Abstract
Prostate cancer (PCa) has long been the leading cause of cancer-associated deaths among male worldwide. Our previous studies have shown that Bloom syndrome protein (BLM) plays a vital role in PCa proliferation, yet the underlying molecular mechanism remains largely obscure. Mechanistically, BLM directly interacted with hepatoma-derived growth factor (HDGF). Functionally, BLM and HDGF knockdown resulted in the higher impairment of PC3 proliferation, clonogenicity, migration and invasion than that their counterpart with either BLM or HDGF knockdown exclusively. Of note, HDGF overexpression expedited, whereas its knockdown suppressed, PC3 proliferation, clonogenicity, migration and invasion. Additionally, the potentiation or attenuation was partially antagonized upon BLM depletion or overexpression. In line with the vitro data, the impact of BLM and HDGF on tumor growth was investigated in mouse xenograft models. ChIP-seq, dual-luciferase reporter and western blotting assays were employed to expound the regulatory network in PC3 cells. The results unveiled that HDGF activated KRAS and suppressed RhoA transcription, and that the function of HDGF was mediated, in part, by interaction with BLM. Accordingly, the MAPK/ERK pathway was activated. Moreover, the regulation of HDGF on KRAS and RhoA had a signal crosstalk. To recapitulate, BLM and HDGF may serve as novel prognostic markers and potential therapeutic targets in PCa.
Keywords: BLM, HDGF, Prostate cancer, Malignancy, MAPK/ERK pathway
Introduction
Prostate cancer (PCa) is the second most frequent male cancer and the fifth leading cause of cancer death. In 2020, an estimated 1.4 million new cases were diagnosed and 375,000 deaths occurred worldwide (Sung et al. 2021). In 1986, the PSA test was approved as a diagnostic, screening and monitoring tool for the early detection of PCa by the United States Food and Drug Administration (Sohn 2015). The main disadvantage of the PSA test is lack of specificity and sensitivity, thus leading to unnecessary biopsy and the diagnosis of indolent PCa. Moreover, the high risk of overdiagnosis and overtreatment has been consequently increasing (Schroder et al. 2009). Practically, other potential prognostic biomarkers of PCa, including PSCA (Gu et al. 2000; Zhao et al. 2009) and uPA (A Helenius et al. 2006; Shariat et al. 2008) have been identified. However, the prognosis of advanced PCa remains poor. Androgen deprivation therapy is still the single most effective treatment for the initial therapy of advanced PCa while progresses to castration-resistant prostate cancer (CRPC) (Mostaghel 2014). Abiraterone and enzalutamide are pivotal AR-inducing drugs for treating CRPC. Unfortunately, some patients are resistant to these drugs and invariably succumb to diseases (Beer et al. 2014; Chi et al. 2019; Clegg et al. 2012). THZ531 and RNASEH2B possess significant synergies with multiple AR antagonists (Tsujino et al. 2021). Metformin, an oral biguanide drug has captured researchers’ attention for its antiproliferative and anticancer effects on PCa. Nevertheless, the relationship between metformin utilization and PCa is far from unambiguous (Ahn et al. 2020; Rothermundt et al. 2014). A deeper elucidation of the latent molecular mechanisms of PCa development and progression may afford novel target therapy for PCa. Thus, the identification of crucial biomarkers for PCa is imperative.
BLM, an important member of the RecQ family of helicases, is associated with an increase in cancer predisposition (Thakkar et al. 2022). It is known for its functions in DNA replication, repair and telomere maintenance (Rezazadeh 2013). Furthermore, as a nucleolus protein, mutation in BLM contributes to severe growth defects and a predisposition toward cancer and other diseases (Weeks et al. 2019). Qun Wang et al. identify seven novel PCa-related genes, and BLM is ranked the first among them (Wang et al. 2015). Our previous studies have shown that cell proliferation was retarded in BLM-silenced PC3 cells. (Chen et al. 2019). Besides, we also found that EZH2 can regulate the expression of MDM2 by interacting with BLM and activating the p53 signaling pathway in PCa (Ruan et al. 2021). Nonetheless, we appeared to have only scratched the surface of BLM function in PCa, let alone its mode of action and regulation details in PC3. Similar to BLM, HDGF is a nucleolar protein (Kishima et al. 2002) that is highly expressed in various malignancies, including hepatocellular carcinoma, non-small-cell lung cancer, pancreatic cancer, gastric cancer and melanoma (Hu et al. 2003; Ren et al. 2004; Tsai et al. 2013; Uyama et al. 2006; Yamamoto et al. 2006). Of note, HDGF, as a transcription factor, has been predicted to bind to the promoter of BLM to facilitate its transcription using the GeneCards database (https://www.genecards.org). As a vital regulator of a broad range of cancer cell activities, HDGF plays crucial roles in cancer cell transformation, apoptosis, angiogenesis, and metastasis as well as interacts with several different protein-binding partners (Bao et al. 2014). Moreover, HDGF is a survival-related protein in PCa oncogenesis (Shetty et al. 2016). Taken together, these studies suggested that existed in a core function regarding BLM and HDGF in PCa, therby warranting further investigation in detail.
In this report, we observed that BLM and HDGF were strikingly high expression and they exhibited remarkably positive interrelation in prostate adenocarcinomas (p = 0.0009). As an aside, BLM directly interacted with HDGF in PC3. In the present study, we determined the expression pattern, functional role and underlying mechanism of BLM. Our study highlighted the importance of BLM in promoting PCa malignancy by interacting with HDGF. Not only that, HDGF-mediated modulation of downstream genes led to KRAS transcriptional activation and RhoA transcriptional supression, thus resulting in the activation of MAPK/ERK pathway.
Materials and methods
Cell culture and reagents
The RWPE-1, WPMY-1, LNCaP, 22RV1, PC3 and DU145 cell lines were obtained from Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China), and HEK293T cells were purchased from Kunming (Kunming, China). Cell line identity was authenticated using short tandem repeat, and cell cultures were routinely assessed for mycoplasma contamination (Yang et al. 2021). RWPE-1 cells were cultured in a customized medium (ZQXZ Bio, Shanghai, China), whereas LNCaP cells were cultured in RPMI 1640 (Gibco, MA, USA) medium supplemented with 1% sodium pyruvate (Life Technologies, CA, USA). 22RV1 and DU145 cells were cultured in RPMI 1640 (Gibco BRL, Grand Island, NY, USA), PC3 cells were cultured in DMEM/F12 (Gibco BRL, Grand Island, NY, USA), and WPMY-1 and HEK293T cells were cultured in DMEM/high glucose (HyClone, Logan, Utah, USA). All media were supplemented with 10% fetal bovine serum (BI, Beit HaEmek, Israel) and 1% penicillin (100 U mL−1)-streptomycin (100 μg mL−1) solution (HyClone, Logan, UT, USA). All cells were cultured in a humidified atmosphere of 5% CO2 at 37 ℃.
Plasmids and short hairpin RNA transfection
BLM (pEGFP-C1-BLM) and HDGF (pDsRed2-C1-HDGF) overexpression plasmids were purchased from TsingKe Biotechnology (Chongqing, China). The different fragments of the KRAS and RhoA promoter regions were cloned into the pGL4.10-basic vector by Genecreat (Wuhan, China). The sequences of short hairpin RNA targeting BLM and HDGF were listed in Table 1. All shRNAs and the negative control shRNA were synthesized by GenePharma Technology (Shanghai, China). All plasmids and shRNAs were then transfected using FuGENE®HD (Promega Corporation, Madison, WI, USA).
