Abstract
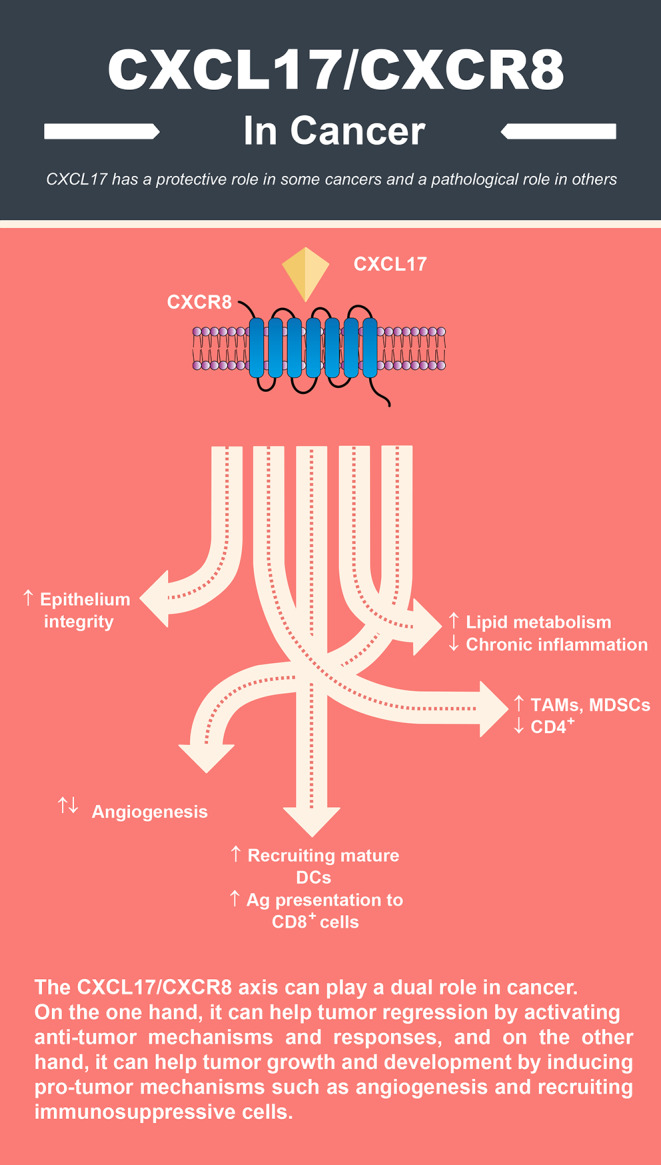
Chemokines are immune system mediators that mediate various activities and play a role in the pathogenesis of several cancers. Among these chemokines, C-X-C motif chemokine 17 (CXCL-17) is a relatively novel molecule produced along the airway epithelium in physiological and pathological conditions, and evidence shows that it plays a homeostatic role in most cases. CXCL17 has a protective role in some cancers and a pathological role in others, such as liver and lung cancer. This chemokine, along with its possible receptor termed G protein-coupled receptor 35 (GPR35) or CXCR8, are involved in recruiting myeloid cells, regulating angiogenesis, defending against pathogenic microorganisms, and numerous other mechanisms. Considering the few studies that have been performed on the dual role of CXCL17 in human malignancies, this review has investigated the possible pro-tumor and anti-tumor roles of this chemokine, as well as future treatment options in cancer therapy.
Graphical Abstract
Keywords: CXCL17, Chemokine, Cancer, CXCR8
Introduction
Cancer is one of the health-related problems worldwide that kills many patients every year and is the leading cause of death before age 70 in many countries (Bray et al. 2021; Sung et al. 2021). With the change of lifestyle in numerous developed and developing countries, even the death rate from cardiovascular diseases and stroke has decreased from cancer, which can be considered a warning (Bray et al. 2021). Despite the extensive research that has been done in the field of mechanisms related to the pathogenesis and progression of cancer, there are still several unknowns, and the existing treatments have been able to give relatively satisfactory results in recent decades, which of course, is still not adequate (Yu et al. 2022). Among the factors involved in tumor progression or regression, the traces of the immune system and its components are always realized. The immune system cells and mediators can have an anti-tumor or tumor-supporting role (Loose and Van de Wiele 2009).
The superfamily of chemokines consists of a large number of ligands that can bind to one or more receptors and exert their effects (Zlotnik and Yoshie 2000; Yoshie et al. 2001). Chemokines are small peptides, while their receptors are class A G protein-coupled receptors. Chemokines are known for regulating the migration of different cells in the body and are considered their most basic function, which is why they are named chemotactic cytokines (Schaerli and Moser 2005; Azin et al. 2012). Over time, these molecules’ importance has become clearer because, in addition to cell migration and chemotaxis, chemokines are involved in several biological and pathological processes in inflammatory conditions, infections, autoimmunity, and cancer (Zlotnik et al. 2006; Rostène et al. 2011; Aminzadeh et al. 2012). The structure of chemokines usually contains four cysteines in conserved positions. In addition, these molecules are small, between 8 and 14 kilodaltons, so they are produced in high amounts by various cells. The justification for the high expression of these molecules is participation in chemotactic mechanisms and migration through the concentration gradient, which has led to the locomotion of specific cells expressing chemokine receptors (Zlotnik et al. 2006). According to the cysteine residue location, chemokines are divided into four subgroups: CC, CXC, CX3C, and C chemokines (Colobran et al. 2007). Some chemokines such as CCL2, CCL3, and CCL5 are produced in large amounts by different cells.
On the other hand, some chemokines such as CCL25, CCL27, CCL28, and CXCL17 can have very high specificity for certain types of cells or tissues. CCL25 is produced in the thymus and gut tissues, CCL27 by skin keratinocytes, CCL28 by mucosal epithelial cells, and CXCL17 by gastrointestinal epithelial cells. Other relatively exclusive features of chemokines include participation in physiological and pathological conditions, regulation of their expression, chromosomal locations of genes encoding chemokines, as well as the binding characteristics of their receptors (Zlotnik et al. 2006).
Among CXC chemokines, CXCL17 was discovered by fold-recognition methods (Pisabarro et al. 2006). CXCL17 is a novel and essential immune molecule produced in mucosal tissues. With its anti-microorganism and anti-inflammatory properties, this chemokine is involved in homeostatic processes (Lee et al. 2013; Srivastava et al. 2018; Xiao et al. 2021). However, the role of CXCL17 in different cancers is entirely different and could have pro-tumor or anti-tumor functions. For example, overexpression of CXCL17 is associated with poor survival in colon, breast, and hepatocellular cancers, while in pancreatic cancer, it has a protective and anti-tumor role because it stimulates anti-tumor responses (Hiraoka et al. 2011; Guo et al. 2017; Yao et al. 2020).
Therefore, this review has investigated the dual role of CXCL17 in human malignancies and discussed the treatment options related to this chemokine.
CXCL17 Biology and Signaling
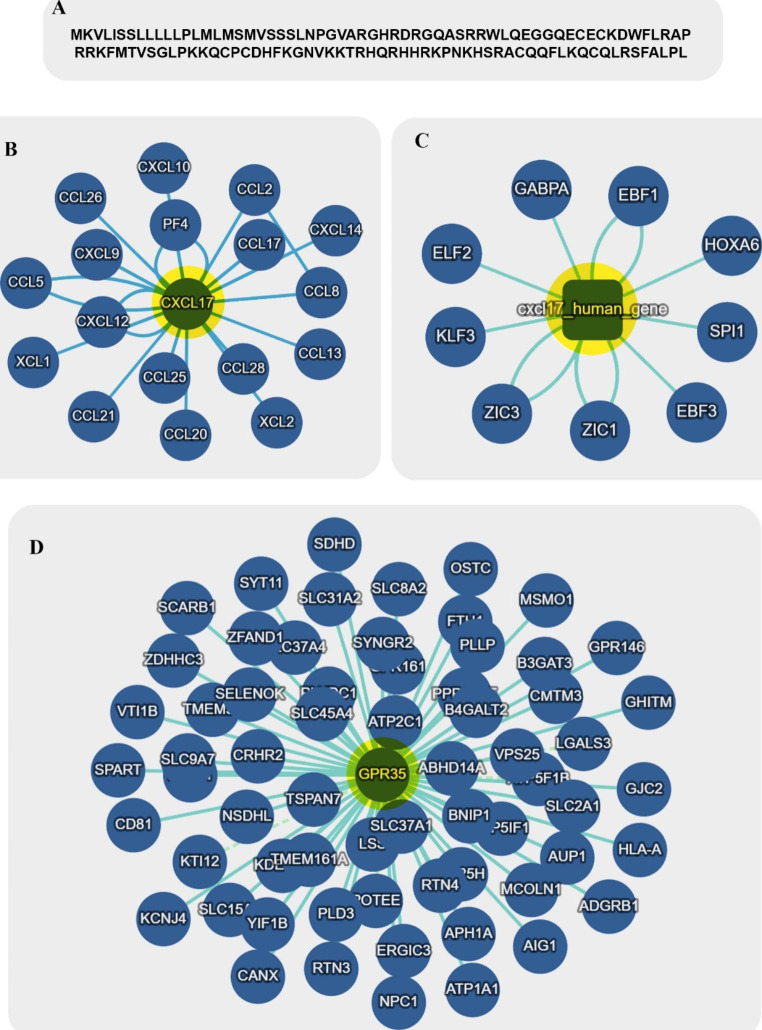
CXCL17 can be considered a member of the mucosal chemokine family, including CXCL14, CCL25 and CCL28. This chemokine is expressed in gastrointestinal and respiratory tissues (Pisabarro et al. 2006; Weinstein et al. 2006). In contrast to CCL25 and CXCL14, which can be detected within the solid parenchyma of different tissues and epithelial barriers, CXCL17 expression is only limited to the epithelial barriers responsible for covering various secretory conduct and hollow organ lumens (Vicari et al. 1997; Hromas et al. 1999; Maerki et al. 2009). The gene comprises four exons involved in coding a propeptide with 119 amino acids (Xiao et al. 2021). The molecular weight of this propeptide is about13,819 Dalton in humans. It has been revealed that there is a similarity between the amino acid sequence of CXCL17 and other CC chemokines such as CCL16 and CCL17 (Weinstein et al. 2006). The CXCL17 gene and protein interaction network are shown in Fig. 1B and C.
