Abstract
Obesity is an important contributing factor to the pathophysiology of atrial fibrillation (AF) and its complications by causing systemic changes, such as altered haemodynamic, increased sympathetic tone, and low-grade chronic inflammatory state. In addition, adipose tissue is a metabolically active organ that comprises various types of fat deposits with discrete composition and localization that show distinct functions. Fatty tissue differentially affects the evolution of AF, with highly secretory active visceral fat surrounding the heart generally having a more potent influence than the rather inert subcutaneous fat. A variety of proinflammatory, profibrotic, and vasoconstrictive mediators are secreted by adipose tissue, particularly originating from cardiac fat, that promote atrial remodelling and increase the susceptibility to AF. In this review, we address the role of obesity-related factors and in particular specific adipose tissue depots in driving AF risk. We discuss the distinct effects of key secreted adipokines from different adipose tissue depots and their participation in cardiac remodelling. The possible mechanistic basis and molecular determinants of adiposity-related AF are discussed, and finally, we highlight important gaps in current knowledge, areas requiring future investigation, and implications for clinical management.
Keywords: Adipokines, Atrial fibrillation, Epicardial adipose tissue, Obesity, NLRP3 inflammasome, Subcutaneous adipose tissue, Visceral adipose tissue
Graphical Abstract
Graphical abstract.
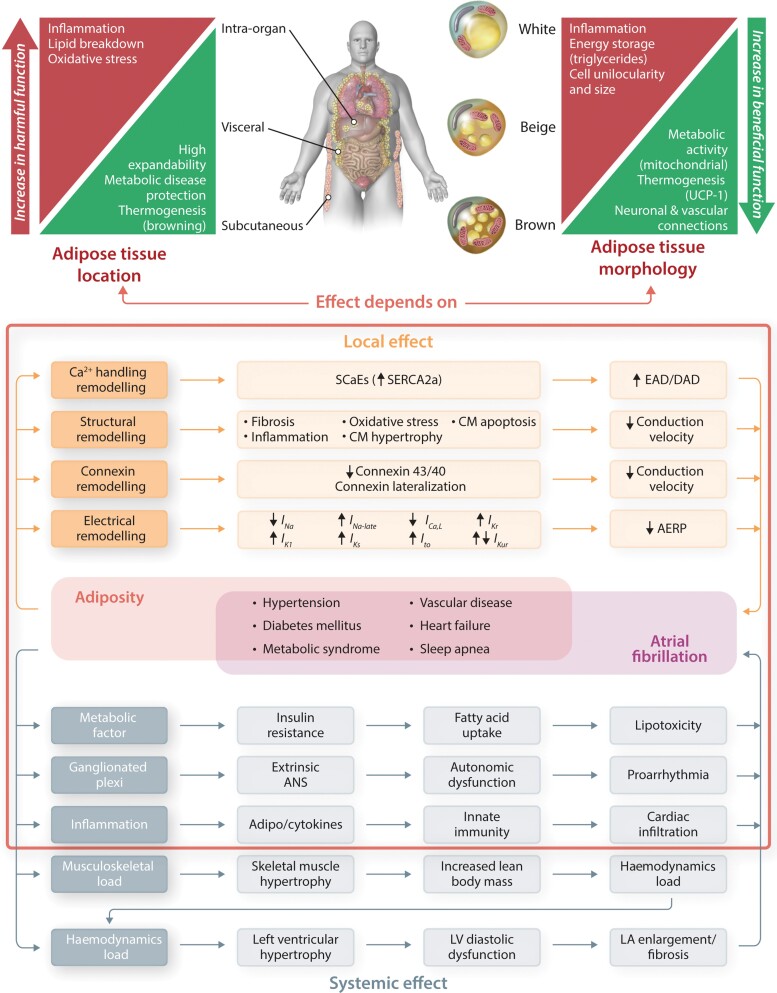
Cellular, localization, and functional heterogeneity of adipose tissue.
CM, cardiomyocyte; see Tables 1 and 2.
Visceral and subcutaneous adipose tissue are two main human fat depots that represent different metabolically and proinflammatory profile (upper left panel). Adipocytes are divided into different cell types with distinct morphological and functional characteristics, including white, beige, and brown adipocytes (upper right panel). Pink adipocytes are not discussed in this review, since they mainly occur in pregnancy.
Adiposity together with accompanying obesity-related comorbidities (metabolic syndrome, diabetes, hypertension, heart failure, sleep apnoea) promote atrial fibrillation through systemic effects (bottom left) via haemodynamic, metabolic, neurohormonal, and proinflammatory factors, and through local effects promoting a proarrhythmic atrial cardiomyopathy involving Ca2+ handling, structural, connexin, and electrical remodelling (bottom right).
Depending on location and morphology, adipose tissue might contribute to proarrhythmia by causing local and systemic effects (red frame).
1. Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a risk to health.1 A body mass index (BMI) over 25 kg/m2 indicates overweight, whereas BMI over 30 kg/m2 indicates obesity.1 The prevalence of overweight/obesity has reached global epidemic status.1 Nearly half of the world population is estimated to be overweight or obese, and for the first time in human history, the number of obese people exceeds those who are underweight.1 The recent scientific statement of the American Heart Association2 positions excess visceral adiposity as a crucial and independent driver of atrial fibrillation (AF).
This review assesses how specific adipose tissue depots, specifically those located around the heart, may contribute causally to AF. We describe the key culprit adipocytokines secreted by distinct fat depots and outline candidate molecular mechanisms and newly discovered metabolic pathways by which these factors, as well as obesity in general, can promote the evolution of an AF-vulnerable arrhythmogenic substrate. Finally, we highlight important gaps in current knowledge and provide perspectives for future exploration of the complex relationship between obesity and AF, as needed to improve clinical management of the arrhythmia.
2. The composition of different fat depots
Mammals, including humans, possess two principal types of fat: white adipose tissue (WAT) and brown adipose tissue (BAT), each with specific cell compositions and secretory effects (Table 1).3 Moreover, brown adipocytes appearing in WAT form the third type of adipose tissue called brite, beige, or brown-in-white.
Table 1.
Key features of white and brown adipose tissue
| White adipose tissue | Brown adipose tissue | |
|---|---|---|
| Macrophages’ markers4 | CD14−, CD16+, CD11c+, CD36+, CD64+, CD86+, HLA-DR+, TL4+, CCR2+ | CD14+, CD16−, CD68+, CD163+, CD204+, CD206+, CD11b+, CD301+ |
| Immune cells5 | CD8+ T-lymphocytes, T-helper type 1 cells | Eosinophils, T-regulatory cells, T-helper Type 2 cells |
| Triggers5 | IFNy, LPS, TNF-α, MCP-1, IL-6, IL-1 | IL-4, IL-10, IL-13, TGF-β |
| Pharmacological interventions that increase particular fat depot | Anti-diabetic drugs (insulin)6 Anti-hypertensive drugs (beta-blockers)7 Anti-psychotic drugs (atypical)8 Anti-depressants (serotonin agents)9 Glucocorticoids10b |
Anti-diabetic drugs (GLP-1R agonists, DPP-4 inhibitors, SGLT2 inhibitors)6 Lipid-lowering drugs6 Anti-obesity drugs (naltrexone + bupropion, phentermine + topiramate)6 |
| Lifestyle interventions that increase particular fat depot | Nutritional changes (vitamin D overconsumption)11 Sleep loss12 Alcohol overconsumption13c Cannabinoids overdose14d Prolonged daily light exposure15 |
Nutritional changes (vitamin A, fish oil overconsumption)6 Increased physical activity6 Bariatric surgery6 Cold exposure6 BAT transplantation6 |
Bolded markers are those distinguishing anti-inflammatory (CD14+ CD16− CD36lowCD163+) from pro-inflammatory macrophages (CD14+ CD16+ CD36high CD163−).4
CXCL, chemokine (C–X–C motif) ligand; DPP-4, dipeptidyl peptidase-4; FGF21, fibroblast growth factor 21; GDF, growth and differentiation factor; GLP-1R, glucagon-like peptide-1 receptor; IFNy, interferon y; IL, interleukin; L-PGDS, lipocalin prostaglandin D synthase; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; RBP-4, retinol-Binding Protein 4; SGLT2, sodium-glucose cotransporter 2; SLIT-C, C-terminal fragment of SLIT2 protein; TGF-β; transforming growth factor beta; TLR, Toll-like receptor; TNF-α, tumour necrosis factor alpha; UCP-1, uncoupling protein 1.
aTGF-β superfamily.
bSpecies-specific effect on adipose tissue.16 In rodents, glucocorticoid treatment decreases activity of BAT, whereas in humans, it acutely increases UCP-1 expression,17 which decreases with chronic (after 48 h) glucocorticoid treatment.18
cChronic alcohol consumption led to a reduction in adipose tissue mass.19
dCannabidiol promotes brown-like phenotype.20
WAT is the predominant form of total body fat. Its primary functions are to store energy for times of higher energy demand and to secrete molecules that regulate food intake and energy turnover.3 White adipocytes are large and unilocular, possessing one large fat droplet that stores energy in the form of triglycerides, and few mitochondria.3 WAT is classified as either subcutaneous adipose tissue (SAT) or visceral adipose tissue (VAT), with VAT located in the intra-abdominal or omental region, and in and around organs such as the heart and vasculature (Figure 1).3 Adipocytes in VAT and SAT arise from different progenitors and exhibit distinct gene expression patterns and functions already in childhood.21 VAT is associated with a high abundance of infiltrating macrophages and a proinflammatory secretome, whereas SAT is typically seen as relatively inert or even beneficial (Graphical Abstract).22 Moreover, abdominal VAT can release its secretory products (e.g. adipokines) into both the systemic circulation and the portal vein, thereby directly worsening or protecting liver function.23 The individual VAT:SAT ratio is higher in the elderly compared with young individuals and lower in females compared with males, and is further determined by genetic background, nutritional health, and the energy homeostasis of the specific depots.3
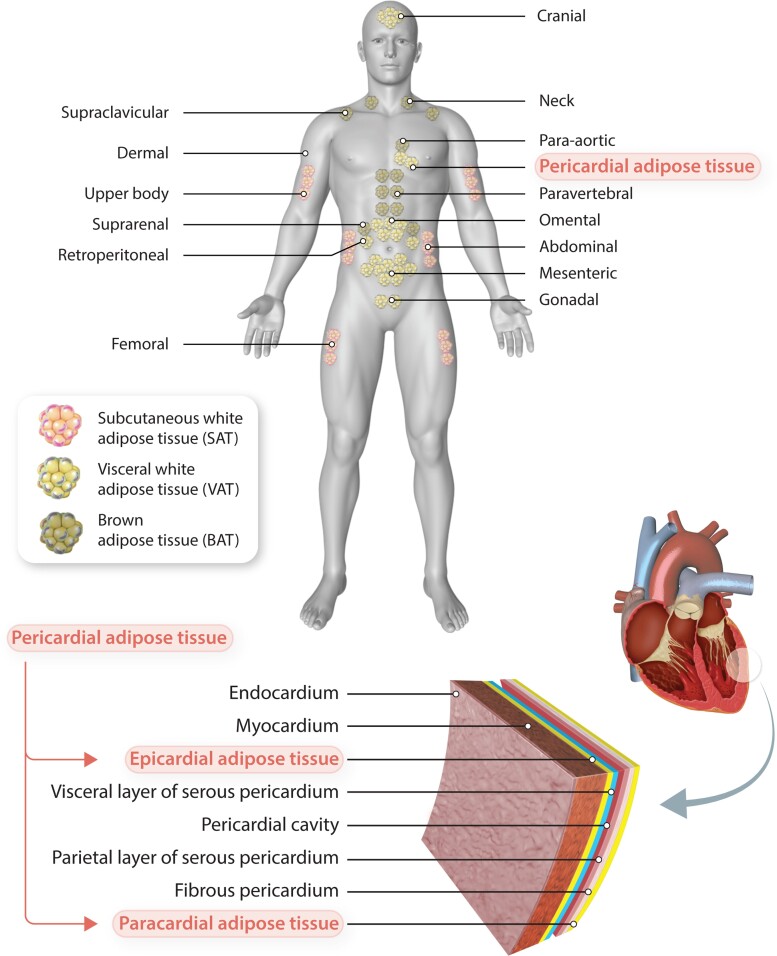
Figure 1.
