Abstract
Background:
A peripheral sensory stimulation named TheraBracelet has recently been shown to have a potential to improve gross manual dexterity following stroke. Upper limb function requires both reach and grasp. It is unknown whether TheraBracelet affects one more than other.
Objective:
Determine whether TheraBracelet improves reaching vs. grasping.
Methods:
Secondary analysis of a pilot randomized controlled trial. Persons with stroke received TheraBracelet (treatment) or no stimulation (control) during task practice therapy (n=6/group). Effects of TheraBracelet on reaching vs. grasping were determined using breakdown of movement times in the Box and Block Test video recordings.
Results:
Improvements in movement times for the treatment compared to control group were more pronounced for grasping than for reaching at both post and follow-up time points.
Conclusions:
TheraBracelet may be beneficial for persons with grasping deficits. This knowledge can guide clinicians for targeted use of TheraBracelet, resulting in effective implementation of the new treatment.
Keywords: stroke, rehabilitation, upper extremity, subliminal stimulation, wearable electronic devices
Introduction
Complete functional recovery of the upper limb occurs in only 11.6% of people 6 months post-stroke, with many persons with stroke requiring ongoing rehabilitative services to continue the recovery process (Kwakkel et al., 2003). A meta-analysis shows that upper extremity motor function improves more when therapy is augmented by peripheral sensory stimulation, compared with therapy alone (Conforto et al., 2018). The scientific rationale is that afferent input is a powerful driver of change in the motor cortex (Baker, 2007; Schabrun et al., 2012).
TheraBracelet is one such peripheral sensory stimulation modality that has recently been developed to improve upper extremity function after stroke (Seo, Woodbury, et al., 2019). TheraBracelet is subthreshold (i.e., imperceptible) random-frequency vibratory stimulation applied to the paretic wrist to enhance sensorimotor cortical activity for paretic upper extremity tasks (Schranz et al., 2022; Seo et al., 2015; Seo, Lakshminarayanan, et al., 2019). Subsequently, it has shown to improve upper extremity sensorimotor function (Enders et al., 2013; Hur et al., 2014; Lakshminarayanan et al., 2015; Seo et al., 2014; Wang et al., 2015) and recovery (Seo, Woodbury, et al., 2019; Vatinno et al., 2022).
Specifically, a recent pilot randomized controlled trial investigated if the use of TheraBracelet during task-practice therapy yielded significantly greater improvements in upper extremity motor outcome compared to task-practice therapy alone (Seo, Woodbury, et al., 2019). Upper extremity motor outcome was assessed by the Box and Block Test (BBT) followed by the Wolf Motor Function Test (WMFT). The pilot trial found large effect sizes on upper extremity outcome measures of the BBT and WMFT time. BBT quantifies the number of blocks that a person can move from one box to the other across a barrier in a minute (Mathiowetz et al., 1985; Platz et al., 2005). The WMFT time is the average movement time to complete 15 functional tasks such as lifting a pencil (Wolf et al., 2001). The pilot trial also found significant improvement in BBT for the treatment group that received TheraBracelet during therapy compared to the control group that received sham (no) TheraBracelet during therapy, but not for the WMFT possibly due to a small sample size and short therapy duration. However, it is currently unknown whether TheraBracelet is more effective in improving reaching or grasping because clinical assessments of upper extremity function typically include both reaching and grasping movements.
The rationale for dissociating the reaching and grasping movements is as follows. Grasping requires control of the distal finger muscles that are predominantly innervated by monosynaptic corticospinal fibers, while reaching requires control of the proximal arm muscles influenced by other resources such as brainstem pathways (Buccolieri et al., 2003; McPherson et al., 2018; Turton & Lemon, 1999). Thus, these movements are differentially impacted by stroke depending on lesion characteristics (Cunningham et al., 2016; Weiller et al., 1993). Furthermore, it has been observed that persons with stroke regain reaching earlier than grasping (Lang et al., 2006).