Fig. 1.
Amino acid sequences of CXCL17 (A) and interaction networks of CXCL17 (B, C) and CXCR8 (D). Copyright © InAct, EMBL-EBI 2022
On the other hand, the structure of CXCL17 in terms of folding is somewhat similar to the structure of CXCL8, which is classified in the CXC chemokines category (Denisov 2021). Analysis of CXCL17 precursor protein demonstrated that four out of six cysteine residues collated between Phe64 and Leu119 are responsible for constructing the CXC motif. The mature form of protein CXCL17 has a molecular weight of about 11,418 Da, obtained after the post-translational cleavage of 22 amino acids ( comprising two cysteine residues) (Lee et al. 2013) (Fig. 1 A). This mature form with four cysteine residues in the amino-terminal sequence has more chemotactic influence on recruiting monocytes than the propeptide form with six cysteine residues (Lee et al. 2013).
The distinctiveness of the receptor of CXCL17 is rather debated. Most studies recognize that CXCL17 has no binding affinity to CXCR2, CXCR3, CXCR4, CXCR7, CCR2, and CCR5 chemokine receptors CXCR8 (GPR35) as the receptor of this chemokine (Maravillas-Montero et al. 2015; White et al. 2021). Previous studies have shown that calcium flux is increased in B cell line cells (Ba/F3) transfected with CXCR8, suggesting that downstream signaling pathways are initiated following the ligation of CXCL17 to CXCR8 (Maravillas-Montero et al. 2015). However, it is still impossible to consider CXCR8 as a specific CXCL17 receptor because some studies have shown that silencing CXCR8 or using antagonists of this receptor cannot have an inhibitory effect on the migration of treated primary and THP-1 monocytes with CXCL17 (Amir et al. 2018; Park et al. 2018). These findings indicate that a specific CXCL17 receptor that has not yet been discovered is probably involved. In humans, CXCR8 is expressed by various tissues, including the small intestine, pancreas, spleen, colon, and immune cells, such as dendritic cells (DCs), neutrophils, monocytes, and T cells. In contrast, the expression of CXCR8 in skeletal muscle, adipose tissue, stomach, liver, thymus, and kidney is very low (Mackenzie et al. 2011). The CXCL17/CXCR8 axis activation can induce cyclic adenosine monophosphate (cAMP), initiating the extracellular signal-regulated kinase 1/2 (ERK1/2) and p38mitogen-activated kinase (MAPK) pathways that are the most frequent stimulated signaling pathways following GPCRs activation (Luttrell 2003). CXCR8 influences the ERK1/2 pathway through modulation of β-arrestins, protein kinase A (by Gαs), protein kinase C (by Gαo and Gαq), and the Gαi subunit βγ dimers (Luttrell 2003; Mackenzie et al. 2011). The interaction network of CXCR8 (GPR35) is illustrated in Fig. 1D.
Role of CXCL17 in the Immune System and related phenomena
Cytokines and chemokines, along with growth factors, play an important and variable role in the immune system (Cameron and Kelvin 2003; Gong et al. 2019; Rojewska et al. 2019, Choreño-Parra et al. 2021) (Fig. 2). CXCL17 is a CXC chemokine involved in the locomotion of immune cells, especially antigen-presenting cells, including DCs, monocytes, and macrophages (Choreño-Parra et al. 2020). However, the recruiting power of CXCL17 is different for different cells. For example, CXCL17 stimulates monocytes more strongly than DCs (Hiraoka et al. 2011). Sensing the luminal antigens in the gastrointestinal and respiratory tracts is essential for regulating mucosal inflammatory responses, and APCs are involved in this phenomenon. CXCL17 promotes the migration of these APCs to these epithelial barriers because this chemokine is expressed in the endothelial cells and mucosa of the mentioned tracts (Hiraoka et al. 2011; Sun et al. 2021). The chemotactic property of CXCL17 is limited to APCs and has no effect on T cells and B cells. Therefore, CXCL17 functions are more manifested in innate immune responses than in adaptive immunity.
Evolving evidence disclose that cytokines, such as IL-1β, TNF-α, and IFN-γ mediate the expression and function of chemokines. For instance, the mentioned cytokines can promote the expression of the CXCL17 gene about 100-fold in epithelial cells. Among these cytokines, IFN-γ acts more strongly in this field than IL-1β and TNF-α. Moreover, IFN-γ can induce the expression and phosphorylation of STAT1 in the JAK/STAT signaling pathway (Yajie and Zhouluo 2015).
A study on Cxcl17−/− mice demonstrated that under both homeostatic and inflammatory states, the recruitment of macrophages into the lungs was impaired compared with wild-type mice. In addition, the number of a novel subset of F4/80+ CDIIcmid macrophage-like cells increased in Cxcl17−/− mice (Burkhardt et al. 2014). This study showed that the recruitment of alveolar macrophages or the precursors of these cells to the lungs in Cxcl17−/− mice is impaired, and their number is also reduced in the lung. Therefore, the theory can be proposed that along with the CCL2/CCR2 chemokinetic axis, which plays a vital role in the migration and recruitment of monocytes and macrophages to various tissues, including the lung, the CXCL17/GPCR35 axis may also be involved in this process with a similar function because this study reported that CXCL17 could not bind to CCR2 (Vakilian et al. 2017; Behfar et al. 2018; Taghavi et al. 2019; Moadab et al. 2021). Endothelial cells also can produce CXCL17 and promote the release of CXCL17 by epithelial cells. Therefore, the migration of monocytes and DCs simplified from the blood vessels to the epithelium by supporting the endothelial-derived CXCL17 and intercellular adhesion molecule 2 (ICAM2) (Hiraoka et al. 2011; Burkhardt et al. 2012).
Based on available knowledge, CXCL14, CXCL17, and CCL28 are mucosal-associated chemokines (Hieshima et al. 2003; Dai et al. 2015). CXCL17 has antimicrobial properties against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella, and Candida albicans dose-dependently (Burkhardt et al. 2012). The fascinating point about CXCL17 is that it preserves the immune-modulator microbiome such as Lactobacillus casei. Accordingly, the gut microbiome regulates inflammatory responses induced by pathogenic bacteria (Ahrne et al. 1998; Llopis et al. 2009). It has been revealed that the carboxyl-terminal domain in CCL28 and the amino-terminal in CXCL14 are responsible for their killing activity. Nonetheless, in the case of CXCL17, the domain associated with killing has not yet been identified (Hieshima et al. 2003; Burkhardt et al. 2012; Dai et al. 2015). The bactericidal property of CXCL17 is probably applied by inducing membrane permeabilization (Burkhardt et al. 2012). The first line of defense against mycotoxins is the intestinal epithelial barrier, and a study reported that a robust release of reactive oxygen species (ROS)-mycotoxins could trigger induced CXCL17 through JNK and p38 signaling pathways, enhancing immunoprotective responses and reducing apoptosis and inflammation via phosphoinositide 3-kinases (PI3Ks)/AKT/ mammalian target of rapamycin (mTOR) pathway in vitro (Caco-2 cell model) (Sun et al. 2021). Chemokines also could participate in angiogenic mechanisms. In this context, CXC chemokines based on the absence or presence of the sequence Glu-Leu-Arg (the ELR motif) are categorized as ELR+ and ELR− (Strieter et al. 1995). Although the ELR motif in CXCL17 has not been identified, this chemokine has angiogenic properties because increased levels of CXCL17 were detected throughout proliferation or tube formation in several human endothelial cell lines (Niiya et al. 2018). Moreover, it has been demonstrated that CXCL17 induced the gene expression of vascular endothelial growth factor (VEGF), angiopoietin 2 (Ang2), urokinase-type plasminogen activator (uPA), uPA receptor (uPAR), and basic fibroblast growth factor (bFGF) involved in neovascularization mechanisms in human umbilical vascular endothelial cells (HUVECs) (Weinstein et al. 2006; Guo et al. 2017). Furthermore, the release of VEGF-A increased following treatment of THP-1 monocytes with CXCL17 (Lee et al. 2013). In several solid tumors such as breast, esophageal, and lung cancers, proangiogenic molecules, including CXCL1, CXCL8, and VEGF, are responsible for CXCL17 expression (Weinstein et al. 2006). Therefore, CXCL17, along with other angiogenic factors, can induce tumor angiogenesis and metastasis (Liu et al. 2020). It has also been reported that in Cxcl17−/− mice the frequency of CD4+ and CD8+ T cells was higher in lymph nodes and spleen than wild-type mice. Moreover, irregular function of T cell may reflect changed migration of myeloid cell, or it could be due to changed developing T cell in the thymus. Therefore, CXCL17 could be involved in regulating T cell function and homeostasis (Hernández-Ruiz et al. 2019). In hyperinflammatory states, CXCL17 can be involved in recruiting immunosuppressive cells to modulate excessive inflammatory responses. An investigation reported that CXCL17 attenuated imiquimod-induced psoriasis-like skin inflammation via recruitment of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) (Oka et al. 2017). In infectious diseases such as influenza A(H1N1) pdm09, study on peripheral blood monocyte-derived macrophages and human alveolar A549 cells showed that levels of CXCL17 significantly elevated and this chemokine can be used as a potential specific diagnostic biomarker for influenza A (H1N1) infections (Choreño-Parra et al. 2021). In asthma, CXCL17 overexpressed following decreasing in the levels of miR221-3p in epithelial and sputum, inhibiting airway eosinophilic inflammation in a protective fashion (Zhang et al. 2018). Therefore, CXCL17 could have an anti-inflammatory role in some inflammatory allergic states, such as asthma.