Types and localization of adipose tissue depots. In humans, BAT is localized mainly around the shoulders and ribs. Visceral WAT surrounds intra-abdominal organs, whereas subcutaneous WAT spreads throughout the body beneath the skin. Pericardial adipose tissue, a subtype of VAT, comprises both the epicardial and paracardial adipose tissue layers. Epicardial fat lies between the visceral pericardium and myocardium, whereas paracardial fat is located external to the fibrous pericardium.
BAT makes up 4% of total body fat. It is located mainly in supraclavicular areas, and to a lesser extent in para-aortic, para-vertebral, and supra-renal regions (Figure 1).3 Its abundance, composition, and endocrine function are highly plastic, depending on nutritional status, sex, age, and adiposity.3 It is higher in the female population and decreases with age.3 BAT exhibits distinct transcriptome, proteome, and secretome signatures compared with WAT and accordingly plays a different physiological role, storing energy to a lesser degree and functioning primarily as a thermoregulatory organ.3 Brown-like adipocytes are smaller, multilocular, and possess a higher number of mitochondria than white adipocytes (Graphical Abstract).24 High expression of uncoupling protein-1 separates oxidative phosphorylation from ATP production, promoting the oxidation of fatty acids for heat production, although additional independent mechanisms may also contribute.25 BAT is classically considered as a thermoregulatory fat depot in newborns only and is only characterized in adult mammals, including humans, just over a decade ago.26 Intriguingly, white shifting of brown adipocytes within cardiac fat correlates with dilatation of the left atrium (LA), a strong predictor of AF.27
A third type of fat can evolve when batches of brown-like adipocytes accumulate in WAT (Table 1).24 This may occur through inducible ‘browning’ of white adipocytes towards the so-called beige, brite, or brown-in-white phenotype in response to cold, β-adrenoceptor stimulation, exercise, or various other endocrine stimuli.6
3. Adipose tissue remodelling in obesity
Obesity causes immune-cell recruitment and infiltration and inflammation of adipose tissue and other organs, with accumulation and activation of M1-macrophages, neutrophils, CD4+ T-helper cells, and CD8+ T cells, while the contribution of M2-macrophages, CD4+ regulatory T cells, regulatory B cells, and eosinophils decreases.28 The contribution of other players, such as natural killer cells and innate lymphoid cells, is just beginning to be explored. Immune-cell recruitment is triggered by chemokines secreted from hypertrophic adipocytes, most notably the monocyte chemoattractant protein-1 (MCP-1),29 which is considered a particular feature of WAT. Recent work employing a wider panel of subset markers to characterize resident macrophages in SAT and VAT from lean and obese human donors, unexpectedly revealed that total macrophage numbers increased with obesity only in SAT, but not in VAT and that M2-macrophages were actually highly prevalent in lean VAT but diminished with obesity.30 Conversely, macrophage expansion in SAT depends largely on increased M2-macrophages, while in VAT, the subset of macrophages that increased numerically with obesity display mixed M1/M2 markers.31 Hence, the classical view that obesity exacerbates the proinflammatory nature of VAT, while SAT is rather inert, is increasingly challenged and requires further in-depth investigations.
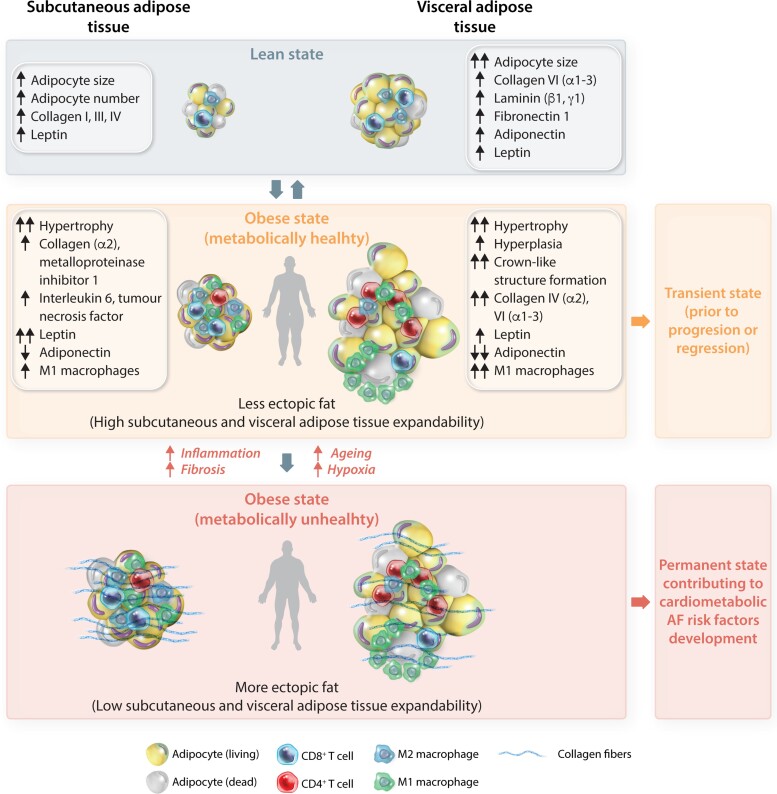
In the lean state, SAT is characterized by a smaller cell size but an increased number of adipocytes as well as differential adipokine secretion compared with VAT (Figure 2). During obesity, VAT adipocytes commence an adipogenic programme encompassing hyperplasia of adipocyte precursors and hypertrophy of mature adipocytes, while subcutaneous adipocytes preferentially undergo hypertrophy.32 Weight gain triggers cell growth of VAT (vs. SAT) adipocytes more rapidly, along with a reduction in adiponectin expression in VAT. As obesity progresses, extracellular matrix rigidity, composition, and remodelling impact adipose tissue expandability by physically limiting adipocyte hypertrophy and hyperplasia. Once the maximal capacity to store lipids is reached, lipids begin to accumulate as increased ectopic fat, defined by excess adipose tissue in locations not classically associated with adipose tissue storage including viscera, heart, and vasculature,33 and promote insulin resistance, apoptosis, and inflammation.23 This process is modulated by genetic and environmental factors, and for VAT is further restricted by the available space in the abdominal cavity.23
Figure 2.
Schematic representation of the cellular dynamics of adipose tissue depots associated with obesity. In lean state (grey), VAT is characterized by large adipocytes, whereas SAT is characterized by a larger number of adipocytes, with VAT and SAT differing in their content of fibrotic factors and adipokines. During obesity, VAT is characterized by hyperplasia of adipocyte precursors and hypertrophy of mature adipocytes, while SAT adipocytes preferentially undergo hypertrophy. Weight gain triggers cell growth of VAT (vs. SAT) adipocytes, along with a reduction in adiponectin expression in VAT. This state is typical for ‘metabolically healthy’ individuals with high expandability of SAT and VAT, resulting in a low degree of ectopic fat storage (yellow). This state can regress or progress. As obesity progresses, extracellular matrix rigidity, composition, and remodelling alter adipose tissue expandability by physically limiting adipocyte hypertrophy and hyperplasia. Once the capacity to store lipids is reached, lipids begin to accumulate at ectopic sites. This process is modulated by genetic and environmental factors, and for VAT is further restricted by the available space in the abdominal cavity. This obese state describes ‘metabolically unhealthy’ individuals with low expandability of SAT and VAT resulting in a high rate of ectopic fat storage (red) that promotes insulin resistance, apoptosis and inflammation in places of residence (heart, liver, pancreas), thereby contributing to the development of cardiometabolic risk factors for AF.
Noteworthy, ectopic accumulation of the fat could be referred not only to the apparent obesity but also ectopic fat accumulation in seemingly lean or slightly overweight patients. These ‘metabolically unhealthy’ individuals are characterized by lower SAT mass, higher VAT mass, adipocyte hypertrophy, a proinflammatory adipose tissue phenotype, and an impaired fat storage capacity of adipose tissue, which may result in ectopic fat deposition (more lipid accumulation in the heart), thereby contributing to the development of cardiometabolic AF risk factors.34 On the other hand, the so-called obesity paradox describes individuals with excess bodyweight and an elevated BMI who are yet ‘metabolically healthy’.34 They are characterized by more abdominal SAT and lower VAT mass, smaller adipocytes and less macrophage infiltration and inflammation in (visceral) adipose tissue, less fat accumulation in organs, and exhibit a lower risk of cardiovascular diseases.34 This may be a transient state, prior to the progression of overt metabolic derangement and the manifestation of cardiovascular diseases.34
The aforementioned data indicate that the key factor driving cardiometabolic health seems to be the VAT compartment, with a low VAT content being associated with a more favourable cardiometabolic risk profile, independently of BMI.34 Of note, the AF-promoting effect of obesity may not be due to adiposity per se,35 but may rather be mediated by the increase in lean body mass caused by overweight.35 The idea that obesity promotes AF by increasing lean body mass requires proof and validation in detailed pathophysiological studies. Because BMI does not differentiate between visceral, ectopic, or subcutaneous fat mass,2 waist circumference might provide a better estimate of the relative abundance of visceral fat.1 Previous work showed that waist circumference correlates much more strongly with visceral fat than BMI and that the addition of waist circumference to BMI explains an additional 11 and 16% of the variation in visceral fat in men and women, respectively.36 This measure could complement BMI in body fat monitoring, and improve health.1
4. Current conceptual model of AF
AF is the most common sustained arrhythmia and is associated with an increased cardiovascular mortality.37 The progressive nature of AF has been attributed to worsening of the underlying atrial cardiomyopathy due to cumulative effects of genetics, chronic cardiovascular diseases and risk factors, as well as atrial remodelling induced by AF itself.38
Mechanistically, the genesis of AF generally requires a trigger and a vulnerable substrate (Figure 3).39,40 The ectopic (triggered) activity is often the result of Ca2+-handling abnormalities leading to abnormal automaticity (spontaneous depolarization of the membrane potential during diastole), or early and delayed afterdepolarizations.41 Early afterdepolarizations are promoted by excessive prolongation of repolarization, providing time for L-type Ca2+-currents to recover from inactivation and contribute additional depolarizing inward current, whereas delayed afterdepolarizations are promoted by spontaneous Ca2+ releases from the intracellular stores of the sarcoplasmic reticulum through ryanodine receptor channels.39 The vulnerable substrate is characterized by short effective refractory period (ERP), typically due to ion-channel remodelling, and slow, heterogeneous conduction velocity resulting from structural remodelling, or remodelling of gap junctions responsible for electrical cell-to-cell coupling and voltage-gated Na+ channels governing cellular excitability.39 In the presence of an ectopic trigger leading to unidirectional block, short ERP and slow heterogeneous conduction promote the occurrence of re-entry, the primary AF-maintaining mechanism.39 Finally, atrial dilatation is a common component of atrial structural remodelling that provides a larger substrate for the maintenance of re-entry.39 The numerous AF-promoting risk factors and wide variety of mechanisms underlying AF create a highly heterogeneous condition that negatively affects the success of most AF therapies, suggesting a need for tailored therapy targeting nodal points of a specific phenotype.39
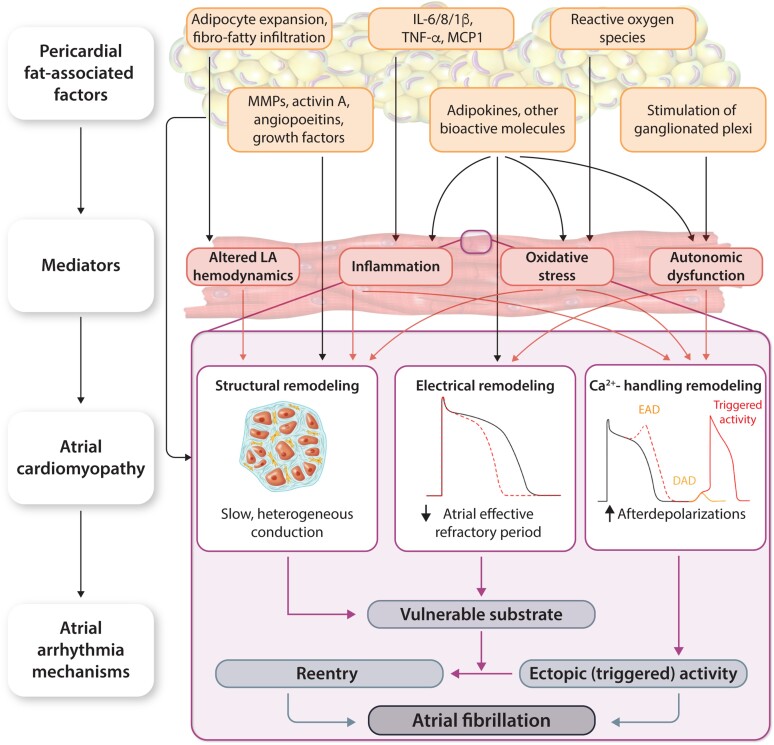
Figure 3.
