Abstract
Urological cancers have obtained much attention in recent years due to their mortality and morbidity. The most common and malignant tumor of urological cancers is prostate cancer that imposes high socioeconomic costs on public life and androgen-deprivation therapy, surgery, and combination of chemotherapy and radiotherapy are employed in its treatment. PI3K/Akt signaling is an oncogenic pathway responsible for migration, proliferation and drug resistance in various cancers. In the present review, the role of PI3K/Akt signaling in prostate cancer progression is highlighted. The activation of PI3K/Akt signaling occurs in prostate cancer, while PTEN as inhibitor of PI3K/Akt shows down-regulation. Stimulation of PI3K/Akt signaling promotes survival of prostate tumor cells and prevents apoptosis. The cell cycle progression and proliferation rate of prostate tumor cells increase by PI3K/Akt signaling induction. PI3K/Akt signaling stimulates EMT and enhances metastasis of prostate tumor cells. Silencing PI3K/Akt signaling impairs growth and metastasis of prostate tumor cells. Activation of PI3K/Akt signaling mediates drug resistance and reduces radio-sensitivity of prostate tumor cells. Anti-tumor compounds suppress PI3K/Akt signaling in impairing prostate tumor progression. Furthermore, upstream regulators such as miRNAs, lncRNAs and circRNAs regulate PI3K/Akt signaling and it has clinical implications for prostate cancer patients.
Keywords: Prostate cancer, PI3K/Akt, Cancer therapy, Anti-cancer agents, Chemoresistance
Graphical abstract
Introduction
One of the most malignant urological cancers is prostate cancer with incidence rate of 1.6 million cases around the world and death rate of 366,000 annually around the world (Pernar et al. 2018). Based on the estimates, prostate cancer is the second most common cancer in USA (Siegel et al. 2020). Since 1990s, a significant reduction has been observed in mortality of prostate cancer; however, mortality of this malignant cancer has been stabilized in 2013–2015 in USA (Lokeshwar et al. 2021). There are a number of reasons responsible for decreased prostate cancer mortality in recent years such as development of novel therapeutic strategies and early diagnosis via detecting PSA in serum (Dickinson et al. 2016). The androgen-dependent nature of prostate cancer was discovered in 1940s and since then, androgen-deprivation therapy (ADT) has been considered as a mainstay for treatment of this malignant disease (Huggins and Hodges 1941; Huggins and Hodges 1972). ADT was beneficial in providing an alternative way for treatment of prostate cancer patients and was preferred to surgical castration. It has been demonstrated that ADT is advantageous in treatment of 80–90% of prostate cancer patients and there has been a sign of decrease in PSA serum levels upon ADT. However, capacity of ADT in prostate cancer treatment declines, as most of the cases progress to castration-resistant prostate cancer (CRPC) within 5 years (Loriot et al. 2018). For improving overall survival of patients with prostate cancer, in addition to ADT, chemotherapy with docetaxel and cabazitaxel, inhibitors of androgen receptor signaling and radiotherapy are used. The clinical studies (phase III) have shown potential of ADT and combination of androgen receptor signaling inhibitors in improving outcomes of patients with prostate cancer. Transrectal ultrasound guided biopsy is employed as an invasive strategy in diagnosis of prostate cancer and it appears that early diagnosis of prostate cancer is completely essential in improving prognosis of patients (Cattrini et al. 2021; Bernstein et al. 2021).
Regardless of therapeutic and diagnostic tools for prostate cancer, there still a much space for developing novel methods for treatment of this disease (Shah et al. 2021; Zaorsky et al. 2021; Habrowska-Górczyńska et al. 2021; Ashrafizadeh et al. 2020a, b, c, d, e; Hussain et al. 2021). It appears that understanding underlying mechanisms involved in prostate cancer progression can greatly help in developing more effective therapeutics compared to conventional methods (Soleymani et al. 2021; Ashrafizadeh et al. 2022; Mirzaei et al. 2022). Increasing evidence has shown role of various molecular pathways in prostate cancer progression. For instance, suppressing STAT3 signaling prevents bone metastasis of prostate tumor cells (Thulin et al. 2021). Acacetin has capacity of binding to STAT3 and its inhibition in prostate cancer therapy (Yun et al. 2021). Down-regulation of Wnt is in favor of in decreasing proliferation and invasion of prostate tumor cells (Ma et al. 2021). Non-coding RNAs including miRNAs, lncRNAs and circRNAs are also involved in regulating prostate of prostate cancer (Kumar et al. 2021; Yu et al. 2022; He et al. 2021a, b, c). The epithelial-to-mesenchymal transition (EMT) is the most well-known mechanism responsible for metastasis of prostate tumor cells (Xu et al. 2021a, b, c, d). Therefore, identification of these factors can result in prostate cancer therapy. In the present review, we focus on PI3K/Akt signaling, as one of the most common signaling networks undergoing abnormal expression in prostate cancer. First of all, we introduce PI3K/Akt signaling and its association with cancer progression in different tumors. Then, an overview of PI3K/Akt inhibitors is provided. At the next sections, we specifically discuss role of PI3K/Akt signaling in growth, metastasis and therapy response of prostate cancer cells. The upstream mediators of PI3K/Akt signaling and its regulation by anti-cancer drugs in prostate cancer are also discussed.
PI3K/Akt signaling and inhibitors
PI3K/Akt signaling
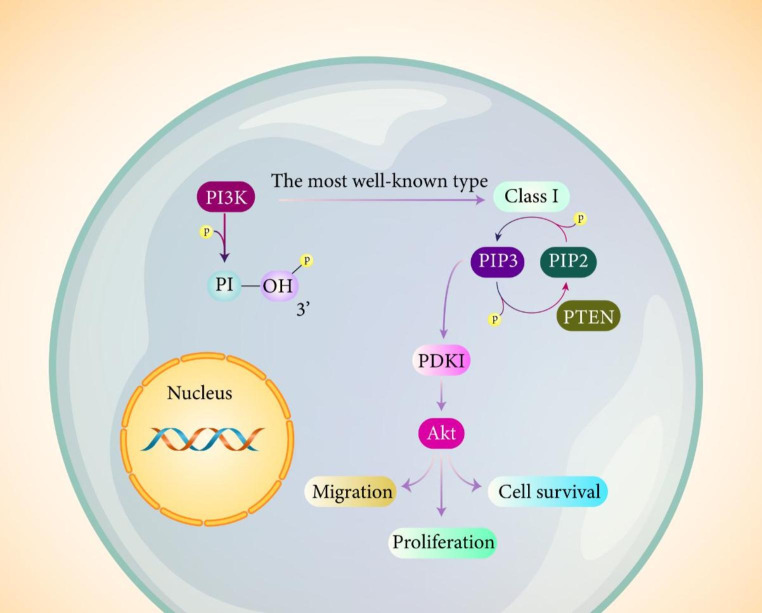
Phosphoinositide 3-kinase (PI3K) is a kind of lipid kinase that has responsibility of phosphorylating 3/-OH group of phosphatidylinositol (PI) at plasma and cell membranes. A number of classifications are considered for PI3K according to structure, binding site and substrate specificity (Bilanges et al. 2019; Gulluni et al. 2019; Hirsch et al. 2020). The most well-known and studied type of PI3K is class I that has ability of producing PIP3 from PIP2. The main site for generation of PIP3 is plasma membrane and it is responsible for recruiting phospholipid effectors such as Akt and PDK-1, as key players of PI3K signaling (Li et al. 2021a, b, c, d, e, f). It has been reported that PIP3 accelerates interaction between PDK-1 and Akt to promote phosphorylation and activation of Akt, resulting in cell proliferation, migration, protein synthesis and cell survival, among others. Furthermore, phosphorylated Akt has capacity of activating mammalian target of rapamycin (mTOR) as downstream target (Bilanges et al. 2019). Akt and PDK-1 contain PH domains and they are capable of binding to PIP3. The activation of PDK-1 by PIP3 leads to phosphorylation of Akt at Thr308 (Xue et al. 2021). Noteworthy, activation of Akt can occur by PDK-2 at Ser473 (Kawakami et al. 2004; Hresko et al. 2003). Phosphatase and tensin homolog (PTEN) is an onco-suppressor that is able to suppress PI3K/Akt signaling and for this purpose, PTEN dephosphorylates PIP3 to PIP2 (Maehama and Dixon 1998; Ashrafizadeh et al. 2020a, b, c, d, e; Abadi et al. 2021). Figure 1 provides a schematic representation of PI3K/Akt signaling.
Fig. 1.
A schematic representation of PI3K/Akt signaling. This pathway exerts an oncogenic role in cancer and is capable of increasing invasion and growth rate of tumor cells. Furthermore, activation of PI3K/Akt signaling stimulates drug resistance in tumor cells (Yang et al. 2020a, b; Dong et al. 2021; Zhang et al. 2021a, b, c; Wu et al. 2020a, b)
Class I PI3K has a heterodimer feature with p110 as catalytic subunit and p85 as regulatory/adaptor subunit. The class I PI3K is further categorized into two major classes including subclass IA and IB. The subclass IA is comprised of PI3Kα, β, and δ and its activation occurs by receptors possessing protein tyrosine kinase activity. The subclass IB is also called PI3Kγ and its stimulation occurs via G proteins’ coupled receptors (Tewari et al. 2019). The most common mutation (30%) occurs in PIK3CA and despite ubiquitous expression of PIK3CB, mutation of this gene is rare (Samuels 2006; Gao et al. 2016; Dbouk et al. 2013). The class II PI3K has regulatory subunits and its activation by cytokine receptors. The class II PI3Ks have a Ras-binding motif and in addition to cytokine receptors, RTKs and integrins can activate them. PIP2 and phosphatidylinositol are considered as subunits of class II PI3Ks and they are involved in biological events including migration, membrane trafficking, receptor internalization and glucose transport (King and Yeomanson 2015; Loh et al. 2013). VPs34 is the only discovered class III PI3K and it is involved in cell survival, proliferation and response to stress (King and Yeomanson 2015; Loh et al. 2013).
PI3K/Akt in oncology
Increasing evidence has evaluated role of PI3K/Akt signaling in cancer progression. Overall, activation of PI3K/Akt signaling is in favor of increasing proliferation and metastasis of tumor cells as well as inducing therapy resistance. The increased metastasis of gastric tumor cells mainly depends on stimulation of EMT mechanism and apolipoprotein C2 (APOC2) stimulates EMT in gastric cancer metastasis via triggering PI3K/Akt/mTOR axis (Wang et al. 2021a, b, c, d, e, f). Another study has revealed that role of GRSF1 in increasing gastric cancer metastasis is mediated via PI3K/Akt signaling. It has been reported that GRSF1 induces PI3K/Akt signaling to stimulate EMT in increasing migration and invasion of gastric tumor cells (Wang et al. 2021a, b, c, d, e, f). On the other hand, ID2 functions as upstream mediator of PI3K/Akt signaling in bladder cancer. ID2 expression demonstrates a decrease in bladder cancer patients and for impairing progression of tumor cells, ID2 suppresses PI3K/Akt signaling (Mao et al. 2021). The interaction among non-coding RNAs can affect PI3K/Akt signaling in cancer. Circ-001422 shows upregulation in osteosarcoma and it reduces expression level of miRNA-195-5p to upregulate FGF2. Then, stimulation of PI3K/Akt signaling occurs to increase migration and invasion of osteosarcoma cells (Yang et al. 2021a, b).
In addition to metastasis, PI3K/Akt signaling participates in proliferation and death of tumor cells. Inorganic pyrophosphatase 1 (PPA1) demonstrates overexpression in colorectal cancer and it induces PI3K/Akt signaling to phosphorylate GSK-3β, leading to a significant increase in growth of tumor cells in vitro and in vivo (Guo et al. 2021a, b). On the other hand, NAT1 shows down-regulation in colorectal cancer and is correlated with unfavorable prognosis. Restoring expression level of NAT1 suppresses proliferation of colorectal tumor cells via inhibiting PI3K/Akt signaling (Cai et al. 2021). Noteworthy, inhibiting PI3K/Akt signaling is associated with apoptosis induction in colorectal tumor cells (Cheng et al. 2021). The activation of PI3K/Akt/mTOR signaling by BCAT1 as upstream mediator can lead to angiogenesis and increasing progression of gastric tumor cells (Shu et al. 2021). Notably, inhibition of PI3K/Akt signaling impairs progression of tumor cells and increases their sensitivity to chemotherapy (Li et al. 2021a, b, c, d, e, f). Therefore, activation of PI3K/Akt signaling increases tumor progression (Qiao et al. 2021; Li et al. 2021a, b, c, d, e, f) and inhibition of this signaling networks using berberine (Li et al. 2021a, b, c, d, e, f) or promethazine (Tan et al. 2021) has been of interest in cancer therapy. The next section focuses on some of the inhibitors of PI3K/Akt signaling in cancer therapy.
