Abstract
Tumor protein D52 (TPD52) is a proto-oncogene overexpressed in prostate cancer (PCa) due to gene amplification and it is involved in the cancer progression of many cancers including PCa. However, the molecular mechanisms underlying the role of TPD52 in cancer progression are still under investigation. In this study, we report that the activation of AMP-activated protein kinase (AMPK) by AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide) inhibited the LNCaP and VCaP cells growth by silencing TPD52 expression. Activation of AMPK inhibited the proliferation and migration of LNCaP and VCaP cells. Interestingly, AICAR treatment to LNCaP and VCaP cells led to the downregulation of TPD52 via activation of GSK3β by a decrease of inactive phosphorylation at Ser9. Moreover, in AICAR treated LNCaP cells, inhibition of GSK3β by LiCl attenuated downregulation of TPD52 indicating that AICAR acts via GSK3β. Furthermore, we found that TPD52 interacts with serine/threonine kinase 11 or Liver kinase B1 (LKB1) a known tumor suppressor and an upstream kinase for AMPK. The molecular modeling and MD simulations indicates that the interaction between TPD52 and LKB1 leads to inhibition of the kinase activity of LKB1 as its auto-phosphorylation sites were masked in the complex. Consequently, TPD52-LKB1 interaction may lead to inactivation of AMPK. Moreover, overexpression of TPD52 is found to be responsible for the reduction of pLKB1 (Ser428) and pAMPK (Thr172). Therefore, TPD52 may be playing its oncogenic role via suppressing the AMPK activation. Altogether, our results revealed a new mechanism of PCa progression in which TPD52 overexpression inhibits AMPK activation by interacting with LKB1. These results support that the use of AMPK activators and/or small molecules that could disrupt the TPD52-LKB1 interaction might be useful to suppress PCa cell growth.
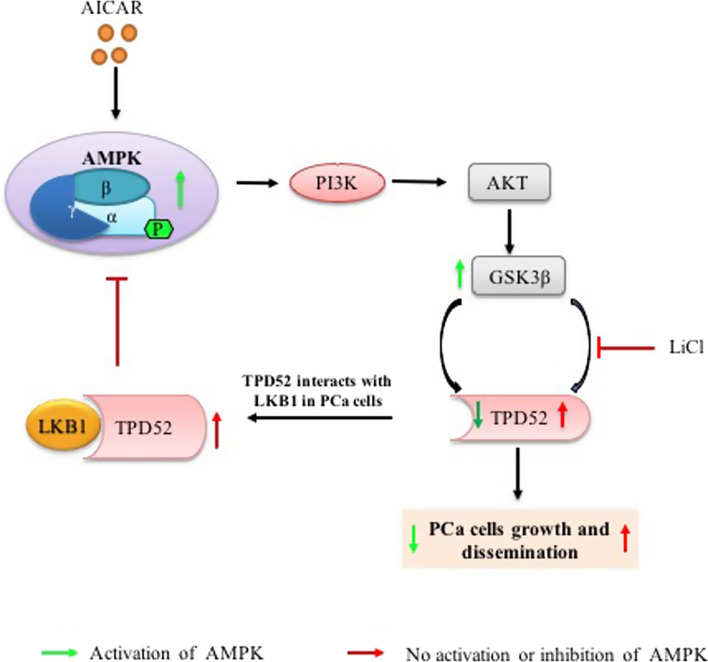
Graphical abstract
TPD52 interacts LKB1 and interfere with activation of AMPK in PCa cells
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-023-00745-y.
Keywords: AMP-activated protein kinase, TPD52 (isoform 3), Liver kinase B1, Protein–protein interactions, Cell proliferation, Prostate cancer
Introduction
Prostate cancer (PCa) cells grow and divide by utilizing cellular energy. The dividing cells require more energy for their continuous proliferation by dysregulating the routine metabolic processes (Hardie et al. 2012; Xiang et al. 2004). The tumor microenvironment regulates aberrant energy metabolism supporting the growth and progression of the lung (William et al. 2012), breast (Gollavilli et al. 2015) and cervical cancers (Yu et al. 2009). AMP-activated protein kinase (AMPK) is a heterotrimeric central metabolic energy regulator consisting of one α catalytic and two regulatory (β and γ) subunits involved in regulation of various biochemical processes (Liang and Mills 2013). In tumor cells, AMPK is activated by an increase in AMP/ATP or ADP/ATP ratio which in turn phosphorylates AMPK at Thr172 of the α subunit (Hardie et al. 2012; Xiang et al. 2004). AMPK suppresses cancer stemness in colorectal cancer via the mevalonate pathway and also in pancreatic cancer by restricting pluripotent factors and EMT (Seo et al. 2020; Sun et al. 2019). In cancer cells, AMPK functions as a tumor suppressor that negatively regulates lymphomagenesis by overcoming Warburg effects (Faubert et al. 2013).
Tumor protein D52 is a proto-oncogene overexpressed in PCa due to gene amplification of specific locus on chromosome 8q21 (Byrne et al. 2014, 1995; Rhodes et al. 2002). High expression of TPD52 in prostate, breast, ovarian and other cancers dysregulates apoptosis and cell migration (Byrne et al. 2005; Rubin et al. 2004; Tennstedt et al. 2014; Ummanni et al. 2008). TPD52 (isoform 3) is a member of the tumor protein D52 family found in high copy numbers in advanced PCa causing growth and proliferation via targeting NF-κB transactivation (Dasari et al. 2017). In Breast cancer cells, TPD52 negatively regulates AMPK signaling through its direct interaction with AMPK (Chen et al. 2022). PrLZ, a TPD52 family protein mediates PCa cell autophagy through LKB1-AMPK (Wang et al. 2004; Zeng et al. 2018). The LKB1-AMPK axis control cell growth, metabolism and polarity (Mirouse and Billaud 2011; Shackelford and Shaw 2009). In cancer and other metabolic diseases, activation of AMPK involves PI3K/Akt/mTOR signaling (El-Masry et al. 2015; Schultze et al. 2012). Moreover, in tumor cells, the coordinated balance of energy intake and expenditure involves GSK3β as an immediate downstream of the AMPK signaling pathway (Mancinelli et al. 2017). However, the detailed role of TPD52 in altering AMPK signaling is still under investigation. Very commonly, AMPK is directly activated by two well-known upstream kinases Liver kinase B1 (LKB1) (Woods et al. 2003) and Calcium/calmodulin-dependent protein kinase kinase II (CAMKKII) (Fogarty et al. 2010). Phosphorylation of AMPKα by these kinases determines the activation of AMPK pathway in regulating cellular energy metabolism. Human LKB1, a serine-threonine Kinase 11 (STK11) functions as a tumor suppressor in assessing cellular energy requirement which accordingly activates its immediate downstream AMPK. The absolute role of AMPK and its net activation in tumor cells depends on various stimuli, upstream kinases and downstream modulators together determine its role in the predisposition of prostate cancer (Khan and Frigo 2017). Hence, we proposed to study whether TPD52 (isoform 3) is involved in disrupting LKB1 and AMPK signaling in prostate cancer cells.
Materials and methods
Cell culture
Prostate cancer LNCaP and VCaP cells were procured from American Type Culture Collection (ATCC, USA) and cultured in base medium RPMI and DMEM respectively, containing 10% (v/v) fetal bovine serum (Gibco, USA), 1% penicillin/streptomycin, sodium bicarbonate, sodium pyruvate (100 mg/ml), glucose (4.5 g/L) and 1% L-glutamine. The cells were maintained at 37 °C in a humified chamber with a 5% CO2 supply (Eppendrof, Germany). Cells were tested periodically for mycoplasma contamination using specific primers in RT-PCR.
Overexpression of TPD52 in PCa cells
The recombinant plasmids for TPD52 overexpression were constructed by cloning the TPD52 isoform 3 (NM 005079.4, Human) into pEGFP-N3 and p3 × Flag-CMV vectors (Clontech Laboratories, USA), as we reported earlier in Ummanni et al. (2008). Transient transfection of plasmids into PCa cells was performed using Lipofectamine™ 3000 (Invitrogen, USA) according to the supplier’s manual. For stable overexpression of TPD52, a doxycycline-inducible expression system was generated by cloning TPD52 (isoform 3) mRNA sequence into the pRetroX-Tet-on system and LNCaP cells were transduced with designated vectors as we described previously (Reddy et al. 2018). The expression of TPD52 in LNCaP cells was induced by the addition of doxycycline (0–50 ng/mL) for 24 h.
