Abstract
This article summarizes important molecular mechanisms that drive aging in human skin from the perspective of dermal fibroblasts. The dermis comprises the bulk of the skin and is largely composed of a collagen‐rich extracellular matrix (ECM). The dermal ECM provides mechanical strength, resiliency, and an environment that supports the functions of ibroblasts and other types of dermal cells. Fibroblasts produce the dermal ECM and maintain its homeostasis. Fibroblasts attach to the ECM and this attachment controls their morphology and function. During aging, the ECM undergoes gradual degradation that is nitiated by matrix metalloproteinases (MMPs). This degradation alters mechanical forces within the dermal ECM and disrupts he interactions between fibroblasts and the ECM thereby generating an aged fibroblast phenotype. This aged fibroblast phenotype is characterized by collapsed morphology, altered mechanosignaling, induction of CCN1, and activation of transcription factor AP‐1, with consequent upregulation of target genes including MMPs and pro‐inflammatory mediators. The TGF‐beta pathway coordinately regulates ECM production and turnover. Altered mechanical forces, due to ECM fragmentation, down-regulate the type II TGF‐beta receptor, thereby reducing ECM production and further increasing ECM breakdown. Thus, dermal aging involves a feed‐forward process that reinforces the aged dermal fibroblast phenotype and promotes age‐related dermal ECM deterioration. As discussed in the article, the expression of the aged dermal fibroblast phenotype involves both adaptive and cell‐autonomous mechanisms.
Graphical abstract
Keywords: Aging, Skin, Extracellular matrix, CCN1, Fibroblasts
The skin has two major compartments, the epidermis, and the dermis. The epidermis is composed of densely packed cells (keratinocytes) that are arranged in layers. Keratinocytes in the bottom layer (basal layer) undergo continuous cell division (Kanitakis 2002; Wong et al. 2016). A portion of these cells migrate toward the surface and undergo terminal differentiation, which produces a non-living barrier at the surface. This barrier protects against evaporation from within and the entry of toxins from the external environment (Jensen and Proksch 2009; Knox and O'Boyle 2021). The dermis resides below the epidermis and comprises the bulk of the skin. The dermis is largely composed of fibrillar collagen, which forms a three-dimensional extracellular matrix (ECM). The dermal ECM confers mechanical stability and forms a bioactive scaffold that supports the functions of dermal cells (Rutter 2000). The vasculature, hair follicles, sebaceous glands, sweat glands, and peripheral nerves are housed within the dermal ECM.
The dermal ECM is produced by dermal fibroblasts, which secrete collagens (primarily type I, type III, and type V collagens) in the form of soluble precursors (procollagens) (Gordon and Hahn 2010; Ricard-Blum 2011). Procollagens contain globular C-terminal and N-terminal domains that confer solubility. These globular domains are proteolytically removed to generate mature collagen, which spontaneously forms micro-fibrils (Gelse et al. 2003). Individual collagen fibrils are enzymatically covalently cross-linked by lysyl oxidase, which converts lysyl amines to aldehydes (Shoulders and Raines 2009). Collagen fibrils can also be cross-linked by non-enzymatic chemistry. Cross-linking is required for mechanical stability and the formation of higher-order bundles of collagen fibrils (Holmes et al. 2018). Collagen cross-links are highly resistant to proteolytic cleavage, which contributes to the low rate of collagen turnover in many connective tissues. Type I collagen in human skin has an extended half-life estimated to be 15 years (Verzijl et al. 2000).
