Abstract
Abstract
Abdominal aortic aneurysms (AAA) have the highest incidence and rupture rate of all aortic aneurysms. The N6 methyladenosine (m6A) modification is closely associated with angiotensin (Ang II)-induced aortic diseases. This study aimed to identify whether the m6A writer METTL3/METTL4 regulates rip3 mRNA expression in AAA. To induce the mouse AAA model, apolipoprotein E-deficient (ApoE-/-) mice were subcutaneously infused with Ang II, and C57BL/6 mice were infused with type I elastase. Vascular smooth muscle cells (VSMCs) were induced with Ang II. Necroptosis was detected using an Annexin V-FITC/PI apoptosis detection kit, and ELISA assays measured inflammatory cytokines. The RNA immunoprecipitation-qPCR determined the methylated rip3 mRNA level. The increased expressions of inflammatory factors, aortic adventitia injury, degradation of elastin, and CD68-positive cells suggested the successful establishment of mouse AAA models. In AAA aorta wall tissues, the m6A modification level and the expression of METTL3/METTL14 were elevated. In Ang II-induced VSMCs, necroptosis and inflammatory cytokines in the supernatants were increased. RNA immunoprecipitation and co-immunoprecipitation assays confirmed the binding between the METTL3–METTL14 complex and rip3 mRNA, the interaction between YTHDF3 and rip3 mRNA, and between the METTL3–METTL14 complex and SMAD2/3. Interference with METTL3/METTL14 attenuated VSMC necroptosis, inflammatory response, and the AAA pathological process in vivo. The METTL3–METTL14 complex, which was increased by the activation of the SMAD2/3, elevated the m6A modification of rip3 mRNA by promoting the binding between YTHDF3 and rip3 mRNA, thus contributing to the progression of AAA.
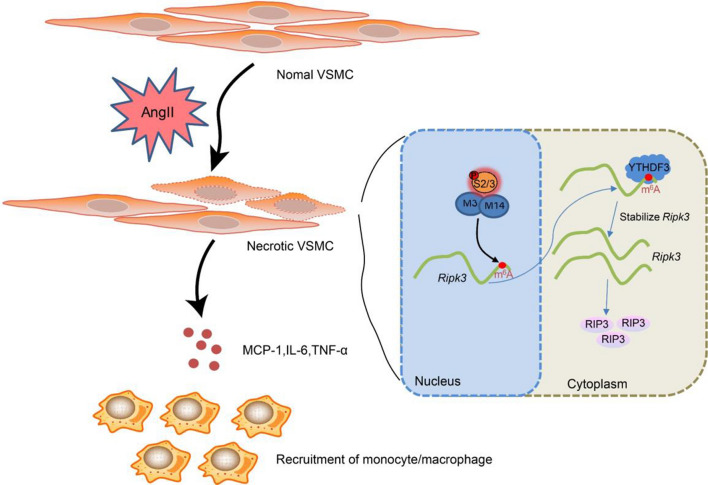
Graphical abstract
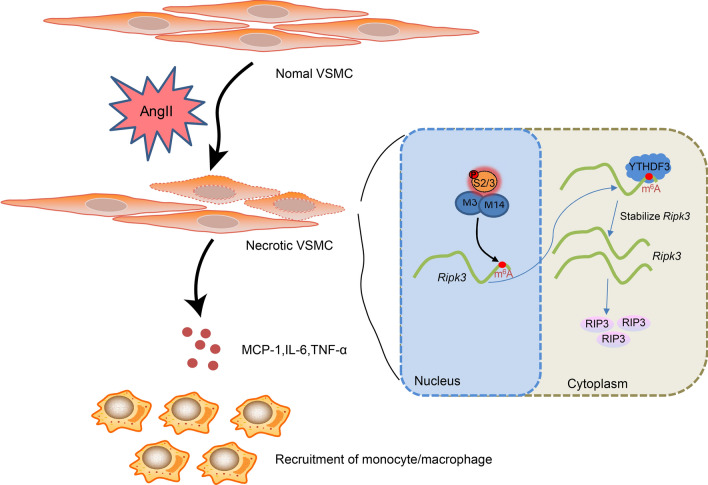
The activation of SMAD2/3 in VSMCs of abdominal aortic wall tissues is stimulated by Ang II. Subsequently, it promotes METTL3 METTL14 complex mediated m6A modification of rip3 mRNA. Meanwhile, the level of rip3 mRNA becomes more stable under the m6A reader of YTHDF3, which increases the protein level of RIP3 and further induces VSMC necroptosis. In addition, cell debris induces inflammatory factors in neighboring VSMCs and recruit monocytes/macrophages to the lesion.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-023-00737-y.
Keywords: m6A modification, Abdominal aortic aneurysm, N6 methyladenosine, RIP3, Necroptosis
Introduction
An aneurysm is a localized abnormal enlargement of a blood vessel, usually caused by thinning of the arterial wall due to vascular injury. Aneurysms can occur in any vessel; among them, the more fatal aneurysms are those of the ring of Willis in the brain, thoracic aorta, and abdominal aorta (DeROO 2022). In contrast, abdominal aortic aneurysms (AAA) have the highest incidence and rupture rate due to their unique hemodynamic and anatomic features(Sun et al. 2022). Chronic vascular wall inflammation, apoptosis of smooth muscle cells, and remodeling of the extracellular matrix are critical histopathological features of abdominal aortic aneurysms(Mangum 2022). Therefore, inhibiting the vascular inflammatory response, reducing smooth muscle cell apoptosis, and maintaining extracellular matrix homeostasis may reduce the occurrence of AAA and improve prognosis.
Vascular smooth muscle cells (VSMCs) are the primary cells that make up the aortic wall’s midmembrane. An imbalance in VSMC proliferation and apoptosis can lead to aortic wall remodeling and AAA progression (Zhong et al. 2020; He et al. 2019). Previous studies have shown that increased apoptosis of VSMCs is closely associated with AAA progression and that impaired VSMC proliferation is a fundamental cause of AAA progression (Chen et al. 2019; Jian et al. 2020). It has been confirmed that promoting VSMC proliferation while inhibiting apoptosis is beneficial in alleviating AAA progression(Sun et al. 2020; Michineau et al. 2014). Our previously published findings showed that VSMC proliferation and apoptosis are regulated by the lncRNA CRNDE and can directly affect AAA progression(Li et al. 2020). However, the underlying molecular mechanisms remain unclear.
Cellular necrosis is a form of cell death with typical morphological features manifested by organelle swelling, cell membrane rupture, and the release of intracellular lysates, causing a local inflammatory response. Cell necrosis is a modifiable mode of cell death. Receptor-interacting protein 3 (RIP3) is a central regulatory protein in the programmed necrosis signaling pathway and mediates the onset of programmed necrosis. RIP3-dependent programmed necrosis is involved in various disease processes, including ischemic injury, acute inflammatory injury, chronic inflammatory diseases, and neurodegenerative diseases. It has been shown that RIP3 expression is significantly elevated in human AAA (especially in smooth muscle cells) (Wang et al. 2017), that RIP3 promotes AAA progression by promoting the necrosis and inflammation of smooth muscle cells (Wang et al. 2015), and that autologous necrotic cell debris induces an inflammasome response in VSMCs (Wortmann et al. 2019). In addition, RIP3 inhibitors reduce the necrosis and inflammation of smooth muscle cells and decrease macrophage infiltration, ultimately alleviating aortic dilation in AAA mice (Zhou et al. 2019). Therefore, further study of the regulatory mechanism of RIP3 expression in AAA may provide new ideas for alleviating the pathological process of AAA.
N6 methyladenosine (m6A) modification is considered the most common posttranscriptional modification of mRNA and non-coding RNA in eukaryotes (Coker et al. 2019). It plays a role in various pathophysiological processes by promoting precursor RNA shearing, mRNA translation, and enhancing RNA stability (Berulava et al. 2020). Recent studies have also revealed that the m6A methylesterase METTL3 can influence the progression of AAA mice by regulating the progression of m6A-dependent initial miRNAs (Zhong et al. 2020). In addition, the m6A methylester METTL14, m6A demethylase FTO, and m6A reader YTHDF3 have also been shown to be aberrantly expressed in AAA patients and associated with processes such as inflammatory infiltration and vascular neogenesis (He et al. 2019). The METTL3–METTL14 complex mediates m6A deposition on nuclear RNA inside mammalian cells (Liu et al. 2014), but the exact mechanisms are unclear. Among them, it has been reported that METTL14 plays an essential role in TNF-α-induced endothelial cell inflammation and atherosclerotic plaque formation by mediating the m6A modification of foxo1, and thus in the process of atherosclerotic plaque formation (Jian et al. 2020). Prediction by bioinformatics software (SRAMP) revealed multiple potential m6A methylation sites in the rip3 mRNA sequence, suggesting that rip3 mRNA may be susceptible to m6A methylation modifications. Therefore, we assumed that the METTL3–METTL14 complex participated in the m6A modification of rip3 mRNA.
Herein, the present study proposed to use the AAA mouse model and angiotensin II (Ang II)-induced VSMCs to explore whether the change in RIP3 expression in VSMCs is related to m6A modification. Meanwhile, this study will investigate whether the METTL3–METTL14 complex regulates VSMC necrosis and inflammation by mediating the m6A modification of rip3 mRNA. The above revelations will provide new ideas for the prevention and treatment of AAA.
