epithelial to mesenchymal transition (EMT) plays a central role in both normal physiological (e.g., embryonic development) and abnormal pathological (e.g., tumor formation and metastasis) processes (Kim et al. 2017). EMT has also been involved in generation of cancer stem cells (CSCs), the subpopulation of cells identified within tumors as having self-renewal, tumor invasion, migration, drug resistance and cancer relapsing capability (Pradella et al. 2017; Siddharth et al. 2017).
The drug efflux efficiency of CSCs comes from the increased expression of ATP-binding cassette (ABC) transporters (Dai et al. 2017). Chemo resistance of stem cells is thought to be provided through ABC transporters, and therefore these ABC transporters have been highly studied in stem cells (Chikazawa et al. 2010). EMT is an important regulator of ABC transporters and EMT-related transcription factors play a major role in regulating ABC transporters expression thereby promoting drug resistance in tumor cells (Jiang et al. 2017).
The side population (SP) cells are defined by their efflux capability of the fluorescent DNA-binding dye Hoechst 33342 through their ABC transporters and are enriched in stem cells, which has self-renewal capacity and obeyed CSCs signaling (WNT/β-catenin, HH-Gli etc.) characteristic (Shimoda et al. 2018). SP cells showed a distinctly higher expression level of ATP binding cassette subfamily G member 2 (ABCG2), an important member of ABC family , sphere formation efficacy (SFE) and growth rate even under hypoxia condition (He et al. 2018). SP cells have a superior anti-apoptotic ability compared with the non-SP cells, which is associated with the expression of ABCG2 (Liu et al. 2014).
Well-known ABC transporters include the multidrug resistance protein (MDR) ATP binding cassette subfamily B member 1 (ABCB1), the MDR-associated protein ATP binding cassette subfamily C member 1 (ABCC1) and the breast cancer resistance protein 1 (BCRP1) ABCG2 (Huang et al. 2013). ABCG2 has been considered as one of the major transporters causing drug resistance in cancer cells (Chen et al. 2010). ABCG2 is capable of effluxing and conferring resistance to most conventional anticancer agents, and its overexpression is often linked to MDR in patients with advanced non-small cell lung cancer (NSCLC) or leukemia (Wu et al. 2017). BCRP1/ABCG2 provides an important cell survival advantage under hypoxia or oxidative stress and plays a pivotal role in modulating the proliferation, differentiation, and survival of these cells (Higashikuni et al. 2010).
Angiogenesis is a biological process of new blood vessel formation and is involved in the process of metastasis (Siddharth et al. 2018). Tumor growth and angiogenesis are codependent; tumor cells secrete several pro-angiogenic molecules that signal endothelial cells to cause neovascularisation (Abhinand et al. 2020). It is reported that, ABCG2 may be associated with angiogenesis and redox regulation in tissues under pathological conditions and in the heart, it is expressed mainly in endothelial cells of micro vessels and plays a pivotal role in cardiac repair after myocardial infarction by promoting angiogenesis (Higashikuni et al. 2012). Previously it is shown that, synchronous co-expression of CD-133 and ABCG2 in stage I NSCLC predicts early recurrence and high level of angiogenesis (Li et al. 2011). A previous result showed that, VEGF A was over-expressed in SP cells accompanied by over-expression of ABCG2 and MDR1 mRNA (Cao et al. 2012). It has already been observed that, there is a synergistic enhancing effect of VEGF and MDR on the invasion of Hep-2 cells (Li et al. 2009) and VEGF is related to the over-expression of MRP1 and MDR in glioblastomas (DeLay et al. 2012). The VEGF/PI3K/Akt pathway is one of the most important signal pathways in cancers, which is vital to angiogenesis, proliferation, invasion, enhancing cell repair motility and migration ability, and inhibition of apoptosis in cancer cells (Yao and Zhang 2019). A study also proved that, mesenchymal stem cells-derived exosomes with low-expressed miR-153-3p notably stimulates the activation of Angiopoietin 1 (ANGPT1) and the VEGF/VEGFR2/PI3K/Akt/eNOS pathways, hence prevents the damaged endothelial cells and cardiomyocytes against hypoxia (Ning et al. 2021).
Curcumin (Cur) is a natural product synthesized from rhizome of Curcuma longa Linn, a well-studied phytochemical that has the potential to suppress the cancer growth (Murakami et al. 2017; Zhang et al. 2018). It is an effective chemo sensitizer that modulates the function of ABCB1, ABCC1, and ABCG2 transporters, inhibits drug efflux and increases the efficacy of anticancer agents in multidrug-resistant cancers (Rao et al. 2014). Cur has shown promising effects in inhibiting cancer angiogenesis, cell migration, invasion, modulates the expression of multiple cell signaling proteins, such as NF-κB, COX-2, and MMP-9 and inhibits EMT (Liu et al. 2019).
Quinacrine (QC) is also a natural bioactive compound, originally used as an antimalarial drug, recently rediscovered its anti-cancer activity against varieties of cancers (Das and Kundu 2021). It inhibited cancer cells growth in in vitro, in vivo and other preclinical model systems (Siddharth et al. 2016; Zhu et al. 2018). In alone or combing with other chemotherapeutic agents, it inhibits topoisomerase activity, decreases major components of cancer cell signaling (WNT/β-catenin, HH-Gli, Notch), increases DNA damage and inhibits the base excision repair pathways (Preet et al. 2012, 2016; Nayak et al. 2017, 2019). The hybrid nanoparticles of silver/gold and QC exhibited an enhanced efficacy against CSCs and inhibitory effect in angiogenesis (Satapathy et al. 2015, 2018).
Very recently, using SP cells (highly CSCs enriched cells), we have shown that Cur and QC combination synergistically kill the breast cancer cells by inhibiting ABCG2. We have reported the detailed apoptotic mechanism of Cur and QC by biochemical assays including in silico approaches in breast cancer cells (Nayak et al. 2020). But no study was performed to prove the effect of Cur + QC in inhibiting ABCG2 mediated metastatic and angiogenic potentiality of CSCs in any in vitro, in vivo or ex vivo system. Here using the in vitro (SP cells as a model system), ex vivo (patient derived CSCs cells) and patient derived xenograft (PDX), we studied the role of ABCG2 in metastasis and angiogenesis and also delineate the detail mechanism of Cur + QC mediated inhibition of angiogenesis in breast cancer.
Materials and methods
Cell culture and reagents
Breast cancer cells were grown in dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 1.5 mM L-glutamine and 1% antibiotic (100 U/ml of penicillin, 10mg/ml of streptomycin) in a humidified atmosphere of 5% CO2 at 37°C. MCF-10A-Tr cells were prepared by the continuous exposure of MCF-10A (normal breast epithelial cells) to cigarette smoke condensate formed from commercially available Indian cigarettes (Mohapatra et al. 2014). These cells are genetically different from their parental cells MCF-10A, more aggressive compared to other breast cancer cells with higher mammosphere forming potentiality and capable of forming visible tumor when implanted to Balb/C mice (Mohapatra et al. 2014). Briefly, MCF-10A-Transformed (MCF-10A-Tr) cells were trypsinized and resuspended in serum free hypoxic medium supplemented with 10 ng/mL basic fibroblast growth factor (bFGF), 20 ng/mL epidermal growth factor (EGF), 5µg/mL insulin, 0.4% bovine serum albumin (BSA) and 20 µM CoCl2 for formation of mammosphere. At first, the cells formed a monolayer culture, stable till 7 passages, termed as Quiescent Tr (Q-Tr). Prolong culture of Q-Tr cells under similar conditions resulted into the development of mammospheres (MAMMO). Upon transfer back to serum containing media (DMEM-F12 (Nutrient mixture F-12) containing 10% FBS, 1.5 mM L-glutamine and 1% antibiotic (100 U/ml of penicillin, 10mg/ml of streptomycin)), the spheroids developed a stable immortal adherent cell line, MCF-10A-Tr-post epithelial to mesenchymal transformed which were already undergone epithelial to mesenchymal transition (MCF-10A-Tr-P-EMT).
Then, CSCs enriched SP cell were isolated from MCF-10A-Tr-P-EMT cells (Nayak et al. 2020). Briefly, 1 × 106 trypsinized cells were washed with 1X PBS followed by addition of Hoechst 33342 (5µg/mL) for 90 min at 37°C. After that, the cells were incubated with 2µg/mL propidium iodide (PI) and analyzed by flow cytometry (BD FACS Aria II, BD Biosciences, San Jose, USA). Hoechst “blue” represented the 450 BP filter and Hoechst “red” was detected at 675 nm. Using Hoechst red and blue axes, a live gate was set on the flow cytometer to exclude dead cells. The SP cells were clearly noted after collecting 104 events within the live gates. SP and non SP cells were categorized into sterile eppendorf tubes containing 100% FBS. Then these cells were grown in culture using DMEM supplemented with 10% FBS, 1.5 mM L-glutamine and 1% antibiotic (100 U/mL of penicillin, 10mg/mL of streptomycin) at 37°C in a humidified atmosphere of 5% CO2 and used for experiments.
Conditioned Media (CM) was prepared following the protocol mentioned earlier (Siddharth et al. 2018). In brief, 1 × 106 cells were seeded in 60 mm tissue culture dishes, incubated for 24 h, then, centrifuged at 1800 rpm at 4°C for 5 min. This supernatant is termed as CM. The protein concentration of CM was measured and used for further experimentation.
