Abstract
Vasculogenic mimicry (VM), defined as an endothelial cell independent alternative mechanism of blood and nutrient supply by dysregulated tumor cells, is associated with poor prognosis in oral squamous cell carcinoma (OSCC). Here we aim to investigate the underlying molecular mechanism of the synergistic effect of phytochemical Lupeol and standard microtubule inhibitor Paclitaxel in reversing the hypoxia induced VM formation in OSCC. The results demonstrated that the hypoxia induced upregulation of HIF-1α led to augmentation of signaling cascade associated with extracellular matrix remodeling and EMT phenotypes that are mechanistically linked to VM. Induction of HIF-1α altered the expression of EMT/CSC markers (E-Cadherin, Vimentin, Snail, Twist and CD133) and enhanced the ability of cell migration/invasion and spheroid formation. Subsequently, the targeted knockdown of HIF-1α by siRNA led to the perturbation of matrigel mediated tube formation as well as of Laminin-5γ2 expression with the down-regulation of VE-Cadherin, total and phosphorylated (S-897) EphA2, pERK1/2 and MMP2. We also observed that Lupeol in association with Paclitaxel resulted to apoptosis and the disruption of VM associated phenotypes in vitro. We further validated the impact of this novel interventional approach in a patient derived tumor explant culture model of oral malignancy. The ex vivo tumor model mimicked the in vitro anti-VM potential of Lupeol-Paclitaxel combination through down-regulating HIF-1α/EphA2/Laminin-5γ2 cascade. Together, our findings elucidated mechanistic underpinning of hypoxia induced Laminin-5γ2 driven VM formation highlighting that Lupeol-Paclitaxel combination may serve as novel therapeutic intervention in perturbation of VM in human OSCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-022-00693-z.
Keywords: Hypoxia, Vasculogenic mimicry, HIF-1α, OSCC, Lupeol, Paclitaxel, Patient derived ex vivo model
Introduction
Oral cancer is one of the leading causes of cancer related mortalities (Sung et al. 2021). Consumption of tobacco (including smokeless tobacco), betel nut chewing, severe alcoholism, poor oral hygiene and human papilloma virus (HPV) infection are the predominant risk factors (Borse et al. 2020). Despite the advances in diagnosis and various treatment modalities, the overall survival of OSCC patients did not improve for more than 50% cases, mostly because of the treatment failure due to the loco-regional recurrence and distant metastasis (Thavarool et al. 2019). The poor survival outcome is also influenced by alternative vascularization strategies associated with resistance in response to anti-angiogenic and anti-neoplastic therapies (Belotti et al. 2021). Hypoxia, one of the most detrimental alterations of the tumor microenvironment mainly in solid tumors including OSCC, is orchestrated owing to lack of oxygen perfusion in tumor core either due to absence of blood vessels or presence of abnormal blood vessels (Muz et al. 2015) and characterized by overexpression of hypoxia inducible factor-1 alpha (HIF-1α). The altered metabolism (glycolytic shift) and high acidic microenvironment due to lactate release ultimately promote survival of cancer cells that is linked to resistance towards chemotherapy and radiotherapy (Patel et al. 2020; Pezzuto et al. 2018) and attribute to tumor metastasis (Lee et al. 2020; Zhou et al. 2017). Under hypoxic condition, vasculogenic mimicry (VM) serves as an unique strategy of utilizing oxygen and essential nutrients independent of angiogenesis and programmed these cells to develop aggressive tumor growth (Maniotis et al. 1999; Folberg et al. 2004), leading to tumor invasion, metastasis and poor prognosis of several cancers (Zhang et al. 2019; Mitra et al. 2020; Ren et al. 2019) including OSCC (Hujanen et al. 2021, Wu et al. 2017, Wang et al. 2018b). Although a variety of proteins and tumor micro-environmental factors have been found to contribute VM formation under hypoxic condition in several cancer models including breast cancer (Wang et al. 2018a; Maroufi et al. 2020), hepatocellular carcinoma (Chen et al. 2019; Zhang et al. 2020), lung adenocarcinoma (Fu et al. 2021), melanoma (Li et al. 2019), glioma (Duan et al. 2018), however, their mechanistic involvement in OSCC remains poorly understood. In a hypoxic tumor microenvironment niche, HIF-1α enhances the differentiation potential of cancer stem cells (CSC) (EmamiNejad et al. 2021) and promotes their phenotypic plasticity to transform cancer cells into more invasive state through epithelial mesenchymal transition (EMT) (Hernández de la Cruz et al. 2020) and remodeling of extracellular matrix (ECM) (Winkler et al. 2020) via degradation of laminin, recruitment of matrix metallo-proteases (Rousselle et al. 2020; Delgado-Bellido et al. 2017) and the formation of PAS + /CD31- infiltrating pseudo-vascular VM network to transport red blood cells and nutrients to the tumor cells (Yue et al. 2021). Previous studies have demonstrated that VM channel forming cells could be fueled by up-regulated expression of VE-Cadherin, EphA2, ERK1/2, MMP2, Laminin-5γ2 genes (Hendrix et al. 2001; Hess et al. 2006; Lu et al. 2013; Larson et al. 2014) in several cancer models. However, their active networking in the context of critical nodal role of HIF-1α has not been established in OSCC. In our earlier study we have depicted the prognostic significance of VM in coordination with Laminin-5γ2 expression in OSCC patient cohorts (Saha et al. 2022). Here, we aim to further investigate the mechanistic link between HIF-1α and Laminin-5γ2 in reshaping of ECM and simultaneous formation of VM architecture in OSCC which may be a potential therapeutic intervention in OSCC with high VM propensity.
Since traditional anti-angiogenic therapy could not inhibit the formation of VM due to lack of potential druggable target, finding new treatment regimens, specifically targeting VM holds a great challenge. To improve prognosis, administration of taxane based drugs such as Paclitaxel in conjunction with the platinum drugs or oncogenic EGFR targeted agent like cetuximab have recently been reported to be effective with lower cytotoxicity and higher tolerability (Ahn et al. 2016; Sawatani et al. 2020) in the treatment of locally advanced OSCC. Paclitaxel is a mitotic inhibitor which stabilizes cytoplasmic microtubules and causes interference of cellular replication, arresting cells from entering to G2/M phases of the cell cycle (Horwitz, 1994). However, its adverse side effects on non-malignant healthy cells remain a safety concern, prompting to develop alternative treatment strategies. Combining the cancer preventive phytochemicals with the conventional chemotherapeutic agents has become an emerging strategy of treating cancer by increasing anti-tumor efficacy and response rate of the anticancer drug and concurrently overcome the drug resistance, dose induced toxicity and adverse side effects (Pezzani et al. 2019; Lee et al. 2020). Hence our next goal is to elucidate the anti-VM efficacy of phytochemical Lupeol which is a pharmacologically active natural triterpene widely found in edible fruits and vegetables (Saleem et al. 2009; Liu et al. 2021) including mango, strawberry, olive, red grapes, white cabbage, cucumber, pepper, tomato and reported to have extensive anti-inflammatory, mutagenesis-inhibiting, anti-neoplastic, anti-arthritis, and anti-diabetic properties in both in vitro and in vivo studies (Bociort et al. 2021; Che et al. 2022; Malekinejad et al. 2022). Moreover, data from in vitro (Pitchai et al. 2014; Nyaboke, et al. 2018) and animal studies (Al-Rehaily et al. 2001; Patocka, 2003; Saleem et al. 2009) provided convincing evidences regarding safety profile of Lupeol. In this context, our study aims to explore the possible chemo-sensitization effect of Lupeol in potentiating the conventional chemotherapeutic drug Paclitaxel and their mechanism of action in human OSCC.
Materials and methods
Reagents
A 30 mM stock solution of Lupeol (Sigma, S957712) was prepared by dissolving in warm alcohol followed by dilution with DMSO (Sigma, D2650) at a ratio of 1:1. For all the treatment protocols, the final concentration of DMSO was < 0.01%. A 50 mM stock solution of Paclitaxel (Sigma, T1912) was prepared by dissolving in DMSO.
Antibodies
The primary and secondary antibodies used for Western blot (WB) and Immuno-histochemistry (IHC) are as follows. Rabbit polyclonal anti-HIF1α (Novus Biologicals, Cat# NB100-479, dilution: 1:500 for WB; 1:100 for IHC), Mouse monoclonal anti-VE-Cadherin (Novus Biologicals, Cat# NB600-1409, dilution: 1:100 for WB; 1:100 for IHC), Rabbit monoclonal anti-EphA2 (Cell signaling technology, Cat# 6997, Clone: D4A2, dilution: 1:1000 for WB; 1:100 for IHC), Rabbit monoclonal anti-pEphA2 (S-897, Cell signaling technology, Cat# 6347, Clone: D9A1, dilution: 1:1000 for WB; 1:100 for IHC),Rabbit monoclonal anti phospho p44/42 MAPK (Erk1/2) (Thr202/Tyr204), from Cell signaling technology, Cat# 4370, Clone: D13.14.4E, dilution: 1:2000 for WB; 1:100 for IHC), Rabbit polyclonal anti Erk1 + Erk2 (Abcam, Cat# ab17942, dilution: 1:1000 for WB), Mouse monoclonal anti-MMP2 (Novus Biologicals, Cat # NB200-114, Clone: 8B4, dilution: 1:1000 for WB; 1:100 for IHC), mouse monoclonal anti-Laminin-5 (Ƴ2 chain) from Merck ( Cat # MAB19562, Clone: D4B5, dilution: 1:1000 for WB; 1:100 for IHC), Rabbit monoclonal anti CD-31 or PECAM-1 (Santa Cruz Biotechnology, Cat# sc-1506-R, Clone: M-20, dilution: 1:100 for IHC), Rabbit monoclonal anti-E-Cadherin (Novus Biologicals, Cat# NBP2-67,540, Clone: ST54-01, dilution: 1:1000 for WB; 1:100 for IHC), Mouse monoclonal anti- Vimentin (Santa Cruz Biotechnology, Cat# sc-6260, Clone:V9, dilution: 1:200 for WB; 1:100 for IHC), Mouse monoclonal anti-Snail (Novus Biologicals, Cat# NBP2-50,300, Clone: 20C8, dilution: 1:1000 for WB; 1:200 for IHC), Mouse monoclonal anti-Twist1 (Novus Biologicals, Cat# NBP2-37,364, Clone: 10E4E6, dilution: 1:1000 for WB; 1:200 for IHC), Rabbit polyclonal anti-CD133 (Novus Biologicals, Cat# NB120-16518, dilution: 1:1000 for WB; 1:100 for IHC), Rabbit monoclonal anti -Bcl2 (Novus Biologicals, Cat# NBP2-07,182, Clone: JF104-8, dilution: 1:1000 for WB), mouse monoclonal anti-Bax (Santa Cruz Biotechnology, Cat # sc-7480, Clone: B9, dilution: 1:200 for WB), Mouse monoclonal anti β-Actin (Santa Cruz Biotechnology, Cat# sc-47778, Clone: C4, dilution: 1:200 for WB) were used as primary antibodies in this study. Horseradish peroxidase (HRP) conjugated Goat Anti Rabbit polyclonal IgG (Sigma Aldrich, Cat# A0545) and Rabbit Anti Mouse IgG (Sigma Aldrich, Cat# A9044) were used as secondary antibodies.