CXCL17 also is involved in fibrotic processes. A study reported that in bleomycin-induced skin fibrosis mice models, CXCL17 could regulate the expression of type I collagen by increasing the expression of miR-29 and matrix metalloproteinase 1 (MMP1) in fibroblasts. Moreover, local injection of CXCL17 diminished bleomycin-induced skin fibrosis in the studied animals (Shimada et al. 2020). Hence, the low CXCL17 expression in systemic sclerosis skin may induce the accumulation of type I collagen.
Moreover, It has been reported that CXCL17 can inhibit CXCR4-mediated signaling and CXCL12 binding by mimicking protamine sulfate and surfen as glycosaminoglycan binders (White et al. 2021). Considering the critical role of the CXCL12/CXCR4 axis in inducing proliferation and angiogenesis of tumor cells, CXCL17 as an endogenous CXCR4 inhibitor can play an anti-tumor and protective role.
Role of CXCL17 in human malignancies
There are several contradictions in the association between CXCL17 and tumorigenesis because this chemokine can have a dual role in cancer (Khandelwal et al. 2019, 2022; Fermin et al. 2021; Hao et al. 2022). Some studies demonstrated that CXCL17 is involved in the tumor progression in breast and colon cancers by inducing angiogenesis and cell proliferation (Weinstein et al. 2006; Mu et al. 2009) (Fig. 3). Another study reported that CXCL17 could induce anti-tumor responses in pancreatic cancer. CXCL17 can recruit and activate DCs to capture tumor antigen and present to cytotoxic T cells in premalignant intraductal papillary mucinous neoplasm (Hiraoka et al. 2011). Another study has also shown that CXCL17 increases the proliferation of HepG2 cells in vitro, although the effect of this chemokine on human hepatocellular carcinoma (HCC) has not yet been entirely determined (Zhou et al. 2012).
Pro-tumoral role
In metastatic breast cancer, it has been shown that by secreting CXCL17, tumor cells lead to the migration of MDSCs with CD11b+Gr-1+ phenotype to the lung. The infiltration of these cells in the lung causes angiogenesis and the growth and survival of cancer cells, promoting tumor metastasis. On the other hand, released CXCL17 can affect MDSCs to produce platelet-derived growth factor (PDGF)-BB. MDSC-derived PDGF-BB induces angiogenesis and forms a pre-metastatic niche in the lung tissue. Additionally, CXCL17 induces CD31+ tumor angiogenesis (Matsui et al. 2012, 2015; Hsu et al. 2019). These findings indicate that CXCL17 can appear as a tumor supporting factor by changing the structure of the vascular network and stimulating angiogenesis, and recruiting MDSCs to the tumor site.
Another study also examined the role of the CXCL17/CXCR8 axis in developing breast cancer in vitro and in vivo. Findings demonstrated that the expression of CXCL17 and CXCR8 was increased in cell line and breast cancer tissue samples, promoting the proliferation and migration of breast cancer cells. Moreover, CXCL17 was associated with lower survival and poor prognosis in breast cancer (Guo et al. 2017). It has been reported that the protein levels of CXCL17 were significantly overproduced in cutaneous squamous cell cancer (cSCC) cell lines, including SCC7, SCC59, SCC12A, and SCC118, than in normal epidermal keratinocytes. CXCL17 participates in skin cancer by activating the AKT/mTOR/STAT3 pathways. In addition, data obtained from patients with cSCC showed that the levels of CXCL17 were significantly higher in metastatic cSCC compared with non-metastatic cSCC (Khandelwal et al. 2022).
It is hypothesized that under physiological conditions, the expression of CXCL17 is steady in gastric mucosa; nonetheless, following the stimulation of pathogen materials, the expression of CXCL17 promoted may contract immune responses and create homeostatic conditions and prevent damaging immune responses. However, the CXCL17/CXCR8 axis may play a pivotal role in gastric cancer (GC) initiation and development, particularly in the intestinal metaplasia to GC phase (Li et al. 2021). A study found that the expression of CXCL17 progressively amplified from non-atrophic gastritis (NAG-NOR), intestinal metaplasia of atrophic gastritis (AG-IM) to IM adjacent to GC (GC-IM), nevertheless; dramatically decreased in GC tissues. These data indicated that reducing CXCL17 may be a crucial molecular occurrence leading to GC development (Li et al. 2021).
CXCL17 can be involved in lung adenocarcinoma-associated spine metastasis because, based on an investigation, the expression of CXCL17 was higher in lung adenocarcinoma cell lines and clinical lung adenocarcinoma samples compared with lung squamous cell carcinoma cell lines and clinical samples. Furthermore, the migration of THP-1 mononuclear macrophages was increased via CXCL17/Src/FAK pathway (Liu et al. 2020).
An immunohistochemical study on HCC tissue specimens showed that intratumoral and peritumoral CXCL17 levels were significantly elevated, and according to these findings, patients were categorized into CXCL17low and CXCL17high groups. Based on this category, the survival rate and 5-year recurrence rate were shorter in CXCL17low patients, while the survival rate was lower in both groups. In addition, the expression of CXCL17 was associated with fewer CD4+ T cells and more CD68+ macrophages infiltration in the TME. These data indicated that dysregulated release of CXCL17 might be associated with tumor-supportive immune cell infiltration and might be a significant target for treating HCC (Li et al. 2014). Emerging evidence has designated that microRNAs (miRNAs) play key roles in regulating tumorigenesis (Zhang et al. 2007; Avvari et al. 2022). CXCL17 is a direct target of miR-325-3p, and in this way, overexpression of miRNA can partially suppress cell proliferation, migration, invasion and angiogenesis via modulating CXCL17/CXCR8 axis in various cancers, such as HCC. In this context, it was reported that the levels of miR-325-3p were downregulated in HCC cell lines and tissues, increasing CXCL17 and tumor development (Li et al. 2021). In oral squamous cell carcinoma (OSCC), miR4513 regulates OSCC cell activities, and this miRNA’s expression was raised in the OSCC cell lines.
Furthermore, CXCL17 could be targeted by miR-4513. Downregulation of miR-4513 prevents cell proliferation, migration, and invasion while enhancing cell apoptosis. A study showed that CXCL17 knockdown repressed the effects of miR-4513 on OSCC cell manners. Therefore, miR-4513 and CXCL17 could have oncogenic roles in OSCC and be considered potential therapeutic targets for treating OSCC (Xu et al. 2019). In GC, long non-coding RNAs (lncRNAs) can be valuable prognostic markers because they are associated with advanced TNM stage and lymphatic and vascular infiltration. It has been revealed that THUMPD3-AS1 was pointedly reduced in GC cell lines and tissues. The overexpression of THUMPD3-AS1 hinders cell proliferation, migration, invasion and intracellular accumulation of ROS in GC cells by regulating CXCL17 and miR-1252-3p. Furthermore, decreasing the expression of THUMPD3-AS1 was accompanied by decreased 5-year overall survival (Tan et al. 2022).
In colorectal cancer (CRC), one of the most significant prognostic factors is lymph node metastasis (Tateishi et al. 2010). On the other hand, CXCL17 is involved in the pathogenesis of CRC, and carcinoembryonic antigen (CEA) could be considered as other prognostic factors in CRC. It has been revealed that the mRNA levels of CXCL17 were upregulated in primary tumors and CEA+ groups. Moreover, CXCL17 mRNA levels were significantly higher in hematoxylin- and eosin-positive (H&E+) lymph nodes than in H&E− nodes. These data indicated that metastatic tumor cells in the lymph nodes of colon tissue express CXCL17, and mRNA of chemokine along with CEA mRNA could be employed as a complementary method to the H&E technique for recognition of aggressive and poorly differentiated tumors (Rashad et al. 2018). In parallel with this study, another group reported that the mRNA levels of CXCL17 were remarkably increased in primary tumors than in normal tissue in colon cancer. The mRNA levels of other chemokines, including CCL2, CXCL9, and CXCL10, were upregulated in tumor tissue, although not as much as CXCL17. According to the significant role of CCL2 and CXCL17 in recruiting monocytes and macrophages, these chemokines’ mRNA levels were associated with tumor development (Ohlsson et al. 2016). It has been speculated that the expression of CXCL17 by tumor cells in CRC contributes to the metastasis and growth of these cells. A study on endometrial adenocarcinoma tissues demonstrated that CXCL17 levels were remarkably elevated, and there is a significant and positive correlation between the expression of CXCL17 and CXCR8 (Hao et al. 2022).
Moreover, increased CXCL17 was associated with low overall survival in patients with endometrial cancer. However, there was no significant association between the expression of CXCR8 and overall survival (Hao et al. 2022). This study suggested that due to the important role of chemokines, especially CXCL17 and CXCL18, in the pathogenesis of endometrial adenocarcinoma, a therapeutic target can be designed to treat this malignancy.
Exposure to ultraviolet B (UVB) radiation is one of the main risk factors for developing cutaneous squamous cell carcinoma (cSCC) (Bottomley et al. 2019). CXCL17 controls the infiltration and polarization of immune cells such as tumor-associated macrophages (TAMs) in the TME. Treating bone marrow-derived macrophages (BDMC) and Raw 264.7 cells (murine macrophages) with CXCL17 led to increased TNF-α, Fizz, Ym2, and arginase expression. This treatment also stimulated the polarization of macrophages towards the M2 phenotype, which can lead to tumor growth and development (Khandelwal et al. 2020).
