Abstract
Human coronaviruses (HuCV) cause common colds. Previous reports suggest that these infectious agents may be neurotropic in humans, as they are for some mammals. With the long-term aim of providing experimental evidence for the neurotropism of HuCV and the establishment of persistent infections in the nervous system, we have evaluated the susceptibility of various human neural cell lines to acute and persistent infection by HuCV-229E. Viral antigen, infectious virus progeny and viral RNA were monitored during both acute and persistent infections. The astrocytoma cell lines U-87 MG, U-373 MG, and GL-15, as well as neuroblastoma SK-N-SH, neuroglioma H4, and oligodendrocytic MO3.13 cell lines, were all susceptible to an acute infection by HuCV-229E. The CHME-5 immortalized fetal microglial cell line was not susceptible to infection by this virus. The MO3.13 and H4 cell lines also sustained a persistent viral infection, as monitored by detection of viral antigen and infectious virus progeny. Sequencing of the S1 gene from viral RNA after ∼130 days of infection showed two point mutations, suggesting amino acid changes during persistent infection of MO3.13 cells but none for H4 cells. Thus, persistent in vitro infection did not generate important changes in the S1 portion of the viral spike protein, which was shown for murine coronaviruses to bear hypervariable domains and to interact with cellular receptor. These results are consistent with the potential persistence of HuCV-229E in cells of the human nervous system, such as oligodendrocytes and possibly neurons, and the virus’s apparent genomic stability.
Coronaviruses are large enveloped positive-stranded RNA viruses. Human coronaviruses (HuCV) are responsible for up to one-third of common colds (49). Other pathologies have occasionally been associated with HuCV, such as pneumonia, meningitis, and diarrhea (57). Two viral serotypes are known in humans. They are designated OC43 and 229E. Even though HuCV-229E was first isolated as a pathogen of the respiratory tract (28), increasing evidence from humans and experiments with other coronaviruses in animal models suggest its neurotropic potential.
Murine hepatitis virus (MHV), a naturally occurring murine coronavirus, gives rise in mice and rats to clinical manifestations resembling multiple sclerosis (MS) in humans. For this reason, it has been studied as an animal model of a virus-induced demyelinating disease of the central nervous system (CNS) (10, 80). The CNS of nonhuman primates is also susceptible to coronavirus infection: a murine virus-like isolate causes a subacute panencephalitis and demyelination (47). Neurotropic strains of MHV enter the brain via the olfactory nerve (4, 37) and then spread within the mouse CNS (53). A similar route might be used by HuCV-229E since it also infects the respiratory tract. Given the fact that this virus can infect human macrophages (51) and human brain endothelial cells (11), HuCV-229E could also use other routes for spreading to the CNS, such as the hematogenous and lymphatic systems, as was shown for MHV in mice (5) and nonhuman primates (12). Neurons, astrocytes, and oligodendrocytes are the target CNS cells for MHV (29). Moreover, we have shown that the 229E prototype strain of HuCV has the capacity to infect human astrocytes and microglia in primary cultures (9). In general, in vitro observations of coronavirus infections corroborate the ones occurring in vivo. For example, Massa and collaborators (43) showed that three MHV-JHM variants, which induce very different pathological patterns in vivo, demonstrated distinct in vitro growth properties in primary cultures of rat glial cells that correlated with the in vivo observations. Moreover, MHV-3 induces an initial ependymitis, meningitis, and encephalitis in animals and has an in vitro affinity for target cells compatible with this clinical outcome: viral growth in neurons, ependymal cells, and meningeal cells, but not astrocytes and oligodendrocytes (78). Therefore, it is reasonable to assume that the characterization of HuCV replication in vitro will provide valuable data regarding its neurotropic properties, given that in vivo experiments could obviously not be performed in humans.
Suggestions of HuCV-229E neurotropism do arise from observations in humans. We recently detected a HuCV-229E receptor (CD13) on human neural cell lines (oligodendrocytes, neurons, and astrocytes), and its presence correlated with virus binding and susceptibility of these cells to infection by HuCV-229E (34). Intrathecal anti-HuCV-229E antibody synthesis was detected in humans, particularly in MS patients (59), suggesting a CNS infection. Moreover, we have repeatedly detected HuCV-229E RNA in human brains from different age groups provided by various brain banks (3, 66). These observations suggest a persistent infection by HuCV-229E in human CNS since it is very unlikely that these humans were all acutely infected just before their death. However, more-extensive studies are needed in order to prove and characterize the persistence of HuCV-229E in human CNS. In the rodent model, MHV RNA can be detected in the brain for a long time after the initial infection (24, 29). This virus is able to persist in the CNS of its host (63) especially in astrocytes, oligodendrocytes (54, 69), or neurons (64) and in various neural cell lines in vitro (40, 67). Viral persistence observed in the CNS may play a role in chronic pathologies observed following a coronaviral infection (39). However, no specific pathology has been clearly associated with the presence of HuCV-229E in the CNS. Nonetheless, we have observed a preferential presence of T lymphocytes cross-reactive with both HuCV-229E and myelin basic protein in the peripheral blood of MS patients compared to that in controls (77).
The route and dose of infection and host factors such as age, species, strain, and immune system status as well as the genetic constitution of the virus influence the outcome of an MHV infection in the CNS (38, 65, 79). Similar results might be expected for HuCV-229E. Coronaviruses possess four structural proteins: spike (S), membrane (M), small membrane (E, formerly sM), and nucleocapsid (N) (35). Among coronavirus structural proteins that might influence the outcome of the infection, the S glycoprotein is particularly interesting. It influences MHV pathogenesis via its multiple biological activities such as receptor ligation (20, 83), mediation of fusion (17), neutralizing antibody domains (17, 18, 75, 81), and cytotoxic-T-lymphocyte epitope domains (7, 13). Vaccination with the S protein (19) or even only peptides of it (74) protected mice from a lethal intracerebral infection by MHV-A59. Moreover, important determinants of neurovirulence reside in regions of the S glycoprotein of MHV (10, 23). In vitro passaging of MHV-4 in cell culture generates heterogeneity in the structure of the S glycoprotein (26). During persistent in vivo CNS infection in mice, multiple point and deletion mutations were shown to arise, mainly concentrated within the S and the N genes (1, 58). Studies regarding the importance of the S glycoprotein for the biology of HuCV-229E, such as the analysis of mutations arising in passaging viruses or during persistent infection, have so far not been reported. However, a study did suggest the existence of serological variants of this virus (56).
Various neural cell lines were shown to be susceptible to infection by MHV (25). However, very limited results (76) concerning the susceptibility to HuCV-229E infection of human immortalized cell lines representative of nervous system cells, which could serve as invaluable in vitro models, are available. We have shown previously that primary cultures of human brain cells can be infected by HuCV-229E (9). In the present study, we used cell lines representative of the different neural cell types (oligodendrocytes, astrocytes, microglia, and neurons) of the human nervous system to evaluate the potential of acute and persistent HuCV-229E infections. Viral antigens, infectious particles, and viral RNA (N and S1 genes) were monitored during an acute and a persistent viral infection. Similar experiments were also performed with HuCV-OC43; these results are presented separately (2).
We show that astrocytoma cell lines (U-87 MG, U-373 MG, and GL-15), neuroblastoma (SK-N-SH), neuroglioma (H4), and immortalized oligodendrocytic (MO3.13) cell lines were all susceptible to an acute HuCV-229E infection. However, only the H4 and MO3.13 cell lines sustained a persistent infection by this virus. Sequencing of the S1 gene showed two point mutations leading to amino acid changes on viral RNA amplified from persistently infected MO3.13 cells. These results are consistent with the potential persistence of HuCV-229E in cells of the human nervous system, mainly oligodendrocytes and possibly neurons.
MATERIALS AND METHODS
Viruses and cell lines.
HuCV-229E was originally obtained from the American Type Culture Collection (ATCC; Manassas, Va.), plaque purified twice, and grown on L-132 cells as described previously (31). The third passage of HuCV-229E from laboratory stocks kept at −90°C, with a titer of 5.5 × 106 50% tissue culture infective doses (TCID50)/ml, was used for all experiments.