Role of pericardial fat in AF promotion. DAD, delayed afterdepolarization; EAD, early afterdepolarization; see Table 1. Pericardial fat-associated factors (adipocyte expansion/infiltration, inflammatory signalling molecules, growth factors, adipokines, reactive oxygen species, and stimulation of ganglionated plexi) stimulate the development of an atrial cardiomyopathy via a wide range of mediators (inflammation, oxidative stress, mechanical, and autonomic dysfunction). The obesity-induced atrial cardiomyopathy includes a vulnerable substrate, consisting of re-entry-promoting structural, connexin, and electrical remodelling, as well as Ca2+-handling remodelling leading to triggered activity via early and delayed afterdepolarizations. Together, these arrhythmia mechanisms promote the initiation and maintenance of AF. IL, interleukin; MCP1, monocyte chemoattractant protein-1; TNF-α, tumour necrosis factor alpha.
5. Clinical evidence and potential mechanisms linking obesity and AF
A recent meta-analysis associated obesity with a >50% increased risk of new-onset AF.42 While mere overweight, in the absence of additional parameters of metabolic syndrome, does not increase the risk of incident AF, obesity with BMI in excess of 30 kg/m2 is per se sufficient to promote AF onset43 and progression from paroxysmal to persistent forms.44 Another meta-analysis of 51 studies including 626 603 patients showed a significant, 10–29% increase in the risk of incident, post-operative, and post-ablation AF, for every 5 kg/m2 increase in BMI, suggesting a linear association between obesity and AF risk.45
Recent studies show that AF may share some genetic loci and susceptibility genes with obesity and BMI. A Mendelian Randomization study showed that a genetic predisposition to childhood obesity is associated with an increased risk of developing AF in adult age.46 Another Mendelian Randomization study of >50 000 European individuals without AF at baseline discovered that a genetic score comprising 39 single-nucleotide polymorphisms identified by genome-wide association studies to be associated with increased BMI was significantly linked to a higher risk of incident AF, even after adjustment for traditional AF risk factors including hypertension, diabetes mellitus, coronary artery disease, and heart failure.47 These data point to a causal relationship between obesity and AF.
The mechanistic basis of the clinical association between AF and obesity remains incompletely understood, but numerous factors have been implicated in proarrhythmic atrial remodelling (Table 2; Figure 3). Among others, several adipokines and other bioactive molecules have been implicated in paracrine fat–heart communication (see Section 7) contributing to proarrhythmic atrial structural, electrical, and Ca2+-handling remodelling. Among patients with AF, those with concomitant obesity exhibit shorter ERPs in both LA and around the pulmonary veins, as well as higher LA pressures and volumes, compared with AF patients with normal BMI.49 The heart is particularly vulnerable to functional changes and end-organ damage induced by obesity and the associated metabolic risk factors hyperglycaemia, dyslipidaemia, and insulin resistance. The increased systemic blood volume that accompanies adipose tissue expansion to ensure adequate perfusion of the excess tissue, increases cardiac output and ventricular wall stress, and causes hypertrophy.74 The resultant diastolic and systolic dysfunction promotes LA enlargement and a rise in pulmonary venous pressures.74 This adaptive remodelling, together with concurrent neurohumoral activation, activation of profibrotic signalling pathways (via regulation of matrix metalloproteases, activin A, angiopoietins, and growth factors), and a sustained inflammatory state (mediated in part by intereleukin (IL)-6/8/1β, tumour necrosis factor alpha, MCP-1), may create a vulnerable substrate for AF promotion (Figure 3).
Table 2.
Available clinical studies with plausible mechanistic data about the obesity-induced remodelling in AF (A) and experimental evidence for the association between adiposity and AF (B)
| Reference | Study population/animal model | Results |
|---|---|---|
| (A) Clinical data | ||
| Schram-Serban et al.48 | Patients without baseline AF undergoing cardiac surgery; obese (n = 106, 74% men, 64 ± 9.6 years, BMI 33 ± 2.9 kg/m2) and non-obese (n = 106, 78% men, 62 ± 12 years, BMI 25 ± 2.4 kg/m2) |
|
| Munger et al.49 | AF patients undergoing catheter ablation; obese (n = 44, 75% men, 57 ± 8.8 years, BMI 35 ± 4.3 kg/m2) and non-obese (n = 19, 58% men, 55 ± 13 years, BMI 24 ± 1.7 kg/m2) |
|
| Fang et al.50 | Patients with AF; obese (n = 14, 50% men, 63 ± 7.0 years, BMI 32 ± 1.6 kg/m2) and non-obese (n = 15, 53% men, 64 ± 7.3 years, BMI 23 ± 1.5 kg/m2) |
|
| Haemers et al.51 | AF patients undergoing cardiac surgery (n = 92, 68% men, 68 ± 10 years, BMI 27 ± 5.9 kg/m2) |
|
| Nalliah et al.52 | AF patients undergoing cardiac surgery (n = 19, 78% men, 64 ± 4.0 years, BMI 30 ± 7.0 kg/m2) |
|
| (B) Experimental data | ||
| Mahajan et al.53 | Sheep with DIO ± weight loss; lean controls |
|
| Scott et al.54 | Sheep ± DIO Mice ± DIO; WT vs. NLRP3−/− |
|
| Haemers et al.51 | Sheep ± RAP-induced AF |
|
| Mahajan et al.55 | Sheep ± DIO |
|
| Abed et al.56 | Sheep ± DIO |
|
| Otsuka et al.57 | Dogs ± DIO |
|
| Okumura et al.58 | Pigs ± DIO |
|
| Lin et al.59 | Rabbit LA myocytes ± human adipocytes or adipocyte-conditioned media |
|
| ||
| Martinez-Mateu et al.60 | Guinea pigs ± DIO |
|
| Bernasochi et al.61 | Young vs. old rodents ± DIO ± 17β-estradiol |
|
| Hohl et al.62 | Rats ± DIO |
|
| McCauley et al.63 | Rats ± DIO |
|
| Fang et al.50 | Mice ± DIO; WT vs. ± cadherin-11−/− |
|
| McCauley et al.64 | Mice ± DIO |
|
| Nalliah et al.52 | HiPSC-CM ± sheep EAT HL-1 cells ± mouse pericardial or inguinal adipose tissue |
|
| Shuai et al.65 | Mice ± DIO; WT vs. ± MD1−/− |
|
| Kondo et al.66 | Mice ± DIO; WT vs. ± IL-10−/− IL-10 supplementation |
|
| Zhang et al.67 | Mice ± DIO ± 4-phenylbutyric acid (antioxidant) |
|
| Zhang et al.68 | Mice ± DIO |
|
| Fu et al.69 | Mice ± DIO |
|
| Feng et al.70 | Mice ± DIO WT vs. ± Nampt−/− |
|
| Ornelas-Loredo et al.71 | Mice ± DIO ± flecainide or sotalol |
|
| Maria et al.72 | Mice ± DIO |
|
| Fukui et al.73 | Mice ± DIO; WT vs. ± leptin−/− |
|
Abbreviations: α-SMA, alpha smooth muscle actin; AF, atrial fibrillation; APD, action potential duration; BMI, body mass index; DIO, diet induced obesity; EAT, epicardial adipose tissue; ERP, effective refractory period; hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; IL, interleukin; ICa,L, L-type Ca2+-current; IKur, ultra-rapid delayed rectifier K+-current; IKr, delayed rectifier K+-current; IK1, inward rectifier K+-current; INa, Na+-current; INa-late, late Na+-current; Ito, transient outward K+-current; LA, left atrium; MD-1; myeloid differentiation; NAD, nicotinamide adenine dinucleotide; Nampt, nicotinamide phosphoribosyltransferase; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domains-containing-3; RA, right atrium; RAP, rapid atrial pacing; WT, wildtype; ↑ indicates increase in function or expression; ↓ indicates reduction in function or expression.
6. Association between cardiac fat and promotion of AF
While some studies demonstrate significant correlations between visceral75 or subcutaneous76 adiposity and LA enlargement, others do not.77 Lee et al.78 found that pericardial and intrathoracic, but not abdominal VAT, were associated with incident AF after adjustment for age and sex, but no longer after adjustment for BMI. The same group had previously reported an association of pericardial, but not intrathoracic or abdominal, VAT with prevalent AF even after adjustment for other risk factors, including BMI.79 The causal role of specialized cardiac VAT depots in AF evolution is the focus of current research, given the accumulating epidemiological evidence consistently correlating cardiac fat with AF type, duration, and recurrence rates after ablation.
6.1. Composition and distribution of cardiac fat depots
Perivascular and cardiac fat are present in humans and other mammals to varying extents and expand in obesity roughly proportional with increases in abdominal VAT, and thus do not reflect ectopic fat deposition, but rather the expansion of normal anatomical structures. This process is modifiable, since weight loss due to dietary restriction or bariatric surgery also reduces the amount of fat around the heart.80 The nomenclature defining the distinct cardiac fat depots is inconsistent, with many terms often used interchangeably. In addition, studies of cardiac fat abundance and its relation to AF do not consistently clarify the specific anatomical and phenotypic nature of the fat under investigation, making the interpretation of how the different cardiac fat depots specifically contribute to AF difficult.
In general, pericardial adipose tissue refers to the VAT deposit over the heart and includes a combination of epicardial adipose tissue (EAT) and paracardial adipose tissue. Paracardial fat designates the total amount of fat on the external surface of the parietal pericardium, while EAT describes the fat located between the myocardium and visceral pericardium, which also penetrates the pericardial sac and has direct contact with the coronary arteries (Figure 1).23 Paracardial fat and EAT arise from different embryonic progenitors, and thus represent distinct types of fat.23 EAT originates from splanchnopleuric mesoderm, while paracardial fat is derived from the primitive thoracic mesenchyme.81 Furthermore, embryonic epicardial cells can be reprogrammed towards adipocytes contributing to fibro-fatty infiltration of the subepicardium of diseased atria.23 Moreover, epicardial progenitor cells can differentiate into myofibroblasts or adipocytes by atrial natriuretic peptides82 and angiotensin-II,83 respectively. This process is mostly absent in the normal adult heart but can be reactivated after myocardial infarction or severe injury.23
Incrementally greater pericardial fat volumes within the pericardial sac are determined by computed tomography (CT) in patients with paroxysmal and persistent forms of AF, and predict the long-term recurrence rate of AF after ablation.84 Of note, although the total EAT volume surrounding the heart is increased in patients with either AF or coronary artery disease, in AF patients EAT accumulation (within the pericardial sac) predominantly occurs around the atria, while ventricle-dominant EAT accumulation is observed primarily in those with coronary artery disease.85 This spatially distinct EAT accumulation (between the visceral pericardium and myocardium) has just been validated in a canine model of AF and concomitant obesity.57
The vast majority of data relating cardiac fat to AF, however, come from studies on EAT. This anatomically and metabolically distinct adipose tissue exhibits increased fatty acid synthesis and a unique inflammation-associated transcriptome. Specific transcriptomic signatures can be attributed to peri-atrial, peri-ventricular, and peri-coronary EAT, implying that each cardiac subdepot displays a unique quality of interaction with adjacent myocardial structures.86 EAT taken from the peri-ventricular area overexpressed genes implicated in Notch/p53, inflammation, ABC transporters, and glutathione metabolism. It could explain the higher sensitivity of peri-ventricular EAT to ‘browning’.23 EAT taken from peri-coronary site overexpressed genes implicated in proliferation, O–N glycan biosynthesis, and sphingolipid metabolism.86 The peri-atrial fat can be distinguished from other localizations by expressing genes implicated in oxidative phosphorylation, muscle contraction, and calcium signalling.86 Peri-ventricular and peri-atrial EAT mainly release proteins related to metabolism, cell growth, transport, and immune response.87 Furthermore, some proteins are found only in peri-atrial EAT including hormone-sensitive lipase and neutrophil proteins.87 These results might suggest a higher association between peri-atrial EAT and AF as the neutrophil to lymphocyte ratio has been proved to be a predictor of AF.88 Also, different patterns of adipokines89 and parasympathetic innervation87 are observed between distinct regions of EAT. The higher acetylcholinesterase activity in peri-atrial EAT points to greater atrial parasympathetic innervation.87
6.2. Relationship between EAT and AF
In the healthy state, EAT has a rather cardioprotective phenotype, can buffer against fatty acid toxicity, provide mechanical protection, dampen inflammatory cytokines and oxidative stress, and support thermogenesis.90 In obesity, altered transcription patterns and increased EAT thickness, particularly near the posterior LA and the atrioventricular groove,23 cause a phenotypical switch with detrimental paracrine consequences to the heart.90
Strong and graded associations have been identified between increasing EAT volume and AF severity, incidence of post-operative AF and the recurrence rates of AF after cardioversion and ablation.91 EAT accumulation (within the pericardial sac) coincides anatomically with regions of low voltage, reduced conduction velocity and electrocardiogram abnormalities, and these indices of atrial electrical and structural remodelling correlate strongly with EAT volume.92 EAT (within the pericardial sac) has also been localized to regions with the highest dominant frequency.93 Few possible explanations for these findings can be proposed (Figure 3).