PI3K/Akt inhibitors
A variety of PI3K/Akt inhibitors have been developed for cancer therapy and some of them are being currently applied in treatment of cancer patients. Overall, pan-PI3K inhibitors, isoform-selective PI3K inhibitors, dual pan-PI3K and mTOR inhibitors, Akt inhibitors, mTOR inhibitors and combination therapy with other inhibitors are used (He et al. 2021a, b, c). Pan-PI3K inhibitors have a number of benefits compared to other kinds of inhibitors such as lower adverse impacts and higher anti-tumor activity. As it was mentioned, p110 is one of the subunits of class I PI3K and pan-PI3K inhibitors target p110 to suppress cancer progression. Pictilisib, buparlisib and pilaralisib are among pan-PI3K inhibitors (Akinleye et al. 2013). The first pan-PI3K inhibitor that was used in clinic is GDC-0941 and it suppresses functions of p110α and p110δ with IC50 value of 3 nM and its combination therapy with other anti-cancer agents increases its capacity in suppressing tumor progression (Folkes et al. 2008; Junttila et al. 2009). However, one of the limitations of pan-PI3K inhibitors is their low specificity towards target. Therefore, other kind of anti-cancer agents, known as isoform-selective PI3K inhibitors were developed and they demonstrated lower off-targeting feature. Hence, high concentration of isoform-selective PI3K inhibitors can be administered and they show high efficacy in cancer therapy (Li et al. 2018a, b). The first agent from this family is BYL-719 that selectively binds to p110α of class I PI3K and it has been used in phase I clinical trial for treatment of cancer patients (Juric et al. 2018). In respect to the fact that both PI3K and mTOR have p110 subunit with similar structures, researchers have focused on developing dual pan-PI3K and mTOR inhibitors for efficient suppression of PI3K/Akt signaling and inhibiting tumor progression. Furthermore, these agents have capacity of full inhibition of p110 subunit and their combination therapy with other inhibitors of PI3K or Akt is not recommended. SF1126, dactolisib, voxtalisib and GSK1059615 are among some of the dual pan-PI3K and mTOR inhibitors (Mayer and Arteaga 2016; Engelman 2009). Perifosine (phase II clinical trial) (Argiris et al. 2006), GSK-690,693 (phase I clinical trial) (Rhodes et al. 2008), VAD-002 (phase I clinical trial) (Sampath et al. 2013) and M2698 (phase I clinical trial) (Machl et al. 2016) are among Akt inhibitors used in clinical course. These are the synthetic small molecules capable of suppressing PI3K/Akt/mTOR signaling and recent experiments have shown that plant derived-natural products such as thidiazuron (Ibrahim et al. 2021), purpurin (Bo et al. 2021), erianin (Xu et al. 2021a, b, c, d) and pinocembrin (Zhu et al. 2021a, b, c) are able to suppress PI3K/Akt signaling in cancer therapy. However, their clinical application appears to be challenging due to their poor bioavailability.
PI3K/Akt in prostate cancer proliferation and metastasis
Among various kinds of urological cancers, prostate cancer is considered the most malignant one. The malignancy of prostate tumor cells is attributed to their capacity in proliferation and invasion. By enhancement in growth and metastasis, a significant decrease occurs in prognosis of prostate cancer patients. Based on the experiments, different underlying molecular pathways are involved in progression (proliferation and migration) of prostate tumor cells. For instance, HNRNPC shows overexpression in prostate cancer and it diminishes infiltration of immune cells. HNRNPC increases prostate tumor growth and invasion, and it is associated with undesirable prognosis in patients (Wang et al. 2021a, b, c, d, e, f). LINC00852 is linked to growth and metastasis of prostate tumor cells. LINC00852 overexpression in prostate cancer enhances proliferation rate and it promotes metastasis via EMT induction (Yi et al. 2021). Silencing oncogenic pathways has been an ideal strategy in impairing prostate cancer progression (Li et al. 2021a, b, c, d, e, f; Xu et al. 2021a, b, c, d; Matsushita et al. 2021). Owing to tumor-promoting function of PI3K/Akt signaling, current section focuses on its role and association with prostate cancer proliferation and metastasis.
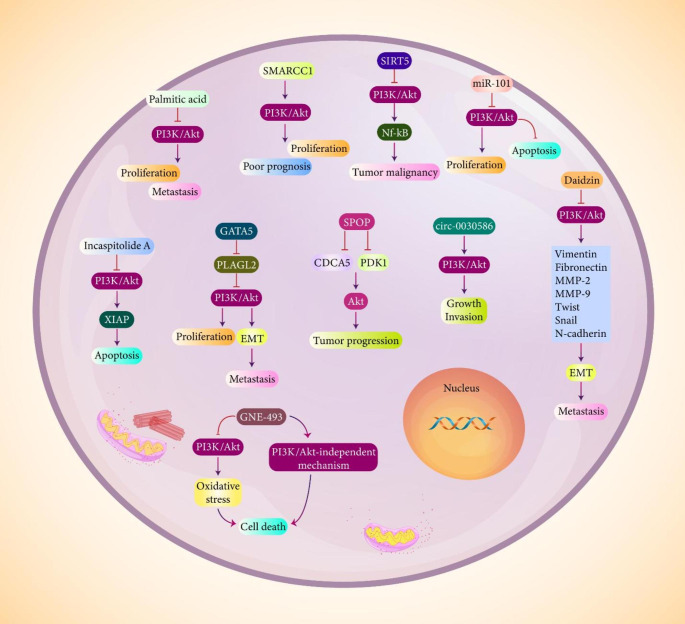
A recent experiment has revealed involvement of PI3K/Akt signaling in increasing growth and invasion of prostate tumor cells. Palmitic acid is an anti-cancer agent that impairs prostate tumor proliferation in vitro and in vivo, and it is associated with cell cycle arrest at G1 phase. Palmitic acid reduces expression levels of cyclin D1 and Rb, while it promotes p27 expression in triggering cell cycle arrest. Palmitic acid has capacity of increasing E-cadherin levels, and decreasing PKCζ and p-Integrinβ1 levels to impair progression of prostate tumor cells. Palmitic acid inhibits PI3K/Akt axis to suppress proliferation and metastasis of prostate tumor cells (Zhu et al. 2021a, b, c). δ-catenin has been implicated in progression of prostate tumor cells and it is linked to PI3K/Akt signaling. p21 is an inhibitor of proliferation and invasion in prostate tumor cells. Upon binding of EGF to its receptor (EGFR) on the surface of prostate tumor cells, activation of Akt signaling occurs that subsequently mediates phosphorylation of δ-catenin at Thr 454. Then, Akt in cooperation with δ-catenin stimulate phosphorylation of p21 at Thr 145 to reduce p21 expression, resulting in an increase in growth and invasion of prostate tumor cells (Shen et al. 2021a, b).
According to tumor-promoting function of PI3K/Akt signaling in prostate cancer, its inhibition can provide a new insight in treatment of this urological cancer. SMARCC1 is a tumor-suppressor factor with low expression in prostate cancer and is correlated with poor prognosis upon down-regulation. Restoring SMARCC1 expression suppresses prostate tumor proliferation and invasion, and inhibits EMT. In vivo experiment on xenograft model has also shown potential of SMARCC1 in suppressing tumor growth. Mechanistically, SMARCC1 suppresses PI3K/Akt signaling in impairing progression of prostate tumor cells (Xiao et al. 2021). SIRT5 is another tumor-suppressor factor in prostate cancer. It appears that PI3K/Akt signaling stimulates NK-kB pathway in increasing progression of prostate tumor. On the other hand, SIRT5 inhibits PI3K/Akt axis to reduce NK-kB pathway expression in impairing prostate tumor malignancy (Choi et al. 2022). GATA5 is suggested to be an inhibitor of cancer progression. It impairs colony formation in colon cancer and is employed as a biomarker (Hellebrekers et al. 2009). Similar to colon cancer, upregulation of GATA5 in prostate cancer is related to apoptosis induction and inhibiting growth and invasion of tumor cells. GATA5 is able to suppress invasion of prostate tumor cells via EMT inhibition. Mechanistically, GATA5 suppresses PI3K/Akt signaling via PLAGL2 down-regulation to impair EMT and proliferation in prostate cancer (Wang et al. 2022a, b).
As it was mentioned, metastasis is an increasing challenge in cancer treatment and it is defined as translocation of cells from their origin region to other distant organs (Dai et al. 2016; Sani et al. 2015; Shay and Lynch 2015; Syn et al. 2016). The ability of tumor cells in disrupting basement membrane has results in metastasis (Monaco et al. 2006; Bacac et al. 2008; Fidler 1989; Ko et al. 2018). Based on the estimates, up to 90% of cancer-related deaths are because of metastasis (Mehlen 2006). The conversion of epithelial cells to mesenchymal ones is considered as a EMT that increases tumor cell metastasis (Hwang et al. 2020; Lee et al. 2020a, b). Various studies have focused on the role of EMT in cancer metastasis and drug resistance (Ashrafizadeh et al. 2020a, b, c, d, e, 2021). Noteworthy, PI3K/Akt signaling demonstrates interaction with EMT mechanism in prostate cancer metastasis. Daidzin is considered as an inhibitor of EMT mechanism in prostate cancer cells. Daidzin is capable of reducing levels of vimentin, fibronectin, MMP-2, MMP-9, Twist, Snail and N-cadherin, while it promotes E-cadherin levels. Exposing prostate tumor cells to daidzin suppresses growth and metastasis. Daidzin inhibits PI3K/Akt/mTOR axis to reverse EMT in decreasing invasion of prostate tumor cells (Yang et al. 2022). On the other hand, there are oncogenic factors in prostate cancer that can stimulate prostate tumor metastasis via affecting EMT mechanism. Hsa-circ-0030586 is attributed to ability in enhancing migration and invasion of prostate tumor cells. Silencing circ-0030586 suppresses growth and invasion of prostate tumor cells. Circ-0030586 stimulates PI3K/Akt signaling to induce EMT in increasing progression and invasion of prostate tumor cells (Luo et al. 2021a, b, c). Since Akt induction promotes prostate cancer metastasis, its inhibition can pave the way for reducing tumor progression. It has been reported that CKB suppresses EMT in reducing prostate cancer invasion. Based on the findings, Akt signaling stimulates EMT in increasing prostate cancer metastasis, while CKB suppresses EMT via Akt down-regulation to decrease invasion of tumor cells (Wang et al. 2021a, b, c, d, e, f).
The studies highlight the fact that PI3K/Akt signaling shows close association with growth and invasion of prostate cancer cells. miRNA-101-3p has interaction in prostate cancer cells in terms of decreasing their proliferation and invasion. However, prostate cancer cells demonstrate low expression level of miRNA-101-3p. Overexpression of miRNA-101-3p is associated with apoptosis induction, proliferation and migration suppression via inhibiting PI3KAkt/mTOR axis (Gu et al. 2021). The PI3K/Akt axis is not the only mechanism responsible for targeting in prostate cancer suppression. In fact, tumor-suppressor factors can inhibit progression of prostate cancer cells in a PI3K-dependnet and -independent mechanisms. A recent experiment has shown that GNE-493 stimulates oxidative stress and necrosis in prostate tumor cells. For this purpose, GNE-493 suppresses PI3K/Akt signaling. However, it has been reported that GNE-493 can also suppress prostate tumor progression in an PI3K-independent mechanism (Jin et al. 2022). The survival of prostate tumor cells mainly depends on PI3K/Akt axis. It has been shown that activation of PI3K/Akt signaling leads to overexpression of XIAP to promote survival rate of prostate tumor cells. Incaspitolide A as anti-cancer agent stimulates apoptosis and suppresses proliferation of cancer cells; for this purpose, this compound inhibits PI3K/Akt signaling to reduce XIAP expression in triggering apoptosis (Huang et al. 2021). In addition to proliferation inhibition, PI3K/Akt inhibition suppresses EMT in retarding progression and invasion of prostate tumor cells (Chang et al. 2021).
SPOP is a new emerging target in prostate cancer therapy and it shows anti-cancer activity. Down-regulation of ATR as upstream mediator leads to stimulation of CDK1/SPOP signaling that increases potential of anti-PD-L1 in prostate cancer suppression (Tang et al. 2021). The mutations occurring in SPOP in prostate cancer can leads to genomic stability and DNA damage repair that are in favor of tumor progression (Jin et al. 2021). Furthermore, SPOP mutations induces Nrf2 signaling and autophagy mechanism in supporting prostate cancer progression (Shi et al. 2022). Upon SPOP mutation, an increase occurs in stability of GLI3 to induce castration resistance feature in prostate cancer (Burleson et al. 2022). Noteworthy, different studies have provided association between SPOP and PI3K/Akt signaling in prostate cancer. CDCA5 is an oncogenic factor in prostate cancer and induces Akt signaling. It has been shown that SPOP increases degradation of CDCA5 to suppress Akt signaling in impairing progression of prostate tumor cells (Luo et al. 2021a, b, c). Besides, PDK1 stimulates Akt signaling in providing prostate cancer progression. As tumor-suppressor factor, SPOP induces degradation and ubiquitination of PDK1 to suppress Akt signaling in preventing progression of prostate tumor cells (Jiang et al. 2021). Taking everything together, these studies highlight the fact that PI3K/Akt signaling is in favor of prostate cancer proliferation and invasion. Upregulation of PI3K/Akt promotes viability of prostate tumor cells and prevents apoptosis in these malignant cells. Furthermore, PI3K/Akt promotes expression of anti-apoptotic factors such as XIAP, while it reduces expression of pro-apoptotic factors in prostate cancer. Based on these studies and discussions, targeting PI3K/Akt signaling is of importance in prostate cancer therapy (Fig. 2) (Chen et al. 2022; Ding et al. 2021a, b; Khalil et al. 2021; Zhou et al. 2021; Liu et al. 2022; Xu et al. 2022; Yang et al. 2021a, b; Sun et al. 2021a, b; Xing et al. 2021).
Fig. 2.
The involvement of PI3K/Akt signaling in proliferation and invasion of prostate tumor cells
PI3K/Akt and angiogenesis
Angiogenesis is formation of new vessels from pre-existing ones and it is a vital mechanism for physiological events such as wound healing and tissue development, among others. However, angiogenesis induction can lead to increased oxygen and nutrient supply to a certain tissue and this is in favor of tumor cells, as they are rapidly proliferating and they need high amount of energy and oxygen (Guo et al. 2021a, b; Liu et al. 2021; Xu et al. 2021a, b, c, d; Shen et al. 2021a, b). A variety of experiments have evaluated role of angiogenesis in prostate cancer progression and related molecular pathways. It has been shown that FOXA1 can promote expression level of angiogenic factors such as EGF, endothelin-1 and Endoglin in triggering angiogenesis and increasing progression of prostate tumor cells (Su et al. 2021). By inhibiting B-RAF/ERK axis, nimbolide suppresses prostate cancer angiogenesis and impairs tumor growth in vivo (Mahmoud et al. 2022). It appears that androgens can induce neo-angiogenesis in prostate cancer. As a naturally occurring compound with anti-cancer activity, atraric acid decreases expression level of angiopoietin 2 to inhibiting angiogenesis in prostate cancer suppression (Ehsani et al. 2022). The current section focuses on the association between PI3K/Akt signaling and angiogenesis in prostate cancer progression.