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cord and were grown following the protocol referred earlier (Siddharth et al. 2018). HUVEC was collected from Kalinga Institute of Medical Sciences Hospital, Bhubaneswar, Odisha, India according to the Institutional Ethical Committee. In brief, HUVECs were grown in M199 (Gibco, Grand Island, NY) growth medium containing 20% FBS and endothelial cell growth supplements (ECGS, BD Biosciences, Bedford, MA). All experiments in HUVECs were done between passages 2–4.
Cell culture reagents and other growth supplements were purchased from Himedia, India. QC (Cat#Q3251) and Cur (#C7727) were procured from Sigma-Aldrich (St Louis, MO). ABCG2 siRNA (#sc-41,151) was purchased from Santa Cruz Biotechnology Inc., CA, USA. ABCG2 plasmid (#25,983) was purchased from Addgene, USA. ABCG2 purified protein (#RPA960Hu01) was purchased from Cloud clone corp., USA. Anti-CXCR-4 (#SAB3500383) was purchased from Sigma-Aldrich (St Louis, MO). Anti-ABCG2 (#42,078), anti-ABCB1 (#13,342), anti-ABCC1 (#14,685), anti-β-catenin (#9587), anti-HIF-1α (#3716), anti-Ang-2 (#2948), anti-c-Myc (#9402), anti-Cyclin D1 (#2922), anti-Chk1 (#2345), anti-E-cadherin (#3195), anti-Vimentin (#5741), anti-Akt (#9272), anti-PI3K (#5292), anti-Sox-2 (#2748), anti-Gli-1 (#2643), anti-eNOS (#32,027), anti-TNF-α (#3711), anti-TGF-β (#3707) anti-mouse IgG (#7076) and anti-rabbit IgG (#7074) were purchased from Cell Signaling Technology, MA, USA. Anti-CD-133 (#E-AB-33,462) is purchased from Elabscience, USA. Anti-Nanog (#sc-376,915), anti-Oct-4 (#sc-5279) and anti-GAPDH (#sc-25,778) were purchased from Santa Cruz Biotechnology Inc., CA, USA. Anti-CD-44 (#ab23557) and anti-Ang-1 (#ab8451) was purchased from Abcam, MA, USA.
Treatment of SP cells with cur and QC
The SP cells were grown and treated with Cur and QC prior to perform the experiments. Before treatment with the drugs, cells were kept for 6–8 h in serum free starvation medium. Both Cur and QC were dissolved in DMSO to make the stock solution. But, in the final treatment, the agents were dissolved in complete media (serum containing). For all the experiments, the cells were treated individually with fixed doses of Cur (8 µM) and QC (5 µM), respectively for 48 h and in combination treatment, cells were pre-treated with a fixed concentration of Cur (8 µM) for 6 h followed by treatment of QC (4 µM) for another 48 h prior to perform the experiments.
Matrigel invasion assay
To study the ability of the cells to attach and invade the matrix and migrate forward, matrigel invasion assay was performed using a 24 transwell plate with a pore size of 8 μm and inserts coated with 20 µL of matrigel (BD Biosciences, 356,234) as referred earlier (Siddharth et al. 2016). Briefly, 3 × 105 cells were resuspended in 100 µL of serum-free media and seeded onto matrigel coated inserts and serum containing complete medium was added to the lower chamber and incubated for 48 h. The non-invaded cells were removed with the help of a cotton swab. The invaded cells were stained with DAPI and counted microscopically at 5 different fields at 40x under the fluorescence microscope (Nikon, Japan). Data is represented as % invasion against cell types and calculated using the following formula,
 |
TCF/LEF promoter activity assay
To measure the promoter activity of the TCF/LEF transcription factor, a luciferase-based reporter gene was used. Cells were grown and divided into two different dishes. In the first set, cells were co-transfected with 2.5 µg TOP Flash, which is a synthetic luciferase-based promoter plasmid (sensitive to the activity of the β-catenin/TCF-4 complex, containing three copies of the TCF-4 binding site upstream of a firefly luciferase reporter gene) and β-galactosidase (β-gal) (0.5µg) using the Lipofectamine 2000® transfection reagent. In the second set, an equal amount of the mutant form of the above promoter (FOP Flash) and β-gal were co-transfected using the same transfection reagent. β-gal was used to monitor the efficacy of transfection in the cells. Similarly in another set of cells, ABCG2 was silenced by siRNA treatment followed by transfection with TOP Flash/FOP Flash plasmid and β-gal and incubated for 24 h post transfection (Siddharth et al. 2017; Nayak et al. 2020). After that, each set was treated with the combined drug for the time period mentioned in the figure legend. Then cells were lysed, and efficiencies of transfection were normalized to β-gal activity. Luciferase activity was measured by micro plate reader (multimode ELISA reader). The relative luciferase activity (TOP Flash/FOP Flash) was calculated from triplicate experiment sets.
Knockdown of ABCG2
Wild-type gene expression of ABCG2 was knocked down in SP cells according to the protocol mentioned earlier (Nayak et al. 2020). Cells were grown in 60 mm cell culture dishes to 70% confluence and were transfected with 4 µg of ABCG2 siRNA and equal amount of scrambled construct using lipofectamine as a transfection reagent. After 8 h, media was replaced and cell growth was allowed for another 24 h. Thereafter, silenced cells were treated with Cur + QC as mentioned in the figure legend. Cells were harvested and ABCG2 level was determined by western blot analysis. Numerical values above each panel of protein represent the band intensity, measured using a UVP GelDoc-It ® 310 Imaging system (UVP, Cambridge, UK).
In ovo blood vessel formation assay
New blood vessel formation in chick embryo was measured in ovo following the protocol described earlier (Satapathy et al. 2015). Briefly, fertilized eggs were incubated for 60 h in a humidified atmosphere at 37°C. Then, under aseptic conditions, windows were made in the egg shell and CM (100µg/ml protein) was added. The progressive increase in vascularity was observed and the pictures were captured photographically.
Overexpression of ABCG2 in SP cells
SP cells were overexpressed with ABCG2 using 4 µg of pSIN4-EF2-ABCG2-IRES-Neo ABCG2 plasmid with Lipofectamine 2000® as transfection reagent following manufacturer’s protocol. Briefly, 1 × 105 SP cells per well were grown in a six well plate and allowed to attain 70% confluence. 4 µg of pSIN4-EF2-ABCG2-IRES-Neo plasmid was transfected by lipofectamine 2000. After 8 h of transfection, serum containing media was added and treatment was performed as mentioned above. After completion of the treatment, cells were harvested and ABCG2 level was determined by western blot analysis. Numerical values above each panel of protein represent the band intensity, measured using a UVP GelDoc-It ® 310 Imaging system (UVP, Cambridge, UK).
ABCG2 purified protein induced angiogenesis
60 h incubated fertilized egg was treated with 1 µg/ml of ABCG2 purified protein for 48 h for in ovo new blood vessel formation.
Gelatin zymography
Expression of MMP-9 and MMP-2 were detected by Gelatin zymography as described earlier (Chatterjee et al. 2021). HUVECs supplemented with metastatic cell (SP cells) CM were grown for 24 h, then, media was replenished with fresh medium and incubated for 24 h. Supernatant of HUVECs was collected and separated on SDS-PAGE containing gelatin co-polymerized with polyacrylamide gel matrix for the detection of MMP-2 and MMP-9. Gels were washed thrice with washing buffer (50 mM Tris HCL, 5 mM CaCl2, 1 µM ZnCl2 and 2.5% Triton X-100 in dH2O, pH 7.4), incubated with incubation buffer (50 mM Tris HCL, 5 mM CaCl2, 1 µM ZnCl2, 1% Triton X-100 in dH2O, pH 7.4) for 16–21 h at 37°C. Then the gels were stained with 1% CBBR-250. Enzyme digested regions appeared as light bands in comparison to the dark background.
Wound healing assay for cell migration
Cell migration was examined using a wound healing assay as described earlier (Nayak et al. 2020). Cells were cultured in 40 mm tissue culture discs until 90% confluence. A sterile micro tip was used to generate a clean wound in the cell monolayer across the centre of the well. Cell debris was removed by washing the plates with fresh medium. The cells were treated in fresh medium with a desired concentration of drugs and then the cells were allowed to migrate in the medium. The wound was assessed by a microscope (Nikon, Japan) at 20x magnification at different time points.
Tube formation assay
A tube formation assay was performed to know the in vitro representation of tube formation (Chatterjee et al. 2021). CM of SP cells was collected and added to 2 × 103 HUVECs in 24 well matrigel coated plates and incubated for 24 h. Tube like structure formation was observed and after Rhodamine Phalloidin staining the structure was captured in five different microscopic fields at 40x magnification using fluorescence microscopes (Evos Fluorescence Microscope, Thermo Fisher Scientific, MA, USA). One set of the representative images was presented.