6.2.1. Autonomic dysfunction
The intrinsic cardiac ganglionated plexi are embedded in the EAT and interact with the extrinsic sympathetic and parasympathetic nervous system.94 They represent the integrative centre of the autonomic nervous system within the heart and fine-tune cardiac electrophysiology. An increasing volume/thickness of EAT might modify the activity and sensitivity of ganglionated plexi by different mechanisms involving endocrine inflammatory mechanisms95 and lipotoxicity.96 Additionally, autonomic nervous system activation might change the endocrine activity of EAT in a feedback system.96 Several animal studies suggest the involvement of ganglionated plexi in the arrhythmogenic effects of risk factors commonly accompanying obesity in AF patients, e.g. sleep apnoea.97 Although ablation of the ganglionated plexi has shown promising results in several AF animal models, the data regarding catheter- or surgical-guided ganglionated plexi ablation as an adjunct approach to pulmonary vein isolation in patients with AF have been inconsistent.98 Thus, future mechanistic clinical and animal studies are required to dissect the precise interaction between EAT and the autonomic nervous system and to identify potential approaches to modulate interactions between EAT and the ganglionated plexi.
6.2.2. Inflammation and fibrosis
EAT from patients with AF exhibits strong inflammatory properties, showing higher 18-fluorodeoxyglucose- positron emission tomography (PET) signal intensity than EAT from patients without AF, or from SAT and thoracic VAT obtained from either group of patients.99 Another key feature of EAT is its highly intrusive nature. Although fatty intrusion into myocardial tissue occurs physiologically, fibro-fatty infiltration of atrial myocardium was recently linked with gap-junction remodelling and impaired impulse conduction.52 This may represent part of a feed-forward cycle, with a population of rate-responsive adipocytes within the atria undergoing transcriptional adaptation to high atrial rate and established AF.100
6.2.3. Lipotoxity
Cardiac VAT exhibits a relatively high lipolytic capacity, which principally governs the release of free fatty acids from triglyceride stores. Recent work highlights fatty acids as potential triggers of atrial inflammation, oxidative stress and AF.54 Clinically, fat depot expansion leads to an increase in circulating non-esterified fatty acids, and AF patients show higher fasting serum fatty free acid levels than controls.101 Patients with AF also show higher atrial expression of fatty acid-binding protein 3, which directs free fatty acid uptake and intracellular transport, together with autophagy-related genes.102 Elevated saturated fatty acids moreover distinguish patients with incident and recurrent AF from those who do not (re)develop AF,103 indicating a role in both onset and progression of AF. A direct causal link between free fatty acids and clinical AF has not yet been demonstrated; however, experimental studies have shown that certain fatty free acid species, such as stearic acid, may disrupt T-tubular structure and remodel membrane ion currents in cardiac myocytes.104 In the contexts of myocardial infarction and sudden cardiac death, the release of free fatty acids from triglyceride stores has been suggested to trigger proarrhythmic metabolic changes that drive ventricular arrhythmias.105 However, whether EAT in or around the atria is the primary source of these fatty acids requires further study.
7. Paracrine communication along the fat–heart axis
Adipocytokines are the major mode of signal transmission between adipose and myocardial compartments.23 The available data on potential contribution of selected adipokines to AF promotion are summarized in Supplementary material online, Table S1. Many adipokines relevant for AF pathophysiology are encapsulated in extracellular vesicles, a key component of the adipocyte secretome.106 These small exosomes arise from the budding of intracellular endosomal membranes and contain distinct cargoes of mediators.106 Extracellular vesicles derived from EAT of AF patients harbour greater amounts of proinflammatory and profibrotic cytokines and micro-RNAs than EAT from control patients.107 These cytokines from EAT of AF patients induce sustained re-entry in monolayers of human-induced pluripotent stem cell (hiPSC)-derived cardiac cells, while EAT-derived extracellular vesicles from control patients had no effect.107
7.1. Adipokines with a potential protective role in the evolution of AF
Adiponectin exerts marked insulinomimetic108 and anti-inflammatory actions, in part by promoting M2-macrophage phenotype transition.109 Human EAT and omental VAT show a lower abundance of adiponectin than SAT,110 consistent with a less benign phenotype, and adiponectin levels correlate inversely with LA size in obesity.111 Adiponectin may blunt interstitial fibrosis and cardiomyocyte hypertrophy, apoptosis, and capillary loss.112 It regulates cardiomyocyte Ca2+ homeostasis by altering sarco/endoplasmic reticulum Ca2+ adenosine triphosphatase-2a and phospholamban expression.113 In dogs, bolus microinjection of adiponectin into the major ganglionated plexi suppressed rapid atrial pacing-evoked neural activity, proinflammatory signalling and macrophage activation, and prevented the abbreviation of the atrial ERP.114 The human data consistently report larger EAT along with increased circulating levels of adiponectin in patients with AF, pointing to a compensatory increase of adiponectin in patients with AF to counteract cardiac remodelling and inflammation.
Omentin (intelectin-1) is a cardioprotective adipokine linked with metabolic control and insulin sensitivity. Omentin is highly expressed in stromal vascular cells including preadipocytes, and to lesser extent in adipocytes of VAT, including EAT, and its abundance is low in SAT.115 Omentin is reduced in both EAT and serum from patients with AF and this appears to correlate with the extent of atrial remodelling, with the lowest levels being observed in patients with permanent AF. Mechanistically, omentin appears to activate AMP-activated protein kinase signalling and suppress mitogenic kinases to prevent myocardial hypertrophy.116
Vaspin, also known as Serpin A12, belongs to serine protease inhibitor (serpin) family and is generally considered to be anti-inflammatory and to possess insulin-sensitizing and anorexigenic effects.117 Vaspin abundance is higher in paracardial fat compared with EAT or SAT, irrespective of rhythm status, with the presence of AF raising vaspin levels in EAT and SAT, while in paracardial fat, its increase is modest.
7.2. Adipocytokines with an inconclusive role in AF development
Resistin (adipocyte-secreted factor or inflammatory zone 3), belongs to the cysteine-rich secretory protein family. Adipocytes are the major source of secreted resistin in rodents; in humans, however, resistin is released mainly by macrophages.118 Resistin impairs cardiac glucose uptake and insulin resistance119 and induces Ca2+-handling remodelling, oxido-inflammatory stress,120 hypertrophy, and fibrosis.121 Clinically, increased plasma or EAT resistin levels have been reported to predict AF risk, particularly of new-onset AF after surgery. However, evidence for a direct proarrhythmic action of resistin is lacking. In isolated human atrial trabeculae, acute application of resistin causes positive inotropic and lusitropic effects, but does not increase the rate of spontaneous contractions.122
Visfatin (pre-B cell-colony enhancing factor-1 or nicotinamide phosphoribosyltransferase) is released from adipocytes, circulating monocytes, and adipose tissue macrophages.123 Recent evidence also indicates its expression in cardiomyocytes,124 but VAT depots including EAT are the major source of circulating visfatin.125 Visfatin promotes cardiac inflammation,126 metabolic derangement,123 hypertrophy, and fibrosis,124 which may explain the association between high plasma visfatin and post-operative AF as well recurrent AF. However, mice with genetic deletion of visfastin are more susceptible to high-fat diet (HFD)-induced AF, through impaired cardiomyocyte Ca2+-handling.70 Clearly, further research is required to define the precise role of visfatin in AF pathophysiology.
Apelin is an insulin-sensitive hormone that is overproduced in obesity,127 supports white adipocyte browning128 and suppresses inflammatory and fibrotic atrial remodelling.129 At the cellular level, apelin counteracts angiotensin-II-induced derangement of autophagy and connexin 43 expression.130 Apelin abundance is lower in right atrial appendages from AF patients compared with sinus rhythm controls and circulating levels correlate inversely with AF burden. Low plasma apelin strongly predicts both post-operative AF and AF recurrences in patients undergoing pulmonary vein isolation or electrical cardioversion. However, apelin stimulates the sarcolemmal Na+/H+ exchanger, leading to intracellular alkalinization with subsequent sensitization of cardiac myofilaments to Ca2+, rendering apelin one of the most potent positive inotropic stimuli.131 Moreover, it shortens the atrial action potential (AP) and refractoriness by modulating multiple ionic channels,132 dysregulates cellular contractility,133 and can augment cardiomyocyte Na+-currents.134 This suggests that the clinical association between apelin levels and AF may be more complex and requires further study and validation.
Leptin is the prototypical adipocyte-secreted adipokine, although it is also released from cardiomyocytes.135 Its primary physiological function is to suppress appetite and enhance energy expenditure, thereby regulating body weight.136 Studies correlating the circulating leptin levels with AF have yielded conflicting results. Similarly, data on the systemic and cellular consequences of elevated leptin levels are also inconsistent. Leptin enhances the production of profibrotic73 and proinflammatory137 factors and stimulates prohypertrophic signalling pathways.138 Yet, leptin can also attenuate cardiac contraction,139 support cardiac metabolism by increasing glucose and fatty acid uptake,140 and counteract lipotoxic cardiomyopathy.141 In isoprenaline-challenged LA rabbit cardiomyocytes, acute leptin exposure ameliorated Ca2+-handling abnormalities and reduced the incidence of delayed afterdepolarizations, along with an increase in Na+-current and decreases in ultra-rapid delayed-rectifier K+ and Na+/Ca2+ exchanger currents.142
The discrepancies between clinical observations and basic research findings might be explained by differences in cardiac expression of adipokines’ receptors between humans and animals. However, to the best of our knowledge, there are no systematic data assessing these interspecies differences.
8. Molecular basis of the association between adiposity and AF
Many experimental studies corroborate the clinical association between weight gain and the development of a proarrhythmic atrial substrate (Table 2). Obesity is linked to conduction abnormalities and both spontaneous and inducible AF. In sheep subjected to an high-fat diet (HFD), weight gain is paralleled by progressive LA enlargement and fibrosis together with increased fibro-fatty infiltration.51,53,55,56 Similar findings were obtained in a canine model, where HFD augments the vulnerability to AF, in close association with the upregulation of profibrotic genes and fibro-fatty infiltration originating from EAT.57
The murine HFD model has proven useful for identifying the molecular determinants of obesity-driven atrial remodelling underlying AF.50, 61,64–66 Atria of HFD-fed mice exhibit increased expression of cadherin-11, a fibroblast-activating factor that promotes atrial fibrosis, which is also upregulated in obese patients with concomitant AF.50 Reduced expression of Myeloid differentiation 1, a negative regulator of the toll-like receptor 4 pathway that is causally involved in obesity-associated cardiac remodelling, oxidative stress and impaired autophagy, was also noted in HFD-fed mice.65
Besides HFD-induced structural remodelling, obese mice also display functional remodelling of various ion currents that support AF inducibility.59,64 McCauley et al.64 found a strong reduction in protein levels of the Na+-channel subunit Nav1.5 concomitant with reduced peak-Na+−current in atrial cardiomyocytes of HFD-fed mice. These findings are consistent with reports in both AF patients143 and animal models of AF,144 in which reduced Na+-current was associated with slowed atrial conduction.144 Abnormal Na+-current can potentially be attributed to increased expression of protein kinase C isoforms α and δ,64 which show atrial upregulation in HFD-fed mice and are known to regulate both the expression and function of Nav1.5.145 Protein kinase Cδ is activated by dietary fat and phosphorylates Nav1.5, thereby reducing Na+-current.145 The HFD-related shortening of the AP could be a result of an upregulation of the ultra-rapid delayed-rectifier K+-current, which is conducted through Kv1.5 channels, as both were increased in HFD-fed mice.64 Conversely, the protein levels of Cav1.2 and the corresponding L-type Ca2+-currents were downregulated in HFD-fed mice, also potentially contributing to AP shortening. Thus, HFD-induced ion-channel remodelling could contribute to obesity-mediated AF arrhythmogenesis by shortening of atrial refractoriness along with conduction slowing, thereby promoting AF-maintaining re-entry.