Cell lines and culture condition
Human oral squamous cell carcinoma cell lines UPCI: SCC154 (ATCC®CRL-3241™) and UPCI: SCC090 (ATCC®CRL-3239™) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). All the cell lines were maintained in Minimum Essential Medium (MEM, Gibco, Life Technologies, USA) supplemented with 10% heat inactivated Fetal Bovine Serum (FBS, Gibco, Life Technologies, USA) and 2 mM of L-glutamine (Thermo-Fisher Scientific) at 37 °C in a humidified incubator with 5% CO2. All the experiments were performed after 3rd passage of cell lines which were maintained in an exponential growth phase. Hypoxic condition was stimulated by incubating cells in a hypoxic chamber flushed with a gas mixture of 1% O2/ 5% CO2/94%N2 and the induction of HIF-1α expression was assessed by Western blot analysis.
Transfection of siRNA
The ON-TARGETplusSMARTpool siRNA targeting HIF-1α (siRNA#1: GAACAAAUACAUGGGAUUA, siRNA#2: AGAAUGAAGUGUACCCUAA, siRNA#3: GAUGGAAGCACUAGACAAA, siRNA#4: CAAGUAGCCUCUUUGACAA) as well as the ON-TARGETplus Non-targeting siRNA (siControl#1: UGGUUUACAUGUCGACUAA, siControl#2: UGGUUUACAUGUUGUGUGA, siControl#3: UGGUUUACAUGUUUUCUGA, siControl#4: UGGUUUACAUGUUUUCCUA) were purchased from Dharmacon (Horizon). The siRNA dried pellets were re-suspended in RNase free 1X siRNA buffer to prepare 20 μM stock solutions. The final concentration of 100 nM of HIF-1α siRNA was used to transfect cells. For transfection, cells were seeded into 6 well plates at 1.5 × 105 cells/well (2 ml) and cultured overnight. The cells (at 50–60% confluency) were transfected with siRNAs using Jetprime (Polyplus) reagents for 48 h according to manufacturer’s instructions and then characterized by Western blot to assess the level of silencing of HIF-1α. The silencing experiments and the downstream assays were performed both under normoxia and hypoxia.
In vitro tube formation assay
Pre-chilled 96 well culture plates were evenly coated with 50 μl of Matrigel matrix (354262, Corning, USA) and incubated at 37 °C for 30 mins to solidify. Tumor cells (2X104 cells/well in 200 μl) were seeded in the Matrigel coated plates and incubated overnight. In case of transfected cells, after 48 h of transfection, cells were trypsinized and then seeded on the Matrigel coated plate. The VM channels formed were observed under inverted microscope and the images were captured at 100X magnification. The images were analyzed to calculate the tube length and number of tubular junctions using Angiogenesis Analyzer compatible with Image J.
Trans-well migration and invasion assay
The migration and invasion assay were carried out using 24 well plate containing trans-well chambers with the polycarbonate filters of 6.5 mm diameter and 8 μm pore size (3422, Corning, USA). Cells suspended in 100 μl of serum free media were seeded in to the upper chambers at a density of 1 × 104/well for migration and invasion. For the invasion assay, the growth factor reduced Matrigel (356231, Corning, USA) was diluted with serum-free medium according to the manufacturer’s instructions at a ratio of 1:5 and 100 μl/well was added to the upper chambers and incubated at 37 °C for 1 h before seeding cells. The lower chambers were filled with 600 μl of complete media containing 10% FBS as chemo-attractant. After the incubation of 24 h the membranes were fixed with methanol for 15 mins and after washing with PBS the membranes were stained with Giemsa stain. The non- migrating or non-invading cells on the upper surface of the membrane were removed using cotton swab. The number of migrating or invading cells was counted and the images were captured at 100X magnification.
Sphere formation assay
For sphere formation assay, single cell suspension in the tumor sphere media (serum free media supplemented with 1X B27, 20 ng/ml epidermal growth factor, 10 ng/ml basic fibroblast growth factor, 5 μg/ml insulin, 0.4% FBS) were seeded at a density of 1000 cells/well (200 μl) in a 96-well ultra-low attachment plate (Corning). After one week of incubation, the resulting spheres were observed under inverted microscope and the sphere forming efficiency was determined by dividing the number of oral spheres by the number of cells seeded and the images were captured at 100X magnification.
Cell viability assay
Cell viability was determined by MTT assay. Cells were seeded at a density of 1 × 104/well (200 μl) in a 96 well plate and exposed to Lupeol (0–200 μM), Paclitaxel (0–150 nM) and Lupeol + Paclitaxel for 48 h under hypoxic condition. Thereafter, 10 μl of MTT solution (2 mg/ml) was added in each well and incubated for 2–4 h at 37 °C. Then 100 μl of DMSO was added to dissolve the formazan crystal and OD was measured at 570 nm using Spectramax i3x microplate reader. The percentage of cell viability was determined with respect to the untreated control.
Determination of combination index (CI)
After the determination of individual and combinatorial inhibitory effect of Lupeol and Paclitaxel, the combination index was calculated according to the method described by Chou et al. (2010). The value of CI implies the quantitative measure of degree of interaction between two drugs. CI < 1 denotes synergistic, CI = 1 denotes additive and CI > 1 denotes antagonistic effects. The dose reduction index (DRI) is the measure of dose reduction of each drug in a synergistic combination of a given level compared with the doses of individual drugs.
Colony formation assay
Colony formation assay was performed as described earlier (Rauth et al. 2016). Approximately 500 cells/well (2 ml) were seeded in a 6 well plate. After overnight incubation, cells were treated with designated doses of Lupeol and Paclitaxel and were grown in fresh medium for another 3 days at 37 °C until the colonies were formed. Cell colonies were fixed with 10% methanol for 15 mins. Colonies were washed with 1X PBS and stained with Harry's hematoxylin. The number of colonies (a single colony denotes > 50 cells) were counted under bright field microscope and photographs were captured at 4X magnification. The percentage of colonies was calculated with respect to the untreated control.
Wound healing assay
Wound healing assay was performed as described earlier (Bhattacharyya et al. 2019). Briefly, cells were seeded at a density of 1 × 106/well in a 6 well plate. When the cells reached more than 80% confluency, a vertical wound was created through cell monolayer using 200 µl pipette tip. Cellular debris and smooth edges of scratch was removed by washing the cells once and replaced with serum free media containing different concentration of Lupeol and Paclitaxel. Wound closure was observed at different time point and photographs were captured at 4X magnification under bright field microscope. The scratched area was analyzed using Image J software.
Evaluation of apoptosis
Cell apoptosis was detected using Annexin V Apoptosis detection kit (Santa Cruz Biotechnology Inc, sc-4252 AK). Briefly, after treatment of individual and combined doses of Lupeol and Paclitaxel for 48 h, the cells were harvested by centrifugation and dissolved in 1X assay buffer. Cells were then stained with Annexin-V-FITC and Propidium Iodide (PI), incubated at room temperature for 15 mins in the dark and the data were acquired by BD FACSVerseTMflow cytometer. Cells without Annexin V-FITC and PI stains were considered as negative controls. The dual parameter dot plot considering the logarithmic fluorescence intensity of FL1-H (X axis- FITC fluorescence) and FL2-H (Y axis- PI fluorescence) was obtained using FlowJo™ v10 software to determine the percentage of total apoptosis (consisting of early apoptosis and late apoptosis).
Apoptotic cells that undergo DNA degradation were detected using TUNEL assay using TACS -2 TdT-Fluor In-situ Apoptosis detection kit (R&D systems, Cat# 4812-30 K) according to the manufacturer’s protocol. After fixation with 3.7% buffered formaldehyde, the cells placed on the sterile coverslips were digested with protease K for 15 mins at room temperature. The cells were then washed and incubated with TUNEL reaction mix (TdT enzyme solution and labeling solution) for 60 mins at 37 °C in humidified chamber. The cells on the coverslips were mounted on clean glass slides and the TUNEL positive apoptotic cells were detected with fluorescence microscope at 400X magnification.
Western blot analysis
Western blot analysis was performed as described earlier (Rauth et al. 2016). Total cell lysates (50 μg) were resolved by 10% SDS-PAGE and subsequently electro-transferred to Polyvinyldene di-fluoride (PVDF) membrane. After blocking with 5% non-fat dry milk for 1 h at room temperature the membranes were incubated with different dilutions of primary antibodies overnight at 4 °C followed by the exposure with appropriate secondary antibodies conjugated with horse radish peroxidase (HRP) for 1 h at room temperature. The signal was visualized using enhanced chemi-luminescence kit (BioRad) and the resulting bands were acquired using Image Lab 5 software (BioRad). The band density was quantified by Image J software. β-actin was used as a loading control.