Enzyme‑linked immunosorbent assay (ELISA)
The experiment was performed as per the protocol referred earlier (Pradhan et al. 2021). Briefly, 30 µg of protein antigen (CM) was mixed with coupling buffer (15 mM Na2CO3 and 25 mM NaHCO3 in PBST), coated onto 96-well micro plates and incubated overnight at 4°C. Then, the solutions were aspirated and each well was washed with wash buffer (1X PBST) and blocked with blocking solution (1% BSA in PBST) and incubated at room temperature for 30 min. Then the blocking solution was removed and the protein specific antibodies (e.g., anti-Ang-1, anti-Ang-2, anti-VEGF A, anti-HIF-1α, anti-TNF-α and anti-TGF-β) were added and incubated for 3 h. Then the wells were again washed with 1X PBST. After that, HRP conjugated secondary antibodies were added and incubated for 2 h. Then the wells were washed with 1X PBST and substrate solution (2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonic acid)) was added in each well and the absorbance of the colored product was measured using micro plate reader (Berthold, Germany) at 405 nm. Besides the above mentioned method, Sandwich ELISA was performed to detect the proliferation marker Ki67 in SP cells using manufacturer’s protocol. Data was calculated and represented graphically as absorbance vs. different sample conditions.
Collection and processing of breast tumor sample for metastasis model development
Breast tumor sample was collected from Acharya Harihar Regional Cancer Centre, Cuttack, Odisha, India following the ethical guidelines of the hospital as per the hospital review board. The samples were collected by following the inclusion criteria (i. The patient must have been confirmed invasive breast cancer histologically, ii. The patient having recurrent breast cancers). Tumor cells were isolated from tumor specimens as per the protocol referred earlier (Pradhan et al. 2021). Briefly tumor tissues were washed in 1X PBS and chopped into pieces in a media containing antibiotics (a mixture containing 0.14 mM ampicillin, 0.26 µM Amphotericin B and 7.54 µM ciprofloxacin) followed by enzymatic treatment with 0.1% collagenase and 50 U/mL dispase in a 37°C water bath for at least 2 h with continuous rotation. After incubation, cells were sieved through 40 μm cell strainer, centrifuged at 1000 rpm for 5 min, seeded onto 60 mm cell culture dishes in DMEM-F12 containing 20% FBS, 1.5 mM L-glutamine and 2% antibiotic (100 U/mL penicillin and 10mg/mL streptomycin) and allowed to grow for 7 days. Then the media was aspirated and fresh media was added and allowed to grow up to 70–80% confluency. After that, the cells were trypsinized and then resuspended in fresh media or was grown for the formation of metastatic model according to the above mentioned protocol.
Histopathological study (H&E staining) and immunohistochemical (IHC) analysis
Histopathology (H&E staining) and IHC study of the patient sample tissue was done as per the procedure described earlier (Sethy et al. 2021). Briefly Paraffin-embedded specimens were sectioned at 3 μm thickness, mounted on slides, heated at 60°C for 30–40 min for melting the wax. Then it was dewaxed with xylene and rehydrated by immersing in different concentrations of alcohol (100%, 90% and 70%, respectively). For H&E staining, the sections were incubated with haematoxylin followed by eosin stain and rinsed in water. After that, the sections were dehydrated by submerging in increasing concentrations of alcohol (70%, 90%, and 100%) followed by xylene for 2 min. For IHC, 3 μm rehydrated tissue sections were washed in 1X PBS and antigen retrieval process was performed by citric acid bufer (pH 6). Then the sections were blocked with 5% FBS for nonspecific binding of the protein and endogenous peroxide activity was blocked with H2O2. Then, sections were incubated with protein specific antibody overnight at 4°C, washed thrice by 1X PBS, incubated by HRP-conjugated secondary antibody for 2 h at room temperature. Next, the sections were washed with 1X PBS and stained with DAB followed by haematoxylin counterstain. Immunoreactivity was observed using a DAB kit (SK-4100, Vector Laboratories, CA, USA) and images were taken at 20x magnification using a bright-field microscope (Leica DM200, USA).
Establishment of patient derived xenograft (PDX) model and blood vessel formation in female Balb/C mice
Female Balb/C mice (6 weeks old) were housed in a proper light/dark cycle of 12/12h and experiments were carried out using the protocol duly approved by the Institutional Animal Ethical Committee, KIIT School of Biotechnology, deemed to be University, Bhubaneswar, Odisha, India. Briefly, breast tumor sample was collected from Acharya Harihar Regional Cancer Centre, Cuttack, Odisha, India following the ethical guidelines of the hospital as per the hospital review board. The tumor tissue was cut into small pieces. Then, a deep incision was made in the anterior region of Balb/C mice using a scissor and then 5–6 chopped tumor tissue pieces were implanted in the anterior peritoneal wall within 3 h of collecting tumor tissues from the patient. For this, we have taken 4 different groups of mice (each group contained 3 mice) along with an equal volume of PBS only for the control. The mice were monitored every other day for 25 days. After that, the treatment of Cur and QC individually and in combination (20 mg/kg/day for each treatment condition) was given orally for at least 2 weeks and then the mice were sacrificed. The implanted site was reopened and blood vessel growth was captured by camera.
Western blot analysis
Western blotting was performed according to the protocol referred earlier (Dash et al. 2022). Approximately, 5 × 105 grown cells were treated with the desired drug as mentioned earlier. Then, cellular lysates were prepared using modified RIPA lysis buffer, and 40 µg of protein was loaded and separated on 10% SDS-PAGE gel electrophoresis (4.5% SDS-PAGE for APC). Proteins were transferred onto PVDF membrane, and western blotting was performed using specific antibody.
Quantification
For quantification of in ovo blood vessel formation, images were analysed using AngioTool software (NIH) and represented graphically using GraphPad Prism 5.0 software. In case of western blot and zymography quantification, numerical value of each panel of protein was the representation of relative fold changes. These fold changes were measured by densitometric analysis using UVP GelDoc-IT® 310.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 5.0 software, USA. The results were represented as the mean ± SD of three separate experiments. The data were analyzed by using one-way Annova followed by Bonferroni multiple comparison test. Statistical significance of difference in the central tendencies compared to control groups was designated as *p < 0.05, ** p < 0.001 and *** p < 0.0001.
Results
Cur and QC combination affects the ABCG2 mediated CSCs signaling in breast cancer cells
CSCs exhibit enhanced DNA repair capacity, chemoresistance and rapid efflux of drugs by upregulating ABC transporters (Siddharth et al. 2016). To study the effect of Cur and QC on highly metastatic breast cancer stem cells (mBCSCs), we first established a metastasis model using MCF-10A-Tr cells and then isolated SP cells (Fig. 1A). The stemness properties and metastatic potentiality of the cells were monitored by measuring some representative characteristics, such as invasive potentiality and expression of representative markers. The invasive potentiality of the cells was increased from EMT to P-EMT and highest invasiveness was noted in SP cells (Fig. 1B). An induction of epithelial marker E-cadherin was noticed in EMT, P-EMT and SP cells and decreased in MAMMO cells whereas a significant reduction of mesenchymal marker Vimentin in EMT, P-EMT and SP cells and reappearance in MAMMO cells was noted which confirmed the model true metastatic in nature (Fig. 1Ci-ii). As both P-EMT and SP cells showed the metastatic potentiality (even SP cells showed higher potentiality in comparison to P-EMT cells) and our previous reports explained the significant expression of ABCG2 in SP cells (Nayak et al. 2020) thus, here we used SP cells for experimentation. Cur and QC and their combination has a cytotoxic and anti-proliferative activity on ABCG2 enriched SP cells (Nayak et al. 2020). Now the expression of ABCG2, ABCC1 and ABCB1 in SP cells after treatment with both the drugs alone and in combination were monitored. The decreased expression of ABCG2, ABCC1 and ABCB1 by 10, 3.3 and 5 fold, respectively in SP cells in respect to control were noted after treatment with Cur + QC (Fig. 1Di-ii). Next, we measured the effect of Cur + QC in the ABC-transporter mediated CSCs signaling (e.g. WNT/β-catenin, HH-Gli) by monitoring the changes in major components of representative CSCs signaling cascade. Decreased protein expression of β-catenin (main intermediate of WNT/β-catenin cascade) and Gli-1 (important component of HH-Gli) and the other associated downstream components were observed after combined exposure of Cur and QC. At optimal combination of the drugs, the expression of the proteins, β-catenin, Cyclin D1, C-Myc, Gli-1 and Chk-1 was reduced by 10, 10, 2, 5 and 3.3 fold with respect to control (Fig. 1Ei-ii). To confirm the ABC-transporter mediated CSCs signaling was affected by Cur + QC, transcription activity of WNT/β-catenin signaling cascade was monitored. Cur + QC combination decreased the transcription activity of WNT/β-catenin transcription factor (TCF/LEF) in compare to untreated cells. The relative luciferase activity of TCF/LEF factor (TOP FLASH/FOP FLASH) was reduced by 3 fold in comparison to untreated one (Fig. 1F) revealed the deregulation of Cur + QC mediated CSCs signaling in breast cancer was modulated by ABC-transporters.
Fig. 1.