Anti-tumoral role
As discussed, in addition to tumor-supportive properties, CXCL17 can also participate in anti-tumor and protective responses (Fig. 2). For instance, in patients with GC, elevated levels of CXCL17 are associated with more prolonged survival through preserving and improving the maturation of gastric mucosa epithelium. Moreover, there is a positive and significant correlation between CXCL17, trefoil factor 1 (TFF1), TFF2 and mucin 5AC (MUC5AC). These molecules preserve the gastric mucosa barrier function and integrity, inhibiting the development of bacterial colonization and chronic inflammation. It has been reported that reduced expression of TFF1, TFF2, and MUC5AC could be associated with an increased risk of GC development (Hoffmann 2015; Heuer et al. 2019).
Furthermore, there is a negative association between the expression of CXCL17, secreted frizzled-related protein 2 (SFRP2) and caudal type homeobox 1 (CDX1) (Van de Bovenkamp et al. 2003; Perez-Vilar et al. 2004; Esposito et al. 2017; Soutto et al. 2019). SFRP2 directly binds to Wnts, regulating the Wnt/β-catenin pathway and inducing cell growth and differentiation (Heinosalo et al. 2018; Zhao et al. 2019; Han et al. 2020). In addition, CDX1, as a DNA-binding protein, is involved in differentiating gastric epithelial cells to the intestinal phenotype. CDX1 also participates in Helicobacter pylori-induced GC (Fujii et al. 2012; Choi et al. 2019). Interestingly, there is a significant synergism between CXCL17 and CCL20 because CCL20 induces the recruitment of Tregs and DCs (Ito et al. 2011; Sebrell et al. 2019; Wang et al. 2019). Furthermore, like CXCL17, CCL20 is involved in mucosal immunity in homeostatic or inflammatory conditions (Li et al. 2021).
Intraductal papillary mucinous neoplasm (IPMN) is a pancreatic cancer intra-epithelial precursor lesion that develops from adenoma to carcinoma. A study investigated the alterations of anti-tumor immune responses during the development of pancreatic cancer. Evaluation of the frequency of tumor-infiltrating lymphocytes (TILs) and DCs as well as the maturation state of DCs in the local lymph nodes during tumor progression showed that anti-tumor immune responses changed to immune tolerance between the phases of intraductal papillary mucinous adenoma (IPMA) and intraductal papillary mucinous carcinoma (IPMC). Moreover, this study showed that the expression of CXCL17 and ICAM2 were upregulated in IPMA while downregulated in the IPMC stage. CXCL17 and ICAM2 promote the infiltration of immature DCs and increase the susceptibility of the tumor cells to CD8+ cytotoxic T cell-induced cytolysis. These data indicated that CXCL17 is involved in immune surveillance during tumor progression in pancreatic cancer (Hiraoka et al. 2011). Another study on patients with colon cancer evaluated the expression of CXCL17 and CXCR8 in the colon and tumor-adjacent clinical samples, and findings showed that protein expression of CXCL17 and CXCR8 was increased, and this increase was associated with longer overall survival. In addition, there was a positive correlation between the expression of CXCL17 and CXCR8. Together, these results propose that the CXCL17/CXCR8 axis may be involved in colon cancer, and upregulation of this ligand/receptor can improve patient outcomes (Yao et al. 2020).
B7-H4 is an immunosuppressive member of the B7 family (Sica et al. 2003). In several human malignancies, B7-H4 is overexpressed by tumor cells in the TME. Interestingly, B7-H4 expression by cancer cells is associated with higher levels of CXCL17 and infiltrating mature APCs (MacGregor et al. 2019).
Altogether, these findings show that CXCL17 can behave differently depending on different environmental signals and stages of cancer. However, understanding the role of dual CXCL17 and CXCR8 in human malignancies requires further studies.
The Targeting CXCL17/CXCR8 in Cancer Therapy
Considering the dual role of the CXCL17/CXCR8 axis in different pathological states, targeting this axis may not always be an appropriate strategy. For example, in pancreatic cancer, as mentioned, this chemokine has a protective and anti-tumor role; therefore, its inhibition can benefit the tumor. Few studies have been conducted in this area, and in this section, the most important studies on CXCL17 and CXCR8 in cancer treatment have been discussed (Table 1).
Table 1.
Possible therapeutic approaches based on CXCL17/CXCR8 targeting
| Strategy | Details of study | Outcomes | Ref |
|---|---|---|---|
| Deletion of CXCL17 |
¬ cSCC ¬ Animal model ¬ Metastatic and non-metastatic cSCC cells |
¬ ↓ Tumor cell proliferation ¬ ↓ Migration and invasion ¬ Suppressing the AKT/mTOR/STAT3 pathways ¬ ↓ The expression of the CD31 biomarker and neovascularization |
(Khandelwal et al. 2022) |
| Inhibiting CXCL17 | ¬ Lung adenocarcinoma |
¬ ↓ Monocyte migration and TAMs to the lung ¬ ↓ The Src/FAK pathway signals ¬ ↓ Polarization of TAMs to the M2 phenotype ¬ ↓ Infiltration of immunosuppressive cells |
(Liu et al. 2020) |
| CXCL17 silencing + CA |
¬ HCC ¬ In vitro |
¬ ↑ LKB1/AMPK pathway ¬ ↑ Autophagy in tumor cells ¬ Inhibiting VEGFR2/Src/FAK/cdc42 axis ¬ ↓ F-actin formation and reducing HCC cell locomotion ¬ ↓ Tumor growth |
(Ku et al. 2015; Wang et al. 2019) |
| CXCL17 targeting by miR-325-3p | ¬ HCC |
¬ ↓ CXCL17 activity ¬ ↓ Tumor progression |
(Li et al. 2021) |
| CXCL17 and miR-1252-3p targeting by THUMPD3-AS1 | ¬ GC |
¬ ↓ CXCL17 activity ¬ ↓ Tumor progression |
(Tan et al. 2022) |
| CXCL17 deletion |
¬ cSCC ¬ In vitro/ animal model ¬ SCCB cells |
¬ ↓ CXCL17 activity ¬ ↓ Infiltration of macrophages, Tregs, and MDSCs ¬ ↓ Tumor progression |
(Khandelwal et al. 2020) |
| Knockdown of CXCR8 |
¬ NSCLC ¬ in vitro and in vivo |
¬ ↓ Tumor cell chemoresistance in NSCLC | (Wang et al. 2018) |
| Genetic depletion of CXCR8 |
¬ Colon cancer ¬ Animal model |
¬ ↓ ion transportation via the Na/K-ATPase channel ¬ ↓ Src signaling ¬ ↓ tumor growth via the epidermal growth factor receptor/Src/Ras/ERK pathway ¬ ↓ Intestinal tumorigenesis |
(Kim et al. 2009; Schneditz et al. 2019) |
| Pepducin |
¬ Colitis ¬ CRC |
¬ ↓ CXCR8 activation ¬ ↓ Tumor burden |
(Quon et al. 2020) |
| Pamoic acid |
¬ Colitis ¬ DSS-induced colitis mouse model |
¬ ↑ CXCR8 activation ¬ ↓ Chronic inflammation ¬ ↑ Lipid metabolism ¬ ↓ The severity of the disease |
(Tsukahara et al. 2017) |
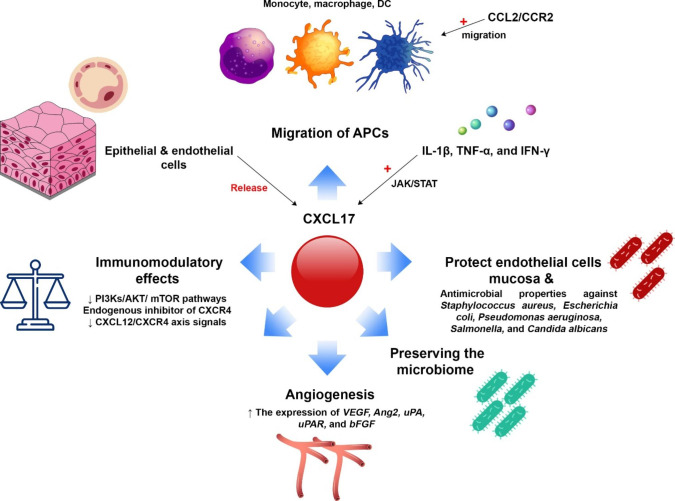
Fig. 2.
Role of CXCL17 in the immune system and related mechanisms. CXCL17 participates in various processes in the immune system. This chemokine can help the migration of APCs such as monocytes, macrophages, and DCs. It also preserves the integrity of the mucosal epithelial layer and leads to the clearance of the infection caused by pathogens. On the other hand, it preserves beneficial bacterial species in the microbiome. This chemokine also has anti-inflammatory properties and induces homeostasis by modulating destructive immune responses. It can also be involved in angiogenic mechanisms depending on the conditions. Cytokines such as IL-1β, TNF-α and specially IFN-γ have been shown to induce the release of CXCL17.
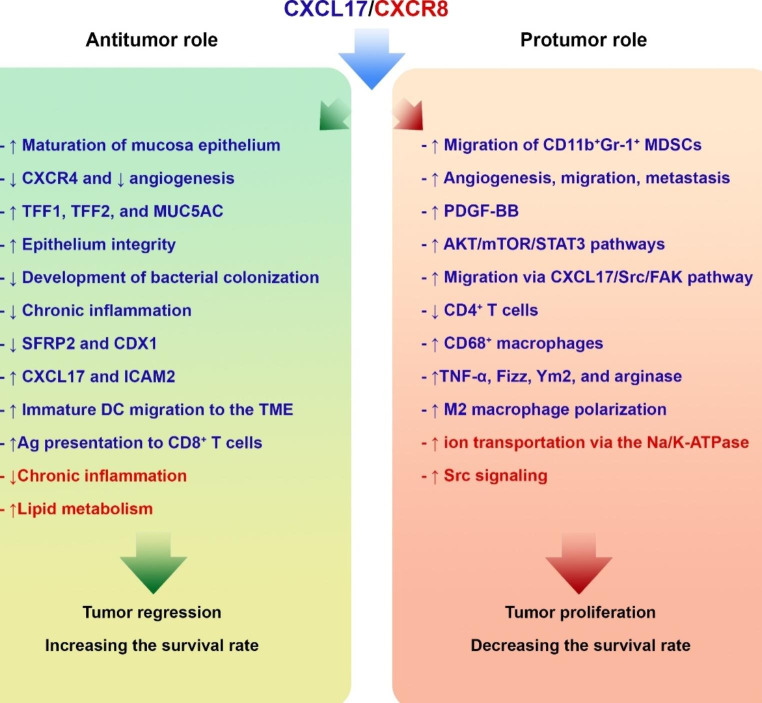
Fig. 3.