Materials and methods
Animals
Male apolipoprotein E-deficient (ApoE-/-) mice on a C57BL/6 background were bought from Cyagen Biosciences (Suzhou, Jiangsu, China). In contrast, Male C57BL/6 mice were purchased from the Shanghai Lab Animal Research Center (Shanghai, China). The mice were housed under pathogen-free conditions with a 12-h dark/light cycle. The mice were given a regular diet with free access to water.
Mouse AAA model
The mouse AAA model was induced with angiotensin II (Ang II) or elastase. ApoE-/- mice (12‒16 weeks old) were used in the Ang II-induced AAA model. They were subcutaneously infused with Ang II (A9525, Sigma, St. Louis, MO, USA) via an implanted osmotic minipump (ALZET, Model 2004, DURECT Corporation, Cupertino, CA, USA) (the Ang II group, n = 4) (Zhong et al. 2020). The control ApoE-/- mice were subcutaneously infused with the same volume of normal saline (Saline group, n = 4). The infusion rate was 1000 ng/kg/min, and the infusion lasted 28 days. A small incision in the dorsum of the neck was cut to insert the minipump under anesthesia.
To establish the Elastase-induced AAA model, type I pancreatic porcine elastase (6 IU/mg; Sigma-Aldrich, St. Louis, MO, USA) was locally applied on C57BL/6 mice for 5 min (the Elastase group, n = 4) (Wang et al. 2015). The abdominal muscle, fascia, and cutaneous muscles were sutured with individual stitches. An equal concentration of heat-inactivated (100 °C for 15 min) elastase was used as the control (the inactive elastase group, n = 4).
The maximal abdominal aortic diameter of the mice in the four groups was detected using the Color Doppler ultrasound system for small animals (M7Vet, Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, Guangdong, China). After two weeks, the mice were sacrificed, and abdominal aortic wall tissues were collected.
Hematoxylin–eosin (HE) staining
Specimens from the dilated aortas in the infra‐renal region of all mice were collected and embedded before being cut into 5 μm serial sections (Wang et al. 2019). The sections were placed in an aqueous hematoxylin solution for several minutes. The sections were then placed in acid and ammonia. Subsequently, the sections were rinsed with running water for 1 h and then placed in distilled water. Dehydration was performed using 70% and 90% alcohol and lasted for 10 min. After that, the sections were stained in an alcoholic eosin staining solution for 2‒3 min. The sections were dehydrated with pure alcohol and then made transparent with xylene. The transparent sections were dripped and sealed with a coverslip. The sections were observed under a microscope and photographed.
Elastic van Gieson (EVG) staining
Sections were dewaxed and stained with prepared Verhof’s staining solution for 1‒3 min until the sections were dark black. The sections were rinsed with water, and a 2% ferric chloride solution was added for 10‒20 s. The elastic fibers were observed as black with a gray background under the microscope. Sections were gently rinsed using water and distilled water, and after 10‒15 s of re-staining using Van Gieson’s solution, the sections were washed with anhydrous ethanol. The sections were dehydrated using anhydrous ethanol, transparent with xylene, and then sealed with neutral gum (Kaneko et al. 2011).
Immunofluorescence staining
Tissues were sliced into 8 μm thick sections for immunofluorescence staining. In brief, at room temperature, the sections were rinsed in 4% paraformaldehyde (PFA) for 10 min. The sections were then washed three times in PBS‐Tween 20 and incubated with 3% goat serum diluted in PBS for 1 h. The anti‐cleaved caspase 3 (8172S, Cell Signaling Technology, Danvers, MA, USA), the anti-α-SMA (ab124964, Abcam, Cambridge, UK), anti-METTL3 (GTX33315, GeneTex, Irvine, CA, USA), and anti-METTL14 (Sigma-Aldrich, St Louis, MO, USA) were added and incubated overnight at 4 °C. DAPI was used to identify the nuclei. After rinsing in PBS, the sections were incubated with secondary antibodies conjugated with Alexa Fluor® 488 (ab150077, Abcam, Cambridge, UK) or Alexa Fluor® 555 (ab150074, Abcam, Cambridge, UK) for 1 h. For double labeling with cleaved caspase‐3, terminal deoxynucleotidyl transferase-mediated dUTP‐biotin nick end labeling (TUNEL) staining (ab66108, Abcam, Cambridge, UK) was applied. The slides were imaged with an inverted fluorescence microscope.
Total mRNA m6A level determination
The total m6A RNA methylation level in abdominal aortic wall tissues was detected using the EpiQuik m6A RNA Methylation Quantification Kit (Epigentek, Farmingdale, NY, USA). The percentage of m6A RNA in total RNA (m6A%) was used to evaluate the m6A RNA methylation status. Briefly, 200 ng of RNA from each sample was pipetted into the wells according to the manufacturer’s protocol. Subsequently, an antibody specific to capturing m6A was added to each well. After washing, the detection antibody was added to the wells. Then, the color development solution was added to each well and placed in the dark room. The intensity of the color was measured at 450 nm using a spectrophotometer. A standard curve was made, and the m6A% was calculated (He et al. 2019). Total m6A RNA methylation level in VSMCs was also detected.
Quantitative real-time PCR (qRT-PCR)
The abdominal aortas were frozen in liquid nitrogen. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Manchester, UK). The QuantiTect Reverse Transcription Kit (Takara, Dalian, Liaoning, China) was used for reverse transcription. The qPCR was performed using the SYBR Green qPCR mix (Applied Biosystems, Foster City, CA, USA) on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primers are shown in Table 1. GAPDH was used as the internal control. Fold changes in gene expression were calculated according to the method of 2−△△Ct.
Table 1.
Primers for the quantitative polymerase chain reaction
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| gapdh | AACTCCCTCAAGATTGTCAGCAA | GTGGTCATGAGCCCTTCCA |
| IL6 | CTTCGGTCCAGTTGCCTTCT | GCCTCTTTGCTGCTTTCACAC |
| tnfa | AAGCCTGTAGCCCACGTCGTA | GGCACCACTAGTTGGTTGTCTTTG |
| ccl2 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| ifng | ACTCAAGTGGCATAGATGTGGAAG | GACGCTTATGTTGTTGCTGATGG |
| mettl3 | TTGTCTCCAACCTTCCGTAGT | CCAGATCAGAGAGGTGGTGTAG |
| mettl14 | GAACACAGAGCTTAAATCCCCA | TGTCAGCTAAACCTACATCCCTG |
| rip3 | TTTTACTCAGGACTCCTCACCG | CTTGGACGAGCCCAGCTTT |
Immunohistochemistry
The sections were dewaxed and rinsed in water, followed by adding 3% H2O2 for 10 min, pouring off the H2O2 and rinsing the sections, adding citric acid buffer, and putting them in the microwave for 3 min, cooling and steaming again. After the sections were cooled and rinsed, they were added to phosphate-buffered saline (PBS) for 5 min. Diluted serum was added and placed in a 37 °C incubator for 30 min. Then, primary antibodies, including anti-CD68 (ab283654, Abcam, Cambridge, UK), anti-CD31 (ab182981, Abcam, Cambridge, UK), and anti-MMP9 (ab228402, Abcam, Cambridge, UK) were added, and they were incubated in the refrigerator at 4 °C overnight. The sections were removed from the refrigerator, washed three times in PBS for 5 min, and incubated with secondary antibody in a 37 °C incubator for 30 min. Diluted streptavidin–biotin complex (SABC) was added and incubated at 37 °C for 30 min. Then, chromogenic diaminobenzidine (DAB), H2O2, and phosphate buffer were added to the sections. The sections were rinsed with water and soaked in hematoxylin for 30 s. Finally, the sections were dehydrated and sealed (Wang et al. 2019).
Western blotting
Total protein was extracted by radioimmunoprecipitation assay (RIPA) lysis buffer with a protease inhibitor cocktail. The BCA protein concentration determination kit (Beyotime, Beijing, China) was used to determine the protein concentration. Samples containing 20 μg of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 5% stacking gel and 10% separation gel. Gel electrophoresis was performed to separate proteins. They were then transferred to polyvinylidene difluoride (PVDF) membranes. Primary antibodies, including anti-TGF-β1 (1/1000, ab215715, Abcam, Cambridge, UK), anti-SMAD2 (1/1000, ab33875, Abcam), anti-p-SMAD2 (1/1000, ab280888, Abcam), anti-SMAD3 (1/1000, ab208182, Abcam), anti-p-SMAD3 (1/2000, ab52903, Abcam), anti-METTL14 (1/1000, ab252562, Abcam), anti-METTL3 (1/1000, ab195352, Abcam), and anti-RIP3 (1 µg/ml, ab62344, Abcam) were added and incubated overnight. After that, the membranes were washed with TRIS-buffered saline with 0.5% Tween 20 (TBST) solution. The membranes were then incubated with a secondary antibody. GAPDH (1/2500, ab9485, Abcam, Cambridge, UK) was used as the internal reference.
Cell culture and treatment
VSMCs from the mouse aorta were bought from Procell Life Science and Technology Co., Ltd. (CP-M076, Wuhan, Hubei, China). They were maintained in a 37 °C, 5% CO2 incubator. VSMCs were divided into two groups: the control group and the Ang II group. In the Ang II group, VSMCs were incubated with 200 nM Ang II (Qin et al. 2020).