Establishment of breast cancer metastasis model and SP cells using MCF-10A-Tr cells. A Morphology of cells at different stages of metastasis. B Matrigel invasion assay in mBCSCs. Ci Expression of E-cadherin and Vimentin in mBCSCs. GAPDH served as the loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Cii is the graphical representation of fig. Ci. Di Expression of ABC transporter proteins after the treatment of Cur and QC individually and in combination in SP cells. GAPDH served as loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Dii is the graphical representation of fig. Di. Ei Expression of WNT/β-catenin signaling proteins in SP cells after the treatment of Cur and QC alone and in combination. GAPDH served as loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Eii is the graphical representation of fig. Ei. F Relative luciferase activity of WNT transcription factor TCF/LEF after the exposure of Cur and QC individually and in combination in SP cells. All the experiments were conducted thrice and representative data were given. Statistical significance was determined by one-way ANOVA where ‘***’, ‘**’, ‘*’ represent statistical significance (P < 0.0001, P < 0.001 and P < 0.05 respectively)
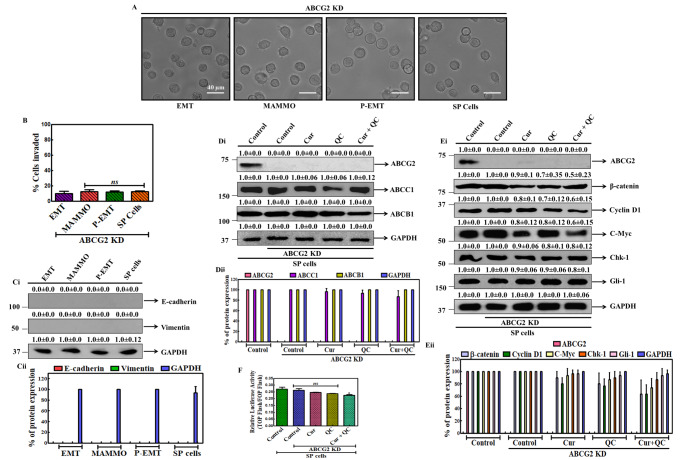
Cur and QC regulates the CSCs signaling through ABCG2
To know the precise role of ABCG2 in mBCSCs formation, we tried to develop a metastasis model using MCF-10A-Tr cells silencing ABCG2. ABCG2 was silenced by transiently transfected with Si-RNAabcg2 plasmid or scrambled siRNA in MCF-10A-Tr or SP cells. There were no distinct EMT, MAMMO and P-EMT phases appeared and deformed unorganized cells were noted. SP cells also did not show P-EMT morphologies rather showed more suspension and deformed structure (Fig. 2A). There were no appreciable changes of invassive potentility of the different phase cells and SP cells was noted (Fig. 2B). The protein expression of representative metastasis marker E-cadhrein, Vimentin was not observed in those cells also suggested that in absence of ABCG2 metastatic potentility of the cells was abolished (Fig. 2Ci-ii). To know whether Cur and QC specifically target ABCG2, an experiment was carried out using SP cells. For this ABCG2 was silenced and then protein expression of other ABC-transporter family proteins (ABCC1 and ABCB1) were monitored using western blot. Interestingly, no significant changes in the expression of ABCC1 and ABCB1 transporter proteins were noted after treatment with Cur and QC individually and in combination (Fig. 2Di-ii). The expression of WNT/β-catenin (β-catenin), HH-Gli (Gli-1) and their downstream signaling components (Cyciln D1, C-Myc, Chk-1) were not altered significantly after treatment with Cur + QC (Fig. 2Ei-ii). Interestingly, no significant alteration of WNT/β-catenin transcription factor (TCF/LEF receptor) was also seen after Cur and QC exposure in ABCG2 knockdown SP cells (Fig. 2F).
Fig. 2.
Development of breast cancer metastasis model using ABCG2 knockdown MCF-10A-Tr and SP cells. A Morphology of different stages of metastasis in ABCG2 knockdown cells. B Invasive potentiality of ABCG2-silenced metastasis cells. Ci Expressions of representative metastasis protein markers in ABCG2 silenced SP cells. GAPDH served as the loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Cii is the graphical representation of fig. Ci. Di Expression of ABC transporter proteins after the treatment of Cur and QC individually and in combination in ABCG2 knockdown SP cells. GAPDH served as loading control. The numerical value above each blot represented the relative fold change in comparison to control analyzed by densitometry. Fig. Dii is the graphical representation of fig. Di. Ei Expression of WNT/β-catenin signaling proteins in Cur + QC treated ABCG2 knockdown SP cells. GAPDH served as loading control. The numerical value above each blot represented the relative fold change in comparison to control analyzed by densitometry. Fig. Eii is the graphical representation of fig. Ei. F Relative luciferase activity of WNT transcription factor TCF/LEF after the exposure of Cur and QC alone and in combination in ABCG2 knockdown SP cells. Data was the mean ± SD of three independent experiments. Statistical significance was determined by one-way ANOVA where ‘*’ represents statistical significance (P < 0.05) and ‘ns’ represents statistical non-significance (P > 0.05)
Cur and QC deregulates metastasis and angiogenesis in SP cells through ABCG2
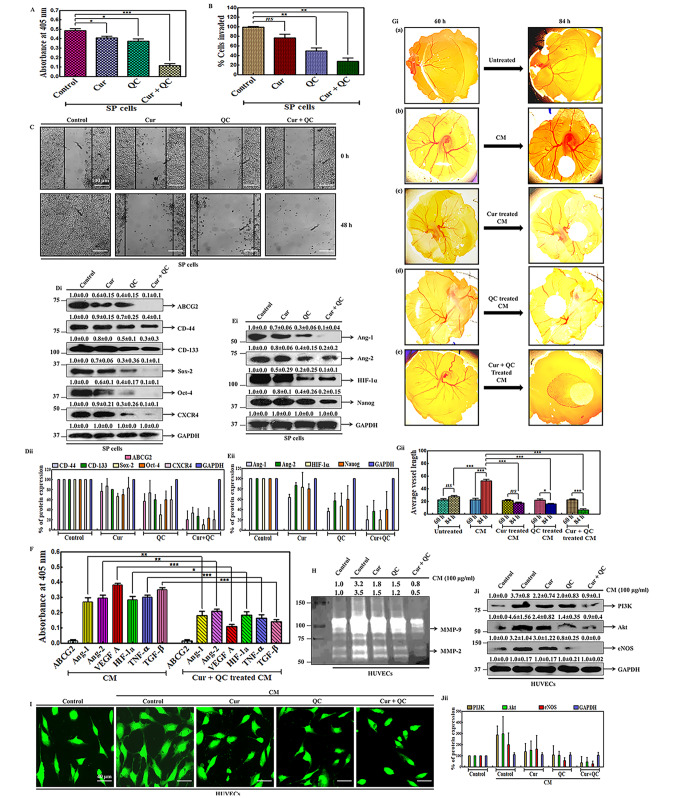
To investigate the effect of Cur + QC in regulating the metastatic properties of SP cells, the cell proliferation, migration and invasive potentialities were measured after the drug exposure. 4.2 fold reduction of representative cell proliferation marker (Ki67), inhibition of cell invasive property and significant reduction of wound healing were noted after Cur and QC treatment (Fig. 3A, B, C). The maximum inhibition of the invasion observed in SP cells after the combination treatment of Cur and QC was 72% as compared to the SP cells treated with Cur (23.5%) and QC (50.5%) individually in compare to untreated control (Fig. 3B). The gap of the wound in treated cells increased with time in contrast to the untreated cells after the drug treatment. In untreated cells, the gap was completely filled at 48 h. The maximum gap of the wound was observed in the combination exposure of Cur and QC in comparison to the individual treatment with Cur or QC (Fig. 3C). After the drug treatment, the expression of CD-44, CD-133, Sox-2 and CXCR4 were decreased by 2.5, 3.3, 10 and 10 fold in compare to untreated control. Interestingly, ABCG2 and Oct-4 expression was totally dissipated after the combined exposure of Cur and QC in compare to control (Fig. 2Di-ii). Next we investigated the angiogenic properties of SP cells after the treatment of Cur + QC. For that, a panel of proteins involved in angiogenesis was examined by western blotting in whole cell lysates of SP cells after drug exposure. Angiogenesis markers (Ang-1 and Ang-2), compared to control, were reduced by 10 fold (Ang-1) and 5 fold (Ang-2). Protein expression of HIF-1α and Nanog reduced approximately 10 fold and 5 fold respectively in combination treatment in compare to control (Fig. 3Ei-ii). It is a well-known fact that, CSCs release angiogenic factors into the cell culture medium which activate the angiogenesis when they bind to surrounding cells. The expression of certain angiogenic markers (e.g. Ang-1, Ang-2, VEGF A, HIF-1α, TNF-α and TGF-β) were observed to be enhanced in the CM of SP cells and decreased after treatment with Cur and QC individually and in combination. Interestingly, the highest expression of VEGF A was found in the CM of SP cells and exact opposite result (lowest expression by 3.5 fold) of VEGF A was noted with the combination of Cur and QC exposure. Interestingly no ABCG2 protein expression was noted in CM (Fig. 3F). Then direct angiogenesis capability of cells was analyzed by measuring the blood vessel formation using fertilized egg embryo (in ovo CAM assay) which showed that, the CM from SP cells triggered new blood vessel formation over 84 h of incubation (Fig. 3Gi b). But a remarkable decrease in number of blood vessel was noted in eggs after the addition of the CM treated with Cur and QC in combination (Fig. 3Gi e) in compare to the addition of CM treated with Cur (Fig. 3Gi c) and QC (Fig. 3Gi d) individually. CSCs secrete certain angiogenic factors that induce a paracrine chemo attractant effect on endothelial cells for angiogenesis. MMPs are traditionally associated with matrix remodeling, cancer invasion and angiogenesis (Shay et al. 2015). Hence the level of representative markers, MMP-9 and MMP-2 were monitored using a gelatin zymography in HUVECs supplemented with CM of SP cells treated with drugs individually and in combination. This showed a 3.2 fold and 3.5 fold increase in MMP-9 and MMP-2 expression in HUVECs supplemented with CM of SP cells, respectively, compared to HUVEC cells without CM. The combination treatment of Cur and QC was found to be more effective in reducing MMPs expression. Approximately, 4 and 7 fold decrease in expression of MMP-9 and MMP-2 were measured in Cur + QC treated CM induced HUVECs respectively (Fig. 3H). In vitro tube formation assay data appeared that, HUVECs supplemented with CM from SP cells exhibited a significant enhancement in the tube formation. The combination of Cur and QC was found to be more effective than their individual treatments in deteriorating the tube formation (Fig. 3I). Then, we anticipated whether there is any mechanism involved in the process, which actually induced angiogenesis. To ascertain that, we performed western blot analysis of a panel of proteins involved in the VEGF A mediated angiogenic pathway. This revealed a 3.7 fold and 4.6 fold increase in PI3K and Akt in HUVECs supplemented with CM of SP cells, respectively, compared to HUVEC cells without CM. But, a decrease in expression of PI3K was observed after the combination treatment of Cur and QC in compare to the individual treatments. Interestingly, eNOS in HUVECs was upregulated by 3.2 fold after supplementation with CM and totally vanished in the combination treatment in compare to the CM supplemented control (Fig. 3Ji-ii).
