Abstract
Objectives:
This study examined the effects of a combination corticosteroid plus long-acting beta2-adrenergic agonist inhaler (IC) on rabbit phonation.
Methods:
White New Zealand male rabbits were assigned randomly to experimental and control groups (n = 11 per group). The experimental group received twice-daily doses of Advair HFA™ (fluticasone propionate 45 mcg and salmeterol 21 mcg) via a veterinary facemask with 1-way valve and spacer; the control group received aerosolized saline. After 8 weeks, animals were euthanized, larynges excised, frozen, and subsequently thawed and mounted on a standard bench apparatus. Phonation was elicited during 15 successive trials, and phonation threshold pressure (PTP; cmH2O) and flow (PTF; L/min) were quantified.
Results:
Repeated measures analysis of variance indicated significant differences between the experimental and control groups (P < .05). Mean PTP and PTF values were higher (worse) for rabbits that received Advair HFA™.
Conclusion:
Following 8-week exposure to ICs, rabbit larynges required greater air pressure and flow to initiate phonation. Because even modest phonation onset differences can have a meaningful clinical impact on voice function, these findings suggest that LABA ICs may put patients at risk for voice disorders. Furthermore, these voice disorders may occur within a relatively short timeframe. The results from this study have important clinical implications for voice care in those who use ICs.
Keywords: PTP, PTF, inhaler, voice, rabbit
LAY SUMMARY
One in 8 people has a chronic lung condition for which inhalers are a lifelong treatment. Over half—including children—may develop prolonged voice disorders. This study showed that some long-acting inhalers impact voice function in as little as 8 weeks.
INTRODUCTION
Approximately 40 million adults and children in the US suffer from chronic airway disease, including asthma, persistent bronchitis, and chronic obstructive pulmonary disease.1,2 The Centers for Disease Control and Prevention report that there are 25 million cases of asthma in the US alone; moreover, the prevalence of asthma increases by approximately 5 million every 10 years.3 Combination corticosteroid plus long-acting beta2-adrenergic agonist (LABA) inhalers (ICs) have emerged as the treatment of choice for the long-term management of asthma. Unfortunately, ICs may be linked to voice disorders.4–9 A meta-analysis of 23 studies suggested that ICs might result in adverse voice effects in approximately 10% of cases.10 One in vitro study found that LABA influences ionic transport, potentially influencing surface vocal fold hydration and contributing to vocal fold injury associated with ICs.11 Contrary to the potential benefits of combination ICs in asthma, numerous studies using animal and human methodologies have reported significant adverse effects of ICs on voice function.12–29 These studies have documented abnormal acoustic, laryngoscopic, and patient self-reported effects on voice function. Therefore, significant opposing health impacts of ICs have been documented for the lower airway versus the larynx.
Among combination ICs, there are different drugs in both the corticosteroid and LABA families that may be included. These fall under the category of daily maintenance inhalers versus albuterol steroid-only rescue inhalers. Metered dose inhalers (MDIs) involve canisters with the drug that is combined with a propellant when activated. MDIs may be combined with facemasks and spacers to facilitate delivery to those who cannot inhale on command. Following the ban of chlorofluorocarbon propellants, dry powder inhalers (DPIs) have emerged as an alternative to MDIs. Typically, DPIs are delivered from a disk that contains individual doses of the drug powder that may be inhaled. The mouthpiece may vary among DPIs, but they are incompatible with spacers. The literature is equivocal on associations between inhaler types and voice function changes.
The presentation of voice disorders associated with ICs is diverse in the literature and based predominantly on case reports. The more frequently reported laryngoscopic changes include vocal fold edema, erythema, stiffness, mass lesions, and the presence of viscous mucus. The length of time required to develop voice disorders after IC initiation is unknown. However, there is preliminary evidence that acoustic and aerodynamic changes—such as onset pressure and airflow measures—might occur in as little as 2 weeks to several months following initiation of treatment. These early changes seem to be reversible in healthy, non-asthmatic individuals,12,14 but may become chronic in people with asthma.
Animal models have contributed significantly to our understanding of laryngeal irritants and their effects on voice function. While vocal folds from several species have similarities to those of humans, rabbits have the advantage of being a small animal model that is ideal for the in vivo administration of combination ICs for the proposed research. Rabbits have similar airway epithelium30 and vocal fold histology to humans,31–34 are common models for voice-related in vivo, ex vivo (bench),34 and in vitro experiments,18,19,35,36 and have been used previously in asthma research.37,38 The current experiment employed a translational rabbit model to determine how ICs affect aerodynamic patterns affecting voice function. This model was ideal to inform our theory-driven hypothesis that 8 weeks of IC use would be associated uniquely with increased (worse) phonation threshold pressure (PTP) and phonation threshold flow (PTF) as compared to controls.
MATERIALS AND METHODS
Animal Procedure
All animal procedures were approved by the Animal Care and Use Committees at The University of Utah and Brigham Young University (Protocol #18-01001) and complied with National Institutes of Health and related guidelines. This work also followed recommendations for Animal Research Reporting of In Vivo Experiments (ARRIVE). All in vivo procedures occurred at The University of Utah, which included on-site veterinary and technician monitoring, husbandry care, and additional daily monitoring by research staff. There were no premature mortalities or adverse events. The study included 22 white New Zealand rabbits (enrollment age = 7-8 months); only male rabbits were included to avoid potential challenges with inhaler delivery due to the anterior laryngeal fat pads that are typically more prominent in female rabbits. Prior to study inclusion, all rabbits were quarantined for one week upon arrival at The University of Utah Comparative Medicine Center; they remained in single housing units throughout the study.