Table 1.
The sequences of short hairpin RNA
| Targeting gene | Sequences |
|---|---|
| sh-BLM-1 | 5'-CACCGGGTCAAGGACTTCTTTAAAATTCAAGAGATTTTAAAGAAGTCCTTGACCCTTTTTTG-3' |
| sh-BLM-2 | 5'-CACCGCCACATGTAGAAAGATATCTTTCAAGAGAAGATATCTTTCTACATGTGGCTTTTTTG-3' |
| sh-BLM-3 | 5'-CACCGCGGCCAATTAAATCAGTATCATTCAAGAGATGATACTGATTTAATTGGCCGTTTTTTG-3' |
| sh-BLM-4 | 5'-CACCGAGCACATCTGTAAATTAATTTCAAGAGAATTAATTTACAGATGTGCTCTTTTTTG-3' |
| sh-HDGF-1 | 5'-CACCGGAATCCAAGGAGAAGTTTGGTTCAAGAGACCAAACTTCTCCTTGGATTCCTTTTTTG-3' |
| sh-HDGF-2 | 5'-CACCGGAAGAAGAGGAGGAGGATGATTCAAGAGATCATCCTCCTCCTCTTCTTCCTTTTTTG-3' |
| sh-HDGF-3 | 5'-CACCGCCGTGAAATCAACAGCCAACTTCAAGAGAGTTGGCTGTTGATTTCACGGCTTTTTTG-3' |
| sh-HDGF-4 | 5'-CACCGGCTACCAAGGAAGATGCTGATTCAAGAGATCAGCATCTTCCTTGGTAGCCTTTTTTG-3' |
Lentivirus-mediated interference
The recombinant lentivirus short hairpin RNA targeting BLM (LV-BLM-RNAi) and HDGF (LV-HDGF-RNAi), the recombinant lentivirus overexpressing BLM (LV-BLM) and HDGF (LV-HDGF) were purchased from Genechem Co., Ltd. (Shanghai, China). The CON254 (Ubi-MCS-SV40-puromycin) was utilized as the control for the overexpression of the empty vector, whereas CON389 (hU6-MCS-CMV-Neomycin) was the control shRNA. These lentiviral particles were transfected into PC3 using HiTransG A. The transfected cells were screened utilizing puromycin (2 μg/mL) or neomycin (400 μg/mL) for at least 1 week.
Co-immunoprecipitation (Co-IP) assay
PC3 cells cultured in a 25-cm2 cell culture flask were lysed with IP lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM DTT, 1 mM PMSF, 1 × protease, and phosphatase inhibitor cocktails. To investigate the interaction between endogenous BLM and HDGF, the supernatants of cell lysates were incubated with protein A/G beads (Thermo Fisher Scientific, Waltham, MA, USA) overnight after the protein A/G beads had been incubated with the appropriate antibodies (Anti-Blooms Syndrome, ab2179, Abcam, Cambridge, USA; HDGF Polyclonal antibody, 11,344–1-AP, Proteintech, Wuhan, China) for 2 h at 4 ℃. The beads were washed with lysis buffer and boiled via SDS-PAGE loading buffer. The precipitated proteins were separated using SDS-PAGE and transferred to PVDF membranes for further analysis.
Silver staining and mass spectrometry (MS)
After the electrophoresis of the samples containing precipitate proteins, the protein gel was stained using a silver staining kit (Solarbio, Beijing, China). Then, the protein bands were visualized and analyzed via MS (Genecreat, Wuhan, China).
Western blotting analysis
Cells were lysed for 30 min on ice with RIPA lysis buffer containing protease inhibitor cocktail and phosphatase inhibitor cocktails (Solarbio, Beijing, China). The protein concentration was measured using the BCA protein assay (Solarbio). Upon determining the concentration, 20 μg of protein was separated via SDS-PAGE, and the following antibodies were used: BLM (Bioss, Beijing, China); HDGF, PCNA, and GAPDH (Proteintech, Wuhan, China); MEK1/2 and Ki67 (Abcam, Cambridge, USA); ERK1/2 (ZEN BIO, Chengdu, China); phospho-MEK1/2 and phospho-ERK1/2 (Cell Signaling Technology, Danvers, MA, USA); KRAS, RhoA, ROCK1, and ROCK2 (Santa Cruz, Dallas, TX, USA).
Agarose gel electrophoresis
2 g of agarose was added into an Erlenmeyer flask. The running buffer TAE (Beyotime, Shanghai, China) was diluted with double distilled water, and 200 mL running buffer was added to the agarose-containing flask. Then, the agarose/buffer mixture was melted by heating in a microwave. Ethidium bromide (EtBr) (Beyotime) was added to the agarose/buffer. The gel tray was placed into the casting apparatus and an appropriate comb was placed into the gel mold to create the wells. The molten agarose was poured into the gel mold and cooled down set at room temperature (RT). The comb was removed and placed the gel in the gel box. The DNA samples were loded into each well in the gel. The power supply was programed to 120 voltage. The gel was exposed to UV-light and the picture was taken with a gel documentation system (Lee et al. 2012).
Immunofluorescence and immunohistochemistry (IHC)
PC3 cells were seeded onto coverslips in a six-well plate. The cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.3% Triton X-100 for 20 min, and then incubated in 5% goat serum albumin for 20 min to block nonspecific protein–protein interactions. After washing, the confocal dishes were then incubated with rabbit anti-BLM (1:200, Proteintech) and mouse anti-HDGF antibodies (1:200, Santa Cruz) for 2 h at 37 °C. Washing once again, the dishes were incubated with goat anti-rabbit IgG (Alexa Fluor® 594) (1:400, Abcam) and goat anti-mouse IgG (Alexa Fluor® 488) antibodies (1:400, Abcam) for 0.5 h at 37 °C and stained with 4,6-diamidino-2-phenylindole (DAPI) for 5 min at RT. The immunofluorescence images were captured using Olympus IX71 Nikon imaging system (200 × magnification). For IHC staining, the tissue chips were purchased from Biomax. Inc. (Lot. PR807c); the chips contained 10 normal prostate tissues, 50 prostate adenocarcinomas, and 20 benign tumor prostate hyperplasias. Employing rabbit anti-BLM Polyclonal antibody and anti-HDGF Polyclonal antibody (AiFang, Hunan, China), the proportion of positively stained cells and IHC signal intensity in the stained tumor sections were evaluated and scored independently by two observers in a blinded manner. The intensity of staining was graded as follows: 0, negative staining; 1, weak staining (light yellow); 2, moderate staining (yellow–brown); and 3, strong staining (brown). The staining index (SI) was calculated by multiplying the proportion score by the staining intensity score. The protein levels of BLM and HDGF in the stained tumor sections were assessed, and their expression indices were further categorized as high or low, according to the cut-off point value determined as the mean SI of all cases (Yang 2021).