Real time PCR
For gene expression analysis total RNA was extracted with Trizol reagent according to manufacturer’s protocol. The complementary DNA (cDNA) was synthesized from 2 μg of total RNA using Roche Evoscript Universal cDNA master kit. Quantitative analysis of cDNA amplification was assessed by incorporating SYBR green nucleic acid stain (Roche FastStart Essential DNA Green Master kit) into double stranded DNA. The specific primers for investigating gene expression are as follows: HIF-1α Forward -5’ GTCTGCAACATGGAAGGTATTG-3’, HIF-1α Reverse- 5’-GCAGGTCATAGGTGGTTTCT -3’, Laminin-5γ2 Forward -5’-GATGGCATTCACTGCGAGAAG-3’, Laminin-5γ2 Reverse 5’-TCGAGCACTAAGAGAACCTTTGG-3’, GAPDH Forward- 5’-GTCAACGGATTTGGTCGTATTG-3’, GAPDH Reverse- 5’- TGTAGTTGAGGTCAATGAAGGG-3’. The PCR condition included denaturation at 95 °C for 1 min, Annealing at 52 °C for 1 min and Extension at 72 °C for 2 mins. All the samples were evaluated in triplicates using Roche Light cycler 96. GAPDH was used as an endogenous control. Quantitative evaluation of data was carried out using 2−ΔΔCT method and Ct (cycle threshold) values were standardized with respect to GAPDH expression.
Patient derived tumor explant culture
The patient derived tumor explant culture was established as described by Majumder et al. (2015). Fresh OSCC tumor specimens were collected from a total of 5 patients (details mentioned in Supplementary Table 2) immediately after surgical resection from Chittaranjan National Cancer Institute, Kolkata. For each patient, non-heparinized blood was collected and serum was separated and stored at − 80 °C for further use. The study was approved by the Institutional Ethics Committee (IEC Ref: A-4.311/53/2014) in accordance with the ethical guidelines of Declaration of Helsinki (1964) and its later amendments. Patients have no history of recurrence, preoperative chemotherapy or radiotherapy. Surgically removed fresh tumor tissues were cut into ~ 2–3mm3 sections and cultured into 24 well culture plates coated with tumor matrix proteins and RPMI medium (Gibco, Life Technologies, USA) supplemented with 2% autologous serum and 8% FBS. Tumor slices were treated with individual and combinatorial doses of Lupeol and Paclitaxel or with DMSO (vehicle control) for 48 h. The formalin fixed paraffin embedded (FFPE) tumor blocks were then used for histological examination.
Immunohistochemistry/PAS dual staining
Immunohistochemistry staining (IHC) was performed according to the manufacturer’s instruction (Millipore, IHC Select DAB150 Immuno-peroxidase secondary detection system kit) on 5-micron sections from the FFPE tumor blocks as per our previously described methods (Saha et al. 2022; Ray et al. 2021; Mitra et al. 2020). Primary antibodies against CD-31 or PECAM-1, HIF-1α, VE-Cadherin, EphA2, pERK1/2, MMP2, Laminin-5γ2, E-Cadherin, Vimentin, Snail, Twist were used for IHC. The final IHC score was determined by considering intensity of staining and proportion (%) of stained cells as described earlier (Saha et al. 2022).
Protein–protein interaction (PPI) and network analysis
For STRING protein–protein interaction (PPI) network analysis (https://string-db.org), all input markers were selected from current study and queried in setting that involves full network (type), confidence (edge meaning), multiple active interaction sources/channels in combination spanning text mining, database, experiments, co-expression, neighborhood, gene fusion and co-occurrence. Minimum required interaction score with high confidence or 0.700 was selected for final analysis. We restricted maximum number of interactions only to queried proteins that have been profiled in the study and no additional layer or second shell was added.
Statistical analysis
All the statistical analyses were performed using GraphPad Prism 7 (GraphPad, USA) software. All the experiments were repeated independently three times and the data were recorded as mean ± SD. One way Analysis of variance (ANOVA) followed by post-hoc comparisons with Tukey test was carried out to assess the significant difference between each treated group and untreated control. P < 0.05 was considered as statistically significant.
Results
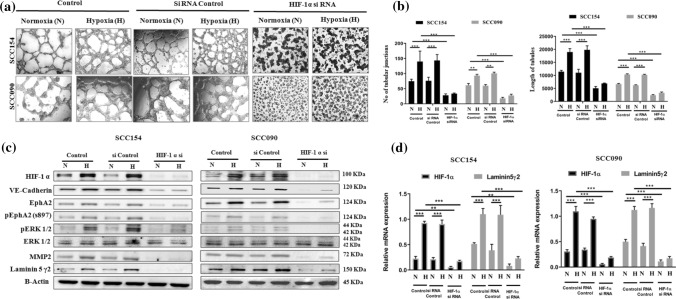
HIF-1α escalates VM forming ability in oral cancer in vitro and positively regulates VM associated EphA2/Laminin-5γ2 axis
To investigate the role of HIF-1α in the enhancement of VM formation, the tube forming ability of OSCC cell lines was monitored in 3D tumor culture by stabilizing HIF-1α under hypoxia and also silencing HIF-1α expression with HIF-1α siRNA. The hypoxic condition triggered the formation of vascular architecture (characterized by the interconnected loops and tubular networks) in all the OSCC cell lines. Further, the cells transfected with HIF-1α siRNA significantly inhibited the tube forming capacity of OSCC cell lines in both normoxia and hypoxia, demonstrating the fractured tubular structure as well as the significant decrease in the length and number of tubular junctions (Fig. 1a, b). These properties remain unperturbed in the non-transfected cells and control siRNA transfected cells under similar conditions. The expression of VM associated signaling molecules was evaluated following the induced and genetically silenced expression of HIF-1α using western blot analysis. Under hypoxia, the expression of HIF-1α protein and its downstream effectors such as VE-Cadherin, EphA2, pEphA2 (S897), pERK1/2, MMP2 and Laminin-5γ2 was significantly increased. In contrast, the knock-down of HIF-1α notably downregulated the expression of these VM associated signaling molecules irrespective of normoxic and hypoxic conditions indicating the role of HIF-1α in regulating the VM via promoting EphA2/Laminin-5γ2 signaling cascade (Fig. 1c). Similarly, the mRNA expression of HIF-1α and Laminin-5γ2 was also markedly reduced in the HIF-1α silenced cells under normoxia and hypoxia (Fig. 1d).
Fig. 1.
siRNA against HIF-1α inhibits vasculogenic mimicry formation and the expression of vasculogenic mimicry related genes in-vitro. (a) Effect of silencing of HIF-1α on the formation of VM in OSCC cells (magnification: 100X) under normoxia and hypoxia. (b) Quantification of VM formation with respect to the number of tubular junction and length of tubules in OSCC cells treated with HIF-1α siRNA under normoxia and hypoxia. (c) Effect of silencing of HIF-1α on the protein expression of VM related genes. (d) Effect of silencing of HIF-1α on the mRNA expression of VM related genes. Each experiment was performed in triplicates. *P value < 0.05, **P value < 0.01 and ***P value < 0.0001 denote statistically significant changes compared to the corresponding control by One Way ANOVA test (P ANOVA < 0.0001) followed by post hoc Tukey’s test
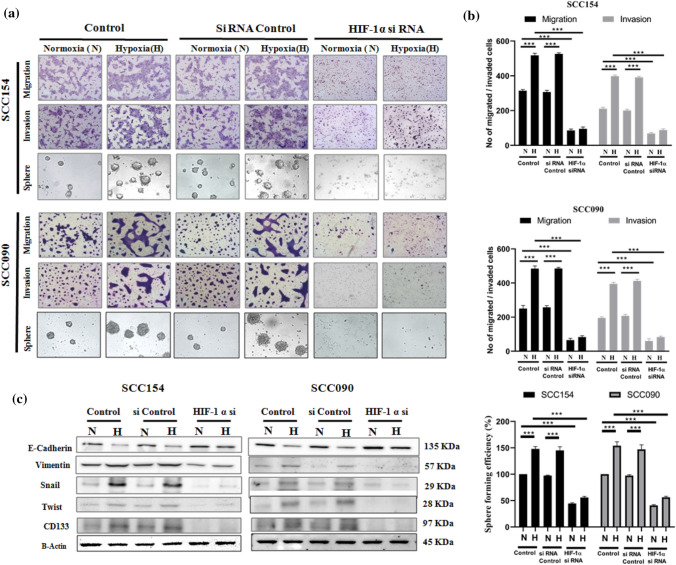
HIF-1α regulates VM related aggressive phenotypes and expression of EMT and CSC markers
Since VM is closely associated with the migration and invasion of tumor cells, we subsequently assessed the migratory and invading potential of OSCC cells. Data from trans-well migration and invasion assay in HIF-1α induced condition and after performing HIF-1α knockdown revealed that, the induction of hypoxia significantly increased the number of migrating and invading cells, whereas the HIF-1α siRNA transfected cells exhibited significant decrease in tumor cell migration and invasion through trans-well chambers compared to the non-transfected cells and control siRNA transfected cells. Hypoxia induced HIF-1α also enhanced the stem like phenotype of tumor cells, characterized by the formation of spherical and non-adherent tumor spheres which was found to be significantly down-regulated in HIF-1α silenced cells (Fig. 2a, b). Since CSC formation is known to accelerate EMT phenotypic state in some cancers (Mani et al. 2008), we also delineated the impact of hypoxia driven CSC and EMT phenomenon through HIF-1α induction. It was found that induction of HIF-1α resulted in the significant decrease in expression of E-Cadherin (epithelial) and concomitant increase in the expression of CD133 as well as related mesenchymal markers (i.e., Vimentin, Snail and Twist). While evaluating the dysregulated expression of the EMT markers in all the OSCC cell lines, it was found that HIF-1α siRNA consistently reversed the effect of hypoxia (Fig. 2c).