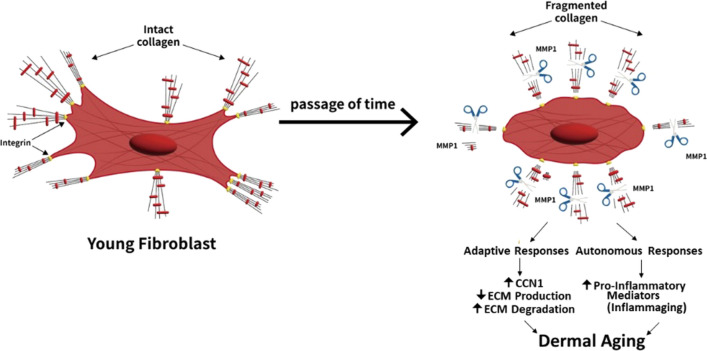
Histologic and ultrastructural studies demonstrate that fibroblasts in young skin reside within and between the bundles of densely packed, intact collagen fibrils. Fibroblasts directly bind to collagen fibrils via specific cell surface integrins. The direct attachment to intact collagen fibrils allows fibroblasts to achieve a stretched morphology, through mechanical forces generated by the assembly of the cytoskeleton (Fisher et al. 2008, 2009, 2014; Qin et al. 2017, 2014b; Quan et al. 2013b) in conjunction with the opposing mechanical resistance exerted by intact collagen bundles. In this state, fibroblasts display a “youthful phenotype”, which achieves a balanced homeostasis that maintains the composition, physical integrity, and function of the dermal ECM.
A prominent feature of aged skin is deleterious alterations of the dermal ECM (Fisher et al. 1996, 1997, 2002, 2008, 2014; Quan and Fisher 2015). During aging, collagen fibrils become fragmented due to rising levels of ECM-degrading matrix metalloproteinases, which are produced by fibroblasts. Collagen fibril cleavage is primarily initiated by matrix metalloproteinase-1 (MMP-1). Following initial cleavage, other members of the MMP family (such as MMP-2, MMP-3, and MMP-9) act to further degrade the fibrils. Collagen fibril proteolysis is incomplete, due in part to cross-linking, which results in the accumulation of residual cross-linked collagen fragments. Age-related proteolytic fragmentation deleteriously alters the organization and the mechanical properties of the dermal ECM. These alterations disrupt the mechanical and physical interactions of dermal fibroblasts with the ECM, thereby negatively impacting fibroblast morphology and function. ECM attachment sites are lost resulting in reduced mechanical forces and a smaller, less stretched conformation. In this state, fibroblasts display an “aged phenotype” that is detrimental to optimal skin health.
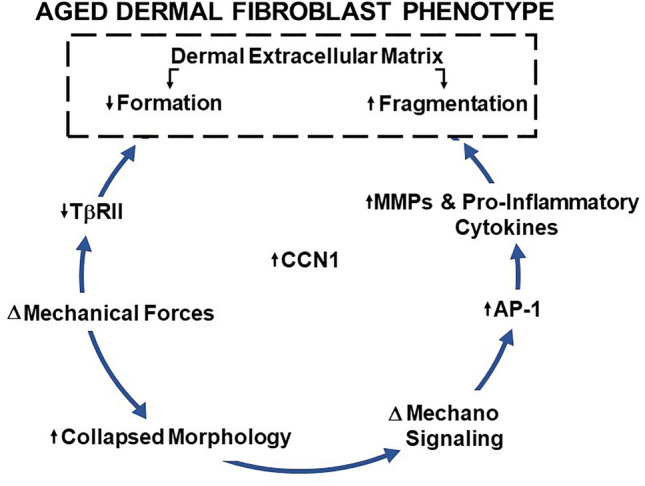
The major features that define the phenotype of fibroblasts in aged skin include (1) increased activity of ECM-degrading MMPs (Fisher et al. 1997, 2009; Qin et al. 2017; Quan and Fisher 2015; Quan et al. 2009, 2013a); (2) reduced collagen production, due to impaired TGF-β/Smad signaling (Purohit et al. 2016; Qin et al. 2018; Quan and Fisher 2015; Varani et al. 2000; Wang et al. 2007); and (3) establishment of an inflammatory dermal microenvironment (inflammaging), due to increased expression of pro-inflammatory cytokines (Pilkington et al. 2021; Qin et al. 2014a; Quan et al. 2011). Alterations of the dermal ECM microenvironment are directly related to age-related skin pathologies, such as increased fragility (Fisher et al. 2009, 2008; Varani et al. 2000), impaired vasculature support (Cheresh and Stupack 2008; Jacob 2003), poor wound healing (Eaglstein 1986, Holt et al. 1992, Valencia et al. 2001), and promotion of skin cancer (Achyut et al. 2013; Bhowmick et al. 2004; Bissell and Hines 2011; Fane and Weeraratna 2020; Pickup et al. 2013).