The nucleotide-binding domain, leucine-rich-containing family, pyrin domains-containing-3 (NLRP3)-inflammasome, a multimeric signalling structure responsible for maturation of IL-1β and IL-18, has been recently implicated in obesity-driven AF initiation and progression. Yao et al.146 provided the first evidence for NLRP3-inflammasome expression in human, canine, and murine atrial cardiomyocytes, and established the causal link to AF. NLRP3-inflammasome activity is also higher in atrial samples of patients who go on to develop post-operative AF after cardiac surgery.147 NLRP3-inflammasome activity rises with increases in BMI of patients and in HFD-fed mice compared with controls,90 mainly through an accelerated ‘triggering’ of the NLRP3-system (assembly of the inflammasome complex with subsequent release of the proinflammatory mediators (caspase-1 and IL-1β). Mechanistically, the NLRP3-inflammasome promotes abnormal diastolic ryanodine receptor type-2-mediated sarcoplasmic reticulum Ca2+ release, which might cause AF-inducing triggered activity, and upregulates ultra-rapid delayed-rectifier K+-currents in cardiomyocytes of HFD-fed mice, thereby reducing atrial AP duration and refractoriness, which promote AF-maintaining re-entry,54 consistent with previous findings in obese sheep.64 NLRP3-inflammasome activity is augmented by leptin and visfastin, but suppressed by adiponectin, apelin, omentin, and vaspin (Supplementary material online, Table S2). However, the upstream mechanisms driving obesity-associated NLRP3-inflammasome activation are unknown and require elucidation in future work.
9. Reversibility of obesity-induced AF and remodelling
Significant weight loss achieved by bariatric surgery reduced the risk of new-onset AF by 29% in both prospective matched cohort Swedish Obese Study (mean weight loss ∼18%)148 and retrospective Bariatric surgery to aLleviate OCcurrence of Atrial Fibrillation Hospitalization-(BLOC-AF) study during 5.5- and 19-year follow-up, respectively.149 In addition, bariatric surgery of obese patients strongly reduced the recurrence rate of AF after ablation during a mean follow-up of 29 months.150 Similarly, weight reduction (median weight loss, 17%) achieved by a structured weight-management programme (low-calorie diet and low-intensity exercise) reduced the burden of symptoms, as well as the number and burden of AF episodes during 12-month follow-up.151 In the 5-year follow-up Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY) study, weight loss of >10% led to a six-fold greater probability of arrhythmia-free survival compared with patients with modest or no weight change.152 In a sub-analysis of the same study cohort (regReSsive Effect of weight-loss and risk factor modification on AF, REVERSE-AF trial), weight loss by >10% was associated with a conversion of persistent to paroxysmal AF in 88% of patients.153 Although weight loss following bariatric surgery and dietary interventions clearly support a causal link between obesity and AF, the precise molecular mechanisms by which weight loss reduces the susceptibility to AF and whether there are differences in the type of fat that is lost following different surgical, pharmacological, or dietary approaches remain unclear. The improvement in LA mechanical function observed in patients who underwent bariatric surgery151,154 and usual (diet) weight loss interventions152 point to reverse atrial molecular remodelling as the potential mechanism, a hypothesis that needs direct verification in experimental and clinical AF paradigms.
Recently, in an HFD-induced obesity sheep model, the reversibility of HFD-induced atrial remodelling by weight loss was clearly demonstrated. Atrial ERPs in obese sheep were shorter compared with lean controls, but this shortening could be reversed with a 30% weight reduction. Similar observations were made for the conduction velocity, which was normalized with weight reduction. Histological assessment revealed increased infiltration of epicardial fat and interstitial fibrosis in obese animals, which could be partially reversed with 30% weight loss. The area occupied by interstitial fibrosis was 7.5 ± 2.4 and 5.5 ± 1.5%, whereas the area infiltrated by epicardial fat was 9.3 ± 1.1 and 6.4 ± 1.1%, in the obese and 30% weight loss groups, respectively.53
10. Limitations in translating animal model findings to man
Mechanistic investigations of obesity-induced AF are typically conducted in HFD animal models. However, pure obesity models do not adequately reproduce the dynamics of an evolving disease state, varied nutrition, and pharmacological interventions in humans.155
Rodent strains show large variations in their level of resistance to obesity and its metabolic sequelae; this is further affected by age-dependent variations in body fat content, composition, and endocrine function.156 In addition, the typical HFD compositions containing on average 40–60% fat may not appropriately recapitulate the general human obesogenic Western diet that contains 30–40% dietary fat and is more complex than the formulated rodent HFDs. Prolonged overnight fasts and a nocturnal lifestyle also impact mouse metabolism, leading to altered insulin-stimulated glucose utilization.157 Changes in pericardial fat and fat infiltration in an obese mouse model induced by at least 20-week HFD are also not as prominent as in humans. Other obese mouse models with genetic modification (mainly targeting the leptin and its receptor)158 usually exhibit severe obesity and may exhibit ectopic fat depots. Finally, inflammatory responses to the HFD may mask other local and systemic effects of obesity and other potential contributors.
Large-animal models of obesity recapitulate key features of human obesity and are instrumental in studying the precise consequences of obesity for human health and disease.159 In addition, techniques for genetic manipulation of large animals are also emerging.159 However, large-animal models are very complex and require highly specialized equipment and facilities for experimentation and monitoring.159 In addition, the animals have a long-life cycle and their cellular and molecular signatures in the heart are not well characterized.159 Even when animals (for example, sheep) are kept obese for nearly 20% of their usual lifespan to mirror long-standing human obesity, the degree of substrate development may still not correlate with what is seen in clinical settings.53 Moreover, the optimal duration to create obesity/metabolic syndrome in large animals has not yet been standardized.159 Finally, sex and age affect the response to the obesogenic diet, with young and male animals being more sensitive to obesity-related comorbidities.159 Future research focusing on humans is, therefore, essential to unravel the key determinants of the obesity–AF axis.
11. Gaps in current knowledge and future perspectives
Since cardiac fat appears to have a more pronounced impact on AF burden than systemic obesity, cardiac fat-targeted interventions could constitute new options to manage AF. Key critical issues that need addressing in future work are summarized in Table 3. Controlled clinical trials targeting pericardial adipose tissue on or around the atria and fatty deposits in the form of fibro-fatty intrusion of EAT deep into the atrial myocardium are required to validate the role of cardiac fat in AF pathophysiology. Studies targeting VAT and SAT are also warranted to assess the contribution of particular fat depots to both weight and AF burden, and the response to reduction. Research comparing obese vs. non-obese patients with incident AF and simultaneous biomarker assessment could help to identify and validate novel biomarkers of adiposity-related AF. Future studies linking genetics and genomics to obesity and AF risk are clearly warranted.
Table 3.
Knowledge gaps and future directions
| Knowledge gap | Future directions |
|---|---|
| Does pericardial fat reduction prevent AF? | Controlled clinical trials targeting pericardial fat |
| What is the effect of different weight loss interventions on particular fat depots (subcutaneous vs. visceral) and the related changes in atrial arrhythmogenesis? | Characterization of changes in specific fat depots in clinical intervention trials comparing different surgical, pharmacological, or dietary weight loss approaches |
| What biomarkers typify adipose-related AF? | Controlled clinical trials of obese vs. non-obese patients with incident AF and simultaneous biomarker assessment |
| Is there a causal link between obesity and AF that can be revealed by genetic determinants? Do obesity and AF share common genetic determinants? | Controlled clinical trials using Mendelian Randomization techniques to link obesity to AF risk |
| What is the exact role of different fat depots and pericardial fat subdepots in AF pathogenesis? | Transgenic animal model with global or adipose tissue-specific deletion and cell culture-based studies |
| Does adipose graft transposition procedure prevent AF? | Animal model-based adipose graft transposition procedure |
| What is the impact of white-to-brown differentiation on AF vulnerability? | Animal model-based white-to-brown global differentiation |
| Which cell types contribute to NLRP3-inflammasome-mediated AF risk? | Animal model and cell culture-based studies |
| How to improve the quantification of pericardial fat subdepots? | Imaging techniques with advanced 3D reconstruction and analysis function |
| What are the mechanisms responsible for ectopic fat formation? | Animal model-based obesity with gradual increases in ectopic fat formation |
AF, atrial fibrillation; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domains-containing-3.
Fine-tuning of transgenic models, such as conditional global knockouts, or atrial vs. adipose tissue-specific deletion of culprit molecules including adipokines may help to delineate the direction and temporal nature of communication between atria and different body fat depots. One approach to dissect the crosstalk between distinct adipose tissue depots and the heart is adipose graft transposition, whereby autologous, vascularized pericardial fat is surgically placed over the myocardial scar.160 Direct evaluation of adipose graft (SAT or BAT) transposition procedure on AF-prone atrial myocardium is clearly warranted. Since hiPSC-derived cardiomyocytes are being increasingly used as models of heart disease,161 testing the potential proarrhythmic effects of individual adipocyte components on hiPSC-derived cardiomyocyte function might provide valuable insights into atrial cardiomyocyte–adipocyte interactions and their consequences for AF susceptibility.161 Conditionally immortalized human atrial cardiomyocytes constitute another novel source for in vitro AF models to study the causal link between obesity and AF.162 The beneficial effect of white-to-brown differentiation on cardiovascular risk needs further investigation in animal models undergoing cold temperature or increased physical activity exposition to assess the consequences for AF susceptibility. The discovery that obesity and enhanced activity of NLRP3-inflammasome are associated with both increased risk and severity of AF opens new avenues of research on underlying mechanisms and potential therapeutic interventions against AF.
Robust quantification of cardiac fat depots and cardiac inflammation remains challenging. Echocardiography quantifies EAT thickness on the ventricular free wall, but 3D volumetric assessment requires CT or cardiac magnetic resonance.163 The latter allows concurrent quantification of EAT and LA volumes,164 but its application is challenging in obese subjects.163 Concurrent metaiodobenzylguanidine scintigraphy and 18-fluorodeoxyglucose-PET/CT gives additional insight into sympathetic and inflammatory activities for improved individual risk prediction. An emerging approach to identify patients with high EAT volume is also provided by near-infrared spectroscopy.165 The constellation of innovative machine learning methods including artificial intelligence, in vivo/ex vivo imaging, and biopsy characterization may facilitate a comprehensive mapping of changes predictive of AF development and the substrate response to intervention, potentially enabling personalised risk projection and identification of innovative treatment options.166 Finally, additional research to understand the biology of cardiac adipose tissue, in particular, the mechanisms responsible for ectopic fat formation and its consequences for AF promotion is required in order to develop novel atrial fat-targeting anti-AF approaches.
12. Conclusion and outlook
Although substantial clinical and epidemiological evidence potentially link different adipose tissue depots with AF, the underlying mechanisms remain poorly understood. Thus, extensive studies are needed to dissect the exact role of different subdepots of pericardial fat and other adipose tissue sources such as VAT and SAT in AF pathogenesis. Genetic studies could be also instrumental to uncover the precise mechanisms linking pericardial fat remodelling to AF promotion. Improved imaging-based cardiac fat quantification should allow a better stratification of AF patients ultimately leading to novel therapeutic approaches.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Contributor Information
Monika Gawałko, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany; 1st Department of Cardiology, Medical University of Warsaw, Banacha 1A, 02-197 Warsaw, Poland; Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Arnela Saljic, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Na Li, Department of Medicine (Section of Cardiovascular Research), Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA; Department of Molecular Physiology and Biophysics, Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA; Cardiovascular Research Institute, Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA.