Fig. 2.
siRNA targeted against HIF-1α inhibits trans-well migration, invasion, sphere formation along with the expression of EMT and CSC related genes in vitro. (a) Effect of silencing of HIF-1α on the trans-well migration, invasion and sphere formation in OSCC cells (magnification: 100X) under normoxia and hypoxia. (b) Quantification of migrated and invaded cells and CSC enriched spheres treated with HIF-1α siRNA under normoxia and hypoxia. (c) Effect of silencing of HIF-1α on the expression of EMT and CSC related proteins. Each experiment was performed in triplicates. *P value < 0.05, **P value < 0.01 and ***P value < 0.0001 denote statistically significant changes compared to the corresponding control by One Way ANOVA test (P ANOVA < 0.0001) followed by post hoc Tukey’s test
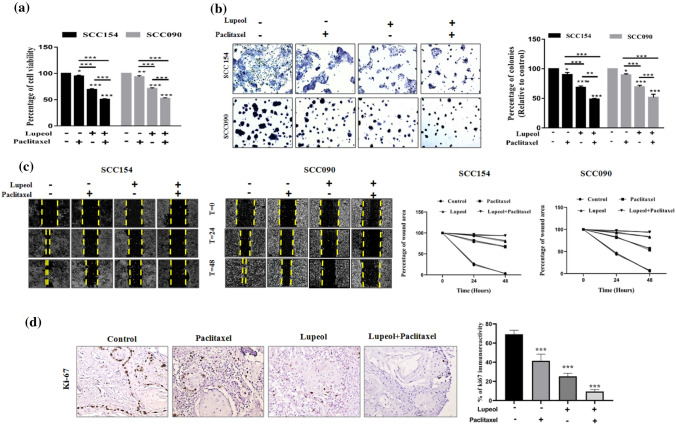
Effect of Lupeol and Paclitaxel on the viability and proliferation of OSCC cells under hypoxia
To ascertain that a combinatorial regimen of Lupeol and Paclitaxel offers synergistic effect in perturbation of VM forming cells, we analyzed the data obtained from MTT assay and observed the cytotoxic effect of Lupeol and Paclitaxel on the OSCC cell lines. The dose dependent cytotoxicity of Lupeol did not differ significantly at 24 h and 48 h in both the UPCI: SCC154 and UPCI: SCC090 cell lines. However, treatment with varying doses of Paclitaxel exhibited notable differences in the cell viability at 48 h when compared the same with 24 h. Hence for all the subsequent assays, the treatment duration of 48 h was considered for both of Lupeol and Paclitaxel. Individual treatment of Lupeol attained the IC50 value of 79.43 μM in UPCI: SCC154 cells and 91.25 μM in UPCI: SCC090 cells during a defined 48 h of treatment window, whereas the treatment of Paclitaxel alone resulted in the IC50 value of 83 nM in UPCI: SCC154 cells and 67 nM in UPCI: SCC090 cells. The simultaneous treatment of cells with Lupeol and sub IC50 doses of Paclitaxel elicited a stronger inhibitory response on cellular viability compared to their individual treatment (Supplementary Table 1 and Supplementary Fig. 1). The Combination index (CI) value for the combinatorial treatment was found to be 0.737 for UPCI: SCC154 cells and 0.815 for UPCI: SCC090 cells indicating the synergistic effects of Lupeol and Paclitaxel in perturbing cell viability. Finally, the combination of 39.43 μM of Lupeol + 20 nM of Paciltaxel (for UPCI: SCC154) and 52.58 μM of Lupeol + 16 nM of Paclitaxel (for UPCI: SCC090) represented the best synergistic inhibition potential (Fig. 3a), which have been designated for further investigations. The combinatorial dose exhibited significantly decreased percentage of colonies (Fig. 3b) and reduced wound healing potential compared to the individual treatment (Fig. 3c).
Fig. 3.
Effect of Lupeol and Paclitaxel on hypoxia induced cellular proliferation, colony formation and wound healing potential. (a) Percentage of cell viability in Lupeol and Paclitaxel treated OSCC cells was measured with respect to the untreated control. (b) Individual and combinatorial effect of Lupeol and Paclitaxel was determined on the hypoxia induced colony formation in OSCC cells (magnification: 40X) and quantification of the percentage of colony formation (relative to control) in OSCC cells treated with Lupeol alone and in combination with Paclitaxel was assessed. (c) Kinetics of wound closure of OSCC cells treated with Lupeol alone and in combination with Paclitaxel (magnification: 40X). (d) Effect of Lupeol and Paclitaxel on regulating the expression of proliferating marker (Ki-67) in ex vivo platform derived from OSCC was determined by IHC (left, 200X magnification). Comparative analysis of IHC scores among groups has been represented (right). Each experiment was performed in triplicates. *P value < 0.05, **P value < 0.01 and ***P value < 0.0001 denote statistically significant changes compared to the corresponding control by One Way ANOVA test (P ANOVA < 0.0001) followed by post hoc Tukey’s test
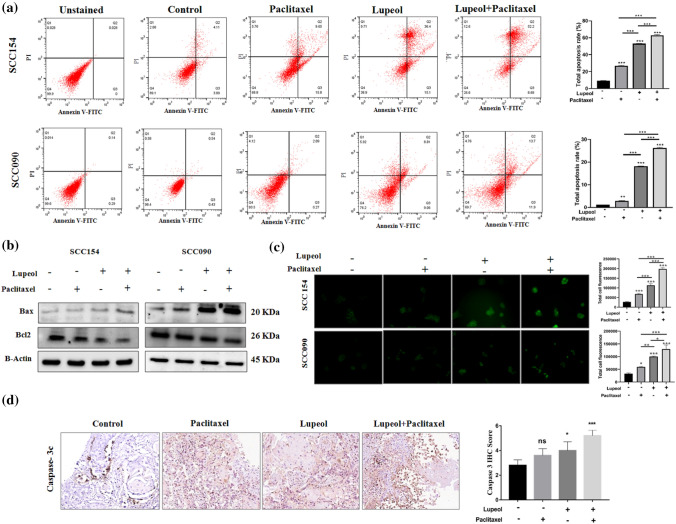
Lupeol treatment enhances the Paclitaxel induced apoptosis in OSCC cell lines under hypoxia
In order to evaluate whether the anticancer property of Lupeol and Paclitaxel are exerted through inducing apoptotic signal, the rate of apoptosis was quantified by flow cytometry using Annexin V-FITC and PI labelling. In both of the OSCC cell lines; though Lupeol or Paclitaxel individually induced apoptosis, their combinatorial treatment demonstrated the significant enhancement of the percentage of apoptotic cells (Fig. 4a). In case of UPCI: SCC154 cell line, the total rate of apoptosis was increased by 52.88%. In case of UPCI: SCC090 cells, the same was found to be enhanced by 24.63% through the synergistic combinatorial treatment of Lupeol and Paclitaxel during hypoxic incubation. Moreover, to understand the underlying molecular mechanisms, the protein expression level of the key regulators of apoptosis (i.e., Bax and Bcl2) was investigated. Significantly up-regulated expression of Bax and down-regulated expression of BCl2 was observed in the cells treated with Lupeol and/or Paclitaxel (Fig. 4b). It is also noteworthy that, the alteration of the apoptotic regulator proteins was found to be more pronounced when both of the compounds were used together than their individual treatment. TUNEL assay also confirmed significantly increased signal intensity of the apoptotic cells in Lupeol and Paclitaxel treated group (Fig. 4c).
Fig. 4.