The GL-15 line, established from a human glioblastoma multiforme, was generated by V. Bocchini (University Medical School, Perugia, Italy) (8). The CHME-5 line was obtained from human fetal microglia by transfection with the large T antigen of simian virus 40 (30). The MO3.13 cell line was obtained from the fusion of a rhabdosarcoma cell (Te671) with an adult human oligodendrocyte (44). The H4, SK-N-SH, U-373 MG, and U-87 MG cell lines were obtained from the ATCC. All of these neural cell lines were grown in Dulbecco’s modified Eagle’s medium, (GIBCO Laboratories, Grand Island, N.Y.) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum and gentamicin (50 μg/ml; GIBCO Laboratories).
Antibodies.
Virus-specific monoclonal antibodies (MAbs) were produced in our laboratory by standard hybridoma technology. MAbs 5-11H.6, directed against the surface S glycoprotein of HuCV-229E, and 1-10C.3, an isotypic control, were used to detect viral antigens in immunoperoxidase and immunofluorescence assays.
Acute infection.
For detection of viral antigens, approximately 1 × 106 to 1.5 × 106 cells (obtained by trypsinization of cell monolayers grown on plastic 75-cm2 flasks, followed by pelleting) were mixed with an equal volume of HuCV-229E virus stock diluted to provide multiplicity of infection (MOI) of 0.1 or 1.0. Twenty-five microliters of this suspension was deposited into each well of a 12-well glass slide (Flow, ICN Biomedical Canada, Ltd., Mississauga, Ontario, Canada), and infection progressed for up to 2 days at 33°C prior to fixation in acetone at −20°C for 30 min. Slides were kept at −70°C prior to revelation of viral antigens by immunofluorescence.
For detection of infectious virus, cell lines at 60 to 80% confluence in 25-cm2 flasks were infected with virus stock diluted to provide an MOI of 0.1. Infection was carried out at 33°C for up to 2 days, and supernatants and cell monolayers taken at different time points were harvested and kept at −70°C until infectious virus titers could be quantitated.
For analysis of viral RNA, cell monolayers at 60 to 80% confluence in 150-cm2 tissue culture-treated petri dishes were infected at an MOI of 0.01 for 1 day at 33°C. Then, cell monolayers were washed twice with phosphate-buffered saline (PBS), pH 7.4, and kept at −90°C until RNA could be extracted.
Persistent infection.
Cell lines at 60 to 80% confluence in 25-cm2 culture flasks were infected with 0.5 ml of viral suspension, providing an MOI of 0.5, and incubated for 2 h at 33°C with periodical agitation. Cell monolayers were then washed with PBS and grown in regular cell culture medium at 37°C. Cells were passaged every 4 to 8 days. Samples of supernatants and cells were kept at −70°C for viral titration by immunoperoxidase at each passage, and cells were kept at −70°C at each fifth passage for RNA extraction. Twenty-five microliters of cell samples from each passage, at a concentration of 0.5 × 106 1.5 × 106 cells/ml, was deposited into each well of a 12-well glass slide (Flow, ICN Biomedical Canada Ltd.), incubated for 24 to 48 h at 37°C, and then fixed in acetone at −20°C for 30 min and kept at −70°C until immunofluorescence assays could be performed.
Detection of viral antigens by immunofluorescence.
Immunofluorescence revelation of viral antigens was performed as described previously (61). Briefly, primary antibody (MAbs 5-11H.6 for HuCV-229E and 1-10C.3 as isotypic control) was added to thawed slides and incubated for 1 h at 37°C. This was followed by three washes in PBS, addition of the fluorescein-conjugated mouse-specific goat secondary antibody (Cappel, Durham, N.C.), and a 30-min incubation at 37°C. After three washes in PBS, slides were mounted with glycerol-PBS (9:1). Slides were stored at 4°C until they could be observed with a Leitz fluorescence microscope (Dialux 20 model).
Immunoperoxidase assay for quantitation of infectious virus titers.
The immunoperoxidase assay for quantitation of infectious virus titers was performed as previously described (61). Briefly, susceptible L-132 cells were inoculated with serial logarithmic dilutions of samples in a 96-well Linbro plate (Flow, ICN Biomedical Canada Ltd.). After 4 to 5 days of incubation at 33°C in 5% (vol/vol) CO2, cells were washed with PBS and fixed with 0.3% (vol/vol) hydrogen peroxide (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada) in methanol for 30 min. After washing with PBS, they were incubated for 2 h at 37°C with an appropriate antiviral MAb (5-11H.6). Cells were then washed five times with PBS, and horseradish peroxidase-conjugated goat anti-mouse immunoglobulins (Cappel) were added and incubated for 2 h at 37°C. Bound antibodies were detected by incubation with 0.025% (wt/vol) 3,3′-diaminobenzidine-tetrahydrochloride (Bio-Rad, Richmond, Calif.) and 0.01% (vol/vol) hydrogen peroxide (Sigma-Aldrich Canada Ltd.) in PBS. The chromogenic reaction was stopped with deionized water. Infectious virus titers were calculated by the Karber method. Negative controls consisted of noninfected cells.
Preparation of RNA, RT, and PCR.
To extract total cellular RNA from acutely or persistently infected or control cells, cell monolayers were lysed with GIT buffer (4 M guanidine isothiocyanate, 2.5 mM sodium acetate, 12 mM β-mercaptoethanol). Lysates were passed through a 26-gauge needle at least six times and then layered onto a cesium chloride cushion (5.7 M cesium chloride, 2.5 mM sodium acetate) for a 12- to 20-h centrifugation at 150,000 × g. Supernatant was removed, and the RNA pellet was resuspended in sterile distilled and deionized H2O. The pair of primers used for amplification of HuCV-229E and the one for the control housekeeping gene (encoding glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) are described in Table 1. Forty picomoles of the inverse-complementary primer or 100 pmol of oligo(dT) (Roche Diagnostics, Laval, Québec, Canada) was incubated with 2 to 5 μg of total cellular RNA at 65°C for 5 min to denature RNA, followed by a slow cooldown to 37°C for annealing. Reverse transcription (RT) with Expand Moloney murine leukemia virus reverse transcriptase (50 U; Roche Diagnostics) was performed at 42°C for 90 min in the presence of 60 U of RNA Guard (Pharmacia, Baie d’Urfé, Québec, Canada), 0.4 mM of each deoxynucleoside triphosphate (Na salt; Roche Diagnostics), 1× reverse transcriptase buffer (50 mM Tris-HCl, 40 mM KCl, 5 mM MgCl2, 0.5% [vol/vol] Tween 20 [pH 8.3]) and 10 mM dithiothreitol (Roche Diagnostics). For PCR, 1 of 10 or 1 of 5 of the synthesized cDNAs was incubated in the presence of 20 pmol (HuCV-229E S1 gene) or 50 pmol (GAPDH or HuCV-229E N gene) of the sense and antisense primers; 1.5 mM (GAPDH gene), 2.0 mM (HuCV-229E N gene), or 3.0 mM (HuCV-229E S1 gene) MgCl2 (Roche Diagnostics); 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, Roche Diagnostics); and 0.4 mM (each) deoxynucleoside triphosphate (Li salt; Roche Diagnostics) at 94°C for 5 min and at 60°C (HuCV-229E N gene), 50°C (GAPDH gene), or 37°C (HuCV-229E S1 gene) for another 5 min. After addition of the Expand high-fidelity PCR system DNA polymerase (Taq and Pwo DNA polymerases) (2.5 U; Roche Diagnostics), 30 cycles of 2 min at 72°C, 1 min at 94°C, and 2 min at 60°C (HuCV-229E N gene), 50°C (GAPDH gene) or 37°C (HuCV-229E S1 gene) were performed, with a final elongation step of 10 min at 72°C. The DNA amplicons were separated by electrophoresis in a 1.5% (wt/vol) agarose gel with ethidium bromide (1 μg/ml).
TABLE 1.