This study was a prospective, double-blind, randomized experimental trial with a synchronous matched control group. Rabbits were randomly assigned to the experimental (IC) or control group and subsequently received twice-daily doses of either Advair HFA™ (fluticasone propionate 45 mcg and salmeterol 21 mcg) or aerosolized isotonic saline (0.9% Na+Cl−). This drug was selected based on previous work documenting adverse voice effects in people with normal voices.39 HFA was used specifically because it is a traditional aerosol inhaler that is compatible with animal delivery based on veterinary care standards. The experimental group received one puff of the IC delivered via a metered dose inhaler (MDI) with spacer and attached facemask; rabbits inhaled transnasally for 18 breaths via the one-way spacer valve as is standard for small animal IC usage. The control group received 18 breaths of aerosolized saline supplied by an ultrasonic nebulizer and identical facemask. After 8 weeks, rabbits were euthanized and larynges extracted, immersed in phosphate-buffered saline, and stored at −80°C. This storage and subsequent thaw practice is a standard methodology for benchtop studies. Additionally, unpublished data from our laboratory indicate that—while the freeze/thaw process results in lower PTP values than fresh larynges—the results are uniform across cohorts and PTF is less sensitive to freeze/thaw.
Ex Vivo Bench Procedure
The larynges were transported to Brigham Young University for ex vivo bench procedures to quantify PTP and PTF in the IC and control groups. Bench apparatus and signal acquisition procedures were based on previously established methodology.40 Upon visual inspection, 1 specimen from the control group had structural damage to the subglottis and trachea, preventing its inclusion in the study. Larynges were finely dissected to the level of the true vocal folds and mounted on a traditional bench apparatus (Figure 1) including a stainless-steel tabletop (Thorlabs, Ann Arbor, MI), 3 micropositioners (Model 1460, Kopf Industries, Tujunga, CA), and a custom foam-filled aluminum pseudolung surrounding subglottal tubing and a custom adapter for rabbit tracheas (Figure 2).
Figure 1:
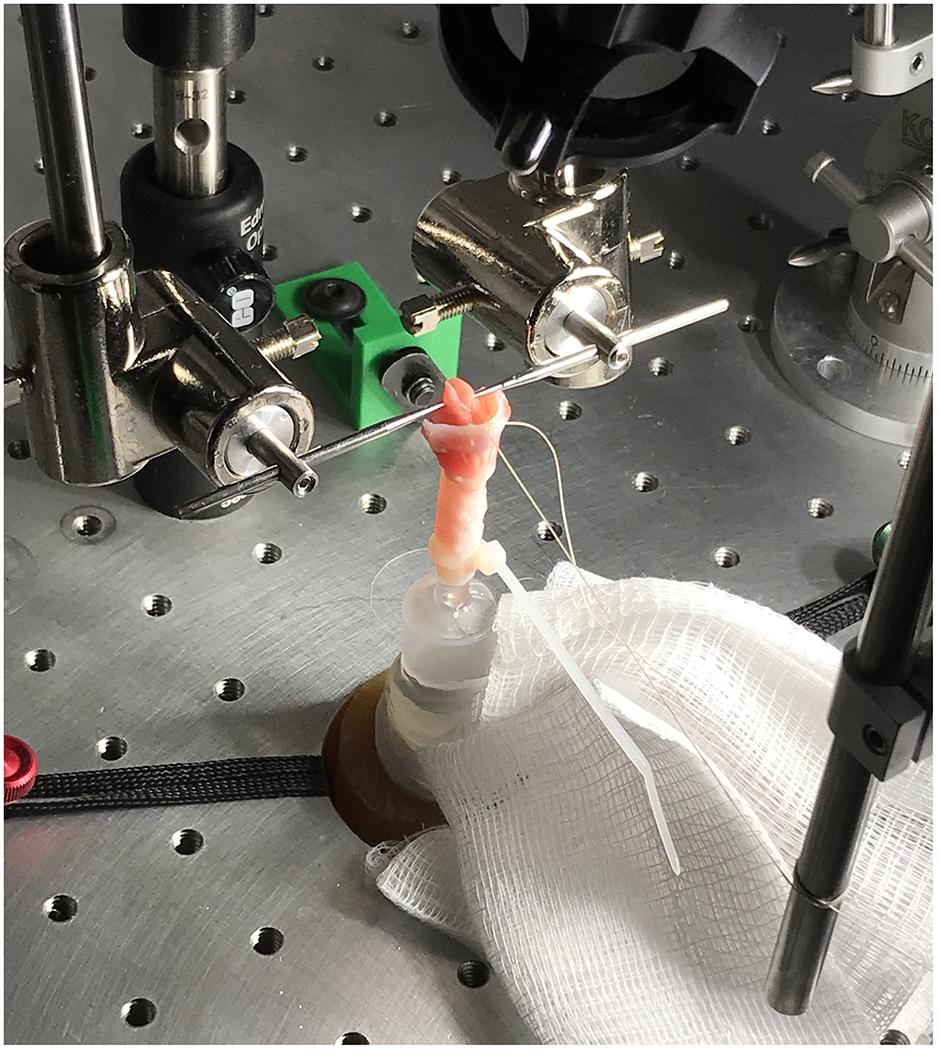
Rabbit Larynx Mounted on Phonation Apparatus, including Custom Micropositioners and Subglottic Tubing
Figure 2:

Benchtop Phonation Apparatus, in the Direction of Airflow: A) Compressed Air Tank, B) Airflow Tubing, C) Airflow Transducer, D) Humidifier, E) Pseudolung, F) Pressure Transducer, and G) Custom Mounting Apparatus
Laryngeal mounting and vocal fold adduction procedures were as follows. Each larynx was secured subglottally to the pseudolung tubing using Teflon™ tape and Zip Ties™ for an air-tight seal. The 3 micropositioners (M1, M2, M3) were secured to the breadboard using ¼−20 custom mounts (Figure 3). Their placement was optimized for rabbit larynges and remained unchanged throughout the study. The white arrows in Figure 3 indicate the 3 manual adjusters for each micropositioner. M1 and M2 are situated as mirror images; adjustments may be made vertically and medially-laterally. M3 may be adjusted anteriorly-posteriorly. The specific locations where M1-M3 adjustments manipulate the vocal folds are circled in red.