Effect of Lupeol and Paclitaxel on induction of apoptosis in vitro and ex vivo culture. (a) Annexin-V/PI staining indicating total apoptosis rate in OSCC cells treated with Lupeol and Paclitaxel. (b) Effect of Lupeol and Paclitaxel on the expression of apoptotic markers. (c) TUNEL assay indicating total fluorescence intensity of apoptotic cells following the treatment of Lupeol and Paclitaxel in comparison with untreated control. (d) Effect of Lupeol and Paclitaxel on regulating the expression of apoptotic marker (Caspase-3c) in ex vivo platform of OSCC by IHC (200X magnification, left). Comparative analysis of IHC scores among groups (right). Each experiment was performed in triplicates. *P value < 0.05, **P value < 0.01 and ***P value < 0.0001 denote statistically significant changes compared to the corresponding control by One Way ANOVA test (P ANOVA < 0.0001) followed by post hoc Tukey’s test
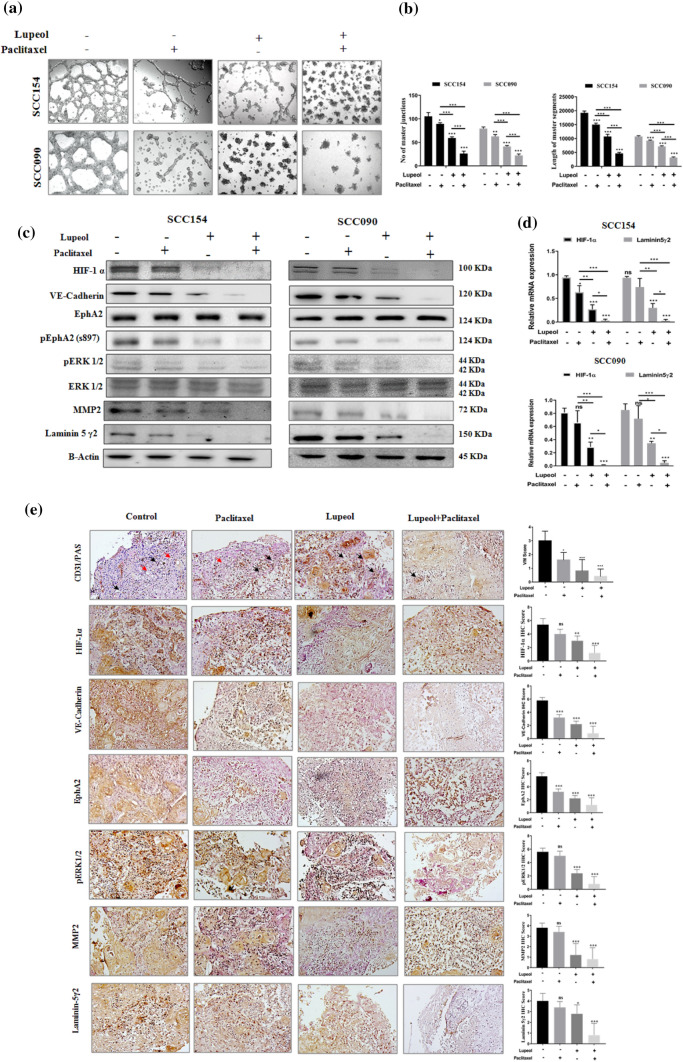
Combined treatment of Lupeol and Paclitaxel hinders the hypoxia induced VM formation and perturbs HIF-1α/EphA2/ Laminin-5γ2 signaling cascade
After ascertaining that hypoxia facilitated VM forming ability of UPCI: SCC154 and UPCI: SCC090 cells (Fig. 1), we further investigated the potential role of Lupeol and Paclitaxel in regulating the hypoxia induced VM forming capacity of these cells (Fig. 5a, b). Significant disruption of VM structures, in conjunction with remarkable reduction of tubular length and numbers were observed in the cells co-treated with Lupeol and Paclitaxel. Though the individual treatment of the compounds notably fractured the in vitro pseudo-vascular architecture, their combinatorial effect is more pronounced affirming their synergistic anticancer potential through mechanistically inhibiting the VM channel formation. To further elucidate the molecular mechanism involved in the reversing effect of Lupeol and Paclitaxel on the hypoxia induced VM formation, the altered expression patterns of HIF-1α and its downstream signaling components were also investigated. The western blot analysis illustrated the significant down-regulation of hypoxia induced HIF-1α and its downstream VM associated regulators such as VE-Cadherin, pEphA2 (S-897), pERK1/2, MMP2 and Laminin-5γ2 in the cells that were treated with Lupeol alone and in combination with Paclitaxel (Fig. 5c). The synergistic association of Lupeol and Paclitaxel also significantly suppressed the hypoxia induced upregulation of the mRNA expression of HIF-1α and Laminin-5γ2 indicating their VM inhibiting potential at the transcription level (Fig. 5d).
Fig. 5.
Effect of Lupeol and Paclitaxel on regulating hypoxia induced VM and the expression of VM related genes in vitro and ex vivo systems. (a) Individual and combinatorial effect of Lupeol and Paclitaxel on the hypoxia induced VM formation in OSCC cells (magnification: 100X). (b) Quantification of VM formation with respect to number of tubular junction and length of tubules in OSCC cells treated with Lupeol alone and in combination with Paclitaxel. (c) Individual and combinatorial effect of Lupeol and Paclitaxel on the protein expression of VM associated genes. (d) Individual and combinatorial effect of Lupeol and Paclitaxel was determined by quantifying the mRNA expression of VM associated genes. (e) Effect of Lupeol and Paclitaxel on regulating the expression of VM associated genes in OSCC derived ex-vivo platform by IHC staining of markers (200X magnification, left). Red arrows in the CD31/PAS staining indicate PAS positive VM architecture and black arrows indicate endothelial structures. Comparative analysis of IHC scores among groups has been represented (right). Each experiment was performed in triplicates. *P value < 0.05, **P value < 0.01 and ***P value < 0.0001 denote statistically significant changes compared to the corresponding control by One Way ANOVA test (P ANOVA < 0.0001) followed by post hoc Tukey’s test
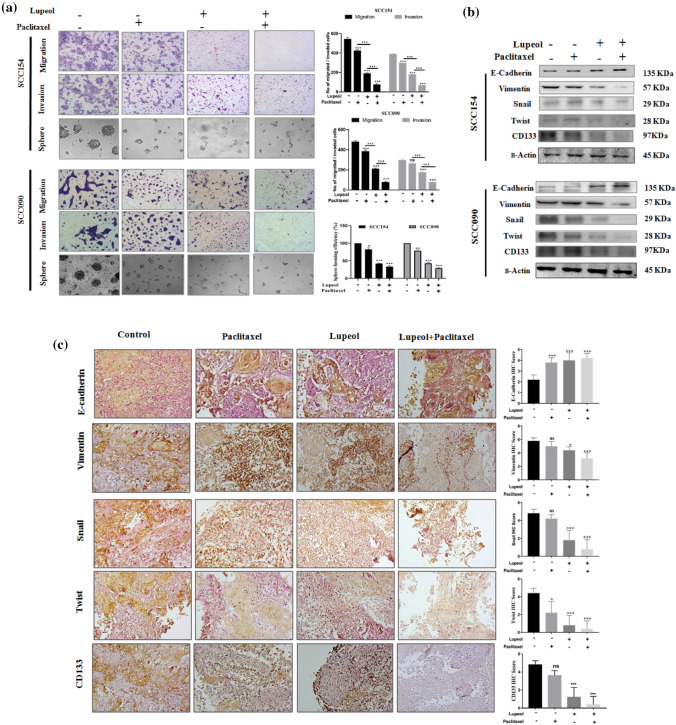
Synergism of Lupeol and Paclitaxel reverses EMT and CSC phenomenon under the influence of hypoxia
In the Fig. 2, we have investigated that the induction of HIF-1α enhanced EMT and CSC phenomenon in OSCC cells which displayed a crucial role in the VM formation. Figure 6a depicted a significantly reduced number of migrated and invaded cells in trans-well setting when co-treated with Lupeol and Paclitaxel indicating their indispensable role in regulating VM associated migration and invasion potential. The combinatorial treatment also caused a significant decrease in the sphere forming potential with the down-regulation of CD133 marker. Furthermore, Lupeol alone as well as in combination with Paclitaxel significantly up-regulated the expression of E-Cadherin and consistently down-regulated the expression of mesenchymal markers including Vimentin, Snail and Twist. Together, these data mechanistically substantiate their reversing effect on the hypoxia promoting EMT (Fig. 6b).
Fig. 6.
Effect of Lupeol and Paclitaxel on hypoxia induced migration, invasion, sphere formation along with alteration of EMT and CSC related genes in vitro and ex vivo. (a) Individual and combinatorial effect of Lupeol and Paclitaxel on the hypoxia induced trans-well migration, invasion and sphere formation in OSCC cells was evaluated (magnification: 100X) and quantification of migrated and invaded OSCC cells and CSC rich sphere forming cells treated with Lupeol alone and in combination with Paclitaxel. (b) Individual and combinatorial effect of Lupeol and Paclitaxel on the protein expression of EMT and CSC associated genes in vitro. (c) Effect of Lupeol and Paclitaxel on regulating the expression of VM associated EMT and CSC markers in ex vivo platform of OSCC. IHC staining for expression of markers (200X magnification, left) and comparative analysis of their IHC scores in Lupeol and Paclitaxel treated tissue specimens (right). Each experiment was performed in triplicates. *P value < 0.05, **P value < 0.01 and ***P value < 0.0001 denote statistically significant changes compared to the corresponding control by One Way ANOVA test (P ANOVA < 0.0001) followed by post hoc Tukey’s test
Synergistic effect of Lupeol and Paclitaxel on regulating hypoxia induced vasculogenic mimicry in patient derived ex vivo culture
Our findings from the in vitro model motivated us to test the same hypothesis in a more complex but relevant patient tumor derived ex vivo culture system. This model preserves the physiological context of tumor microenvironment and therefore represents a close approximation of native tumor niche state (Majumder et al. 2015). In order to elucidate the efficacy of the combinatorial drugs in ex vivo system, the alteration of VM associated regulators along with the proliferative and apoptotic markers was assessed at 48 h post treatment. Tissue architecture of untreated control exhibited relatively compact tumor cells whereas the Lupeol and Paclitaxel co-treated group was found to have more disintegrated structures. CD31/PAS staining showed a significantly decreased number of VM structures in Lupeol and Paclitaxel treated tumor fragments, compared to the untreated control (Fig. 5e). Lupeol and Paclitaxel treatment group also displayed a significant decrease in the expression of the cell proliferation marker Ki67 (Fig. 3d) as well as VM associated HIF-1α and its downstream regulators VE-Cadherin, pEphA2 (S-897), pERK1/2, MMP2 and Laminin-5γ2, compared to the untreated control (Fig. 5e). The significantly upregulated expression of active Caspase 3c in Lupeol and Paclitaxel treated group confirmed that their augmented anticancer potential is exerted by the induction of apoptosis (Fig. 4d). Furthermore, the immuno-histochemical profiling of the expression of EMT and CSC markers showed reduced expression of CSC marker CD133 and mesenchymal markers including Vimentin, Snail, Twist and significantly increased expression of the epithelial marker E-Cadherin in the tissue sections treated with Lupeol alone and in combination with Paclitaxel, indicating the potential of this drug combination in disrupting EMT phenomenon in personalized ex vivo setting (Fig. 6c).
