Abstract
Trophoblast cell surface antigen 2 (TROP2) is a calcium-transducing transmembrane protein mainly involved in embryo development. The aberrant expression of TROP2 is observed in numerous cancers, including triple-negative breast cancer, gastric, colorectal, pancreatic, squamous cell carcinoma of the oral cavity, and prostate cancers. The main signaling pathways mediated by TROP2 are calcium signaling, PI3K/AKT, JAK/STAT, MAPKs, and β-catenin signaling. However, collective information about the TROP2-mediated signaling pathway is not available for visualization or analysis. In this study, we constructed a TROP2 signaling map with respect to its role in different cancers. The data curation was done manually by following the NetPath annotation criteria. The described map consists of different molecular events, including 8 activation/inhibition, 16 enzyme catalysis, 19 gene regulations, 12 molecular associations, 39 induced-protein expressions, and 2 protein translocation. The data of the TROP2 pathway map is made freely accessible through the WikiPathways Database (https://www.wikipathways.org/index.php/Pathway:WP5300).
Graphical abstract
Development of TROP2 signaling pathway map
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-023-00742-1.
Keywords: Cancer, Signalling pathway, Protein–protein interactions, WikiPathways, Triple-negative breast cancer
Introduction
The Trophoblast cell surface antigen 2 (TROP2), a calcium transducing glycoprotein, originally identified in trophoblasts and fetal tissues (Vranic and Gatalica 2022). It has stem-cell-like properties and is involved in activities like cell migration, proliferation, self-renewal, and survival (Ripani et al. 1998; Shvartsur and Bonavida 2015). It is also known to play a role in embryonic development with its expression reported in the intestinal epithelium of a 14-day embryo (Shvartsur and Bonavida 2015). The TROP2 protein is highly expressed on the surface of trophoblast cells, which can invade uterine decidua during placental implantation (Lipinski et al. 1981). TROP2 expression can also be seen at the tips of newly formed ileum and villi during the 15–16 days of an embryo. The TROP2-expressing cells then gradually disappear in the period from the 16th embryonic day till birth (Mustata et al. 2013). In contrast, it has also been observed that TROP2 is expressed in various cancers such as gastric cancer, pancreatic cancer, prostate cancer, and Triple-negative breast cancer (TNBC), and its expression has been associated with poor prognosis (Fong et al. 2008; Zhao et al. 2018, 2019; Hsu et al. 2020).
The TROP2, is encoded by the Tumor-associated calcium signal transducer 2 (TACSTD2) gene, which is located on the short arm of chromosome 1 at position 1p32 (Calabrese et al. 2001; Cubas et al. 2009). It is a 35 kDa, 323 amino acids long transmembrane glycoprotein. It comprises an extracellular, transmembrane, and intracellular domain with a cytoplasmic tail required for signaling (Linnenbach et al. 1989; Cubas et al. 2009). The Human TROP2 consists of a HIKE domain, a Phosphatidylinositol 4,5-bisphosphate (PIP2) binding site, and also a serine at 303, which can be phosphorylated by Protein Kinase C (PKC) (Goldenberg et al. 2018). PIP2 can get cleaved/hydrolysed by Phospholipase C (PLC) upon binding to the cytoplasmic tail of TROP2, leading to an increase in inositol 1,4,5-triphosphate (IP3) which will be followed by calcium release from the endoplasmic reticulum (Cubas et al. 2009). This free calcium can activate PKCs via a positive feedback loop, which leads to the phosphorylation of TROP2 and subsequent activation of Raf and NF-κB pathways. The release of calcium activates Mitogen-Activated Protein Kinase (MAPK) signaling which promotes the cell cycle (Cubas et al. 2010).