Cell proliferation and clonogenic assays
LNCaP cells were seeded (1x103) in 96 well plate and treated with different concentrations of AICAR (0–1 mM) and analyzed at various time points (0–72 hrs). Cell proliferation was determined using cell counting kit-8 (Sigma-Aldrich, USA). The OD of the samples was measured in a multimode Infinite M200 Pro plate reader (TECAN, Switzerland) at 450nm. The experiments were performed in triplicates in three independent experiments. To see anchorage-independent growth of LNCaP cells in-vitro, we performed a clonogenic assay using low melting soft agar. A 6-well plate coated with 1.5 ml base agar (0.6%) was overlaid with 1.5 ml top agar (0.3%) containing 7 × 103 cells per well. After 24 h of incubation, 500 μl of the feeding media supplemented with or without AICAR was added to the designated wells to determine its effect on cell growth. The feeding media was replenished every third day and after two weeks of experimentation, the colonies were stained with crystal violet (0.05% w/v dissolved in 20% methanol) for 30 min. The excess dye was removed by destaining carefully with water. The images were captured in Olympus Xi71 microscope (Olympus, Japan) and the colonies with spheroid diameter of > 70 µM were counted using ImageJ and average number of colonies was plotted as graphs from average colony numbers ± SD. The experiment was performed independently twice in triplicates (p < 0.05).
Migration assay
A Transwell migration assay was performed using the Boyden chamber (Corning, USA) to analyze the migration of LNCaP cells stimulated with AICAR at different concentrations. On top of the chamber, 5x104 cells suspended in migration buffer (1 mM MgCl2, 0.2 mM MnCl2, 2 mM CaCl2, and 0.5% BSA) were seeded and incubated for 24 hrs in humidified incubator with constant 5% CO2 supply at 37 °C. The cells migrated across the membrane were fixed with 4% PFA and stained using 0.5% crystal violet. The migrated cells were counted manually from Olympus IX73 inverted microscope (Olympus, Japan). All experiments were performed in triplicates in three independent experiments; the mean values are represented with ± SD.
Semi-quantitative RT-PCR and agarose gel electrophoresis
Experimental cells were collected in TRIzol reagent (Sigma-Aldrich, USA) and RNA was isolated as per the manufacturer’s protocol. Subsequently, after cDNA synthesis (Bio-Rad, USA), mRNA expression was analyzed by RT-PCR with 25 reaction cycles as reported previously by Dasari et al. (2019). Primer details for RT-PCR are mentioned in (Supplementary information Table 1).
Western blotting
Cells were harvested by scraping in RIPA buffer (Sigma-Aldrich, USA) supplemented with protease inhibitor cocktail (Tocris Bioscience, UK) and lysed by passing through 29 gauge insulin syringes. The clear lysates obtained were used in western blotting as reported previously by Dasari et al. (2019). The details of the antibodies and their dilutions for the detection of target proteins are provided in the (Supplementary information Table 2).
Double immunofluorescence
Double immunofluorescence was performed to examine the distribution pattern and colocalization of TPD52 and LKB1 in VCaP & LNCaP cells. The cells (5 × 104) were seeded in 2-chambered glass slides (Genetix Biotech, India) and allowed to become 70% confluent. The cells were fixed with 4% PFA (w/v) for 15min and permeabilized by treating with Triton X-100 (0.25% v/v) for 10min. After washing with ice-cold PBS, slides were incubated in 2% BSA to avoid the non-specific binding of antibodies. The slides were incubated with mouse anti-TPD52 (Sigma-Aldrich, USA) and rabbit anti-LKB1 (Cell Signaling Technology, USA) antibodies diluted (1:200) in 2% BSA and incubated at 4 °C overnight. Following incubation, slides were washed in TBST to remove excess primary antibody. Followed by secondary antibody incubation and detected with anti-mouse (conjugated with Alexa Fluor 555) and anti-rabbit (conjugated with Alexa Fluor 488) diluted at 1:1000 in 2% BSA for two hours in dark with gentle shaking. After removing excess antibody using TBST, the coverslips were detached from the glass chamber and mounted on glass slides using FluoroshieldTM (Sigma-Aldrich, USA). The images were visualized under Flouview1000, a confocal imaging system (Olympus, Japan) and co-localization were measured by Pearson correlation coefficient obtained using FV10-ASW 3.0 software. The images were produced from three individual experiments by capturing at least five different fields each time.
Protein–protein docking
For homology modeling, a FASTA sequence of TPD52 isoform 3 or TPD52L3 was obtained from the Uniport database (https://www.uniprot.org/). The 3D structure of TPD52L3 was generated using the I-TASSER server (https://zhanggroup.org/). The finest acquired model displayed TM score = 0.46 ± 0.15, RMSD =10.0 ± 4.6 Å, C-score = 2.15 & cluster density around 0.4 with decoys 4504 (SPECKER program based Monte Carlo simulations). The final resulted model consisting of 68.7% favoured and 89.0% allowed residues was validated through MoLProbity (http://molprobity.manchester.ac.uk/) (Chen et al. 2010). The obtained TPD52L3 model was taken as a template for protein-protein docking with LKB1 structure reported as heterotrimeric complex (PDB: 2WTK, Zeqiraj et al. 2009) consisting of LKB1, STRADα and MO25α using ClusPro 2.0 server (https://cluspro.org/help.php). From ClusPro outputs (PPI complexes), interaction between TPD52 and LKB1 was subjected to further analysis. To investigate the specificity of the interaction between TPD52 and LKB1, alanine mutagenesis was carried out using default parameters of BioLuminate module (https://www.schrodinger.com/products/bioluminate), which calculates the stability and affinity of residues by refining the side chain with backbone minimization at cut off 0.0 Å. In this method, we have enabled the affinity maturation workflow and Monte Carlo optimization method to predict the parameters (Beard et al. 2013).
Co-immunoprecipitation
Cells were directly lysed and homogenized in RIPA (Sigma-Aldrich, USA) containing protease inhibitor cocktail (Tocris Bioscience, UK). The homogenates were clarified by centrifugation for 20 min at 13,000 g and 4 °C. The cell lysates (~1mg) were pre-cleared by incubation with protein A/G agarose beads (20 μl/mg lysate) (Santa Cruz Biotechnology, USA) for 1 hr with constant mixing in a rotating shaker at 4 °C. The beads adsorbed with non-specific antigens was discarded and the resultant pre-cleared protein sample was quantified for immunoprecipitation. An equal amount of ~1 mg of total protein was incubated with 10 μg of either anti-LKB1 or anti-TPD52 antibody. For control, IgG antibody was used and all tubes were incubated overnight at 4 °C. The antibody-antigen complexes were pulled down with 50μl of protein-A magnetic beads (Bio-Rad, USA) and incubated 2 h at 4 °C. The beads were washed with mild RIPA buffer (20 mM HEPES, 150 mM NaCl, 0.5% NP40, 10% Glycerol, 1 mM EDTA) containing protease inhibitors. The beads captured with antigen-antibody complexes were purified using a magnetic rack (Bio-Rad, USA) and resuspended in 2X lamellae buffer. These samples along with input control were heated at 95°C for 10min and used for western blotting. Followed by western blotting, HRP-linked anti-rabbit IgG specific to light chain (Bio-Rad, USA) and anti-mouse IgG specific to heavy and light chain (Cell Signaling Technology, USA) secondary antibodies were used for detection of respective target proteins.