RT-PCR and sequencing primers
| RNA amplified or sequenced | Primer sequence | Primer designation | Nucleotides |
|---|---|---|---|
| RT-PCR HuCV-229E N gene | 5′-TCTGCCAAGAGTCTTGCTCG-3′ | E7 sense | 819–838a |
| 5′-AGCATAGCAGCTGTTGACGG-3′ | E9 antisense | 1035–1054a | |
| RT-PCR HuCV-229E S1 gene portion | 5′-GTTGCATATTGCTGGTTGTCAAAC-3′ | 229E-S1PH sense | 68–91b |
| 5′-CCTGGCGCTATTTCTTAAGGC-3′ | 229E-S1PI antisense | 1944–1964b | |
| RT-PCR GAPDH gene | 5′-GTGAAGGTCGGAGTCAACG-3′ | GAPDH-H sense | 10–68c |
| 5′-CACCTGGTGCTCAGTGTAGC-3′ | GAPDH-I antisense | 824–843c | |
| Sequencing S1 gene portion | 5′-GGAACCATTTTGTTTAAAACATC-3′ | S-229E-A sense | 426–448b |
| 5′-GTACGTTGACTTCAAACCTCAG-3′ | S-229E-B sense | 960–981b | |
| 5′-CTCCTTGTAACCCACCAGATC-3′ | S-229E-C sense | 1522–1542b | |
| 5′-GGTTACAAGGAGTGATAGAGTAG-3′ | S-229E-D antisense | 1511–1533b | |
| 5′-CGTACAACACAATAAACGTATG-3′ | S-229E-E antisense | 933–944b | |
| 5′-CAAAATGGTTCCACGTCTAAGG-3′ | S-229E-F antisense | 414–435b |
Cloning and nucleotide sequencing.
HuCV-229E S1 gene PCR products were ligated into pGEM-T or pGEM-T Easy vector (Promega, Madison, Wis.) according to the manufacturer’s instructions. The ligation mixture was then transformed into competent XL-1 blue bacteria. Many individual bacterial colonies were screened for the presence of the amplicons. Plasmid DNA was prepared from at least three individual clones for each cloning experiment, with a plasmid mini kit (Qiagen, Mississauga, Ontario, Canada), and sequenced with an automated sequencer (ABI 310 genetic analyzer; Perkin-Elmer) in both directions by using a universal primer, SP6, and internal primers (Table 1). Nucleotide sequences and predicted amino sequences were analyzed with Geneworks software for Macintosh (version 2.5.1; Oxford Molecular Ltd., Oxford, United Kingdom).
RESULTS
Acute infection of neural cell lines.
Even though primary cultures of human brain cells are infectable by HuCV-229E (9), their limited life span does not allow the study of the effects of a long-standing coronavirus infection. Although the susceptibility of different neural cell lines to infection by coronaviruses, particularly MHV, has been extensively characterized, very limited results are available concerning the susceptibility to HuCV-229E infection of human continuous cell lines representative of cells of the nervous system (76). Therefore, we first characterized the susceptibility to HuCV-229E infection of various available neural cell lines representative of different cell types found in the nervous system. This virus was initially adapted in our laboratory to replicate in the L-132 cell line, which is of human embryonic fibroblastic origin. For this reason, these cells were used as a positive control for infection. We first performed a detection of HuCV-229E S antigen by indirect immunofluorescence on cells acutely infected at an MOI of 0.1. Representative results of at least three independent experiments are shown in Fig. 1. The specificity of the indirect immunofluorescence test was verified with an isotypic control MAb on the same slide as well as with noninfected cells. In both cases, no signal could be detected for any cell line (data not shown). Results obtained with the reference L-132 cell line are shown in Fig. 1H. The indirect immunofluorescence test was found suitable for the detection of an HuCV-229E infection because of its specificity and since a characteristic cytoplasmic signal was observed (41). Figures 1A and B show the results obtained with two neuronal cell lines, SK-N-SH and H4. Both cell lines were susceptible to HuCV-229E infection; however, a higher percentage of SK-N-SH cells were infected compared to that observed for the H4 cell line (Table 2). We determined that the SK-N-SH cell line was at least as susceptible to acute infection by HuCV-229E as our reference L-132 cell line. Astrocytic cell lines (U-373 MG, U-87 MG, and GL-15 [Fig. 1C to E, respectively) were all susceptible to HuCV-229E infection. The GL-15 cell line was more easily infected at a lower MOI than the other two astrocytic cell lines. The oligodendrocytic MO3.13 cell line (Fig. 1F) was infected by HuCV-229E at a level comparable to that of the H4 neuronal and the GL-15 astrocytic cell lines (Table 2). The CHME-5 microglial cell line (Fig. 1G) was the only neural cell line tested not susceptible to HuCV-229E infection. No viral antigen was detected with this cell line, even at different times postinfection or higher MOIs. The percentage of infected cells progressed with time for all susceptible cell lines, from 12 to 40 h, and was augmented by increasing the MOI (data not shown). This is concordant with previously reported results (76).
FIG. 1.
Detection of HuCV antigens by indirect immunofluorescence on cells acutely infected by 229E strain, using 229E-specific MAb (5-11H.6). (A) H4 cells; (B) SK-N-SH cells; (C) U-373 MG cells; (D) U-87 MG cells; (E) GL-15 cells; (F) MO3.13 cells; (G) CHME-5 cells; (H) L-132 cells.
TABLE 2.
Susceptibility of neural cell lines to acute and persistent infection by HuCV 229E
| Cell line
|
Infectiona
|
||
|---|---|---|---|
| Designation | Phenotype | Acute | Persistent |
| U-87 MG | Astrocytoma | 1+ | − |
| U-373 MG | Astrocytoma (grade III) | 1+ | − |
| GL-15 | Astrocytic (glioblastoma multiforme positive for glial fibrillary acidic protein marker) | 2+ | − |
| MO3.13 | Oligodendrocytic (adult oligodendrocyte fused with a rhabdosarcoma) | 2+ | 2+ |
| CHME-5 | Microglial (fetal microglia transfected with large T antigen of simian virus 40) | − | − |
| H4 | Neuroglial (ganglioglioma) | 2+ | 1+ |
| SK-N-SH | Neuroblastoma | 3+ | − |
Results obtained from an indirect immunofluorescence assay and an infectious virus titer assay. −, noninfectable or no persistence after fifth passage; +, <10% of cells infected; 2+, between 10 and 50% of cells infected; 3+, >50% of cells infected.
HuCV-229E infection of the various cell lines was productive since infectious virions were produced by all the susceptible cell lines. An assay to determine the kinetics of virion release was performed between 2 and 60 h of infection (data not shown): a strong increase in viral titers was mainly seen during the first 15 to 20 h, and then a plateau effect with a slow decrease was observed during the remainder of the infection. Most of the time, extracellular virus production paralleled that of intracellular virions but was at a higher level. Maximal virion production was achieved at 30 to 40 h postinfection. Titers observed (data not shown) were due not to the virus inoculum but rather to new virus production, since an increase in viral titer with time was observed for all the cell lines tested: SK-N-SH, H4, MO3.13, U-87 MG, and U-373 MG. Finally, we could establish a correlation between production of infectious virions and the percentage of infected cells. Cell lines showing a low percentage of infection produced low titers of virions: for example, the U-373 MG cell line produced a maximum of 4 log TCID50/ml when an MOI of 1 was used; however, the MO3.13 and H4 cell lines produced, under the same conditions, maxima of 6.5 and 7 log TCID50/ml, respectively.
Detection of coronaviral antigens in neural cell lines during persistent infection.
It is well known that MHV, the murine counterpart of HuCV, can establish a persistent infection in different cell lines, with various effects (40, 50, 67). In vivo persistence has even been observed in animals (1, 63). Since in vivo experiments are obviously not possible in humans and also because primary cultures of human brain cells have a limited life span, we verified the ability of immortalized neural cell lines to sustain a persistent infection. The various cell lines we showed to be susceptible to an acute infection were infected and then cultured for several passages. At each cell passage, cells were collected for the detection of coronaviral antigens by indirect immunofluorescence (Fig. 2). The L-132 cells (Fig. 2C) sustained a persistent infection for over 42 cell passages, as was previously shown (14). At all passages tested, HuCV-229E antigens were detected by an indirect immunofluorescence assay. Among cell lines of the neuronal type, only H4 cells (Fig. 2A) sustained a persistent viral infection; however the percentage of positive cells was low (<5%). Surprisingly, the SK-N-SH cell line was negative after the second passage of HuCV-229E infection (data not shown), even though this cell line was extensively infected during an acute infection. No astrocytic cell lines (U-87 MG, U-373 MG, or GL-15) were susceptible to a persistent infection: no viral antigen could be detected at the second passage and further passages (data not shown). On the other hand, the oligodendrocytic cell line MO3.13 sustained a persistent infection for at least 44 cell passages (Fig. 2B). At all cell passages tested, HuCV-229E antigens could be detected in these persistently infected cells at a level comparable to that observed in persistently infected L-132 reference cells. Persistent infection did not significantly influence the proliferation of H4 and MO3.13 cells. However, L-132 cells showed a slight cytopathic effect due to virus at the second passage but recovered at the following passage (data not shown). Overall, among the neural cell lines tested, only the neuronal H4 cell line and the oligodendrocytic cell line MO3.13 showed a persistent presence of HuCV-229E antigens. Finally, the susceptibility to HuCV-229E acute infection did not necessarily correlate with the establishment of a persistent infection by the same viral strain.