Issam Abu-Taha, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany.
Thomas Jespersen, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Dominik Linz, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark; Centre for Heart Rhythm Disorders, Royal Adelaide Hospital, University of Adelaide, Port Road, SA 5000 Adelaide, Australia; Department of Cardiology, Radboud University Medical Centre, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, The Netherlands.
Stanley Nattel, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany; Medicine and Research Center, Montréal Heart Institute and University de Montréal, 3655 Promenade Sir William Osler, Montreal, QC H3G 1Y6, Canada; IHU LIRYC Institute, Avenue du Haut Lévêque, 33600 Pessac, Bordeaux, France.
Jordi Heijman, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Anke Fender, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany.
Dobromir Dobrev, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany; Department of Molecular Physiology and Biophysics, Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA; Medicine and Research Center, Montréal Heart Institute and University de Montréal, 3655 Promenade Sir William Osler, Montreal, QC H3G 1Y6, Canada.
Funding
Canadian Institutes of Health Research (148401 to S.N.) and Heart and Stroke Foundation of Canada (18-0022032 to S.N.). National Institutes of Health (R01HL136389, R01HL147108 to N.L., R01HL136389, R01HL131517, R01HL089598, and R01HL163277 to D.D.), the German Research Foundation (DFG, Do 769/4-1 to D.D.), and the Horizon 2020 programme of the European Union (large-scale integrative project Machine Learning Artificial Intelligence Early Detection Stroke Atrial Fibrillation, MAESTRIA, No. 865286 to D.D.
References
- 1. Maffetone PB, Rivera-Dominguez I, Laursen PB. Overfat and underfat: new terms and definitions long overdue. Front Public Health 2016;4:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council . Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;143:e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol 2018;221:jeb162958. [DOI] [PubMed] [Google Scholar]
- 4. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018;155:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omran F, Christian M. Inflammatory signaling and brown fat activity. Front Endocrinol(Lausanne) 2020;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He Y, Ma N, Tang M, Jiang ZL, Liu H, Mei J. The differentiation of beige adipocyte in pericardial and epicardial adipose tissues induces atrial fibrillation development. Eur Rev Med Pharmacol Sci 2017;21:4398–4405. [PubMed] [Google Scholar]
- 7. Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, Cecchini MP, Kershaw EE, Barbatelli G, Haemmerle G, Zechner R, Cinti S. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 2018;59:784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oh JE, Cho YM, Kwak SN, Kim JH, Lee KW, Jung H, Jeong SW, Kwon OJ. Inhibition of mouse brown adipocyte differentiation by second-generation antipsychotics. Exp Mol Med 2012;44:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rozenblit-Susan S, Chapnik N, Froy O. Serotonin prevents differentiation of brown adipocytes by interfering with their clock. Obesity(Silver Spring) 2019;27:2018–2024. [DOI] [PubMed] [Google Scholar]
- 10. Kong X, Yu J, Bi J, Qi H, Di W, Wu L, Wang L, Zha J, Lv S, Zhang F, Li Y, Hu F, Liu F, Zhou H, Liu J, Ding G. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes 2015;64:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheung WW, Ding W, Hoffman HM, Wang Z, Hao S, Zheng R, Gonzalez A, Zhan JY, Zhou P, Li S, Esparza MC, Lieber RL, Mak RH. Vitamin D ameliorates adipose browning in chronic kidney disease cachexia. Sci Rep 2020;10:14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heyde I, Begemann K, Oster H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 2021;162:bqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang B, Zhang F, Zhang H, Wang Z, Ma YN, Zhu MJ, Du M. Alcohol intake aggravates adipose browning and muscle atrophy in cancer-associated cachexia. Oncotarget 2017;8:100411–100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verty AN, Evetts MJ, Crouch GJ, McGregor IS, Stefanidis A, Oldfield BJ. The cannabinoid receptor agonist THC attenuates weight loss in a rodent model of activity-based anorexia. Neuropsychopharmacology 2011;36:1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TC, Lucassen EA, Sips HC, Chatzispyrou IA, Houtkooper RH, Meijer JH, Coomans CP, Biermasz NR, Rensen PC. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci USA 2015;112:6748–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee RA, Harris CA, Wang JC. Glucocorticoid receptor and adipocyte biology. Nucl Receptor Res 2018;5:101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barclay JL, Agada H, Jang C, Ward M, Wetzig N, Ho KK. Effects of glucocorticoids on human brown adipocytes. J Endocrinol 2015;224:139–147. [DOI] [PubMed] [Google Scholar]
- 18. Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN, van Beek EJ, Morton NM, Walker BR, Stimson RH. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 2016;24:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 2007;282:28465–28473. [DOI] [PubMed] [Google Scholar]
- 20. Parray HA, Yun JW. Cannabidiol promotes browning in 3T3-L1 adipocytes. Mol Cell Biochem 2016;416:131–139. [DOI] [PubMed] [Google Scholar]
- 21. Vidal H. Gene expression in visceral and subcutaneous adipose tissues. Ann Med 2001;33:547–555. [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–18. [DOI] [PubMed] [Google Scholar]
- 23. Gaborit B, Sengenes C, Ancel P, Jacquier A, Dutour A. Role of epicardial adipose tissue in health and disease: a matter of fat? Compr Physiol 2017;7:1051–1082. [DOI] [PubMed] [Google Scholar]
- 24. Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 2013;154:2992–3000. [DOI] [PubMed] [Google Scholar]
- 25. Cohen P, Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol 2021;22:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez N, Moreno-Villegas Z, Gonzalez-Bris A, Egido J, Lorenzo O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol 2017;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan S, Chan YT, Revelo XS, Winer DA. The immune landscape of visceral adipose tissue during obesity and aging. Front Endocrinol(Lausanne) 2020;11:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dommel S, Bluher M. Does C-C Motif Chemokine Ligand 2(CCL2) link obesity to a pro-inflammatory state? Int J Mol Sci 2021;22:1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care 2011;14:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giron-Ulloa A, Gonzalez-Dominguez E, Klimek RS, Patino-Martinez E, Vargas-Ayala G, Segovia-Gamboa NC, Campos-Pena V, Rodriguez-Arellano ME, Meraz-Rios MA, Campos-Campos SF, Sanchez-Torres C. Specific macrophage subsets accumulate in human subcutaneous and omental fat depots during obesity. Immunol Cell Biol 2020;98:868–882. [DOI] [PubMed] [Google Scholar]
- 32. Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009;27:2563–2570. [DOI] [PubMed] [Google Scholar]
- 33. Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation 2011;124:e837–e841. [DOI] [PubMed] [Google Scholar]
- 34. Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts 2017;10:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nattel S. Atrial fibrillation and body composition: is it fat or lean that ultimately determines the risk? J Am Coll Cardiol 2017;69:2498–2501. [DOI] [PubMed] [Google Scholar]
- 36. Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr 2002;75:683–688. [DOI] [PubMed] [Google Scholar]
- 37. Heijman J, Guichard JB, Dobrev D, Nattel S. Translational challenges in atrial fibrillation. Circ Res 2018;122:752–773. [DOI] [PubMed] [Google Scholar]
- 38. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm 2017;14:e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 41. Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res 2017;120:1969–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asad Z, Abbas M, Javed I, Korantzopoulos P, Stavrakis S. Obesity is associated with incident atrial fibrillation independent of gender: a meta-analysis. J Cardiovasc Electrophysiol 2018;29:725–732. [DOI] [PubMed] [Google Scholar]
- 43. Nystrom PK, Carlsson AC, Leander K, de Faire U, Hellenius ML, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS One 2015;10:e0127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J 2008;29:2227–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, Twomey D, Ganesan AN, Rangnekar G, Roberts-Thomson KC, Lau DH, Sanders P. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626, 603 individuals in 51 studies. JACC Clin Electrophysiol 2015;1:139–152. [DOI] [PubMed] [Google Scholar]
- 46. Chen W, Yao D, Yan H, Wang M, Pan Y. Genetically predicted childhood obesity and adult atrial fibrillation: a mendelian randomization study. Nutr Metab Cardiovasc Dis 2021;32:1019–1026. [DOI] [PubMed] [Google Scholar]
- 47. Chatterjee NA, Giulianini F, Geelhoed B, Lunetta KL, Misialek JR, Niemeijer MN, Rienstra M, Rose LM, Smith AV, Arking DE, Ellinor PT, Heeringa J, Lin H, Lubitz SA, Soliman EZ, Verweij N, Alonso A, Benjamin EJ, Gudnason V, Stricker BHC, Van Der Harst P, Chasman DI, Albert CM. Genetic obesity and the risk of atrial fibrillation: causal estimates from mendelian randomization. Circulation 2017;135:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schram-Serban C, Heida A, Roos-Serote MC, Knops P, Kik C, Brundel B, Bogers A, de Groot NMS. Heterogeneity in conduction underlies obesity-related atrial fibrillation vulnerability. Circ Arrhythm Electrophysiol 2020;13:e008161. [DOI] [PubMed] [Google Scholar]
- 49. Munger TM, Dong YX, Masaki M, Oh JK, Mankad SV, Borlaug BA, Asirvatham SJ, Shen WK, Lee HC, Bielinski SJ, Hodge DO, Herges RM, Buescher TL, Wu JH, Ma C, Zhang Y, Chen PS, Packer DL, Cha YM. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol 2012;60:851–860. [DOI] [PubMed] [Google Scholar]
- 50. Fang G, Cao W, Chen L, Song S, Li Y, Yuan J, Fei Y, Ge Z, Chen Y, Zhou L, Xiao Y, Wan Y, Wang Y, Wang Q. Cadherin-11 deficiency mitigates high-fat diet-induced inflammatory atrial remodeling and vulnerability to atrial fibrillation. J Cell Physiol 2021;236:5725–5741. [DOI] [PubMed] [Google Scholar]
- 51. Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, Claus P, LePrince P, Nicoletti A, Jalife J, Wolke C, Lendeckel U, Jais P, Willems R, Hatem SN. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J 2017;38:53–61. [DOI] [PubMed] [Google Scholar]
- 52. Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, Lui E, Sim CB, Larobina M, O’Keefe M, Goldblatt J, Royse A, Lee G, Porrello ER, Watt MJ, Kistler PM, Sanders P, Delbridge LMD, Kalman JM. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol 2020;76:1197–1211. [DOI] [PubMed] [Google Scholar]
- 53. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Wood JPM, Manavis J, Samuel CS, Patel KP, Finnie JW, Alasady M, Kalman JM, Sanders P. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 2021;7:630–641. [DOI] [PubMed] [Google Scholar]
- 54. Scott L Jr, Fender AC, Saljic A, Li L, Chen X, Wang X, Linz D, Lang J, Hohl M, Twomey D, Pham TT, Diaz-Lankenau R, Chelu MG, Kamler M, Entman ML, Taffet GE, Sanders P, Dobrev D, Li N. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res 2021;117:1746–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 56. Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, Sanders P. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 57. Otsuka N, Okumura Y, Arai M, Kurokawa S, Nagashima K, Watanabe R, Wakamatsu Y, Yagyu S, Ohkubo K, Nakai T, Hao H, Takahashi R, Taniguchi Y, Li Y. Effect of obesity and epicardial fat/fatty infiltration on electrical and structural remodeling associated with atrial fibrillation in a novel canine model of obesity and atrial fibrillation: a comparative study. J Cardiovasc Electrophysiol 2021;32:889–899. [DOI] [PubMed] [Google Scholar]