The TROP2 overexpression in osteosarcoma cells activates the mechanisms through Phosphoinositide 3-Kinase (PI3K), Akt serine/threonine kinase family (Akt) signaling pathway and regulates cell proliferation and migration (Gu et al. 2018). Phosphorylation of Janus Kinase 2 (JAK2) at (Y1007/1008) and Signal Transducer and Activator of Transcription 3 (STAT3) at (Y705) by TROP2 increases proliferation and metastasis in glioblastoma tissues and cell lines (Hou et al. 2019). Hsu et al (2020), reported that TROP2 overexpression in the neuroendocrine phenotype of metastatic prostate cancer (NEPC) induces tumor growth and metastasis by the upregulation of Poly ADP-Ribose Polymerase 1 (PARP1) (Hsu et al. 2020). TROP2 has been a target of interest for developing antibody–drug conjugates (ADC) for several cancers. Sacituzumab Govitecan (Trodelvy), an ADC has been approved by Food and Drug Administration (FDA) to treat TNBC patients with metastatic or unresectable tumors. TROP2 ADCs are also being tested against other epithelial cancers such as gastric, ovarian, renal and lung cancer to name a few (Bardia et al. 2021). The activation and aberrant expression of TROP2 is significantly associated with oncogenesis in various cancers, which has been reported across literature. We assembled all the molecular events involving activation/inhibition, enzyme catalysis, gene regulation, protein expression, molecular association and translocation events from the available literature. In this study, the molecular events that occur based on TROP-2 over expression and upon activation by cleavage have been compiled from the available literature. We compiled all the TROP-2 mediated molecular signaling events to develop a comprehensive signaling pathway map, similar to the previously published signaling pathways (Raju et al. 2014; Abhinand et al. 2016; Rex et al. 2020; Dagamajalu et al. 2021; Aravind et al. 2022).
Methodology
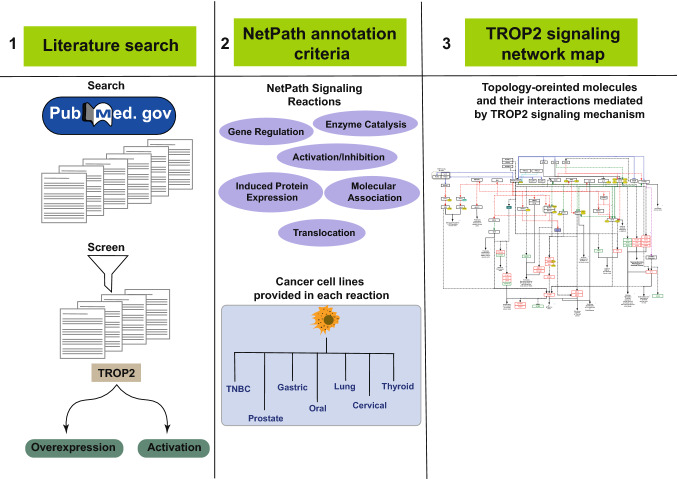
We carried out a literature survey for the research articles pertaining to the TROP2 mediated signaling molecules in the PubMed. The research articles were fetched from PubMed using the search term “TROP2”. A manual screening was performed to identify the articles with information regarding the signaling events mediated by TROP2. The molecular events were manually curated based on NetPath annotation criteria (Kandasamy et al. 2010). We curated only those signaling reactions which were triggered by the overexpression or activation of TROP2. These TROP2-mediated downstream reactions were classified into five events (1) gene regulation, (2) induced protein expression, (3) enzyme catalysis, (4) protein–protein interactions, (5) translocation, and (6) activation and inhibition events. Additional information such as the cell lines used in the study and the type of the experiment performed was also curated. Each signaling event described in the pathway were hyperlinked to the research articles from where they were retrieved. PathVisio software was used to develop the pathway map of TROP2-mediated signaling events (Kutmon et al. 2015).