Fig. 3.
Cur and QC modulates the ABCG2 mediated metastasis and angiogenesis in SP cells A Expression of proliferation marker Ki67 in whole cell lysates of SP cells. B Invasive capacities of the cells after drug treatment. C Anchorage-dependent cell migration of SP cells after drug treatment. Di Expression of representative metastatic markers in SP cells after the exposure of Cur and QC individually and in combination . GAPDH served as loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Dii is the graphical representation of fig. Di. Ei Expression of representative angiogenic markers in treated SP cells. GAPDH served as loading control. The numerical value above each blot represented the relative fold change in comparison to control analyzed by densitometry. Fig. Eii is the graphical representation of fig. Ei. F Expression of the representative soluble angiogenic markers in CM of SP cells after Cur and QC treatment measured by ELISA. Gi CAM assay showing the effect of CM from ABCG2 enriched SP cells in inducing new blood vessel formation and reducing new blood vessel formation in ABCG2 enriched SP cells treated with Cur and QC alone and in combination in ovo. Fig. Gii is the graphical representation of fig. Gi using angiotool64 0.6a Software. H Expression of MMP-9 and MMP-2 in HUVECs supplemented with CM of SP cells after treatment with Cur and QC. I In vitro tubule formation of HUVECs supplemented with CM of SP cells after the treatment of drugs. Ji Expression of PI3K, Akt and eNOS in whole cell lysates of HUVECs supplemented with CM of SP cells. GAPDH served as loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Jii is the graphical representation of fig. Ji. All the experiments were conducted thrice and representative data were given. Statistical significance was determined by one-way ANOVA where ‘***’, ‘**’, ‘*’ represent statistical significance (P < 0.0001, P < 0.001 and P < 0.05 respectively) and ‘ns’ represents statistical non-significance (P > 0.05)
Knockdown of ABCG2 reduces the metastasis and angiogenesis in ovo
Further to confirm the role of ABCG2 in metastatic properties, ABCG2 was knocked down in SP cells and treated with Cur + QC and the proliferation, invasion, migration were accessed. Non-significant alternations of Ki67 expression, invassion and migration potentiality of cells were noted after Cur and QC treatment (Fig. 4A, B, C). The expression of metastatic markers CD-44, CD-133, Sox-2, Oct-4 and CXCR4 were slightly changed in the combination treatment of Cur and QC (Fig. 4Di-ii). Similarly, the expression of representative markers for angiogenesis was analyzed in ABCG2 knockdown SP cells. A non-significant change in the protein expression was found in both individual and combination treatments of Cur and QC (Fig. 4Ei-ii). An unaltered expression of representative angiogenic markers in the CM of ABCG2 knockdown SP cells and in the Cur + QC treated CM of ABCG2 knockdown SP cells were also noticed (Fig. 4F). When the CM of ABCG2 knockdown SP cells and the CM of ABCG2 knockdown SP cells treated with Cur and QC individually and in combination was incubated to the chick embryo, no considerable change in the angiogenesis was observed (Fig. 4Gi). Likewise, no distinguished alterations in MMP-9 and MMP-2 expression was found in HUVECs supplemented with the CM of ABCG2 knockdown SP cells and after the supplementation of the CM of ABCG2 knockdown SP cells treated with Cur and QC individually and in combination (Fig. 4H). Then the CM of ABCG2 silenced SP cells was supplemented to HUVECs and incubated for 48 h, no distinctive tube formation was observed. In the combination treatment of Cur and QC, nonsignificant morphological changes of HUVECs were found (Fig. 4I). Interestingly no remarkable change in the expression of angiogenic signaling cascade in HUVECs was observed in compare to control (Fig. 4Ji-ii).
Fig. 4.
Cur + QC treatment did not alter metastasis and angiogenesis in ABCG2 knock down SP cells. A Expression of proliferation marker Ki67 in whole cell lysates of ABCG2 knock down SP cells. B Matrigel invasion assay in ABCG2 knockdown SP cells after drug treatment. C Anchorage-dependent cell migration of ABCG2 knockdown SP cells after drug treatment. Di Expression of some representative metastatic markers after the treatment of Cur and QC individually and in combination in ABCG2 knockdown SP cells. GAPDH served as loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Dii is the graphical representation of fig. Di. Ei Expression of some angiogenic markers in treated ABCG2 knockdown SP cells. GAPDH served as loading control. The numerical value above each blot represented the relative fold change in comparison to control analyzed by densitometry. Fig. Eii is the graphical representation of fig. Ei. F Expression of some angiogenic markers in CM from ABCG2 knockdown SP cells and same CM treated with Cur and QC alone and in combination measured by ELISA. Gi CAM assay showing the effects of CM from ABCG2 knockdown SP cells treated with Cur and QC alone and in combination in ovo. Fig. Gii is the graphical representation of fig. Gi using angiotool64 0.6a Software. H Expression of MMP-9 and MMP-2 in HUVECs supplemented with CM of ABCG2 knockdown SP cells after treatment with Cur and QC individually and in combination. I In vitro tubule formation of HUVECs incubated with CM from ABCG2 silenced SP cells after the treatment with desired drugs. Ji Expression of PI3K, Akt and eNOS in whole cell lysates of HUVECs supplemented with the CM of ABCG2 knockdown SP cells. GAPDH served as loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Jii is the graphical representation of fig. Ji. All the experiments were conducted thrice and representative data were given. Statistical significance was determined by one-way ANOVA where ‘***’, ‘**’, ‘*’ represent statistical significance (P < 0.0001, P < 0.001 and P < 0.05 respectively) and ‘ns’ represents statistical non-significance (P > 0.05)
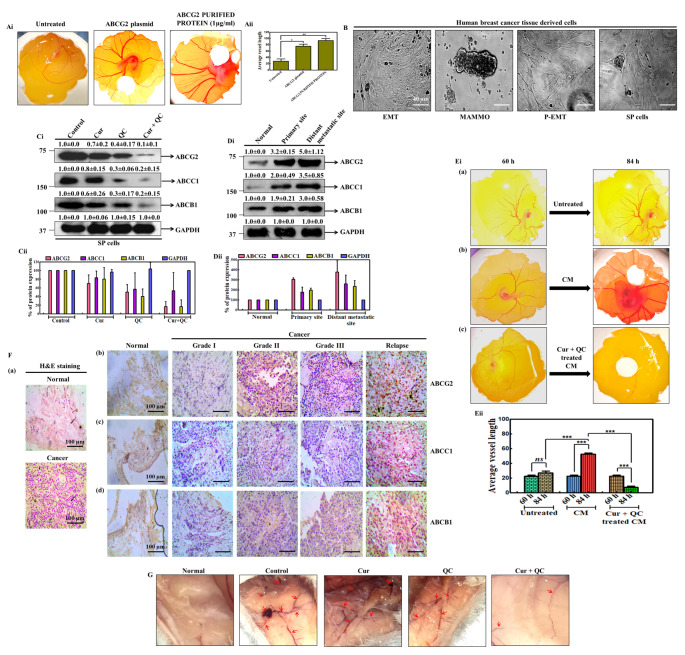
Cur and QC in combination decreased the angiogenesis provoked by ABCG2 in patient derived samples
To study the role of ABCG2 in detail; we have carried out two sets of experiments. In one set, ABCG2 gene was overexpressed and CM was collected from the over-expressed cells and the blood vessel formation in egg was measured. In another set, purified ABCG2 protein was used to study the vascularization. Interestingly, it was noted that CM of ABCG2 over-expressed cells and purified protein increased blood vessel in similar manner (Fig. 5Ai). Quantification of blood vessel length showed higher average length in eggs treated with CM from over-expressed cells and purified ABCG2 compared to untreated eggs (Fig. 5Aii). Then a metastasis model was developed by using the patient derived breast tumor tissue cells and the angiogenesis was studied (Fig. 5B). The expression of ABCG2 including ABCC1 and ABCB1 was accessed in the cell lysate of the cultured human breast cancer tissue derived cells (SP cells) after treating with Cur and QC individually and in combination. In the combination treatment of Cur and QC, highest decrease in the expression of ABCG2, ABCC1 and ABCB1 was found by 10 fold, 5 fold and 5 fold respectively in compare to control (Fig. 5Ci-ii). Then we measured the expression of above proteins in distant relapsed samples (DRS), their primary tumors and normal adjacent tissues by western blotting. Similar to the above data, expression of all the markers (ABCG2, ABCC1 and ABCB1) was increased by 5, 3.5 and 3 fold, respectively, in DRS and elevated up to 3.2, 2 and 1.9 fold in primary tumors as compared to the paired adjacent normal tissues (Fig. 5Di-ii). In ovo data revealed that, CM from cultured human breast cancer tissue derived cells (SP cells) increased angiogenesis (Fig. 5Ei b) compared to untreated eggs (Fig. 5Ei a). The highest angiogenesis was noted over 84 h time course in compare to untreated eggs of 84 h (Fig. 5Ei b). After addition of Cur and QC, decrease in the angiogenesis was noted (Fig. 5Ei c). Higher expression of ABCG2 was detected by IHC in human breast cancer tissue in compare to ABCC1 and ABCB1 (Fig. 5F b-d). H&E staining showed characteristic cancerous nature and marked differences in paired adjacent normal tissues (Fig. 5F a). Finally to study the effect of Cur + QC in vivo, a PDX model was established after implanting the tumor tissue into the Balb/C mice. The group of mice implanted with tumor tissue displayed aggressive new blood vessel formation compared to the saline treated group. But new blood vessel formation was diminished significantly after the combination treatment of Cur and QC (Fig. 5G).