Dual role of the CXCL17/CXCR8 in the pathogenesis of tumor. CXCL17 (blue) and CXCR8 (red) could have both tumor-supportive and antitumor roles in cancer via mediating several mechanisms
CXCR17
An animal cSCC model showed that deletion of CXCL17 in metastatic and non-metastatic cSCC cells could meaningfully decrease tumor cell proliferation, migration, and invasion via suppressing the AKT/mTOR/STAT3 pathways. According to the significant role of CXCL17 in angiogenic mechanisms, the expression of the CD31 biomarker revealed that CXCL17 deletion reduced tumor neovascularization (Khandelwal et al. 2022). In lung adenocarcinoma, hyperproduction of CXCL17 lead to monocyte migration and TAMs to the lung via inducing the Src/FAK pathway. The polarization of TAMs to the M2 phenotype led to an immunosuppressive milieu, promoting tumor growth and progression. Therefore, targeting CXCL17 or downstream molecules in the Src/FAK pathway might be a potential therapeutic approach in lung adenocarcinoma (Liu et al. 2020).
A study showed that CXCL17 levels were increased in HCC, promoting tumor cell proliferation, invasion, and migration. In addition, following the hyperproduction of CXCL17, the expression of autophagic activity markers, such as LC3B and p62, were reduced. CXCL17 silencing could induce liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) pathway, stimulating autophagy in tumor cells (Wang et al. 2019). Moreover, it has been reported that corosolic acid (CA) can suppress the vascular endothelial growth factor receptor 2 (VEGFR2)/Src/FAK/cdc42 axis, decreasing F-actin formation and reducing HCC cell locomotion in vitro. In HCC models, 5 mg/kg/day of CA significantly inhibited tumor growth. Regarding the role of CXCL17 in the stimulation of this axis and angiogenesis and its tumor supportive missions in HCC design, combination therapy using CA and a CXCL17 inhibitor or silencing CXCL17 may be effective for treating HCC or other solid tumors in a synergistic manner (Ku et al. 2015).
The use of microRNAs that target CXCL17 can also be an attractive treatment option. As discussed in HCC, the level of miR-325-3p is significantly downregulated, and this decrease is associated with increased CXCL17 activity and tumor progression (Li et al. 2021). THUMPD3-AS1 is a long non-coding RNA (lncRNA), and its levels are reduced in GC. Furthermore, CXCL17 and miR-1252-3p are the targets of THUMPD3-AS, and it is hypothesized that they could be a potential therapeutic target for treating GC (Tan et al. 2022). However, due to these molecules’ different and sometimes conflicting roles in different malignancies, it may not be possible to apply a single prescription for all cancers. For example, it has been reported that THUMPD3-AS1 plays a protumoral role in inducing self-renewal by regulating the expression of one cut domain family member 2 (ONECUT2) and miR-543 in non-small cell lung cancer (NSCLC), resulting in tumor growth and development (Hu et al. 2019). An investigation reported that following CXCL17 deletion in SCCB cells and inoculation in FVB mice, the analysis of isolated tumor tissue revealed that the infiltration of macrophages, Tregs, and MDSCs was markedly reduced at the tumor site. These data indicated that targeting CXCL17 could consider a therapeutic tactic for treating cSCC (Khandelwal et al. 2020). However, these studies were conducted in the preclinical phase and needed more studies in the human phases.
CXCR8
Evidence shows that, like CXCL17, CXCR8 expression is associated with malignancies such as GC, colon, and breast cancer, although its exact role is still unclear (Okumura et al. 2004; Guo et al. 2017; Wang et al. 2018; Ali et al. 2019). In patients with NSCLC, the increased expression of CXCR8 is associated with poor prognosis, and knockdown of CXCR8 pointedly reduces tumor cell chemoresistance in NSCLC in vitro and in vivo (Wang et al. 2018). This evidence shows that CXCR8 can play a significant role in tumor progression and metastasis, and targeting it may benefit cancer treatment. However, considering the diverse roles of CXCL17 and CXCR8 in different cancers and different grades of malignancies, targeting these molecules may act as a double-edged sword. A study on colon cancer cells revealed that CXCR8 induces ion transportation via the Na/K-ATPase channel, activating Src signaling, responsible for tumor growth via the epidermal growth factor receptor/Src/Ras/ERK pathway (Kim et al. 2009; Schneditz et al. 2019). Another investigation demonstrated that genetic depletion of CXCR8 reduced intestinal tumorigenesis in colon cancer models (Schneditz et al. 2019). Treatment of animals with pepducin, a lipid-coupled peptide, could also selectively inhibit CXCR8 activation and decrease tumor burden in colitis-associated mice models (Quon et al. 2020).
Moreover, there is an association between hypoxia-inducible factor-1α (HIF-1α) and the expression of CXCR8 in some pathologic states, such as cardiac failure (Ronkainen et al. 2014). Regarding the role of HIF-1α in hypoxia and angiogenesis in solid tumors, inhibition of HIF-1α can also affect the expression of CXCR8 and be useful in cancer treatment. According to these studies, inhibition of CXCR8 using its antagonists may be a therapeutic strategy in CXCR8-induced malignancies. So far, few CXCR8 antagonists have been introduced and studied, the most important of which are CID2745687 (methyl-5-[(tert-butylcarbamothioylhydrazinylidene)methyl]-1-(2,4-difluorophenyl)pyrazole-4-carboxylate, ML144 (CID-1,542,103) 1-[(4-Chlorophenyl)methyl]-4-[4-(2-methylphenyl)-1-piperazinyl]pyrazolo[3,4-d]pyrimidine, ML145 (CID-2,286,812) 2-hydroxy-4-[4-[(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]butanoylamino]benzoic acid, and ML-194 (1-(2,4-difluorophenyl)-5-[[2-[[(1,1-dimethylehyl)amino]thioxomethyl]hydrazinylidene]methyl]-1 H-pyrazole-4-carboxylic acid methyl ester) (Zhao et al. 2010; Sharmin et al. 2020). Although their antagonistic effect and specificity in different species (human, mouse, and rat) are still debated, no study has used these antagonists in cancer treatment.
On the other hand, CXCR8 agonists can also be relevant in cancer treatment. According to the role of chronic inflammation and lipid metabolism in tumorigenesis and the inhibitory effect of CXCR8 on inflammatory responses as well as its stimulatory effect on lipid metabolism, CXCR8 agonists such as zaprinast, lodoxamide, bufrolin, kynurenic acid, amlexanox, pamoic acid, flavonoid glycosides, and 6-bromo-8-(4-[(3)H] methoxybenzamido)-4-oxo-4 H-chromene-2-carboxylic acid ([(3)H]PSB-13,253) might also have a repressive effect on tumor development and progression (Cosi et al. 2011; Thimm et al. 2013; MacKenzie et al. 2014; Wang et al. 2019). For instance, an investigation reported that administration of pamoic acid to DSS-induced colitis mice models reduced the severity of the disease (Tsukahara et al. 2017). Since ulcerative colitis is associated with CRC, it is possible that using CXCR8 agonists can help prevent chronic inflammatory-induced CRC (Yashiro 2014).
Concluding remarks
Based on the available evidence, CXCL17 can have various activities, and in most cases, including mucosal infections, it has a protective role. In cancer, however, this chemokine has a mysterious and dual role because sometimes it acts against the tumor and sometimes as a tumor-supportive molecule. For example, recruiting monocytes, macrophages, and MDSCs to the tumor site, inhibiting apoptosis and autophagy, and inducing proliferation and angiogenesis are among its most important protumoral activities. On the other hand, in some other cancers, CXCL17 plays an anti-tumor role by inhibiting signaling pathways leading to tumor growth or inhibiting molecules involved in tumor development and metastasis. CXCR8 is also involved in anti-tumor and protumor processes, making targeting CXCL17 and CXCR8 challenging for cancer therapy.
On the other hand, CXCR8 cannot be the specific receptor of CXCL17. As a result, combination therapies may be more effective than monotherapy. Another problem in cancer treatment by targeting the CXCL17/CXCR8 axis is the possibility of increased susceptibility to infections because, as discussed, CXCL17 plays a protective role in innate immunity against various pathogens. Regarding the recruiting of myeloid cells such as macrophages, monocytes, and MDSCs, it is important to mention that the CCL2/CCR2 chemokine axis is also actively involved in this process, and it cannot be expected that only by inhibiting the CXCL17/CXCR8 axis, the migration of these cells to the tumor or inflammation site will be completely disrupted. Accordingly, the design of dual antagonists capable of inhibiting CCL2 and CXCL17 can be an attractive option for cancer treatment and future studies.