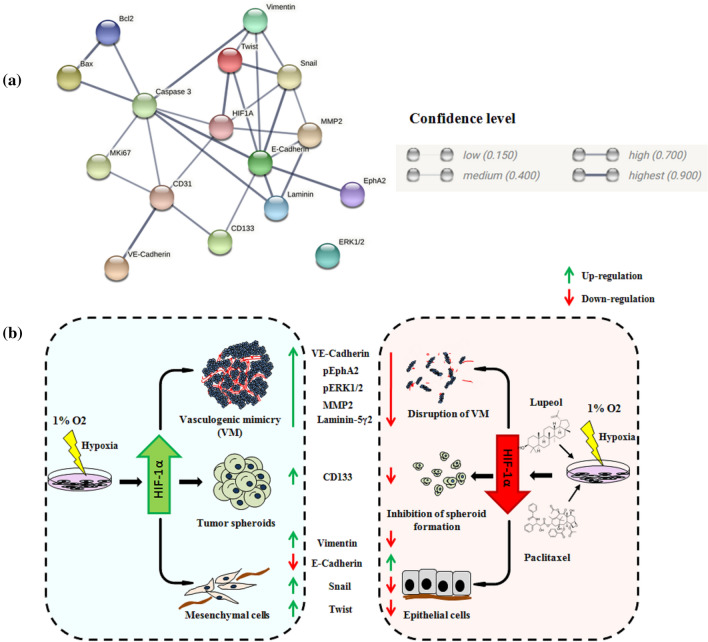
PPI analysis of VM interacting proteins
To investigate the functional link among the differentially expressed proteins in the hypothesized network, protein- protein interactions (PPI) were analyzed via STRING. In our study the STRING analysis identified the main network proteins implicated cellular proliferation, extracellular matrix remodeling, EMT, CSC phenotype and apoptosis. The different grade of association was denoted via confidence level (low-0.150, medium-0.400, high-0.700, highest- 0.900) and edge thickness. Our key network protein HIF-1α exhibited highest degree of association with Twist (0.979), followed by the same with MMP2 (0.754) and Snail (0.727). Laminin also indicated strong association (0.925) with MMP2 and Caspase 3 (0.907). All other possible associations are depicted in Fig. 7a.
Fig. 7.
Model depicting the involvement of HIF1α and key interacting partners in VM and intervention by Lupeol and Paclitaxel. (a) Protein–protein interaction network analysis using input proteins from the present study on STRING platform showing interacting molecules and confidence level in the network. Legends of confidence level have been taken from the STRING database. (b) Schematic diagram representing cellular phenotypes, molecular drivers and synergistic effect of Lupeol and Paclitaxel on perturbing hypoxia induced Vasculogenic mimicry in OSCC.
Discussion
Despite the recent advances in tumor biology, the treatment outcome of OSCC patient remains poor. Resistance to anti-angiogenesis and anti-metastatic drugs due to the tumor perfusion through alternative vascularization pathways emerged as a new treatment challenge. VM has been shown to be associated with tumor size, grade, metastasis and poor prognosis in OSCC patients which emphasizes the urgent need for developing novel anti-vascular therapeutic agents that specifically target VM.
Recent studies indicated the prognostic significance of a numbers of VM associated markers including SOX7 (Hong et al. 2021), LGR5 (Wu et al. 2017), ALDH1, Beclin1, p16 (Wang et al. 2018b), NC11 domain of Collagen XVI (Bedal et al. 2015) in OSCC. However, the molecular mechanisms, underpinning the VM formation, in coordination with other intracellular factors remain elusive. The current study demonstrated an association between hypoxic induction of HIF-1α and VM formation mainly through modulating the expression of extracellular matrix remodeling protein Laminin-5γ2 and elucidated a network that represents the up-regulation of an array of critical players like VE-Cadherin, EphA2, pEphA2 (S-897), pERK1/2 and MMP2. During the siRNA mediated knockdown of HIF-1α, a disrupted VM formation along with inhibited potential of cellular migration, invasion and stemness was evident under both normoxia and hypoxia in SCC154 and SCC090 cell lines. Hypoxic induction of VM has been reported in lung adenocarcinoma (Fu et al. 2021), hepatocellular carcinoma (Zhang et al. 2020) and breast cancer (Wang et al. 2018a). However, none of the studies have focused upon the hypoxia induced remodeling of ECM components. From these perspectives, our findings suggested a narrative of multifactorial signaling in the hypoxic tumor microenvironment that influences the ECM degradation and metastatic VM formation in OSCC model. We further demonstrated that the hypoxic induction of HIF-1α augmented EMT in concurrence with stem-like phenotype in OSCC cells through altered expression of E-Cadherin, Vimentin, Snail, Twist and CD133. Previous work by Wang et al. (2019) highlighted the role of VEGFA in hypoxia induced VM formation through regulating EMT and stemness in salivary adenoid cystic carcinoma (SACC). Another study from the same group also indicated the VM forming potential of CD133 + cancer stem like cells in SACC (Wang et al. 2016) highlighting the critical roles of these complementary cellular contexts. The crucial role of EMT in hypoxia induced VM formation has been demonstrated in several other independent studies involving various cancer models (Maroufi et al. 2020, Chen et al. 2019, Li et al. 2019, Wang et al. 2014) covering diverse indications. However, no evidence supporting hypoxia induced VM formation via EMT has been reported yet in OSCC. From the perspectives of examining therapeutic challenges and opportunities of targeting this key phenotypic cluster, our study also revealed the potential synergistic partnership of Lupeol and Paclitaxel in perturbing the hypoxia induced VM tube formation through the down-regulation of HIF-1α mediated and Laminin-5γ2 driven signaling cascade both in vitro and in patient derived ex vivo system. Several studies have demonstrated that phytochemicals and active compounds from dietary supplements such as green tea extract, hyperforin and silibinin synergistically enhance Paclitaxel response in different cancer models including breast cancer (Barathan et al. 2021), ovarian cancer (Panji et al. 2021), gastric cancer (Zhang et al. 2018). The anticancer activity of Lupeol has been explored in head and neck cancer by inhibiting oncogenic EGFR cascade (Rauth et al. 2016), inducing the intrinsic apoptotic pathway (Bhattacharyya et al. 2017) and downregulating NF-κβ activity (Lee et al. 2007). Though the cytotoxic effect of Lupeol has been studied on various cancer models (Min et al. 2019; Sinha et al. 2019; Malekinejad et al. 2022) the chemo-sensitization potential of Lupeol to amplify the effectiveness of Paclitaxel has not been elucidated yet. To the best of our knowledge, our investigation is the first preclinical study assessing the synergistic potential of Lupeol and common anti-neoplastic drug Paclitaxel in suppressing the vasculogenic mimicry induced by hypoxia both in in vitro and ex vivo OSCC models. Our data comprehensively highlights that Lupeol and Paclitaxel individually exhibited growth inhibitory effects on OSCC cells in a dose dependent manner and the combination index analysis demonstrated that the combination regimen with Lupeol and lower dose of Paclitaxel significantly induced antitumor effects. We have observed synergistic anti-tumor efficacy (CI < 1) with approximately two fold reduction of IC50 dose of Lupeol and four fold reduction of Paclitaxel IC50 dose. The flow cytometric analysis using Annexin-V/PI staining in parallel with fluorescence imaging of TUNEL assay validated that the combined cytotoxic effect of Lupeol/Paclitaxel is causally associated with apoptotic cell death pathway which was consistent with the western blot results showing the alteration of Bax/Bcl2 ratio following the combined treatment of Lupeol and Paclitaxel. Our study further revealed that Lupeol, as a single agent has the potential to inhibit hypoxia induced VM in in vitro and in patient derived ex vivo OSCC model, and its synergistic cooperation with Paclitaxel exhibited an enhanced anti VM effect against OSCC. Earlier the study by Bhattacharyya et al. (2019) indicated the reversing effect of Lupeol on the progression of VM in murine melanoma model by downregulating CD133 expression and Paclitaxel also exhibited an in vitro VM inhibitory potential in murine glioma model (Liu et al. 2015). However, the exact molecular mechanistic approach that underlines the destruction of VM tubes remained un-explored. In this regard, for the first time our study portrayed that, along with the structural disruption of VM tubes in UPCI: SCC154 and UPCI: SCC090 cell lines, Lupeol decreased the expression of HIF-1α, leading to the concurrent inhibition of VE-Cadherin, pEphA2 (S-897), pERK1/2, MMP2 and Laminin-5γ2. Indeed, its co-treatment with lower dose of Paclitaxel enhanced the down-regulation of key proteins suggesting that Lupeol/Paclitaxel synergism hinders hypoxia induced VM formation in OSCC model and the mechanism may be related to inhibition of HIF-1α mediated EphA2-Laminin-5γ2 axis. Previous studies by Li et al. (2018) and Liu et al. (2018) reported the VM inhibitory effect of Niclosamide and Melatonin in OSCC via up-regulation of miR-124, down-regulation of STAT-3 and through the blockade of ROS reliant AKT/ERK signaling pathways respectively. IL-17F also inhibited the in vitro VM formation in OSCC cells (Almahmoudi et al. 2021). Corresponding to these findings, our study proposed an additional novel therapeutic intervention strategy targeting this potentially actionable perturbation leading to the pseudo-vascular features. Furthermore, our findings also unfolded that, compared to the individual treatment, the combinatorial dose of Lupeol and Paclitaxel revealed pronounced inhibitory effect on the expression of markers related to EMT (Vimentin, Snail, Twist) and CSC (CD133) phenotypes which are critically required for VM formation (Sun et al. 2019; Jue et al. 2017). Consistent with the outcomes of in vitro model, we also demonstrated that a combined treatment of Lupeol and Paclitaxel can proficiently reduce the formation of VM channels in patient derived ex vivo platform via CD31/PAS staining and the immuno-histochemical expression of VM associated markers were also significantly down-regulated in the combination group compared to the individually treated groups. Mechanistically, in this context, the ex vivo data provides key translational insights which will be beneficial for the prediction of therapeutic response in a personalized settings and identifying the most appropriate treatment regimen for individual patients. Collectively, we conjectured from the above findings that Paclitaxel in concert with Lupeol may inhibit hypoxia induced VM formation via down-regulating HIF-1α-EphA2-Laminin-5γ2 cascade and associated EMT and CSC phenomenon in OSCC. Further in vivo investigation in appropriate animal models is critically required to understand their translatability and assess their anti-VM potential for OSCC treatment.