Figure 3:
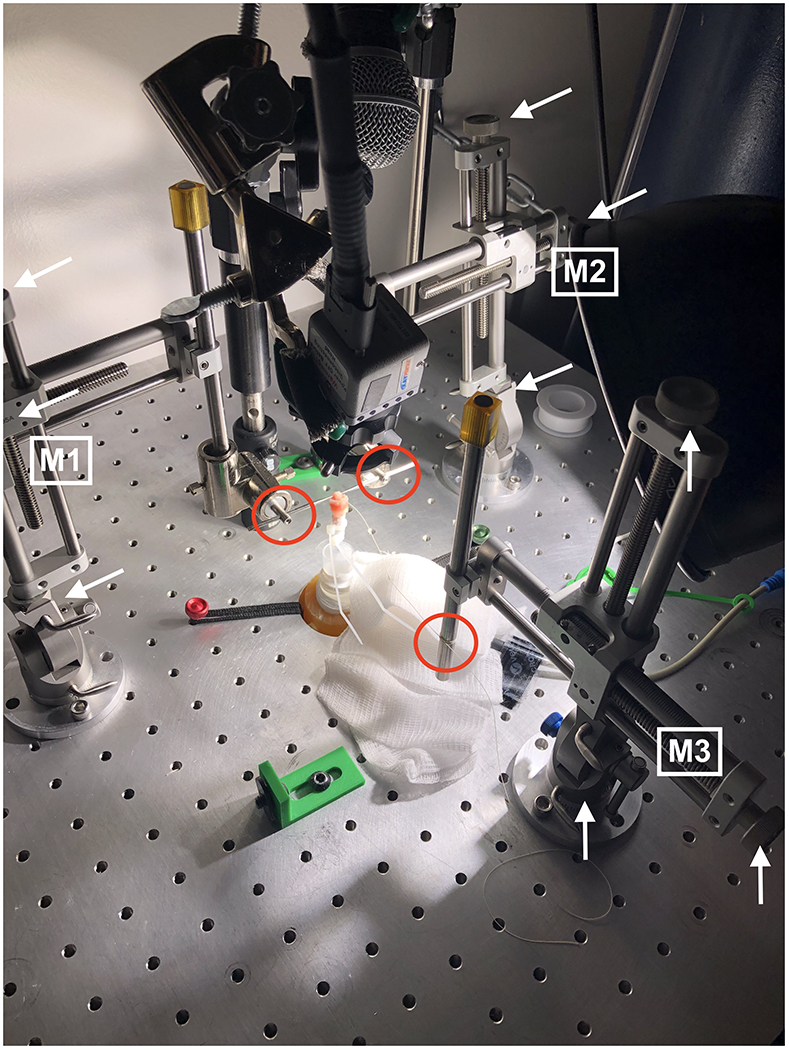
Micropositioner Uniformity in Vocal Fold Adduction
During mounting, M1 and M2 are adjusted as above so that the micrometers (i.e., custom needles used for adduction) are inserted into the arytenoid cartilages bilaterally. The trachea must not be compressed, and there must be no vocal fold vertical plane difference. The arytenoids are adducted until the vocal processes touch and there is no gap between the arytenoids posteriorly (i.e., so no air can pass between the arytenoids posteriorly). M3 is used to add tension40 via a suture thread from the anterior thyroid cartilage to the vocal folds until glottic closure is visually complete. Airflow is supplied to ensure that the vocal folds vibrate and any M1, M2, or M3 adjustments are finalized. Once this process is complete, no changes are made to adduction, abduction, or elongation.