Results
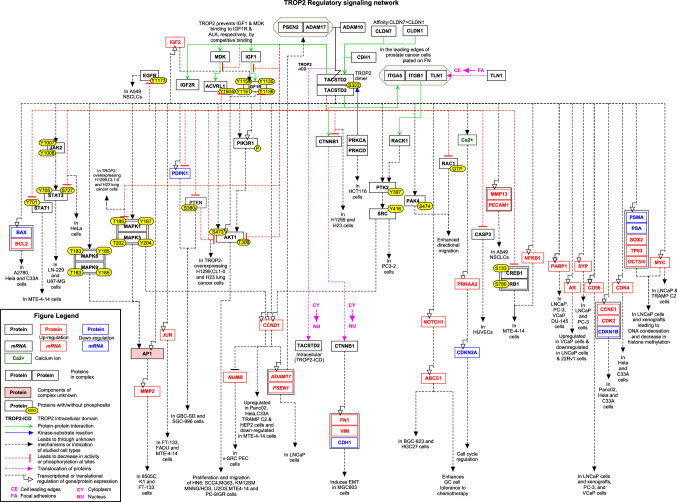
The PubMed search with query terms fetched 388 research articles. Manual screening of the research articles resulted in 28 articles, which had information pertinent to TROP2 mediated signaling. The curation of the signaling reactions yielded 19 gene regulations, 39 induced protein expression, 8 activation/inhibition, 16 enzyme catalysis, 12 molecular association and 2 translocation events (Supplementary data S1). These signaling events were assembled into a comprehensive TROP-2 signaling pathway map (Fig. 1).
Fig. 1.
Schematic representation of TROP2 mediated signaling mechanism in different types of cancers
Summary of TROP2 signaling pathway
The TROP2 protein has emerged as a therapeutic target due to its overexpression in several cancers such as TNBC, gastric, prostate, lung and pancreatic cancers. In prostate cancer cells, TROP2 is activated by regulated intramembrane proteolysis (RIP) by proteins such as ADAM Metallopeptidase Domain 17 (ADAM17) and Presenilin 1 (PSEN1) which separates the intracellular domain (ICD) from the extracellular domain (ECD) of TROP2. The activated ICD associates with Catenin Beta 1 (CTNNB1) and translocates into the nucleus where it upregulates cyclin D1 (CCND1), which promotes transformation through its self-renewal activity (Stoyanova et al. 2012). Further, it has also been reported that Cyclin D1 (CCND1) expression by the kinase activity of SRC induces the TROP2 ICD nuclear accumulation by the TROP2 cleavage complex (ADAM17 and PSEN1) and attenuated NUMB Endocytic Adaptor Protein (NUMB) expression, which is an inhibitor of TROP2 proteolytic complex. This demonstrated increased nuclear TROP2 ICD and attenuated NUMB expression results into reduced recurrence-free survival probability in human prostate cancer (Ju et al. 2016). Trerotola et al. (2021) reported that ADAM Metallopeptidase Domain 10 (ADAM10) also cleaves TROP2 between the arginine and threonine residues (R87 and T88), which triggers the progression of malignant tumors of skin, ovary, colon, lung, and pancreas (Pavsic 2021; Trerotola et al. 2021). The study in human colorectal and pancreatic cancer cell lines reported that TROP2 phosphorylation (S322) by Protein Kinase C α/δ has a causal effect on the interaction with claudin-7 (CLDN7), leading to its mislocalization, which results into the loss of membrane-localized CLDN7, which enhances cell motility (Mori et al. 2019).
The TROP2 activates MAPK pathway in several cancers to fuel their growth. Upon activation of MAPK pathway due to TROP2, STAT1 and STAT3 were activated via phosphorylation leading to upregulation of Jun, NF-κB, CREB1, Rb, STAT1 and STAT3 through activation of the MAPK1/3 (T202/Y204) phosphorylation and increased expression of CCND1, which promoted cancer growth in MTE 4–14 cells (Guerra et al. 2013). In thyroid cancer cells, TROP2 overexpression induces the phosphorylation of MAPK3/MAPK1 (T202/Y204) and MAPK8 (T183) which upregulated the expression of MMP2 through the activation of transcription factor AP1 and promotes the invasion and migration (Guan et al. 2017). The overexpression of TROP2 in human colorectal cancer cell lines induces the upregulation of CCND1, CCNE1, CDK2, activating transcription factor AP1 and downregulate CDKN1B via MAPK1/3 (T202/Y204) pathway which are involved in cancer cell growth and survival (Cubas et al. 2010).