Aging is the highest risk factor for most human diseases (de Magalhaes 2013; Fane and Weeraratna 2020). The majority of aging research focuses on cell-autonomous mechanisms of aging such as DNA damage, mitochondrial dysfunction, impairment of gene transcription, oxidative damage of proteins and lipids, etc. These mechanisms often encompass or lead to a state of cellular senescence. Although a strict definition of senescence is elusive and a matter of current debate, senescence generally describes a state of irreversible loss of proliferative potential, resistance to death, altered metabolism, and elevated expression of secreted factors that promote inflammation, suppress immunity, and support the growth of transformed cells. However, all cells in vivo directly adhere to or exist close to connective tissues, which are largely comprised of ECM. The connective tissue ECM critically supports organ mechanical integrity/form and function. Importantly, it is becoming increasingly clear that aberrant ECM homeostasis is a hallmark of age-related disorders. For example, dysregulation of ECM composition, structure, abundance, and mechanical properties contributes to many age-related diseases including osteoarthritis (van der Kraan 2017), fibrosis (Rockey et al. 2015; Schaefer 2018), cardiovascular degeneration, and cancer (Fane and Weeraratna 2020; Goruppi and Dotto 2013; Ng and Brugge 2009; Vanharanta and Massague 2012). Since fibroblasts are primarily responsible for ECM homeostasis, these findings highlight the importance of the interplay between the ECM and fibroblasts in the biology of aging.
As described above, dermal aging involves the degeneration of the dermal ECM that is the result of age-related phenotypic alterations of dermal fibroblasts. Dermal aging is a feed-forward self-perpetuating process that is the manifestation of the inseparable connections between the phenotype of fibroblasts and their extracellular environment. Given this perspective of dermal aging, what are the mechanisms that give rise to the phenotype of fibroblasts in aged skin? In broad terms, there are two distinct possibilities: reversible adaptation to the degeneration of the surrounding dermal ECM environment or irreversible cell-autonomous responses to the passage of time. Evidence indicates that both adaptive and cell-autonomous mechanisms are involved in the establishment of the aged phenotype of fibroblasts. Interestingly, these two mechanisms appear to act primarily on distinct fibroblast functions.
There is abundant evidence that fibroblasts in aged skin can be stimulated to produce and maintain new dermal ECM. The “rejuvenation” of dermal fibroblasts is most evident in the context of anesthetic procedures that cause controlled wounding. Typically, these procedures employ lasers that cause superficial thermal wounding of aged facial skin. This wounding evokes the skin’s natural healing response, which in its later phases includes the deposition and remodeling of new dermal ECM by fibroblasts (Martin and Nunan 2015; Werner and Grose 2003). The wound environment converts fibroblasts from an aged to a youthful phenotype, with respect to ECM homeostasis. Similar “rejuvenation “of fibroblasts in aged skin is observed following injection of cross-linked hyaluronic acid dermal filler, which introduces mechanical forces within the dermis through its space-filling properties. These forces cause fibroblasts to stretch. Fibroblasts perceive stretch through mechanical sensing pathways that stimulate the production of new ECM (Chen et al. 2021; Rogers et al. 2021). “Rejuvenation” of fibroblasts in aged skin is also observed following topical application of all-trans retinoic acid, the active metabolite of vitamin A (Cho et al. 2005; Kafi et al. 2007). All-trans retinoic acid acts through nuclear receptors that regulate the transcription of specific genes. In aged skin, topical application of all trans retinoic acid re-programs dermal fibroblasts to produce a new extracellular matrix (Griffiths et al. 1993, Kang et al. 2001). Taken together, the above studies support the concept that the phenotype of fibroblasts in aged skin, with respect to dermal ECM homeostasis, is reversible and therefore likely to reflect adaptive responses to the age-related degeneration of the dermal ECM environment.