FIG. 2.
Detection of HuCV antigens by indirect immunofluorescence on cells persistently infected by 229E strain, using 229E-specific MAb (5-11H.6). (A) H4 cells, passage 37; (B) MO3.13 cells, passage 40; (C) L-132 cells, passage 40.
Detection of infectious virions during persistent infection.
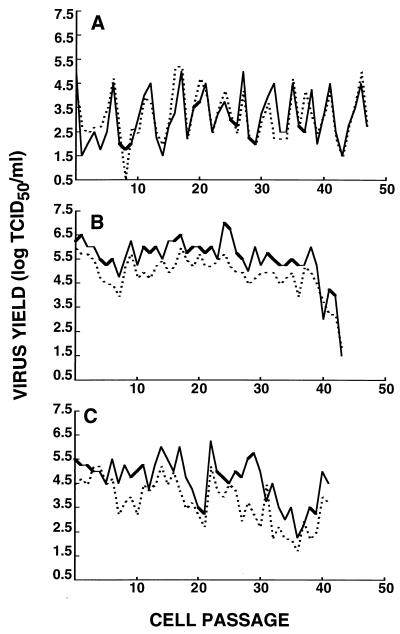
Since a persistent infection does not necessarily correlate with production of infectious virus (52), we verified the presence of such infectious viral particles in the supernatant and in the intracellular compartment of all persistently infected cell lines at every cell passage. Infectious virions were detected by an immunoperoxidase test (Fig. 3). As observed with the immunofluorescence assay, only the H4 and MO3.13 neural cell lines and the L-132 reference cell line were susceptible to persistent infection by HuCV-229E. In general, release of extracellular virus paralleled the production of intracellular infectious virions. Viral titers varied with time, probably because of the varying percentages of infected cells and also depending on the cell cycle of the cell population. The maximal titer observed in persistently infected H4 cells was 5.25 log TCID50/ml, while in MO3.13 cells it was 7.0 log TCID50/ml. In the latter case, it was even higher than for the L-132 reference cell line (5.75 log TCID50/ml). The levels of infectious virions produced by the persistently infected cells correlated with the percentage of infected cells as estimated by indirect immunofluorescence. Approximately 1% of persistently infected H4 cells were positive for HuCV-229E antigen, and this cell line produced lower maximal infectious titers than the L-132 cell line. On the other hand, around 5 to 10% of persistently infected MO3.13 were positive in the same test and produced a maximum of 100 times more infectious virions (Table 2 and Fig. 3). The persistent infection resulted not only in a persistent presence of HuCV-229E antigen in the cell culture but also in the release of infectious virions at all cell passages during the persistent infection, which was monitored for ∼130 days. The parental immortalized Te671 cell line used to generate the MO3.13 cell line was also persistently infected by HuCV-229E, as shown by immunofluorescence detection of viral antigen and the production of infectious virions in the intracellular and extracellular compartments (data not shown).
FIG. 3.
Yield of infectious virions from persistent HuCV-229E infections of different cell lines. (A) H4 cells; (B) MO3.13 cells; (C) L-132 cells. Solid lines, supernatant (extracellular virus); dotted lines, cell lysate (intracellular virus).
Detection of RNA during persistent infection.
We looked for the presence of coronavirus RNA in the cell culture as another indication of viral replication. Since total RNA was taken from cell culture, every viral genome could be amplified, even defective ones. The RT-PCR assay being more sensitive than indirect immunofluorescence (61), it also allowed us to verify the presence of viral RNA even when no antigens or virions were detected. We chose to look for the N protein gene since it is usually present at high levels during a coronavirus infection. Moreover, the 3′-coterminal nested set structure of subgenomic coronavirus mRNAs results in the presence of this gene on all viral RNAs (35). Total RNA was extracted, reverse transcribed, and amplified by RT-PCR for a portion of the N protein gene, by using the primers described in Table 1. The PCR products were of the expected size (Fig. 4). No amplification products were detected when RNA from noninfected cells was used, while the GAPDH RNA was amplified from the same sample preparation (data not shown). Results from MO3.13 (Fig. 4B), H4 (Fig. 4A), and L-132 (Fig. 4C) cell lines were similar. Acutely and persistently infected cells were positive for viral RNA at all passages tested. Thus, persistent infection resulted in the persistence of coronavirus RNA at an apparently constant level.
FIG. 4.
Detection of the N protein gene by RT-PCR during persistent HuCV-229E infection of various cell lines. One tenth of the PCR amplicon obtained was loaded on a 1.5% (wt/vol) agarose gel for electrophoretic separation. (A) H4 cells; (B) MO3.13 cells; (C) L-132 cells. Lanes: N, noninfected cells; A, acutely infected cells; -, negative control for reverse transcriptase and for PCR; numbers, cell passages.
Sequencing of S1 gene and a region of the N gene in persistently infected neural cell lines.
During an in vivo persistent CNS infection by MHV in mice, multiple point and deletion mutations were previously shown to arise, mainly concentrated in the S and N genes (1, 58). Important determinants of neurovirulence reside in the S1 region of the S glycoprotein of MHV (10, 23). For these reasons, we evaluated possible molecular variations occurring in the amplified N gene portion and the S1 portion of the S gene during persistent HuCV-229E infections. The N gene amplicons obtained (Fig. 4) were cloned and sequenced in both directions for three clones for each cell line. Compared to the published sequence (60), two point mutations were present in every clone from acute and persistent infections as well as in our initial virus stock. They were located at positions 879 (ATC to ATG [ATC→ATG] [amino acid change: I→M]) and 880 (GAA to CAA [GAA→CAA] [amino acid change: E→Q]). Importantly, another sequence of the N gene, which bears exactly the same mutations as those observed in our clones, is available (60a). We conclude that these mutations are shared by HuCV-229E viruses studied in other laboratories.
An RT-PCR amplification of the S1 gene portion was first performed, and then the 1,897-bp amplicons were cloned and sequenced in both orientations (see description of internal primers in Table 1) to reduce possible errors introduced by automatic sequence analysis. Changes observed were compared to the published sequence (55) (Table 3). Results were confirmed by reading both nucleic acid strands and characterizing three clones for each cell line and infection. In certain cases, even amplicons obtained from different RT-PCRs were cloned and sequenced. No sequence differences between independent cloned amplicons were observed. Sequencing of the S1 gene from persistent passages 42 for L-132 cells, 48 for H4 cells, and 40 for MO3.13 cells as well as the acute HuCV-229E infection of these same cell types was performed. A point mutation compared to the published sequence was observed in all clones sequenced (55). This point mutation, at position 727, would lead to an amino acid change from a cysteine to a phenylalanine. However, we found in the GenBank database a sequence of the S1 gene from the ATCC isolate of HuCV-229E that bears the same point mutation at position 727 as our sequences (accession no. Y09923 [28a]). We conclude that the sequence published in 1990 contains a discrepancy with our sequence, which is also observed by other laboratories. Coronavirus RNA obtained at the 42nd cell passage of persistently infected L-132 cells and at the 40th cell passage of persistently infected MO3.13 cells presented a mutation at position 571, leading to an amino acid change from a threonine to an isoleucine in all three clones sequenced for each cell line. In the case of viral sequences from persistently infected MO3.13 cells, an additional mutation was observed at position 592, leading also to an amino acid change, from an alanine to a valine. Thus, we have identified a residue in S1 that was mutated in more than one cell line; however, this mutation was not necessary for the establishment of persistence, since HuCV-229E from persistently infected H4 cells did not show this mutation. Mutations found in one out of three sequenced clones were also observed for all cell lines either from acutely or persistently infected cells (data not shown). The frequency of these isolated mutations varied between 8.1 × 10−4 and 1.1 × 10−3 and were similar in acute and persistent infections. Moreover, no isolated mutation was found in more than one cell line or infection. Thus, we conclude that these isolated point mutations were probably induced by the RT-PCR and not by the persistent infection.