The TROP2 being an oncogene is involved in the activation of MAPK1/3 pathway in Non-Small Cell Lung Cancer (NSCLC), thyroid cancer and cervical cancer (Howland and Wang 2008; Zuo et al. 2013; Guan et al. 2017; Guo et al. 2017). TROP2 overexpression in thyroid cancer cells promotes cell growth, migration and invasion by the increased expression of CCND1, CCNE1, CDK2, CDK4, BCL2 and attenuated expression of CDKN1B, BAX and CDH1 through phosphorylation of MAPK1/3 (T202 and Y204) (Liu et al. 2013). On the other hand, in lung adenocarcinoma cell line H1299, the downregulation of TROP2 expression by the hypermethylation of promoter region increases the binding of Insulin Like Growth Factor (IGF-1) to IGF-1R leading to downstream signaling through AKT and MAPK pathways. The increased phosphorylation of AKT1 and MAPK1/3, elevated the expression and nuclear translocation of CTNNB1 induces the enhanced expression of Snail Family Transcriptional Repressor 2 (SNAI2) and promotes cell proliferation and colony formation. Whereas, the 5-Aza-2'-deoxycytidine treatment on lung cancer cell (CL) lines induces enhanced expression of TROP2 and reduced AKT/CTNNB1 and MAPK signaling and decreased expression of SNAI2, which inhibits cell proliferation and colony forming efficiency (Lin et al. 2012).
The study by Sin et al. (2019) reported that upon overexpression of TROP2 in cervical cancer cells, it binds to Midkine (MDK) which is a ligand for Activin A Receptor Like Type 1 (ACVRL1) and also IGF1, which inhibits phosphorylation of ACVRL1 (Y1604) and IGF1R (Y1135/1136, Y1150/1151) respectively, in HeLa cells. This interaction between TROP2 with MDK and IGF1 eventually leads to decreased STAT3 and AKT1 phosphorylations, thereby reducing the oncogenecity of cervical cancer cells (Sin et al. 2019). Overexpression of TROP2 in lung squamous cell carcinoma cell line upregulated the expression of MKI67, CD34, PECAM1 and MMP13 and downregulated cleaved Caspase 3 (CASP3) via phosphorylation of MAPK1/3, which resulted in increased neovascularisation and angiogenesis of the lung squamous carcinoma cells (Wang et al. 2017). The overexpression of TROP2 in PC-9/GR lung squamous cell carcinoma cell line, has also been studied. TROP2 binds to IGF2R with the help of IGF-2 and induces the phosphorylation of IGF1R and AKT1 which increases the gefitinib drug resistance in NSCLC PC-9/GR cell line (Sun et al. 2021).
The overexpression of TROP2 in human breast cancer cells enhances tumor growth by activating AKT1 through the phosphorylation (S473 and T308) (Guerra et al. 2016). Guerra et al. 2021, reported that TROP2 tightly interacts with ADAM10 to cleave Cadherin 1 (CDH1) in KM12SM cells. The cleavage of CDH1 results in activation of CTNNB1 which promotes anti-apoptotic signaling and increased cell migration and survival in colon cancer cells (Guerra et al. 2021). Overexpression of TROP2 in OSCC cell lines SCC4 cells induces the phosphorylation of AKT1, elevated expression of PIK3R1, PDPK1 and downregulation of PTEN which mediated OSCC proliferation, migration and invasion of cells (Tang et al. 2019). Li et al 2017, reported that TROP2 overexpression in gall bladder cancer cells increased the expression of VIM, downregulated CDH1, PDPK1 and PTEN through PI3K/AKT (T308) pathway resulting in the proliferation, migration and metastasis and induction of EMT in GBC-SD and SGC-996 cells (Li et al. 2017).