Conclusion
From this study we may conclude that HIF-1α is a key regulator of OSCC in terms of VM formation and for targeting its downstream signaling components, the synergistic combination of Lupeol and paclitaxel will emerge as a new treatment modality for clinically aggressive oral cancers following evaluation through proper clinical trials in future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The study was supported in part by Chittaranjan National Cancer Institute (CNCI), Kolkata, India. Dr. Nabendu Murmu sincerely thanks to Dr. JayantaChakrabarti, Director, CNCI, Kolkata, India for his active support. Ms Depanwita Saha was supported by a Senior Research Fellowship (SRF) from Indian Council of Medical Research (ICMR), Government of India, Sanction No: 3/2/2/112/2022-NCD-III.
Author contributions
DS and NM conceptualized and designed the study. DS, DM performed the experiments and analyzed the data. NA, SS supplied tissue specimens. SMM validated the IHC data and provided clinical insights. PKM provided valuable insights into the manuscript. DS, BM and NM drafted the manuscript. BM, PKM and NM reviewed and edited the manuscript. All the authors approved the submitted version of the manuscript.
Funding
This work was financially supported by the Science and Engineering Research Board (SERB), Government of India under grant (Project No: EEQ/2016/000345).
Declarations
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn MJ, D’Cruz A, Vermorken JB, Chen JP, Chitapanarux I, Dang HQT, Guminski A, Kannarunimi D, Lin TY, Ng WT. Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: a literature review. Oral Oncol. 2016;53:10–16. doi: 10.1016/j.oraloncology.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Almahmoudi R, Salem A, Hadler-Olsen E, Svineng G, Salo T, Al-Samadi A. The effect of interleukin-17F on vasculogenic mimicry in oral tongue squamous cell carcinoma. Cancer Sci. 2021;112:2223–2232. doi: 10.1111/cas.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rehaily AJ, El-Tahir KEH, Mossa JS, Rafatullah S. Pharmacological studies of various extracts and the major constituent, Lupeol, obtained from hexane extract of Teclea nobilis in rodents. Nat Prod Sci. 2001;7:76–82. [Google Scholar]
- Barathan M, Zulpa AK, Mee Hoong S, Vellasamy KM, Vadivelu J. Synergistic effect of hyperforin and Paclitaxel on growth inhibition, apoptotic mediator activation in MCF-7 human breast cancer cells. J Taibah Univ Sci. 2021;15:918–927. doi: 10.1080/16583655.2021.2010910. [DOI] [Google Scholar]
- Bedal KB, Grässel S, Spanier G, Reichert TE, Bauer RJ. The NC11 domain of human collagen XVI induces vasculogenic mimicry in oral squamous cell carcinoma cells. Carcinogenesis. 2015;36:1429–1439. doi: 10.1093/carcin/bgv141. [DOI] [PubMed] [Google Scholar]
- Belotti D, Pinessi D, Taraboletti G. Alternative vascularization mechanisms in tumor resistance to therapy. Cancers (basel) 2021;13:1912. doi: 10.3390/cancers13081912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Sekar V, Majumde B, Mehrotra DG, Banerjee S, Bhowmick AK, Alam N, Mandal GK, Biswas J, Majumder PK. CDKN2A-p53 mediated antitumor effect of Lupeol in head and neck cancer. Cell Oncol (dordr) 2017;40:145–155. doi: 10.1007/s13402-016-0311-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Mitra D, Ray S, Biswas N, Banerjee S, Majumder B, Mustafi SM, Murmu N. Reversing effect of Lupeol on vasculogenic mimicry in murine melanoma progression. Microvasc Res. 2019;121:52–62. doi: 10.1016/j.mvr.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Bociort F, Macasoi IG, Marcovici I, Motoc A, Grosu C, Pinzaru I, Petean C, Avram S, Dehelean CA. Investigation of lupeol as anti-melanoma agent: an in vitro-in Ovo perspective. Curr Oncol. 2021;28:5054–5066. doi: 10.3390/curroncol28060425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sens Int. 2020;1:100046. doi: 10.1016/j.sintl.2020.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che S, Wu S, Yu P. Lupeol induces autophagy and apoptosis with reduced cancer stem-like properties in retinoblastoma via phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin inhibition. J Pharm Pharmacol. 2022;74:208–215. doi: 10.1093/jpp/rgab060. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lin W, Yin Z, Zou Y, Liang S, Ruan S, Chen P, Li S, Shu Q, Cheng B. Melittin inhibits hypoxia-induced vasculogenic mimicry formation and epithelial-mesenchymal transition through suppression of HIF-1α/Akt pathway in liver cancer. Evid Based Complement Alternat Med. 2019;2019:9602935. doi: 10.1155/2019/9602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Delgado-Bellido D, Serrano-Saenz S, Fernández-Cortés OFJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer. 2017;16:65. doi: 10.1186/s12943-017-0631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S. Silencing the autophagy-specific gene Beclin-1 contributes to attenuated hypoxia-induced vasculogenic mimicry formation in glioma. Cancer Biomark. 2018;21:565–574. doi: 10.3233/CBM-170444. [DOI] [PubMed] [Google Scholar]
- Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy Javanmard S, Taherian M, Ahmadlou M. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21:62. doi: 10.1186/s12935-020-01719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS. 2004;112(7–8):508–525. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [DOI] [PubMed] [Google Scholar]
- Fu R, Du W, Ding Z, Wang Y, Li Y, Zhu J, Zeng Y, Zheng Y, Liu Z, Huang J. HIF-1α promoted vasculogenic mimicry formation in lung adenocarcinoma through NRP1 upregulation in the hypoxic tumor microenvironment. Cell Death Dis. 2021;12:1–11. doi: 10.1038/s41419-021-03682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández de la Cruz ON, López-González JS, García-Vázquez R, Salinas-Vera YM, Muñiz-Lino MA, Aguilar-Cazares D, López-Camarillo C, Carlos-Reyes Á. Regulation networks driving vasculogenic mimicry in solid tumors. Front Oncol. 2020;9:1419. doi: 10.3389/fonc.2019.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor REB, Hendrix MJC. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- Hong KO, Oh KY, Yoon HJ, Swarup N, Jung M, Shin JA, Kim JH, Chawla K, Lee JI, Cho SD. SOX7 blocks vasculogenic mimicry in oral squamous cell carcinoma. J Oral Pathol Med. 2021;50:766–775. doi: 10.1111/jop.13176. [DOI] [PubMed] [Google Scholar]
- Horwitz SB. Taxol (Paclitaxel): mechanisms of action. Ann Oncol. 1994;5(Suppl 6):S3–6. [PubMed] [Google Scholar]
- Hujanen R, Almahmoudi R, Salo T, Salem A. Comparative analysis of vascular mimicry in head and neck squamous cell carcinoma in vitro and in vivo approaches. Cancers. 2021;13:4747. doi: 10.3390/cancers13194747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue C, Lin C, Zhisheng Z, Yayun Q, Feng J, Min Z, Haibo W, Youyang S, Hisamitsu T, Shintaro I. Notch1 promotes vasculogenic mimicry in hepatocellular carcinoma by inducing EMT signaling. Oncotarget. 2017;8:2501–2513. doi: 10.18632/oncotarget.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AR, Lee CW, Lezcano C, Zhan Q, Huang J, Fischer AH, Murphy GF. Melanoma spheroid formation involves laminin-associated vasculogenic mimicry. Am J Pathol. 2014;184:71–78. doi: 10.1016/j.ajpath.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TY, Tseng YH. The potential of phytochemicals in oral cancer prevention and therapy: a review of the evidence. Biomolecules. 2020;10:E1150. doi: 10.3390/biom10081150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Poon RTP, Wo JY, Ma S, Guan XY, Myers JN, Altevogt P, Yuen APW. Lupeol suppresses cisplatin-induced nuclear factor-kappaB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2007;67:8800–8809. doi: 10.1158/0008-5472.CAN-07-0801. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y. LRIG1 acts as a critical regulator of melanoma cell invasion, migration, and vasculogenic mimicry upon hypoxia by regulating EGFR/ERK-triggered epithelial-mesenchymal transition. Biosci Rep. 2019;39:bsr20181165. doi: 10.1042/BSR20181165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang Z, Han Z, Wen Y, Ma Z, Wang Y. Niclosamide acts as a new inhibitor of vasculogenic mimicry in oral cancer through upregulation of miR-124 and downregulation of STAT3. Oncol Rep. 2018;39:827–833. doi: 10.3892/or.2017.6146. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang X, Xie L, Deng M, Chen H, Song J, Long J, Li X, Luo J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol Res. 2021;164:105373. doi: 10.1016/j.phrs.2020.105373. [DOI] [PubMed] [Google Scholar]
- Liu R, Wang HL, Deng MJ, Wen XJ, Mo YY, Chen FM, Zou CL, Duan WF, Li L, Nie X. Melatonin inhibits reactive oxygen species-driven proliferation, epithelial-mesenchymal transition, and vasculogenic mimicry in oral cancer. Oxid Med Cell Longev. 2018;2018:3510970. doi: 10.1155/2018/3510970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mei L, Yu Q, Xu C, Qiu Y, Yang Y, Shi K, ZhangQ GH, Zhang Z. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7:16792–16801. doi: 10.1021/acsami.5b04596. [DOI] [PubMed] [Google Scholar]
- Lu XS, Sun W, Ge CY, Zhang WZ, Fan YZ. Contribution of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. Int J Oncol. 2013;42:2103–2115. doi: 10.3892/ijo.2013.1897. [DOI] [PubMed] [Google Scholar]
- Majumder B, Baraneedharan U, Thiyagarajan S, Radhakrishnan P, Narasimhan H, Dhandapani M, Brijwani N, Pinto DD, Prasath A, Shanthappa BU. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat Commun. 2015;6:6169. doi: 10.1038/ncomms7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad F, Kheradmand F, Khadem-Ansari MH, Malekinejad H. Lupeol synergizes with doxorubicin to induce anti-proliferative and apoptotic effects on breast cancer cells. Daru. 2022 doi: 10.1007/s40199-022-00436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe'er J, Trent JM, Meltzer PS, Hendrix MJC. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroufi NF, Amiri M, Dizaji BF, Vahedian V, Akbarzadeh M, Roshanravan N, Haiaty S, Nouri M, Rashidi MR. Inhibitory effect of melatonin on hypoxia-induced vasculogenic mimicry via suppressing epithelial-mesenchymal transition (EMT) in breast cancer stem cells. Eur J Pharmacol. 2020;881:173282. doi: 10.1016/j.ejphar.2020.173282. [DOI] [PubMed] [Google Scholar]
- Min TR, Park HJ, Ha KT, Chi GY, Choi YH, Park SH. Suppression of EGFR/STAT3 activity by lupeol contributes to the induction of the apoptosis of human non-small cell lung cancer cells. Int J Oncol. 2019;55:320–330. doi: 10.3892/ijo.2019.4799. [DOI] [PubMed] [Google Scholar]
- Mitra D, Bhattacharyya S, Alam N, Sen S, Mitra S, Mandal S, Vignesh S, Majumder B, Murmu N. Phosphorylation of EphA2 receptor and vasculogenic mimicry is an indicator of poor prognosis in invasive carcinoma of the breast. Breast Cancer Res Treat. 2020;179:359–370. doi: 10.1007/s10549-019-05482-8. [DOI] [PubMed] [Google Scholar]
- Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (auckland, N.z.) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyaboke HO, Moraa M, Omosa LK, Mbaveng AT, Vaderament-Alexe, NN, Masila V, Okemwa E, Efferth T, Kuete V (2018) Cytotoxicity of Lupeol from the Stem Bark of Zanthoxylum gilletii against Multi-factorial Drug Resistant Cancer Cell Lines.