Cell viability assay, EdU cell proliferation assay, and cell colony assay
PC3 cells (2 × 103 cells/mL) were seeded in 96-well tissue culture plates and incubated until cells were attached to the wells. Next, the PC3 cells viability was measured at 24, 48, and 72 h upon transfection with shBLM, shHDGF, control shRNA(GenePharma, Shanghai, China), pEGFP-C1-BLM, pDsRed2-C1-HDGF (TsingKe Biotech, Chongqing, China) according to the manufacturer’s instructions using Cell Counting Kit-8 (CCK-8) assay (APE × BIO, Houston, TX, USA). Furthermore, EdU was employed to assess cell proliferation after PC3 transfection for 48 h as per the manufacturer’s instructions (RiboBio, Guangzhou, China). Each treatment had six independent replicates. For the cell colony assay, 500 PC3 cells were seeded in six-well plates and cultured for 2 weeks after transfection. The formative colonies were fixed with 4% formalin for 20 min and stained with crystal violet. The colonies were enumerated using ImageJ 1.8.0 software (National Institutes of Health, Bethesda, MD, USA).
Wound healing assay and transwell invasion assay
The ibidi Culture-Insert 4 Well (ibidi LLC, Madison, WI, USA) was used for the wound healing assay. Cell suspension was prepared, and that 110 μL was added to each well. After appropriate cell attachment (24 h), the Culture-Insert 4 Well was gently removed using sterile tweezers. ‘Wound closure’ was observed at 0, 24, and 48 h and digital images were captured under an inverted microscope. The wound area was measured using ImageJ software to calculate the fold change in the area covered in the wells. The transwell assay was performed with BD BioCoat 9 Matrigel Invasion Chambers (BD Biosciences, Franklin Lakes, NJ, USA). Matrigel was diluted with DMED/F12 medium and added to the upper chambers for 12 h at 37 ℃. Then, a total of 5 × 104 homogeneous single-cell suspensions in 100 μL of serum-free MDED/F12 medium were added to the upper compartment of a chamber, and the lower compartment was filled with 500 μL of DMED/F12 medium containing 10% FBS. The incubation time for PC3 was 48 h.
Chromatin immunoprecipitation and deep SEQuencing (ChIP-seq)
PC3 cells were crosslinked with 1% formaldehyde (final concentration) for 10 min at RT and quenched with 0.125 M glycine for 5 min. The cell pellets were washed repeatedly with PBS. The pellets were then lysed using lysis buffer (50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.1% sodium deoxycholate, and 1% Triton X-100, and complemented with protease inhibitor cocktail) for 10 min. After centrifugation, the supernatant was discarded and the pellet was lysed in lysis buffer and sonicated. Sheared chromatin was incubated overnight with HDGF antibody bound to PierceTM Protein A/G Agarose Beads (Thermo Fisher Scientific, Waltham, MA, USA) overnight, followed by elution and reversed crosslinking at 65 °C overnight. TE buffer (10 mM Tris–HCl and 1 mM EDTA) was added to DNA elution buffer followed by RNase treatment (0.5 mg/mL) at 37 °C for 30 min and by proteinase K treatment (0.3 mg/mL) at 51 °C for 1 h. DNA was subsequently isolated and purified. Purified DNA was used to construct a DNA library, and the concentration and purity of the DNA library were detected by a Qubit 1 × dsDNA Assay Kit (Thermo Fisher). Finally, the DNA library was sent for sequencing to Genecreat (Wuhan, China).
Dual-luciferase reporter assay
The pGL4.10 luciferase vector was used as the positive control for the luciferase reporter system. A series of truncations of KRAS and RhoA promoter regions were inserted between the KpnI and NheI sites of the pGL4.10 luciferase vector. Briefly, 293 T cells were plated in 96-well plates and transfected with luciferase plasmid and shNC or shHDGF. To normalize the transfection efficiency, the cells were cotransfected with the Renilla luciferase control reporter pRL-TK vector by using EndoFectin™ Max (GeneCopoeia). Upon transfection for 48 h, passive lysis buffer (20 μL per well) was added to cells. Then, 110 μL LARII was applied to each well for detecting the luciferase activity. Finally, 100 μL Stop & Glo® was applied to each well for detecting the Ranilla luciferase activity. The luciferase reporter assay was used the Dual-Luciferase Reporter Assay System (Promega).
In vivo tumor model
Four-week old female BALB/c nude mice were purchased from Tengxin Biotechnology Co., Ltd. After 1 week of adaptive feeding, a total of 4 × 106 cells/mouse in 100 μL of DMEM/F12 were subcutaneously injected into the left flank of each nude mice, and they were sacrificed under anesthesia 28 days later. Tumor volumes were recorded every 7 days and calculated according to the following equation: volume = (width2 × length)/2 (Tomayko and Reynolds 1989). The tumor xenografts were harvested, weighed, and processed for IHC staining.
Statistical analysis
The data were expressed as the mean ± sd. from at least three independent experiments. Correlation between BLM and HDGF expression was assessed using the Spearman rank correlation analysis. Statistical significance was compared by Student’s two-tailed t-test for two groups and one-way ANOVA for multiple groups. All analyses were performed by GraphPad Prism 5.0. All statistical tests were two-sided, and a P value of < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001).
Results
Recognition of HDGF as a protein-binding partner for BLM
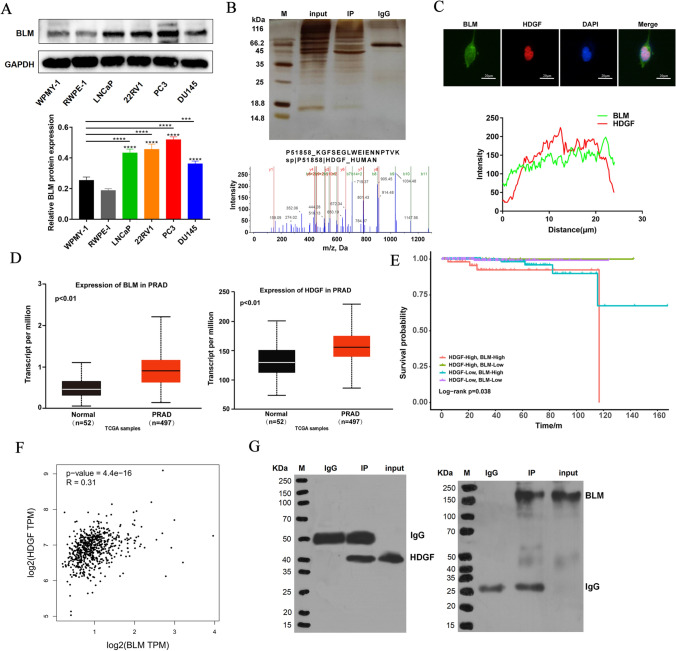
BLM protein level in PCa cells (LNCaP, 22RV1, PC3 and DU145) was strikingly higher compared to that in the normal prostate cells (WPMY-1 and RWPE-1), and the results also showed that the highest level of BLM was in PC3 (Fig. 1A). The new protein-binding partners of BLM were identified by Co-IP assay accompanying with MS in PC3. SDS-PAGE was visualized with colloidal silver staining. And MS/MS analysis was used to determine protein bands that differed from the IgG control group via MALDI-TOF/TOF; the MS/MS data were analyzed using the ProteinPilot™ software by the Mascot search. HDGF was caught as a candidate protein that interacted with BLM; the peptide-spectrum sequence was KGFSEGLWEIENNPTVK (Fig. 1B). The subcellular localization images of BLM and HDGF were examined under a fluorescence microscope. Immunofluorescence analysis displayed that BLM (green) and HDGF (red) co-localized in the nucleus (Fig. 1C). Based on these data, the expression profile bioinformatic analysis of BLM and HDGF (http://ualcan.path.uab.edu/analysis.html, http://gepia.cancer-pku.cn) was performed. BLM and HDGF were remarkably highly expressed in PCa tissues compared with normal tissues (Fig. 1D). Samples from PRAD in TCGA database were divided into 4 groups according to the optimal cut off and the expression of BLM and HDGF at mRNA level. The relationship between survival time and gene expression in each group was shown as follows (Fig. 1E). We found that high expression of BLM and HDGF was negatively correlated with patients’ survival. And Pearson correlation analysis indicated that HDGF was significantly related to BLM (Fig. 1F). Next, we performed Co-IP assay to validate BLM interaction with HDGF by anti-BLM or anti-HDGF antibodies, respectively. Namely, the BLM IP group examined the expression of HDGF (Fig. 1G, left), in turn, the HDGF IP group measured the expression of BLM (Fig. 1G, right). To summarize, BLM can physically associated with HDGF.