The overexpression of TROP2 in OSCC cell lines and human tissues promoted intracellular calcium ion release resulting in the induction of S phase of cell cycle and positively regulating cell proliferation and division (Jia et al. 2020). The increased expression of TROP2 in glioblastoma tissues and glioblastoma cell lines induces the phosphorylation of JAK2 (Y1007/1008) and STAT3 (Y705) and elevated expression of CCND1, BIRC5, MMP2 and VEGFR2, which promotes proliferation and metastasis, and inhibits apoptosis (Hou et al. 2019). TROP2 overexpression has been associated with poor prognosis in gastric cancer (Zhao et al. 2016; Kushiyama et al. 2021). Kuai et al (2020) reported that the effects of TROP2 overexpression in HGC27 gastric cancer cells leads to multi drug resistance through elevated expression of ABCC1 (Kuai et al. 2020). TROP2 has also been reported to induce EMT in gastric cancer cells mediated through CTNNB1. Overexpression of TROP2 in gastric cancer cells lead to the nuclear accumulation of CTNNB1 encouraging EMT by increasing the expression of fibronectin and VIM and decreasing the expression of CDH1 (Zhao et al. 2019).
Trerotola et al. 2012 have carried several research studies to find out the role and signaling mechanism of TROP2 in prostate cancer. They reported that TROP2 is mainly involved in inhibition of prostate cancer cell adhesion to fibronectin by facilitating the interaction between Rack1 and ITGB1 which results in activation of SRC (Y416) and PTK2 (Y397) (Trerotola et al. 2012). TROP2 has been reported to enhance directional migration of PC3-2 cells on fibronectin through the interaction with ITGB1, ITGA5 and TLN1 and downregulation of RAC1-GTP (Trerotola et al. 2015). TROP2 induces the activation of PAK4 (S474), a kinase involved in increasing focal adhesion turnover via ITGB1 pathway and translocation of TLN1 from the leading edges to focal adhesions of the cells, which promotes metastasis in prostate cancer cells (Trerotola et al. 2013). In addition, Hsu et al (2020), demonstrated that TROP2 overexpression induces the upregulation of PARP1, neuroendocrine markers such as CD56, SYP, CHGA, proteins associated with lineage plasticity such as Sox2, Ezh2, c-myc, Oct3/4 and downregulation of AR, CK8, PSA and PSMA which are involved in the driving the metastatic prostate cancer with neuroendocrine phenotype (Hsu et al. 2020).
Conclusions
The TROP2 is mainly involved in diverse biological processes such as cell proliferation, apoptosis and drug resistance. The TROP2 signaling pathway was generated by assembling the information of signaling events triggered by TROP2 overexpression and activation from the literature. This pathway resource can help in identifying drug targets for diseases stimulated by TROP2 expression. We believe that the TROP2 signaling pathway will offer a base for accelerating further research which can help scientists in discerning disease progression as a result of TROP2 activation and design better therapeutics.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka, India, for the support to Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). Rajesh Raju is a recipient of the Young Scientist Award (YSS/2014/000607) from the Science and Engineering Research Board, Department of Science and Technology (DST), Government of India.
Declarations
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shubham Sukerndeo Upadhyay, Email: shubham@yenepoya.edu.in.
Rex Devasahayam Arokia Balaya, Email: rexprem@yenepoya.edu.in.
Sakshi Sanjay Parate, Email: sakship@yenepoya.edu.in.
Shobha Dagamajalu, Email: shobha_d@yenepoya.edu.in.
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in
Rohan Shetty, Email: shettyrohan@rediffmail.com.
Rajesh Raju, Email: rajeshraju@yenepoya.edu.in.