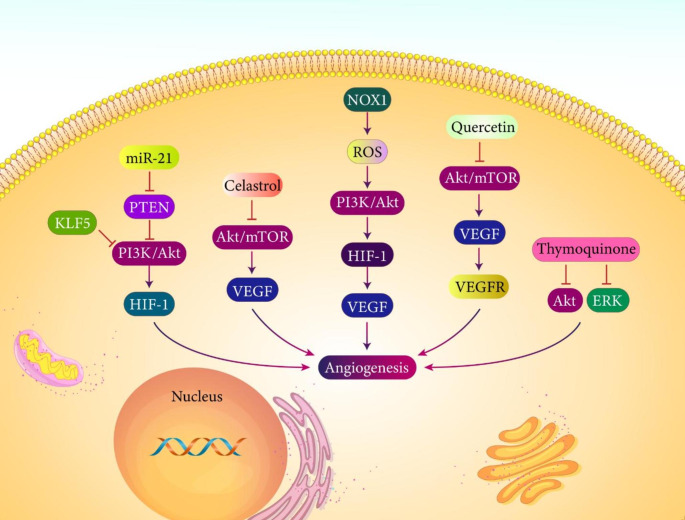
It has been reported that activation of PI3K/Akt signaling is associated with angiogenesis induction and enhanced progression of prostate tumor cells. Overexpression of PTEN as negative regulator of PI3K/Akt signaling inhibits angiogenesis in prostate cancer. Furthermore, overexpression of Akt dominant negative mutant prevents angiogenesis and reduces expression levels of HIF-1α and VEGF in prostate cancer (Fang et al. 2007). A recent experiment has shown that overexpression of both VEGF receptor 2 (VEGFR2) and induction of PI3K/Akt/mTOR axis are essential for angiogenesis induction in prostate cancer. As anti-cancer agent, kochia scoparia seed extract prevents VEGF-mediated angiogenesis in prostate cancer via down-regulation of VEGFR2 and inhibition of PI3K/Akt/mTOR axis to reduce progression of tumor cells (Cho et al. 2019). As it was discussed in previous section, PTEN can suppress PI3K/Akt signaling in prostate cancer to impair progression of tumor cells. On the other hand, PI3K/Akt axis is involved in angiogenesis induction in prostate tumor cells. It has been shown that PTEN deficient leads to Akt activation in prostate tumor that subsequently stimulates angiogenesis. The ability of PI3K/Akt signaling in angiogenesis induction is attributed to enhanced accumulation of HIF-1α in prostate tumor. As tumor-suppressor factor, KLF5 decreases Akt expression to reduce accumulation of HIF-1α in suppressing angiogenesis and retarding progression of prostate tumor cells (Ci et al. 2015).
Different aspects of tumor progression including growth and metastasis are affected by NOX-dependent ROS (Brar et al. 2002; Arbiser et al. 2002; Yamaura et al. 2009). It has been reported that ROS overgeneration by NOX can lead to activation of STAT, PI3K/Akt and RTK pathways in increasing tumor progression (Mahadev et al. 2004; Mesquita et al. 2010; Lee et al. 2007; Naughton et al. 2009). NOX1 is implicated in triggering PI3K/Akt signaling and angiogenesis in prostate cancer. Down-regulation of NOX1 is associated with a significant decrease in progression (growth and metastasis) of prostate tumor cells. It has been reported that NOX1 promotes ROS generation to induce Akt signaling. Then, overexpression of HIF-1α occurs to upregulate VEGF in triggering angiogenesis and increasing prostate tumor progression (Li et al. 2013). The interactions occurring between PTEN, PI3K/Akt signaling, HIF-1α and VEGF can affect angiogenesis induction in prostate tumor cells (Zhong et al. 2000).
Increasing evidence reveals potential of miRNAs in regulating angiogenesis in cancer cells. miRNAs can affect various downstream targets such as SOX9 and ROCK in regulating angiogenesis and they are also affected by upstream mediators such as circRNAs to modulate angiogenesis and affect tumor progression (Zhu et al. 2021a, b, c; You et al. 2021; Hu et al. 2021). Overexpression of miRNA-21 in prostate tumor results in angiogenesis and increased tumor progression. miRNA-21 down-regulation prevents angiogenesis in prostate cancer. PTEN is a target of miRNA-21 and PTEN overexpression abrogates angiogenesis in prostate cancer. miRNA-21 has ability of reducing PTEN expression to induce Akt and ERK signaling pathways. Then, HIF-1α and VEGF overexpression occurs, leading to angiogenesis in prostate tumor cells (Liu et al. 2011). There are anti-cancer agents affecting angiogenesis that can be beneficial in treatment of prostate cancer. Thymoquinone is an anti-tumor agents that suppresses angiogenesis in different caners such as osteosarcoma, thyroid cancer and breast cancer via modulating varous molecular pathways such a s NF-κB and VEGF (Bhattacharya et al. 2020; Ozturk et al. 2018; Peng et al. 2013). Thymoquinone is capable of suppressing angiogenesis in prostate cancer. Activation of Akt and ERK signaling pathways results in angiogenesis and prostate cancer progression. Thymoquinone inhibits angiogenesis in vitro and in vivo, and it demonstrated high safety profile. Thymoquinone suppresses Akt and ERK molecular pathways in impairing angiogenesis in prostate tumor (Yi et al. 2008). Another new emerging anti-tumor compound is quercetin with capacity of triggering apoptosis and retarding progression of different cancers such as breast cancer, esophagus cancer, colon cancer and it also demonstrate chemo-preventive effects (Chen et al. 2021a, b; Trinh et al. 2022; Jing et al. 2021; Wang et al. 2022a, b; Umar et al. 2022). Quercetin has shown high potential in treatment of prostate cancer and it can inhibit angiogenesis for this purpose (Pratheeshkumar et al. 2012). Quercetin suppresses angiogenesis in prostate cancer and it prevents VEGFR2 induction by VEGF. After that, expression levels of Akt and mTOR decrease to suppress prostate tumor growth and to decrease its volume in vivo (Pratheeshkumar et al. 2012). Another anti-cancer agent is celastrol capable of capable of impairing progression of brain, thoracic and gastrointestinal tumors, among others. Celastrol induces apoptosis and it prevents angiogenesis in tumor suppression (Lim et al. 2021). Celastrol retards growth of prostate tumor cells and induces apoptosis. It has been reported that celastrol suppresses Akt/mTOR axis in decreasing VEGF expression and impairing angiogenesis in prostate tumor suppression (Pang et al. 2010). Overall, studies highlight the fact that angiogenesis is in favor of prostate tumor progression, and its regulation by anti-cancer agents and signaling pathways especially PI3K/Akt pathway is of importance in prostate cancer (Fig. 3) (Huang et al. 2022; Sheng et al. 2022; Nalla et al. 2011).
Fig. 3.
PI3K/Akt signaling and angiogenesis in prostate cancer
PI3K/Akt and cancer stem cells
Cancer stem cells (CSCs) are new emerging targets in tumor suppression and they are present in different kinds of cancers including breast cancer, lung cancer, liver cancer, pancreatic cancer and leukemia, among others (Chu et al. 2021; Yang et al. 2020a, b; Jariyal et al. 2019). CSCs possess various characteristics including self-renewal ability, migration and differentiation (Martin-Belmonte and Perez-Moreno 2012; Deldar Abad Paskeh et al. 2021). There are a number of markers for CSCs including CD24, CD34, CD44, CD133 and ALDH1. Various cancers have different CSC markers for isolation and validation (Visvader 2008). CSCs demonstrate close association with proliferation, metastasis and drug resistance in tumors (Shibue and Weinberg 2017; Lambert et al. 2021; Wilson et al. 2020). Therefore, their targeting is of importance in cancer therapy (Lee et al. 2021a, b; Raghav and Mann 2021; Cui et al. 2021). The current section focuses on the role of PI3K/Akt signaling in affecting CSCs in prostate cancer.
Histone acetylation and stimulation of PI3K/Akt signaling are considered as factors responsible for preserving CSC features in prostate cancer (Wu et al. 2020a, b). KLF4 stimulates PI3K/Akt signaling to decrease p21 expression in increasing stemness of prostate tumor cells. It has been shown that miRNA-7 functions as tumor-suppressor and its reduces KLF4 expression to inhibit PI3K/Akt signaling in upregulating p21 expression and impairing stemness of prostate tumor cells (Chang et al. 2015). In tumor spheres, an increase occurs in expression level of PI3K. Down-regulation of PTEN by shRNA results in an increase in colony formation capacity of prostate cancer cells due to activation of PI3K/Akt signaling. Noteworthy, prostate cancer cells demonstrate PTEN down-regulation to induce PI3K/Akt signaling in improving stemness features (Dubrovska et al. 2009). Another experiment also reveals that PTEN down-regulation leads to induction of PI3K/Akt signaling and increased population of cancer-stem cells in prostate tumor (Kim et al. 2014). THBS4 shows overexpression in prostate cancer and it promotes growth and self-renewal capacity of tumor cells. Furthermore, THBS4 overexpression prevents apoptosis and increases carcinogenesis in vivo. Noteworthy, THBS4 increases stemness of prostate cancer cells via activating PI3K/Akt signaling (Hou et al. 2020). The CSCs participate in drug resistance in prostate cancer. It has been reported that acetyl-11-keto-β-boswellic acid inhibits STAT3 and Akt signaling pathways in impairing CSCs and reversing drug resistance in prostate cancer (Liu et al. 2019). These studies highlight the fact that PI3K/Akt signaling activation is in favor of prostate cancer growth and invasion as well as therapy resistance and its targeting should be considered in reversing stemness in prostate tumor (Wang et al. 2019; Chang et al. 2013; Lee et al. 2021a, b; Huang et al. 2018; Ohnishi et al. 2016).
PI3K/Akt in therapy resistance
Prostate cancer cells are considered to various types of therapeutic modalities to diminish tumor progression and improve patient survival. Among different kinds of treatments, chemotherapy is the most well-known one, although other therapeutics such as immunotherapy and radiotherapy have been also employed for this malignant disease. The challenging point is the resistance of prostate tumor cells to therapy and highlighting factors involved in this process (Ding et al. 2021a, b; Jamroze et al. 2021; Qi et al. 2022; Zhang et al. 2021a, b, c; Lu et al. 2021; He et al. 2021a, b, c; Annala et al. 2018). There are two types of therapeutic resistance in prostate cancer including intrinsic and extrinsic resistance. In intrinsic one, prostate cancer cells demonstrate some features and activation of oncogenic pathways to develop therapy resistance. For instance, EGFR overexpression promotes proliferation and colony formation capacity of prostate tumor cells. It has been shown that EGFR enhances Rad51 expression to stimulate EMT and DNA damage repair mechanisms in triggering intrinsic resistance to therapy (Rajput et al. 2021). In prostate cancer pathogenesis, Wnt/β-catenin demonstrates interactions with androgen receptor and MMP-7 protein. Noteworthy, induction of Wnt/β-catenin signaling is correlated with therapy resistance in prostate cancer (Wang et al. 2021a, b, c, d, e, f). N-Myc increases TEM8 expression to induce growth and angiogenesis in prostate cancer cells, and is associated with therapy resistance (Li et al. 2021a, b, c, d, e, f). The current section focuses on the role of PI3K/Akt signaling in therapy resistance in prostate cancer.
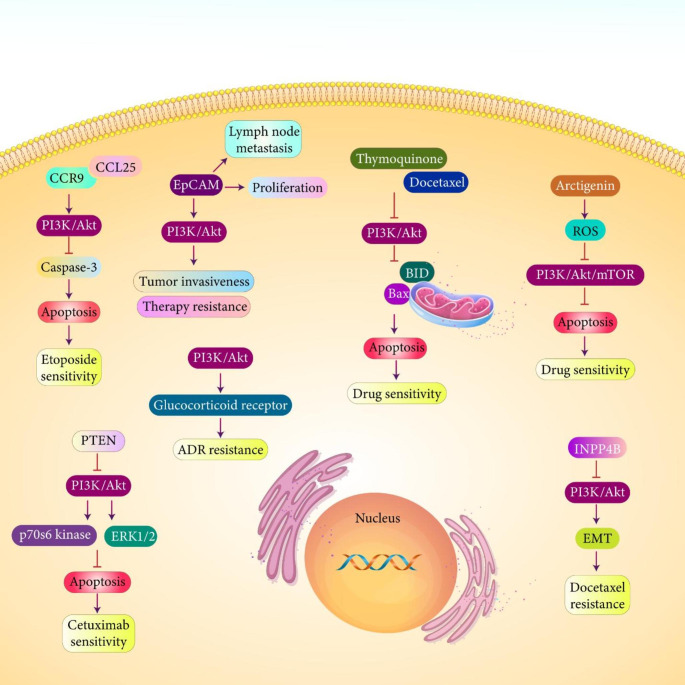
The strategy applied by chemotherapeutic agents in cancer therapy is apoptosis induction. This is an optional way in decreasing viability and survival of prostate tumor cells and impairing their progression. However, prostate tumor cells activate PI3K/Akt signaling as an anti-apoptotic mechanism in triggering etoposide resistance. Hence, revealing association between PI3K/Akt signaling with antiapoptotic signals and upstream mediators are of importance in prostate cancer therapy. CCR9 binds to its ligand CCL25 to stimulate PI3K/Akt signaling that decreases caspase-3 expression in preventing apoptosis in prostate tumor and decreasing sensitivity to etoposide. Therefore, inhibition of PI3K/Akt can be considered as a promising strategy in elevating prostate cancer sensitivity to etoposide chemotherapy (Sharma et al. 2010). The upregulation of EpCAM is associated with lymph node metastasis and proliferation ability of prostate tumor cells. Silencing EpCAM reduces colony formation capacity of prostate tumor cells and increases chemo- and radio-sensitivity. Notably, EpCAM stimulates PI3K/Akt signaling to increase invasiveness of prostate tumor cells and to mediate therapy resistance (Ni et al. 2013).