Therefore, rehabilitation treatments should target the specific reach vs. grasp deficit of a person depending on their stage of recovery and impairment characteristics. Likewise, development of new rehabilitation technologies should have a clear indication of the specific benefits they intend to have. Rehabilitation therapists report that they are more likely to adopt a new rehabilitation technology if they know that the new intervention targets the client’s specific need (Chen & Bode, 2011; Morrow et al., 2021).
Consequently, the objective of this study was to determine whether use of TheraBracelet results in greater improvement in reaching vs. grasping. TheraBracelet stimulation has been shown to affect the sensorimotor cortex activity (Schranz et al., 2022; Seo et al., 2015; Seo, Lakshminarayanan, et al., 2019), which has greater influence over the distal hand muscles used for grasping than proximal arm muscles used for reaching (Buccolieri et al., 2003). In addition, previous evidence shows TheraBracelet’s effect on dexterous hand function (Seo et al., 2014). Based on this, we hypothesized that TheraBracelet would result in greater improvement in grasping than reaching. The knowledge obtained in the present study could improve effective use of the TheraBracelet treatment by providing clinicians with a more targeted approach to rehabilitation of the upper limb following stroke.
Materials and methods
Study design
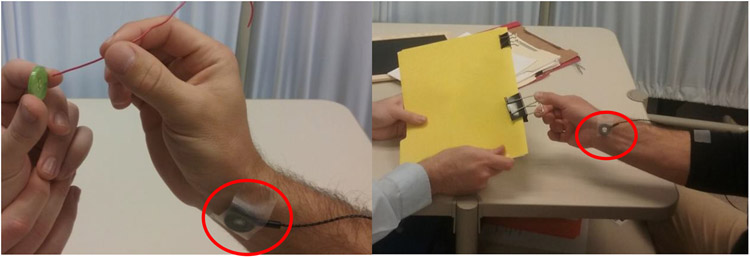
This study is a secondary analysis of a triple-blind pilot randomized controlled trial that showed use of TheraBracelet during therapy can improve gross manual dexterity in persons with stroke compared to therapy alone (Seo, Woodbury, et al., 2019). In this trial, participants were randomly assigned to either a treatment group or control group (n=6/group). Participants, therapists administering task-practice therapy, and assessors were blinded to group allocation. The treatment group received a 2-week task-practice therapy while a vibrator applied TheraBracelet subthreshold random-frequency vibratory stimulation to the paretic wrist (Figure 1). The control group received the same 2-week task-practice therapy but no stimulation from the vibrator.
Figure 1.
A vibrator was placed on the paretic wrist for both groups. The vibrator delivered subthreshold vibratory TheraBracelet stimulation for the treatment group and no stimulation for the control group during therapy.
The therapy involved repetitive practice of tasks requiring primarily unilateral dexterous hand and finger motions in sitting. The therapy was manualized following the EXCITE trial manual (Wolf et al., 2006) and task-specific training manual (Lang & Birkenmeier, 2014). Participants and therapists collaboratively chose meaningful tasks from the task menu to facilitate applicability of learned skills to the participants’ real-world tasks and goals. Task difficulty was graded to achieve optimal challenge for the participant, as described by Lang & Birkenmeier (2014).
For assessment, a trained study personnel administered BBT. BBT was completed by participants using the paretic hand prior to the intervention (pre), on average 6 days after the completion of the 2-week intervention (post), and on average 19 days after the intervention completion (follow-up). All BBT assessments were videotaped from the top/sagittal view at 30 frames per second.
Participants
A total of 12 adults who were at least 6 months post stroke were recruited, to control for the confounding effects of time since stroke and spontaneous recovery (Colombo et al., 2013). Participants had mild to moderate upper limb impairment based on the well-established upper limb impairment scale of Fugl-Meyer Assessment Upper Extremity, with scores 30-60 out of 66 (Fugl-Meyer et al., 1975; Woodbury et al., 2013) . All participants had cognitive abilities to follow instructions and participate in the task-practice therapy. No participants had botulinum toxin injection in the paretic upper limb within 3 months of or during enrollment. Participants were 7 males and 5 females, on average 63 years old (SD=8), and on average 5 years post stroke (SD=5). Their average Fugl-Meyer Assessment Upper Extremity score was 48 (SD=8). The demographic and clinical characteristics were comparable between the two groups (Table 1) (Seo, Woodbury, et al., 2019). This study was approved by the Institutional Review Board. Informed consent was obtained from all participants.