Fig. 1.
BLM interacted with HDGF. A Basic expression of BLM in human normal prostate cells (WPMY-1 and RWPE-1) and human PCa cell lines (LNCaP, 22RV1, PC3 and DU145). B BLM-interacting proteins were obtained using Co-IP under physiological conditions. After SDS-PAGE and silver staining, IP and IgG control group were analyzed using MS; secondary MS of HDGF. C PC3 cells were immunostained with anti-BLM (green) and anti-HDGF (red) antibodies and visualized. Scale bar: 20 µm. D The expression of BLM and HDGF in the PRAD TCGA database. E The relationship between survival time and the expression of BLM and HDGF. F The mRNA levels of BLM and HDGF were significantly correlated in the PRAD TCGA database. G Interaction between BLM and HDGF was confirmed via Co-IP in PC3. The presented results were representative of experiments repeated at least three times. Data were represented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
BLM and HDGF may serve as poor prognostic biomarkers for PCa patients
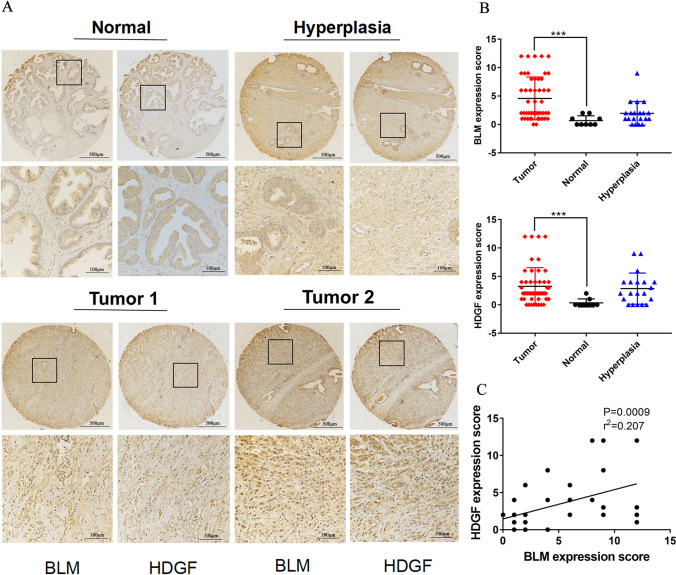
The expression of BLM and HDGF was evaluated in prostate tissue chips containing 50 prostate adenocarcinomas, 20 benign prostate hyperplasias and 9 normal prostate tissues via IHC analysis. In addition, both BLM and HDGF were localized in the nucleus, and patients with strong BLM staining were likely inclined to show strong HDGF staining (Fig. 2A). The expression of BLM and HDGF was tellingly higher in prostate adenocarcinoma tissues than that in normal tissues (Fig. 2B; Tables 2 and 3). The Spearman rank correlation analysis revealed that the expression of BLM and HDGF was positively correlated (r = 0.207, P = 0.0009) (Fig. 2C). It was important to note that the higher expression of BLM and HDGF was correlated with the T status (P = 0.058, P = 0.095), clinical stage (P = 0.058, P = 0.095), Gleason grade (P = 0.001, P = 0.009) and Gleason score (P = 0.000, P = 0.016) in tumor tissues but not with age or the NM status (P > 0.05) (Table 4).
Fig. 2.
Expressions of BLM and HDGF were detected using IHC. A Representative images of BLM and HDGF expression in prostate adenocarcinoma, hyperplasia and adjacent normal prostate tissues. B Expressions of BLM and HDGF in PCa tissues, normal prostate tisssues and hyperplasia prostate tissues. C BLM and HDGF expressions were positively correlated in PCa tissues. The presented results were representative of experiments repeated at least three times. Data were represented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Table 2.
The expression of BLM protein in different types of prostate tissues
| Histopathological type | N | BLM | P value (Compare with cancer tissue) | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive expression rate (%) | |||
| Normal tissue | 9 | 2 | 7 | 22.2 | 0.112 |
| Hyperplasia tissue | 20 | 10 | 10 | 50.0 | 0.135 |
| Cancer tissue | 50 | 38 | 12 | 76.0 | / |
Table 3.
The expression of HDGF protein in different types of prostate tissues
| Histopathological type | N | HDGF | P value (Compare with cancer tissue) | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive expression rate (%) | |||
| Normal tissue | 9 | 1 | 8 | 11.1 | 0.112 |
| Hyperplasia tissue | 20 | 13 | 7 | 65.0 | 0.112 |
| Cancer tissue | 50 | 36 | 14 | 72.0 | / |
Table 4.