- 58. Okumura Y, Watanabe I, Nagashima K, Sonoda K, Sasaki N, Kogawa R, Takahashi K, Iso K, Ohkubo K, Nakai T, Takahashi R, Taniguchi Y, Mitsumata M, Nikaido M, Hirayama A. Effects of a high-fat diet on the electrical properties of porcine atria. J Arrhythm 2015;31:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin YK, Chen YC, Chen JH, Chen SA, Chen YJ. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic Res Cardiol 2012;107:293. [DOI] [PubMed] [Google Scholar]
- 60. Martinez-Mateu L, Saiz J, Aromolaran AS. Differential modulation of IK and ICa, L channels in high-fat diet-induced obese guinea pig atria. Front Physiol 2019;10:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bernasochi GB, Boon WC, Curl CL, Varma U, Pepe S, Tare M, Parry LJ, Dimitriadis E, Harrap SB, Nalliah CJ, Kalman JM, Delbridge LMD, Bell JR. Pericardial adipose and aromatase: a new translational target for aging, obesity and arrhythmogenesis? J Mol Cell Cardiol 2017;111:96–101. [DOI] [PubMed] [Google Scholar]
- 62. Hohl M, Lau DH, Muller A, Elliott AD, Linz B, Mahajan R, Hendriks JML, Bohm M, Schotten U, Sanders P, Linz D. Concomitant obesity and metabolic syndrome add to the atrial arrhythmogenic phenotype in male hypertensive rats. J Am Heart Assoc 2017;6:e006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McCully BH, Hasan W, Streiff CT, Houle JC, Woodward WR, Giraud GD, Brooks VL, Habecker BA. Sympathetic cardiac hyperinnervation and atrial autonomic imbalance in diet-induced obesity promote cardiac arrhythmias. Am J Physiol Heart Circ Physiol 2013;305:H1530–H1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McCauley MD, Hong L, Sridhar A, Menon A, Perike S, Zhang M, da Silva IB, Yan J, Bonini MG, Ai X, Rehman J, Darbar D. Ion channel and structural remodeling in obesity-mediated atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shuai W, Kong B, Fu H, Shen C, Jiang X, Huang H. MD1 deficiency promotes inflammatory atrial remodelling induced by high-fat diets. Can J Cardiol 2019;35:208–216. [DOI] [PubMed] [Google Scholar]
- 66. Kondo H, Abe I, Gotoh K, Fukui A, Takanari H, Ishii Y, Ikebe Y, Kira S, Oniki T, Saito S, Aoki K, Tanino T, Mitarai K, Kawano K, Miyoshi M, Fujinami M, Yoshimura S, Ayabe R, Okada N, Nagano Y, Akioka H, Shinohara T, Akiyoshi K, Masaki T, Teshima Y, Yufu K, Nakagawa M, Takahashi N. Interleukin 10 treatment ameliorates high-fat diet-induced inflammatory atrial remodeling and fibrillation. Circ Arrhythm Electrophysiol 2018;11:e006040. [DOI] [PubMed] [Google Scholar]
- 67. Zhang Y, Yang S, Fu J, Liu A, Liu D, Cao S. Inhibition of endoplasmic reticulum stress prevents high-fat diet mediated atrial fibrosis and fibrillation. J Cell Mol Med 2020;24:13660–13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang F, Hartnett S, Sample A, Schnack S, Li Y. High fat diet induced alterations of atrial electrical activities in mice. Am J Cardiovasc Dis 2016;6:1–9. [PMC free article] [PubMed] [Google Scholar]
- 69. Fu Y, Jiang T, Sun H, Li T, Gao F, Fan B, Li X, Qin X, Zheng Q. Necroptosis is required for atrial fibrillation and involved in aerobic exercise-conferred cardioprotection. J Cell Mol Med 2021;25:8363–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Feng D, Xu D, Murakoshi N, Tajiri K, Qin R, Yonebayashi S, Okabe Y, Li S, Yuan Z, Aonuma K, Ieda M. Nicotinamide phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide(NAD) axis suppresses atrial fibrillation by modulating the calcium handling pathway. Int J Mol Sci 2020;21:4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ornelas-Loredo A, Kany S, Abraham V, Alzahrani Z, Darbar FA, Sridhar A, Ahmed M, Alamar I, Menon A, Zhang M, Chen Y, Hong L, Konda S, Darbar D. Association between obesity-mediated atrial fibrillation and therapy with sodium channel blocker antiarrhythmic drugs. JAMA Cardiol 2020;5:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maria Z, Campolo AR, Scherlag BJ, Ritchey JW, Lacombe VA. Dysregulation of insulin-sensitive glucose transporters during insulin resistance-induced atrial fibrillation. Biochim Biophys Acta Mol Basis Dis 2018;1864:987–996. [DOI] [PubMed] [Google Scholar]
- 73. Fukui A, Ikebe-Ebata Y, Kondo H, Saito S, Aoki K, Fukunaga N, Shinohara T, Masaki T, Teshima Y, Takahashi N. Hyperleptinemia exacerbates high-fat diet-mediated atrial fibrosis and fibrillation. J Cardiovasc Electrophysiol 2017;28:702–710. [DOI] [PubMed] [Google Scholar]
- 74. Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev 2019;8:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging 2017;10:e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Katulska K, Milewska A, Wykretowicz M, Krauze T, Przymuszala D, Piskorski J, Stajgis M, Guzik P, Wysocki H, Wykretowicz A. Arterial stiffness, body fat compartments, central hemodynamics, renal function and left atrial size. Scand J Clin Lab Invest 2013;73:563–568. [DOI] [PubMed] [Google Scholar]
- 77. Aronis KN, Wang N, Phillips CL, Benjamin EJ, Marcus GM, Newman AB, Rodondi N, Satterfield S, Harris TB, Magnani JW; Health ABC Study . Associations of obesity and body fat distribution with incident atrial fibrillation in the biracial health aging and body composition cohort of older adults. Am Heart J 2015;170:498–505.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee JJ, Yin X, Hoffmann U, Fox CS, Benjamin EJ. Relation of pericardial fat, intrathoracic fat, and abdominal visceral fat with incident atrial fibrillation (from the Framingham Heart Study). Am J Cardiol 2016;118:1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the framingham heart study. Circ Arrhythm Electrophysiol 2010;3:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev 2015;16:406–415. [DOI] [PubMed] [Google Scholar]
- 81. Wu Y, Zhang A, Hamilton DJ, Deng T. Epicardial fat in the maintenance of cardiovascular health. Methodist Debakey Cardiovasc J 2017;13:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Suffee N, Moore-Morris T, Farahmand P, Rucker-Martin C, Dilanian G, Fradet M, Sawaki D, Derumeaux G, LePrince P, Clement K, Dugail I, Puceat M, Hatem SN. Atrial natriuretic peptide regulates adipose tissue accumulation in adult atria. Proc Natl Acad Sci USA 2017;114:E771–E780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Suffee N, Moore-Morris T, Jagla B, Mougenot N, Dilanian G, Berthet M, Proukhnitzky J, Le Prince P, Tregouet DA, Puceat M, Hatem SN. Reactivation of the epicardium at the origin of myocardial fibro-fatty infiltration during the atrial cardiomyopathy. Circ Res 2020;126:1330–1342. [DOI] [PubMed] [Google Scholar]
- 84. Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol 2010;56:784–788. [DOI] [PubMed] [Google Scholar]
- 85. Hasebe H, Yoshida K, Nogami A, Ieda M. Difference in epicardial adipose tissue distribution between paroxysmal atrial fibrillation and coronary artery disease. Heart Vessels 2020;35:1070–1078. [DOI] [PubMed] [Google Scholar]
- 86. Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, Clement K. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res 2015;108:62–73. [DOI] [PubMed] [Google Scholar]
- 87. Couselo-Seijas M, Lopez-Canoa JN, Agra-Bermejo RM, Diaz-Rodriguez E, Fernandez AL, Martinez-Cereijo JM, Duran-Munoz D, Bravo SB, Velo A, Gonzalez-Melchor L, Fernandez-Lopez XA, Martinez-Sande JL, Garcia-Seara J, Gonzalez-Juanatey JR, Rodriguez-Manero M, Eiras S. Cholinergic activity regulates the secretome of epicardial adipose tissue: association with atrial fibrillation. J Cell Physiol 2019;234:10512–10522. [DOI] [PubMed] [Google Scholar]
- 88. Shao Q, Chen K, Rha SW, Lim HE, Li G, Liu T. Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res 2015;46:199–206. [DOI] [PubMed] [Google Scholar]
- 89. Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb 2010;17:115–130. [DOI] [PubMed] [Google Scholar]
- 90. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 2015;11:363–371. [DOI] [PubMed] [Google Scholar]
- 91. Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, Pathak RK, Wong DT, Selvanayagam JB, Sanders P, Clarke R. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9:e004378. [DOI] [PubMed] [Google Scholar]
- 92. Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, Carbone A, Teo K, Agbaedeng T, Linz D, de Groot JR, Kalman JM, Lau DH, Sanders P. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol 2018;4:1529–1540. [DOI] [PubMed] [Google Scholar]
- 93. Maeda M, Oba K, Yamaguchi S, Arasaki O, Sata M, Masuzaki H, Shimabukuro M. Usefulness of epicardial adipose tissue volume to predict recurrent atrial fibrillation after radiofrequency catheter ablation. Am J Cardiol 2018;122:1694–1700. [DOI] [PubMed] [Google Scholar]
- 94. Linz D, Elliott AD, Hohl M, Malik V, Schotten U, Dobrev D, Nattel S, Bohm M, Floras J, Lau DH, Sanders P. Role of autonomic nervous system in atrial fibrillation. Int J Cardiol 2019;287:181–188. [DOI] [PubMed] [Google Scholar]
- 95. Yu L, Meng G, Huang B, Zhou X, Stavrakis S, Wang M, Li X, Zhou L, Wang Y, Wang M, Wang Z, Deng J, Po SS, Jiang H. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol 2018;255:92–98. [DOI] [PubMed] [Google Scholar]
- 96. Couselo-Seijas M, Rodriguez-Manero M, Gonzalez-Juanatey JR, Eiras S. Updates on epicardial adipose tissue mechanisms on atrial fibrillation. Obes Rev 2021;22:e13277. [DOI] [PubMed] [Google Scholar]
- 97. Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Levy P, Kalman JM, Sanders P. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol 2018;3:532–540. [DOI] [PubMed] [Google Scholar]
- 98. Yan F, Zhao S, Wu W, Xie Z, Guo Q. Different effects of additional ganglion plexus ablation on catheter and surgical ablation for atrial fibrillation: a systemic review and meta-analysis. J Cardiovasc Electrophysiol 2019;30:3039–3049. [DOI] [PubMed] [Google Scholar]
- 99. Mazurek T, Kiliszek M, Kobylecka M, Skubisz-Gluchowska J, Kochman J, Filipiak K, Krolicki L, Opolski G. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am J Cardiol 2014;113:1505–1508. [DOI] [PubMed] [Google Scholar]
- 100. Chilukoti RK, Giese A, Malenke W, Homuth G, Bukowska A, Goette A, Felix SB, Kanaan J, Wollert HG, Evert K, Verheule S, Jais P, Hatem SN, Lendeckel U, Wolke C. Atrial fibrillation and rapid acute pacing regulate adipocyte/adipositas-related gene expression in the atria. Int J Cardiol 2015;187:604–613. [DOI] [PubMed] [Google Scholar]
- 101. Khawaja O, Bartz TM, Ix JH, Heckbert SR, Kizer JR, Zieman SJ, Mukamal KJ, Tracy RP, Siscovick DS, Djousse L. Plasma free fatty acids and risk of atrial fibrillation (from the cardiovascular health study). Am J Cardiol 2012;110:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shingu Y, Takada S, Yokota T, Shirakawa R, Yamada A, Ooka T, Katoh H, Kubota S, Matsui Y. Correlation between increased atrial expression of genes related to fatty acid metabolism and autophagy in patients with chronic atrial fibrillation. PLoS ONE 2020;15:e0224713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pellegrini CN, Buzkova P, Lichtenstein AH, Matthan NR, Ix JH, Siscovick DS, Heckbert SR, Tracy RP, Mukamal KJ, Djousse L, Kizer JR. Individual non-esterified fatty acids and incident atrial fibrillation late in life. Heart 2021;107:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. O’Connell RP, Musa H, Gomez MS, Avula UM, Herron TJ, Kalifa J, Anumonwo JM. Free fatty acid effects on the atrial myocardium: membrane ionic currents are remodeled by the disruption of T-tubular architecture. PLoS ONE 2015;10:e0133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Oliver MF. Fatty acids and the risk of death during acute myocardial ischaemia. Clin Sci (Lond) 2015;128:349–355. [DOI] [PubMed] [Google Scholar]
- 106. Gomez-Serrano M, Ponath V, Preusser C, Pogge von Strandmann E. Beyond the extracellular vesicles: technical hurdles, achieved goals and current challenges when working on adipose cells. Int J Mol Sci 2021;22:3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, Ziv T, Lewis N, Schary Y, Levin-Kotler LP, Volvovitch D, Zuroff EM, Amunts S, Regev-Rudzki N, Sternik L, Raanani E, Gepstein L, Leor J. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation 2021;143:2475–2493. [DOI] [PubMed] [Google Scholar]