Table 1.
Participant characteristics
| Treatment group (n=6) | Control group (n=6) | p | |
|---|---|---|---|
| Age mean (SD) in years | 61 (10) | 64 (8) | 0.73 |
| Male/female | 5/1 | 2/4 | 0.24 |
| Ischemic/hemorrhagic stroke | 5/1 | 6/0 | 1.00 |
| Time since stroke range in years | 1-16 | 1-6 | 0.24 |
| Fugl-Meyer Assessment Upper Extremity Score mean (SD) | 45 (9) | 51 (7) | 0.59 |
Movement time analysis
To determine relative effects of TheraBracelet on reaching vs. grasping, change in reaching and grasping time during BBT from pre- to post-intervention was compared between the two groups. BBT was used because the movement time analysis for BBT has been established in the literature (Seo & Enders, 2012; Slota et al., 2014). In addition, BBT involves repeated measurements for one task of moving one block at a time, thus providing more accurate estimate of measurement variability. Following the established movement time analysis method (Seo & Enders, 2012; Slota et al., 2014), video recordings of BBT were analyzed in the following way. The motions of BBT were broken down into 6 movement components required to complete BBT (Figure 2, adapted from Seo & Enders, 2012 and Slota et al., 2014). The start of each movement component was defined as follows. (1) Reach to Grasp: starts when the hand crosses the barrier toward the next block. (2) Contact to Lift: starts when the fingers have contacted the block. (3) Transport: starts when the block has been lifted off the floor of the box. (4) Release: starts when the block crosses the barrier. (5) Free Fall: starts when the block has left contact with the fingers. (6) Return: starts when the hand begins moving toward the barrier. For each block moved for BBT, one examiner who was blind to the group assignment determined the movement component times by counting the number of frames used for each movement component using QuickTime (Apple Inc., Cupertino, CA) and multiplying it by 1000/30 to compute time in milliseconds. These 6 movement components were further classified as either reaching or grasping (Figure 2).
Figure 2.
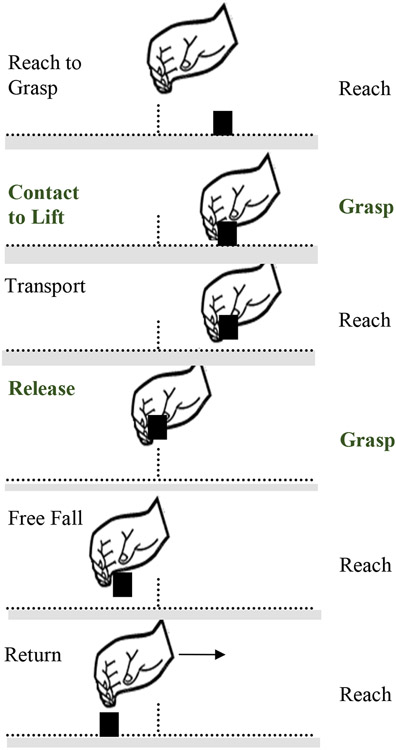
Illustration of six movement components that are required to grasp and move each block (black square) across the barrier (vertical line in the middle) in the Box and Block Test (adapted from Seo & Enders 2012). The designation of each of the six movement components to the reaching vs. grasping class (in bold) is also noted.