Correlations between BLM, HDGF expression and clinicopathological characteristics of PCa patients
| Clinicopathological characteristics | N | BLM | X2 | P value | HDGF | X2 | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | ||||||
| Age (years) | |||||||||
| ≤ 69 | 24 | 16(66.7) | 8(33.3) | 2.204 | 0.138 | 17(70.8) | 7(29.1) | 0.031 | 0.86 |
| > 69 | 26 | 22(84.6) | 4(16.4) | 19(73.1) | 7(26.9) | ||||
| T status | |||||||||
| T1–T2 | 30 | 20(70.0) | 10(30.0) | 3.582 | 0.058 | 19(63.3) | 11(36.7) | 2.794 | 0.095 |
| T3–T4 | 20 | 18(95.0) | 2(5.0) | 17(85.0) | 3(15.0) | ||||
| Clinical stage | |||||||||
| I–II | 30 | 20(70.0) | 10(30.0) | 3.5828 | 0.058 | 19(63.3) | 11(36.7) | 2.794 | 0.095 |
| III–IV | 20 | 18(95.0) | 2(5.0) | 17(85.0) | 3(15.0) | ||||
| Gleason score | |||||||||
| < 7 | 13 | 5(38.5) | 8(61.5) | 13.572 | 0.000 | 6(46.2) | 7(53.8) | 5.821 | 0.016 |
| ≥ 7 | 37 | 33(89.2) | 4(10.8) | 30(81.1) | 7(18.9) | ||||
| Gleason grade | |||||||||
| 1–3 | 21 | 11(52.4) | 10(47.6) | 11.074 | 0.001 | 11(52.4) | 10(47.6) | 6.913 | 0.009 |
| 4–5 | 29 | 27(93.1) | 2(6.9) | 25(86.2) | 4(13.8) | ||||
| N-regional lymph nodes | |||||||||
| N0 | 44 | 33(77.5) | 11(25.0) | 1.923 | 0.166 | 31(70.5) | 13(29.5) | 0.434 | 0.510 |
| N1 | 6 | 6(100.0) | 0(0.0) | 5(83.3) | 1(16.7) | ||||
| M-Distant metastasis | |||||||||
| M0 | 49 | 38(77.6) | 11(22.4) | 0.288 | 0.592 | 35(71.4) | 14(28.6) | 0.397 | 0.529 |
| M1 | 1 | 1(100.0) | 0(0.0) | 1(100.0) | 0(0.0) | ||||
P value is from χ2-test
BLM and HDGF depletion inhibited PC3 proliferation, clonogenicity, migration and invasion
Firstly, shBLM4 and shHDGF1 as the most efficacious shRNAs, knocked down BLM and HDGF expression levels in the following assays (Fig. 3A). The shRNAs-mediated silencing of BLM and HDGF, downregulated the expression of BLM and HDGF in PC3. Besides, BLM knockdown nearly failed to influence HDGF expression. Likewise, BLM expression was almost unaffected by HDGF silencing (Fig. 3B). To validate the biological roles of BLM and HDGF in the PC3, cells proliferation, clonogenicity, migration, and invasion were assessed. The EdU and CCK-8 assays showed that the PC3 proliferation was more impaired with both BLM and HDGF knockdown compared with that BLM or HDGF knockdown alone (Fig. 3C, D). The clonogenicity of PC3 displayed an apparent attenuation with both BLM and HDGF silencing versus that BLM or HDGF knockdown solely (Fig. 3E). PC3 migration and invasion were more notably inhibited after combining BLM and HDGF knockdown compared with that BLM or HDGF knockdown singly (Fig. 3F, G). Overall, these results demonstrated that both BLM and HDGF were key factors in the biological regulation of PC3.
Fig. 3.
BLM and HDGF deleption influenced the biological rs of PC3. A Silencing efficacy of shRNA-targeted BLM and HDGF were detected respectively. B Expressions of BLM and HDGF were inhibited in BLM- and HDGF-silenced PC3 cells. C–G. Knockdown of BLM and HDGF attenuated PC3 proliferation, clonogenicity, migration and invasion. The presented results were representative of experiments repeated at least three times. Data were represented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
HDGF activated KRAS and suppressed RhoA transcription
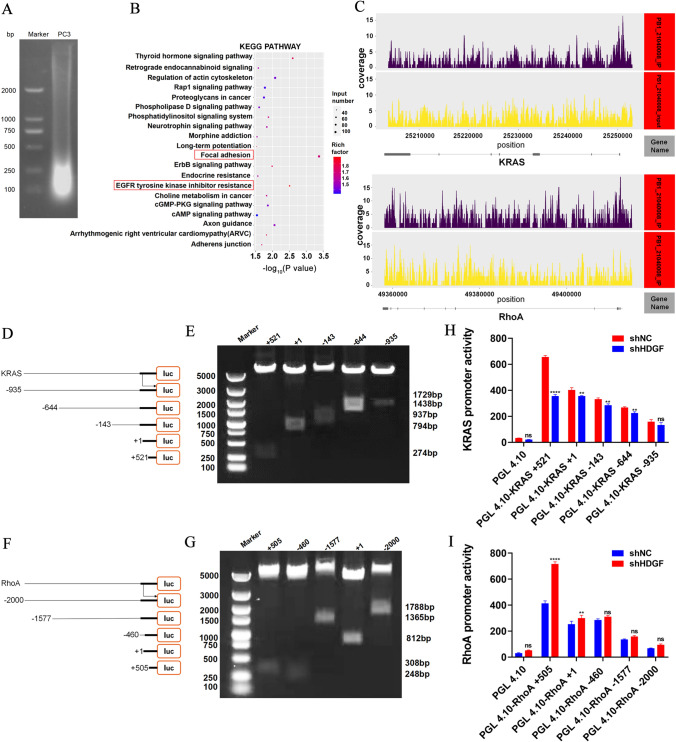
To explore the downstream gene of HDGF, DNA of PC3 was segmented into sizes between 100 and 500 bp for chip enrichment (Fig. 4A). Then, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment was performed following DNA sequencing, and the focal adhesion and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor resistance signaling pathways were enriched as the primary pathways in PC3 (Fig. 4B). KRAS and RhoA were captured as the most potential candidates that bound to HDGF promoter. The map of READS on Peak associated genes including KRAS and RhoA, horizontal axis showed the gene location, and the vertical axis showed the depth of reads coverage (Fig. 4C). Futher analysis demonstrated that HDGF bound to KRAS promoter at position 25,250,136–25,250,409 in the negative strand of chromosome 12, HDGF bound to RhoA promoter at two positions 49,412,189–49,412,436 and 49,411,165–49,411,472 in the negative strand of chromosome 3 via ChIP-seq assay. In order to determine KRAS and RhoA were required for HDGF transcriptional regulation, a series of KRAS and RhoA gene promoter fragments were amplified by PCR and cloned into the pGL4.10-basic vector to construct dual-fluorescence reporter plasmids (Fig. 4D, F). Agarose gel electrophoresis showed that the size of each truncated promoter fragment coincided with the sequence designed in this study (Fig. 4E, G). HDGF depletion notably attenuated pGL4.10-basic-KRAS + 521 promoter transcriptional activity, whereas augmented pGL4.10-basic-RhoA + 505 promoter transcriptional activity by dual-luciferase reporter assay (Fig. 4H, I). These findings not only unveiled that HDGF transcriptionally activated KRAS, but also expounded that HDGF transcriptionally suppressed RhoA. In short, HDGF could directly bound to the promoter region of the KRAS and RhoA, thus indicating that the BLM-HDGF/KRAS and BLM-HDGF/RhoA axes may exist in PC3.
Fig. 4.