For each larynx, phonation was induced during 15 successive trials. Repeated trials were incorporated into the operational procedures given emerging literature that this results in more representative voice parameter data.41,42 Subglottal air was supplied from pressurized tanks containing standardized medical grade air (<1% RH) that was then heated and humidified using a TheraHeat™ humidifier (Model RC7000, Smiths Medical, Dublin, OH) prior to pseudolung entry. Larynges were sprayed with a fine saline mist every 2-3 trials to maintain topical hydration. Acoustic and aerodynamic signals were acquired using PowerLab™ (AD Instruments, Colorado Springs, CO). The pressure transducer (Model MLT844) was inserted below the trachea and the respiratory airflow head was in-line with the tubing between the compressed air tank and humidifier. A dynamic microphone (Model SM-48, Shure, Niles, IL) was positioned at a 45° angle above the vocal folds. Acoustic, pressure, and airflow waveforms were recorded in LabChart™ (version 8).
The bench laboratory is equipped with room temperature control and reverberation reduction. Temperature and relative humidity (RH) were recorded before and after each larynx using the AcuRite™ Hygrometer (Model 01083M). The mean pre-experiment room temperature was 77°F (SD = 2.2, range = 75-80); mean post-experiment room temperature was unchanged, 77°F (SD = 2.1, range = 75-80). The mean pre-experiment RH was 22% (SD = 9, range = 11-38) and the mean post-experiment was 23% (SD = 9%, 12-39).
Data Analysis
Phonation onset was identified visually using acoustic waveforms and subsequently confirmed via audio playback in LabChart™. Employing 2:1 zoom, a cursor was placed at the peak of the 2nd quasi-periodic phonation onset waveform for each of the 15 trials. The time-aligned acoustic, pressure, and airflow waveform data were exported as .txt files and then imported into a custom Matlab™ program (The MathWorks Inc, 2019) developed by the 5th author; the 5 ms before and after each onset cursor were averaged to derive PTP and PTF for each phonation trial. This analysis methodology was optimized for rabbit phonation during the pilot phase of this project and resulted in the highest accuracy for PTP and PTF measurement.
Statistical Analysis and Reliability