Further evidence for the adaptive nature of the phenotype of fibroblasts in aged skin comes from a recent study that quantified global gene expression of dermal fibroblasts cultured from young and aged skin (Cui et al. 2022). At early passage, primary cultures of dermal fibroblasts from young (20–30 years of age) and aged (> 80 years of age) individuals displayed similar rates of growth and strikingly similar levels of expression of genes that encode for proteins that comprise the dermal extracellular matrix. These ECM-related genes included 42 members of the collagen family, 29 genes that encode proteoglycans, and 157 genes that encode glycoproteins. Although genes that encode ECM-related proteins were similarly expressed in cultured fibroblasts from young and aged skin, differences in gene expression were detected. A total of 477 genes were differentially expressed: 300 upregulated and 177 downregulated in fibroblasts from aged versus young skin. Interestingly, in silico analyses of upregulated genes and the potential protein–protein interactions among their products revealed statistically significant enrichment in cytokine and chemokine-mediated signaling pathways, including cytokine-cytokine receptor interactions, TNF signaling pathway, and chemokine activity. Down-regulated genes and the protein–protein interactions among their products were enriched in the pathways that regulate lipid biosynthesis, and fatty acid metabolism. The finding that fibroblasts, which have been removed from their in vivo dermal environment and placed in culture, exhibit age-dependent differences in gene expression suggests that cell-autonomous mechanisms, in addition to adaptive mechanisms described above, contribute to the phenotype of fibroblasts in aged skin. The possibility that adaptive mechanisms and cell-autonomous mechanisms regulate distinct features of the aged phenotype, i.e., ECM homeostasis and pro-inflammatory pathways (termed inflammation), respectively, is especially intriguing. Optimal therapeutic approaches to improve the health of aged skin may need to employ a combination of strategies that target adaptive and cell-autonomous mechanisms.
As stated above, aging is a major risk factor for cancer. This connection between aging and cancer is especially true in the case of epithelial (keratinocyte) skin cancer, which is the most common form of human cancer, with over three million people diagnosed annually (more than 50% of all cancers in the USA) (de Magalhaes 2013). It is estimated that 40–50% of Americans who live to age 65 will have keratinocyte skin cancer at least once, according to the NCI (Kaufman 2009). Although oncogenic mutations are necessary for the development of keratinocyte skin cancer, the accumulation of mutations with age does not fully explain why keratinocyte skin cancer is so common in the elderly. Multiple key somatic driver mutations such as mutations in Notch and p53 are readily detected in physiologically healthy normal skin (Jonason et al. 1996, Liggett and DeGregori 2017, Martincorena et al. 2015, Rozhok and DeGregori 2015). Beyond the accumulation of mutations, there must be other, unknown age-associated mechanisms that drive the formation of keratinocyte cancer. Emerging evidence indicates that alterations in the stromal extracellular matrix and adaptive responses of stromal cells can precede the development of carcinoma. Indeed, recent findings have demonstrated that precancerous stroma can play a critical role in both cancer formation and initiation (field cancerization) (Bhowmick et al. 2004; Bissell and Hines 2011; Fane and Weeraratna 2020; Hu et al. 2012; Kaur et al. 2019; Nelson and Bissell 2006; Vanharanta and Massague 2012).