Acknowledgements
Rafsanjan University of Medical Sciences supported this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold A, Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85(1):88–94. doi: 10.1046/j.1365-2672.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- Ali H, AbdelMageed M, Olsson L, Israelsson A, Lindmark G, Hammarström M-L, Hammarström S, Sitohy B. Utility of G protein-coupled receptor 35 expression for predicting outcome in colon cancer. Tumor Biology. 2019;41(6):1010428319858885. doi: 10.1177/1010428319858885. [DOI] [PubMed] [Google Scholar]
- Aminzadeh F, Ghorashi Z, Nabati S, Ghasemshirazi M, Arababadi MK, Shamsizadeh A, Karimabad MN, Khorramdelazad H, Darakhshan S, Hassanshahi G. Differential Expression of CXC Chemokines CXCL 10 and CXCL 12 in Term and Pre-term Neonates and Their Mothers. Am J Reprod Immunol. 2012;68(4):338–344. doi: 10.1111/j.1600-0897.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- Amir NABM, Mackenzie AE, Jenkins L, Boustani K, Hillier MC, Tsuchiya T, Milligan G, Pease JE. Evidence for the Existence of a CXCL17 Receptor Distinct from GPR35. J Immunol. 2018;201(2):714–724. doi: 10.4049/jimmunol.1700884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvari S, Prasad D, Khan IA (2022) Role of MicroRNAs in cell growth proliferation and tumorigenesis. Role of MicroRNAs in Cancers. Springer, pp 37–51
- Azin H, Vazirinejad R, Ahmadabadi BN, Khorramdelazad H, Zarandi ER, Arababadi MK, Karimabad MN, Shamsizadeh A, Rafatpanah H, Hassanshahi G. The SDF-1 3′ a genetic variation of the chemokine SDF-1α (CXCL12) in parallel with its increased circulating levels is associated with susceptibility to MS: a study on Iranian multiple sclerosis patients. J Mol Neurosci. 2012;47(3):431–436. doi: 10.1007/s12031-011-9672-6. [DOI] [PubMed] [Google Scholar]
- Behfar S, Hassanshahi G, Nazari A, Khorramdelazad H. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. ” Cytokine. 2018;110:226–231. doi: 10.1016/j.cyto.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Bottomley MJ, Thomson J, Harwood C, Leigh I (2019) The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci 20(8): 2009 [DOI] [PMC free article] [PubMed]
- Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- Burkhardt AM, Maravillas-Montero JL, Carnevale CD, Vilches-Cisneros N, Flores JP, Hevezi PA, Zlotnik A. CXCL17 is a major chemotactic factor for lung macrophages. J Immunol. 2014;193(3):1468–1474. doi: 10.4049/jimmunol.1400551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuñiga J, Selman M. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J Immunol. 2012;188(12):6399–6406. doi: 10.4049/jimmunol.1102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MJ, Kelvin DJ. Cytokines and chemokines-their receptors and their genes: an overview. Adv Exp Med Biol. 2003;520:8–32. doi: 10.1007/978-1-4615-0171-8_2. [DOI] [PubMed] [Google Scholar]
- Choi SI, Yoon C, Park MR, Lee D, Kook M-C, Lin J-X, Kang JH, Ashktorab H, Smoot DT, Yoon SS. CDX1 Expression Induced by CagA-Expressing Helicobacter pylori Promotes Gastric Tumorigenesis. Mol Cancer Res. 2019;17(11):2169–2183. doi: 10.1158/1541-7786.MCR-19-0181. [DOI] [PubMed] [Google Scholar]
- Choreño-Parra JA, Dunlap MD, Swanson R, Jiménez-Álvarez LA, Muñoz-Torrico M, Guzmán-Beltrán S, Zúñiga J, Khader SA (2021) CXCL17 Is Dispensable during hypervirulent Mycobacterium tuberculosis HN878 infection in mice. ImmunoHorizons. 5:752–7599 [DOI] [PMC free article] [PubMed]
- Choreño-Parra JA, Jiménez-Álvarez LA, Ramírez-Martínez G, Sandoval-Vega M, Salinas-Lara C, Sánchez-Garibay C, Luna-Rivero C, Hernández-Montiel EM, Fernández-López LA, Cabrera-Cornejo MF. CXCL17 is a specific diagnostic biomarker for severe pandemic influenza A (H1N1) that predicts poor clinical outcome. Front Immunol. 2021;12:633297. doi: 10.3389/fimmu.2021.633297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choreño-Parra JA, Thirunavukkarasu S, Zúñiga J, Khader SA. The protective and pathogenic roles of CXCL17 in human health and disease: Potential in respiratory medicine. Cytokine Growth Factor Rev. 2020;53:53–62. doi: 10.1016/j.cytogfr.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colobran R, Pujol-Borrell R, Armengol MP, Juan M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin Experimental Immunol. 2007;148(2):208–217. doi: 10.1111/j.1365-2249.2007.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosi C, Mannaioni G, Cozzi A, Carlà V, Sili M, Cavone L, Maratea D, Moroni F. G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: Studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology. 2011;60(7–8):1227–1231. doi: 10.1016/j.neuropharm.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Dai C, Basilico P, Cremona TP, Collins P, Moser B, Benarafa C, Wolf M. CXCL14 displays antimicrobial activity against respiratory tract bacteria and contributes to clearance of Streptococcus pneumoniae pulmonary infection. J Immunol. 2015;194(12):5980–5989. doi: 10.4049/jimmunol.1402634. [DOI] [PubMed] [Google Scholar]
- Denisov SS (2021) CXCL17: The black sheep in the chemokine flock. Front Immunol :2811 [DOI] [PMC free article] [PubMed]
- Esposito R, Morello S, Vllahu M, Eletto D, Porta A, Tosco A. Gastric TFF1 expression from acute to chronic Helicobacter infection. Front Cell Infect Microbiol. 2017;7:434. doi: 10.3389/fcimb.2017.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin JM, Alam MM, Gu X, Asarkar A, Nathan C-A, Khandelwal AR. CXCL17 as a prognostic biomarker for aggressive cutaneous squamous cell carcinoma. Cancer Res. 2021;81(13Supplement):647–647. doi: 10.1158/1538-7445.AM2021-647. [DOI] [Google Scholar]
- Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, Yamagata Y, Seto Y, Aburatani H, Hatakeyama M (2012) CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proceedings of the National Academy of Sciences 109(50): 20584–20589 [DOI] [PMC free article] [PubMed]
- Gong F-h, Xiao X-q, Zhang X-p, Long L, Huang S, Wang X-s Z.-l. Shu and Y.-s. Yang (2019). Association between unstable angina and CXCL17: a new potential biomarker. Open Medicine 14(1):939–944 [DOI] [PMC free article] [PubMed]
- Guo YJ, Zhou YJ, Yang XL, Shao ZM, Ou ZL. The role and clinical significance of the CXCL17-CXCR8 (GPR35) axis in breast cancer. Biochem Biophys Res Commun. 2017;493(3):1159–1167. doi: 10.1016/j.bbrc.2017.09.113. [DOI] [PubMed] [Google Scholar]
- Han M, Wang S, Fritah S, Wang X, Zhou W, Yang N, Ni S, Huang B, Chen A, Li G. Interfering with long non-coding RNA MIR22HG processing inhibits glioblastoma progression through suppression of Wnt/β-catenin signalling. Brain. 2020;143(2):512–530. doi: 10.1093/brain/awz406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Gao X, Wang Y-p, Liu Q, Zhu H, Zhao S-j, Qin Q-h, Meng J, Li L-L, Lin S-C. "Expression and clinical significance of CXCL17 and GPR35 in endometrial carcinoma". Anticancer Drugs. 2022;33(5):467–477. doi: 10.1097/CAD.0000000000001280. [DOI] [PubMed] [Google Scholar]
- Heinosalo T, Gabriel M, Kallio L, Adhikari P, Huhtinen K, Laajala T, Kaikkonen E, Mehmood A, Suvitie P, Kujari H. Secreted frizzled-related protein 2 (SFRP2) expression promotes lesion proliferation via canonical WNT signaling and indicates lesion borders in extraovarian endometriosis. Hum Reprod. 2018;33(5):817–831. doi: 10.1093/humrep/dey026. [DOI] [PubMed] [Google Scholar]
- Hernández-Ruiz M, Othy S, Herrera C, Nguyen HT, Arrevillaga‐Boni G, Catalan‐Dibene J, Cahalan MD, Zlotnik A. Cxcl17–/– mice develop exacerbated disease in a T cell‐dependent autoimmune model. J Leukoc Biol. 2019;105(5):1027–1039. doi: 10.1002/JLB.3A0918-345RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer F, Stürmer R, Heuer J, Kalinski T, Lemke A, Meyer F, Hoffmann W. Different forms of TFF2, a lectin of the human gastric mucus barrier: In vitro binding studies. Int J Mol Sci. 2019;20(23):5871. doi: 10.3390/ijms20235871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170(3):1452–1461. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Yamazaki–Itoh R, Ino Y, Mizuguchi Y, Yamada T, Hirohashi S, Kanai Y. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140(1):310–321. doi: 10.1053/j.gastro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int J Oncol. 2015;47(3):806–816. doi: 10.3892/ijo.2015.3090. [DOI] [PubMed] [Google Scholar]
- Hromas R, Broxmeyer HE, Kim C, Nakshatri H, Christopherson II K, Azam M, Hou Y-H. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem Biophys Res Commun. 1999;255(3):703–706. doi: 10.1006/bbrc.1999.0257. [DOI] [PubMed] [Google Scholar]