Statistical analysis
Linear mixed model analysis was performed using SAS (SAS Institute Inc., Cary, NC) to determine the effect of TheraBracelet on the movement component times. Factors included in the model were group (treatment and control), time (pre, post, and follow-up), and BBT movement component. To account for within-participant correlations, a random intercept was included in the model. An autoregressive (AR(1)) structure was used for the correlations over time. Diagnostics were performed to verify assumptions and to choose the appropriate structure for the within-subject correlations over time. A log transformed led to adequacy of assumptions such as normality. P-values smaller than 0.05 were deemed statistically significant. When interactions were significant, linear contrasts with Tukey adjustment were used to make post-hoc pairwise comparisons.
Results
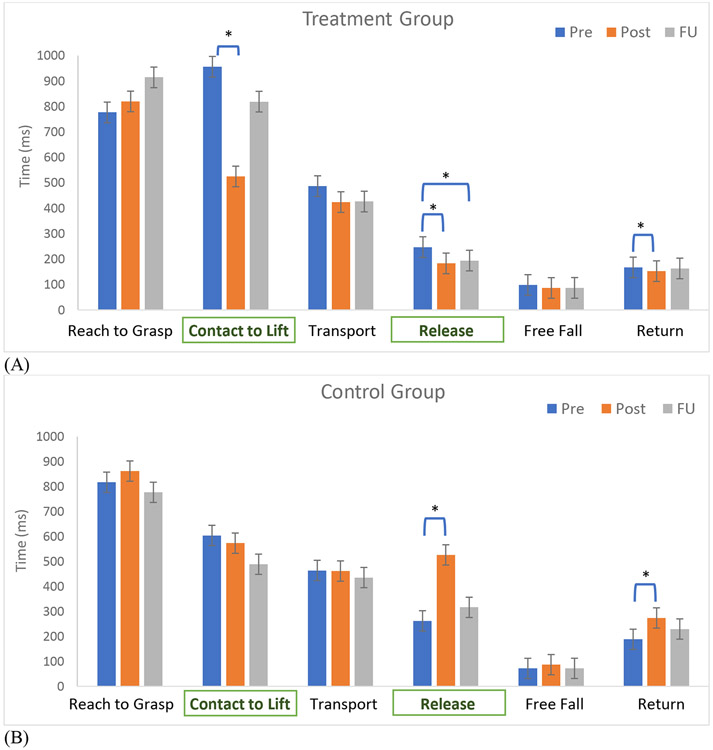
Movement time changed differently over time by group and movement component (group x time x movement component interaction p<.0001, Figure 3). For the treatment group, movement became significantly faster from pre to post for the two grasp movement components of Contact to Lift (p=0.0003) and Release (p<.0001). Contact to Lift time improved by 45% and Release time improved by 26% from pre to post. Release time also improved from pre to follow-up by 21% (p<.0001). Return time also significantly improved from pre to post for the treatment group (p=0.0039). However, the mean difference was only 15 milliseconds, less than our measurement increment. Movement time for other components did not significantly change with the time (p>0.05).
Figure 3.
Movement time for each component at 3 timepoints (pre, post, and follow-up) for the treatment group (A) and control group (B). Error bars show standard errors. Asterisks denote significant changes based on pairwise comparisons with Tukey post hoc adjustment. Movement components for grasp are in bold in rectangles (Contact to Lift and Release), while those for reach are not.
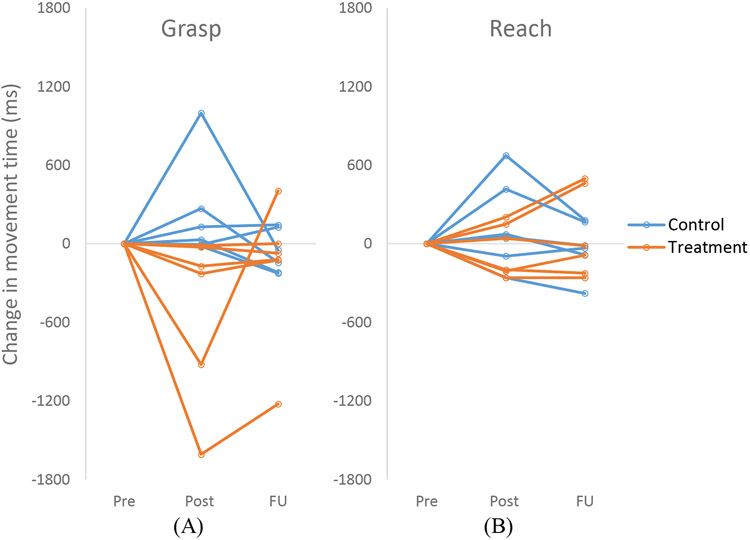
In the control group, movement became significantly slower for Release (p=0.0025) and Return (p=0.0329) from pre to post. Release time became slower by two folds. Return time became slower by 45%. All other movement component time comparisons were not significant including the between-group movement time comparison at pre (p>0.05). Individual participants’ changes in the movement component time across the three time points are presented in Figure 4.