Final data sets included all repeated phonation trials for larynges in the IC group and the control group. Data were examined for central tendency and variability, including box plots and summary statistics. The effects of ICs were examined using repeated measures ANOVAs for mean PTP and PTF across the 15 trials; group differences were evaluated using Student Newman-Keuls post-hoc tests (alpha = .05). Although PTP and PTF measurement was automated, reliability was calculated to account for potential differences in onset cursor placement. Based on 10% reanalysis of randomly selected samples, intrajudge (Pearson > .98) and interjudge (ICC > .94) reliability were within acceptable ranges. All analyses were conducted by the 4th author—project biostatistician—using the Statistical Analysis System (version 9.4, SAS Institute Inc., Cary, NC, 2012).
RESULTS
Mean PTP and PTF for each of the 15 successive phonation trials are graphed with corresponding regression lines in Figures 4 and 5, respectively. The results from repeated measures ANOVAs indicated that IC exposure significantly increased PTP [F(35, 279) = 124.09, P < 0.0001] and PTF [F(35, 279) = infinity (too large to estimate), P < 0.0001] across the 15 phonation trials. Post-hoc Student-Neuman-Keuls tests indicated significant differences between the IC and control groups for PTP (P < .05) and PTF (P < .05). For the IC group, mean PTP was 7.82 cmH2O (95% CI = 7.68-7.94) and mean PTF was 0.103 L/min (95% CI = 0.101-0.104). For the control group, mean PTP was 7.44 cmH2O (95% CI = 7.29-7.58) and mean PTF was 0.068 L/min (95% CI = 0.067-0.069).
Figure 4:
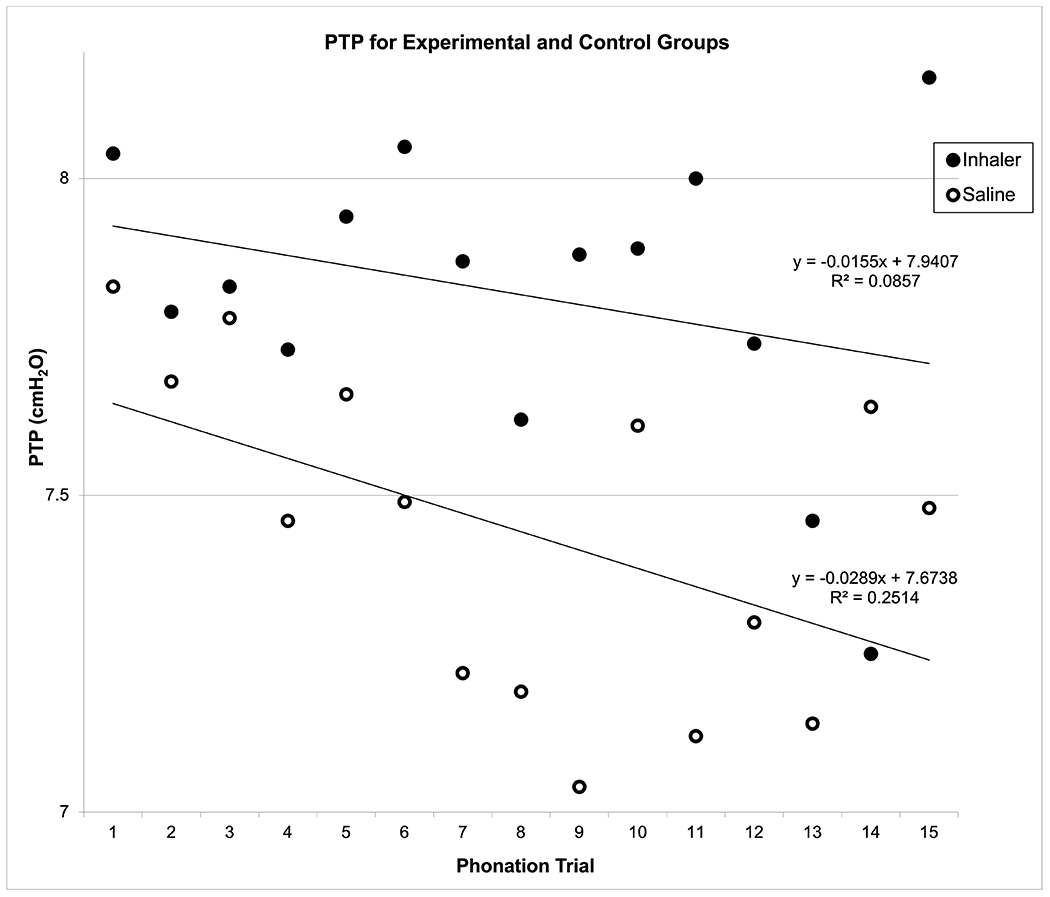
Mean Phonation Threshold Pressure (PTP) for Experimental (Inhaler) versus Control (Saline) Groups across 15 Phonation Trials
Figure 5:
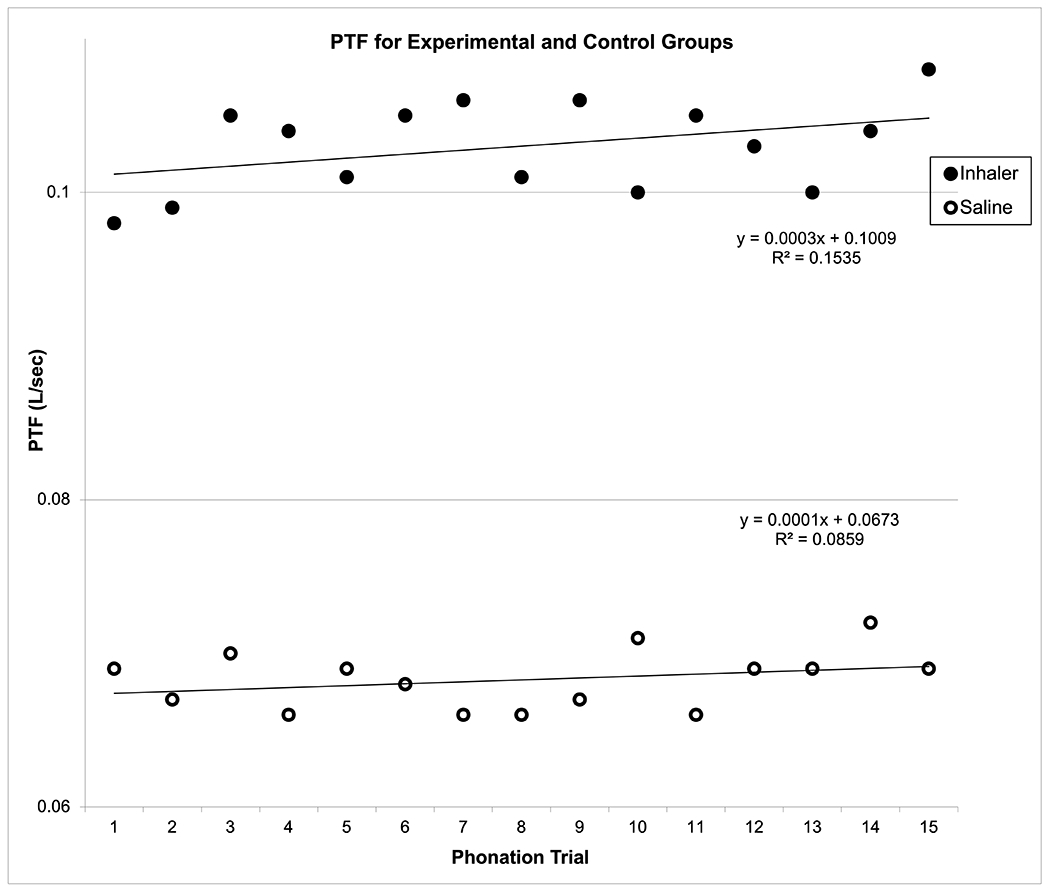
Mean Phonation Threshold Flow (PTF) for Experimental (Inhaler) versus Control (Saline) Groups across 15 Phonation Trials
DISCUSSION
This study aimed to quantify the effects of corticosteroid plus LABA combination ICs on aerodynamic patterns during rabbit phonation. Following 8 weeks of twice-daily doses of ICs or saline, larynges were excised and mounted on a phonation apparatus and voicing elicited. The results indicated that ICs adversely and uniquely affected the aerodynamic characteristics of phonation onset, causing significant increases in PTP and PTF as compared to controls. Despite precise mechanical controls, greater SDs were also observed for the IC group, indicating less predictability that is also often associated with aerodynamic measures associated with voice disorders in humans. The results from this study provide confirmatory evidence for a direct link between the use of ICs and their adverse impact on voice function. These findings have important implications for clinical voice care in patients with asthma.