Flow cytometry analysis
An Annexin V-FITC/PI Apoptosis Detection Kit (40302ES20, Yeasen Biotechnology, Shanghai, China) was used. To determine the necroptosis of VSMCs, single-cell suspensions were stained with 5 μl Annexin V-FITC and 10 μl PI solution. The VSMCs were then sorted by flow cytometry (FACSCanto II; BD Biosciences, San Jose, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-6, TNF-α, MCP-1, and IFN-γ in VSMC supernatants were detected using the Mouse IL-6 ELISA Kit (E-EL-M0044c, Elabscience Biotechnology, Wuhan, Hubei, China), the Mouse TNF-α ELISA Kit (SEKM-0034, Solarbio Life Sciences, Beijing, China), the Mouse MCP-1 ELISA Kit (ab100722, Abcam, Cambridge, UK), and the Mouse IFN-γ ELISA kit (Beyotime, Beijing, China) according to the manufacturer’s instructions.
VSMC transfection
Small interfering RNAs against mettl3 (si-mettl3) and mettl14 (si-mettl14) were synthesized using RiboBio Technology (Guangzhou, Guangdong, China). The sequences of si-mettl14 are GCAGCACCUCGAUCAUUUATT, and the negative control (NC) siRNA sequences are UUCUCCGAACG UGUCACGUTT. VSMCs were transfected with si-mettl3, si-mettl14, or NC (10 nM) using Lipofectamine RNAiMax (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. After 24 h, Ang II was added and incubated for 72 h. Interference with ythdf3 in VSMCs was also performed using si-ythdf3 (RiboBio Technology, Guangzhou, Guangdong, China).
VSMCs were transfected with si-NC, si-mettl3, or si-mettl14 using Metafectene Pro (Biontex, Martinsried, Germany) according to the manufacturer’s instructions. After 6 h, the cells were transduced with 100 MOI adenovirus Ad-rip3 (Hanbio Technology, Shanghai, China) for 2 h. After removing viral solutions, the cells were cultured in 10% FBS DMEM and treated with Ang II for 72 h. The cells were then analyzed by flow cytometry, and the cell supernatant was collected for the Raw264.7 transwell assay.
Transwell assay
Macrophage migration assay was performed using 8.0 μm transwell plates (#CLS3464, Sigma-Aldrich, St Louis, MO, USA). The conditioned medium of VSMCs was collected and added to the lower chambers of the transwell plates. At the same time, 2 × 105 RAW 264.7 cells (CL-0190, Procell Technology, Wuhan, Hubei, China) were starved overnight and seeded in the upper chambers. Six hours later, cells on the upper surface of the transwell insert were removed by gently scraping with a cotton swab, and the membranes were fixed and stained with 0.1% crystal violet (#C0775, Sigma-Aldrich, St Louis, MO, USA).
Anti-mettl3/mettl14-treated AAA mouse model
ApoE-/- mice (12‒16 weeks old) were subcutaneously infused with Ang II (A9525, Sigma, St. Louis, MO, USA) via an implanted osmotic minipump (ALZET, Model 2004, DURECT Corporation, Cupertino, CA, USA), as described above (n = 10 in each group). Before the Ang II treatment, the ApoE-/- Mice were infected with AAV9-mediated vectors, including AAV9-si-NC, AAV9-si-mettl3, or AAV9-si-mettl14 (Hanbio Technology, Shanghai, China) with 1 × 1011 vg through tail vein injection. Mice were sacrificed, and the abdominal aortic wall tissues were collected on 28d after the Ang II treatment. Abdominal aorta diameter was detected using Doppler ultrasonography (MYLAB™ Sigma VET, Shanghai, China).
Methylated RNA immunoprecipitation (MeRIP)-qPCR
The MeRIP procedure was performed using a Magna MeRIP™ m6A kit (#17–10,499, Millipore, Temecula, CA, USA (Wan et al. 2022). In brief, purified mRNA was digested by DNase I. They were fragmented into ~ 100 nt via RNA fragmentation reagent at 94 °C. After adding the stop buffer, standard ethanol precipitation was performed. The 12 μg of anti-m6A was pre-incubated with 50 μl beads in IP buffer containing 0.1% NP-40, 10 mM Tris–HCl, and 150 mM NaCl, pH 7.4) for 1 h at room temperature. Then, 6 μg of fragment mRNAs were added to the mixture at 4 °C and incubated for 4 h. The high concentration of proteinase K digested the immunoprecipitated mixture. The phenol–chloroform method and ethanol precipitation were used to extract the RNAs. They were used for qPCR analysis. qPCR analysis determined the modification of m6A.
Detection of rip3 mRNA stability
The RNA synthesis inhibitor actinomycin D (Beyotime, Beijing, China) was added to the VSMCs. The expression of rip3 mRNA was detected using qRT-PCR at 0, 3, and 6 h.
Co-immunoprecipitation (Co-IP)
Ang II-treated VSMCs were washed twice with prechilled PBS. Pre-cooled RIPA buffer was added to the sample, and the cells were scraped off the culture dish with a pre-cooled cell scraper. The cell suspension was transferred to a 1.5 EP tube, shaken for 15 min at 4 °C, and centrifuged at 14,000 g for 15 min. The supernatant was transferred to a new centrifuge tube. The beads were washed twice with PBS, and a 50% concentration was prepared with PBS. Then, 100 μl protein A agarose beads were added to every 1 ml of total protein, and they were shaken at 4 ℃ for 10 min, centrifuged at 14,000 g for 15 min, and transferred to a new centrifuge tube. Protein A beads were removed, the protein standard curve was plotted, and the protein concentration was determined. The total protein was diluted to approximately 1 μg/μl with PBS, the rabbit antibody was added, and the antigen–antibody mixture was shaken overnight at 4 °C. After that, 100 μl protein A agarose beads were added to capture the antigen–antibody complex, and they were shaken overnight at 4 °C and centrifuged at 14,000 g for 5 s. The agarose beads–antigen–antibody complex was collected, and the supernatant was removed. They were washed three times with pre-cooled RIPA buffer, and 60 μl 2 × sampling buffer was added and mixed well. The sample was boiled for 5 min, and the supernatant was collected for electrophoresis.
RNA Immunoprecipitation (RIP)
Cells (1 × 107) were centrifuged at 1000 g for 5 min at 4 °C, and the supernatant was discarded. The samples were washed twice with pre-cooled PBS, and the supernatant was discarded. The sample was added to a 1 × PLB lysis solution and ice bath for 5 min. Protein A agarose beads were shaken and mixed, and 40 μl beads were aspirated into a 1.5 mL centrifuge tube. The beads were washed twice with 0.5 ml NT-2. Protein A agarose beads were resuspended with 100 μl NT-2, and 5 μg of the METTL3/METTL14 antibody, YTHDF1/2/3 antibody, or IgG antibody was added. The samples were incubated for 1 h at room temperature. The samples were centrifuged at 4000 g for 1 min at 4℃, and the supernatant was removed. Protein A–antibody complexes were resuspended with 1 ml of NT-2 and centrifuged at 4000 g for 1 min at 4 °C. The supernatant was removed. The procedure was repeated five times for a total of six washes. Subsequently, protein A–antibody complexes were resuspended with 900 μl NT-2. Cell lysates were thawed on ice and centrifuged at 17,000 g for 10 min at 4 °C. Then, 10 μl of lysate supernatant was taken as input and frozen at -80 °C, and 100 μl of supernatant was added to 900 μl of protein A-antibody complex and incubated overnight at 4 °C. Protein A complexes were resuspended with 1 mL of precooled NT-2. The protein A complex was resuspended with 150 μl Proteinase K buffer, and 107 μl NT-2, 15 μl 10% SDS, and 18 μl Proteinase K were added to the input sample and incubated at 55 °C for 30 min. Then, 1 ml Trizol buffer was added to the sample for RNA extraction. Reverse transcription and qPCR detection were performed.
Dual-luciferase reporter assay
The two predicted m6A sites were mutated, and a dual luciferase plasmid vector containing wild-type (WT) and mutant (Mut) rip3 3’UTR was constructed by RiboBio Technology (Guangzhou, Guangdong, China), which was cotransfected with plasmids of HA-mettl3, Flag-mettl14, or His-ythdf3 in 293 T cells. A dual-luciferase reporter assay system (Promega, Madison, WI, USA) measured luciferase activity in the cell lysates. Renilla luciferase was used for the normalized luciferase values.
Statistical analysis
Data are presented as mean ± standard deviation (SD). The difference between the two groups was compared using the student’s t‐test. The difference among multiple groups was compared using the one-way analysis of variance (ANOVA), followed by the LSD post hoc test. SPSS 18.0 (IBM, Armonk, NY, USA) and Graphpad Prism 8 were used to analyze the data. If P < 0.05, the differences were considered statistically significant.