According to the discussions, activation of PI3K/Akt signaling is an impediment towards anti-cancer activity of chemotherapeutic agents and developing drug resistance. Although previous studies demonstrated that CCR9/CCL25 axis and EpCAM as oncogenic factors stimulate PI3K/Akt signaling in prostate cancer progression and drug resistance, restoring expression level of tumor-suppressor factors is beneficial in drug sensitivity and PI3K/Akt signaling inhibition. It has been reported that overexpression of PTEN in prostate cancer inhibits PI3K/Akt signaling. Then, a significant decrease occurs in expression levels of GSK-3β, P70S6 kinase and ERK1/2 as downstream targets, leading to apoptotic cell death in prostate tumor and improving response to cetuximab (Bouali et al. 2009). As it was mentioned, ADT is employed for prostate cancer suppression, but there are reports of resistance. Glucocorticoid receptor (GR) hyperactivation has been linked to therapy resistance in prostate cancer (Arora et al. 2013; Xie et al. 2015; Le et al. 2022; O’Reilly et al. 2022) and some clinical trials have focused on GR inhibition along with enzalutamide in prostate cancer therapy. Notably, inhibition of PI3K/Akt signaling diminishes proliferation rate of prostate tumor cells. Overexpression of GR stimulates ADR resistance in prostate tumor (in vitro and in vivo) and based on the results of a recent experiment, inhibition of PI3K/Akt signaling prevents resistance via GR down-regulation (Adelaiye-Ogala et al. 2020). Therefore, an ideal strategy is co-administration of chemotherapeutic agents and other anti-tumor factors in prostate cancer therapy. The anti-cancer drug should be capable of targeting PI3K/Akt signaling in prostate cancer therapy. Thymoquinone is naturally occurring compound with potent anti-cancer activity. Thymoquinone has been employed in treatment of various cancers such as lung tumor, breast tumor and colorectal tumor, among others. Thymoquinone can affect various signaling networks in cancer therapy that PI3K/Akt signaling is among them and it can be used as a chemo-sensitizer agent (Alam et al. 2022; Karim et al. 2022; Thabet et al. 2021; Abd-Rabou et al. 2021). A recent experiment has employed docetaxel and thymoquinone in treatment of prostate cancer. This combination therapy stimulates apoptosis in prostate cancer cells and abrogates their progression. Docetaxel and thymoquinone combination suppress PI3K/Akt signaling to increase expression levels of BID and Bax as pro-apoptotic factors and decreasing prostate tumor viability (Singh et al. 2019). Another anti-cancer agent is arctigenin that can decrease growth and metastasis of tumor cells. This compound stimulates apoptosis and decreases viability of tumor cells. It affects various molecular pathways such as STAT3 and β-catenin in impairing tumor progression (Shi et al. 2020; Sun et al. 2018a, b; Janthamala et al. 2021; Luo et al. 2021a, b, c). Acidic tumor microenvironment induces drug resistance in prostate cancer. Arctigenin increases ROS levels to suppress PI3K/Akt/mTOR axis in suppressing tumor progression. Reducing ROS levels abrogates potential of arctigenin in prostate cancer suppression (Lee et al. 2018). Therefore, application of anti-cancer agents capable of triggering apoptosis in prostate tumor (Qian et al. 2021) can be used for purpose of drug sensitivity.
The activation of PI3K/Akt signaling appears to be vital for cell cycle progression of prostate tumor cells and its inhibition can promote drug sensitivity. PI3K-C2β is essential for cell cycle progression in prostate cancer. Reducing expression level of PI3K-C2β by siRNA prevents mitosis in prostate tumor and reduces colony formation capacity of cancer cells in vitro. For in vivo experiment, reducing PI3K-C2β expression decreases tumor formation and delays it. Upon down-regulation of PI3K-C2β, an increase occurs in ability of docetaxel in suppressing progression of prostate tumor cells (Cisse et al. 2019). Activation of PI3K/Akt signaling and EMT mechanism can stimulate docetaxel resistance in prostate cancer. Overexpression of INPP4B inhibits PI3K/Akt signaling to prevent EMT and to reverse docetaxel resistance in prostate tumor (Chen et al. 2016). These studies demonstrate potential of PI3K/Akt signaling in triggering therapy resistance in prostate cancer (Fig. 4).
Fig. 4.
The involvement of PI3K/Akt signaling in therapy resistance in prostate cancer
Anti-cancer drugs
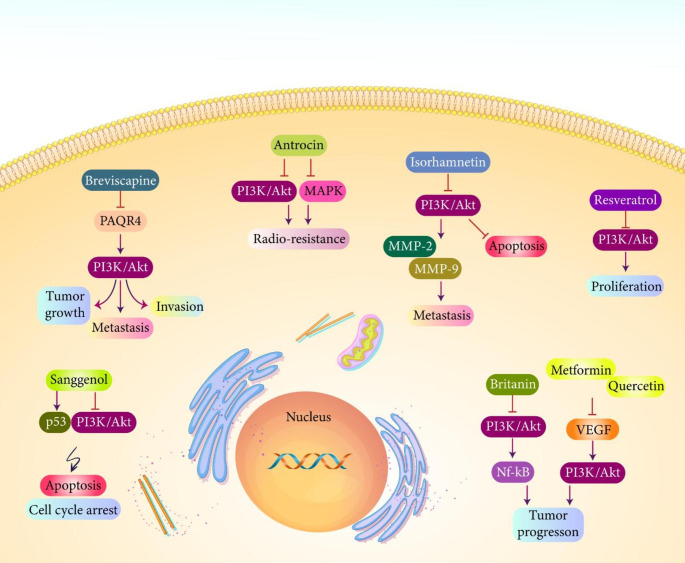
The previous experiments revealed role of PI3K/Akt signaling as oncogenic factor in prostate cancer (Zhan et al. 2020). Increasing evidence demonstrates potential of anti-cancer agents in targeting PI3K/Akt signaling for purpose of prostate cancer therapy. Breviscapine (BRE) extract has demonstrated potential in suppressing progression of prostate tumor cells. Administration of BRE extract is associated with a reduction in growth and invasion of prostate tumor cells. Normally, PAQR4 stimulates PI3K/Akt signaling in increasing growth and metastasis (EMT) in prostate cancer. Administration of BRE extract inhibits PI3K/Akt signaling via PAQR4 down-regulation to suppress prostate tumor progression (Ye et al. 2020a, b). Antrocin is another anti-cancer agent that can promote sensitivity of prostate tumor cells to radiotherapy. Based on a recent experiment, antrocin suppresses PI3K/Akt and MAPK molecular pathways to impair progression of prostate cancer cells and to increase their sensitivity to radiotherapy (Chen et al. 2018). As it was mentioned, ADT resistance is found in prostate cancer and tumor cells have capacity of progressing in an androgen-independent manner. Isorhamnetin impairs growth and metastasis of prostate tumor cells. Isorhamnetin inhibits PI3K/Akt signaling to induce apoptosis and reduce expression levels of MMP-2 and MMP-9 in impairing metastasis of tumor cells (Cai et al. 2020). Both p53 and PI3K/Akt/mTOR molecular pathways are affected by sanggenol in prostate cancer treatment. By increasing p53 expression and suppressing PI3K/Akt/mTOR axis, sanggenol stimulates apoptosis and cell cycle arrest in prostate tumor cells (Won and Seo 2020).
Resveratrol is a plant-derived natural compound applied in treatment of prostate cancer. Resveratrol inhibits AR and CXCR4 molecular pathways in inhibiting progression of prostate tumor cells (Jang et al. 2019). Resveratrol impairs proliferation and induces expression of pro-apoptotic factors via overexpression of SUMO-1 (Cheng et al. 2018). Based on the studies, resveratrol is an ideal candidate in suppressing growth and metastasis of prostate tumor cells (Wilson et al. 2017; Khusbu et al. 2020). A recent experiment has shown that resveratrol has ability of targeting PI3K/Akt signaling in impairing prostate cancer progression. Resveratrol administration suppresses prostate cancer progression in a time- and concentration-dependent manner. Resveratrol inhibits PI3K/Akt signaling to prevent proliferation of prostate tumor cells (Ye et al. 2020a, b). Another experiment focuses on application of britanin in treatment of prostate cancer. Based on the results of this study, britanin suppresses PI3K/Akt signaling to reduce expression level of its downstream target NF-κB, leading to growth inhibition in vitro and in vivo (Zeng et al. 2020).
VEGF can function as upstream mediator of PI3K/Akt signaling in increasing prostate cancer progression. A combination of metformin and quercetin inhibits PI3K/Akt signaling via VEGF down-regulation to suppress tumor proliferation in vitro and in vivo (Sun et al. 2018a, b). Arctigenin is another anti-cancer compound employed in treatment of prostate cancer. Arctigenin reduces expression levels of VEGF, EGF and FGF-β in impairing progression of prostate tumor cells in vitro and in vivo (Wang et al. 2017). Arctigenin promotes CCN1 expression and mediates mitochondrial dysfunction to induce necroptosis in impairing progression of prostate tumor cells (Lee et al. 2020a, b). VEGF and IGF-1 undergo down-regulation by arctigenin in suppressing prostate cancer progression (Hao et al. 2020). Based on these studies, arctigenin has capacity of impairing progression of prostate tumor cells. Arctigenin administration induces cell cycle arrest at G0/G1 phase and it induces apoptosis and autophagy. For this purpose, arctigenin inhibits PI3K/Akt/mTOR axis in impairing prostate cancer progression (Sun et al. 2021a, b). According to studies, inhibition of PI3K/Akt axis by anti-cancer agents induces mitochondrial dysfunction and mediates apoptosis and cell cycle arrest (Wu et al. 2018a, b). Furthermore, inhibition of PI3K/Akt signaling by anti-tumor agents suppresses migration and invasion of prostate cancer cells (Zhu et al. 2018). The anti-tumor agents employed in treatment of prostate cancer and suppressing PI3K/Akt signaling are phytochemicals (Zhang et al. 2017; Atmaca et al. 2022; Zhu et al. 2019; Ran et al. 2020; Li et al. 2022a, b). Their limitation is poor bioavailability and more studies are required in developing nanocarriers for their targeted delivery in prostate cancer therapy via targeting PI3K/Akt signaling. Overall, studies advocate impact of anti-cancer agents in impairing PI3K/Akt signaling in prostate cancer suppression (Fig. 5) (Abd Wahab et al. 2021; Li et al. 2018a, b, 2020; Long et al. 2021; Zhang et al. 2021a, b, c; Chan et al. 2017; Raasmaja et al. 2019; Sachan et al. 2018).
Fig. 5.
The anti-tumor agents targeting PI3K/Akt signaling in prostate cancer therapy
Clinical application
A number of experiments have evaluated role of PI3K/Akt signaling as biomarker in prostate cancer. As number of studies is low, but they can provide novel insights for prostate cancer prognosis and diagnosis. The polymorphisms and mutations in PI3K and Akt are found in prostate cancer patients (Nóbrega et al. 2020). Compared to normal tissues, high phosphorylation of Akt-1 occurs in prostate cancer tissues and can lead to recurrence (Ayala et al. 2004). Although such studies have evaluated prognostic function of PI3K/Akt (Torrealba et al. 2020; Sande et al. 2005), they lack large sample size and future studies should focus on their specificity and sensitivity in diagnosis of prostate cancer patients. Furthermore, more studies should be performed in combining PI3K/Akt with other prognostic factors in designing a signature for prostate cancer diagnosis and prognosis. Table 1 provides a summary of PI3K/Akt signaling association in prostate cancer progression.
Table 1.