Fig. 5.
Cur and QC disrupts the angiogenesis in patient derived samples. Ai CAM assay showing the effects of pSIN4-EF2-ABCG2-IRES-Neo plasmid and purified ABCG2 protein in inducing new blood vessel formation in ovo. Aii Graphical representation of CAM assay of fig. Ai. B Morphology of human breast cancer tissue derived cells at different stages of metastasis. Ci Western blot analysis of MDR proteins in the lysate of cultured human breast cancer tissue derived cells after the treatment of Cur and QC alone and in combination. GAPDH served as the loading control. The numerical value above each blot indicated the relative fold change in comparison to control analyzed by densitometry. Fig. Cii is the graphical representation of fig. Ci. Di Expression of representative MDR markers in different primary and distant sites. GAPDH served as the loading control. The numerical value above each blot represented the relative fold change in comparison to control analyzed by densitometry. Fig. Dii is the graphical representation of fig. Di. Ei CAM assay showing new blood vessel formation in eggs after induction of CM from cultured tumor tissue derived SP cells. Eii Graphical representation of in ovo blood vessel formation from fig. Ei at three different microscopic fields using angiotool64 0.6a Software. F Expression of ABCG2, ABCC1 and ABCB1 in primary breast cancer tissue sample a histopathology of H&E staining of human breast samples. Rows b–d show the expression of ABCG2, ABCC1 and ABCB1 by IHC analysis in different stages of sample. The images were taken by bright-field microscopy at 20x magnification. Scale bar represents 20μm. Images are representative of three independent experiments. G Decrease in In vivo blood vessel formation in female Balb/C mice after the treatment of Cur and QC individually and in combination. Photographs are representative of three independent experiments. All the experiments were conducted thrice and representative data were given. Statistical significance was determined by one-way ANOVA where ‘***’, ‘**’, ‘*’ represent statistical significance (P < 0.0001, P < 0.001 and P < 0.05 respectively) and ‘ns’ represents statistical non-significance (P > 0.05)
Discussion
Although the function of ABCG2 in drug efflux, EMT transition, drug resistance and persistence of CSCs are well accepted fact in cancer biology, but the role of ABCG2 in cancer metastasis and angiogenesis and its regulation in those processes are unknown. Tumor angiogenesis research and anti-angiogenesis drug development efforts have remarkably increased the awareness of the importance of the tumor microenvironment with respect to tumor progression and aggressiveness. SP cells share the fundamental features of CSCs such as self-renewal, high tumorigenicity, differentiation potentiality, chemotherapeutic resistance, hence the identification and characterization of SP cells help to target and kill the CSCs (Yu et al. 2015; Shang et al. 2016). Here, using in vitro (SP cells), ex vivo (patient derived CSCs), PDX model and multiple biochemical assays, systemically we have showed that ABCG2 has potential role in cancer angiogenesis and Cur and QC treatment inhibits the process. Under hypoxia, activated ABCG2 releases VEGF A (an angiogenic factor) into surrounding medium and these released VEGF A interacts with receptors in endothelial cells (e.g., HUVECs) and promotes the angiogenesis process via PI3K-Akt-eNOS signaling cascade. Cur and QC inhibits ABCG2 and disrupts VEGF A secretion as a result the angiogenesis process halts.
To reach a conclusion, we systematically performed series of experiments using several systems starting from in vitro cell culture to ex vivo and finally in vivo PDX model. At first, (i) a metastatic mBCSCs model was established using MCF-10A-Tr cells and SP cells. SP cells exhibited the highest invasiveness and CSCs characteristics with highest over-expression of ABCG2 protein. These cells also offered highest migration, proliferation, induction of expression of representative MDR proteins, increased expression of CSCs signaling proteins, induced WNT-transcription factor and served as a true model for metastasis (Fig. 1).
Next, (ii) The specific role of ABCG2 in mBCSCs was evaluated. The ABCG2 silencing cells neither formed the defined P-EMT cells nor showed metastatic characteristics. These deformed cells did not show any significant effect after Cur + QC treatment (Fig. 2). (iii) But the combination treatment of Cur and QC exhibited a reduction in the proliferation capabilities in SP cells (reduction of Ki67 protein expression), diminished invasiveness, decreased anchorage-dependent cell migration and inhibited expression of representative metastatic markers (e.g. ABCG2, CD-44, CD-133, Sox-2, Oct-4 and CXCR4), which, confirmed the anti-metastatic property of the agents (Fig. 3A-Di-ii). But non-significant reduction and unaltered expression of those proteins after Cur + QC exposure in ABCG2 silenced cells further confirmed that these agents act though ABCG2 dependent manner (Fig. 4A-Di-ii).
-
(iv)
An induction of blood vascularization in fertilized egg embryo, tube formation in HUVEC cells, MMP-9, MMP-2 expression and increased expression of representative angiogenesis markers as well as increased components of angiogenesis signaling cascade (PI3K, Akt, eNOS) after incubation of CM derived from SP cells into HUVECs were noticed (Fig. 3Ei-ii-Ji-ii). (v) But significant reduction of representative angiogenesis markers (Ang-1, Ang-2, VEGF A, HIF-1α, TNF-α, TGF-β) in the CM of SP cells and the subsequent reduction of vascularization in fertilized egg embryo, MMP-9, MMP-2, cell tubular formation in HUVECs after Cur + QC treatment revealed the involvement of ABCG2 in angiogenesis and Cur + QC modulated this process (Fig. 3Ei-ii-Ji-ii). Un-alteration of the above fact in ABCG2 silenced cells or Cur + QC treated ABCG2 knockdown cells further confirmed the role of ABCG2 in angiogenesis and the effectiveness of the combination drug treatment (Fig. 4Ei-ii-Ji-ii).
-
(vi)
To further confirm the ABCG2 dependency in angiogenesis, ABCG2 was overexpressed in cells and the CM of ABCG2 over-expressed cells or direct ABCG2 purified protein was incubated in fertilized egg embryo for visualization of changing vascularization. The data of Fig. 5Ai-ii is showing an increased vascularization in embryo which further strengthens the above fact. (vii) Next, highly metastatic CSCs were isolated from patient derived primary cells. In hypoxia, these cells also increased ABCG2 and offered metastatic properties. Increased vascularization was also noticed after incubation of CM derived from these cells into fertilized egg embryo (Fig. 5B, Ei-ii). (Viii) The level of ABCG2 and associated proteins were monitored in the cell lysate of the cultured human breast cancer tissue derived cells (SP cells). Increased protein expression of ABCG2 in increasing grade of tumor suggests that ABCG2 is involved in cancer progression and metastasis (Fig. 5Ci-ii, Di-ii). Higher expression of ABCG2 was detected by IHC in human breast cancer tissue in compare to ABCC1 and ABCB1 (Fig. 5F). (ix) Finally, for monitoring the direct vascularization and effect of Cur + QC in this process, a PDX model was established after implanting the tumor tissue into the Balb/C mice. The increased blood vessel formation in the xenograft model was observed visually. Interestingly fragmented damage of blood vessel in tumor tissue implanted mice confirmed that Cur + QC combination inhibits angiogenesis. Thus taken together, data suggests Cur + QC mediated reduction of angiogenesis through ABCG2 in breast cancer CSCs (Fig. 5G).
To study the effect of ABCG2 in metastasis and angiogenesis in breast cancer and their regulation by Cur + QC, we have developed two well planned and well designed model systems. Breast cancer stem cells (mBCSCs) were developed either by growing the cells first with serum deprived condition till the formation of mesenchymal characteristics, sphere formation followed by serum endowment condition or by isolating the CSCs population (SP cells) by FACS sorting from the bulk of cancer cells (Nayak et al. 2020). Both the cells were highly CSC populated cells and bear CSCs characteristics and obeyed the metastatic capability. Using the SP cells model system, data showed that, ABCG2 activates the metastasis by increasing the cell migration, proliferation, invasion and changing the representative metastatic markers in cancer cells and Cur + QC deregulates metastasis via ABCG2 by decreasing the above parameters.