Results
The level of m6A modification is elevated during the AAA pathological process
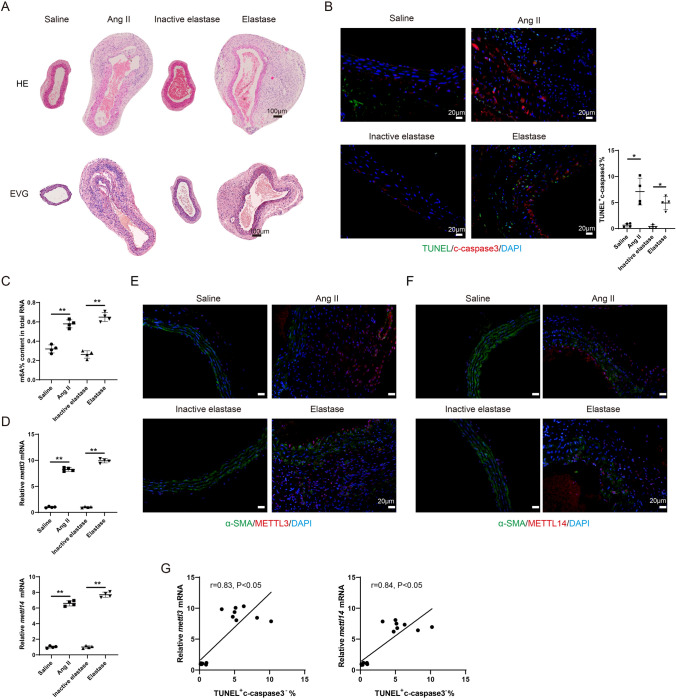
The AAA mouse model was established using Ang II or Elastase. In the Ang II group and the elastase group, flattening, fragmentation, and degeneration of the elastic laminae in the medial layer, as well as thickening and remodeling in the aortic adventitia, were observed from H&E staining compared with the corresponding control groups (Fig. 1A). The EVG staining results showed severe elastin degradation in Ang II- and Elastase-induced mice (Fig. 1A). In the Ang II group and the Elastase group, the expressions of inflammatory factors, including IL6, tnfa, ccl2, and ifng, were elevated in mouse aortic tissues compared with the corresponding control groups (all P < 0.05, Fig. S1A–S1D). The increased CD31 positive cells suggested increased angiogenesis, and the elevated MMP9 positive cells suggested an increased remodeling of the extracellular matrix (Fig. S1E). The elevated level of CD68-positive cells in AAA mouse models suggested a significant increase in macrophage infiltration (P < 0.01 and P < 0.05, Fig. S1F). These findings suggest that the Ang II-induced and the Elastase-induced AAA mouse models were successfully established.
Fig. 1.
The level of m6A modification is elevated during the AAA pathological process. The mouse AAA model was induced with Angiotensin II (Ang II) or Elastase. ApoE − / − mice were subcutaneously infused with Ang II via an implanted osmotic minipump (the Ang II group, n = 4). The control ApoE − / − mice were subcutaneously infused with the same volume of normal saline (the Saline group, n = 4). The infusion rate was 1000 ng/kg/min and the infusion lasted for 28 days. Type I pancreatic porcine elastase (6 IU/mg) was locally applied on C57BL/6 mice for 5 min (the Elastase group, n = 4). An equal concentration of heat-inactivated (100 °C for 15 min) elastase was used as the control (the Inactive elastase group, n = 4). A HE staining and the EVG staining of the mouse aortic tissues. B Immunofluorescence staining of the mouse aortic tissues using anti-c-caspase 3 and TUNEL assay (Brown-Forsythe and Welch ANOVA test with Games-Howell’s post-hoc test). C The m6A% content in total RNA in abdominal aortic wall tissues was detected using the EpiQuik m6A RNA Methylation Quantification Kit (**P < 0.01, one-way ANOVA with Tukey’s post-hoc test). D Relative mRNA expressions of mettl3 and mettl14 in mouse aortic tissues were detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (**P < 0.01, one-way ANOVA with Tukey’s post-hoc test). E–F Immunofluorescence staining of the mouse aortic tissues using anti-α-SMA, anti-METTL3, and anti-METTL14 (Scale Bar = 20 μm). G The relationship between the expression of mettl3/mettl14 and the percentage of TUNEL+ c-caspase 3− cells were determined using Pearson’s correlation analysis (n = 16, Pearson r test)
Meanwhile, cell necrosis was found in AAA mouse models as the number of TUNEL+ cleaved-Caspase 3− (c-caspase 3−) cells was increased (Fig. 1B). As shown in Fig. 1C, in the Ang II-induced and the Elastase-induced AAA mouse models, the m6A% content in total RNA was markedly elevated (both P < 0.05). The mRNA expressions of mettl3 and mettl14 were elevated in the model mice (all P < 0.01, Fig. 1D-1F). In addition, the expressions of mettl3 and mettl14 were positively related to the percentage of TUNEL+ c-caspase 3− cells (both P < 0.05, Fig. 1G).
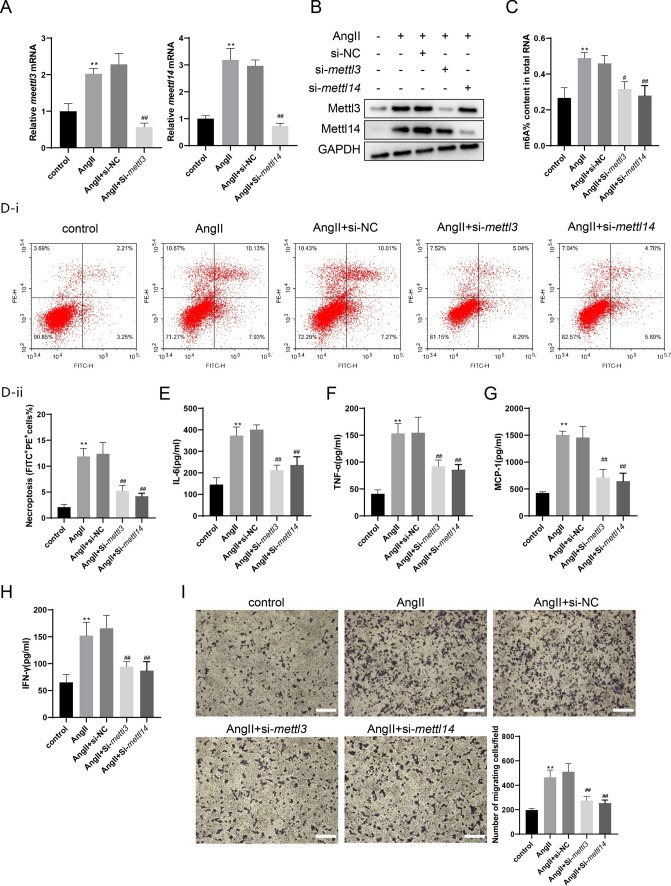
Interference with mettl3 or mettl14 reduces necroptosis and inflammatory response of Ang II-treated VSMCs.
Ang II-treated mouse VSMCs were used to mimic the AAA pathological process. The Ang II treatment increased the mRNA expressions of mettl3 and mettl14 (both P < 0.01), while the interference with mettl3 or mettl14 negated such a response (both P < 0.01, Fig. 2A). The protein expressions of METTL3 and METTL14 were elevated by Ang-II treatment and reduced by interference with mettl3 or mettl14 (Fig. 2B). The Ang-II treatment also elevated the m6A% content in total RNA (P < 0.01) and was reduced by the interference with mettl3 or mettl14 (P < 0.05 and P < 0.01, Fig. 2C). The percentage of necroptosis (FITC+ PI+) of VSMCs was increased by Ang II treatment (P < 0.01), while the interference decreased with mettl3 or mettl14 (both P < 0.01, Fig. 2D). The levels of inflammatory factors in cell supernatants, including IL-6, TNF-α, MCP-1, and IFN-γ, were also reduced by the interference with mettl3 or mettl14 (all P < 0.01), which was raised by the Ang II treatment (all P < 0.01, Fig. 2E–H). The VSMC cell supernatants were used to incubate RAW264.7 cells. The interference with mettl3 or mettl14 inhibited the migration of RAW264.7 cells (both P < 0.01, Fig. 2I).
Fig. 2.