Aerodynamic measurements are used frequently to identify voice differences that can arise from vocal fold health versus disease.43,44 Voice onset measures can become elevated as a first sign of change in the vocal fold epithelia, including those relating to viscosity and inflammation.45–47 Other characteristics that explain differences in onset measures include mechanical factors such as prephonatory glottal width, configuration, and mucosal wave velocity, which are often affected by structural changes in the vocal folds. In people with airway disease, both structural and behavioral factors might contribute to the development and propagation of IC-related voice disorders. It is also likely that certain characteristics of the disease itself are predictive for voice problems in people with asthma. However, it is critical to underscore that biomechanical voice changes occurred in a minimally phonatory, nonasthmatic animal in this study. These changes were also detectable as soon as 8 weeks after commencing IC treatment, which has important clinical implications for follow up and referral practices.
It is notable that inhaled particle delivery is inefficient for both humans and animals, including obligatory nasal and obligatory oral breathers. In all species, the majority of particles deposit in the nasal cavity and/or oropharynx and larynx. Drug dosages are scaled to account for this in all species during the clinical trials phase. The dosages for small animals are similar to those in small children; both use an oral-nasal mask with a spacer for delivery. For this study, dosage for rabbits was based on veterinary medicine including guidelines for other similar nasal breathers, such as cats. Similarly in patients with asthma, only a small fraction of combination IC particulates reach the lungs to relax smooth muscle and reduce airway inflammation. The vast majority (>75%) deposit in the aerodigestive tract, including the oropharynx, larynx, and vocal folds.48 These particulates permeate airway surface fluid and rapidly absorb into epithelial cells. Despite evidence that combination ICs cause voice disorders, the pathophysiology of these laryngeal changes is undetermined. Voice disorders may be caused by laryngeal inflammation or maladaptive voicing behaviors due to laryngeal irritation that cause laryngeal edema. Furthermore, vocal fold response to combination ICs is likely mediated by the asthma phenotype. Understanding vocal fold epithelial response to combination ICs will offer significant insight into the depth of vocal fold penetration.12,19,49–51 The vocal folds are covered by stratified squamous epithelium whereas the lower airway is lined with ciliated pseudostratified columnar epithelium. The results from this study lay important groundwork for hypothesis-driven quantification of IC effect differences in the larynx versus bronchi.
The magnitude of PTP and PTF differences is commensurate with those from other studies quantifying aerodynamic changes associated with voice problems in humans. Because PTP has been the primary outcome measure for many aerodynamic studies, there are more data supporting the clinical significance of these differences. Further, work by Titze52 indicates that voice production is most efficient when PTP is low and the vocal folds are healthy. Because it requires a doubling of subglottic pressure over threshold to increase glottal source power by approximately 6 dB, small magnitude changes in PTP are associated with substantial effects on the voice.
There are several limitations to the current study that warrant attention. First, it is important to acknowledge that only male rabbits were included in this study. As such, the effects of biological sex cannot be accounted for in this experiment. Translational human subjects work will be needed to determine the potential influence of sex as a biological variable. Second, only healthy, vocally normal rabbits were included in this experiment. Currently, animal asthma models are constrained predominantly to allergic phenotypes, involving exposure to an allergen and then waiting for an asthma response to occur. At the same time, it is notable that adverse voice function effects occurred in otherwise healthy animals. Third, the 8-week experimental end point for this study was selected based on previous literature regarding the initial presentation of voice problems after IC treatment initiation. It remains unknown if ICs result in detectable voice changes sooner than 8 weeks. The rationale for the 8-week timepoint involved the range of times voice changes were reported in the literature. Although voice changes might occur in as little as 2 weeks, we used a conservative approach at the upper range reported to avoid potential animal wastage and the possibility of a Type II statistical error. This study addressed our research hypothesis that ICs would produce changes in voice function in healthy animals. Additional studies are needed to examine different durations of exposure, IC dosages, and specific drug combinations. Future work should address these points using additional methodologies such as histological analysis and the translation to human subjects. The quantification of immunohistochemical and other tissue differences is needed to identify the specific causative factors associated with IC-related changes in voice function. Ultimately, some of the main challenges in this work will involve controlling for the many covariates in human studies, such as voice use patterns, other health conditions, and IC dosage.
CONCLUSION
Combination LABA plus corticosteroid inhalers impair aerodynamic voice function in a healthy animal model. Additional experimentally controlled studies are needed to determine the potential structural and physiological etiologies for these changes. Understanding how the vocal fold epithelia responds uniquely to ICs is essential for disorder management and prevention. The results from this study suggest that asthma patients with voice problems should receive expedited care that avoids 8 weeks of unchanged IC exposure. Judicious follow up referrals are critical to prevent potentially irreversible voice disorders in at-risk populations. Ultimately, we must identify the underlying pathogenesis and pathophysiology for IC-related voice problems so that we can develop voice-sparing drugs and novel protocols for asthma management.
Acknowledgements:
The authors thank students Shauntel Anderson, Brittany Jones, Joren Larsen, Miriam Bake, Brendan Olsen, and Christina Pang for their assistance during data collection.