HDGF was characterized as KRAS and RhoA promoter-binding protein. A DNA was segmented into sizes between 100 and 500 bp for chip enrichment. B The focal adhesion and EGFR tyrosine kinase inhibitor resistance pathways were enriched according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. C The map of READS on peak associated genes was KRAS and RhoA. D–G Truncated fragments of KRAS and RhoA genes were designed to construct dual-fluorescence reporter plasmids; the reporter plasmids were performed by double enzyme digestion. H, I The promoter activity of various reporter plasmids was measured using the dual-luciferase reporter assay. The presented results were representative of experiments repeated at least three times. Data were represented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
The crosstalk of KRAS and RhoA was regulated by HDGF interaction with BLM, which activated the MAPK/ERK signaling pathway
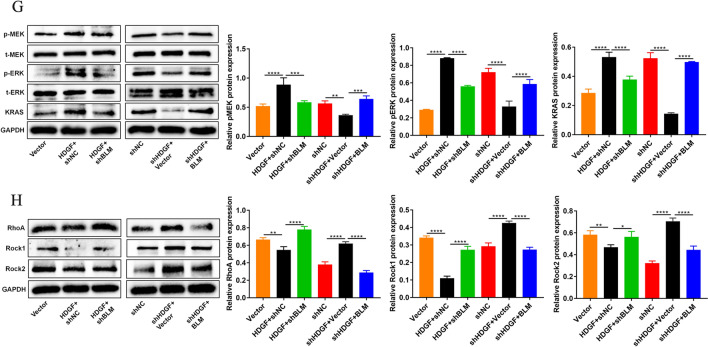
For this experiment, the rescue assay was performed. BLM expression was almost impervious upon HDGF knockdown or overexpression. Equally, HDGF expression was nearly insusceptible to BLM siliencing or overexpression. Importantly, these findings were in keeping with the previous result in Fig. 3B. It is important to note that the intranuclear proteins Ki67 and PCNA are closely associated of cell proliferation in PCa (Spratt 2018; Taftachi et al. 2005; Wilkins et al. 2018). Our results showed that Ki67 and PCNA were upregulated in HDGF-overexpressed PC3 cells, while Ki67 and PCNA were downregulated in HDGF-silenced PC3 cells. Besides, the upregulation or downregulation was partly rescued by BLM depletion or BLM overexpression (Fig. 5A). Functionally, HDGF overexpression partially abolished the promotion effects on cell invasion, clonogenicity, migration and proliferation elicited by BLM deficiency in PC3. Conversely, silencing HDGF impeded PC3 invasion, clonogenicity, migration and proliferation. Nonetheless, the inhibition was alleviated following BLM overexpression. (Fig. 5B–F). The present study expounded that the interplay BLM with HDGF modulated biological behaviors of PC3. To uncover the HDGF downstream signaling pathway related to KRAS and RhoA, the quantification of KRAS and RhoA, coupled with their signaling pathway proteins, were detected by western blotting (Fig. 5G, H). Finally, HDGF overexpression increased KRAS and diminished RhoA expression, these roles could be partially reversed upon BLM knockdown. And in turn, KRAS expression was diminished while that of RhoA increased in HDGF-dificient PC3 cells. Futhermore, these effects were rescued by BLM overexpression. Meanwhile, the results evinced that HDGF overexpression, increased the expressions of key targets involving in pMEK and pERK in the MAPK/ERK signaling pathway, yet decreased the expressions of key targets covering Rock1 and Rock2 in the Rho-Rac signaling pathway. Instead, HDGF depletion caused the reduction of pMEK and pERK expression, the increased expression of Rock1 and Rock2. In brief, these appeared to offer compelling evidence of the existence of a signal crosstalk between HDGF-KRAS and HDGF-RhoA in PC3.
Fig. 5.
BLM promoted clonogenicity, invasion, migration and proliferation in PC3. A Expressions of BLM, HDGF, Ki67 and PCNA in PC3 were assessed using western blotting after HDGF and BLM knockdown or overexpression. B-F Clonogenicity, invasion, migration and proliferation of PC3 were identified. G Expressions of proteins related to MAPK/ERK pathway were detected. H Expressions of proteins concerned with the Rho–Rac signaling pathway were investigated; GAPDH was used as the loading control. The presented results were representative of experiments repeated at least three times. Data were represented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
The expression and function of KRAS and RhoA were modulated by HDGF interaction with BLM in vivo
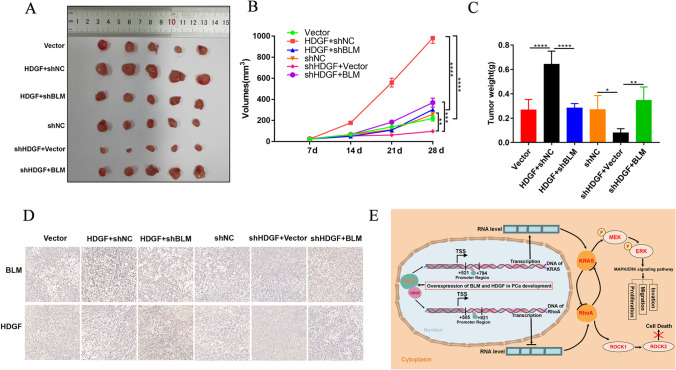
Whether the impact of BLM interaction with HDGF on tumor growth as per the in vitro data was then evaluated for consistency in mouse xenograft models. PC3 cells infected with lentivirus were subcutaneously injected into the left flanks of nude mice, and tumor growth was monitored every 7 days. After approximately 4 weeks, tumors were excised and weighed, and subsequently processed for IHC staining (Fig. 6A–D). The results showed that HDGF overexpression expedited tumor growth and that the effect could be reversed via BLM knockdown. By contrast, HDGF knockdown retarded tumor growth. Moreover, the inhibition effect could be rescued upon BLM overexpression. These results, completely, afforded in vivo support of the observations in vitro.
Fig. 6.
The expression and function of KRAS and RhoA were modulated via HDGF interaction with BLM in vivo. A Images of PCa tumor xenografts from each mouse (n = 5 for each group). B, C Tumor volumes (mm3) were monitored weekly and tumor weights (g) were measured. D Expressions of BLM and HDGF in tumor tissues were analyzed using IHC staining. E Schematic model of BLM and HDGF promoted PCa malignancy via the MAPK/ERK signaling pathway. The presented results were representative of experiments repeated at least three times. Data were represented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Discussion
PCa is one of the most ubiquitous male cancers causing death worldwide (Sung et al. 2021). Although the PSA test is the main clinical practice for PCa screening and detection, PSA screening does more harm than good as it fails to meet public health requirements (Pezaro et al. 2014). As an vital member of the RecQ helicases, BLM may be predisposed to body cancer upon its defect (Mosedale and Hickson 2013). BLM is a predominant nucleolus protein that functions in DNA replication, repair and telomere maintenance (Rezazadeh 2013). Nevertheless, mutations in BLM lead to severe growth defects and predisposition to cancers as well as other diseases. Previous research confirmed that BLM was dramatically relevant to PCa and could be a prospective biomarker of PCa for target therapy (Wang 2015; Qian et al. 2017). Nonetheless, interrogating the molecular mechanism and regulatory network of BLM in PCa remains largely imperative.
Initially, BLM protein expression in PCa cell lines (LNCaP, 22RV1, PC3 and DU145) and human normal prostate cell lines (WPMY-1 and RWPE-1) was quantified. The results showed that BLM had the highest expression in PC3. Hereafter, the Co-IP assay coupled with MS was executed to obtain BLM interaction proteins, and HDGF was one of the candidates that intrigued us the most. A large set of studies, have ascribed HDGF mutiple regulatory functions through its PWWP region, for instance in ribosome biogenesis, DNA damage repair and transcriptional regulation via interaction with various protein-binding partners (Stec et al. 2000; Bao et al. 2014). Furthermore, it has been reported that HDGF is highly expressed in PCa (Morelli et al. 2004; Nazarenko et al. 2010). IFA analysis confirmed that both BLM and HDGF were colocalized in the nucleus. The Co-IP assay following western blotting indicated that BLM directly interacted with HDGF. IHC revealed higher BLM and HDGF expressions in PCa tissues compared with normal tissues. Importantly, the high expression of BLM and HDGF was positively correlated with the T status, clinical stage, Gleason grade and Gleason score in tumor tissues, suggesting that BLM and HDGF were engaged in the progression of PCa. Therefore, it is plausible that both BLM and HDGF, served as indicators, can be ultilized to predict the prognosis of patients for PCa. In PC3 loss-of-function assay was performed to probe the explicit role of BLM interaction with HDGF. The inhibitory influence of BLM accompanied with HDGF depletion on PC3 proliferation, migration and invasion were greater than that BLM or HDGF depletion exclusively. And thus, we conjectured the existence of a positive correlation between BLM and HDGF.