TABLE 3.
Characterization of sequence changes detected in the S1 region of the surface glycoprotein during persistent HuCV-229E infection
DISCUSSION
HuCV are recognized respiratory pathogens, causing up to 30% of common colds (49). Increasing data suggesting their neurotropic potential has accumulated. Human neural cell lines and primary cultures of human brain cells are susceptible to infection by the 229E strain of HuCV (9, 76) and by MHV (46). However, very limited results (76) concerning the susceptibility of human continuous cell lines representative of nervous system cells to infection by HuCV-229E, which could provide an in vitro model for studying persistent infection, are available.
In the present study, we have shown that cell lines representative of astrocytes (U-87 MG, U-373 MG, and GL-15), oligodendrocytes (MO3.13), and neurons (SK-N-SH and H4) were all susceptible to infection, albeit at various levels (see summary of results in Table 2), to an acute infection by HuCV-229E, as monitored by the detection of viral antigen and by the release of infectious virions (Fig. 1 and 2). On the other hand, the CHME-5 cell line, which is representative of microglia and is to our knowledge the only available human microglial cell line, was not susceptible to infection by HuCV-229E. Indeed, no viral antigens could be detected in this cell line even when longer times of infection were investigated or higher MOIs were used. Among the numerous cell lines susceptible to acute infection, the oligodendrocytic cell line (MO3.13) and one neuronal cell line (H4) also demonstrated a persistent infection by this virus.
Astrocytes were shown to be susceptible to infection by HuCV-229E but to a lesser extent than to HuCV-OC43 infection since, in general, the percentage of infected cells was lower in immortalized cell lines for HuCV-229E compared to the other strain, OC43 (2). Moreover, HuCV-229E infection of primary cultures of astrocytes could be detected only by RT-PCR in a previous study (9), suggesting an abortive infection and explaining why these cells could not sustain a long-term infection. In fact, the astrocytic cell lines we tested did not sustain a persistent HuCV-229E infection. On the other hand, the murine strain MHV-JHM was reported to infect brain astrocytes in mice (70) and in nonhuman primates (48). Our results are consistent with a potential role of astrocytes in HuCV-229E infection of the CNS during an acute infection but not a persistent infection. Thus, HuCV-229E may not use these cells as a reservoir for its persistence in human CNS, contrary to what was observed for MHV-JHM in mice (54).
The CHME-5 microglial cell line was not susceptible to an acute HuCV-229E infection, even though primary cultures of human adult microglia were previously shown to be susceptible to infection by the same virus (9). The CHME-5 cells were generated by immortalization of human fetal microglia, while primary cultures used by Bonavia et al. (9) were from adult brain. However, the infection levels in primary cultures were not very high and the infection seemed to be abortive since no virus progeny was observed and only viral RNA, not viral antigen, could be detected (9). Interestingly, recent work in our laboratory has shown that the HuCV-229E receptor, aminopeptidase N or CD13 (82), is also utilized by HuCV-229E as an attachment site to neural cells used in the present study (34). In that study, it was also found that the CHME-5 cells did not express CD13, as shown by cytofluorometry, which explains their noninfectability by HuCV-229E (34). We do not know if primary cultures of human microglia express this cellular receptor, although we do expect they would since this molecule is known to be expressed on monocyte/macrophage-type cells, most likely also including microglial cells that share most of the markers found on these cells. Nevertheless, based on our previous results with primary cultures and current results with immortalized cell lines, we conclude that HuCV-229E apparently would not easily infect microglia.
The MO3.13 oligodendrocytic cell line was susceptible to acute and persistent infection by both HuCV strains (2). However, we previously showed that primary cultures of oligodendrocytes were not positive for viral antigen detection when infected with HuCV (9). Moreover, the susceptibilities to HuCV-OC43 infection of oligodendrocytes were different between immortalized and primary cells. These variations could be due to the different degree of differentiation of the neural cells we have used as immortalized cell lines compared to that of primary cultures. Also, it is possible that the sensitivities of our assays were insufficient to detect a low level of virus infection in primary cultures. Nevertheless, we cannot rule out the possibility that immortalized cell lines have acquired new properties, such as susceptibility to HuCV infections.
For neuronal infections, we evaluated two different immortalized cell lines: SK-N-SH and H4. Both cell lines were susceptible to acute infection by HuCV-229E, as shown by viral antigen detection and production of infectious virions. However, unlike the H4 cell line, the SK-N-SH cell line did not sustain a persistent HuCV-229E infection. In our previous study with primary cultures (9), no HuCV-229E RNA detection could be performed on primary cultures of neurons and no viral antigen was detected after an infection (9). Thus, it is difficult to compare our results relating to neuronal cell lines to already-published work in which the infectability of neurons by HuCV-229E could not be ruled out, given that persistence of MHV in neurons was previously shown (63). Moreover, since the H4 cell line is from a neuroglioma (ganglioglioma) that consists of a mixture of cells of neuronal and glial origins and since, to our knowledge, no human neuronal cell lines from the CNS are available (neuroblastomas are from the peripheral nervous system), we have performed our experiments with this cell line. More experiments should be performed when a CNS neuronal cell line becomes available, to validate the possibility that neurons could serve as a reservoir for HuCV-229E.
Differences in the genetic constitution of various viral strains and variants could affect the neurotropism and the pathology observed and these differences could appear during an in vivo or in vitro infection (71, 72). It has been shown that MHV-4 infects mainly neurons of mice (22), whereas MHV-JHM is found mainly in oligodendrocytes of the spinal cord and brain of the same animals (73). Finally, MHV-A59 infects principally neurons and neural processes and terminals (37). Given this variety of results obtained with MHV strains, it was important to look at genetic variations of HuCV in the context of potential in the human nervous system. The viral N and S genes are of interest since, in persistently infected mice, RNA analysis showed a diverse population of viral sequences for these two genes (1, 58). Also, important molecular determinants of MHV neuropathogenesis are located on the S protein (42, 45).
The portion of the N gene amplified in our study (nucleotides 819 to 1054) showed two point mutations in all persistently infected cell lines that were identical to a submitted sequence (60a). We have also looked at the S1 gene portion since most of the mutations observed with MHV were concentrated on this apparently hypervariable portion of the spike protein. One point mutation at position 571 was observed on HuCV-229E RNA from persistently infected MO3.13 and L-132 cells, leading to an amino acid change from a threonine to an isoleucine, an amino acid that is less charged. An additional mutation, at position 592, was observed on RNA from persistently infected MO3.13 cells, leading to an amino acid change from an alanine to a valine, a larger amino acid. In the case of HuCV-OC43, the MO3.13 cell line was also prone to more point mutations than the other cell lines and particularly at different positions than in other cell lines (2). Thus, interaction of HuCV with this cell line is apparently more prone to the appearance of virus mutants.
It is difficult at the moment to suggest a role for these mutations within the S1 gene of HuCV-229E, since no particular function has been attributed to this region of the HuCV-229E S protein. However, it has been shown for porcine transmissible gastroenteric virus, a coronavirus belonging to the same antigenic group as HuCV-229E, that the S1 gene is responsible for receptor binding and also that enterovirulence is associated with a region within the N-terminal end of S1 (36). This strongly suggests that the S1 gene of HuCV-229E plays a role in receptor binding and influences viral tropism. Since viruses do not have to face immune system pressure in vitro, we could then argue that these mutations did not occur for avoiding recognition by the immune system, but we can speculate that they favor viral replication. Interestingly, we observed a higher maximal virus yield for infected MO3.13 and L-132 cells (bearing the point mutation at position 571) compared to infected H4 cells. Moreover, the absence of immune response may explain the constant shedding of infectious virus during the persistent infection, even after ∼130 days, since no mechanism acted to eliminate viruses or infected cells. We can speculate that the point mutation observed in two distinct cell lines is at a position prone to mutation compared to other regions of this protein. Other genomic regions should be sequenced to verify the possibility that other regions are more predisposed to mutation, even though the S1 region was predicted from work with MHV to be more variable. It will be interesting to compare HuCV-229E, with and without certain point mutations within the S1 gene, with regard to their capacity to infect and replicate in neural cells.