The PI3K/Akt signaling in prostate cancer progression
| Signaling networks | Remarks | Refs |
|---|---|---|
| CCR9/CCL25/PI3K/Akt |
CCR9 binds to CCL25 to induce PI3K/Akt signaling Apoptosis inhibition Reducing anti-cancer activity of etoposide |
(Sharma et al. 2010) |
| EpCAM/PI3K/Akt/mTOR |
Association with prostate cancer metastasis Mediating drug resistance and radio-resistance EpCAM induces PI3K/Akt signaling |
(Ni et al. 2013) |
| PTEN/PI3K/Akt | PTEN suppresses PI3K/Akt signaling to promote cell death caused by cetuximab | (Bouali et al. 2009) |
| PI3K/Akt/GR | Inhibition of PI3K/Akt signaling reduces GR expression in reversing enzalutamide resistance in prostate cancer | (Adelaiye-Ogala et al. 2020) |
| PI3K/Akt | A combination of docetaxel and thymoquinone can stimulate apoptosis in prostate cancer via inhibition of PI3K/Akt signaling | (Singh et al. 2019) |
| ROS/PI3K/Akt/mTOR | Arctigenin promotes ROS generation to suppress PI3K/Akt/mTOR axis in inducing mitochondrial dysfunction and impairing tumor progression | (Lee et al. 2018) |
| PI3K/C2β | Inhibition of PI3K/C2β reduces cell division in prostate cancer and increases docetaxel sensitivity of tumor cells | (Cisse et al. 2019) |
| INNP4B/PI3K/Akt | INPP4B suppresses PI3K/Akt signaling in inhibiting docetaxel resistance | (Chen et al. 2016) |
| PI3K/Akt/mTOR | Radiation induces autophagy and promotes drug sensitivity via suppressing PI3K/Akt/mTOR axis | (Wu et al. 2018a, b) |
|
PI3K/mTOR PIM |
Co-inhibition of PI3K/mTOR and PIM is promising in suppressing prostate cancer progression | (Luszczak et al. 2020) |
| PI3K/Akt/NF-κB | Apigenin suppresses PI3K/Akt axis to reduce NF-κB expression in impairing prostate cancer progression | (Erdogan et al. 2016) |
| PI3K/Akt/mTOR | Rottlerin stimulates apoptosis and autophagy in prostate tumor cells | (Kumar et al. 2014) |
|
PI3K/Akt MAPK |
Overexpression of PI3K/Akt and MAPK stimulates drug resistance in prostate cancer | (Liu et al. 2015) |
| Midkine/PI3K/Akt | Down-regulation of midkine suppresses PI3K/Akt signaling and promotes efficacy of quercetin in prostate tumor inhibition | (Erdogan et al. 2018) |
| SOX2/PI3K/Akt | SOX2 stimulates PI3K/Akt signaling in mediating paclitaxel resistance in prostate cancer | (Li et al. 2014) |
| CNTN-1/PI3K/Akt | CNTN-1 induces EMT and PI3K/Akt signaling to mediate docetaxel resistance in prostate cancer | (Chen et al. 2021a, b) |
| PI3K/Akt | Quercetin promotes docetaxel sensitivity of prostate tumor cells via PI3K/Akt inhibition | (Lu et al. 2020) |
| miRNA-4638-5p/PI3K/Akt | miRNA-4638-5p prevents development of castration resistance in prostate cancer via suppressing PI3K/Akt signaling | (Wang et al. 2016) |
| SPP1/PI3K/Akt | SPP1 induces PI3K/Akt signaling to mediate EMT and enzalutamide resistance in prostate cancer | (Pang et al. 2021) |
| CamKII/Akt/mTOR | Citrate suppresses CamKII/Akt/mTOR axis in inducing autophagic cell death in prostate tumor | (Fan et al. 2021) |
| miRNA-1297/PTEN/Akt/ERK | miRNA-1297 induces Akt/ERK axis via PTEN down-regulation to increase proliferation and metastasis of prostate tumor cells | (Wang et al. 2021a, b, c, d, e, f) |
| Akt | Solamargine inhibits proliferation of prostate tumor cells and enhances docetaxel sensitivity via Akt signaling inhibition | (Ge et al. 2022) |
| LIFR/PDPK1/Akt/GCN5 | LIFR induces Akt signaling and related molecular pathways in increasing progression of prostate tumor cells | (Ding et al. 2022) |
| FBXO31/DUSP6/PI3K/Akt | FBXO31 down-regulation in prostate cancer promotes DUSP6 expression to induce PI3K/Akt signaling in promoting tumor formation | (Duan et al. 2021) |
| CMTM5/EGFR/PI3K/Akt | CMTM5 inhibits PI3K/Akt signaling via EGFR down-regulation to decrease proliferation and colony formation in prostate tumor | (Li et al. 2022a, b) |
| miRNA-130b/PI3K/Akt | miRNA-130b as tumor-suppressor factor, suppresses PI3K/Akt signaling in reducing metastasis of prostate cancer cells | (Jia et al. 2022) |
| - | Inhibiting PI3K p110β prevents development of castration resistance in prostate cancer | (Gao et al. 2022) |
| PLK4/PI3K/Akt | Fraxetin inhibits PI3K/Akt signaling via PLK4 down-regulation to impair growth and metastasis of prostate tumor cells | (Ma et al. 2022) |
| miRNA-192-5p/PI3K/CHOP | BK002 as anti-cancer agent, promotes miRNA-192-5p expression to inhibit PI3K signaling, leading to CHOP overexpression and apoptosis induction in prostate tumor cells | (Park et al. 2022) |
| KDM5A/miRNA-330-3p/PI3K/Akt | KDM5A reduces miRNA-330-3p expression to induce PI3K/Akt signaling in increasing prostate cancer tumorigenesis | (Mi et al. 2022) |
Conclusion and remarks
The treatment of urological cancers especially prostate cancer has been an ideal aim for physicians and researchers around the world. The heterogenic nature of prostate cancer has made it difficult to find an appropriate treatment for this disease and various studies have focused on genetic and epigenetic mutations occurring in prostate cancer. Epigenetic and genetic mutations are associated with each other in prostate cancer and they can affect each other activation or inhibition. The current review focused on PI3K/Akt signaling targeting in prostate cancer. Similar to other cancers, PI3K/Akt signaling demonstrates an oncogenic function in prostate cancer and its overexpression is observed in this malignant disease. Induction of PI3K/Akt signaling promotes hallmarks of prostate cancer. It has been reported that PI3K/Akt signaling prevents apoptosis in prostate cancer. Furthermore, PI3K/Akt signaling activation enhances proliferation rate of prostate tumor cells and mediates their cell cycle progression. Silencing PI3K/Akt signaling induces apoptosis and cell cycle arrest at G phase. Furthermore, PI3K/Akt inactivation decreases growth rate of prostate tumor in vitro and in vivo.
Notably, PI3K/Akt signaling increases metastasis of prostate cancer cells and it can induce EMT mechanism. Furthermore, PI3K/Akt signaling promotes expression level of MMPs in increasing motility of prostate tumor cells. PI3K/Akt signaling activation induces drug resistance and radio-resistance in prostate tumor. Various anti-cancer agents that are mainly phytochemicals can suppress PI3K/Akt signaling in impairing prostate tumor progression. Various upstream mediators including non-coding RNAs regulate PI3K/Akt signaling in prostate cancer that are promising therapeutic targets. Furthermore, PI3K/Akt can be considered as biomarker for diagnosis and prognosis of prostate cancer. Future studies can focus on revealing other signaling networks related to PI3K/Akt signaling in modulating progression of prostate tumor cells.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sepideh Mirzaei, Email: sepidehmirzaei.smv@gmail.com.
Maliheh Entezari, Email: mentezari@iautmu.ac.ir.
Saeed Samarghandian, Email: samarghandians1@nums.ac.ir.
References
- Abadi AJ et al (2021) Small in size, but large in action: microRNAs as potential modulators of PTEN in breast and lung cancers. Biomolecules 11(2):304 [DOI] [PMC free article] [PubMed]
- Abd Wahab NA et al (2021) Diarylpentanoid (1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadiene-3-one) (MS13) Exhibits Anti-proliferative, Apoptosis Induction and Anti-migration Properties on Androgen-independent Human Prostate Cancer by Targeting Cell Cycle-Apoptosis and PI3K Signalling Pathways. Front Pharmacol 12:707335 [DOI] [PMC free article] [PubMed]
- Abd-Rabou AA, et al. Thymoquinone Crosstalks with DR5 to Sensitize TRAIL Resistance and Stimulate ROS-Mediated Cancer Apoptosis. Asian Pac J Cancer Prev. 2021;22(9):2855–2865. doi: 10.31557/APJCP.2021.22.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelaiye-Ogala R, et al. Targeting the PI3K/AKT Pathway Overcomes Enzalutamide Resistance by Inhibiting Induction of the Glucocorticoid Receptor. Mol Cancer Ther. 2020;19(7):1436–1447. doi: 10.1158/1535-7163.MCT-19-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinleye A, et al. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6(1):88. doi: 10.1186/1756-8722-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S, et al. Thymoquinone and quercetin induce enhanced apoptosis in non-small cell lung cancer in combination through the Bax/Bcl2 cascade. J Cell Biochem. 2022;123(2):259–274. doi: 10.1002/jcb.30162. [DOI] [PubMed] [Google Scholar]
- Annala M, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov. 2018;8(4):444–457. doi: 10.1158/2159-8290.CD-17-0937. [DOI] [PubMed] [Google Scholar]
- Arbiser JL et al (2002) Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA 99(2):715–720 [DOI] [PMC free article] [PubMed]
- Argiris A et al (2006) A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther 5:766–770 [DOI] [PubMed]
- Arora VK et al (2013) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155(6):1309–1322 [DOI] [PMC free article] [PubMed]
- Ashrafizadeh M et al (2020a) Progress in delivery of siRNA-based therapeutics employing nano-vehicles for treatment of prostate cancer. Bioengineering (Basel) 7(3):91 [DOI] [PMC free article] [PubMed]
- Ashrafizadeh M et al (2020b) PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol 881:173226 [DOI] [PubMed]
- Ashrafizadeh M et al (2020c) PTEN, a barrier for proliferation and metastasis of gastric cancer cells: from molecular pathways to targeting and regulation. Biomedicines 8:264 [DOI] [PMC free article] [PubMed]
- Ashrafizadeh M et al (2020d) Association of the epithelial–mesenchymal transition (EMT) with cisplatin resistance. Int J Mol Sci 21(11):4002 [DOI] [PMC free article] [PubMed]
- Ashrafizadeh M et al (2020e) MicroRNAs and their influence on the ZEB family: mechanistic aspects and therapeutic applications in cancer therapy. Biomolecules 10(7):1040 [DOI] [PMC free article] [PubMed]
- Ashrafizadeh M et al (2021) New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed Pharmacother 141:111824 [DOI] [PubMed]
- Ashrafizadeh M et al (2022) Targeting autophagy in prostate cancer: preclinical and clinical evidence for therapeutic response. J Exp Clin Cancer Res 41(1):105 [DOI] [PMC free article] [PubMed]
- Atmaca H, Camli Pulat C, Cittan M. Liquidambar orientalis Mill. gum extract induces autophagy via PI3K/Akt/mTOR signaling pathway in prostate cancer cells. Int J Environ Health Res. 2022;32(5):1011–1019. doi: 10.1080/09603123.2020.1818187. [DOI] [PubMed] [Google Scholar]
- Ayala G, et al. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10(19):6572–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- Bacac M, Stamenkovic I. Metastatic cancer cell. 2008;3:221–247. doi: 10.1146/annurev.pathmechdis.3.121806.151523. [DOI] [PubMed] [Google Scholar]
- Bernstein DE, et al. Prostate cancer and microfluids. Urologic Oncology: Seminars and Original Investigations. 2021;39(8):455–470. doi: 10.1016/j.urolonc.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J Control Release. 2020;322:357–374. doi: 10.1016/j.jconrel.2020.03.033. [DOI] [PubMed] [Google Scholar]
- Bilanges B, Posor Y, Vanhaesebroeck B (2019) PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol 20(9):515–534 [DOI] [PubMed]
- Bo S, et al. Purpurin, a anthraquinone induces ROS-mediated A549 lung cancer cell apoptosis via inhibition of PI3K/AKT and proliferation. J Pharm Pharmacol. 2021;73(8):1101–1108. doi: 10.1093/jpp/rgab056. [DOI] [PubMed] [Google Scholar]
- Bouali S, et al. PTEN expression controls cellular response to cetuximab by mediating PI3K/AKT and RAS/RAF/MAPK downstream signaling in KRAS wild-type, hormone refractory prostate cancer cells. Oncol Rep. 2009;21(3):731–735. [PubMed] [Google Scholar]
- Brar SS et al (2002) An NAD (P) H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol 282(6):C1212–C1224 [DOI] [PubMed]
- Burleson M, et al. GLI3 Is Stabilized by SPOP Mutations and Promotes Castration Resistance via Functional Cooperation with Androgen Receptor in Prostate Cancer. Mol Cancer Res. 2022;20(1):62–76. doi: 10.1158/1541-7786.MCR-21-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai F et al (2020) Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci Rep 40(3):BSR20192826 [DOI] [PMC free article] [PubMed]
- Cai J, et al. NAT1 is a critical prognostic biomarker and inhibits proliferation of colorectal cancer through modulation of PI3K/Akt/mTOR. Future Oncol. 2021;17(19):2489–2498. doi: 10.2217/fon-2020-0992. [DOI] [PubMed] [Google Scholar]
- Cattrini C et al (2021) Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers (Basel) 13(8):45221 [DOI] [PMC free article] [PubMed]
- Chan ML, et al. Enantiomerically pure β-dipeptide derivative induces anticancer activity against human hormone-refractory prostate cancer through both PI3K/Akt-dependent and -independent pathways. Oncotarget. 2017;8(57):96668–96683. doi: 10.18632/oncotarget.18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4(10):e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YL, et al. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6(27):24017–24031. doi: 10.18632/oncotarget.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, et al. Total Flavonoids of Litchi Seed Attenuate Prostate Cancer Progression Via Inhibiting AKT/mTOR and NF-kB Signaling Pathways. Front Pharmacol. 2021;12:758219. doi: 10.3389/fphar.2021.758219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li H, Chen Q. INPP4B reverses docetaxel resistance and epithelial-to-mesenchymal transition via the PI3K/Akt signaling pathway in prostate cancer. Biochem Biophys Res Commun. 2016;477(3):467–472. doi: 10.1016/j.bbrc.2016.06.073. [DOI] [PubMed] [Google Scholar]
- Chen YA et al (2018) Antrocin Sensitizes Prostate Cancer Cells to Radiotherapy through Inhibiting PI3K/AKT and MAPK Signaling Pathways. Cancers (Basel) 11(1):34 [DOI] [PMC free article] [PubMed]
- Chen X, et al. EGFR and ERK activation resists flavonoid quercetin-induced anticancer activities in human cervical cancer cells in vitro. Oncol Lett. 2021;22(5):754. doi: 10.3892/ol.2021.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, et al. CNTN-1 promotes docetaxel resistance and epithelial-to-mesenchymal transition via the PI3K/Akt signaling pathway in prostate cancer. Arch Med Sci. 2021;17(1):152–165. doi: 10.5114/aoms.2020.92939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. Lycopene enhances the sensitivity of castration-resistant prostate cancer to enzalutamide through the AKT/EZH2/ androgen receptor signaling pathway. Biochem Biophys Res Commun. 2022;613:53–60. doi: 10.1016/j.bbrc.2022.04.126. [DOI] [PubMed] [Google Scholar]