References
- Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10(4):347–354. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind A, Palollathil A, Rex DAB, Kumar KMK, Vijayakumar M, Shetty R, Codi JAK, Prasad TSK, Raju R. A multi-cellular molecular signaling and functional network map of C-C motif chemokine ligand 18 (CCL18): a chemokine with immunosuppressive and pro-tumor functions. J Cell Commun Signal. 2022;16(2):293–300. doi: 10.1007/s12079-021-00633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, Moroose R, Santin AD, Kalinsky K, Picozzi V, O'Shaughnessy J, Gray JE, Komiya T, Lang JM, Chang JC, Starodub A, Goldenberg DM, Sharkey RM, Maliakal P, Hong Q, Wegener WA, Goswami T, Ocean AJ. Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32(6):746–756. doi: 10.1016/j.annonc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- Calabrese G, Crescenzi C, Morizio E, Palka G, Guerra E, Alberti S. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet Cell Genet. 2001;92(1–2):164–165. doi: 10.1159/000056891. [DOI] [PubMed] [Google Scholar]
- Cubas R, Li M, Chen C, Yao Q. TROP2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796(2):309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Cubas R, Zhang S, Li M, Chen C, Yao Q. TROP2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagamajalu S, Rex DAB, Palollathil A, Shetty R, Bhat G, Cheung LWT, Prasad TSK. Correction to: a pathway map of AXL receptor-mediated signaling network. J Cell Commun Signal. 2021;15(1):149. doi: 10.1007/s12079-020-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, Gastl G, Spizzo G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99(8):1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–29006. doi: 10.18632/oncotarget.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu QZ, Nijiati A, Gao X, Tao KL, Li CD, Fan XP, Tian Z. TROP2 promotes cell proliferation and migration in osteosarcoma through PI3K/AKT signaling. Mol Med Rep. 2018;18(2):1782–1788. doi: 10.3892/mmr.2018.9083. [DOI] [PubMed] [Google Scholar]
- Guan H, Guo Z, Liang W, Li H, Wei G, Xu L, Xiao H, Li Y. TROP2 enhances invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways. BMC Cancer. 2017;17(1):486. doi: 10.1186/s12885-017-3475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, Lattanzio R, Piantelli M, Alberti S. The trop-2 signalling network in cancer growth. Oncogene. 2013;32(12):1594–1600. doi: 10.1038/onc.2012.151. [DOI] [PubMed] [Google Scholar]
- Guerra E, Trerotola M, Tripaldi R, Aloisi AL, Simeone P, Sacchetti A, Relli V, D'Amore A, La Sorda R, Lattanzio R, Piantelli M, Alberti S. Trop-2 induces tumor growth through AKT and determines sensitivity to AKT inhibitors. Clin Cancer Res. 2016;22(16):4197–4205. doi: 10.1158/1078-0432.CCR-15-1701. [DOI] [PubMed] [Google Scholar]
- Guerra E, Trerotola M, Relli V, Lattanzio R, Tripaldi R, Vacca G, Ceci M, Boujnah K, Garbo V, Moschella A, Zappacosta R, Simeone P, de Lange R, Weidle UH, Rotelli MT, Picciariello A, Depalo R, Querzoli P, Pedriali M, Bianchini E, Angelucci D, Pizzicannella G, Di Loreto C, Piantelli M, Antolini L, Sun XF, Altomare DF, Alberti S. Trop-2 induces ADAM10-mediated cleavage of E-cadherin and drives EMT-less metastasis in colon cancer. Neoplasia. 2021;23(9):898–911. doi: 10.1016/j.neo.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhu X, Zhao L, Li X, Cheng D, Feng K. Tumor-associated calcium signal transducer 2 regulates neovascularization of non-small-cell lung cancer via activating ERK1/2 signaling pathway. Tumour Biol. 2017;39(3):1010428317694324. doi: 10.1177/1010428317694324. [DOI] [PubMed] [Google Scholar]
- Hou J, Lv A, Deng Q, Zhang G, Hu X, Cui H. TROP2 promotes the proliferation and metastasis of glioblastoma cells by activating the JAK2/STAT3 signaling pathway. Oncol Rep. 2019;41(2):753–764. doi: 10.3892/or.2018.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Hsu EC, Rice MA, Bermudez A, Marques FJG, Aslan M, Liu S, Ghoochani A, Zhang CA, Chen YS, Zlitni A, Kumar S, Nolley R, Habte F, Shen M, Koul K, Peehl DM, Zoubeidi A, Gambhir SS, Kunder CA, Pitteri SJ, Brooks JD, Stoyanova T. TROP2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci U S A. 2020;117(4):2032–2042. doi: 10.1073/pnas.1905384117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Wang T, Ding G, Kuai X, Wang X, Wang B, Zhao W, Zhao Y. TROP2 inhibition of P16 expression and the cell cycle promotes intracellular calcium release in OSCC. Int J Biol Macromol. 2020;164:2409–2417. doi: 10.1016/j.ijbiomac.2020.07.234. [DOI] [PubMed] [Google Scholar]