A controversy has arisen in recent scientific publications concerning the possible emergence of quasispecies during persistent coronavirus infections within the CNS. Stühler and colleagues reported that no quasispecies arose during a persistent MHV-JHM infection within the CNS of rats (68). Moreover, another study showed little evidence for the selection of specific mutations within the M protein, N–cytotoxic-T-lymphocyte epitope, and encapsidation sequence with time after infection in mice (6). On the other hand, others have shown that within the N and S genes, multiple point and deletion mutations occurred during a 42-day period postinfection in mice, suggesting rapid viral genomic RNA evolution and the emergence of quasispecies (1, 58). However, even though they were common, these variants were not required for persistent infections in mice (1, 58). The selection of virus variants with distinct localized mutations and the evolution of more virulent phenotypes for cell lines persistently infected with MHV was previously shown (15, 16, 27). However, mutation frequencies and the rate at which they appeared were different at various genomic positions (16).
The error rate of retroviral reverse transcriptase enzymes has been estimated at 10−4 on complex RNA templates (62). The Expand high-fidelity PCR system DNA polymerase (Taq and Pwo DNA polymerases) we used has a calculated error rate of 8.5 × 10−6 (Roche Diagnostics). Since we have performed 30 amplification cycles, the total error rate for our RT-PCR could be estimated at 3.6 × 10−4 [=1 × 10−4 + (8.5 × 10−6 × 30 cycles)]. Variations between clones of virus genome generated by RT-PCR should be greater than that expected from the error rates of reverse transcriptase and Taq (62) to be considered as mutations representative of the viral population. Substitutions present in more than one clone are likely to represent mutations actually present in the virus population (62). The error rate of isolated mutations (found on only one out of three sequenced clones for each cell line) varied between 8.1 × 10−4 and 1.1 × 10−3, which corresponds to a 2.2- to 3.0-fold increase compared to the theoretically expected error rate calculated for our experiments because of reverse transcriptase- and Taq polymerase-induced errors. Based on these results we cannot make a conclusion on the presence of quasispecies among the population of HuCV-229E viruses. Moreover, these rates of isolated mutations were comparable between S1 sequenced from acute and persistent infections, meaning that the persistent infection did not seem to involve a quasispecies evolution. If we agree to describe quasispecies as a whole population of phylogenetically related variants observed within a single infected cell population (62), we did not in our study sequence enough clones to make a conclusion about the whole population, but our results do not suggest such variants at the level of the S1 gene. It would be necessary to sequence a larger number of clones to clearly establish the absence of a diverse population of mutant viral RNA. Again, we should mention that, in our study, viruses were not exposed to immune responses and so did not have to escape from immune recognition.
The neurotropism of murine coronaviruses has been well documented by in vitro and in vivo experiments. However, the neurotropism of HuCV remains to be confirmed. In rodents, a coronavirus infection does not necessarily result in severe pathological symptoms but could be manifested as a clinically silent subacute demyelinating disease (79). Indeed, MHV-JHM can persist and replicate within neurons and oligodendrocytes of rats without causing cell degeneration and death or eliciting an immune attack which may lead to tissue necrosis and suppression of the infection (63). On the other hand, the persistent infection could lead to a pathological outcome: an MHV-JHM mutant was shown to establish a reproducible persistent infection in mice and to induce progression from an acute demyelinating disease to a chronic recurring form (32). MHV-JHM could also establish a persistent infection when injected intravitreally, i.e., into another immunologically privileged site in the body, where viral RNA was detected by in situ hybridization up to 60 days postinfection (33).
Other viruses, such as Borna disease virus, human immunodeficiency virus, polyomavirus JC, and measles virus, have been shown to persist within the human CNS with or without accompanying pathological manifestations. A persistent HuCV-229E infection might not lead directly to pathology since no cytopathic effects were observed in any of the neural cell lines tested in the present study. However, the presence of coronavirus antigens within the CNS could lead to localized and potentially detrimental immune responses. For example, it has been shown that a MHV-JHM infection of rats leads to T-cell-mediated autoimmune myelin reactivity, presumably through immune cross-reactivity with both virus and myelin antigens (80). Interestingly, we have previously reported the preferential presence of T cells cross-reactive to both HuCV-229E and myelin basic protein in the peripheral blood of MS patients compared to controls (77).
Viral RNA could be detected in both the rodent and human CNS for long periods after the primary infection. It is unlikely that all human brain tissues positive for HuCV RNA, originating from different laboratories (3, 46, 66), were from humans acutely infected by HuCV just prior to their deaths, in part because of differences in age and geographical origin at the time of autopsy. Coronaviruses might provide low levels of viral antigens in order to escape the immune system during a persistent infection, since most often only viral RNA and neither infectious viruses nor viral antigens are detected for such long periods. However, sensitivities of techniques used might influence the observations made. Detailed in situ detection of HuCV in human brain sections would confirm the tropism observed in vitro with primary and immortalized cultures and provide clues as to the impact of the presence of HuCV-229E in human CNS. These studies are in progress. The results of our present study favor oligodendrocytes, and possibly neurons, as potential HuCV-229E reservoirs within the human CNS.
ACKNOWLEDGMENTS
This work was supported by grant MT-9203 from the Medical Research Council of Canada to P.J.T., who also gratefully acknowledges a senior scholarship award from the Fonds de la Recherche en Santé du Québec (FRSQ). N.A. is grateful to the Institut Armand-Frappier as well as to the Multiple Sclerosis Society of Canada for studentship support.
We thank Francine Lambert for excellent technical assistance. We thank Julie Edwards for critically reviewing the manuscript.
REFERENCES
- 1.Adami C, Pooley J, Glomb J, Stecker E, Fazal F, Fleming J O, Baker S C. Evolution of mouse hepatitis virus (MHV) during chronic infection: quasispecies nature of the persisting MHV RNA. Virology. 1995;209:337–346. doi: 10.1006/viro.1995.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbour N, Côté G, Lachance C, Tardieu M, Cashman N R, Talbot P J. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbour, N., and P. J. Talbot. 1998. Unpublished data.