Figure 4.
Change in the movement time for the grasp (A) vs. reach (B) for individual participants in the treatment and control group.
Discussion
This study examined how the use of TheraBracelet affected reaching vs. grasping time. For the treatment group, movement became significantly faster in the grasping components of BBT (Contact to Lift and Release) rather than the reaching components. These changes in the movement time corresponded to improvement in the BBT score exceeding the minimum detectable change (Chen et al., 2009) in the previous trial (Seo, Woodbury, et al., 2019). For the control group, neither reach nor grasp movements became faster. These results suggest that TheraBracelet may be more beneficial in targeting grasping deficits than reaching. This study provides in-depth understanding of how TheraBracelet was able to improve gross manual dexterity in persons with chronic hemiparesis after stroke.
These results support the hypothesis and its rationale that the TheraBracelet stimulation may affect the neural activity in the sensorimotor cortex (Schranz et al., 2022; Seo et al., 2015; Seo, Lakshminarayanan, et al., 2019) which has a greater influence over the distal hand muscles used for grasping than proximal arm muscles used for reaching (Buccolieri et al., 2003; McPherson et al., 2018; Turton & Lemon, 1999). We speculate that this rationale may explain why movement became faster only for the grasping components and not for the reaching components in the treatment group. It is interesting to note that this finding was obtained even though the TheraBracelet stimulation was applied to the wrist, not to the hand.
Another potential explanation for the finding is that TheraBracelet enhanced grasping more than reaching because the task-practice therapy in this study focused on dexterous manipulation of objects with the hand and finger. Peripheral sensory stimulation along with other stimulation modalities is utilized as a tool to prime the brain and nervous system and enhance the effect of the subsequent behavioral therapy (Carrico, Chelette, Westgate, Powell, et al., 2016; Carrico, Chelette, Westgate, Salmon-Powell, et al., 2016; Cassidy et al., 2014; Celnik et al., 2007; Conforto et al., 2007). However, all practiced therapy tasks involved activities of daily living which inherently involve some reaching in addition to grasping (Lang & Birkenmeier, 2013). Reaching during therapy tasks did not involve full shoulder flexion and elbow extension, but neither does BBT.
Clinically, the results of this study offer an important insight into the benefits of TheraBracelet use. Specifically, the results of this study can be used to assist clinicians in improving outcomes by providing a guide for the persons TheraBracelet can be best used for. Occupational therapy is a discipline founded on activity analysis, in which each activity is broken down into the small individual components needed to complete the activity. Similarly, treatment modalities need to specify what components they target to improve overall upper extremity function (Chen & Bode, 2011; Morrow et al., 2021). The results of this study demonstrate that TheraBracelet not only improves overall upper limb function (Seo, Woodbury, et al., 2019), but it can also be used as a targeted treatment approach for persons with stroke who are experiencing challenges in completing daily activities specifically because of grasping deficits. Thus, TheraBracelet as an addition to task specific therapy targeting grasping deficits may benefit upper limb recovery post stroke.
Strengths of this study include the blinding of all parties. In addition to participants, therapist providing the therapy, and the study personnel administering BBT, the examiner who determined the movement component times was blind to the group assignment of individual participants. Furthermore, an established movement time analysis method was applied to tease out the effect of an intervention on reach vs. grasp separately. These movement components are typically lumped in clinical assessments in intervention trials and the dissociation in the present study provides in-depth understanding of how the new intervention may improve the upper extremity function.