It is well documented fact that, cancer cells release some soluble factors into surrounding medium which binds to the nearby endothelial cell surface receptor and activates the angiogenic signals in endothelial cells and hence induces angiogenesis. To study the angiogenesis and specifically find out the role of ABCG2 in this process, we have developed an angiogenesis model system using SP cells (epithelial cells) and HUVEC cells (endothelial cells). This is an extremely good model to study the exact role of any protein in angiogenesis and the specific soluble factors responsible for angiogenesis. In hypoxic condition, the SP cells (which have high ABCG2 expression) release angiogenic factors such as Ang-1, Ang-2, VEGF A, HIF-1α, TNF-α, TGF-β, and induced the angiogenesis after binding of these factors with the receptor on endothelial cell (HUVECs) surface receptors. Induction of ABCG2 and simultaneous increase in VEGF A in CM and as well as increased angiogenesis after the incubation of the CM in HUVEC was noted. But reduction of ABCG2 and concomitant decreased VEGF A followed by reduction of angiogenesis was noted after Cur + QC exposure. Thus, data revealed that Cur + QC reduced the angiogenesis process via ABCG2 through VEGF A.
Now question arises whether above phenomena is only true for in vitro system or same thing is replicated in in vivo too. To address the question, we have systematically carried out many experiments using patient derived cancer cells system as well as PDX model. The CSCs generated from patient derived cells (SP cells) also showed the metastatic properties and after addition of Cur + QC, ABCG2 expression was decreased. The CM of these cells (SP cells) also increased the angiogenesis and Cur + QC treated CM reduced the vascularization which proved that ABCG2 mediated angiogenesis is not only true for in vitro cell culture system but also holds for the same consequences in tumor microenvironment (TME). The reduction of blood vessel formation in PDX mice strengthens the agreement of in vitro data.
Next, experiments were carried out to decipher the detail mechanism by which ABCG2 induced the angiogenesis. Data showed that, ABCG2 or any soluble fraction of ABCG2 was not secreted into CM of SP cells along with other angiogenic factors such as VEGF A, Ang-1, Ang-2 etc. But vascularization and/or angiogenesis potentiality was shown after addition of CM into HUVECs (endothelial cells). Thus, it is clear from the observed data that, ABCG2 did not directly bind to the receptor of endothelial cell surface rather it indirectly induced angiogenesis. Interestingly, soluble angiogenesis factor VEGF A was increased in hypoxic condition but after Cur + QC exposure it decreased significantly and concomitantly reduced the angiogenesis. Upregulated VEGF A promoted angiogenesis via activating PI3K-Akt-eNOS axis. Thus, data suggested that Cur + QC treatment decreased the ABCG2 expression and decreased ABCG2 leads to reduction of VEGF A expression which ultimately inhibits the angiogenesis. But the detailed mechanism of ABCG2 mediated VEGF A regulation has to be studied more for a concrete conclusion.
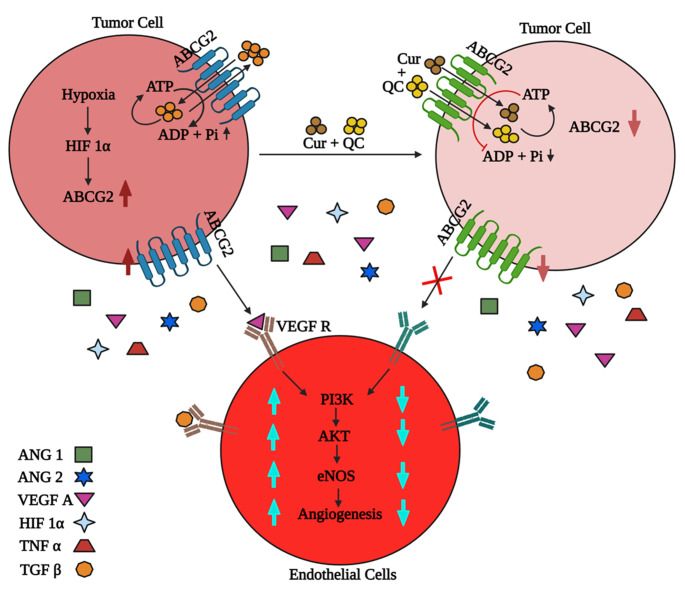
Finally, we summarized the result and provided a flow diagram of the mechanism of ABCG2 mediated angiogenesis in CSCs in Fig. 6. In hypoxic condition, ABCG2 enriched SP cells help to release VEGF A (angiogenic factor). VEGF A binds to the receptor on endothelial cell (HUVECs) surface. Induction of ABCG2 and simultaneous increase in VEGF A in CM promotes angiogenesis via PI3K-Akt-eNOS cascade. But, Cur + QC treatment in SP cells increases the drug accumulation in cells by inhibiting ABCG2. Simultaneous decrease in the expression of VEGF A and inhibition of angiogenesis was observed. Hence, collectively the data suugest that Cur + QC reduced the angiogenesis process via ABCG2 through VEGF A and we propose that ABCG2 is a potential anti-angiogenesis target in metastatic breast cancer.
Fig. 6.
Schematic representation of ABCG2 induced angiogenesis. VEGF A from ABCG2 enriched SP cells interacts with VEGF R and activates tumor induced angiogenesis via PI3K, Akt and eNOS
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to sincerely thank the Indian Council of Medical Research (ICMR), Government of India, for providing a research fellowship (Reference number: 3/2/2/35/2018/Online Onco Fship/NCD-III) to DN.
Authors’ contributions
DN performed the majority of the experiments and wrote the initial draft of the manuscript. SP, CD, SB helped in performing some of the experiments and analyzed the data. CNK conceived the scientific idea, designed the experiments, and wrote the final version of the manuscript.
Funding
Not applicable.
Data Availability
Experimental data generated during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethical Approval and Consent to participate
The mice experiments were carried out according to the guidelines of CPCSEA, New Delhi and approved by the Institutional Animal Ethics Committee (IAEC), KIIT, Deemed to be University (Ethical clearance approval Regd. #1577/PO/Re/S/2011/CPCSEA). The experiments using patient tissue samples were conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Acharya Harihar Regional Cancer Centre, Cuttack, Odisha, India (Ethical clearance approval Regd. #ECR/297/Inst/OR/2013). The experiments using HUVECs were performed according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Kalinga Institute of Medical Sciences hospital, Bhubaneswar, Odisha, India (Ethical clearance approval Regd. #KIMS/KIIT/IEC/55/2016).
Consent for publication
Not applicable.