- 4.Barnett E M, Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 1993;194:185–191. doi: 10.1006/viro.1993.1248. [DOI] [PubMed] [Google Scholar]
- 5.Barthold S W, Smith A L. Viremic dissemination of mouse hepatitis virus-JHM following intranasal inoculation of mice. Arch Virol. 1992;122:35–44. doi: 10.1007/BF01321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann C, Dimacali E, Stohl S, Wei W, Lai M M C, Tahara S, Marten N. Variability of persisting MHV RNA sequences constituting immune and replication-relevant domains. Virology. 1998;244:563–572. doi: 10.1006/viro.1998.9147. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann C C, Yao Q, Lin M, Stohlman S A. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted cytotoxic T cell response. J Gen Virol. 1996;77:315–325. doi: 10.1099/0022-1317-77-2-315. [DOI] [PubMed] [Google Scholar]
- 8.Bocchini V, Casalone R, Collini P, Rebel G, Lo Curto F. Changes in glial fibrillary acidic protein and karyotype during culturing of two cell lines established from human glioblastoma multiforme. Cell Tissue Res. 1991;265:73–81. doi: 10.1007/BF00318141. [DOI] [PubMed] [Google Scholar]
- 9.Bonavia A, Arbour N, Yong V W, Talbot P J. Infection of primary cultures of human neural cells by human coronaviruses 229E and OC43. J Virol. 1997;71:800–806. doi: 10.1128/jvi.71.1.800-806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchmeier M J, Dalziel R G, Koolen M J M, Lampert P W. Molecular determinants of CNS virulence of the coronavirus mouse hepatitis virus-4. In: Brinton M A, Rueckert R R, editors. Positive-strand RNA viruses. New York, N.Y: Alan R. Liss, Inc.; 1987. pp. 409–422. [Google Scholar]
- 11.Cabirac G F, Murray R S, McLaughlin L B, Skolnick D M, Hogue B, Dorovini-Zis K, Didier P J. In vitro interaction of coronaviruses with primate and human brain microvascular endothelial cells. Adv Exp Med Biol. 1995;380:79–88. doi: 10.1007/978-1-4615-1899-0_11. [DOI] [PubMed] [Google Scholar]
- 12.Cabirac G F, Soike K F, Zhang J-Y, Hoel K, Butunoi C, Cai G-Y, Johnson S, Murray R S. Entry of coronavirus into primate CNS following peripheral infection. Microb Pathog. 1994;16:349–357. doi: 10.1006/mpat.1994.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro R F, Perlman S. CD8+ T-cell epitopes within the surface glycoprotein of a neurotropic coronavirus and correlation with pathogenicity. J Virol. 1995;69:8127–8131. doi: 10.1128/jvi.69.12.8127-8131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaloner-Larsson G, Johnson-Lussenburg C M. Establishment and maintenance of a persistent infection of L132 cells by human coronavirus strain 229E. Arch Virol. 1981;69:117–120. doi: 10.1007/BF01315155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Baric R S. Molecular anatomy of mouse hepatitis virus persistence: coevolution of increased host cell resistance and virus virulence. J Virol. 1996;70:3947–3960. doi: 10.1128/jvi.70.6.3947-3960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Baric R S. Function of a 5′-end genomic RNA mutation that evolves during persistent mouse hepatitis virus infection in vitro. J Virol. 1995;69:7529–7540. doi: 10.1128/jvi.69.12.7529-7540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins A R, Knobler R L, Powell H, Buchmeier M J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell-cell fusion. Virology. 1982;119:358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel C, Anderson R, Buchmeier M J, Fleming J O, Spaan W J M, Wege H, Talbot P J. Identification of an immunodominant linear neutralization domain of the S2 portion of the murine coronavirus spike glycoprotein and evidence that it forms part of a complex tridimensional structure. J Virol. 1993;67:1185–1194. doi: 10.1128/jvi.67.3.1185-1194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel C, Talbot P J. Protection from lethal coronavirus infection by affinity-purified spike glycoprotein of murine hepatitis virus, strain A59. Virology. 1990;174:87–94. doi: 10.1016/0042-6822(90)90057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dveksler G S, Pensiero M N, Cardellichio C B, Williams R K, Jiang G S, Holmes K V, Dieffenbach C W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988;263:15335–15341. [PubMed] [Google Scholar]
- 22.Fazakerley J K, Parker S E, Bloom F, Buchmeier M J. The V5A13.1 envelope glycoprotein deletion mutant of mouse hepatitis virus type-4 is neuroattenuated by its reduced rate of spread in the central nervous system. Virology. 1992;187:178–188. doi: 10.1016/0042-6822(92)90306-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming J O, Trousdale M D, Bradbury J, Stohlman S A, Weiner L P. Experimental demyelination induced by coronavirus JHM (MHV-4): molecular identification of a viral determinant of paralytic disease. Microb Pathog. 1987;3:9–20. doi: 10.1016/0882-4010(87)90033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming J O, Houtman J J, Alaca H, Hinze H C, McKenzie D, Aiken J, Bleasdale T, Baker S. Persistence of viral RNA in the central nervous system of mice inoculated with MHV-4. Adv Exp Med Biol. 1994;342:327–332. doi: 10.1007/978-1-4615-2996-5_50. [DOI] [PubMed] [Google Scholar]
- 25.Flintoff W F, Van Dinter S. Several rat cell lines share a common defect in their inability to internalize murine coronaviruses efficiently. J Gen Virol. 1989;70:1713–1724. doi: 10.1099/0022-1317-70-7-1713. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher T M, Parker S E, Buchmeier M J. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J Virol. 1990;64:731–741. doi: 10.1128/jvi.64.2.731-741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gombold J L, Hingley S T, Weiss S R. Fusion-defective mutants of mouse hepatitis virus A59 contain a mutation in the spike protein cleavage signal. J Virol. 1993;67:4504–4512. doi: 10.1128/jvi.67.8.4504-4512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamre D, Procknow J J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 28a.Hays, J. P. 1997. GenBank accession no. Y09923.
- 29.Houtman J J, Fleming J O. Pathogenesis of mouse hepatitis virus-induced demyelination. J Neurovirol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- 30.Janabi N, Peudenier S, Héron B, Ng K H, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglia cells with the SV40 large T antigen. Neurosci Lett. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- 31.Jouvenne P, Mounir S, Stewart J N, Richardson C D, Talbot P J. Sequence analysis of human coronavirus 229E mRNAs 4 and 5: evidence for polymorphism and homology with myelin basic protein. Virus Res. 1992;22:125–141. doi: 10.1016/0168-1702(92)90039-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knobler R L, Lampert P W, Oldstone M B A. Virus persistence and recurring demyelination produced by a temperature-sensitive mutant of MHV-4. Nature. 1982;298:279–280. doi: 10.1038/298279a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komurasaki Y, Nagineni C N, Wang Y, Hooks J J. Virus RNA persists within the retina in coronavirus-induced retinopathy. Virology. 1996;222:446–450. doi: 10.1006/viro.1996.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lachance C, Arbour N, Cashman N R, Talbot P J. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronaviruses 229E. J Virol. 1998;72:6511–6519. doi: 10.1128/jvi.72.8.6511-6519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai M M C, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laude H, Godet M, Bernard S, Gelfi J, Duarte M, Delmas B. Functional domains in the spike protein of transmissible gastroenteritis virus. Adv Exp Med Biol. 1995;380:299–304. doi: 10.1007/978-1-4615-1899-0_48. [DOI] [PubMed] [Google Scholar]
- 37.Lavi E, Fishman P S, Highkin P S, Weiss S R. Limbic encephalitis after inhalation of a murine coronavirus. Lab Investig. 1988;58:31–36. [PubMed] [Google Scholar]
- 38.Lavi E, Gilden D H, Highkin M K, Weiss S R. The organ tropism of mouse hepatitis virus A59 in mice is dependent on dose and route of inoculation. Lab Anim Sci. 1986;36:130–135. [PubMed] [Google Scholar]
- 39.Lavi E, Gilden D H, Highkin M K, Weiss S R. Persistence of mouse hepatitis virus A59 RNA in a slow virus demyelinating infection in mice as detected by in situ hybridization. J Virol. 1984;51:563–566. doi: 10.1128/jvi.51.2.563-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas A, Coulter M, Anderson R, Dales S, Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978;88:325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macnaughton M R, Thomas B J, Davies H A, Patterson S. Infectivity of human coronavirus strain 229E. J Clin Microbiol. 1980;12:462–468. doi: 10.1128/jcm.12.3.462-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makino S, Fleming J O, Keck J G, Stohlman S A, Lai M M C. RNA recombination of coronaviruses: localization of neutralizing epitopes and neuropathogenic determinants on the carboxyl terminus of peplomers. Proc Natl Acad Sci USA. 1987;84:6567–6571. doi: 10.1073/pnas.84.18.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massa P T, Wege H, ter Meulen V. Growth pattern of various JHM coronavirus isolates in primary rat glial cell cultures correlates with differing neurotropism in vivo. Virus Res. 1988;9:133–144. doi: 10.1016/0168-1702(88)90028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaurin J, Trudel G C, Shaw I T, Antel J P, Cashman N R. A human glial hybrid cell line differentially expressing genes subserving oligodendrocyte and astrocyte phenotype. J Neurobiol. 1995;26:283–293. doi: 10.1002/neu.480260212. [DOI] [PubMed] [Google Scholar]