- 108. Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev 2005;6:13–21. [DOI] [PubMed] [Google Scholar]
- 109. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 2010;285:6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ybarra J, Resmini E, Planas F, Navarro-Lopez F, Webb S, Pou JM, Santos A, Ballesta-Lopez C. Relationship between adiponectin and left atrium size in uncomplicated obese patients: adiponectin, a link between fat and heart. Obes Surg 2009;19:1324–1332. [DOI] [PubMed] [Google Scholar]
- 112. Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie 2012;94:2143–2149. [DOI] [PubMed] [Google Scholar]
- 113. Tanaka K, Wilson RM, Essick EE, Duffen JL, Scherer PE, Ouchi N, Sam F. Effects of adiponectin on calcium-handling proteins in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhou Z, Li S, Sheng X, Liu Z, Lai Y, Wang M, Wang Z, Zhou L, Meng G, Chen H, Zhou H, Zhou X, Jiang H. Interactions between metabolism regulator adiponectin and intrinsic cardiac autonomic nervous system: a potential treatment target for atrial fibrillation. Int J Cardiol 2020;302:59–66. [DOI] [PubMed] [Google Scholar]
- 115. Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, Carter RA, Tichansky DS, Madan AK. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32:810–815. [DOI] [PubMed] [Google Scholar]
- 116. Matsuo K, Shibata R, Ohashi K, Kambara T, Uemura Y, Hiramatsu-Ito M, Enomoto T, Yuasa D, Joki Y, Ito M, Hayakawa S, Ogawa H, Kihara S, Murohara T, Ouchi N. Omentin functions to attenuate cardiac hypertrophic response. J Mol Cell Cardiol 2015;79:195–202. [DOI] [PubMed] [Google Scholar]
- 117. Dimova R, Tankova T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed Res Int 2015;2015:823481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kyrou I, Mattu HS, Chatha K, Randeva HS. Fat hormones, adipokines. Endocrinol Heart Health Dis 2017;7:167–205. [Google Scholar]
- 119. Graveleau C, Zaha VG, Mohajer A, Banerjee RR, Dudley-Rucker N, Steppan CM, Rajala MW, Scherer PE, Ahima RS, Lazar MA, Abel ED. Mouse and human resistins impair glucose transport in primary mouse cardiomyocytes, and oligomerization is required for this biological action. J Biol Chem 2005;280:31679–31685. [DOI] [PubMed] [Google Scholar]
- 120. Chemaly ER, Hadri L, Zhang S, Kim M, Kohlbrenner E, Sheng J, Liang L, Chen J, K-Raman P, Hajjar RJ, Lebeche D. Long-term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J Mol Cell Cardiol 2011;51:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol 2008;45:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Aitken-Buck HM, Babakr AA, Fomison-Nurse IC, van Hout I, Davis PJ, Bunton RW, Williams MJA, Coffey S, Jones PP, Lamberts RR. Inotropic and lusitropic, but not arrhythmogenic, effects of adipocytokine resistin on human atrial myocardium. Am J Physiol Endocrinol Metab 2020;319:E540–E547. [DOI] [PubMed] [Google Scholar]
- 123. Romacho T, Sanchez-Ferrer CF, Peiro C. Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm 2013;2013:946427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pillai VB, Sundaresan NR, Kim G, Samant S, Moreno-Vinasco L, Garcia JG, Gupta MP. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol 2013;304:H415–H426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. [DOI] [PubMed] [Google Scholar]
- 126. Yu XY, Qiao SB, Guan HS, Liu SW, Meng XM. Effects of visfatin on proliferation and collagen synthesis in rat cardiac fibroblasts. Horm Metab Res 2010;42:507–513. [DOI] [PubMed] [Google Scholar]
- 127. Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, Audigier Y, Saulnier-Blache JS, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005;146:1764–1771. [DOI] [PubMed] [Google Scholar]
- 128. Than A, He HL, Chua SH, Xu D, Sun L, Leow MK, Chen P. Apelin enhances brown adipogenesis and browning of white adipocytes. J Biol Chem 2015;290:14679–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lv W, Zhang L, Cheng X, Wang H, Qin W, Zhou X, Tang B. Apelin inhibits Angiotensin II-induced atrial fibrosis and atrial fibrillation via TGF-beta1/Smad2/alpha-SMA pathway. Front Physiol 2020;11:583570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chen Y, Qiao X, Zhang L, Li X, Liu Q. Apelin-13 regulates Angiotensin II-induced Cx43 downregulation and autophagy via the AMPK/mTOR signaling pathway in HL-1 cells. Physiol Res 2020;69:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, Marczin N, Szokodi I, Yacoub MH, Terracciano CM. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun 2007;357:889–895. [DOI] [PubMed] [Google Scholar]
- 132. Cheng CC, Weerateerangkul P, Lu YY, Chen YC, Lin YK, Chen SA, Chen YJ. Apelin regulates the electrophysiological characteristics of atrial myocytes. Eur J Clin Invest 2013;43:34–40. [DOI] [PubMed] [Google Scholar]
- 133. Chamberland C, Barajas-Martinez H, Haufe V, Fecteau MH, Delabre JF, Burashnikov A, Antzelevitch C, Lesur O, Chraibi A, Sarret P, Dumaine R. Modulation of canine cardiac sodium current by Apelin. J Mol Cell Cardiol 2010;48:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kim YM, Lakin R, Zhang H, Liu J, Sachedina A, Singh M, Wilson E, Perez M, Verma S, Quertermous T, Olgin J, Backx PH, Ashley EA. Apelin increases atrial conduction velocity, refractoriness, and prevents inducibility of atrial fibrillation. JCI Insight 2020;5:e126525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. McGaffin KR, Moravec CS, McTiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circ Heart Fail 2009;2:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Friedman JM. Leptin and the regulation of body weight. Keio J Med 2011;60:1–9. [DOI] [PubMed] [Google Scholar]
- 137. Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J 2001;15:2565–2571. [DOI] [PubMed] [Google Scholar]
- 138. Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res 2003;93:277–279. [DOI] [PubMed] [Google Scholar]
- 139. Nickola MW, Wold LE, Colligan PB, Wang GJ, Samson WK, Ren J. Leptin attenuates cardiac contraction in rat ventricular myocytes. Role of NO. Hypertension 2000;36:501–505. [DOI] [PubMed] [Google Scholar]
- 140. Palanivel R, Eguchi M, Shuralyova I, Coe I, Sweeney G. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism 2006;55:1067–1075. [DOI] [PubMed] [Google Scholar]
- 141. Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci USA 2004;101:13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lin YK, Chen YC, Huang JH, Lin YJ, Huang SS, Chen SA, Chen YJ. Leptin modulates electrophysiological characteristics and isoproterenol-induced arrhythmogenesis in atrial myocytes. J Biomed Sci 2013;20:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol 2010;55:2330–2342. [DOI] [PubMed] [Google Scholar]
- 144. Gaspo R, Bosch RF, Bou-Abboud E, Nattel S. Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ Res 1997;81:1045–1052. [DOI] [PubMed] [Google Scholar]
- 145. Liu M, Shi G, Yang KC, Gu L, Kanthasamy AG, Anantharam V, Dudley SC Jr. Role of protein kinase C in metabolic regulation of the cardiac Na(+) channel. Heart Rhythm 2017;14:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Muller FU, El-Armouche A, Tony Eissa N, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Kunzel S, El-Armouche A, Reichenspurner H, Kamler M, Nikolaev V, Ravens U, Li N, Nattel S, Wehrens XHT, Dobrev D. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res 2020;127:1036–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjostrom L, Karason K. Bariatric surgery and the risk of new-onset atrial fibrillation in swedish obese subjects. J Am Coll Cardiol 2016;68:2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Srivatsa UN, Malhotra P, Zhang XJ, Beri N, Xing G, Brunson A, Ali M, Fan D, Pezeshkian N, Chiamvimonvat N, White RH. Bariatric surgery to aLleviate OCcurrence of atrial fibrillation hospitalization-BLOC-AF. Heart Rhythm O2 2020;1:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Donnellan E, Wazni O, Kanj M, Hussein A, Baranowski B, Lindsay B, Aminian A, Jaber W, Schauer P, Saliba W. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol 2019;12:e007598. [DOI] [PubMed] [Google Scholar]
- 151. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 152. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 153. Middeldorp ME, Pathak RK, Lau DH, Sanders P. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study-authors’ reply. Europace 2019;21:990–991. [DOI] [PubMed] [Google Scholar]
- 154. Strzelczyk J, Kalinowski P, Zieniewicz K, Szmigielski C, Byra M, Styczynski G. The influence of surgical weight reduction on left atrial strain. Obes Surg 2021;31:5243–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Giles ED, Jackman MR, MacLean PS. Modeling diet-induced obesity with obesity-prone rats: implications for studies in females. Front Nutr 2016;3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Islam MT, Henson GD, Machin DR, Bramwell RC, Donato AJ, Lesniewski LA. Aging differentially impacts vasodilation and angiogenesis in arteries from the white and brown adipose tissues. Exp Gerontol 2020;142:111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr Diabetes 2014;4:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hall ME, Harmancey R, Stec DE. Lean heart: role of leptin in cardiac hypertrophy and metabolism. World J Cardiol 2015;7:511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Suleiman JB, Mohamed M, Bakar ABA. A systematic review on different models of inducing obesity in animals: advantages and limitations. J Adv Vet Anim Res 2019;7:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gastelurrutia P, Galvez-Monton C, Camara ML, Bustamante-Munguira J, Garcia-Pavia P, Avanzas P, Alberto San Roman J, Pascual-Figal D, Teresa E, Crespo-Leiro MG, Manito N, Nunez J, Fernandez-Aviles F, Caballero A, Teis A, Lupon J, Brugada R, Martin C, Silva J, Revilla-Orodea A, Canovas SJ, Melero JM, Cuenca-Castillo JJ, Gonzalez-Pinto A, Bayes-Genis A. Rationale and design of a multicentre, prospective, randomised, controlled clinical trial to evaluate the efficacy of the adipose graft transposition procedure in patients with a myocardial scar: the AGTP II trial. BMJ Open 2017;7:e017187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, Pahnke A, Protze S, Lee JH, Davenport Huyer L, Jekic D, Wickeler A, Naguib HE, Keller GM, Vunjak-Novakovic G, Broeckel U, Backx PH, Radisic M. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019;176:913–927.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Harlaar N, Dekker SO, Zhang J, Snabel RR, Veldkamp MW, Verkerk AO, Fabres CC, Schwach V, Lerink LJS, Rivaud MR, Mulder AA, Corver WE, Goumans M, Dobrev D, Klautz RJM, Schalij MJ, Veenstra GJC, Passier R, van Brakel TJ, Pijnappels DA, de Vries AAF. Conditional immortalization of human atrial myocytes for the generation of in vitro models of atrial fibrillation. Nat Biomed Eng 2022;6:389–402. [DOI] [PubMed] [Google Scholar]
- 163. Monti CB, Codari M, De Cecco CN, Secchi F, Sardanelli F, Stillman AE. Novel imaging biomarkers: epicardial adipose tissue evaluation. Br J Radiol 2020;93:20190770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhou Y, Yu M, Cui J, Hu F, Yang Z, Yuan J, Qiao S. The predictive value of epicardial adipose tissue volume assessed by cardiac magnetic resonance for atrial fibrillation in patients with hypertrophic obstructive cardiomyopathy. Int J Cardiovasc Imaging 2021;37:1383–1393. [DOI] [PubMed] [Google Scholar]
- 165. Kawamorita T, Kuronuma K, Yagi T, Tachibana E, Sugai S, Hayashida S, Iso K, Iida K, Atsumi W, Kunimoto S, Suzuki Y, Tani S, Matsumoto N, Okumura Y, Sakatani K. Application of peripheral near infrared spectroscopy to assess risk factors in patient with coronary artery disease: part 1. Adv Exp Med Biol 2020;1232:331–337. [DOI] [PubMed] [Google Scholar]
- 166. Lu Y, Wang T, Zhan R, Wang X, Ruan X, Qi R, Huang S. Effects of epicardial adipose tissue volume and density on cardiac structure and function in patients free of coronary artery disease. Jpn J Radiol 2020;38:666–675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.