Declaration of competing interests
The authors have no competing financial and/or non-financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abhinand CS, Athira PA, Soumya SJ, Sudhakaran PR. Multiple targets directed multiple ligands: An in silico and in vitro approach to evaluating the effect of triphala on angiogenesis. Biomolecules. 2020;10:177. doi: 10.3390/biom10020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Jia J, Ma L, et al. Recombinant human endostatin could eliminate the pro-angiogenesis priority of SP cells sorted from non-small cell lung cancer cells. Clin Transl Oncol. 2012;14:575–585. doi: 10.1007/s12094-012-0844-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Sinha S, Molla S, et al. PARP inhibitor Veliparib (ABT-888) enhances the anti-angiogenic potentiality of Curcumin through deregulation of NECTIN-4 in oral cancer: Role of nitric oxide (NO) Cell Signal. 2021;80:109902. doi: 10.1016/j.cellsig.2020.109902. [DOI] [PubMed] [Google Scholar]
- Chen Z, Liu F, Ren Q, et al. Suppression of ABCG2 inhibits cancer cell proliferation. Int J Cancer. 2010;126:841–851. doi: 10.1002/ijc.24796. [DOI] [PubMed] [Google Scholar]
- Chikazawa N, Tanaka H, Tasaka T, et al. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30:2041–2048. [PubMed] [Google Scholar]
- Dai Y, Liu S, Zhang W-Q, et al. YAP1 regulates ABCG2 and cancer cell side population in human lung cancer cells. Oncotarget. 2017;8:4096. doi: 10.18632/oncotarget.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Kundu CN. Anti-cancer stem cells potentiality of an anti-malarial agent quinacrine: an old wine in a new bottle. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti -Cancer Agents) 2021;21:416–427. doi: 10.2174/1871520620666200721123046. [DOI] [PubMed] [Google Scholar]
- Dash SR, Chatterjee S, Sinha S et al (2022) NIR irradiation enhances the apoptotic potentiality of quinacrine-gold hybrid nanoparticles by modulation of HSP-70 in oral cancer stem cells. Nanomed-Nanotechnol 40:102502 [DOI] [PubMed]
- DeLay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18:2930–2942. doi: 10.1158/1078-0432.CCR-11-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemitabar S, Yazdian-Robati R, Hashemi M, et al. ABCG2 aptamer selectively delivers doxorubicin to drug-resistant breast cancer cells. J Biosci. 2019;44:39. doi: 10.1007/s12038-019-9854-x. [DOI] [PubMed] [Google Scholar]
- He C, Zhang H, Wang B, et al. SDF-1/CXCR4 axis promotes the growth and sphere formation of hypoxic breast cancer SP cells by c-Jun/ABCG2 pathway. Biochem Biophys Res Commun. 2018;505:593–599. doi: 10.1016/j.bbrc.2018.09.130. [DOI] [PubMed] [Google Scholar]
- Higashikuni Y, Sainz J, Nakamura K, et al. The ATP-binding cassette transporter BCRP1/ABCG2 plays a pivotal role in cardiac repair after myocardial infarction via modulation of microvascular endothelial cell survival and function. Arterioscler Thromb Vasc Biol. 2010;30:2128–2135. doi: 10.1161/ATVBAHA.110.211755. [DOI] [PubMed] [Google Scholar]
- Higashikuni Y, Sainz J, Nakamura K, et al. The atp-binding cassette transporter abcg2 protects against pressure overload–induced cardiac hypertrophy and heart failure by promoting angiogenesis and antioxidant response. Arterioscler Thromb Vasc Biol. 2012;32:654–661. doi: 10.1161/ATVBAHA.111.240341. [DOI] [PubMed] [Google Scholar]
- Huang L, Perrault C, Coelho-Martins J, et al. Induction of acquired drug resistance in endothelial cells and its involvement in anticancer therapy. J Hematol Oncol. 2013;6:1–12. doi: 10.1186/1756-8722-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z-S, Sun Y-Z, Wang S-M, Ruan J-S. Epithelial-mesenchymal transition: potential regulator of ABC transporters in tumor progression. J Cancer. 2017;8:2319. doi: 10.7150/jca.19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Xing T, Yang Z, et al. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017;7:1. doi: 10.3390/jcm7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jiang AC, Dong P, et al. MDR1/P-gp and VEGF synergistically enhance the invasion of Hep-2 cells with multidrug resistance induced by taxol. Ann Surg Oncol. 2009;16:1421–1428. doi: 10.1245/s10434-009-0395-7. [DOI] [PubMed] [Google Scholar]
- Li F, Zeng H, Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol. 2011;28:1458–1462. doi: 10.1007/s12032-010-9646-5. [DOI] [PubMed] [Google Scholar]
- Liu H-B, Meng Q-H, Du D-W, et al. The effects of ABCG2 on the viability, proliferation and paracrine actions of kidney side population cells under oxygen-glucose deprivation. Int J Med Sci. 2014;11:1001. doi: 10.7150/ijms.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang P, Hou X, et al. Hybrid curcumin–phospholipid complex-near-infrared dye oral drug delivery system to inhibit lung metastasis of breast cancer. Int J Nanomed. 2019;14:3311. doi: 10.2147/IJN.S200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra P, Preet R, Das D, et al. The contribution of heavy metals in cigarette smoke condensate to malignant transformation of breast epithelial cells and in vivo initiation of neoplasia through induction of a PI3K–AKT–NFκB cascade. Toxicol Appl Pharmcol. 2014;274:168–179. doi: 10.1016/j.taap.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ohnuma S, Fukuda M, et al. Synthetic analogs of curcumin modulate the function of multidrug resistance–linked ATP-binding cassette transporter ABCG2. Drug Metab Dispos. 2017;45:1166–1177. doi: 10.1124/dmd.117.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Siddharth S, Das S, et al. Nanoquinacrine caused apoptosis in oral cancer stem cells by disrupting the interaction between GLI1 and β catenin through activation of GSK3β. Toxicol Appl Pharmcol. 2017;330:53–64. doi: 10.1016/j.taap.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Nayak A, Das S, Nayak D, et al. Nanoquinacrine sensitizes 5-FU-resistant cervical cancer stem-like cells by down-regulating Nectin-4 via ADAM-17 mediated NOTCH deregulation. Cell Oncol. 2019;42:157–171. doi: 10.1007/s13402-018-0417-1. [DOI] [PubMed] [Google Scholar]
- Nayak D, Tripathi N, Kathuria D, et al. Quinacrine and curcumin synergistically increased the breast cancer stem cells death by inhibiting ABCG2 and modulating DNA damage repair pathway. Int J Biochem Cell Biol. 2020;119:105682. doi: 10.1016/j.biocel.2019.105682. [DOI] [PubMed] [Google Scholar]
- Ning W, Li S, Yang W, et al. Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell Signal. 2021;77:109812. doi: 10.1016/j.cellsig.2020.109812. [DOI] [PubMed] [Google Scholar]
- Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16:1–19. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan R, Chatterjee S, Hembram KC, et al. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J Nutr Biochem. 2021;92:108624. doi: 10.1016/j.jnutbio.2021.108624. [DOI] [PubMed] [Google Scholar]
- Preet R, Mohapatra P, Mohanty S, et al. Quinacrine has anticancer activity in breast cancer cells through inhibition of topoisomerase activity. Int J Cancer. 2012;130:1660–1670. doi: 10.1002/ijc.26158. [DOI] [PubMed] [Google Scholar]
- Preet R, Siddharth S, Satapathy SR, et al. Chk1 inhibitor synergizes quinacrine mediated apoptosis in breast cancer cells by compromising the base excision repair cascade. Biochem Pharmacol. 2016;105:23–33. doi: 10.1016/j.bcp.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Rao DK, Liu H, Ambudkar SV, Mayer M. A combination of curcumin with either gramicidin or ouabain selectively kills cells that express the multidrug resistance-linked ABCG2 transporter. J Biol Chem. 2014;289:31397–31410. doi: 10.1074/jbc.M114.576819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapathy SR, Siddharth S, Das D, et al. Enhancement of cytotoxicity and inhibition of angiogenesis in oral cancer stem cells by a hybrid nanoparticle of bioactive quinacrine and silver: implication of base excision repair cascade. Mol Pharm. 2015;12:4011–4025. doi: 10.1021/acs.molpharmaceut.5b00461. [DOI] [PubMed] [Google Scholar]
- Satapathy SR, Nayak A, Siddharth S et al (2018) Metallic gold and bioactive quinacrine hybrid nanoparticles inhibit oral cancer stem cell and angiogenesis by deregulating inflammatory cytokines in p53 dependent manner. Nanomedicine: Nanotechnology, Biology and Medicine 14:883–896 [DOI] [PubMed]
- Sethy C, Goutam K, Das B, et al. Nectin-4 promotes lymphangiogenesis and lymphatic metastasis in breast cancer by regulating CXCR4-LYVE-1 axis. Vascul Pharmacol. 2021;140:106865. doi: 10.1016/j.vph.2021.106865. [DOI] [PubMed] [Google Scholar]
- Shang H-G, Yu H-L, Ma X-N, Xu X. Multidrug resistance and tumor-initiating capacity of oral cancer stem cells. JBUON. 2016;21:461–465. [PubMed] [Google Scholar]
- Shay G, Lynch CC, Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M, Ota M, Okada Y (2018) Isolation of cancer stem cells by side population method. Cancer Stem Cells. Springer, pp 49–59 [DOI] [PubMed]
- Siddharth S, Nayak D, Nayak A, et al. ABT-888 and quinacrine induced apoptosis in metastatic breast cancer stem cells by inhibiting base excision repair via adenomatous polyposis coli. DNA Repair. 2016;45:44–55. doi: 10.1016/j.dnarep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Siddharth S, Goutam K, Das S, et al. Nectin-4 is a breast cancer stem cell marker that induces WNT/β-catenin signaling via Pi3k/Akt axis. Int J Biochem Cell Biol. 2017;89:85–94. doi: 10.1016/j.biocel.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Siddharth S, Nayak A, Das S, et al. The soluble nectin-4 ecto-domain promotes breast cancer induced angiogenesis via endothelial Integrin-β4. Int J Biochem Cell Biol. 2018;102:151–160. doi: 10.1016/j.biocel.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Taheri T, Jamialahmadi K, Khadijeh F. Unexpected Lower Expression of Oncoprotein Gankyrin in Drug Resistant ABCG2 Overexpressing Breast Cancer Cell Lines. Asian Pac J Cancer Prev. 2017;18:3413–3418. doi: 10.22034/APJCP.2017.18.12.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-P, Hsiao S-H, Murakami M, et al. Alpha-mangostin reverses multidrug resistance by attenuating the function of the multidrug resistance-linked ABCG2 transporter. Mol Pharm. 2017;14:2805–2814. doi: 10.1021/acs.molpharmaceut.7b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Zhang Z. The reversal of MRP1 expression induced by low-frequency and low-intensity ultrasound and curcumin mediated by VEGF in brain glioma. OncoTargets and therapy. 2019;12:3581. doi: 10.2147/OTT.S195205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-J, Ou J-H, Wang M-L, et al. Elevated survivin mediated multidrug resistance and reduced apoptosis in breast cancer stem cells. J BUON. 2015;20:1287–1294. [PubMed] [Google Scholar]
- Zhang H, Zhang Y, Cheng Y, et al. Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol Carcinog. 2018;57:44–56. doi: 10.1002/mc.22718. [DOI] [PubMed] [Google Scholar]
- Zhu S, Chen Z, Wang L, et al. A combination of SAHA and Quinacrine is effective in inducing cancer cell death in upper gastrointestinal cancers. Clin Cancer Res. 2018;24:1905–1916. doi: 10.1158/1078-0432.CCR-17-1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental data generated during the current study are available from the corresponding author upon reasonable request.