Limitations of this study include the limited sample size. Although the vibrator’s wire did not appear to impede participants’ ability to complete the tabletop therapy activities in this study, a wireless vibrator (Seo et al., 2020) is expected to improve usability. In addition, outcome measures of the previous trial were based on movement time, not quality. In subsequent research, we plan to use other standardized assessments or 3-dimentional kinetic and kinematic analysis to investigate not only the speed but also quality of reaching and grasping components in more functional tasks. Moreover, a larger trial for TheraBracelet will use therapy balanced for both in-hand manipulation and reaching (Seo et al., 2022). Thus, the finding will be re-examined with the future trial data with therapy balanced for reaching and grasping and a larger sample size. Future work will also examine clinical meaningfulness of the intervention associated with faster movement time by assessing participants’ perceived meaningfulness (Seo et al., 2022).
Conclusion
Functional upper limb recovery requires recovery of both reach and grasp. This study shows that TheraBracelet may be a beneficial treatment tool to use in adjunct with task practice therapy for persons with deficits in grasping in particular. This knowledge can guide clinicians for targeted use of TheraBracelet, resulting in effective implementation of the new treatment.
Acknowledgments:
The authors would like to acknowledge Jenna Blaschke, BA, OTD, and Laura Judy, BS, OTD for their contributions to this project.
Funding:
This research was supported by the NIH/NIGMS P20GM109040 and NIH/NICHD R01HD094731.
Footnotes
Declaration of Interest: N.J. Seo is an inventor of a patent regarding the investigated sensory stimulation. The other authors report no conflicts of interest.
Ethics Approval: The study protocol was approved by the Institutional Review Board at the Medical University of South Carolina (approval id: Pro42759).
Clinical trial registration number: NCT02675764
References
- Baker SN (2007, Dec). Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol, 17(6), 649–655. 10.1016/j.conb.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccolieri A, Avanzino L, Trompetto C, & Abbruzzese G (2003, Feb). Relaxation in distal and proximal arm muscles: a reaction time study. Clin Neurophysiol, 114(2), 313–318. 10.1016/s1388-2457(02)00379-6 [DOI] [PubMed] [Google Scholar]
- Carrico C, Chelette KC 2nd, Westgate PM, Powell E, Nichols L, Fleischer A, & Sawaki L (2016, Jul). Nerve Stimulation Enhances Task-Oriented Training in Chronic, Severe Motor Deficit After Stroke: A Randomized Trial. Stroke, 47(7), 1879–1884. 10.1161/STROKEAHA.116.012671 [DOI] [PubMed] [Google Scholar]
- Carrico C, Chelette KC 2nd, Westgate PM, Salmon-Powell E, Nichols L, & Sawaki L (2016, Jun). Randomized Trial of Peripheral Nerve Stimulation to Enhance Modified Constraint-Induced Therapy After Stroke. Am J Phys Med Rehabil, 95(6), 397–406. 10.1097/PHM.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JM, Gillick BT, & Carey JR (2014, Jan). Priming the brain to capitalize on metaplasticity in stroke rehabilitation. Phys Ther, 94(1), 139–150. 10.2522/ptj.20130027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, Wolk R, & Cohen LG (2007, Nov). Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil, 88(11), 1369–1376. 10.1016/j.apmr.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Chen CC, & Bode RK (2011, May). Factors influencing therapists' decision-making in the acceptance of new technology devices in stroke rehabilitation. Am J Phys Med Rehabil, 90(5), 415–425. 10.1097/PHM.0b013e318214f5d8 [DOI] [PubMed] [Google Scholar]
- Chen HM, Chen CC, Hsueh IP, Huang SL, & Hsieh CL (2009, Jun). Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair, 23(5), 435–440. 10.1177/1545968308331146 [DOI] [PubMed] [Google Scholar]