- Ju X, Jiao X, Ertel A, Casimiro MC, Di Sante G, Deng S, Li Z, Di Rocco A, Zhan T, Hawkins A, Stoyanova T, Ando S, Fatatis A, Lisanti MP, Gomella LG, Languino LR, Pestell RG. v-Src oncogene induces TROP2 proteolytic activation via cyclin D1. Cancer Res. 2016;76(22):6723–6734. doi: 10.1158/0008-5472.CAN-15-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11(1):R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai X, Jia L, Yang T, Huang X, Zhao W, Zhang M, Chen Y, Zhu J, Feng Z, Tang Q. TROP2 promotes multidrug resistance by regulating notch1 signaling pathway in gastric cancer cells. Med Sci Monit. 2020;26:e919566. doi: 10.12659/MSM.919566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiyama S, Yashiro M, Yamamoto Y, Sera T, Sugimoto A, Nishimura S, Togano S, Kuroda K, Yoshii M, Tamura T, Toyokawa T, Tanaka H, Muguruma K, Nakada H, Ohira M. Clinicopathologic significance of TROP2 and phospho-TROP2 in gastric cancer. Mol Clin Oncol. 2021;14(5):105. doi: 10.3892/mco.2021.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutmon M, van Iersel MP, Bohler A, Kelder T, Nunes N, Pico AR, Evelo CT. PathVisio 3: an extendable pathway analysis toolbox. PLoS Comput Biol. 2015;11(2):e1004085. doi: 10.1371/journal.pcbi.1004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Teng S, Zhang Y, Zhang W, Zhang X, Xu K, Yao H, Yao J, Wang H, Liang X, Hu Z. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget. 2017;8(29):47052–47063. doi: 10.18632/oncotarget.16789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang YL, Jou YS, Hong TM, Yang PC. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med. 2012;4(6):472–485. doi: 10.1002/emmm.201200222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnenbach AJ, Wojcierowski J, Wu SA, Pyrc JJ, Ross AH, Dietzschold B, Speicher D, Koprowski H. Sequence investigation of the major gastrointestinal tumor-associated antigen gene family, GA733. Proc Natl Acad Sci U S A. 1989;86(1):27–31. doi: 10.1073/pnas.86.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981;78(8):5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu Y, Bao X, Tian J, Liu Y, Yang X. Overexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathway. PLoS ONE. 2013;8(9):e75864. doi: 10.1371/journal.pone.0075864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Akita K, Ojima K, Iwamoto S, Yamashita T, Morii E, Nakada H. Trophoblast cell surface antigen 2 (Trop-2) phosphorylation by protein kinase C alpha/delta (PKCalpha/delta) enhances cell motility. J Biol Chem. 2019;294(30):11513–11524. doi: 10.1074/jbc.RA119.008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustata RC, Vasile G, Fernandez-Vallone V, Strollo S, Lefort A, Libert F, Monteyne D, Perez-Morga D, Vassart G, Garcia MI. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 2013;5(2):421–432. doi: 10.1016/j.celrep.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Pavsic M. TROP2 forms a stable dimer with significant structural differences within the membrane-distal region as compared to EpCAM. Int J Mol Sci. 2021;22(19):10640. doi: 10.3390/ijms221910640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Palapetta SM, Sandhya VK, Sahu A, Alipoor A, Balakrishnan L, Advani J, George B, Kini KR, Geetha NP, Prakash HS, Prasad TS, Chang YJ, Chen L, Pandey A, Gowda H. A network map of FGF-1/FGFR Signaling System. J Signal Transduct. 2014;2014:962962. doi: 10.1155/2014/962962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DAB, Agarwal N, Prasad TSK, Kandasamy RK, Subbannayya Y, Pinto SM. A comprehensive pathway map of IL-18-mediated signalling. J Cell Commun Signal. 