GST pull down assays
GST tagged full length TPD52 or its truncated forms were expressed and purified using the pGEX-6P1 vector as reported previously (Dasari et al. 2019). Briefly, the expression of GST or GST fusion proteins was induced by adding 0.5mM of IPTG when culture OD600 reached nearly 0.6–0.8 for 4 h at 30 °C. For pull down assays, the cell pellet was resuspended in lysis buffer (50 mM Tris base, 500 mM NaCl, 1 mM EDTA, 0.1% Triton X, 10% glycerol, and 0.1mM PMSF) and lysed by sonication for 7 min (40% amplitude with 5sec ON and 8 s OFF cycles) until homogenous lysate is obtained. The lysate was cleared by centrifugation and kept for 30 min, 12000 rpm at 4 °C. For purification of complexes, Glutathione Sepharose beads (GE Healthcare, USA) were incubated with clear lysate (> 5 mg protein/ml slurry) for 30 min at 4 °C in a rotating shaker to capture prey proteins (GST & GST fusion proteins). The immobilized beads were washed with mild lysis buffer to get rid of non-specific proteins. A small fraction (~5%) of immobilized beads were run through SDS-PAGE gel and stained with
Coomassie brilliant blue for detection of all GST & GST fusion proteins. Protein immobilized with Glutathione beads was normalized from SDS-PAGE gel using ImageJ software. Equal quantity of prey protein immobilized beads were resuspended in 1.5 mg of LNCaP cells homogenate and incubated for ~16–20 h at 4 °C in a rotator shaker. Prey proteins bound to GST and other GST fusion proteins were captured after washing with mild lysis buffer followed by direct lysis in 2X lamellae buffer at 95 °C for 10 min. Eluted fractions along with positive control were used in western blotting for the detection of target proteins.
Statistical analysis
All the experiments were analyzed for statistical significance of the results by paired student’s t-test using GraphPad Prism 8 software. The individual experiments were performed three times in triplicates except the colony formation assay which was performed twice in triplicates. The p-values < 0.05 were considered statistically significant and represented as * = p < 0.05, ** = p < 0.01 and *** = p < 0.001. Error bars in the graphs were created using mean ± standard deviation (SD) values.
Results
AICAR inhibits PCa cell proliferation
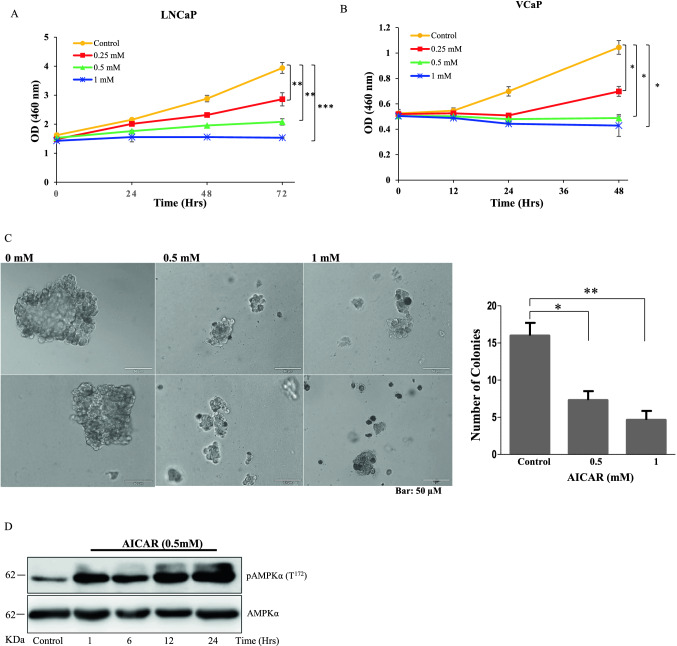
Earlier studies indicated that activation of AMPK target tumor cell proliferation (Gollavilli et al. 2015; Xiang et al. 2004; Yu et al. 2009). Since, the above reports did not reveal the mechanism, in this study we determined the effect of AICAR, an AMPK activator on proliferation of PCa cells. AICAR inhibited proliferation of LNCaP and VCaP cells in both concentration (0–1 mM) and time (0–72 h) dependently (Fig. 1A & B). To confirm AICAR effects on anchorage-independent cell growth, colony formation assay was performed and it was found that the colony formation ability of the LNCaP cells was decreased by more than 60% with 1 mM AICAR kept for 14 days (Fig. 1C). These results indicates that AICAR restricts proliferation efficiency of PCa cells in in-vitro. Concurrently, we also checked the activation status of AMPKα in AICAR stimulated LNCaP cells and observed that the pAMPK levels are elevated with respect to time of stimulation (Fig. 1D).
Fig. 1.
AICAR inhibits PCa cell proliferation and anchorage independent growth. (A) LNCaP & (B) VCaP cells treated with AICAR at different concentrations and incubated for various time points. The cell proliferation rate was determined using the CCK8 kit. Data displayed are mean ± SD of three individual experiments performed in triplicates. (C) In colony formation assay, LNCaP cells were cultured in soft agar with media containing AICAR for 14 days. Representative images taken from two individual experiments performed in triplicate. The enlarged images show changes in colony morphology induced by increasing concentration of AICAR. The number of colonies stained positively with crystal violet on termination of assay are plotted. Results were statistically significant and measured by paired student t-test (P < 0.05). (D) In western blotting, pAMPKα (T172) and AMPKα levels were determined in LNCaP cells treated with 0.5 mM of AICAR for various time points from 1 to 24 h
AMPK activation downregulates TPD52 overexpression in PCa cells
TPD52 is found be overexpressed in various cancers (Xiang et al. 2004) and it was also shown to induce proliferation in PCa (Rubin et al. 2004; Ummanni et al. 2008). However, the mechanisms which regulates TPD52 in cancers is still under investigation. With supporting evidence that TPD52 is involved in cancer progression, we hypothesized that the activation of AMPK might negatively regulates TPD52 expression in PCa cells. To investigate this, LNCaP and VCaP cells were treated with AICAR and expression of TPD52 was determined. AICAR significantly downregulated TPD52 expression in both LNCaP and VCaP cells (Fig. 2A, B & E). To determine whether the decreased TPD52 levels were due to gene downregulation, TPD52 mRNA level was estimated in AICAR treated cells. The TPD52 mRNA is significantly decreased in LNCaP cells treated with 0.5 mM AICAR for 24 h (Fig. 2C) and VCaP cells treated with 1 mM AICAR for 36 h (Fig. 2D) confirming that the activation of AMPK downregulates TPD52 expression. To further ascertain that the activation of AMPK inversely regulates TPD52 expression, PCa cells were treated with Compound C, a potent AMPK inhibitor and TPD52 expression at protein level was measured in western blotting. Inactivation of AMPKα leads to further overexpression of TPD52 in LNCaP cells (Fig. 2F & G). These results revealed that AICAR might exhibits its anti tumor activity through by downregulation of TPD52 expression.
Fig. 2.
AICAR targets TPD52 expression in PCa Cells. PCa cells LNCaP (A) & VCaP (B) were treated with AICAR for activation of AMPK by AICAR (0.5 and 1.0 mM) treatment and downregulation of TPD52 was determined by immunoblotting. The TPD52 mRNA was determined in LNCaP (C) and VCaP (D) cells treated with 0.5 mM AICAR for different time periods. The Mean ± SD values are plotted with significance using student’s t-test. (E) Same as (C) except that the cells were lysed for isolation of total proteins and the pAMPK and TPD52 levels are determined. The LNCaP cells were treated with different concentrations of Compound C (0—1 μM) for 24 h (F) or 0.5 μM for different time points (24 and 48 h) (G) and pAMPK and TPD52 levels are determined
AMPK activation reverses TPD52 mediated LNCaP cell migration
To acquire further insight into the physiological role of AICAR in TPD52 overexpression in PCa cells, we have used EGFP-TPD52 plasmid construct for exogenous overexpression of TPD52 as previously reported and treated with different concentrations of AICAR. The western blot results showed that EGFP-TPD52 over expression was depleted in presence of AICAR compared to control cells with EGFP alone (Fig. 3A, B). Ummanni et al. (2008) showed that TPD52 promotes PCa cell migration for the dissemination of tumor cells. Thereafter, we have checked the effect of AICAR on the invasion potential of LNCaP cells. AICAR significantly inhibited the invasive potential of LNCaP cells (Fig. 3C, D). The western blot results showed that pAMPK levels significantly reduced under exogenous overexpression of EGFP-TPD52 when compared to control cells and the same was reversed by the addition of AICAR (Fig. 3E). Taken together, these results indicate that AICAR remarkably inhibits proliferation and invasion of LNCaP cells by minimizing the TPD52 oncogenic properties.
Fig. 3.