Financial Support:
This work was supported by NIH/NIDCD grants 1R01DC016269-01A1 and 2R01DC009616-06A1, and the David O. McKay School of Education, Brigham Young University
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Presentation Disclosure: A portion of this work was accomplished as part of the 2nd author’s master’s thesis and presented at the American Speech-Language-Hearing Association Annual Convention, November 2021, Washington, D.C.
Level of Evidence: N/A
REFERENCES
- 1.Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998-2009. NCHS Data Brief 2011:1–8. [PubMed] [Google Scholar]
- 2.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000; 160:1683–1689. [DOI] [PubMed] [Google Scholar]
- 3.Asthma. Available at: https://www.niaid.nih.gov/diseases-conditions/asthma.
- 4.Galvan CA, Guarderas JC. Practical considerations for dysphonia caused by inhaled corticosteroids. Mayo Clin Proc 2012; 87:901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza N, Kasper Schwartz S, Antin-Ozerkis D. Laryngeal findings in users of combination corticosteroid and bronchodilator therapy. Laryngoscope 2004; 114:1566–1569. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura K, Koyama H, Ikeda A et al. The effect of high-dose inhaled beclomethasone dipropionate in patients with stable COPD. Chest 1999; 115:31–37. [DOI] [PubMed] [Google Scholar]
- 7.Toogood JH, Jennings B, Greenway RW, Chuang L. Candidiasis and dysphonia complicating beclomethasone treatment of asthma. J Allergy Clin Immunol 1980; 65:145–153. [DOI] [PubMed] [Google Scholar]
- 8.Williamson IJ, Matusiewicz SP, Brown PH, Greening AP, Crompton GK. Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. Eur Respir J 1995; 8:590–592. [PubMed] [Google Scholar]
- 9.Erickson E, Sivasankar M. Evidence for adverse phonatory change following an inhaled combination treatment. J Speech Lang Hear Res 2010; 53:75–83. [DOI] [PubMed] [Google Scholar]
- 10.Rachelefsky GS, Liao Y, Faruqi R. Impact of inhaled corticosteroid-induced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol 2007; 98:225–238. [DOI] [PubMed] [Google Scholar]
- 11.Sivasankar M, Blazer-Yost B. Effects of long-acting beta adrenergic agonists on vocal fold ion transport. Laryngoscope 2009; 119:602–607. [DOI] [PubMed] [Google Scholar]
- 12.Catten M, Gray SD, Hammond TH, Zhou R, Hammond E. Analysis of cellular location and concentration in vocal fold lamina propria. Otolaryngol Head Neck Surg 1998; 118:663–667. [DOI] [PubMed] [Google Scholar]
- 13.DelGaudio JM. Steroid inhaler laryngitis: dysphonia caused by inhaled fluticasone therapy. Arch Otolaryngol Head Neck Surg 2002; 128:677–681. [DOI] [PubMed] [Google Scholar]
- 14.Leikin JB, Davis A, Klodd DA et al. Selected topics related to occupational exposures. Dis Mon 2000; 46:240–322. [DOI] [PubMed] [Google Scholar]
- 15.Elliott DDP. Guide to Aerosol Delivery Device for Physicians, Nurses, Pharmacists, and Other Health Care Professionals: American Association for Respiratory Care, 2011. [Google Scholar]
- 16.Fernandez Tena A, Casan Clara P. Deposition of inhaled particles in the lungs. Arch Bronconeumol 2012; 48:240–246. [DOI] [PubMed] [Google Scholar]
- 17.Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med 2010; 42:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol 2005; 114:19–24. [DOI] [PubMed] [Google Scholar]
- 19.Branski RC, Rosen CA, Verdolini K, Hebda PA. Biochemical markers associated with acute vocal fold wound healing: a rabbit model. J Voice 2005; 19:283–289. [DOI] [PubMed] [Google Scholar]
- 20.Thibeault SGS. Vocal fold injury and repair. San Diego, CA: Plural Publishing, 2005. [Google Scholar]
- 21.Leydon C, Imaizumi M, Yang D, Thibeault SL, Fried MP. Structural and functional vocal fold epithelial integrity following injury. Laryngoscope 2014; 124:2764–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alper R, Fu X, Erickson-Levendoski E, Zheng W, Sivasankar M. Acute stress to excised vocal fold epithelium from reactive oxygen species. Laryngoscope 2011; 121:2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branski RC, Zhou H, Kraus DH, Sivasankar M. The effects of cigarette smoke condensate on vocal fold transepithelial resistance and inflammatory signaling in vocal fold fibroblasts. Laryngoscope 2011; 121:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duarte JL, de Faria FA, Ceolin DS, Cestari TM, de Assis GF. Effects of passive smoke inhalation on the vocal cords of rats. Braz J Otorhinolaryngol 2006; 72:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.E EL SM. Functional changes in porcine vocal folds induced by a short duration pollutant challenge International Conference on Voice Physiology and Biomechanics. Erlangen, Germany, 2012. [Google Scholar]