2020;14(2):257–266. doi: 10.1007/s12079-019-00544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripani E, Sacchetti A, Corda D, Alberti S. Human trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76(5):671–676. doi: 10.1002/(SICI)1097-0215(19980529)76:5<671::AID-IJC10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Shvartsur A, Bonavida B. TROP2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6(3–4):84–105. doi: 10.18632/genesandcancer.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin STK, Li Y, Liu M, Ma S, Guan XY. TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol Oncol. 2019;152(1):185–193. doi: 10.1016/j.ygyno.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Stoyanova T, Goldstein AS, Cai H, Drake JM, Huang J, Witte ON. Regulated proteolysis of TROP2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signaling. Genes Dev. 2012;26(20):2271–2285. doi: 10.1101/gad.196451.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Jia L, Wang T, Zhang Y, Zhao W, Wang X, Chen H. TROP2 binding IGF2R induces gefitinib resistance in NSCLC by remodeling the tumor microenvironment. J Cancer. 2021;12(17):5310–5319. doi: 10.7150/jca.57711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Tang Q, Jia L, Chen Y, Lin L, Kuai X, Gong A, Feng Z. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway. Int J Mol Med. 2019;44(6):2161–2170. doi: 10.3892/ijmm.2019.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M, Li J, Alberti S, Languino LR. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the beta1 integrin-RACK1 axis. J Cell Physiol. 2012;227(11):3670–3677. doi: 10.1002/jcp.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A, Languino LR. Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res. 2013;73(10):3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M, Ganguly KK, Fazli L, Fedele C, Lu H, Dutta A, Liu Q, De Angelis T, Riddell LW, Riobo NA, Gleave ME, Zoubeidi A, Pestell RG, Altieri DC, Languino LR. Trop-2 is up-regulated in invasive prostate cancer and displaces FAK from focal contacts. Oncotarget. 2015;6(16):14318–14328. doi: 10.18632/oncotarget.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M, Guerra E, Ali Z, Aloisi AL, Ceci M, Simeone P, Acciarito A, Zanna P, Vacca G, D'Amore A, Boujnah K, Garbo V, Moschella A, Lattanzio R, Alberti S. Trop-2 cleavage by ADAM10 is an activator switch for cancer growth and metastasis. Neoplasia. 2021;23(4):415–428. doi: 10.1016/j.neo.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic S, Gatalica Z. Trop-2 protein as a therapeutic target: a focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers. Bosn J Basic Med Sci. 2022;22(1):14–21. doi: 10.17305/bjbms.2021.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Guo Y, Wang M, Zhou T, Xue Y, Du G, Wei X, Wang J, Qi L, Zhang H, Li L, Ye L, Guo X, Wu X. The glial cell-derived neurotrophic factor (GDNF)-responsive phosphoprotein landscape identifies raptor phosphorylation required for spermatogonial progenitor cell proliferation. Mol Cell Proteomics. 2017;16(6):982–997. doi: 10.1074/mcp.M116.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhu H, Zhang S, Yong H, Wang W, Zhou Y, Wang B, Wen J, Qiu Z, Ding G, Feng Z, Zhu J. TROP2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget. 2016;7(5):6136–6145. doi: 10.18632/oncotarget.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kuai X, Zhou X, Jia L, Wang J, Yang X, Tian Z, Wang X, Lv Q, Wang B, Zhao Y, Huang W. TROP2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol Rep. 2018;40(2):759–766. doi: 10.3892/or.2018.6496. [DOI] [PubMed] [Google Scholar]
- Zhao W, Jia L, Kuai X, Tang Q, Huang X, Yang T, Qiu Z, Zhu J, Huang J, Huang W, Feng Z. The role and molecular mechanism of TROP2 induced epithelial-mesenchymal transition through mediated beta-catenin in gastric cancer. Cancer Med. 2019;8(3):1135–1147. doi: 10.1002/cam4.1934. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zuo T, Qin JY, Chen J, Shi Z, Liu M, Gao X, Gao D. Involvement of N-cadherin in the protective effect of glial cell line-derived neurotrophic factor on dopaminergic neuron damage. Int J Mol Med. 2013;31(3):561–568. doi: 10.3892/ijmm.2013.1226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.