- Hsu Y-L, Yen M-C, Chang W-A, Tsai P-H, Pan Y-C, Liao S-H, Kuo P-L. CXCL17-derived CD11b + Gr-1 + myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 2019;21(1):1–13. doi: 10.1186/s13058-019-1114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Chen Y, Li X, Miao H, Li R, Chen D, Wen Z. THUMPD3-AS1 is correlated with non-small cell lung cancer and regulates self-renewal through miR-543 and ONECUT2. OncoTargets Ther. 2019;12:9849. doi: 10.2147/OTT.S227995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Carson IV WF, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317(5):613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal AR, Alam MM, Moore-Medlin T, Savage HA, Nathan C-AO. Role of the CXCL17-CXCR8 (GPR35) axis in cutaneous squamous cell carcinoma. Cancer Res. 2019;79(13Supplement):1969–1969. doi: 10.1158/1538-7445.AM2019-1969. [DOI] [Google Scholar]
- Khandelwal AR, Kandula RA, Alam MM, Fermin JM, Moore-Medlin T, DiGiovanni J, Nathan C-AO. Targeting CXCL17 (CXC Motif Chemokine Ligand 17) inhibits cutaneous squamous cell carcinoma via modulating angiogenesis. Cancer Res. 2022;82(12Supplement):231–231. doi: 10.1158/1538-7445.AM2022-231. [DOI] [Google Scholar]
- Khandelwal AR, Paralikar AA, Soleja RQ, Temple ZB, Robert MM, Alam M, Nathan C-AO. CXCL17 modulates macrophage polarization and immune cell infiltrate in cutaneous squamous cell carcinoma. Cancer Res. 2020;80(16Supplement):3859–3859. doi: 10.1158/1538-7445.AM2020-3859. [DOI] [Google Scholar]
- Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat reviews Clin Oncol. 2009;6(10):587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- Ku C-Y, Wang Y-R, Lin H-Y, Lu S-C, Lin J-Y. Corosolic acid inhibits hepatocellular carcinoma cell migration by targeting the VEGFR2/Src/FAK pathway. PLoS ONE. 2015;10(5):e0126725. doi: 10.1371/journal.pone.0126725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-Y, Wang C-J, Lin T-Y, Hsiao C-L, Luo C-W. CXCL17, an orphan chemokine, acts as a novel angiogenic and anti-inflammatory factor. Am J Physiology-Endocrinology Metabolism. 2013;304(1):E32–E40. doi: 10.1152/ajpendo.00083.2012. [DOI] [PubMed] [Google Scholar]
- Li L, Ji Y, Chen Y-C, Zhen Z-J. MiR-325-3p mediate the CXCL17/CXCR8 axis to regulate angiogenesis in hepatocellular carcinoma. Cytokine. 2021;141:155436. doi: 10.1016/j.cyto.2021.155436. [DOI] [PubMed] [Google Scholar]
- Li L, Yan J, Xu J, Liu C-Q, Zhen Z-J, Chen H-W, Ji Y, Wu Z-P, Hu J-Y, Zheng L. CXCL17 expression predicts poor prognosis and correlates with adverse immune infiltration in hepatocellular carcinoma. PLoS ONE. 2014;9(10):e110064. doi: 10.1371/journal.pone.0110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-z, Liu A-r, Liu S-y, Yan L-r, Yuan Y, Xu Q (2021) The Involvement of CXCL17-GPR35 in Gastric Cancer Initiation and Development. [DOI] [PMC free article] [PubMed]
- Liu W, Xie X, Wu J. Mechanism of lung adenocarcinoma spine metastasis induced by CXCL17. Cell Oncol. 2020;43(2):311–320. doi: 10.1007/s13402-019-00491-7. [DOI] [PubMed] [Google Scholar]
- Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espín-Basany E, Guarner F, Malagelada JR. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15(2):275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- Loose D, Van de Wiele C. The immune system and cancer. Cancer Biother Radio. 2009;24(3):369–376. doi: 10.1089/cbr.2008.0593. [DOI] [PubMed] [Google Scholar]
- Luttrell L. ’Location, location, location’: activation and targeting of MAP kinases by G protein-coupled receptors. J Mol Endocrinol. 2003;30(2):117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- MacGregor HL, Garcia-Batres C, Sayad A, Elia A, Berman HK, Toker A, Katz SR, Shaw PA, Clarke BA, Crome SQ. Tumor cell expression of B7-H4 correlates with higher frequencies of tumor-infiltrating APCs and higher CXCL17 expression in human epithelial ovarian cancer. Oncoimmunology. 2019;8(12):e1665460. doi: 10.1080/2162402X.2019.1665460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A, Lappin J, Taylor D, Nicklin S, Milligan G. GPR35 as a novel therapeutic target. Front Endocrinol. 2011;2:68. doi: 10.3389/fendo.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie AE, Caltabiano G, Kent TC, Jenkins L, McCallum JE, Hudson BD, Nicklin SA, Fawcett L, Markwick R, Charlton SJ. The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35. Mol Pharmacol. 2014;85(1):91–104. doi: 10.1124/mol.113.089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerki C, Meuter S, Liebi M, Mühlemann K, Frederick MJ, Yawalkar N, Moser B, Wolf M. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol. 2009;182(1):507–514. doi: 10.4049/jimmunol.182.1.507. [DOI] [PubMed] [Google Scholar]
- Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. "Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17". J Immunol. 2015;194(1):29–33. doi: 10.4049/jimmunol.1401704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Morikawa S, Ezaki T. Possible roles of CXCL17 in angiogenesis during tumor progression. FASEB J. 2015;29:926912. doi: 10.1096/fasebj.29.1_supplement.926.12. [DOI] [Google Scholar]
- Matsui A, Yokoo H, Negishi Y, Endo-Takahashi Y, Chun NA, Kadouchi I, Suzuki R, Maruyama K, Aramaki Y, Semba K (2012) CXCL17 expression by tumor cells recruits CD11b + Gr1highF4/80 – cells and promotes tumor progression. [DOI] [PMC free article] [PubMed]
- Moadab F, Khorramdelazad H, Abbasifard M. Role of CCL2/CCR2 axis in the immunopathogenesis of rheumatoid arthritis: Latest evidence and therapeutic approaches. Life Sci. 2021;269:119034. doi: 10.1016/j.lfs.2021.119034. [DOI] [PubMed] [Google Scholar]
- Mu X, Chen Y, Wang S, Huang X, Pan H, Li M. Overexpression of VCC-1 gene in human hepatocellular carcinoma cells promotes cell proliferation and invasion. Acta Biochim Biophys Sin. 2009;41(8):631–637. doi: 10.1093/abbs/gmp051. [DOI] [PubMed] [Google Scholar]
- Niiya K, Ohara H, Isono M, Sheikh AM, Matsuo H, Fujikawa K, Isomura M, Kato N, Nabika T. Further dissection of QTLs for salt-induced stroke and identification of candidate genes in the stroke-prone spontaneously hypertensive rat. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-27539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson L, Hammarström M-L, Lindmark G, Hammarström S, Sitohy B. Ectopic expression of the chemokine CXCL17 in colon cancer cells. Br J Cancer. 2016;114(6):697–703. doi: 10.1038/bjc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Sugaya M, Takahashi N, Takahashi T, Shibata S, Miyagaki T, Asano Y, Sato S. CXCL17 attenuates imiquimod-induced psoriasis-like skin inflammation by recruiting myeloid-derived suppressor cells and regulatory T cells. J Immunol. 2017;198(10):3897–3908. doi: 10.4049/jimmunol.1601607. [DOI] [PubMed] [Google Scholar]
- Okumura S, i. H, Baba T, Kumada K, Nanmoku H, Nakajima Y, Nakane K, Hioki, Ikenaka K. Cloning of a G-protein‐coupled receptor that shows an activity to transform NIH3T3 cells and is expressed in gastric cancer cells. Cancer Sci. 2004;95(2):131–135. doi: 10.1111/j.1349-7006.2004.tb03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee SJ, Nam SY, Im DS. GPR35 mediates lodoxamide-induced migration inhibitory response but not CXCL17‐induced migration stimulatory response in THP‐1 cells; is GPR35 a receptor for CXCL17? Br J Pharmacol. 2018;175(1):154–161. doi: 10.1111/bph.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vilar J, Randell SH, Boucher RC. "C-Mannosylation of MUC5AC and MUC5B Cys subdomains. ” Glycobiology. 2004;14(4):325–337. doi: 10.1093/glycob/cwh041. [DOI] [PubMed] [Google Scholar]
- Pisabarro MT, Leung B, Kwong M, Corpuz R, Frantz GD, Chiang N, Vandlen R, Diehl LJ, Skelton N, Kim HS. Cutting edge: novel human dendritic cell-and monocyte-attracting chemokine-like protein identified by fold recognition methods. J Immunol. 2006;176(4):2069–2073. doi: 10.4049/jimmunol.176.4.2069. [DOI] [PubMed] [Google Scholar]
- Quon T, Lin L-C, Ganguly A, Tobin AB, Milligan G. Therapeutic opportunities and challenges in targeting the orphan G protein-coupled receptor GPR35. ACS Pharmacol Translational Sci. 2020;3(5):801–812. doi: 10.1021/acsptsci.0c00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashad Y, Olsson L, Israelsson A, Öberg Ã, Lindmark G, Hammarström M-L, Hammarström S, Sitohy B. Lymph node CXCL17 messenger RNA: A new prognostic biomarker for colon cancer. Tumor Biology. 2018;40(9):1010428318799251. doi: 10.1177/1010428318799251. [DOI] [PubMed] [Google Scholar]
- Rojewska E, Ciapała K, Mika J. Kynurenic acid and zaprinast diminished CXCL17-evoked pain-related behaviour and enhanced morphine analgesia in a mouse neuropathic pain model. Pharmacol Rep. 2019;71(1):139–148. doi: 10.1016/j.pharep.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Ronkainen V-P, Tuomainen T, Huusko J, Laidinen S, Malinen M, Palvimo JJ, Ylä-Herttuala S, Vuolteenaho O, Tavi P. Hypoxia-inducible factor 1-induced G protein-coupled receptor 35 expression is an early marker of progressive cardiac remodelling. Cardiovascular Res. 2014;101(1):69–77. doi: 10.1093/cvr/cvt226. [DOI] [PubMed] [Google Scholar]
- Rostène W, Dansereau MA, Godefroy D, Van Steenwinckel J, Goazigo ARL, Mélik-Parsadaniantz S, Apartis E, Hunot S, Beaudet N, Sarret P. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem. 2011;118(5):680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Moser B. Immunologic research. 2005;31(1):57–74. doi: 10.1385/IR:31:1:57. [DOI] [PubMed] [Google Scholar]