- 45.Morris V L, Tieszer C, Mackinnon J, Percy D. Characterization of coronavirus JHM variants isolated from Wistar Furth rats with a viral-induced demyelinating disease. Virology. 1989;169:127–136. doi: 10.1016/0042-6822(89)90048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray R S, Cai G Y, Hoel K, Johnson S, Cabirac G F. Coronaviruses and multiple sclerosis. Adv Exp Med Biol. 1993;342:353–357. doi: 10.1007/978-1-4615-2996-5_54. [DOI] [PubMed] [Google Scholar]
- 47.Murray R S, Cai G-Y, Hoel K, Zhang J-Y, Soike K F, Cabirac G F. Coronavirus infects and causes demyelination in primate central nervous system. Virology. 1992;188:274–284. doi: 10.1016/0042-6822(92)90757-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray R S, Cai G Y, Soike K F, Cabirac G F. Further observations on coronavirus infection of primate CNS. J Neurovirol. 1997;3:71–75. doi: 10.3109/13550289709015795. [DOI] [PubMed] [Google Scholar]
- 49.Myint S H. Human coronaviruses—a brief review. Rev Med Virol. 1994;4:35–46. [Google Scholar]
- 50.Okumura A, Machii K, Azuma S, Toyoda Y, Kyuwa S. Maintenance of pluripotency in mouse embryonic stem cells persistently infected with murine coronavirus. J Virol. 1996;70:4146–4149. doi: 10.1128/jvi.70.6.4146-4149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson S, Macnaughton M R. Replication of human respiratory coronavirus strain 229E in human macrophages. J Gen Virol. 1982;60:307–314. doi: 10.1099/0022-1317-60-2-307. [DOI] [PubMed] [Google Scholar]
- 52.Pearson J, Mims C A. Differential susceptibility of cultured neural cells to the human coronavirus OC43. J Virol. 1985;53:1016–1019. doi: 10.1128/jvi.53.3.1016-1019.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perlman S, Evans G, Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med. 1990;172:1127–1132. doi: 10.1084/jem.172.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perlman S, Ries D. The astrocyte is a target cell in mice persistently infected with mouse hepatitis virus, strain JHM. Microb Pathog. 1987;3:309–314. doi: 10.1016/0882-4010(87)90064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raabe T, Schelle-Prinz B, Siddell S G. Nucleotide sequence of the gene encoding the spike glycoprotein of human coronavirus HCV 229E. J Gen Virol. 1990;71:1065–1073. doi: 10.1099/0022-1317-71-5-1065. [DOI] [PubMed] [Google Scholar]
- 56.Reed S E. The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: evidence of heterogeneity among 229E-related strains. J Med Virol. 1984;13:179–192. doi: 10.1002/jmv.1890130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Resta S, Luby J P, Rosenfeld C R, Siegel J D. Isolation and propagation of a human enteric coronavirus. Science. 1985;229:978–981. doi: 10.1126/science.2992091. [DOI] [PubMed] [Google Scholar]
- 58.Rowe C L, Baker S C, Nathan M J, Fleming J O. Evolution of mouse hepatitis virus: detection and characterization of spike deletion variants during persistent infection. J Virol. 1997;71:2959–2969. doi: 10.1128/jvi.71.4.2959-2969.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salmi A, Ziola B, Hovi T, Reunanen M. Antibodies to coronaviruses OC43 and 229E in multiple sclerosis patients. Neurology. 1982;32:292–295. doi: 10.1212/wnl.32.3.292. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber S S, Kamahora T, Lai M M C. Sequence analysis of the nucleocapsid protein gene of human coronavirus 229E. Virology. 1989;169:142–151. doi: 10.1016/0042-6822(89)90050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60a.Siddell, S. 1993. GenBank accession no. X51325.
- 61.Sizun J, Arbour N, Talbot P J. Comparison of immunofluorescence with monoclonal antibodies and RT-PCR for the detection of human coronaviruses 229E and OC43 in cell culture. J Virol Methods. 1998;72:145–152. doi: 10.1016/S0166-0934(98)00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith D B, McAllister J, Casino C, Simmonds P. Virus ‘quasispecies’: making a mountain out of a molehill? J Gen Virol. 1997;78:1511–1519. doi: 10.1099/0022-1317-78-7-1511. [DOI] [PubMed] [Google Scholar]
- 63.Sorensen O, Coulter-Mackie M B, Puchalski S, Dales S. In vivo and in vitro models of demyelinating disease. IX. Progression of JHM virus infection in the central nervous system of the rat during overt and asymptomatic phases. Virology. 1984;137:347–357. doi: 10.1016/0042-6822(84)90227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorensen O, Dales S. In vivo and in vitro models of demyelinating disease: JHM virus in the rat central nervous system localized by in situ cDNA hybridization and immunofluorescent microscopy. J Virol. 1985;56:434–438. doi: 10.1128/jvi.56.2.434-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorensen O, Dugre R, Percy D, Dales S. In vivo and in vitro models of demyelinating disease: endogenous factors influencing demyelinating disease caused by mouse hepatitis virus in rats and mice. Infect Immun. 1982;37:1248–1260. doi: 10.1128/iai.37.3.1248-1260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart J N, Mounir S, Talbot P J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stohlman S A, Weiner L P. Stability of neurotropic mouse hepatitis virus (JHM strain) during chronic infection of neuroblastoma cells. Arch Virol. 1978;57:53–61. doi: 10.1007/BF01315637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stühler A, Flory E, Wege H, Lassmann H, Wege H. No evidence for quasispecies populations during persistence of the coronavirus mouse hepatitis virus JHM: sequence conservation within the surface glycoprotein S in Lewis rats. J Gen Virol. 1997;78:747–756. doi: 10.1099/0022-1317-78-4-747. [DOI] [PubMed] [Google Scholar]
- 69.Sun N, Grzybicki D, Castro R F, Murphy S, Perlman S. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213:482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun N, Perlman S. Spread of a neurotropic coronavirus to spinal cord white matter via neurons and astrocytes. J Virol. 1995;69:633–641. doi: 10.1128/jvi.69.2.633-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taguchi F, Massa P T, ter Meulen V. Characterization of a variant virus isolated from neural cell culture after infection of mouse coronavirus JHMV. Virology. 1986;155:267–270. doi: 10.1016/0042-6822(86)90187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taguchi F, Siddell S G, Wege H, ter Meulen V. Characterization of a variant virus selected in rat brains after infection by coronavirus mouse hepatitis virus JHM. J Virol. 1985;54:429–435. doi: 10.1128/jvi.54.2.429-435.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi K, Goto N, Ishida T, Katami K, Fujiwara K. Acute demyelination in mice inoculated intraspinally with mouse hepatitis virus, JHM strain. Jpn J Exp Med. 1981;51:323–330. [PubMed] [Google Scholar]
- 74.Talbot P J, Dionne G, Lacroix M. Vaccination against lethal coronavirus-induced encephalitis with a synthetic decapeptide homologous to a domain in the predicted peplomer stalk. J Virol. 1988;62:3032–3036. doi: 10.1128/jvi.62.8.3032-3036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talbot P J, Salmi A A, Knobler R L, Buchmeier M J. Topographical mapping of epitopes on the glycoproteins of murine hepatitis virus-4 (strain JHM): correlation with biological activities. Virology. 1984;131:250–260. doi: 10.1016/0042-6822(84)90032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Talbot P J, Ékandé S, Cashman N R, Mounir S, Stewart J N. Neurotropism of human coronavirus 229E. Adv Exp Med Biol. 1994;342:339–346. doi: 10.1007/978-1-4615-2996-5_52. [DOI] [PubMed] [Google Scholar]
- 77.Talbot P J, Paquette J S, Ciurli C, Antel J P, Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol. 1996;39:233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tardieu M, Boespflug O, Barbé T. Selective tropism of a neurotropic coronavirus for ependymal cells, neurons, and meningeal cells. J Virol. 1986;60:574–582. doi: 10.1128/jvi.60.2.574-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watanabe R, Wege H, ter Meulen V. Comparative analysis of coronavirus JHM-induced demyelinating encephalomyelitis in Lewis and Brown Norway rats. Lab Investig. 1987;57:375–384. [PubMed] [Google Scholar]
- 80.Watanabe R, Wege H, ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983;305:150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wege H, Winter J, Meyermann R. The peplomer protein E2 of coronavirus JHM as a determinant of neurovirulence: definition of critical epitopes by variant analysis. J Gen Virol. 1988;69:87–98. doi: 10.1099/0022-1317-69-1-87. [DOI] [PubMed] [Google Scholar]
- 82.Yeager C L, Ashmun R A, Williams R K, Cardellichio C B, Shapiro L H, Look A T, Holmes K V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yokomori K, Lai M M C. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992;66:6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]