- Cheng TM, et al. Resveratrol induces sumoylated COX-2-dependent anti-proliferation in human prostate cancer LNCaP cells. Food Chem Toxicol. 2018;112:67–75. doi: 10.1016/j.fct.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Cheng Y, et al. Periplocymarin Induced Colorectal Cancer Cells Apoptosis Via Impairing PI3K/AKT Pathway. Front Oncol. 2021;11:753598. doi: 10.3389/fonc.2021.753598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HD, et al. Kochia scoparia seed extract suppresses VEGF-induced angiogenesis via modulating VEGF receptor 2 and PI3K/AKT/mTOR pathways. Pharm Biol. 2019;57(1):684–693. doi: 10.1080/13880209.2019.1672753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, et al. SIRT5 Directly Inhibits the PI3K/AKT Pathway in Prostate Cancer Cell Lines. Cancer Genomics Proteomics. 2022;19(1):50–59. doi: 10.21873/cgp.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M et al (2021) Targeting cancer stem cells by nutraceuticals for cancer therapy. Semin Cancer Biol [DOI] [PubMed]
- Ci X, et al. KLF5 inhibits angiogenesis in PTEN-deficient prostate cancer by attenuating AKT activation and subsequent HIF1α accumulation. Mol Cancer. 2015;14:91. doi: 10.1186/s12943-015-0365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse O, et al. Downregulation of class II phosphoinositide 3-kinase PI3K-C2β delays cell division and potentiates the effect of docetaxel on cancer cell growth. J Exp Clin Cancer Res. 2019;38(1):472. doi: 10.1186/s13046-019-1472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, et al. Curr Med Chem. 2021;28(33):6773–6804. doi: 10.2174/0929867328666210405111913. [DOI] [PubMed] [Google Scholar]
- Dai X et al (2016) Ascochlorin enhances the sensitivity of doxorubicin leading to the reversal of epithelial-to-mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther 15(12):2966–2976 [DOI] [PubMed]
- Dbouk HA et al (2013) Characterization of a tumor-associated activating mutation of the p110β PI 3-kinase. PLos One 8(5):e638335 [DOI] [PMC free article] [PubMed]
- Deldar Abad Paskeh M, et al. Int J Mol Sci. 2021;22(21):11669. doi: 10.3390/ijms222111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J et al (2016) Trends in prostate cancer incidence and mortality in Canada during the era of prostate-specific antigen screening. CMAJ Open 4(1):E73–E79 [DOI] [PMC free article] [PubMed]
- Ding X, et al. Muscleblind-like 1 antisense RNA 1 inhibits cell proliferation, invasion, and migration of prostate cancer by sponging miR-181a-5p and regulating PTEN/PI3K/AKT/mTOR signaling. Bioengineered. 2021;12(1):803–814. doi: 10.1080/21655979.2021.1890383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, et al. Role of noncoding RNA in drug resistance of prostate cancer. Cell Death Dis. 2021;12(6):590. doi: 10.1038/s41419-021-03854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, et al. Leukemia inhibitory factor receptor homodimerization mediated by acetylation of extracellular lysine promotes prostate cancer progression through the PDPK1/AKT/GCN5 axis. Clin Transl Med. 2022;12(2):e676. doi: 10.1002/ctm2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, et al. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front Pharmacol. 2021;12:628690. doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, et al. Loss of FBXO31-mediated degradation of DUSP6 dysregulates ERK and PI3K-AKT signaling and promotes prostate tumorigenesis. Cell Rep. 2021;37(3):109870. doi: 10.1016/j.celrep.2021.109870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsani M et al (2022) The natural compound atraric acid suppresses androgen-regulated neo-angiogenesis of castration-resistant prostate cancer through angiopoietin 2. Oncogene 41(23):3263-3277 [DOI] [PMC free article] [PubMed]
- Engelman J (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9(8):550–562 [DOI] [PubMed]
- Erdogan S, et al. The flavonoid apigenin reduces prostate cancer CD44(+) stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016;162:77–86. doi: 10.1016/j.lfs.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Erdogan S, et al. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed Pharmacother. 2018;107:793–805. doi: 10.1016/j.biopha.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Fan X, et al. Citrate activates autophagic death of prostate cancer cells via downregulation CaMKII/AKT/mTOR pathway. Life Sci. 2021;275:119355. doi: 10.1016/j.lfs.2021.119355. [DOI] [PubMed] [Google Scholar]
- Fang J, et al. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19(12):2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. Cytometry. 1989;10(6):673–680. doi: 10.1002/cyto.990100602. [DOI] [PubMed] [Google Scholar]
- Folkes AJ et al (2008) The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin>-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 51(18):5522–5532 [DOI] [PubMed]
- Gao Y, Yuan CY, Yuan WJSCI (2016) Will targeting PI3K/Akt/mTOR signaling work in hematopoietic malignancies? Stem Cell Investig 3:31 [DOI] [PMC free article] [PubMed]
- Gao X, et al. Blocking PI3K p110β Attenuates Development of PTEN-Deficient Castration-Resistant Prostate Cancer. Mol Cancer Res. 2022;20(5):673–685. doi: 10.1158/1541-7786.MCR-21-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, et al. Solamargine Inhibits Prostate Cancer Cell Growth and Enhances the Therapeutic Efficacy of Docetaxel via Akt Signaling. J Oncol. 2022;2022:9055954. doi: 10.1155/2022/9055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, et al. Inhibition of MicroRNA miR-101-3p on prostate cancer progression by regulating Cullin 4B (CUL4B) and PI3K/AKT/mTOR signaling pathways. Bioengineered. 2021;12(1):4719–4735. doi: 10.1080/21655979.2021.1949513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulluni F et al (2019) Class II PI3K functions in cell biology and disease. Trends Cell Biol 29(4):339–359 [DOI] [PubMed]
- Guo C, et al. PPA1 Promotes Breast Cancer Proliferation and Metastasis Through PI3K/AKT/GSK3β Signaling Pathway. Front Cell Dev Biol. 2021;9:730558. doi: 10.3389/fcell.2021.730558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, et al. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol Cancer. 2021;20(1):93. doi: 10.1186/s12943-021-01372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habrowska-Górczyńska DE et al (2021) FOXO3a and Its Regulators in Prostate Cancer. Int J Mol 22(22):12530 [DOI] [PMC free article] [PubMed]
- Hao Q, et al. Arctigenin inhibits prostate tumor growth in high-fat diet fed mice through dual actions on adipose tissue and tumor. Sci Rep. 2020;10(1):1403. doi: 10.1038/s41598-020-58354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YD, et al. A urine extracellular vesicle circRNA classifier for detection of high-grade prostate cancer in patients with prostate-specific antigen 2–10 ng/mL at initial biopsy. Mol Cancer. 2021;20(1):96. doi: 10.1186/s12943-021-01388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target therapy. 2021;6(1):425–425. doi: 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He MX, et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat Med. 2021;27(3):426–433. doi: 10.1038/s41591-021-01244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellebrekers DM, et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res. 2009;15(12):3990–3997. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Gulluni F. Adv Biol Regul. 2020;75:100693. doi: 10.1016/j.jbior.2020.100693. [DOI] [PubMed] [Google Scholar]
- Hou Y, Li H, Huo W. THBS4 silencing regulates the cancer stem cell-like properties in prostate cancer via blocking the PI3K/Akt pathway. Prostate. 2020;80(10):753–763. doi: 10.1002/pros.23989. [DOI] [PubMed] [Google Scholar]
- Hresko RC, Murata H, Mueckler M. Phosphoinositide-dependent kinase-2 is a distinct protein kinase enriched in a novel cytoskeletal fraction associated with adipocyte plasma membranes. J Biol Chem. 2003;278(24):21615–21622. doi: 10.1074/jbc.M302937200. [DOI] [PubMed] [Google Scholar]
- Hu K et al (2021) Exosome circCMTM3 promotes angiogenesis and tumorigenesis of hepatocellular carcinoma through miR-3619-5p/SOX9. Hepatol Res 51(11):1139–1152 [DOI] [PubMed]
- Huang B, et al. Suppressed epithelial-mesenchymal transition and cancer stem cell properties mediate the anti-cancer effects of ethyl pyruvate via regulation of the AKT/nuclear factor-κB pathway in prostate cancer cells. Oncol Lett. 2018;16(2):2271–2278. doi: 10.3892/ol.2018.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. Incaspitolide A isolated from Carpesium cernuum L. inhibits the growth of prostate cancer cells and induces apoptosis via regulation of the PI3K/Akt/xIAP pathway. Oncol Lett. 2021;21(6):477. doi: 10.3892/ol.2021.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TF et al (2022) 4-Acetylantroquinonol B Suppresses Prostate Cancer Growth and Angiogenesis via a VEGF/PI3K/ERK/mTOR-Dependent Signaling Pathway in Subcutaneous Xenograft and In Vivo Angiogenesis Models. Int J Mol Sci 23(3):1446 [DOI] [PMC free article] [PubMed]
- Huggins C, Hodges CVJCr. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases. metastatic carcinoma of the prostate. 1941;1(4):293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- Huggins C, Hodges, CV (1972) Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 22(4):232–240 [DOI] [PubMed]
- Hussain Y et al (2021) Quercetin and its nano-scale delivery systems in prostate cancer therapy: paving the way for cancer elimination and reversing chemoresistance. Cancers (Basel) 13(7):1602 [DOI] [PMC free article] [PubMed]
- Hwang ST et al (2020) Corilagin represses epithelial to mesenchymal transition process through modulating Wnt/β-catenin signaling cascade. Biomolecules 10(10):1406 [DOI] [PMC free article] [PubMed]
- Ibrahim HM, et al. Thidiazuron suppresses breast cancer via targeting miR-132 and dysregulation of the PI3K-Akt signaling pathway mediated by the miR-202-5p-PTEN axis. Biochem Cell Biol. 2021;99(3):374–384. doi: 10.1139/bcb-2020-0377. [DOI] [PubMed] [Google Scholar]
- Jamroze A, Chatta G, Tang DG. Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett. 2021;518:1–9. doi: 10.1016/j.canlet.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YG, et al. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J Steroid Biochem Mol Biol. 2019;192:105406. doi: 10.1016/j.jsbmb.2019.105406. [DOI] [PubMed] [Google Scholar]
- Janthamala S, et al. Arctigenin inhibits cholangiocarcinoma progression by regulating cell migration and cell viability via the N-cadherin and apoptosis pathway. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(10):2049–2059. doi: 10.1007/s00210-021-02123-0. [DOI] [PubMed] [Google Scholar]
- Jariyal H, et al. Advancements in cancer stem cell isolation and characterization. 2019;15(6):755–773. doi: 10.1007/s12015-019-09912-4. [DOI] [PubMed] [Google Scholar]
- Jia L, et al. miR-130b suppresses the invasion and migration of prostate cancer via inhibiting DLL1 and regulating the PI3K/Akt pathways. Exp Ther Med. 2022;23(1):98. doi: 10.3892/etm.2021.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, et al. SPOP-mediated ubiquitination and degradation of PDK1 suppresses AKT kinase activity and oncogenic functions. Mol Cancer. 2021;20(1):100. doi: 10.1186/s12943-021-01397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, et al. Prostate cancer-associated SPOP mutations lead to genomic instability through disruption of the SPOP-HIPK2 axis. Nucleic Acids Res. 2021;49(12):6788–6803. doi: 10.1093/nar/gkab489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, et al. GNE-493 inhibits prostate cancer cell growth via Akt-mTOR-dependent and -independent mechanisms. Cell Death Discov. 2022;8(1):120. doi: 10.1038/s41420-022-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, et al. Quercetin inhibiting the PD-1/PD-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytother Res. 2021;35(11):6441–6451. doi: 10.1002/ptr.7297. [DOI] [PubMed] [Google Scholar]
- Junttila TT et al (2009) Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15(5):429–440 [DOI] [PubMed]
- Juric D, et al. Phosphatidylinositol 3-kinase α–selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study -human study. 2018;36(13):1291. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S et al (2022) PI3K-AKT Pathway Modulation by Thymoquinone Limits Tumor Growth and Glycolytic Metabolism in Colorectal Cancer. Int J Mol Sci 23(4):2305 [DOI] [PMC free article] [PubMed]
- Kawakami Y, et al. Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004;279(46):47720–47725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- Khalil MI, et al. Interaction of TLK1 and AKTIP as a Potential Regulator of AKT Activation in Castration-Resistant Prostate Cancer Progression. Pathophysiology. 2021;28(3):339–354. doi: 10.3390/pathophysiology28030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusbu FY, et al. Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int J Biochem Cell Biol. 2020;118:105644. doi: 10.1016/j.biocel.2019.105644. [DOI] [PubMed] [Google Scholar]
- Kim RJ, et al. PTEN loss-mediated Akt activation increases the properties of cancer stem-like cell populations in prostate cancer. Oncology. 2014;87(5):270–279. doi: 10.1159/000363186. [DOI] [PubMed] [Google Scholar]
- King D, Yeomanson D, Bryant, HE (2015) PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol 37:245–2514 [DOI] [PubMed]
- Ko J-H et al (2018) Bergamottin suppresses metastasis of lung cancer cells through abrogation of diverse oncogenic signaling cascades and epithelial-to-mesenchymal transition. Molecules 23(7):1601 [DOI] [PMC free article] [PubMed]
- Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014;343(2):179–189. doi: 10.1016/j.canlet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Kumar S, et al. Long non-coding RNA regulating androgen receptor signaling in breast and prostate cancer. Cancer Lett. 2021;504:15–22. doi: 10.1016/j.canlet.2020.11.039. [DOI] [PubMed] [Google Scholar]
- Lambert AW, R.A.J.N.R C, Weinberg Nat Rev Cancer. 2021;21(5):325–338. doi: 10.1038/s41568-021-00332-6. [DOI] [PubMed] [Google Scholar]
- Le TT, et al. The ADAM9/UBN2/AKR1C3 axis promotes resistance to androgen-deprivation in prostate cancer. Am J Cancer Res. 2022;12(1):176–197. [PMC free article] [PubMed] [Google Scholar]