Overexpression of TPD52 inactivates AMPK and promotes PCa cell migration. LNCaP cells were transfected with EGFP empty vector (A) or EGFP-TPD52 (B) and treated with AICAR (0.5 and 1.0 mM) for 24 h. Overexpression of EGFP-TPD52 in LNCaP was confirmed by western blotting. (C) For Transwell migration assay, LNCaP cells were transfected and treated similarly to (A) & (B) and the migration potential of treated cells was determined. (D) The number of migrated cells was counted manually and plotted with mean ± SD of three independent experiments. (E) The pAMPK levels indicating AMPK activation in LNCaP cells transfected with EGFP or EGF-TPD52 and treated with AICAR (0.5 and 1.0 mM) was determined by using western blot
AICAR mediate TPD52 downregulation through PI3K/AKT signaling
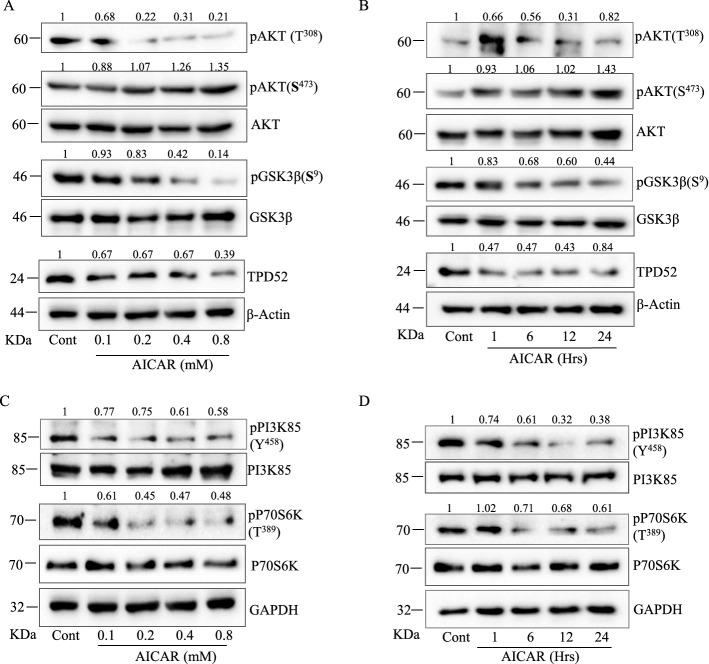
Previously, it has been reported that the activation of AKT regulates TPD52 expression and TPD52 controls PCa cell survival through AKT (El-Masry et al. 2015; Meijer and Dubbelhuis 2004). Therefore, to obtain more mechanistic information on how AICAR downregulates TPD52, first we determined phosphorylation of AKT in AICAR treated LNCaP cells. AICAR reduced AKT (Thr308) phosphorylation in both time and concentration dependent manner. In contrary, phosphorylation of AKT (Ser473) was elevated in AICAR treated LNCaP cells (Fig. 4A & B). To obtain more insights into the mechanism for AMPK mediated downregulation of TPD52, we tested AICAR activity on AKT downstream target GSK3β. We found a significant decrease in pGSK3β (Ser9) as expected in correlation with reduced pAKT (Thr308) levels in PCa cells (Fig. 4A & B). The GSK3β is a critical downstream element of the PI3K/AKT pathway which gets hyper-activated in various human cancers (Jiang et al. 2020) and phosphorylation of Ser9 inhibits its activity. Hence, AMPK is activating GSK3β by decreasing its Ser9 phosphorylation in PCa cells. More to that, the above results indicate that AKT (Thr308) might also regulate the activation status of GSK3β in LNCaP cells. Therefore, to investigate whether AMPK regulate the activity of AKT through PI3K, cells were exposed to AICAR for different time and concentrations. Interestingly, AICAR treatment decreased phosphorylation of the p85 subunit of PI3K both time and concentration dependently in LNCaP cells (Fig. 4C & D). Further, we examined the effect of AMPK activation on AKT downstream signaling in AICAR treated cells and the results showed that AICAR significantly inhibited p70S6K, a downstream target of AKT/mTOR signaling pathway in LNCaP cells. This indicates that activation of AMPK also regulates the mTOR signaling by inhibiting p70S6K in LNCaP cells (Fig. 4C & D).
Fig. 4.
AICAR mediates downregulation of TPD52 via PI3K/AKT signaling. LNCaP cells treated with AICAR for 12 h at various concentrations (0—0.8 mM) for 24 h (A) or different time points (0—24 h) with 0.5 mM (B) and analyzed in western blot for AKT, pAKT(S473), pAKT(T308), GSK3β, pGSK3β(S9) and TPD52. (C) & (D) In similar experimental conditions as (A) & (B), the other targets PI3K85, pPI3K85, P70S6K and pP70S6K was measured
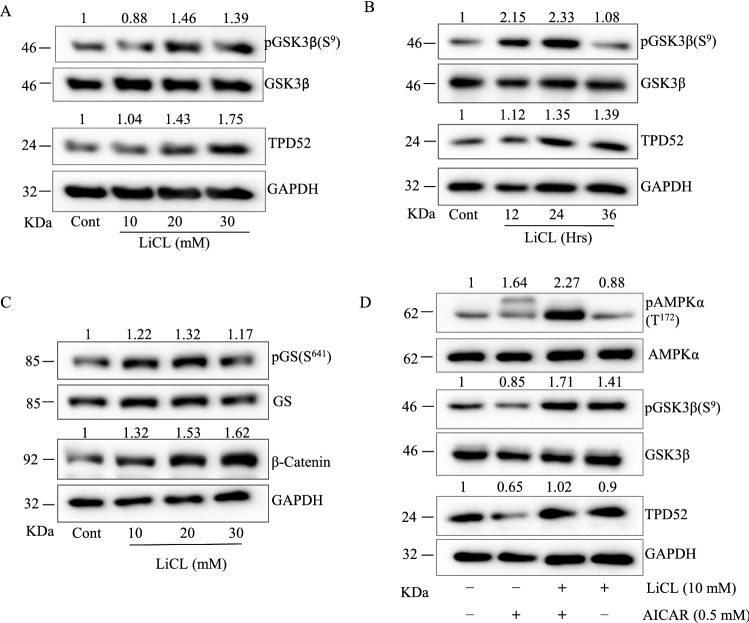
As earlier studies have shown that TPD52 determines the activation of PI3K/AKT, we hypothesized that AICAR is targeting TPD52 downregulation via activation of GSK3β. Therefore, we have measured expression of TPD52 in LNCaP cells treated with known GSK3β inhibitor LiCl (0–30 mM) for different time points (0–36 h) and compared to control untreated cells. TPD52 was found to be significantly upregulated in presence of LiCl, while pGSK3β (Ser9) levels were increased as expected (Fig. 5A & B). Conclusively, to determine the role of GSK3β on AMPK induced downregulation of TPD52, LNCaP cells were treated with AICAR with or without LiCl. From the results, we observed that the inhibition of GSK3β by LiCl attenuated AICAR mediated downregulation of TPD52. Earlier studies have shown that TPD52 displays oncogenic functions and promote PCa progression (Dasari et al. 2019; Rubin et al. 2004; Tennstedt et al. 2014). In the present study we found that that AICAR down regulates TPD52 via GSK3β in LNCaP and VCaP cells. Hence, we hypothesized that the tumorigenic potential of TPD52 may negatively regulates GSK3β axis for inducing tumor cell proliferation and metastasis. In LNCaP cells, inhibition of GSK3β by LiCl led to increased phosphorylation of its substrate glycogen synthase (pGS(Ser649)) and stabilization of its downstream target β-Catenin as expected (Fig. 5C). Importantly, when LNCaP cells are co-treated with AICAR and LiCl in combination, downregulation of TPD52 is attenuated along with inactivation of GSK3β evidenced by increased pGSK3β (Ser9) (Fig. 5D). These results shows that GSK3β is involved in the regulation of TPD52 expression in LNCaP and VCaP cells.
Fig. 5.