The interference with METTL3 or METTL14 reduced necroptosis and inflammatory response of Ang II-treated VSMCs. VSMCs were divided into the Control group and the Ang II group. In the Ang II group, VSMCs were incubated with 200 nM Ang II. VSMCs were also transfected with si-mettl3, si-mettl14, or NC (10 nM) for 24 h, followed by incubation with Ang II for 72 h. A The mRNA expressions of mettl3 and mettl14 were detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, ##P < 0.01 vs Ang II + si-NC). B The protein expressions of METTL3 and METTL14 were determined by western blotting. C The m6A% content in total RNA in VMSCs was detected using the EpiQuik m6A RNA Methylation Quantification Kit (one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, #P < 0.05, ##P < 0.01 vs Ang II + si-NC). D The necroptosis of VSMCs was detected using the Annexin V-FITC/PI Apoptosis Detection Kit (one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, ##P < 0.01 vs Ang II + si-NC). E–H The levels of IL-6, TNF-α, MCP-1, and IFN-γ in VSMC supernatants were detected using the Mouse IL-6 ELISA Kit, the Mouse TNF-α ELISA Kit, the Mouse MCP-1 ELISA Kit, and the Mouse IFN-γ ELISA Kit (one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, ##P < 0.01 vs Ang II + si-NC). I The conditioned medium of VSMCs was collected and added to the lower chambers of the transwell plates while 2 × 105 RAW 264.7 cells were seeded in the upper chambers. Macrophage migration assay was performed using 8.0 µm transwell plates (one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, ##P < 0.01 vs Ang II + si-NC, Scale Bar = 200 μm)
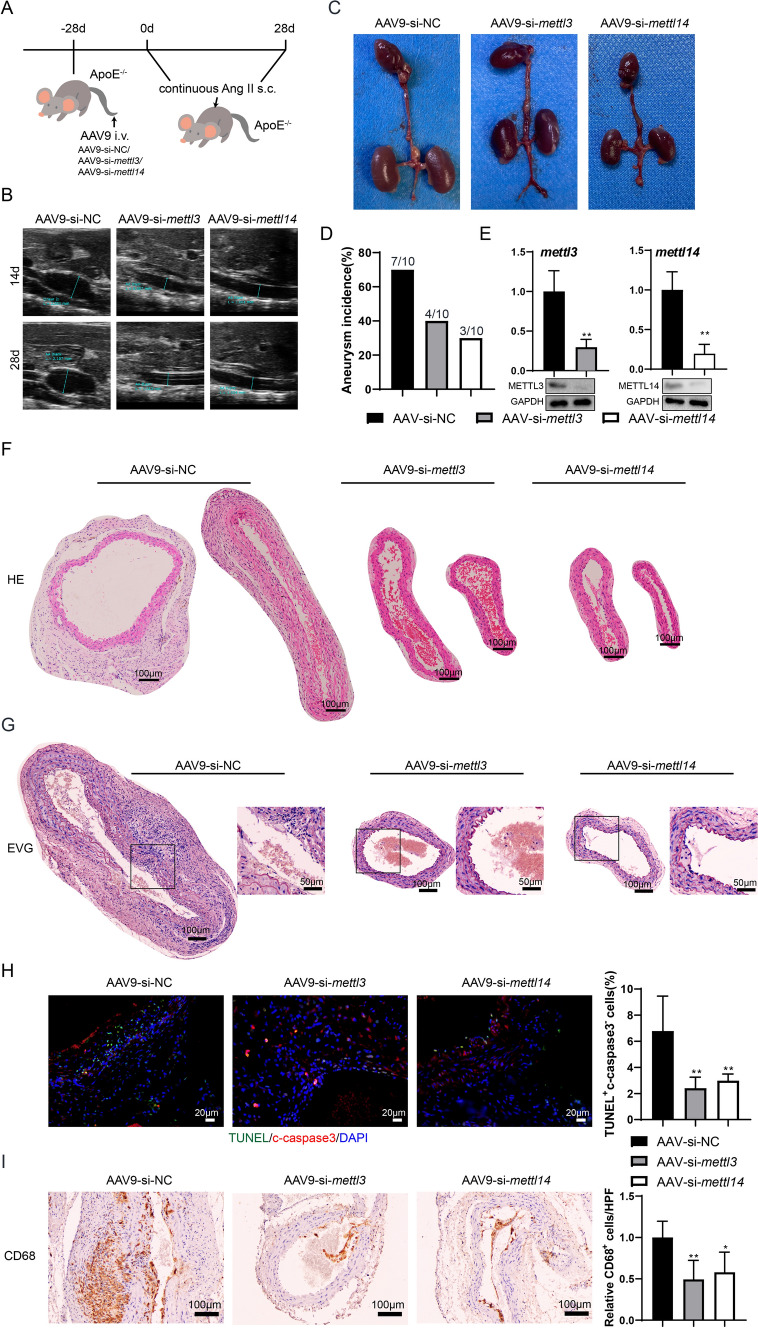
The interference with mettl3 or mettl14 attenuates AAA in vivo
The interference with mettl3 or mettl14 in ApoE-/- mice was mediated by AAV vectors (Fig. 3A). Compared with the AAV9-si-NC group, interference with mettl3 or mettl14 could reduce the diameter of the aorta both at 14 d and 28 d (Fig. 3B). The Fig. 3C showed representative images of the aorta in the three groups. The AAA incidence was 7/10, 4/10, and 3/10 in the three groups, respectively (Fig. 3D). The results of qRT-PCR and western blotting confirmed the decrease of mettl3/mettl14 mRNA levels and METTL3/METTL14 protein levels in the abdominal aortic wall tissues (P < 0.05, Fig. 3E). The HE and EVG staining indicated that interference with mettl3 or mettl14 could attenuate the pathological change of AAA (Fig. 3F-3G). In addition, the interference with mettl3 or mettl14 reduced the percentage of TUNEL+ c-caspase 3− cells (both P < 0.01, Fig. 3H) and CD68 positive cells (P < 0.01 and P < 0.05, Fig. 3I). These data suggest that the interference with mettl3 or mettl14 has a therapeutic effect on AAA in vivo.
Fig. 3.
The interference with mettl3 or mettl14 attenuates AAA in vivo. ApoE-/- mice were subcutaneously infused with Ang II. Before the Ang II treatment, the ApoE-/- Mice were infected with AAV9-mediated vectors, including AAV9-si-NC, AAV9-si-mettl3, or AAV9-si-mettl14, with 1 × 1011 vg through tail vein injection (n = 10 in each group). A The experimental protocol. B Abdominal aorta diameter was detected at 14d and 28d using Doppler ultrasonography. C The image of the aorta in three groups. D The AAA incidence. E The mRNA expressions of mettl3 and mettl14 were detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (n = 5, unpaired two-tailed t-test, **P < 0.01). The protein expressions of METTL3 and METTL14 were detected using western blotting. F-G HE staining and the EVG staining of the mouse aortic tissues (n = 5). H Immunofluorescence staining of the mouse aortic tissues using anti-c-caspase 3 and TUNEL assay (n = 5, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs AAV-si-NC). I Immunohistochemistry of the mouse aortic tissues using anti-CD68 (n = 5, one-way ANOVA with Tukey’s post-hoc test, *P < 0.05, **P < 0.01 vs AAV-si-NC)
The interference with mettl3 or mettl14 reduces necroptosis and the inflammatory response of Ang II-treated VSMCs by promoting the degradation of rip3 mRNA
As shown in Fig. 4A, the results of qRT-PCR and western blotting showed that Ang II upregulated RIP3 and p-MLKL (P < 0.01) and downregulated by interference with mettl3/mettl14 (both P < 0.01) in vitro. The same trends were also observed in the in vivo experiments (Fig. 4B). In VSMCs, the rip3 enrichment in the MeRIP assay was elevated by Ang II (P < 0.01) and was reduced by the interference with mettl3/mettl14 (both P < 0.01, Fig. 4C), suggesting that the m6A level of rip3 could be regulated by METTL3/METTL14. Meanwhile, interference with mettl3/mettl14 increased the degradation of rip3 mRNA at 3 and 6 h (Fig. 4D). As METTL3 and METTL14 proteins are more stable as a complex, the Co-IP assay showed binding between METTL3 and METTL14 in Ang II-treated VSMCs (Fig. 4E). After interfering mettl3/mettl14, the METTL3/METTL14-enriched rip3 mRNA level was reduced (both P < 0.01, Fig. 4F). In addition, the interference with mettl3/mettl14 in VSMCs, followed by overexpression of rip3, reversed the inhibitory effect of the mettl3/mettl14 interference on Ang II-induced necroptosis and inflammation response (Fig. 4G-4J).
Fig. 4.
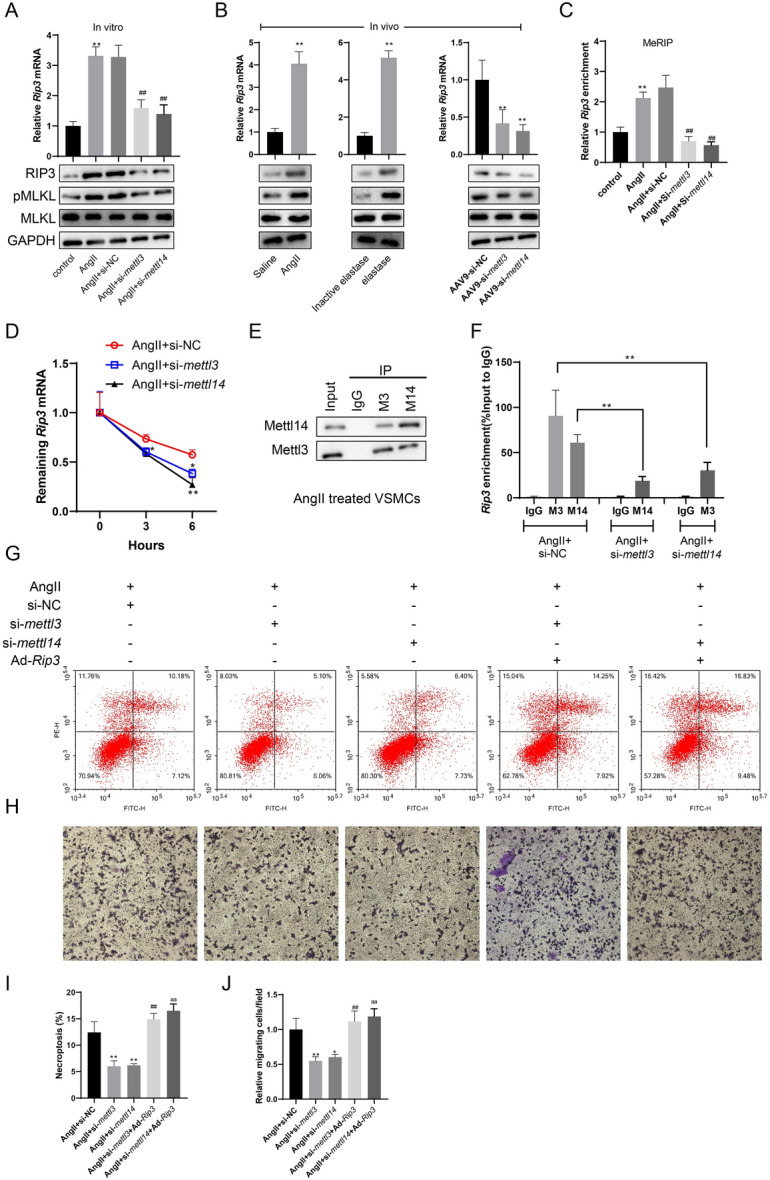