- Lee JK et al (2007) NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenenterology 133(5):1637–1648 [DOI] [PubMed]
- Lee YJ, Oh JE, Lee SH. Arctigenin shows preferential cytotoxicity to acidity-tolerant prostate carcinoma PC-3 cells through ROS-mediated mitochondrial damage and the inhibition of PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;505(4):1244–1250. doi: 10.1016/j.bbrc.2018.10.045. [DOI] [PubMed] [Google Scholar]
- Lee JH et al (2020a) Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J Adv Res 26:83–94 [DOI] [PMC free article] [PubMed]
- Lee YJ, et al. Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis. Mol Cell Biochem. 2020;467(1–2):45–56. doi: 10.1007/s11010-020-03699-6. [DOI] [PubMed] [Google Scholar]
- Lee TK-W et al (2021a) Cancer stem cells in hepatocellular carcinoma—From origin to clinical implications. Nat Rev Gastroenterol Hepatol 19(1):26-44 [DOI] [PubMed]
- Lee JE, Lim YH, Kim JH (2021b) UCH-L1 and UCH-L3 regulate the cancer stem cell-like properties through PI3†K/Akt signaling pathway in prostate cancer cells. Anim Cells Syst (Seoul) 25(5):312–322 [DOI] [PMC free article] [PubMed]
- Li Q, et al. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim Biophys Acta. 2013;1833(12):3375–3385. doi: 10.1016/j.bbamcr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Li D, et al. Sox2 is involved in paclitaxel resistance of the prostate cancer cell line PC-3 via the PI3K/Akt pathway. Mol Med Rep. 2014;10(6):3169–3176. doi: 10.3892/mmr.2014.2630. [DOI] [PubMed] [Google Scholar]
- Li M et al (2018a) Phosphoinositide 3-kinase gamma inhibition protects from anthracycline cardiotoxicity and reduces tumor growth. Circulation 138(7):696–711 [DOI] [PubMed]
- Li X et al (2018b) Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Mar Drugs 16(9) [DOI] [PMC free article] [PubMed]
- Li S, et al. The Impact of Icariside II on Human Prostate Cancer Cell Proliferation, Mobility, and Autophagy via PI3K-AKT-mTOR Signaling Pathway. Drug Des Devel Ther. 2020;14:4169–4178. doi: 10.2147/DDDT.S268524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H et al (2021a) Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers (Basel) 13(14):3517 [DOI] [PMC free article] [PubMed]
- Li H, et al. Halofuginone Sensitizes Lung Cancer Organoids to Cisplatin via Suppressing PI3K/AKT and MAPK Signaling Pathways. Front Cell Dev Biol. 2021;9:773048. doi: 10.3389/fcell.2021.773048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. E2F2 inhibition induces autophagy via the PI3K/Akt/mTOR pathway in gastric cancer. Aging. 2021;13(10):13626–13643. doi: 10.18632/aging.202891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, et al. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells. Pharm Biol. 2021;59(1):21–30. doi: 10.1080/13880209.2020.1865407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. Exosomal lncAY927529 enhances prostate cancer cell proliferation and invasion through regulating bone microenvironment. Cell Cycle. 2021;20(23):2531–2546. doi: 10.1080/15384101.2021.1992853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, et al. N-Myc promotes angiogenesis and therapeutic resistance of prostate cancer by TEM8. Med Oncol. 2021;38(10):127. doi: 10.1007/s12032-021-01575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X et al (2022a) Gypenoside-Induced Apoptosis via the PI3K/AKT/mTOR Signaling Pathway in Bladder Cancer. Biomed Res Int 2022:9304552 [DOI] [PMC free article] [PubMed]
- Li L et al (2022b) CMTM5 inhibits the development of prostate cancer via the EGFR/PI3K/AKT signaling pathway. Mol Med Rep 25(1) [DOI] [PMC free article] [PubMed]
- Lim HY, et al. Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Lett. 2021;521:252–267. doi: 10.1016/j.canlet.2021.08.030. [DOI] [PubMed] [Google Scholar]
- Liu LZ, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS ONE. 2011;6(4):e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. The Upregulation of PI3K/Akt and MAP Kinase Pathways is Associated with Resistance of Microtubule-Targeting Drugs in Prostate Cancer. J Cell Biochem. 2015;116(7):1341–1349. doi: 10.1002/jcb.25091. [DOI] [PubMed] [Google Scholar]
- Liu YQ, et al. Acetyl-11-keto-β-boswellic acid suppresses docetaxel-resistant prostate cancer cells in vitro and in vivo by blocking Akt and Stat3 signaling, thus suppressing chemoresistant stem cell-like properties. Acta Pharmacol Sin. 2019;40(5):689–698. doi: 10.1038/s41401-018-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Quercetin inhibits invasion and angiogenesis of esophageal cancer cells. Pathol Res Pract. 2021;222:153455. doi: 10.1016/j.prp.2021.153455. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. SLC4A4 promotes prostate cancer progression in vivo and in vitro via AKT-mediated signalling pathway. Cancer Cell Int. 2022;22(1):127. doi: 10.1186/s12935-022-02546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh AH et al (2013) Dissecting the PI3K signaling axis in pediatric solid tumors: novel targets for clinical integration. Front Onc 3:93 [DOI] [PMC free article] [PubMed]
- Lokeshwar SD, Klaassen Z, Saad FJNRU (2021) Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol 18(7):433–442 [DOI] [PubMed]
- Long Y et al (2021) [Xihuang Pills and its main components inhibit PI3K/Akt/mTOR signaling pathways and promote the apoptosis of prostate cancer cells in PC-3 tumor-bearing mice]. Zhonghua Nan Ke Xue 27(4):340–346 [PubMed]
- Loriot Y et al (2018) Management of non-metastatic castrate-resistant prostate cancer: A systematic review. Cancer Treat Rev 70:223–231 [DOI] [PubMed]
- Lu X, et al. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int J Biol Sci. 2020;16(7):1121–1134. doi: 10.7150/ijbs.41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, et al. KAT2A-mediated AR translocation into nucleus promotes abiraterone-resistance in castration-resistant prostate cancer. Cell Death Dis. 2021;12(8):787. doi: 10.1038/s41419-021-04077-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo GC, et al. Hsa_circ_0030586 promotes epithelial-mesenchymal transition in prostate cancer via PI3K-AKT signaling. Bioengineered. 2021;12(2):11089–11107. doi: 10.1080/21655979.2021.2008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, et al. SPOP promotes CDCA5 degradation to regulate prostate cancer progression via the AKT pathway. Neoplasia. 2021;23(10):1037–1047. doi: 10.1016/j.neo.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, et al. Arctigenin inhibits human breast cancer cell proliferation, migratory and invasive abilities and epithelial to mesenchymal transition by targeting 4EBP1. Exp Ther Med. 2021;21(6):547. doi: 10.3892/etm.2021.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczak S, et al. Co-targeting PIM and PI3K/mTOR using multikinase inhibitor AUM302 and a combination of AZD-1208 and BEZ235 in prostate cancer. Sci Rep. 2020;10(1):14380. doi: 10.1038/s41598-020-71263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, et al. PHLDA3 exerts an antitumor function in prostate cancer by down-regulating Wnt/β-catenin pathway via inhibition of Akt. Biochem Biophys Res Commun. 2021;571:66–73. doi: 10.1016/j.bbrc.2021.07.038. [DOI] [PubMed] [Google Scholar]
- Ma Z, Sun Y, Peng W. Fraxetin down-regulates polo-like kinase 4 (PLK4) to inhibit proliferation, migration and invasion of prostate cancer cells through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway. Bioengineered. 2022;13(4):9345–9356. doi: 10.1080/21655979.2022.2054195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machl A et al (2016) M2698 is a potent dual-inhibitor of p70S6K and Akt that affects tumor growth in mouse models of cancer and crosses the blood-brain barrier. Am J Cancer Resn 6(4):806–818 [PMC free article] [PubMed]
- Maehama T, Dixon, JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3, 4, 5-trisphosphate. J Biol Chem 273(22):13375–13378 [DOI] [PubMed]
- Mahadev K et al (2004) The NAD (P) H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24(5):1844–1854 [DOI] [PMC free article] [PubMed]
- Mahmoud N, et al. Nimbolide inhibits 2D and 3D prostate cancer cells migration, affects microtubules and angiogenesis and suppresses B-RAF/p.ERK-mediated in vivo tumor growth. Phytomedicine. 2022;94:153826. doi: 10.1016/j.phymed.2021.153826. [DOI] [PubMed] [Google Scholar]
- Mao W, et al. ID2 Inhibits Bladder Cancer Progression and Metastasis via PI3K/AKT Signaling Pathway. Front Cell Dev Biol. 2021;9:738364. doi: 10.3389/fcell.2021.738364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno MJNRC. Nat Rev Cancer. 2012;12(1):23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- Matsushita M, et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Prostate Cancer Growth via IGF1 Signaling. Cancer Res. 2021;81(15):4014–4026. doi: 10.1158/0008-5472.CAN-20-4090. [DOI] [PubMed] [Google Scholar]
- Mayer IA, Arteaga, CL (2016) The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med 67:11–28 [DOI] [PubMed]
- Mehlen P (2006) A.J.N.r.c. Puisieux, Metastasis: a question of life or death. Nat Rev Cancer 6(6):449–458 [DOI] [PubMed]
- Mesquita FS et al (2010) Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod 82(2):341–351 [DOI] [PMC free article] [PubMed]
- Mi Y et al (2022) Lysine demethylase 5A promotes prostate adenocarcinoma progression by suppressing microRNA-330-3p expression and activating the COPB2/PI3K/AKT axis in an ETS1-dependent manner. J Cell Commun Signal. 10.1007/s12079-022-00671-5 [DOI] [PMC free article] [PubMed]
- Mirzaei S et al (2022) Transforming growth factor-beta (TGF-β) in prostate cancer: A dual function mediator? Int J Biol Macromol 206:435-452 [DOI] [PubMed]
- Monaco S et al (2006) Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci 15(12):2805–2815 [DOI] [PMC free article] [PubMed]
- Nalla AK, et al. N-cadherin mediates angiogenesis by regulating monocyte chemoattractant protein-1 expression via PI3K/Akt signaling in prostate cancer cells. Exp Cell Res. 2011;317(17):2512–2521. doi: 10.1016/j.yexcr.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Naughton R et al (2009) Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia 23(8):1432–1440 [DOI] [PubMed]
- Ni J, et al. Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int J Biochem Cell Biol. 2013;45(12):2736–2748. doi: 10.1016/j.biocel.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Nóbrega M, et al. Association of polymorphisms of PTEN, AKT1, PI3K, AR, and AMACR genes in patients with prostate cancer. Genet Mol Biol. 2020;43(3):e20180329. doi: 10.1590/1678-4685-gmb-2018-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly D, et al. CaV1.3 enhanced store operated calcium promotes resistance to androgen deprivation in prostate cancer. Cell Calcium. 2022;103:102554. doi: 10.1016/j.ceca.2022.102554. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y, et al. Lapatinib-resistant cancer cells possessing epithelial cancer stem cell properties develop sensitivity during sphere formation by activation of the ErbB/AKT/cyclin D2 pathway. Oncol Rep. 2016;36(5):3058–3064. doi: 10.3892/or.2016.5073. [DOI] [PubMed] [Google Scholar]
- Ozturk SA, et al. The effects of thymoquinone and genistein treatment on telomerase activity, apoptosis, angiogenesis, and survival in thyroid cancer cell lines. J Cancer Res Ther. 2018;14(2):328–334. doi: 10.4103/0973-1482.202886. [DOI] [PubMed] [Google Scholar]
- Pang X, et al. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70(5):1951–1959. doi: 10.1158/0008-5472.CAN-09-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X et al (2021) SPP1 Promotes Enzalutamide Resistance and Epithelial-Mesenchymal-Transition Activation in Castration-Resistant Prostate Cancer via PI3K/AKT and ERK1/2 Pathways. Oxid Med Cell Longev 2021:5806602 [DOI] [PMC free article] [PubMed]
- Park MN, et al. BK002 Induces miR-192-5p-Mediated Apoptosis in Castration-Resistant Prostate Cancer Cells via Modulation of PI3K/CHOP. Front Oncol. 2022;12:791365. doi: 10.3389/fonc.2022.791365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol Rep. 2013;29(2):571–578. doi: 10.3892/or.2012.2165. [DOI] [PubMed] [Google Scholar]
- Pernar CH, et al. Cold Spring Harb Perspect Med. 2018;8(12):a030361. doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheeshkumar P, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE. 2012;7(10):e47516. doi: 10.1371/journal.pone.0047516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, et al. Overcoming resistance to immune checkpoint therapy in PTEN-null prostate cancer by intermittent anti-PI3Kα/β/δ treatment. Nat Commun. 2022;13(1):182. doi: 10.1038/s41467-021-27833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, et al. Zingerone suppresses cell proliferation via inducing cellular apoptosis and inhibition of the PI3K/AKT/mTOR signaling pathway in human prostate cancer PC-3 cells. J Biochem Mol Toxicol. 2021;35(1):e22611. doi: 10.1002/jbt.22611. [DOI] [PubMed] [Google Scholar]
- Qiao TY, et al. Claudin14 promotes colorectal cancer progression via the PI3K/AKT/mTOR pathway. Neoplasma. 2021;68(5):947–954. doi: 10.4149/neo_2021_210210N203. [DOI] [PubMed] [Google Scholar]
- Raasmaja A, Stenius U, Ghalali A (2019) The Water Extract of Juniperus communis L. Induces Cell Death and Sensitizes Cancer Cells to Cytostatic Drugs through p53 and PI3K/Akt Pathways. Int J Mol Sci 20(9) [DOI] [PMC free article] [PubMed]
- Raghav PK, Mann ZJLS. Life Sci. 2021;277:119465. doi: 10.1016/j.lfs.2021.119465. [DOI] [PubMed] [Google Scholar]
- Rajput M, et al. EGFR-mediated Rad51 expression potentiates intrinsic resistance in prostate cancer via EMT and DNA repair pathways. Life Sci. 2021;286:120031. doi: 10.1016/j.lfs.2021.120031. [DOI] [PubMed] [Google Scholar]
- Ran L, et al. Antitumor effects of pollen polysaccharides from Chinese wolfberry on DU145 cells via the PI3K/AKT pathway in vitro and in vivo. Int J Biol Macromol. 2020;152:1164–1173. doi: 10.1016/j.ijbiomac.2019.10.206. [DOI] [PubMed] [Google Scholar]
- Rhodes N et al (2008) Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res 68(7):2366–2374 [DOI] [PubMed]
- Sachan R, et al. Afrocyclamin A, a triterpene saponin, induces apoptosis and autophagic cell death via the PI3K/Akt/mTOR pathway in human prostate cancer cells. Phytomedicine. 2018;51:139–150. doi: 10.1016/j.phymed.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Sampath D et al (2013) Phase I clinical, pharmacokinetic, and pharmacodynamic study of the Akt-inhibitor triciribine phosphate monohydrate in patients with advanced hematologic malignancies. Leuk Res 37(11):1461–1467 [DOI] [PMC free article] [PubMed]