- Colombo R, Sterpi I, Mazzone A, Delconte C, & Pisano F (2013). Robot-aided neurorehabilitation in sub-acute and chronic stroke: does spontaneous recovery have a limited impact on outcome? NeuroRehabilitation, 33(4), 621–629. 10.3233/NRE-131002 [DOI] [PubMed] [Google Scholar]
- Conforto AB, Cohen LG, dos Santos RL, Scaff M, & Marie SK (2007, Mar). Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol, 254(3), 333–339. 10.1007/s00415-006-0364-z [DOI] [PubMed] [Google Scholar]
- Conforto AB, Dos Anjos SM, Bernardo WM, Silva AAD, Conti J, Machado AG, & Cohen LG (2018, Oct). Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabil Neural Repair, 32(10), 863–871. 10.1177/1545968318798943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham P, Turton AJ, Van Wijck F, & Van Vliet P (2016, Aug). Task-specific reach-to-grasp training after stroke: development and description of a home-based intervention. Clin Rehabil, 30(8), 731–740. 10.1177/0269215515603438 [DOI] [PubMed] [Google Scholar]
- Enders LR, Hur P, Johnson MJ, & Seo NJ (2013, Oct 11). Remote vibrotactile noise improves light touch sensation in stroke survivors' fingertips via stochastic resonance. J Neuroeng Rehabil, 10(1), 105. 10.1186/1743-0003-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, & Steglind S (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med, 7(1), 13–31. https://www.ncbi.nlm.nih.gov/pubmed/1135616 [PubMed] [Google Scholar]
- Hur P, Wan YH, & Seo NJ (2014, Jun). Investigating the role of vibrotactile noise in early response to perturbation. IEEE Trans Biomed Eng, 61(6), 1628–1633. 10.1109/TBME.2013.2294672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, van der Grond J, & Prevo AJ (2003, Sep). Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke, 34(9), 2181–2186. 10.1161/01.STR.0000087172.16305.CD [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan K, Lauer AW, Ramakrishnan V, Webster JG, & Seo NJ (2015, Jul 14). Application of vibration to wrist and hand skin affects fingertip tactile sensation. Physiol Rep, 3(7), e12465. 10.14814/phy2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, & Birkenmeier RL (2013). Upper-extremity task-specific training after stroke or disability: A manual for occupational therapy and physical therapy (1st ed ed.). AOTA Press. [Google Scholar]
- Lang CE, & Birkenmeier RL (2014). Upper-extremity task-specific training after stroke or disability: A manual for occupational therapy and physical therapy. [Google Scholar]
- Lang CE, Wagner JM, Edwards DF, Sahrmann SA, & Dromerick AW (2006, Dec). Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair, 20(4), 444–454. 10.1177/1545968306289299 [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Volland G, Kashman N, & Weber K (1985, Jun). Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther, 39(6), 386–391. 10.5014/ajot.39.6.386 [DOI] [PubMed] [Google Scholar]
- McPherson JG, Chen A, Ellis MD, Yao J, Heckman CJ, & Dewald JPA (2018, Apr 1). Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J Physiol, 596(7), 1211–1225. 10.1113/JP274968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CM, Johnson E, Simpson KN, & Seo NJ (2021). Determining Factors that Influence Adoption of New Post-Stroke Sensorimotor Rehabilitation Devices in the USA. IEEE Trans Neural Syst Rehabil Eng, 29, 1213–1222. 10.1109/TNSRE.2021.3090571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, & Johnson G (2005, Jun). Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil, 19(4), 404–411. 10.1191/0269215505cr832oa [DOI] [PubMed] [Google Scholar]