To investigate the downstream genes that could be modulated by HDGF, the ChIP-seq was performed and detailed sequencing of IP products which yielded interesting results. The KEGG pathway enriched the focal adhesion and EGFR tyrosine kinase inhibitor resistance signaling pathways as the primary pathways in PC3. Strikingly, the KRAS and RhoA captured our attention in the two pathways. KRAS is the first cancer gene identified in human cancer cells (CJ et al. 1982). Furthermore, previous research evince that KRAS and RhoA are homologous genes to RAS (Hodge et al. 2020). In the present study, we identified that the HDGF could directly bind to the region of KRAS and RhoA promoter. Accordingly, HDGF was postulated to have a bidirectional modulation on KRAS and RhoA. To corroborate this postulation, the dual-luciferase reporter assay results unmasked that the promoter activity of KRAS was restrained, whereas that of RhoA was potentiated in HDGF-silenced 293 T cells. Reiterating recapitulation, these results displayed that transcriptional promotion on KRAS and restraint on RhoA of HDGF in PC3.
In parallel, the predominance of BLM interplaying with HDGF in mediating the oncogenic properties was determined by rescue assays, the study showcased that HDGF overexpression fostered PC3 proliferation, migration and invasion, whereas BLM knockdown partially countered these results. Furthermore, these biological behaviors of PC3 were repressed after HDGF knockdown, and this impairment influence was at least in part abrogated via BLM overexpression. Ki67 and PCNA proteins are hallmarks of PCa cell proliferation (Spratt 2018; Taftachi 2005; Wilkins 2018). As reported previously, in this report, another intriguing observation was that Ki67 and PCNA exhibited consistently expressed in HDGF/BLM silenced- or overexpressed-PC3 cells. Hence, BLM and HDGF may serve as biomarkers and new promising targets for therapeutic intervention in PCa.
The abnormal activation of the RAS signaling pathway is associated with many tumors and implicated in various cellular activities of carcinogenesis (Imperial et al. 2019). This aberrant activation can contribute to cell cycle progression, prompt the expression of cyclin D1 expression, facilitate cell cycle transition from G1 phase to S phase, and cause uncontrolled cell proliferation (Shen et al. 2019). The MAPK and PI3K are two significant downstream signaling pathways of RAS (de Sousa Mesquita et al. 2017). More importantly, Rho-Rac participates in RAS effector pathways, which plays an important role in promoting cytoskeleton construction, epithelial-mesenchymal transition, cell cycle and cell proliferation (Tang et al. 2018). ROCK, key proteins including ROCK1 and ROCK2 in Rho-Rac pathway, mediates cell death owing to its serine/threonine protein kinase activity (Zeng et al. 2020). In aggregate, we postulated that existed HDGF-related MAPK and Rho-Rac signaling pathways in PC3.
Intriguingly, KRAS expression was upregulated, whereas RhoA was downregulated when HDGF was overexpressed, and the results were counteracted via BLM knockdown. By contrast, HDGF knockdown resulted in KRAS decrease while RhoA increase, and this phenomenon was partly reversed after BLM overexpression. Meanwhile, the results exhibited that HDGF overexpression, enhanced the expressions of key targets encompassing pMEK and pERK in the MAPK/ERK signaling pathway, whilst lessened the expressions of crucial targets covering Rock1 and Rock2 in the Rho-Rac signaling pathway. Upon HDGF knockdown and vice versa. These results implicated that BLM functioned in PC3 in part via the MAPK/ERK signaling pathway.
Previously, Chew et al. have confirmed KRAS and RhoA signal crosstalk in vivo and its essential role in regulating liver overgrowth, hepatocyte transformation and cancer mortality (Chew et al. 2014). Simultaneously, another study shows that KRAS overexpression attenuates RhoA activity in pancreatic cancer cells (Dreissigacker et al. 2006). Herein, a model of relationship between BLM and HDGF in PCa development (Fig. 6E) showing crosstalk between KRAS and RhoA via HDGF regulation through BLM interaction was established. The tumor growth drived by BLM was also interrogated in mouse xenograft models. In accord with the vitro data, suggesting that BLM promoted malignancy of PCa by interacting with HDGF.
Altogether, the present study not only unravelled that BLM contributed to PCa malignancy by interacting with HDGF but also that the positive regulation of HDGF on KRAS and negative regulation on RhoA. Ultimately, the targeting of KRAS and RhoA by HDGF had a signal crosstalk, and, as such, the MAPK/ERK pathway was activated. The study results supported the potential of double targeting BLM and HDGF as promising therapeutic avenues for PCa.
Acknowledgements
Not applicable.
Author contributions
Y Ruan conceived the project. M Huang prepared all the materials. Y Guo carried out the in vitro and in vitro experiments. All authors participated in the discussion and evaluation of experimental results. Y Guo completed the first draft and drew the schematic figures. H Xu finalized the manuscript.