The interference with mettl3 or mettl14 reduces necroptosis and inflammatory response of Ang II-treated VSMCs by promoting the degradation of rip3 mRNA. A VSMCs were divided into the Control group and the Ang II group. In the Ang II group, VSMCs were incubated with 200 nM Ang II. VSMCs were also transfected with si-mettl3, si-mettl14, or NC (10 nM) for 24 h, followed by incubation with Ang II for 72 h. The mRNA expression of rip3 was detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, ##P < 0.01 vs Ang II + si-NC). The protein expression of RIP3, MLKL, and p-MLKL was determined by western blotting. B The mRNA expression of rip3 in mouse aortic wall tissues was detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (left two panels: n = 4, unpaired two-tailed t-test; right panel: n = 5, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs saline, inactive elastase, or AAV9-si-NC). The protein expression of RIP3, MLKL, and p-MLKL was determined by western blotting. C The level of methylated rip3 mRNA in VSMCs was determined by the Methylated RNA immunoprecipitation (MeRIP)-qPCR (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01 vs control, ##P < 0.01 vs Ang II + si-NC). D The RNA synthesis inhibitor, actinomycin D, was added to VSMCs. The expression of rip3 mRNA was detected using qRT-PCR at 0, 3, and 6 h (n = 3, two-way ANOVA with Sidak’s post-hoc test, *P < 0.05, **P < 0.01 vs AAV9-si-NC). E The binding between METTL3 and METTL14 in Ang II-induced VSMCs was confirmed by Co-IP. F The binding between METTL3/METTL14 and rip3 mRNA was confirmed by the RIP assay (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01). VSMCs were transfected with si-NC, si-mettl3, or si-mettl14 for 6 h. They were transduced with 100 MOI adenovirus Ad-rip3 for 2 h. After the removal of viral solutions, cells were treated with Ang II for 72 h. Then, the cells were analyzed by flow cytometry G, I, and the cell supernatant was collected for Raw264.7 transwell assay H, J (n = 3, one-way ANOVA with Tukey’s post-hoc test, *P < 0.05, **P < 0.01 vs Ang II + si-NC, ##P < 0.01 vs Ang II + si-mettl3, aaP < 0.01 vs AngII + si-mettl14)
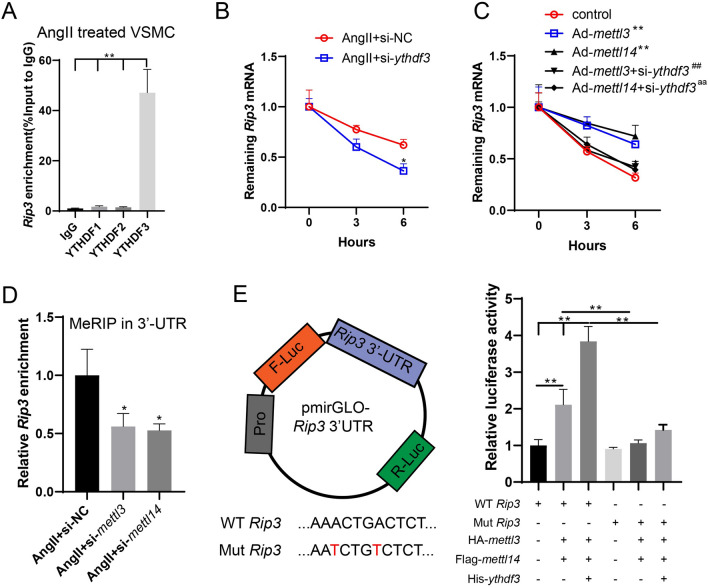
YTHDF3 promotes the stability of rip3 mRNA by recognizing the METTL3–METTL14 complex-mediated m6A binding sites
As shown in Fig. 5A, YTHDF3 could bind with rip3 mRNA (P < 0.01), and the interference with ythdf3 promoted the degradation of rip3 mRNA (P < 0.05, Fig. 5B). Meanwhile, the overexpression of mettl3/mettl14 and the interference with ythdf3 promoted the degradation of rip3 mRNA, which was inhibited by the overexpression of mettl3/mettl14 alone (both P < 0.01, Fig. 5C). These findings suggest that YTHDF3 might be the m6A reader of the m6A-modified rip3 mRNA and were mediated by the METTL3-METTL14 complex. By predicting the m6A binding site using the SRAMP software (http://www.cuilab.cn/sramp/), we found a high-confidence region in human and mouse rip3 mRNAs (Fig. S2A). In the 3’UTR of rip3, there are conserved and classical RRACU sites (Fig. S2B). Meanwhile, after interference with mettl3/mettl14, the m6A-bound rip3 mRNA enrichment in the 3’UTR was markedly reduced (both P < 0.05, Fig. 5D). To clarify the specific m6A binding site, we mutated the two predicted m6A sites and constructed a dual luciferase plasmid vector containing wild-type (WT) and mutant (Mut) rip3 3’UTR, which was cotransfected with plasmids of HA-mettl3, Flag-mettl14, or His-ythdf3 in 293 T cells. It was found that mettl3/mettl14 overexpression promoted the fluorescence activity of the WT vector, and the addition of His-ythdf3 significantly increased the activity. In contrast, no significant changes were observed in the Mut vector, suggesting that the two sites in the 3’UTR region were involved in the m6A modification of rip3 by the mettl3–mettl14 complex. In addition, these sites were recognized by YTHDF3, thereby promoting the stability of rip3 mRNA (Fig. 5E).
Fig. 5.
YTHDF3 promotes the stability of rip3 mRNA by recognizing the METTL3-METTL14 complex-mediated m6A binding sites. A The binding between YTHDF1/2/3 and rip3 mRNA in Ang II-treated VSMCs was confirmed by the RIP assay (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01). B The RNA synthesis inhibitor, actinomycin D, was added to VSMCs. The expression of rip3 mRNA was detected using qRT-PCR at 0, 3, and 6 h (n = 3, two-way ANOVA with Sidak’s post-hoc test, *P < 0.05 vs Ang II + si-NC). C VSMCs were transfected with Ad-mettl3/mettl14 or co-transfected with si-ythdf3. The RNA synthesis inhibitor, actinomycin D, was added to VSMCs. The expression of rip3 mRNA was detected using qRT-PCR at 0, 3, and 6 h (n = 3, unpaired two-tailed t-test, **P < 0.01 vs control, ##P < 0.01 vs Ad-mettl3, aaP < 0.01 vs Ad-mettl14). D The m6A-bound rip3 enrichment in 3’UTR was detected using MeRIP (n = 3, one-way ANOVA with Tukey’s post-hoc test, *P < 0.05 vs Ang II + si-NC). E The two predicted m6A sites were mutated and a dual luciferase plasmid vector containing wild-type (WT) and mutant (Mut) rip3 3’UTR was constructed, which was co-transfected with plasmids of HA-mettl3, Flag-mettl14, or His-ythdf3 in 293 T cells. The fluorescence activity was determined (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01)
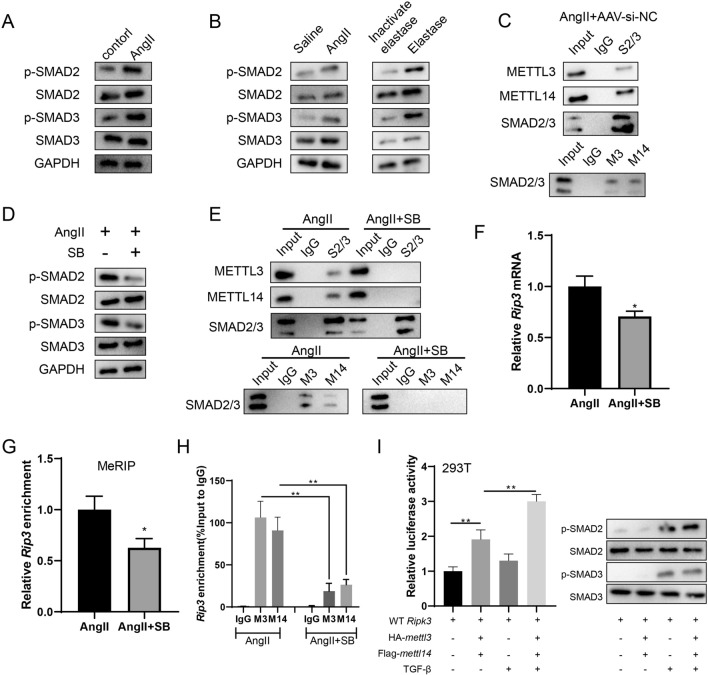
The activation of SMAD2/3 promotes the METTL3/METTL14-mediated m6A modification of rip3 mRNA
In the in vitro (Fig. 6A) and in vivo (Fig. 6B) experiments, p-SMAD2 and p-SMAD3 protein levels were elevated by Ang II or elastase. Meanwhile, in aortic wall tissues from the Ang II + AAV-si-NC group, the Co-IP assay confirmed binding between SMAD2/3 and METTL3/METTL14 (Fig. 6C). In VSMCs, after the inhibition of SMAD2/3 phosphorylation using 10 μM SB431542 (SB), the binding between SMAD2/3 and METTL3/METTL14 was reduced (Fig. 6D–6E), and the rip3 mRNA expression was also decreased (P < 0.05, Fig. 6F). In addition, the inhibition of SMAD2/3 phosphorylation inhibited the m6A-bound rip3 mRNA (P < 0.05, Fig. 6G), and it also inhibited the binding between rip3 mRNA with METTL3/METTL14 (P < 0.01, Fig. 6H). After the activation of SMAD2/3 using 100 pM TGF-β1, the binding between rip3 mRNA with METTL3/METTL14 was promoted (Fig. 6I). These data indicate that the activation of SMAD2/3 could promote the METTL3/METTL14-mediated m6A modification of rip3 mRNA.