Inhibition of GSK3β increases TPD52 expression in PCa cells. (A) LNCaP cells were treated with different concentrations of LiCl (0 -30 mM) or 10 mM LiCl for different time points (B) time points (0- 36 h) and analyzed for pGSk3β and TPD52 in western blot. (C) LNCaP cells treated with either LiCl (10 to 30 mM) or (D) in combination with AICAR (0.5 mM) and determined the expression of target proteins such as AMPK, pAMPK, GSK3β, p-GSK3β (S9) and TPD52
TPD52 interacts with LKB1 and decreases downstream AMPKα activation
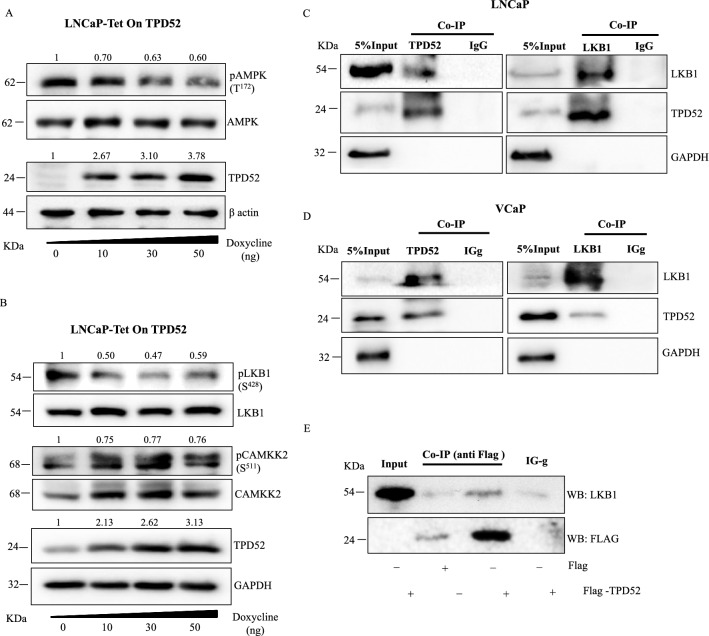
After confirming that the activation of AMPK suppresses TPD52 mediated oncogenic properties and TPD52 overexpression in many other types of cancers including PCa, we investigated whether TPD52 has any adverse effects on the AMPK signaling network. We generated LNCaP cells in which overexpression of TPD52 can be induced by doxycycline using the Tet-On system. Stable overexpression of TPD52 in LNCaP cells treated with doxycycline (0–50 ng) was confirmed by western blot. Interestingly, pAMPK levels were significantly reduced with overexpression of TPD52 in LNCaP cells (Fig. 6A). More importantly, we also observed a significant reduction in pLKB1 but not in pCAMKK2 which are upstream kinases of AMPK (Fig. 6B). Therefore, we pursue to identify how TPD52 negatively regulates LKB1 in order to inhibit AMPK signaling and suppress its anti-tumorigenic potential. First, we hypothesized that TPD52 might interact with LKB1 and determine its activity in cancer cells. Thus, immunoprecipitation was performed for the pull down of TPD52 and LKB1 complexes in both LNCaP and VCaP cell lines. Immunoprecipitation of TPD52 resulted in co-precipitation of LKB1 and likewise, immunoprecipitation of LKB1 also co-precipitated TPD52 in both LNCaP and VCaP cells. This result highlights that TPD52 interacts with LKB1 in PCa cells (Fig. 6C, D). As TPD52 has multiple isoforms, we further attempted to verify this interaction by overexpression of FLAG-TPD52 (isoform 3) in LNCaP cells followed by immunoprecipitation of complexes using anti-FLAG antibody. In line with the above results, pull down with anti-FLAG antibody followed by detection of target proteins in western blot confirmed the interaction between exogenous FLAG-TPD52 and LKB1 (Fig. 6E). Further, to confirm and validate the intracellular interaction between TPD52 and LKB1 in PCa cells (LNCaP and VCaP), immunocytochemistry was performed. The double immunofluorescence results displayed co-localization of both TPD52 and LKB1, also the graph denotes a positive correlation between green and red fluorescence signals in confocal microscopy confirmed their interaction in LNCaP and VCaP cells (Fig. 7A, B).
Fig. 6.
TPD52 interacts with LKB1 in PCa cells. (A) Activation of AMPK was determined in LNCaP cells with stable overexpression of TPD52 using the Tet-On system induced by doxycycline. (B) In the same cells as (A), activation of LKB1 and CAMKKII, upstream kinases for AMPK activation was determined. (C) & (D) Co-Immunoprecipitation of TPD52 and LKB1 in LNCaP and VCaP cell lysate using anti-LKB1and anti-TPD52 followed by detection of respective targets using specific antibody in western blotting. IgG was used negative control. (E) LNCaP cells transfected for overexpression of FLAG-TPD52 fusion protein followed by immunoprecipitation with anti-FLAG antibody for pull down of bait FLAG-TPD52 along with prey protein LKB1which is subsequently detected in western blot using anti-LKB1 antibody
Fig. 7.
Double immunofluorescence showing colocalization of TPD52 & LKB1. (A) Representative images of LNCaP & (B) VCaP cells cultured in chambered glass slides for 24 h incubated and immunoflurosecence was performed for cellular co-localization of TPD52 (red) and LKB1 (green). DAPI staining was performed for visualization of nucleus. In merged images arrows indicates colocalization of LKB1 and TPD52 (yellow). In scattered plots, Pearson correlation coefficient for co-localization of LNCaP and VCaP was found to be r = 0.98 and r = 0.78 respectively
TPD52 isoform 3 mainly interact with LKB1 kinase
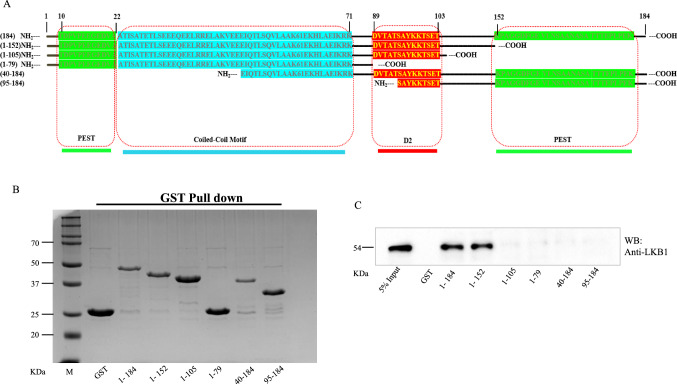
TPD52 family proteins have certain well conserved domains such as PEST, coiled-coil motif and D2. These regions may interact with various other proteins for functional activation. As the above results endorse the physical interaction of TPD52 with LKB1, we mapped the regions of TPD52 that are involved in its interaction with LKB1. For this, a set of vectors were designed, TPD52 full length and short sequences were cloned as reported in the literature (Byrne et al. 1996; Dasari et al. 2019). TPD52 characteristic D2-motif, PEST sequences, coiled-coil motif and phosphorylation sites were shown in Fig. 8a. The GST-TPD52 (1-184) or GST-TPD52 fragments consisting of N-terminal PEST motif (1–79), (1–105), (1–152) and other fragments with C-terminal PEST motif (40–184) and (95–184) were expressed using pGEX-6P1 vector in E.coli and purified using GST beads. The expression of purified GST-TPD52 or GST tagged TPD52 fragments were tested in SDS-PAGE gel (Fig. 8B). Further pull down assay results have clearly shown that LKB1 bound to GST-TPD52 (1-184) and GST-TPD52 (1-152) (Fig. 8C). The specific interaction between GST-TPD52 (1–152) and LKB1only indicates that the N-terminal PEST, coiled-coil and D2-motif flanking with C-terminal tail are involved in their interaction in PCa cells.
Fig. 8.