Funding
The authors acknowledge the financial support received from the National Natural Science Foundation of China (Grant Numbers 31860242).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval
All animal experimental procedures were approved by the Laboratory Animal Ethics Committee of Guizhou University (License Number: EAE-GZU-2022-P001).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn HK, Lee YH, et al. Current status and application of metformin for prostate cancer: a comprehensive review. Int J Mol Sci. 2020;21(22):8540. doi: 10.3390/ijms21228540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C, Wang J, et al. HDGF: a novel jack-of-all-trades in cancer. Future Oncol. 2014;10(16):2675–2685. doi: 10.2217/fon.14.194. [DOI] [PubMed] [Google Scholar]
- Beer TM, Armstrong AJ, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xu H, et al. Bloom syndrome protein activates AKT and PRAS40 in prostate cancer cells. Oxid Med Cell Longev. 2019;2019:1–19. doi: 10.1155/2019/3685817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew TW, Liu XJ, et al. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33(21):2717–2727. doi: 10.1038/onc.2013.240. [DOI] [PubMed] [Google Scholar]
- Chi KN, Agarwal N, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- Clegg NJ, Wongvipat J, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Can Res. 2012;72(6):1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Mesquita AP, de Araújo Lopes S, et al. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis. 2017;22(9):1116–1137. doi: 10.1007/s10495-017-1392-0. [DOI] [PubMed] [Google Scholar]
- Der CJ, Krontiris TG, et al. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci USA. 1982;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreissigacker U, Mueller MS, et al. Oncogenic K-Ras down-regulates Rac1 and RhoA activity and enhances migration and invasion of pancreatic carcinoma cells through activation of p38. Cell Signal. 2006;18(8):1156–1168. doi: 10.1016/j.cellsig.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Gu Z, Thomas G, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19(10):1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- Helenius MA, Savinainen KJ, et al. Amplification of the urokinase gene and the sensitivity of prostate cancer cells to urokinase inhibitors. BJU Int. 2006;97(2):404–409. doi: 10.1111/j.1464-410X.2005.05912.x. [DOI] [PubMed] [Google Scholar]
- Hodge RG, Schaefer A, et al. RAS and RHO family GTPase mutations in cancer: twin sons of different mothers? Crit Rev Biochem Mol Biol. 2020;55(4):386–407. doi: 10.1080/10409238.2020.1810622. [DOI] [PubMed] [Google Scholar]
- Hu T, Huang C, et al. Expression of hepatoma-derived growth factor in hepatocellular carcinoma. Cancer. 2003;98(7):1444–1456. doi: 10.1002/cncr.11653. [DOI] [PubMed] [Google Scholar]
- Imperial R, Toor OM, et al. Comprehensive pancancer genomic analysis reveals (RTK)-RAS-RAF-MEK as a key dysregulated pathway in cancer: Its clinical implications. Semin Cancer Biol. 2019;54:14–28. doi: 10.1016/j.semcancer.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Kishima Y, Yamamoto H, et al. Hepatoma-derived growth factor stimulates cell growth after translocation to the nucleus by nuclear localization signals. J Biol Chem. 2002;277(12):10315–22. doi: 10.1074/jbc.M111122200. [DOI] [PubMed] [Google Scholar]
- Lee PY, Costumbrado J, et al. Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp. 2012;62:1–5. doi: 10.3791/3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- Mosedale G, Hickson ID. Encyclopedia of biological chemistry. Cambridge: Academic Press; 2013. pp. 43–49. [Google Scholar]
- Mostaghel EA. Abiraterone in the treatment of metastatic castration-resistant prostate cancer. Cancer Manag Res. 2014;6:39–51. doi: 10.2147/CMAR.S39318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko I, Rana S, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- Pezaro C, Woo HH, et al. Prostate cancer: measuring PSA. Intern Med J. 2014;44(5):433–440. doi: 10.1111/imj.12407. [DOI] [PubMed] [Google Scholar]
- Qian X, Feng S, et al. RecQ helicase BLM regulates prostate cancer cell proliferation and apoptosis. Oncol Lett. 2017;14(4):4206–4212. doi: 10.3892/ol.2017.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Tang X, et al. Expression of hepatoma-derived growth factor is a strong prognostic predictor for patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2004;22(16):3230–3237. doi: 10.1200/JCO.2004.02.080. [DOI] [PubMed] [Google Scholar]
- Rezazadeh S. On BLM helicase in recombination-mediated telomere maintenance. Mol Biol Rep. 2013;40(4):3049–3064. doi: 10.1007/s11033-012-2379-0. [DOI] [PubMed] [Google Scholar]
- Rothermundt C, Hayoz S, et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09) Eur Urol. 2014;66(3):468–474. doi: 10.1016/j.eururo.2013.12.057. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Xu H, et al. BLM interaction with EZH2 regulates MDM2 expression and is a poor prognostic biomarker for prostate cancer. Am J Cancer Res. 2021;11(4):1347–1368. [PMC free article] [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Karam JA, et al. Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res. 2008;14(12):3785–3791. doi: 10.1158/1078-0432.CCR-07-4969. [DOI] [PubMed] [Google Scholar]
- Shen N, Li L, et al. A missense variant in PTPN12 associated with the risk of colorectal cancer by modifying Ras/MEK/ERK signaling. Cancer Epidemiol. 2019;59:109–114. doi: 10.1016/j.canep.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Shetty A, Dasari S, et al. Hepatoma-derived growth factor: a survival-related protein in prostate oncogenesis and a potential target for vitamin K2. Urol Oncol Semin Orig Investig. 2016;34(11):483.e1–483.e8. doi: 10.1016/j.urolonc.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn E. Screening: diagnostic dilemma. Nature. 2015;528(7582):S120–S122. doi: 10.1038/528S120a. [DOI] [PubMed] [Google Scholar]
- Spratt DE. Ki-67 remains solely a prognostic biomarker in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2018;101(3):513–515. doi: 10.1016/j.ijrobp.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Stec I, Nagl SB, et al. The PWWP domain: a potential protein-protein interaction domain in nuclear proteins influencing differentiation? FEBS Lett. 2000;473(1):1–5. doi: 10.1016/S0014-5793(00)01449-6. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Taftachi R, Ayhan A, et al. Proliferating-cell nuclear antigen (PCNA) as an independent prognostic marker in patients after prostatectomy: a comparison of PCNA and Ki-67. BJU Int. 2005;95(4):650–654. doi: 10.1111/j.1464-410X.2005.05356.x. [DOI] [PubMed] [Google Scholar]
- Tang L, Dai F, et al. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol Res. 2018;133:201–212. doi: 10.1016/j.phrs.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Thakkar MK, Lee J, et al. RecQ Helicase Somatic alterations in Cancer. Front Mol Biosci. 2022;9:887758. doi: 10.3389/fmolb.2022.887758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- Tsai HE, Liu GS, et al. Downregulation of hepatoma-derived growth factor contributes to retarded lung metastasis via inhibition of epithelial-mesenchymal transition by systemic POMC gene delivery in melanoma. Mol Cancer Ther. 2013;12(6):1016–1025. doi: 10.1158/1535-7163.MCT-12-0832. [DOI] [PubMed] [Google Scholar]
- Tsujino T, Komura K, et al. CRISPR screen contributes to novel target discovery in prostate cancer. Int J Mol Sci. 2021;22(23):12777. doi: 10.3390/ijms222312777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama H, Tomita Y, et al. Hepatoma-derived growth factor Is a novel prognostic factor for patients with pancreatic cancer. Clin Cancer Res. 2006;12(20):6043–6048. doi: 10.1158/1078-0432.CCR-06-1064. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lv H, et al. Genome-wide haplotype association study identifies BLM as a risk gene for prostate cancer in Chinese population. Tumor Biol. 2015;36(4):2703–2707. doi: 10.1007/s13277-014-2893-x. [DOI] [PubMed] [Google Scholar]
- Weeks SE, Metge BJ, et al. The nucleolus: a central response hub for the stressors that drive cancer progression. Cell Mol Life Sci. 2019;76(22):4511–4524. doi: 10.1007/s00018-019-03231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AC, Gusterson B, et al. Ki67 is an independent predictor of recurrence in the largest randomized trial of 3 radiation fractionation schedules in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2018;101(2):309–315. doi: 10.1016/j.ijrobp.2018.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Tomita Y, et al. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis and prognosis of gastric carcinoma. Clin Cancer Res. 2006;12(1):117–122. doi: 10.1158/1078-0432.CCR-05-1347. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma Y, et al. A novel HDGF-ALCAM axis promotes the metastasis of Ewing sarcoma via regulating the GTPases signaling pathway. Oncogene. 2021;40(4):731–745. doi: 10.1038/s41388-020-01485-8. [DOI] [PubMed] [Google Scholar]
- Zeng C, Zeng B, et al. Rho-ROCK signaling mediates entotic cell death in tumor. Cell Death Discov. 2020;6(1):1–3. doi: 10.1038/s41420-020-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Liu J, et al. Prostate stem cell antigen mRNA expression in preoperatively negative biopsy specimens predicts subsequent cancer after transurethral resection of the prostate for benign prostatic hyperplasia. Prostate. 2009;69(12):1292–1302. doi: 10.1002/pros.20973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.