Fig. 6.
The activation of SMAD2/3 promotes the METTL3/METTL14-mediated m6A modification of rip3 mRNA. A VSMCs were divided into the Control group and the Ang II group. In the Ang II group, VSMCs were incubated with 200 nM Ang II. The protein expressions of SMAD2, p-SMAD2, SMAD3, and p-SMAD3 were determined by western blotting. B The protein expressions of SMAD2, p-SMAD2, SMAD3, and p-SMAD3 in mouse aortic wall tissues were determined by western blotting. C The binding between SMAD2/3 and METTL3/METTL14 in aortic wall tissues from the Ang II + AAV-si-NC group was confirmed using the Co-IP assay. D In VSMCs, after the inhibition of SMAD2/3 phosphorylation using 10 μM SB431542 (SB), the protein expressions of SMAD2, p-SMAD2, SMAD3, p-SMAD3 were determined by western blotting. E In VSMCs, after the inhibition of SMAD2/3 phosphorylation using 10 μM SB, the binding between SMAD2/3 and METTL3/METTL14 was determined using the Co-IP assay. F In VSMCs, after the inhibition of SMAD2/3 phosphorylation using 10 μM SB, the mRNA expression of rip3 was detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (n = 3, unpaired two-tailed t-test, *P < 0.05 vs Ang II). G In VSMCs, after the inhibition of SMAD2/3 phosphorylation using 10 μM SB, the m6A-bound rip3 enrichment in 3’UTR was detected using MeRIP (n = 3, unpaired two-tailed t-test, *P < 0.05 vs Ang II). H In VSMCs, after the inhibition of SMAD2/3 phosphorylation using 10 μM SB, the binding between rip3 mRNA with METTL3/METTL14 was detected using the RIP assay (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01). I In 293 T cells, after the activation of SMAD2/3 using 100 pM TGF-β1, the binding between rip3 mRNA with METTL3/METTL14 was detected using the dual-luciferase reporter assay (n = 3, one-way ANOVA with Tukey’s post-hoc test, **P < 0.01)
Discussion
The m6A methylation, representing the methylated adenosine at the N6 position, has been demonstrated to be the most prevalent epigenetic modification on all RNAs. As reported, the m6A methylation level increased along with the progression of AAA (He et al. 2019). The m6A writers, including METTL3, METTL14, and WTAP, are the main components of the m6A methylase complex, activating m6A modification (Yao et al. 2018; Wu et al. 2018). The m6A erasers, including ALKBH5 and FTO, are demethylases that could reverse the m6A modification (Zhang et al. 2017, 2016). The m6A readers, including YTHDF1, YTHDF2, and YTHDF3, are vital m6A-binding proteins that could recognize m6A-modified mRNAs and transmit the code to downstream effectors (Meng et al. 2019; Koranda et al. 2018). In the current study, we found that Ang II stimulated the activation of SMAD2/3 in VSMCs of abdominal aortic wall tissues and promoted METTL3-METTL14 complex-mediated m6A modification of rip3 mRNA. Meanwhile, the level of rip3 mRNA became more stable under the m6A reader of YTHDF3, increasing the protein level of RIP3 and further inducing VSMC necroptosis. VMSC debris induced inflammatory factors in neighboring VSMCs and recruited monocytes or macrophages to the lesion, thus contributing to the progression of AAA (Fig. 7).
Fig. 7.
The activation of SMAD2/3 in VSMCs of abdominal aortic wall tissues is stimulated by Ang II. Subsequently, it promotes METTL3-METTL14 complex-mediated m6A modification of rip3 mRNA. Meanwhile, the level of rip3 mRNA becomes more stable under the m6A reader of YTHDF3, which increases the protein level of RIP3 and further induces VSMC necroptosis. In addition, cell debris induces inflammatory factors in neighboring VSMCs and recruit monocytes/macrophages to the lesion
The pathophysiology of AAA remains incompletely understood. Studies showed that the inflammatory response was severe, and infiltrating inflammatory cells were abundant in human specimens and animal models in the aneurysmal aorta (Kaiser et al. 2011; Thompson et al. 2006). Consistent with these studies, we found that in the Ang II-induced AAA mouse model and the Elastase-induced AAA mouse model, the mRNA expressions of inflammatory factors, including IL6, tnfa, ccl2, and ifng, were significantly increased. Meanwhile, in Ang II-induced VSMCs, the levels of these inflammatory factors in supernatants were also elevated. The inflammatory cells, such as macrophages, are reported to be the primary source of both matrix-degrading enzymes and proinflammatory cytokines (Thompson et al. 2006). Therefore, the CD68 positive cells, the macrophage surface markers, in the Ang II-induced AAA mouse model and the Elastase-induced AAA mouse model were more than those in the corresponding groups. However, other inflammatory cells, such as mast cells, also contribute to the release of proinflammatory cytokines. Whether m6A modification regulates these cells deserves further investigation.
Cell death includes two opposite forms: necrosis and apoptosis (Dang et al. 2022). Necrosis is associated with rapid loss of cell membrane integrity and inflammation. In contrast, apoptosis is regulated by the sequential activation of caspases, which do not trigger immune responses. Recent evidence indicates that well-orchestrated signaling networks regulate necrosis. It could be considered programmed necrosis or necroptosis (Liu et al. 2022). RIP3 is a closely related kinase during necroptosis and can initiate the necroptosis process (Beretta et al. 2022). The elevation of RIP3 expression and necroptosis was observed in the Ang II-induced AAA mouse model and the Elastase-induced AAA mouse model, consistent with a previous study (Wang et al. 2015). Besides, the RIP assay confirmed the binding between rip3 mRNA and YTHDF3 in Ang II-treated VSMCs. In addition, the overexpression of ythdf3 increased the stability of rip3 mRNA, therefore increasing both rip3 mRNA and RIP3 protein expression.
The TGF-β signaling pathway is an essential regulator of vascular remodeling. This could affect the synthesis and degradation of the vascular extracellular matrix (Jones et al. 2009). It was reported that the dysfunction of the TGF-β signaling pathway, such as the mutations in TGF-β receptors, was related to the onset of thoracic aortic aneurysm syndromes (Pannu et al. 2005). Although thoracic aortic aneurysms and AAA sites are different, the pathological processes are similar. Therefore, dysregulated TGF-β signaling pathway also plays a vital role in AAA formation. For example, Baas et al. found that genetic variations in TGF-β receptors, TGFBR1 and TGFBR2, were associated with AAA in the Dutch population (Baas et al. 2010). A recent study found that the interaction between SMAD2/3 in the TGF-β signaling pathway can increase the binding of the m6A methyltransferase complex and promote the methylation process.(Bertero et al. 2018) In our research, in the Ang II-induced AAA mouse model and the Elastase-induced AAA mouse model, the protein expressions of p-SMAD2 and p-SMAD3 were both elevated, suggesting the activation of the TGF-β signaling pathway. In contrast, the anti-TGF-β treatment could reduce rip3 mRNA expression and m6A-bound rip3 mRNA enrichment. Interestingly, the SMAD2/3-mediated Co-IP assay confirmed the binding between SMAD2/3 and the METTL3-METTL14 complex, which is consistent with the study conducted by Bertero and his colleagues.(Bertero et al. 2018) They also found that SMAD2/3 was involved in functional interaction with the METTL3-METTL14-WTAP complex, which deposits m6A. Since there are many signaling molecules in the TGF-β signaling pathway, we believed that other molecules might participate in the TGF-β-mediated m6A modification, which could exist in our subsequent investigations.
In conclusion, our study first identified that the METTL3-METTL14 complex was involved in the m6A modification of rip3 mRNA during AAA progression. Meanwhile, METTL3-METTL14 complex-mediated rip3 m6A modification was regulated by the m6A reader of YTHDF3. The SMAD2/3-mediated activation of the TGF-β signaling pathway also participated in the m6A modification. Our study suggested that the anti-activation of SMAD2/3 could be a potential therapeutic strategy for AAA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- AAA
Abdominal aortic aneurysms
- TGF-β
Transforming growth factor β
- Ang II
Angiotensin II
- VSMC
Vascular smooth muscle cell
- RIP3
Receptor-interacting protein 3
- m6A
N6 methyladenosine
- ApoE-/-
Apolipoprotein E-deficient
- qRT-PCR
Quantitative real-time PCR
- HE
Hematoxylin–eosin
- EVG
Elastic van Gieson
- PBS
Phosphate buffer saline
- SABC
Streptavidin–biotin complex
- DAB
Diaminobenzidine
- RIPA
Radioimmunoprecipitation assay
- SDS-PAGE
Sulfate–polyacrylamide gel electrophoresis
- PVDF
Polyvinylidene difluoride
- TBST
TRIS-buffered saline with 0.5% Tween 20
- Co-IP
Co-Immunoprecipitation
- ELISA
Enzyme-linked immunosorbent assay
- MeRIP
Methylated RNA immunoprecipitation
- RNA
Immunoprecipitation (RIP)
- SD
Standard deviation
- ANOVA
Analysis of variance
Authors' contributions
KL and HL put forward the concept of the study, designed the study, prepared the manuscript, and contributed to the statistical analysis. DZ designed the study, prepared the manuscript, and contributed to the statistical analysis. SZ contributed to the data acquisition. HW analyzed the data and interpretation and edited the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number 81301328) and the Scientific and Technological Research Project of the Department of Science and Technology of Henan province (Grant Number 192102310063).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
All authors declare that they have no competing interests.