Different TPD52 fragments shows variable interactions with LKB1. (A) Amino acid sequence of full length and different TPD52 fragments along with their structural features highlighted in different colours. (B) Expression and purification of GST tagged TPD52 or truncated fragments were shown in Coomassie Brilliant Blue stained gel (C) Pull down assay followed by western blotting showing LKB1 is co-purified with TPD52 full length and its truncated fragment consisting N-terminal amino acid residues 1–152
Protein–protein interaction between TPD52 and LKB1
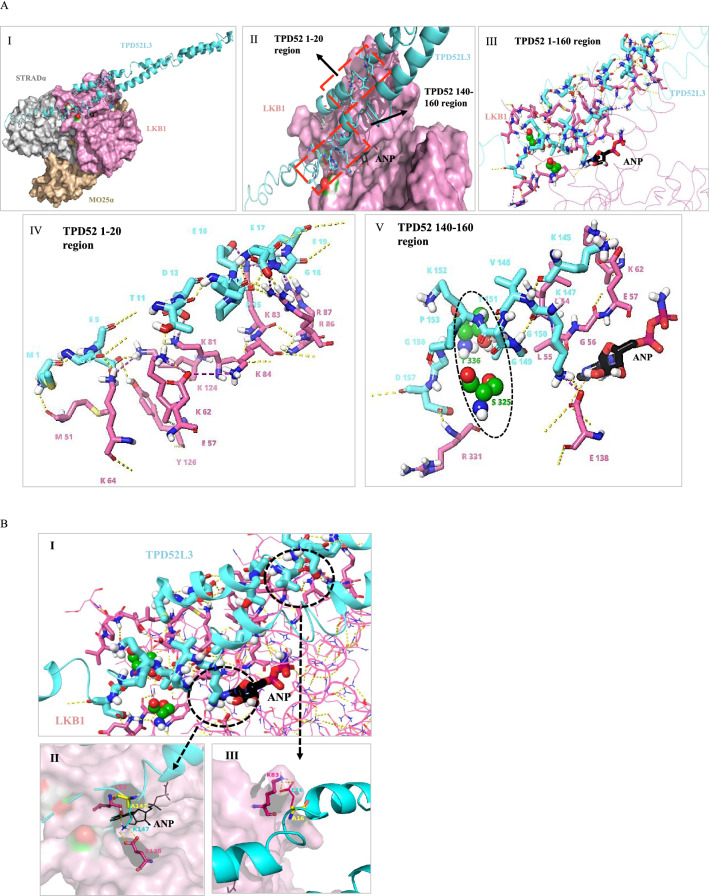
From the above evidence that TPD52 interacts with LKB1, with a molecular docking approach, we modeled the finest template for TPD52 isoform 3 as a ligand (Fig. 9A I & II), and LKB1 heterotrimeric complex with STRADα and MO25α chains (PDB: 2WTK) as a receptor protein to validate protein-protein interaction (PPI) by using ClusPro 2.0 PPI server. To understand the binding pattern of the TPD52L3 sequence with LKB1, we studied the binding sites and the results obtained were analyzed by focusing on their alignments. The PPI complexes of LKB1-TPD52 are sorted and a single complex exhibiting the desired pose with the lowest energy (balanced score = −792.9) was found to be in close proximity to experimental values (Fig. 9A I, II & III). The residues 1–20 and 140–160 of TPD52 form hydrogen bonds with LKB1 residues (D300, R331, T336, S325, D53, G52, Y340, E138, L55, K64, K62, E57, Y126, K81, K124, K84, R87, R86, and K83) (Fig. 9A IV &V). Among these interacting residues S325 and T336 of LKB1 comprise of C-terminal flanking tail (CFTL), which serves as an auto-phosphorylating site for binding with other regulatory units, T336 associated with CFTL- based phosphorylation forms a hydrogen bond with T151 of the TPD52. In addition, Y340 of CFTL exhibited a π-cation frame with M1 of TPD52 (Fig. 9A III & V).
Fig. 9.
Homology modelling and protein–protein docking of TPD52L3 & LKB1. (A-I) Ribbon diagram of TPD52L3 (cyan) & surface model of heterotrimeric complex LKB1 (pink), STRADα (grey), MO25α (yellow). (A-II) At LKB1 surface TPD52 region 1–20 and 140–160 interacts with region LKB1, highlighted in red dashed lines. (A-III) Model illustrates complete interaction of TPD52 with LKB1 represented in thick sticks whereas extended ribbons are TPD52 & LKB1 non-interacting residues (A-IV & V). Specific TPD52 region, 1 to 20 & 140 to 160 interacts with LKB1. Space-filling model signifies the LKB1 auto-phosphorylating residue S325 and T336 (green), highlighted in black dashed line. ANP is represented in black thick sticks. Hydrogen bonds in yellow dotted lines. (B) Mutations showing loss of interaction. (B-I) The ribbon diagram depicts total interacting residues and two important regions are highlighted. (B-II) Overlapping residues showing native and mutated interactions (yellow) K147 A (B-III) same as (II) only E16 A. Yellow dotted lines represent polar contacts
Unexpectedly, we also observed that TPD52 positioned on the surface of LKB1 that interacts with ANP (Adenylate ester, phosphor amino phosphonic acid), a natural ligand of LKB1 and ATP analogue, which is also reported as AMP-PNP (Fig. 8D V) (Dauter and Dauter 2011; Zeqiraj et al 2009). Other residues such as G155, G156, and D157 of TPD52 formed a hydrogen bond network with D330 and R331 of LKB1. Further analysis predicted that the residues N252, E290 and R362 of the STRADα within the complex also interact with TPD52 (from region 170–184) on the surface (supplementary figure 1A). Surprisingly, none of the TPD52 residues were found to be interacting with MO25α chain or mutation prone region of LKB1 (E182, L177, L164, G242, F157, L285 & W308) (Supplementary figure 1B). From, the PPI studies, we noticed that TPD52 residues from 1–20 and 140–160 were interacting with LKB1. These observations provided evidence for the interaction between TPD52L3 and LKB1 that determine the kinase activity of LKB1 in PCa cells (Fig. 10).
Fig. 10.
A schematic representation of molecular signaling in response to reduced cellular AMPK activity in prostate cancer where AMPK inactivation suppresses PI3K/AKT signaling and GSK3β signaling which upregulates TPD52 expression. This overexpressed TPD52 interacts with LKB1 tumor suppressor and dysregulates AMPK activation which leads to prostate cancer cell growth and dissemination
In-silico mutational analysis of TPD52L3 interacting residues
To determine the binding specificity and affinity of TPD52 to LKB1, a single point mutation using alanine scanning was carried out employing 1-20 and 140-160 domains of TPD52 (Supplementary Figure 2). In identifying the key residues, we found that the residues M1, L8, E16, K147 and T151 are necessary for its interaction with LKB1. However, residue analysis showed that the amino acids D2, E5, E16 and E147 form contacts with LKB1 based on net changes in binding potential and interaction energies (Supplementary Table 3). Together, these results highlighted that the residues E5, L8, E16, K147 and T151 are essential for establishing polar contacts with LKB1. These five active TPD52 residues involved in its interaction with LKB1 were further subjected to mutagenesis in-silico by employing a series of single point mutations (Supplementary Table 4). The mutants with K147A and E16A significantly lost their polar interactions with E138 and K83 of LKB1 respectively. The mutation T151A led to the loss of key hydrogen bonding with T336, an auto-phosphorylation site of LKB1 (Fig. 9B I, II and III). These results confirmed the N-terminal PEST and D2-motif are important for TPD52 interaction with LKB1.
Discussion
Considering the fact that the uncontrolled proliferation of cancer cells involve several metabolic pathways to meet cellular energy requirements. AMPK is one amongst the signaling pathways that regulates cellular metabolism by sensing the energy requirement of tumor cells. In this regard, AMPK is found to be directly regulating growth inhibitory pathways in several diseases including cancers (Luo et al 2010). In NSCLC, myeloid leukemia and some non-malignant diseases, AMPK signaling activity was inhibited due to loss of function of tumor suppressor LKB1 (Green et al. 2010; Han et al. 2013; Matsumoto et al. 2007). In cancer cells, cancer associated ubiquitin ligases target AMPK for inactivation of cell growth inhibitory mechanism (Pineda et al. 2015). In a recent study, it has been reported that activation of AMPK by AICAR sensitizes LNCaP and PC3 cells to radiotherapy (Rae et al. 2019). Therapeutics directly targeting AMPK is inappropriate and not advised due to their part in various diversified and miscellaneous biochemical processes. However, activation of AMPK in the regulation of oncogenic signal transduction remained elusive. In the present study, we show that activation of AMPK suppresses the expression of TPD52 in PCa cells. Importantly, the oncogenic role of TPD52 inactivated AMPK via inhibition of its upstream kinase LKB1 through its direct interaction. TPD52 is one of the six oncogenes associated with PCa progression (Ross-Adams et al. 2015) which promotes metastasis in various cancers and its expression positively correlates with the growth of cancer cells and determines apoptosis (Byrne et al. 2005; Chen et al, 2021; Dasari et al. 2019; Dasari et al. 2017; Wang et al. 2004). Clinical testing of tissue samples also reported consistent elevated levels of TPD52 in localized and metastatic prostate cancer (Rubin et al. 2004). In murine model, a protein based vaccine developed against TPD52 produce an adaptive immune response to overcome elevated TPD52 mediated tumorigenesis (Payton et al. 2008). TPD52 isoforms are found to be involved in IL-6 induced neuroendocrine differentiation of PCa cells (Moritz et al. 2016) and docetaxel resistance via impeding LKB1-AMPK mediated autophagy in PCa (Wang et al. 2004). Although TPD52 expression and its multifaceted functions are well correlated with cancer progression in particular prostate and breast cancers, whereas its molecular role is still incomprehensible. In the present study, we observed that AICAR decreased PCa cell proliferation and migration as a result of active AMPK. The results showed that the AICAR an activator of AMPK by phosphorylation significantly downregulated TPD52 expression in both LNCaP and VCaP cells. While investigating AICAR effect on inhibition of cell growth and migration, it was found that the activation of AMPK led to decreased pAKT (Thr308) through inactivation of PI3K. Interestingly, pAKT (Ser473) levels were significantly increased in AICAR treated cells which may be due to the fact that rictor-mTOR complex regulates AKT/PKB phosphorylation. In line with these results, we also found that activation of AMPK leads to the activation of GSK3β which might cause decreased TPD52 expression in PCa cells. The GSK3β is a serine/threonine kinase that regulates several proteins involved in various signaling pathways including transcription factors, cell cycle/survival regulators and proto-oncogenes. Though recent studies suggest that GSK3β may promote tumorigenesis in many cancer types (Mancinelli et al. 2017), in our study, inhibition GSK3β by LiCl led to elevated TPD52 expression and LiCl restored TPD52 in AICAR treated cells. Moreover, these results showed decreased mTOR activity in AICAR treated LNCaP and VCaP cells. Hence, it appears that AMPK activation mediates TPD52 expression through PI3K/mTOR axis and thereby inhibiting PCa cell growth.