- Schabrun SM, Ridding MC, Galea MP, Hodges PW, & Chipchase LS (2012). Primary sensory and motor cortex excitability are co-modulated in response to peripheral electrical nerve stimulation. PLoS One, 7(12), e51298. 10.1371/journal.pone.0051298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz C, Vatinno AA, Ramakrishnan V, & Seo NJ (2022). Neuroplasticity after upper extremity rehabilitation therapy with sensory stimulation in chronic stroke survivors. Brain Communication , In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, & Enders LR (2012, Oct-Dec). Hand grip function assessed by the box and block test is affected by object surfaces. J Hand Ther, 25(4), 397–404; quiz 405. 10.1016/j.jht.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Seo NJ, Enders LR, Fortune A, Cain S, Vatinno AA, Schuster E, Ramakrishnan V, & Feng W (2020, Apr). Phase I Safety Trial: Extended Daily Peripheral Sensory Stimulation Using a Wrist-Worn Vibrator in Stroke Survivors. Transl Stroke Res, 11(2), 204–213. 10.1007/s12975-019-00724-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Kosmopoulos ML, Enders LR, & Hur P (2014). Effect of remote sensory noise on hand function post stroke. Front Hum Neurosci, 8, 934. 10.3389/fnhum.2014.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Lakshminarayanan K, Bonilha L, Lauer AW, & Schmit BD (2015, Nov). Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials - an EEG study. Physiol Rep, 3(11), e12624. 10.14814/phy2.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Lakshminarayanan K, Lauer AW, Ramakrishnan V, Schmit BD, Hanlon CA, George MS, Bonilha L, Downey RJ, DeVries W, & Nagy T (2019, Mar). Use of imperceptible wrist vibration to modulate sensorimotor cortical activity. Exp Brain Res, 237(3), 805–816. 10.1007/s00221-018-05465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Ramakrishnan V, Woodbury ML, Bonilha L, Finetto C, Schranz C, Scronce G, Coupland K, Blaschke J, Baker A, Howard K, Meinzer C, Velozo CA, & Adams RJ (2022, Apr 5). Concomitant sensory stimulation during therapy to enhance hand functional recovery post stroke. Trials, 23(1), 262. 10.1186/s13063-022-06241-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Woodbury ML, Bonilha L, Ramakrishnan V, Kautz SA, Downey RJ, Dellenbach BHS, Lauer AW, Roark CM, Landers LE, Phillips SK, & Vatinno AA (2019, Mar 1). TheraBracelet Stimulation During Task-Practice Therapy to Improve Upper Extremity Function After Stroke: A Pilot Randomized Controlled Study. Phys Ther, 99(3), 319–328. 10.1093/ptj/pzy143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slota GP, Enders LR, & Seo NJ (2014, Jul). Improvement of hand function using different surfaces and identification of difficult movement post stroke in the Box and Block Test. Appl Ergon, 45(4), 833–838. 10.1016/j.apergo.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Turton A, & Lemon RN (1999, Dec). The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res, 129(4), 559–572. 10.1007/s002210050926 [DOI] [PubMed] [Google Scholar]
- Vatinno AA, Hall L, Cox H, Fluharty A, Taylor C, Wease A, Davis A, Cain S, Ramakrishnan V, Woodbury M, & Seo NJ (2022, Jan). Using Subthreshold Vibratory Stimulation During Poststroke Rehabilitation Therapy: A Case Series. OTJR (Thorofare N J), 42(1), 30–39. 10.1177/15394492211042275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lakshminarayanan K, Slota GP, Seo NJ, & Webster JG (2015, Jan). An MRI-compatible hand sensory vibrotactile system. Physiol Meas, 36(1), N15–21. 10.1088/0967-3334/36/1/N15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, & Frackowiak RS (1993, Feb). Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol, 33(2), 181–189. 10.1002/ana.410330208 [DOI] [PubMed] [Google Scholar]
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, & Piacentino A (2001, Jul). Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke, 32(7), 1635–1639. 10.1161/01.str.32.7.1635 [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D, & Investigators E (2006, Nov 1). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA, 296(17), 2095–2104. 10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]
- Woodbury ML, Velozo CA, Richards LG, & Duncan PW (2013, Aug). Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch Phys Med Rehabil, 94(8), 1527–1533. 10.1016/j.apmr.2013.03.007 [DOI] [PubMed] [Google Scholar]