Ethical approval
This study was approved by the Institute Research Medical Ethics Committee of Henan Provincial People’s Hospital. All animal experiments complied with the ARRIVE guidelines and were carried out following the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kun Li and Dongbin Zhang have contributed equally to this work.
References
- Baas AF, Medic J. Association of the TGF-beta receptor genes with abdominal aortic aneurysm. Eur J Human Genet EJHG. 2010;18(2):240–244. doi: 10.1038/ejhg.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta GL, Zaffaroni N. Necroptosis and prostate cancer: molecular mechanisms and therapeutic potential. Cells. 2022;11(7):1221. doi: 10.3390/cells11071221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, Kadiwala J, Hubner NC, de Los Mozos IR, Sadée C, Lenaerts AS, Nakanoh S, Grandy R, Farnell E, Ule J, Stunnenberg HG, Mendjan S, Vallier L. The SMAD2/3 interactome reveals that TGFβ controls m(6)A mRNA methylation in pluripotency. Nature. 2018;555(7695):256–259. doi: 10.1038/nature25784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berulava T, Buchholz E, Elerdashvili V, Pena T, Islam MR, Lbik D, Mohamed BA, Renner A, von Lewinski D, Sacherer M, Bohnsack KE, Bohnsack MT, Jain G, Capece V, Cleve N, Burkhardt S, Hasenfuss G, Fischer A, Toischer K. Changes in m6A RNA methylation contribute to heart failure progression by modulating translation. Eur J Heart Fail. 2020;22(1):54–66. doi: 10.1002/ejhf.1672. [DOI] [PubMed] [Google Scholar]
- Chen J, Ning Y, Zhang H, Song N, Gu Y, Shi Y, Cai J, Ding X, Zhang X. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239:117034. doi: 10.1016/j.lfs.2019.117034. [DOI] [PubMed] [Google Scholar]
- Coker H, Wei G, Brockdorff N. m6A modification of non-coding RNA and the control of mammalian gene expression. Biochim Biophys Acta. 2019;1862(3):310–318. doi: 10.1016/j.bbagrm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Dang X, Huan X, Du X, Chen X, Bi M, Yan C, Jiao Q, Jiang H. Correlation of ferroptosis and other types of cell death in neurodegenerative diseases. Neurosci Bull. 2022;38:938. doi: 10.1007/s12264-022-00861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRoo E, Stranz A, Yang H, Hsieh M, Se C, Zhou T. Endothelial dysfunction in the pathogenesis of abdominal aortic aneurysm. Biomolecules. 2022;12(4):509. doi: 10.3390/biom12040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Xing J, Wang S, Xin S, Han Y, Zhang J. Increased m6A methylation level is associated with the progression of human abdominal aortic aneurysm. Ann Transl Med. 2019;7(24):797. doi: 10.21037/atm.2019.12.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian D, Wang Y, Jian L, Tang H, Rao L, Chen K, Jia Z, Zhang W, Liu Y, Chen X, Shen X, Gao C, Wang S, Li M. METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics. 2020;10(20):8939–8956. doi: 10.7150/thno.45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Spinale FG, Ikonomidis JS. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J Vasc Res. 2009;46(2):119–137. doi: 10.1159/000151766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Anzai T, Takahashi T, Kohno T, Shimoda M, Sasaki A, Shimizu H, Nagai T, Maekawa Y, Yoshimura K, Aoki H, Yoshikawa T, Okada Y, Yozu R, Ogawa S, Fukuda K. Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm. Cardiovasc Res. 2011;91(2):358–367. doi: 10.1093/cvr/cvr080. [DOI] [PubMed] [Google Scholar]
- Koranda JL, Dore L, Shi H, Patel MJ, Vaasjo LO, Rao MN, Chen K, Lu Z, Yi Y, Chi W, He C, Zhuang X. Mettl14 Is essential for epitranscriptomic regulation of striatal function and learning. Neuron. 2018;99(2):283–292.e285. doi: 10.1016/j.neuron.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Cui M, Zhang K, Wang G, Zhai S. LncRNA CRNDE affects the proliferation and apoptosis of vascular smooth muscle cells in abdominal aortic aneurysms by regulating the expression of Smad3 by Bcl-3. Cell Cycle. 2020;19(9):1036–1047. doi: 10.1080/15384101.2020.1743915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hong M, Li Y, Chen D, Wu Y, Hu Y. Programmed cell death tunes tumor immunity. Front Immunol. 2022;13:847345. doi: 10.3389/fimmu.2022.847345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangum K, Gallagher K, Davis FM. The role of epigenetic modifications in abdominal aortic aneurysm pathogenesis. Biomolecules. 2022;12(2):172. doi: 10.3390/biom12020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng TG, Lu X, Guo L, Hou GM, Ma XS, Li QN, Huang L, Fan LH, Zhao ZH, Ou XH, OuYang YC, Schatten H, Li L, Wang ZB, Sun QY. Mettl14 is required for mouse postimplantation development by facilitating epiblast maturation. FASEB J : off Pub Federation Am Soc Exp Biol. 2019;33(1):1179–1187. doi: 10.1096/fj.201800719R. [DOI] [PubMed] [Google Scholar]
- Michineau S, Franck G, Wagner-Ballon O, Dai J, Allaire E, Gervais M. Chemokine (C-X-C motif) receptor 4 blockade by AMD3100 inhibits experimental abdominal aortic aneurysm expansion through anti-inflammatory effects. Arterioscler Thromb Vasc Biol. 2014;34(8):1747–1755. doi: 10.1161/ATVBAHA.114.303913. [DOI] [PubMed] [Google Scholar]
- Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, Shete S, Willing MC, Raman CS, Milewicz DM. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112(4):513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- Qin F, Liu X, Chen J, Huang S, Wei W, Zou Y, Liu X, Deng K, Mo S, Chen J, Chen X, Huang Y, Liang W. Anti-TGF-β attenuates tumor growth via polarization of tumor associated neutrophils towards an anti-tumor phenotype in colorectal cancer. J Cancer. 2020;11(9):2580–2592. doi: 10.7150/jca.38179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Wu Z, Wang X, Wang Y, Hu X, Qin W, Lu S, Xu D, Wu Y, Chen Q, Ding X, Guo H, Li Y, Wang Y, Fu B, Yao W, Wei M, Wu H. LNC942 promoting METTL14-mediated m(6)A methylation in breast cancer cell proliferation and progression. Oncogene. 2020;39(31):5358–5372. doi: 10.1038/s41388-020-1338-9. [DOI] [PubMed] [Google Scholar]
- Sun W, Zheng J, Gao Y. Targeting platelet activation in abdominal aortic aneurysm: current knowledge and perspectives. Biomolecules. 2022;12(2):206. doi: 10.3390/biom12020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- Wan W, Ao X, Chen Q, Yu Y, Ao L, Xing W, Guo W, Wu X, Pu C, Hu X, Li Z, Yao M, Luo D, Xu X. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N(6)-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21(1):60. doi: 10.1186/s12943-021-01447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu Z, Ren J, Morgan S, Assa C, Liu B. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116(4):600–611. doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhou T, Liu Z, Ren J, Phan N, Gupta K, Stewart DM, Morgan S, Assa C, Kent KC, Liu B. Inhibition of Receptor-Interacting Protein Kinase 1 with Necrostatin-1s ameliorates disease progression in elastase-induced mouse abdominal aortic aneurysm model. Sci Rep. 2017;7:42159. doi: 10.1038/srep42159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen C, Wang Q, Cao Y, Xu L, Qi R. Inhibitory effects of cycloastragenol on abdominal aortic aneurysm and its related mechanisms. Br J Pharmacol. 2019;176(2):282–296. doi: 10.1111/bph.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann M, Skorubskaya E, Peters AS, Hakimi M, Böckler D, Dihlmann S. Necrotic cell debris induces a NF-κB-driven inflammasome response in vascular smooth muscle cells derived from abdominal aortic aneurysms (AAA-SMC) Biochem Biophys Res Commun. 2019;511(2):343–349. doi: 10.1016/j.bbrc.2019.02.051. [DOI] [PubMed] [Google Scholar]
- Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang Y, Li J, Sheng R, Deng P, Wang Y, Zheng R, Jiang Y, Ye L, Chen Q, Zhou X, Lin S, Yuan Q. Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9(1):4772. doi: 10.1038/s41467-018-06898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao QJ, Sang L, Lin M, Yin X, Dong W, Gong Y, Zhou BO. Mettl3-Mettl14 methyltransferase complex regulates the quiescence of adult hematopoietic stem cells. Cell Res. 2018;28(9):952–954. doi: 10.1038/s41422-018-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo F, Zhao R. Hepatic expression of FTO and fatty acid metabolic genes changes in response to lipopolysaccharide with alterations in m(6)A modification of relevant mRNAs in the chicken. Br Poult Sci. 2016;57(5):628–635. doi: 10.1080/00071668.2016.1201199. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, Majumder S, He C, Huang S. m(6)A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell. 2017;31(4):591–606.e596. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, He X, Song H, Sun Y, Chen G, Si X, Sun J, Chen X, Liao W, Liao Y, Bin J. METTL3 Induces AAA development and progression by modulating N6-methyladenosine-dependent primary miR34a processing. Mol Ther Nucleic Acids. 2020;21:394–411. doi: 10.1016/j.omtn.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Wang Q, Phan N, Ren J, Yang H, Feldman CC, Feltenberger JB, Ye Z, Wildman SA, Tang W, Liu B. Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models. Cell Death Dis. 2019;10(3):226. doi: 10.1038/s41419-019-1468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.