As AMPK activation status is determining the expression levels of TPD52, further attention was given to TPD52 due to its broad oncogenic role in multiple carcinomas. To investigate TPD52 as a probable target, we hypothesized that it might interact negatively with AMPK complex and/or its upstream tumor suppressor proteins. Conspicuously, differentially expressed proteins associated with cancers may interact with other subcellular proteins and advances growth and survival of malignant cells (Garner and Janda 2011). Along these lines, stable overexpression of TPD52 caused the inactivation of AMPK in PCa cells. Most importantly, decreased pLKB1 (Ser428) indicates that the oncogenic activity of TPD52 regulates tumor suppressor LKB1 to suppress anti-proliferative role of AMPK in PCa cells. In our quest to further understand the TPD52 regulation of the LKB1/AMPK axis, we found a direct interaction between TPD52 and LKB1 in both LNCaP and VCaP cells which was validated by co-immunoprecipitation followed by western blotting to capture and detect prey proteins respectively. Pull down assays for FLAG-TPD52 and immunoblotting for LKB1 substantiated these results. Besides, immunocytochemistry results confirmed co-localization of TPD52 and LKB1 in LNCaP and VCaP cells. Furthermore, our results showed that TPD52 interacts with LKB1 through a coiled-coil motif and D52-motif between 1 and 152 amino acid residues. This is in line with the previous reports that both these motifs are involved in TPD52 family protein’s interactions with other proteins (Shahheydari et al. 2014). The reduced pAMPK in TPD52 positive cells indicates that the interaction between TPD52 and LKB1 might inhibit the kinase activity of LKB1 which directly phosphorylates AMPK as a tumor suppressor in cancer cells. To determine the role of TPD52 on LKB1 activity, an In-silico molecular modeling approach revealed that TPD52L3 residues interact with auto-phosphorylation sites S325 and T336 of LKB1. Interestingly, we also observed that the extended region of TPD52 (170-184) interacts with STRADα which also determines the activity of LKB1 heterotrimeric complex (Zeqiraj et al. 2009). Additionally in In-silico mutagenesis through alanine scan confirmed that the N-terminal PEST and D2-motif are essential for TPD52 interaction with LKB1. These interactions provided evidence for binding TPD52 with LKB1 and causing inhibition of its kinase activity leading to the inactivation of AMPK and dysregulating its downstream effectors. As retaining and engaging enzymes to their active conformation assist proper interactions and activation of their dependable substrates and kinases (Cowan-Jacob et al. 2014). LKB1 as a prime kinase activates 13 other kinases of the AMPK subfamily and its inhibition or loss of function causes Peutz-Jeghers Syndrome (PJS) a rare autosomal disease associated with gastrointestinal cancers and promotes tumorigenesis in different cancers (Lizcano et al. 2004; Mehenni et al. 1998; Woods et al. 2003). With our findings that binding of TPD52 to LKB1 interferes with its tumor suppressor function, the discovery of small molecules disrupting the TPD52-LKB1 interaction might be useful to suppress tumor cell growth.
Conclusions
In the present study we report a new regulation of TPD52 expression in LNCaP and VCaP cells by AMPK through PI3K/GSK3β axis and also revealed a new mechanism that the oncogenic role of TPD52 is mediated through its interaction with LKB1. The interaction between LKB1 and TPD52 is found to be crucial in activation status of AMPK as molecular modeling describes that TPD52 binds to phosphorylation sites of LKB1 which determines its kinase activity essential for activation of its substrate AMPK. Moreover, it has been reported that AMPK signaling pathway controls proliferation and survival of PCa cells. Hence, the use of AMPK activators or newer therapeutics targeting TPD52 and/or LKB1 might offer effective therapy in combination with other chemotherapeutic drugs, where TPD52 is involved in cancer progression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the HCP-040 project funded by the Council of Scientific and Industrial Research (CSIR). PK acknowledges CSIR for graduate student fellowship. CSIR-IICT manuscript communication no: IICT/Pubs./2022/241.
Authors contributions
RU and PK: Study design and planning of experiments. PK, SK, AP, SN and SSJ: Experimentations, data acquisition, data analysis. RU and PK: Manuscript writing, review and editing of the manuscript.