CCN1 is a multi-functional, secreted ECM-associated matricellular protein (Chen and Du 2007; Lau 2011). It is highly expressed in dermal fibroblasts in aged human skin (Cole et al. 2018; Quan et al. 2011). Genetically modified mice that express elevated levels of CCN1 in dermal fibroblasts exhibit accelerated dermal aging, characterized by loss and fragmentation of the dermal ECM (Quan et al. 2021). In these CCN1 mice, fibroblasts exhibit an aged phenotype characterized by increased expression of MMPs, reduced production of collagen, a contracted morphology, and increased levels of pro-inflammatory mediators, thereby mimicking the aged phenotype of fibroblasts in aged humans. CCN1 mice are an ideal model to investigate the role of dermal ECM aging on the formation of keratinocyte skin cancer. Importantly, CCN1 mice exhibit greatly enhanced skin tumor formation, in two independent experimental models of skin carcinogenesis. In both models, tumors resembling squamous cell cancer (SCC) occurred only in CCN1-expressing mice. No SCC-like tumors were detected in age and sex-matched littermates that did not express CCN1. In both models, enhanced tumor formation was dependent on the development of the age-related dermal ECM phenotype. In addition, fibroblast-specific knockout of CCN1 significantly reduced tumor formation. These data support the concept that the age-related fibroblast phenotype and consequent alterations of the dermal microenvironment are critical mediators of the development of keratinocyte cancer. This concept provides a mechanistic connection between aging and cancer incidence and helps to explain the high prevalence of skin cancer in the elderly.
In summary, the dermal ECM is primarily composed of bundles of collagen fibrils, which comprise the bulk of skin. Collagen fibrils are stabilized by covalent cross-links that have an exceedingly long half-life (Verzijl et al. 2000). Dermal fibroblasts produce the dermal ECM and reside within the bundles of collagen fibrils. Direct binding of fibroblasts to collagen fibrils establishes communication, among fibroblasts and the ECM, that is mediated by biochemical and mechanical signaling pathways. These pathways regulate fibroblast phenotype, i.e., morphology and functions, that determine the condition of the dermal ECM.
Our working model for the process of skin aging from the perspective of dermal fibroblasts is depicted in Fig. 1. During aging, the dermal ECM is gradually cleaved by proteases thereby deleteriously altering the composition, organization, and mechanical properties of the ECM and the phenotype fibroblasts residing within the ECM. The adaptive responses of fibroblasts to the degeneration of their ECM environment result in the upregulation of matrix metalloproteinases and downregulation of ECM structural proteins. Thereby, driving further ECM degradation in a self-perpetuating cycle. Coincident with adaptation to the degeneration ECM environment fibroblasts also undergo cell autonomous alterations that result in the upregulation of inflammatory mediators. environment ECM degradation and a pro-inflammatory milieu act in concert to create a dermal environment that promotes age-related skin diseases including keratinocyte cancer. The above scenario provides a useful framework for further investigations of mechanisms that regulate fibroblast functions during aging, especially those involved in mechano-sensing and epigenetic processes. Perhaps information gained from investigating these topics will shed light on the molecular pathways that cause fibroblasts to acquire a phenotype that reinforces rather than reverses the damaging effects of aging. Such knowledge could lead to therapeutic strategies that would yield healthy skin throughout a lifetime.
Fig. 1.