- Schneditz G, Elias JE, Pagano E, Zaeem Cader M, Saveljeva S, Long K, Mukhopadhyay S, Arasteh M, Lawley TD, Dougan G. GPR35 promotes glycolysis, proliferation, and oncogenic signaling by engaging with the sodium potassium pump. Sci Signal. 2019;12(562):eaau9048. doi: 10.1126/scisignal.aau9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebrell TA, Hashimi M, Sidar B, Wilkinson RA, Kirpotina L, Quinn MT, Malkoç Z, Taylor PJ, Wilking JN, Bimczok D. A novel gastric spheroid co-culture model reveals chemokine-dependent recruitment of human dendritic cells to the gastric epithelium. Cell Mol Gastroenterol Hepatol. 2019;8(1):157–171. doi: 10.1016/j.jcmgh.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmin O, Abir AH, Potol A, Alam M, Banik J, Rahman A, Tarannum N, Wadud R, Habib ZF, Rahman M. Activation of GPR35 protects against cerebral ischemia by recruiting monocyte-derived macrophages. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-66417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Makino K, Jinnin M, Sawamura S, Kawano Y, Ide M, Kajihara I, Makino T, Fukushima S, Ihn H. CXCL17-mediated downregulation of type I collagen via MMP1 and miR-29 in skin fibroblasts possibly contributes to the fibrosis in systemic sclerosis. J Dermatol Sci. 2020;100(3):183–191. doi: 10.1016/j.jdermsci.2020.09.010. [DOI] [PubMed] [Google Scholar]
- Sica GL, Choi I-H, Zhu G, Tamada K, Wang S-D, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immun. 2003;18(6):849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- Soutto M, Chen Z, Bhat AA, Wang L, Zhu S, Gomaa A, Bates A, Bhat NS, Peng D, Belkhiri A. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat Commun. 2019;10(1):1–15. doi: 10.1038/s41467-019-11011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Hernández-Ruiz M, Khan AA, Fouladi MA, Kim GJ, Ly VT, Yamada T, Lam C, Sarain SA, Boldbaatar U. "CXCL17 Chemokine–Dependent Mobilization of CXCR8 + CD8 + Effector Memory and Tissue-Resident Memory T Cells in the Vaginal Mucosa Is Associated with Protection against Genital Herpes". J Immunol. 2018;200(8):2915–2926. doi: 10.4049/jimmunol.1701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270(45):27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Sun C, Shen H, Cai H, Zhao Z, Gan G, Feng S, Chu P, Zeng M, Deng J, Ming F. Intestinal guard: Human CXCL17 modulates protective response against mycotoxins and CXCL17-mimetic peptides development. Biochem Pharmacol. 2021;188:114586. doi: 10.1016/j.bcp.2021.114586. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Taghavi Y, Hassanshahi G, Kounis NG, Koniari I, Khorramdelazad H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: latest evidence and clinical considerations. J cell communication Signal. 2019;13(4):451–462. doi: 10.1007/s12079-018-00500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Liu L, Zhang X, Xue Y, Gao J, Zhao J, Chi N, Zhu Y (2022) THUMPD3-AS1 is correlated with gastric cancer and regulates cell function through miR-1252-3p and CXCL17. Crit Rev Eukaryot Gene Expr 32(8): 69-80 [DOI] [PubMed]
- Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23(8):1068–1072. doi: 10.1038/modpathol.2010.88. [DOI] [PubMed] [Google Scholar]
- Thimm D, Funke M, Meyer A, Müller CE. 6-Bromo-8-(4-[3H] methoxybenzamido)-4-oxo-4 H-chromene-2-carboxylic Acid: A Powerful Tool for Studying Orphan G Protein-Coupled Receptor GPR35. J Med Chem. 2013;56(17):7084–7099. doi: 10.1021/jm4009373. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Hamouda N, Utsumi D, Matsumoto K, Amagase K, Kato S. G protein-coupled receptor 35 contributes to mucosal repair in mice via migration of colonic epithelial cells. Pharmacol Res. 2017;123:27–39. doi: 10.1016/j.phrs.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Vakilian A, Khorramdelazad H, Heidari P, Rezaei ZS, Hassanshahi G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int. 2017;103:1–7. doi: 10.1016/j.neuint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Van de Bovenkamp JH, Mahdavi J, Korteland-Van Male AM, Büller HA, Einerhand AW, Borén T, Dekker J. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter. 2003;8(5):521–532. doi: 10.1046/j.1523-5378.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert D, Jenkins NA, Bacon KB. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immun. 1997;7(2):291–301. doi: 10.1016/S1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- Wang D, Yang L, Yu W, Wu Q, Lian J, Li F, Liu S, Li A, He Z, Liu J. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-κB signaling. J Immunother Cancer. 2019;7(1):1–15. doi: 10.1186/s40425-019-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen L, Qu L, Li K, Zhao Y, Wang Z, Li Y, Zhang X, Jin Y, Liang X. Isolation and bioactive evaluation of flavonoid glycosides from Lobelia chinensis Lour using two-dimensional liquid chromatography combined with label-free cell phenotypic assays. J Chromatogr A. 2019;1601:224–231. doi: 10.1016/j.chroma.2019.04.073. [DOI] [PubMed] [Google Scholar]
- Wang L, Li H, Zhen Z, Ma X, Yu W, Zeng H, Li L. CXCL17 promotes cell metastasis and inhibits autophagy via the LKB1-AMPK pathway in hepatocellular carcinoma. Gene. 2019;690:129–136. doi: 10.1016/j.gene.2018.12.043. [DOI] [PubMed] [Google Scholar]
- Wang W, Han T, Tong W, Zhao J, Qiu X. Overexpression of GPR35 confers drug resistance in NSCLC cells by β-arrestin/Akt signaling. OncoTargets Ther. 2018;11:6249. doi: 10.2147/OTT.S175606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein EJ, Head R, Griggs DW, Sun D, Evans RJ, Swearingen ML, Westlin MM, Mazzarella R. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. 2006;350(1):74–81. doi: 10.1016/j.bbrc.2006.08.194. [DOI] [PubMed] [Google Scholar]
- White CW, Kilpatrick LE, Dale N, Abhayawardana RS, Dekkers S, Stocks MJ, Pfleger KD, Hill SJ (2021) CXCL17 is an endogenous inhibitor of CXCR4 via a novel mechanism of action. bioRxiv [DOI] [PMC free article] [PubMed]
- Xiao S, Xie W, Zhou L. Mucosal chemokine CXCL17: What is known and not known. Scand J Immunol. 2021;93(2):e12965. doi: 10.1111/sji.12965. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sun J, Xiao W, Liu Y, Yue J, Xue L, Deng J, Zhi K, Wang Y. MiR-4513 mediates the proliferation and apoptosis of oral squamous cell carcinoma cells via targeting CXCL17. Eur Rev Med Pharmacol Sci. 2019;23(9):3821–3828. doi: 10.26355/eurrev_201905_17809. [DOI] [PubMed] [Google Scholar]
- Yajie G, Zhouluo O. IFN-γ increased expression of CXCL17 by breast epithelial cells via a JAK-STAT1-dependent pathway. China Oncology. 2015;25(5):321–325. [Google Scholar]
- Yao H, Lv Y, Bai X, Yu Z, Liu X. Prognostic value of CXCL17 and CXCR8 expression in patients with colon cancer. Oncol Lett. 2020;20(3):2711–2720. doi: 10.3892/ol.2020.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterology: WJG. 2014;20(44):16389. doi: 10.3748/wjg.v20.i44.16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie O, Imai T, Nomiyama H. Adv immunol. 2001;78:57–110. doi: 10.1016/S0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- Yu JB, Schrag D, Robin Yabroff K. Health economics research in cancer treatment: current challenges and future directions. JNCI Monogr. 2022;2022(59):51–56. doi: 10.1093/jncimonographs/lgac009. [DOI] [PubMed] [Google Scholar]
- Zhang K, Liang Y, Feng Y, Wu W, Zhang H, He J, Hu Q, Zhao J, Xu Y, Liu Z. Decreased epithelial and sputum miR-221-3p associates with airway eosinophilic inflammation and CXCL17 expression in asthma. Am J Physiology-Lung Cell Mol Physiol. 2018;315(2):L253–L264. doi: 10.1152/ajplung.00567.2017. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dahlberg JE, Tam W. MicroRNAs in tumorigenesis: a primer. Am J Pathol. 2007;171(3):728–738. doi: 10.2353/ajpath.2007.070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Chen Y, Yang N, Chen Q, Bao Z, Liu M, Hu S, Li J, Wu X. miR-218‐5p regulates skin and hair follicle development through Wnt/β‐catenin signaling pathway by targeting SFRP2. J Cell Physiol. 2019;234(11):20329–20341. doi: 10.1002/jcp.28633. [DOI] [PubMed] [Google Scholar]
- Zhao P, Sharir H, Kapur A, Cowan A, Geller EB, Adler MW, Seltzman HH, Reggio PH, Heynen-Genel S, Sauer M. Targeting of the orphan receptor GPR35 by pamoic acid: a potent activator of extracellular signal-regulated kinase and β-arrestin2 with antinociceptive activity. Mol Pharmacol. 2010;78(4):560–568. doi: 10.1124/mol.110.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Lu X, Zhu P, Zhu W, Mu X, Qu R, Li M. VCC-1 over-expression inhibits cisplatin-induced apoptosis in HepG2 cells. Biochem Biophys Res Commun. 2012;420(2):336–342. doi: 10.1016/j.bbrc.2012.02.160. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7(12):1–11. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]