Data availability
All the raw data and material is available with the corresponding author.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Consent for publication
All the authors reviewed the data, read the manuscript and consented to publication of the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Beard H, Cholleti A, Pearlman D, Sherman W, Loving KA. Applying physics-based scoring to calculate free energies of binding for single amino acid mutations in protein-protein complexes. PloS One. 2013;8(12):e82849. doi: 10.1371/journal.pone.0082849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JA, Tomasetto C, Garnier JM, Rouyer N, Mattei MG, Bellocq JP, Rio MC, Basset P. A screening method to identify genes commonly overexpressed in carcinomas and the identification of a novel complementary DNA sequence. Can Res. 1995;55(13):2896–2903. [PubMed] [Google Scholar]
- Byrne JA, Mattei M-G, Basset P. Definition of the tumor protein D52 (TPD52) gene family through cloning of D52Homologues in human (hD53) and mouse (mD52) Genomics. 1996;35(3):523–532. doi: 10.1006/geno.1996.0393. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Balleine RL, Fejzo MS, Mercieca J, Chiew YE, Livnat Y, Heaps L, Peters GB, Byth K, Karlan BY, Slamon DJ. Tumor protein D52 (TPD52) is overexpressed and a gene amplification target in ovarian cancer. Int J Can. 2005;117(6):1049–1054. doi: 10.1002/ijc.21250. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Frost S, Chen Y, Bright RK. Tumor protein D52 (TPD52) and cancer—oncogene understudy or understudied oncogene? Tumor Biology. 2014;35(8):7369–7382. doi: 10.1007/s13277-014-2006-x. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66(1):12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Peng C, Tan W, Yu J, Zayas J, Peng Y, Lou Z, Pei H, Wang L. Tumor protein D52 (TPD52) affects cancer cell metabolism by negatively regulating AMPK. Cancer Med. 2022 doi: 10.1002/cam4.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan-Jacob SW, Jahnke W, Knapp S. Novel approaches for targeting kinases: allosteric inhibition, allosteric activation and pseudokinases. Future Med Chem. 2014;6(5):541–561. doi: 10.4155/fmc.13.216. [DOI] [PubMed] [Google Scholar]
- Dasari C, Yaghnam DP, Walther R, Ummanni R. Tumor protein D52 (isoform 3) contributes to prostate cancer cell growth via targeting nuclear factor-κB transactivation in LNCaP cells. Tumor Biology. 2017;39(5):1010428317698382. doi: 10.1177/1010428317698382. [DOI] [PubMed] [Google Scholar]
- Dasari C, Reddy K, Natani S, Murthy T, Bhukya S, Ummanni R (2019) Tumor protein D52 (isoform 3) interacts with and promotes peroxidase activity of Peroxiredoxin 1 in prostate cancer cells implicated in cell growth and migration. Biochim Biophys Acta (BBA) Mol Cell Res 1866(8):1298–309 [DOI] [PubMed]
- Dauter M, Dauter Z. Deprotonated imidodiphosphate in AMPPNP-containing protein structures. Acta Crystall Sect D Biolog Crystall. 2011;67(12):1073–1075. doi: 10.1107/S0907444911046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Masry OS, Al-Sakkaf K, Brown BL, Dobson PR. Differential crosstalk between the AMPK and PI3K/Akt pathways in breast cancer cells of differing genotypes: Leptin inhibits the effectiveness of AMPK activation. Oncol Rep. 2015;34(4):1675–1680. doi: 10.3892/or.2015.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG. Calmodulin-dependent protein kinase kinase-β activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010;426(1):109–118. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner LA, Janda DK. Protein-protein interactions and cancer: targeting the central dogma. Curr Top Med Chem. 2011;11(3):258–80. doi: 10.2174/156802611794072614. [DOI] [PubMed] [Google Scholar]
- Gollavilli PN, Kanugula AK, Koyyada R, Karnewar S, Neeli PK, Kotamraju S. AMPK inhibits MTDH expression via GSK 3β and SIRT 1 activation: potential role in triple negative breast cancer cell proliferation. FEBS J. 2015;282(20):3971–3985. doi: 10.1111/febs.13391. [DOI] [PubMed] [Google Scholar]
- Green AS, Chapuis N, Trovati Maciel T, Willems L, Lambert M, Arnoult C, Boyer O, Bardet V, Park S, Foretz M, Viollet B. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116(20):4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- Han D, Li S-J, Zhu Y-T, Liu L, Li M-X. LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer. Asian Pac J Cancer Prev. 2013;14(7):4033–4039. doi: 10.7314/apjcp.2013.14.7.4033. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020;47(6):4587–4629. doi: 10.1016/j.bbrc.2003.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AS, Frigo DEA. Spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat Rev Urol. 2017;14(3):164–180. doi: 10.1038/nrurol.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Mills GB. AMPK: A Contextual Oncogene or Tumor Suppressor? AMPK Regul Can Metabol Can Res. 2013;73(10):2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23(4):833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6(3):457–470. doi: 10.7314/apjcp.2013.14.7.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E, Giampietri C. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxidat Med Cell Longev. 2017 doi: 10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Iwakawa R, Takahashi K, Kohno T, Nakanishi Y, Matsuno Y, Suzuki K, Nakamoto M, Shimizu E, Minna JD, Yokota J. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene. 2007;26(40):5911–5918. doi: 10.1038/sj.onc.1210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehenni H, Gehrig C, Nezu JI, Oku A, Shimane M, Rossier C, Guex N, Blouin JL, Scott HS, Antonarakis SE. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Human Genet. 1998;63(6):1641–1650. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313(2):397–403. doi: 10.1016/j.bbrc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett. 2011;585(7):981–985. doi: 10.1016/j.febslet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Moritz T, Venz S, Junker H, Kreuz S, Walther R, Zimmermann U. Isoform 1 of TPD52 (PC-1) promotes neuroendocrine transdifferentiation in prostate cancer cells. Tumor Biol. 2016;37(8):10435–10446. doi: 10.1007/s13277-016-4925-1. [DOI] [PubMed] [Google Scholar]
- Payton LA, Lewis JD, Byrne JA, Bright RK. Vaccination with metastasis-related tumor associated antigen TPD52 and CpG/ODN induces protective tumor immunity. Cancer Immunol Immunother. 2008;57(6):799–811. doi: 10.1007/s00262-007-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda CT, Ramanathan S, Tacer KF, Weon JL, Potts MB, Ou YH, White MA, Potts PR. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell. 2015;160(4):715–728. doi: 10.1016/j.cell.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Mairs RJ. AMPK activation by AICAR sensitizes prostate cancer cells to radiotherapy. Oncotarget. 2019;10(7):749–759. doi: 10.18632/oncotarget.26598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KR, Dasari C, Duscharla D, Supriya B, Ram NS, Surekha MV, Kumar JM, Ummanni R. Dimethylarginine dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in prostate cancer, and its overexpression conveys tumor growth and angiogenesis by metabolizing asymmetric dimethylarginine (ADMA) Angiogenesis. 2018;21(1):79–94. doi: 10.1007/s10456-017-9587-0. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Can Res. 2002;62(15):4427–4433. [PubMed] [Google Scholar]
- Ross-Adams H, Lamb AD, Dunning MJ, Halim S, Lindberg J, Massie CM, Egevad LA, Russell R, Ramos-Montoya A, Vowler SL, Sharma NL. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: a discovery and validation cohort study. EBioMed. 2015;2(9):1133–1144. doi: 10.1016/j.cell.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MA, Varambally S, Beroukhim R, Tomlins SA, Rhodes DR, Paris PL, Hofer MD, Storz-Schweizer M, Kuefer R, Fletcher JA, Hsi BL. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Can Res. 2004;64(11):3814–3822. doi: 10.1158/0008-5472.CAN-03-3881. [DOI] [PubMed] [Google Scholar]
- Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Exp Rev. Mol Med. 2012 doi: 10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- Seo Y, Kim J, Park SJ, Park JJ, Cheon JH, Kim WH, Kim TI. Metformin suppresses cancer stem cells through AMPK activation and inhibition of protein prenylation of the mevalonate pathway in colorectal cancer. Cancers. 2020;12(9):2554. doi: 10.3390/cancers12092554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahheydari H, Frost S, Smith BJ, Groblewski GE, Chen Y, Byrne JA. Identification of PLP2 and RAB5C as novel TPD52 binding partners through yeast two-hybrid screening. Mol Biol Rep. 2014;41(7):4565–4572. doi: 10.1007/s11033-014-3327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Cao J, Chen K, Cheng L, Zhou C, Yan B, Qian W, Li J, Duan W, Ma J, Qi D. Betulinic acid inhibits stemness and EMT of pancreatic cancer cells via activation of AMPK signaling. Int J Oncol. 2019;54(1):98–110. doi: 10.3892/ijo.2018.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennstedt P, Bölch C, Strobel G, Minner S, Burkhardt L, Grob T, Masser S, Sauter G, Schlomm T, Simon R. Patterns of TPD52 overexpression in multiple human solid tumor types analyzed by quantitative PCR. Int J Oncol. 2014;44(2):609–615. doi: 10.3892/ijo.2013.2200. [DOI] [PubMed] [Google Scholar]
- Ummanni R, Teller S, Junker H, Zimmermann U, Venz S, Scharf C, Giebel J, Walther R. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J. 2008;275(22):5703–5713. doi: 10.1111/j.1742-4658.2008.06697.x. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu J, Saramäki O, Visakorpi T, Sutherland WM, Zhou J, Sen B, Lim SD, Mabjeesh N, Amin M, Dong JT. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer research. 2004;64(5):1589–1594. doi: 10.1158/0008-5472.can-03-3331. [DOI] [PubMed] [Google Scholar]
- William WN, Kim JS, Liu DD, Solis L, Behrens C, Lee JJ, Lippman SM, Kim ES, Hong WK, Wistuba II, Lee HY. The impact of phosphorylated AMP-activated protein kinase expression on lung cancer survival. Ann Oncol. 2012;23(1):78–85. doi: 10.1093/annonc/mdr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321(1):161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- Yu SY, Chan DW, Liu VW, Ngan HY. Inhibition of cervical cancer cell growth through activation of upstream kinases of AMP-activated protein kinase. Tumor Biol. 2009;30(2):80–85. doi: 10.1159/000216843. [DOI] [PubMed] [Google Scholar]
- Zeng J, Liu W, Fan YZ, He DL, Li L. PrLZ increases prostate cancer docetaxel resistance by inhibiting LKB1/AMPK-mediated autophagy. Theranostics. 2018;8(1):109. doi: 10.7150/thno.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeqiraj E, Filippi BM, Deak M, Alessi DR, Van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326(5960):1707–1711. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw data and material is available with the corresponding author.