- 26.Gaafar HA, Al-Mansour AH. The effect of cigarette smoke on the vocal cord mucosa of the rabbit: An electron microscopic study. J Laryngol Otol 1981; 95:721–729. [DOI] [PubMed] [Google Scholar]
- 27.Isik AC, Kalender Y, Yardimci S, Ergun A. Environmental tobacco smoke in rats. J Otolaryngol 2004; 33:382–386. [PubMed] [Google Scholar]
- 28.Leonard R, Charpied G, Faddis B. Effects of chronic ozone (O3) exposure on vocal-fold mucosa in bonnet monkeys. J Voice 1995; 9:443–448. [DOI] [PubMed] [Google Scholar]
- 29.Marcelino FC, Oliveira DT. Histopathological changes of vocal folds induced by chronic pollutant exposure: an experimental study. J Voice 2005; 19:529–533. [DOI] [PubMed] [Google Scholar]
- 30.Uhlik J, Vajner L, Adaskova J, Konradova V. Effect of inhalation of single dose of beclomethasone on airway epithelium. Ultrastruct Pathol 2007; 31:221–232. [DOI] [PubMed] [Google Scholar]
- 31.Flint PW, Corio RL, Cummings CW. Comparison of soft tissue response in rabbits following laryngeal implantation with hydroxylapatite, silicone rubber, and Teflon. Ann Otol Rhinol Laryngol 1997; 106:399–407. [DOI] [PubMed] [Google Scholar]
- 32.Dufresne AM, Lafreniere D. Soft tissue response in the rabbit larynx following implantation of LactoSorb (PLA/PGA copolymer) prosthesis for medialization laryngoplasty. J Voice 2000; 14:387–397. [DOI] [PubMed] [Google Scholar]
- 33.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice 2002; 16:96–104. [DOI] [PubMed] [Google Scholar]
- 34.Maytag AL, Robitaille MJ, Rieves AL, Madsen J, Smith BL, Jiang JJ. Use of the rabbit larynx in an excised larynx setup. J Voice 2013; 27:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awan SN, Novaleski CK, Rousseau B. Nonlinear analyses of elicited modal, raised, and pressed rabbit phonation. J Voice 2014; 28:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojima T, Valenzuela CV, Novaleski CK et al. Effects of phonation time and magnitude dose on vocal fold epithelial genes, barrier integrity, and function. Laryngoscope 2014; 124:2770–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keir S, Page C. The rabbit as a model to study asthma and other lung diseases. Pulm Pharmacol Ther 2008; 21:721–730. [DOI] [PubMed] [Google Scholar]
- 38.Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy 2007; 37:973–988. [DOI] [PubMed] [Google Scholar]
- 39.Gallivan GJ, Gallivan KH, Gallivan HK. Inhaled corticosteroids: hazardous effects on voice-an update. J Voice 2007; 21:101–111. [DOI] [PubMed] [Google Scholar]
- 40.Mills RD, Dodd K, Ablavsky A, Devine E, Jiang JJ. Parameters From the Complete Phonatory Range of an Excised Rabbit Larynx. J Voice 2017; 31:517 e519–517 e517. [DOI] [PubMed] [Google Scholar]
- 41.Scherer RC, Vail VJ, Guo CG. Required number of tokens to determine representative voice perturbation values. J Speech Hear Res 1995; 38:1260–1269. [DOI] [PubMed] [Google Scholar]
- 42.Shrivastav R, Sapienza CM, Nandur V. Application of psychometric theory to the measurement of voice quality using rating scales. J Speech Lang Hear Res 2005; 48:323–335. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman MR, Rieves AL, Budde AJ, Surender K, Zhang Y, Jiang JJ. Phonation instability flow in excised canine larynges. J Voice 2012; 26:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang J, O’Mara T, Conley D, Hanson D. Phonation threshold pressure measurements during phonation by airflow interruption. Laryngoscope 1999; 109:425–432. [DOI] [PubMed] [Google Scholar]
- 45.Plexico LW, Sandage MJ, Faver KY. Assessment of phonation threshold pressure: a critical review and clinical implications. Am J Speech Lang Pathol 2011; 20:348–366. [DOI] [PubMed] [Google Scholar]
- 46.Jiang JJ, Titze IR. A methodological study of hemilaryngeal phonation. Laryngoscope 1993; 103:872–882. [DOI] [PubMed] [Google Scholar]
- 47.Jiang JJ, Tao C. The minimum glottal airflow to initiate vocal fold oscillation. J Acoust Soc Am 2007; 121:2873–2881. [DOI] [PubMed] [Google Scholar]
- 48.Hassen HE, Abo Hasseba AM. Voice evaluation in asthma patients using inhaled corticosteroids. Kulak Burun Bogaz Ihtis Derg 2016; 26:101–108. [DOI] [PubMed] [Google Scholar]
- 49.Gray SD. Cellular physiology of the vocal folds. Otolaryngol Clin North Am 2000; 33:679–698. [DOI] [PubMed] [Google Scholar]
- 50.Hirano M, Sato K, Nakashima T. Fibroblasts in human vocal fold mucosa. Acta Otolaryngol 1999; 119:271–276. [DOI] [PubMed] [Google Scholar]
- 51.Levendoski EE, Leydon C, Thibeault SL. Vocal fold epithelial barrier in health and injury: a research review. J Speech Lang Hear Res 2014; 57:1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Titze IR. Principles of Voice Production. Englewood Cliffs, NJ: Prentice Hall, 1994. [Google Scholar]