- Panji M, Behmard V, Zare Z, Malekpour M, Nejadbiglari H, Yavari S, Nayerpourdizaj T, Safaeian A, Bakhshi A, Abazari O. Synergistic effects of green tea extract and Paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene. 2021;787:145638. doi: 10.1016/j.gene.2021.145638. [DOI] [PubMed] [Google Scholar]
- Patel U, Pandey M. Kannan S (2020) Prognostic and predictive significance of nuclear HIF1α expression in locally advanced HNSCC patients treated with chemoradiation with or without nimotuzumab. Br J Cancer. 2020;123:1757–1766. doi: 10.1038/s41416-020-01064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patočka J. Biologically active pentacyclic triterpenes and their current medicine signification. J Appl Biomed. 2003;1:7–12. doi: 10.32725/jab.2003.002. [DOI] [Google Scholar]
- Pezzani R, Salehi B, Vitalini S, Iriti M, Zuñiga FA, Sharifi-Rad J, Martorell M, Martins N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: an update on the cancer perspective. Medicina (kaunas) 2019;55:110. doi: 10.3390/medicina55040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. A Review Curr Mol Med. 2018;18(6):343–351. doi: 10.2174/1566524018666181109121849. [DOI] [PubMed] [Google Scholar]
- Pitchai D, Roy A, Ignatius C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J Adv Pharm Technol Res. 2014;5:179–184. doi: 10.4103/2231-4040.143037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth S, Ray S, Bhattacharyya S, Mehrotra DG, Alam N, Mondal G, Nath P, Roy A, Biswas J, Murmu N. Lupeol evokes anticancer effects in oral squamous cell carcinoma by inhibiting oncogenic EGFR pathway. Mol Cell Biochem. 2016;417:97–110. doi: 10.1007/s11010-016-2717-y. [DOI] [PubMed] [Google Scholar]
- Ray S, Saha D, Alam N, Mitra Mustafi S, Mandal S, Sarkar A, Majumder B, Murmu N. Exposure to chewing tobacco promotes primary oral squamous cell carcinoma and regional lymph node metastasis by alterations of SDF1α/CXCR4 axis. Int J Exp Pathol. 2021;102:80–92. doi: 10.1111/iep.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren HY, Shen JX, Mao XM, Zhang XY, Zhou P, Li SY, Zheng ZW, Shen DY, Meng JR. Correlation between tumor vasculogenic mimicry and poor prognosis of human digestive cancer patients: a systematic review and meta-analysis. Pathol Oncol Res. 2019;25:849–858. doi: 10.1007/s12253-018-0496-3. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Scoazec JY. Laminin 332 in cancer: When the extracellular matrix turns signals from cell anchorage to cell movement. Semin Cancer Biol. 2020;62:149–165. doi: 10.1016/j.semcancer.2019.09.026. [DOI] [PubMed] [Google Scholar]
- Saha D, Mitra D, Alam N, Sen S, Mitra Mustafi S, Mandal S, Majumder B, Murmu N. Orchestrated expression of vasculogenic mimicry and laminin-5γ2 is an independent prognostic marker in oral squamous cell carcinoma. Int J Exp Pathol. 2022;103:54–64. doi: 10.1111/iep.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatani Y, Komiyama Y, Nakashiro KI, Uchida D, Fukumoto C, Shimura M, Hasegawa T, Kamimura R, Hitomi-Koide M, Hyodo T. Paclitaxel potentiates the anticancer effect of cetuximab by enhancing antibody-dependent cellular cytotoxicity on oral squamous cell carcinoma cells in vitro. Int J Mol Sci. 2020;21:E6292. doi: 10.3390/ijms21176292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K, Chowdhury S, Banerjee S, Mandal B, Mandal M, Majhi S, Brahmachari G, Ghosh J, Sil PC. Lupeol alters viability of SK-RC-45 (Renal cell carcinoma cell line) by modulating its mitochondrial dynamics. Heliyon. 2019;5:e02107. doi: 10.1016/j.heliyon.2019.e02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Yao N, Cheng S, Li L, Liu S, Yang Z, Shang G, Zhang D, Yao Z. Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer. Cancer Biol Med. 2019;16:299–311. doi: 10.20892/j.issn.2095-3941.2018.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Thavarool SB, Muttath G, Nayanar S, Duraisamy K, Bhat P, Shringarpure K, Nayak P, Tripathy JP, Thaddeus A, Philip S. Improved survival among oral cancer patients: findings from a retrospective study at a tertiary care cancer centre in rural Kerala. India World J Surg Oncol. 2019;17:15. doi: 10.1186/s12957-018-1550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lin P, Sun B, Zhang S, Cai W, Han C, Li L, Lu H, Zhao X (2014) Epithelial-mesenchymal transition regulated by EphA2 contributes to vasculogenic mimicry formation of head and neck squamous cell carcinoma. Biomed Res Int 2014:803914. 10.1155/2014/803914 [DOI] [PMC free article] [PubMed]
- Wang HF, Wang SS, Zheng M, Dai LL, Wang K, Gao XL, Cao MX, Yu XH, Pang X, Zhang M. Hypoxia promotes vasculogenic mimicry formation by vascular endothelial growth factor A mediating epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Cell Prolif. 2019;52:e12600. doi: 10.1111/cpr.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Gao XL, Liu X, Gao SY, Fan YL, Jiang YP, Ma XR, Jiang J, Feng H, Chen QM. CD133+ cancer stem-like cells promote migration and invasion of salivary adenoid cystic carcinoma by inducing vasculogenic mimicry formation. Oncotarget. 2016;7:29051–29062. doi: 10.18632/oncotarget.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun H, Zhang D, Fan D, Zhang Y, Dong X, Liu S, Yang Z, Ni C, Li Y. TP53INP1 inhibits hypoxia-induced vasculogenic mimicry formation via the ROS/snail signalling axis in breast cancer. J Cell Mol Med. 2018;22:3475–3488. doi: 10.1111/jcmm.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Zhang Y, Yu L, Zhu B, Wu S, Wang D. Vasculogenic mimicry and expression of ALDH1, Beclin1, and p16 correlate with metastasis and prognosis in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2018;11:1599–1609. [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Song W, Cheng Z, Yang D, Yu L. Expression of LGR5 in oral squamous cell carcinoma and its correlation to vasculogenic mimicry. Int J Clin Exp Pathol. 2017;10:11267–11275. [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Lou Y, Liu X, Peng X. Vasculogenic mimicry in head and neck tumors: a narrative review. Transl Cancer Res. 2021;10:3044–3052. doi: 10.21037/tcr-21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Zhou HM, Zhang X, Mu W, Hu JN, Liu GL, Li Q. Hypoxic induction of vasculogenic mimicry in hepatocellular carcinoma: role of HIF-1 α, RhoA/ROCK and Rac1/PAK signaling. BMC Cancer. 2020;20:32. doi: 10.1186/s12885-019-6501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ge Y, Ping X, Yu M, Lou D, Shi W. Synergistic apoptotic effects of silibinin in enhancing Paclitaxel toxicity in human gastric cancer cell lines. Mol Med Rep. 2018;18:1835–1841. doi: 10.3892/mmr.2018.9129. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Imani S, Shasaltaneh MD, Hosseinifard H, Zou L, Fan Y, Wen Q. The role of vascular mimicry as a biomarker in malignant melanoma: a systematic review and meta-analysis. BMC Cancer. 2019;19:1134. doi: 10.1186/s12885-019-6350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Huang S, Wang L, Yuan X, Dong Q, Zhang D, Wang X. Clinical and prognostic significance of HIF-1α overexpression in oral squamous cell carcinoma: a meta-analysis. World J Surg Oncol. 2017;15:104. doi: 10.1186/s12957-017-1163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.