Working model for the process of skin aging from the perspective of dermal fibroblasts as described above
Acknowledgements
This work was supported by the National Institute of Health (AG054835 to GJ Fisher & T Quan; AG051849 to GJ Fisher & T Quan and P30AR075043 to Gudjonsson).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achyut BR, Bader DA, Robles AI, Wangsa D, Harris CC, Ried T, et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-beta signaling. PLoS Genet. 2013;9(2):e1003251. doi: 10.1371/journal.pgen.1003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100(6):1337–1345. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- Chen K, Kwon SH, Henn D, Kuehlmann BA, Tevlin R, Bonham CA, et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat Commun. 2021;12(1):5256. doi: 10.1038/s41467-021-25410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene. 2008;27(48):6285–6298. doi: 10.1038/onc.2008.304. [DOI] [PubMed] [Google Scholar]
- Cho S, Lowe L, Hamilton TA, Fisher GJ, Voorhees JJ, Kang S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J Am Acad Dermatol. 2005;53(5):769–774. doi: 10.1016/j.jaad.2005.06.052. [DOI] [PubMed] [Google Scholar]
- Cole MA, Quan T, Voorhees JJ, Fisher GJ. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Commun Signal. 2018;12(1):35–43. doi: 10.1007/s12079-018-0459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Worthen C, Haas R, Grill S, Shi M, Tsoi LC, et al. The phenotype of dermal fibroblasts in young vs. aged human skin: adaptation to dermal extracellular matrix deterioration and cell autonomous responses. J Investig Dermatol. 2022;142(8):S24. doi: 10.1016/j.jid.2022.05.148. [DOI] [Google Scholar]
- de Magalhaes JP. How ageing processes influence cancer. Nat Rev Cancer. 2013;13(5):357–365. doi: 10.1038/nrc3497. [DOI] [PubMed] [Google Scholar]
- Eaglstein WH. Wound healing and aging. Dermatol Clin. 1986;4(3):481–484. doi: 10.1016/S0733-8635(18)30811-8. [DOI] [PubMed] [Google Scholar]
- Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174(1):101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Sachs DL, Voorhees JJ. Ageing: collagenase-mediated collagen fragmentation as a rejuvenation target. Br J Dermatol. 2014;171(3):446–449. doi: 10.1111/bjd.13267. [DOI] [PubMed] [Google Scholar]
- Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339(1):247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goruppi S, Dotto GP. Mesenchymal stroma: primary determinant and therapeutic target for epithelial cancer. Trends Cell Biol. 2013;23(12):593–602. doi: 10.1016/j.tcb.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329(8):530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Holmes DF, Lu Y, Starborg T, Kadler KE. Collagen fibril assembly and function. Curr Top Dev Biol. 2018;130:107–142. doi: 10.1016/bs.ctdb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A. Effect of age on wound healing in healthy human beings. Surgery. 1992;112(2):293–297. [PubMed] [Google Scholar]
- Hu B, Castillo E, Harewood L, Ostano P, Reymond A, Dummer R, et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149(6):1207–1220. doi: 10.1016/j.cell.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57(5–6):195–202. doi: 10.1016/S0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Proksch E. The skin's barrier. G Ital Dermatol Venereol. 2009;144(6):689–700. [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA. 1996;93(24):14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, Hamilton TA, et al. Improvement of naturally aged skin with vitamin A (retinol) Arch Dermatol. 2007;143(5):606–612. doi: 10.1001/archderm.143.5.606. [DOI] [PubMed] [Google Scholar]
- Kang S, Fisher GJ, Voorhees JJ. Photoaging: pathogenesis, prevention, and treatment. Clin Geriatr Med. 2001;17(4):643–659. doi: 10.1016/S0749-0690(05)70091-4. [DOI] [PubMed] [Google Scholar]
- Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12(4):390–399. [PubMed] [Google Scholar]
- Kaufman SR. Developments in age-related macular degeneration: diagnosis and treatment. Geriatrics. 2009;64(3):16–19. [PubMed] [Google Scholar]
- Kaur A, Ecker BL, Douglass SM, Kugel CH, 3rd, Webster MR, Almeida FV, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9(1):64–81. doi: 10.1158/2159-8290.CD-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox S, O'Boyle NM. Skin lipids in health and disease: a review. Chem Phys Lipids. 2021;236:105055. doi: 10.1016/j.chemphyslip.2021.105055. [DOI] [PubMed] [Google Scholar]
- Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68(19):3149–3163. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett LA, DeGregori J. Changing mutational and adaptive landscapes and the genesis of cancer. Biochim Biophys Acta Rev Cancer. 2017;1867(2):84–94. doi: 10.1016/j.bbcan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348(6237):880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MR, Brugge JS. A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell. 2009;16(6):455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13(11):788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington SM, Bulfone-Paus S, Griffiths CEM, Watson REB. Inflammaging and the Skin. J Invest Dermatol. 2021;141(4S):1087–1095. doi: 10.1016/j.jid.2020.11.006. [DOI] [PubMed] [Google Scholar]
- Purohit T, He T, Qin Z, Li T, Fisher GJ, Yan Y, et al. Smad3-dependent regulation of type I collagen in human dermal fibroblasts: impact on human skin connective tissue aging. J Dermatol Sci. 2016;83(1):80–83. doi: 10.1016/j.jdermsci.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Qin Z, Okubo T, Voorhees JJ, Fisher GJ, Quan T. Elevated cysteine-rich protein 61 (CCN1) promotes skin aging via upregulation of IL-1beta in chronically sun-exposed human skin. Age (dordr) 2014;36(1):353–364. doi: 10.1007/s11357-013-9565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Voorhees JJ, Fisher GJ, Quan T. Age-associated reduction of cellular spreading/mechanical force up-regulates matrix metalloproteinase-1 expression and collagen fibril fragmentation via c-Jun/AP-1 in human dermal fibroblasts. Aging Cell. 2014;13(6):1028–1037. doi: 10.1111/acel.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Balimunkwe RM, Quan T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br J Dermatol. 2017;177(5):1337–1348. doi: 10.1111/bjd.15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Fisher GJ, Voorhees JJ, Quan T. Actin cytoskeleton assembly regulates collagen production via TGF-beta type II receptor in human skin fibroblasts. J Cell Mol Med. 2018;22(9):4085–4096. doi: 10.1111/jcmm.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61(5):427–434. doi: 10.1159/000371708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14(1):20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Robichaud P, Voorhees JJ, Fisher GJ. CCN1 contributes to skin connective tissue aging by inducing age-associated secretory phenotype in human skin dermal fibroblasts. J Cell Commun Signal. 2011;5(3):201–207. doi: 10.1007/s12079-011-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Little E, Quan H, Qin Z, Voorhees JJ, Fisher GJ. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J Invest Dermatol. 2013;133(5):1362–1366. doi: 10.1038/jid.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Wang F, Shao Y, Rittie L, Xia W, Orringer JS, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133(3):658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Xiang Y, Liu Y, Qin Z, Yang Y, Bou-Gharios G, et al. Dermal fibroblast CCN1 expression in mice recapitulates human skin dermal aging. J Invest Dermatol. 2021;141(4S):1007–1016. doi: 10.1016/j.jid.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Bell PD, Hill JA. Fibrosis–a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- Rogers JD, Holmes JW, Saucerman JJ, Richardson WJ. Mechano-chemo signaling interactions modulate matrix production by cardiac fibroblasts. Matrix Biol plus. 2021;10:100055. doi: 10.1016/j.mbplus.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhok AI, DeGregori J. Toward an evolutionary model of cancer: considering the mechanisms that govern the fate of somatic mutations. Proc Natl Acad Sci USA. 2015;112(29):8914–8921. doi: 10.1073/pnas.1501713112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter N. The dermis. Semin Neonatol. 2000;5(4):297–302. doi: 10.1053/siny.2000.0016. [DOI] [PubMed] [Google Scholar]
- Schaefer L. Decoding fibrosis: mechanisms and translational aspects. Matrix Biol. 2018;68–69:1–7. doi: 10.1016/j.matbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):401–421. doi: 10.1067/mjd.2001.111633. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat Rev Rheumatol. 2017;13(3):155–163. doi: 10.1038/nrrheum.2016.219. [DOI] [PubMed] [Google Scholar]
- Vanharanta S, Massague J. Field cancerization: something new under the sun. Cell. 2012;149(6):1179–1181. doi: 10.1016/j.cell.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114(3):480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25(2):92–98. doi: 10.1111/exd.12832. [DOI] [PubMed] [Google Scholar]