- Samuels Y (2006) K.J.C.o.i.o. Ericson, Oncogenic PI3K and its role in cancer. Curr Opin Oncol 18(1):77–82 [DOI] [PubMed]
- Sani IK, Marashi SH, Kalalinia FJTiV (2015) Solamargine inhibits migration and invasion of human hepatocellular carcinoma cells through down-regulation of matrix metalloproteinases 2 and 9 expression and activity. Toxicol In Vitro 29(5):893–900 [DOI] [PubMed]
- Shah S et al (2021) BRCA mutations in prostate cancer: assessment, implications and treatment considerations. Int J Mol Sci 22(23):12628 [DOI] [PMC free article] [PubMed]
- Sharma PK, et al. CCR9 mediates PI3K/AKT-dependent antiapoptotic signals in prostate cancer cells and inhibition of CCR9-CCL25 interaction enhances the cytotoxic effects of etoposide. Int J Cancer. 2010;127(9):2020–2030. doi: 10.1002/ijc.25219. [DOI] [PubMed] [Google Scholar]
- Shay G, Lynch CC. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y et al (2021a) δ-Catenin Participates in EGF/AKT/p21(Waf) Signaling and Induces Prostate Cancer Cell Proliferation and Invasion. Int J Mol Sci 22(10) [DOI] [PMC free article] [PubMed]
- Shen Y, et al. STAT3-YAP/TAZ signaling in endothelial cells promotes tumor angiogenesis. Sci Signal. 2021;14(712):eabj8393. doi: 10.1126/scisignal.abj8393. [DOI] [PubMed] [Google Scholar]
- Sheng D, et al. Scutellaria barbata D.Don (SBD) extracts suppressed tumor growth, metastasis and angiogenesis in Prostate cancer via PI3K/Akt pathway. BMC Complement Med Ther. 2022;22(1):120. doi: 10.1186/s12906-022-03587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H et al (2020) Arctigenin Attenuates Breast Cancer Progression through Decreasing GM-CSF/TSLP/STAT3/β-Catenin Signaling. Int J Mol Sci 21(17) [DOI] [PMC free article] [PubMed]
- Shi Q et al (2022) SPOP mutations promote p62/SQSTM1-dependent autophagy and Nrf2 activation in prostate cancer. Cell Death Differ 29(6):1228-1239 [DOI] [PMC free article] [PubMed]
- Shibue T, Weinberg, RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. 14(10):611–629 [DOI] [PMC free article] [PubMed]
- Shu X, et al. BCAT1 Activates PI3K/AKT/mTOR Pathway and Contributes to the Angiogenesis and Tumorigenicity of Gastric Cancer. Front Cell Dev Biol. 2021;9:659260. doi: 10.3389/fcell.2021.659260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30 [DOI] [PubMed]
- Singh SK et al (2019) Docetaxel Combined with Thymoquinone Induces Apoptosis in Prostate Cancer Cells via Inhibition of the PI3K/AKT Signaling Pathway. Cancers (Basel) 11(9) [DOI] [PMC free article] [PubMed]
- Soleymani L et al (2021) Role of ZEB family members in proliferation, metastasis, and chemoresistance of prostate cancer cells: Revealing signaling networks. Curr Cancer Drug Targets 21(9):749–767 [DOI] [PubMed]
- Su Y, et al. FOXA1 promotes prostate cancer angiogenesis by inducing multiple pro-angiogenic factors expression. J Cancer Res Clin Oncol. 2021;147(11):3225–3243. doi: 10.1007/s00432-021-03730-3. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Arctigenin Inhibits Liver Cancer Tumorigenesis by Inhibiting Gankyrin Expression via C/EBPα and PPARα. Front Pharmacol. 2018;9:268. doi: 10.3389/fphar.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, et al. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene. 2018;664:50–57. doi: 10.1016/j.gene.2018.04.045. [DOI] [PubMed] [Google Scholar]
- Sun W et al (2021a) Long non-coding DANCR targets miR-185-5p to upregulate LIM and SH3 protein 1 promoting prostate cancer via the FAK/PIi>3K/AKT/GSK3β/snail pathway. J Gene Med 23(7):e3344 [DOI] [PubMed]
- Sun BL, et al. Arctigenin Triggers Apoptosis and Autophagy via PI3K/Akt/mTOR Inhibition in PC-3 M Cells. Chem Pharm Bull (Tokyo) 2021;69(5):472–480. doi: 10.1248/cpb.c21-00021. [DOI] [PubMed] [Google Scholar]
- Syn N et al (2016) Exosome-mediated metastasis: from epithelial–mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci 37(7):606–617 [DOI] [PubMed]
- Tan X, et al. Promethazine inhibits proliferation and promotes apoptosis in colorectal cancer cells by suppressing the PI3K/AKT pathway. Biomed Pharmacother. 2021;143:112174. doi: 10.1016/j.biopha.2021.112174. [DOI] [PubMed] [Google Scholar]
- Tang Z, et al. ATR Inhibition Induces CDK1-SPOP Signaling and Enhances Anti-PD-L1 Cytotoxicity in Prostate Cancer. Clin Cancer Res. 2021;27(17):4898–4909. doi: 10.1158/1078-0432.CCR-21-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D et al (2019) Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Seminars in cancer biology. Elsevier [DOI] [PubMed]
- Thabet NA, et al. Thymoquinone chemosensitizes human colorectal cancer cells to imatinib via uptake/efflux genes modulation. Clin Exp Pharmacol Physiol. 2021;48(6):911–920. doi: 10.1111/1440-1681.13476. [DOI] [PubMed] [Google Scholar]
- Thulin MH, et al. Inhibition of STAT3 prevents bone metastatic progression of prostate cancer in vivo. Prostate. 2021;81(8):452–462. doi: 10.1002/pros.24125. [DOI] [PubMed] [Google Scholar]
- Torrealba N, et al. TGF-β/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male. 2020;23(5):801–811. doi: 10.1080/13685538.2019.1597840. [DOI] [PubMed] [Google Scholar]
- Trinh NT et al (2022) Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway. Pharmaceuticals (Basel) 15(3) [DOI] [PMC free article] [PubMed]
- Umar SM, et al. Quercetin Impairs HuR-Driven Progression and Migration of Triple Negative Breast Cancer (TNBC) Cells. Nutr Cancer. 2022;74(4):1497–1510. doi: 10.1080/01635581.2021.1952628. [DOI] [PubMed] [Google Scholar]
- Van de Sande T, et al. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol. 2005;206(2):214–219. doi: 10.1002/path.1760. [DOI] [PubMed] [Google Scholar]
- Visvader JE. and G.J.J.N.r.c. Lindeman. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. MiR-4638-5p inhibits castration resistance of prostate cancer through repressing Kidins220 expression and PI3K/AKT pathway activity. Oncotarget. 2016;7(30):47444–47464. doi: 10.18632/oncotarget.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, et al. Arctigenin inhibits prostate tumor cell growth in vitro and in vivo. Clin Nutr Exp. 2017;13:1–11. doi: 10.1016/j.yclnex.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Oncolytic Herpes Simplex Virus and PI3K Inhibitor BKM120 Synergize to Promote Killing of Prostate Cancer Stem-like Cells. Mol Ther Oncolytics. 2019;13:58–66. doi: 10.1016/j.omto.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, et al. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin Transl Med. 2021;11(8):e522. doi: 10.1002/ctm2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, et al. GRSF1 promotes tumorigenesis and EMT-mediated metastasis through PI3K/AKT pathway in gastric cancer. Biochem Biophys Res Commun. 2021;555:61–66. doi: 10.1016/j.bbrc.2021.03.121. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. HNRNPC Promotes Proliferation, Metastasis and Predicts Prognosis in Prostate Cancer. Cancer Manag Res. 2021;13:7263–7276. doi: 10.2147/CMAR.S330713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. CKB inhibits epithelial-mesenchymal transition and prostate cancer progression by sequestering and inhibiting AKT activation. Neoplasia. 2021;23(11):1147–1165. doi: 10.1016/j.neo.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chen Q, Xu H. Wnt/β-catenin signal transduction pathway in prostate cancer and associated drug resistance. Discov Oncol. 2021;12(1):40. doi: 10.1007/s12672-021-00433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Silencing miRNA-1297 suppresses the invasion and migration of prostate cancer cells via targeting modulation of PTEN and blocking of the AKT/ERK pathway. Exp Ther Med. 2021;22(1):768. doi: 10.3892/etm.2021.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q et al (2022a) Overexpression of GATA5 Inhibits Prostate Cancer Progression by Regulating PLAGL2 via the FAK/PI3K/AKT Pathway. Cancers (Basel) 14(9) [DOI] [PMC free article] [PubMed]
- Wang Y, et al. Quercetin Antagonizes Esophagus Cancer by Modulating miR-1-3p/TAGLN2 Pathway-Dependent Growth and Metastasis. Nutr Cancer. 2022;74(5):1872–1881. doi: 10.1080/01635581.2021.1972125. [DOI] [PubMed] [Google Scholar]
- Wilson S, et al. Resveratrol enhances polyubiquitination-mediated ARV7 degradation in prostate cancer cells. Oncotarget. 2017;8(33):54683–54693. doi: 10.18632/oncotarget.18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MM et al (2020) Emerging mechanisms by which EMT programs control stemness. Trends Cancer 6(9):775–780 [DOI] [PubMed]
- Won YS, Seo KI (2020) Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells. Nutrients 12(2) [DOI] [PMC free article] [PubMed]
- Wu X, et al. Royleanone diterpenoid exhibits potent anticancer effects in LNCaP human prostate carcinoma cells by inducing mitochondrial mediated apoptosis, cell cycle arrest, suppression of cell migration and downregulation of mTOR/PI3K/AKT signalling pathway. J buon. 2018;23(4):1055–1060. [PubMed] [Google Scholar]
- Wu Y, et al. Low-frequency ultrasound enhances chemotherapy sensitivity and induces autophagy in PTX-resistant PC-3 cells via the endoplasmic reticulum stress-mediated PI3K/Akt/mTOR signaling pathway. Onco Targets Ther. 2018;11:5621–5630. doi: 10.2147/OTT.S176744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. PI3K/AKT/mTOR pathway-related long non-coding RNAs: roles and mechanisms in hepatocellular carcinoma. Pharmacol Res. 2020;160:105195. doi: 10.1016/j.phrs.2020.105195. [DOI] [PubMed] [Google Scholar]
- Wu J, et al. Development of human prostate cancer stem cells involves epigenomic alteration and PI3K/AKT pathway activation. Exp Hematol Oncol. 2020;9:12. doi: 10.1186/s40164-020-00168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZM, et al. SMARCC1 Suppresses Tumor Progression by Inhibiting the PI3K/AKT Signaling Pathway in Prostate Cancer. Front Cell Dev Biol. 2021;9:678967. doi: 10.3389/fcell.2021.678967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie N et al (2015) The expression of glucocorticoid receptor is negatively regulated by active androgen receptor signaling in prostate tumors. Int J Cancer 136(4):E27–E38 [DOI] [PubMed]
- Xing P et al (2021) Knockdown of lncRNA MIR4435–2HG and ST8SIA1 expression inhibits the proliferation, invasion and migration of prostate cancer cells in vitro and in vivo by blocking the activation of the FAK/AKT/β–catenin signaling pathway. Int J Mol Med 47(6) [DOI] [PMC free article] [PubMed]
- Xu F, et al. HOXD13 suppresses prostate cancer metastasis and BMP4-induced epithelial-mesenchymal transition by inhibiting SMAD1. Int J Cancer. 2021;148(12):3060–3070. doi: 10.1002/ijc.33494. [DOI] [PubMed] [Google Scholar]
- Xu Y et al (2021b) Erianin induces triple-negative breast cancer cells apoptosis by activating PI3K/Akt pathway. Biosci Rep 41(6) [DOI] [PMC free article] [PubMed]
- Xu B, et al. [EEFSEC knockdown inhibits proliferation, migration and invasion of prostate cancer cells in vitro] Nan Fang Yi Ke Da Xue Xue Bao. 2021;41(12):1787–1794. doi: 10.12122/j.issn.1673-4254.2021.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, et al. Endothelial deletion of SHP2 suppresses tumor angiogenesis and promotes vascular normalization. Nat Commun. 2021;12(1):6310. doi: 10.1038/s41467-021-26697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, et al. Cabazitaxel suppresses the proliferation and promotes the apoptosis and radiosensitivity of castration-resistant prostate cancer cells by inhibiting PI3K/AKT pathway. Am J Transl Res. 2022;14(1):166–181. [PMC free article] [PubMed] [Google Scholar]
- Xue C et al (2021) Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduct Target Ther 6(1):400 [DOI] [PMC free article] [PubMed]
- Yamaura M et al (2009) NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res 69(6):2647–2654 [DOI] [PubMed]
- Yang J, et al. Sirt6 promotes tumorigenesis and drug resistance of diffuse large B-cell lymphoma by mediating PI3K/Akt signaling. J Exp Clin Cancer Res. 2020;39(1):142. doi: 10.1186/s13046-020-01623-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. Transduct Target Ther. 2020;5(1):1–35. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B et al (2021a) Circular RNA circ_001422 promotes the progression and metastasis of osteosarcoma via the miR-195-5pi>/FGF2/PI3K/Akt axis. J Exp Clin Cancer Res 40(1):235 [DOI] [PMC free article] [PubMed]
- Yang G, et al. lncRNA ADAMTS9-AS1 promotes bladder cancer cell invasion, migration, and inhibits apoptosis and autophagy through PI3K/AKT/mTOR signaling pathway. Int J Biochem Cell Biol. 2021;140:106069. doi: 10.1016/j.biocel.2021.106069. [DOI] [PubMed] [Google Scholar]
- Yang MH, et al. Daidzin targets epithelial-to-mesenchymal transition process by attenuating manganese superoxide dismutase expression and PI3K/Akt/mTOR activation in tumor cells. Life Sci. 2022;295:120395. doi: 10.1016/j.lfs.2022.120395. [DOI] [PubMed] [Google Scholar]
- Ye J, et al. Breviscapine suppresses the growth and metastasis of prostate cancer through regulating PAQR4-mediated PI3K/Akt pathway. Biomed Pharmacother. 2020;127:110223. doi: 10.1016/j.biopha.2020.110223. [DOI] [PubMed] [Google Scholar]
- Ye M, et al. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/AKT signaling pathway in LNCaP prostate cancer cells. Oncol Lett. 2020;20(5):169. doi: 10.3892/ol.2020.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7(7):1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Li G, Sun B. Overexpression of LINC00852 promotes prostate cancer cell proliferation and metastasis. Asia Pac J Clin Oncol. 2021;17(6):435–441. doi: 10.1111/ajco.13418. [DOI] [PubMed] [Google Scholar]
- You LN, et al. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021;28(6):719–736. doi: 10.1038/s41417-020-00269-2. [DOI] [PubMed] [Google Scholar]
- Yu YZ et al (2022) Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer 21(1):12 [DOI] [PMC free article] [PubMed]
- Yun S et al (2021) Acacetin Inhibits the Growth of STAT3-Activated DU145 Prostate Cancer Cells by Directly Binding to Signal Transducer and Activator of Transcription 3 (STAT3). Molecules 26(20) [DOI] [PMC free article] [PubMed]
- Zaorsky NG, et al. Nat Rev Urol. 2021;18(11):643–668. doi: 10.1038/s41585-021-00497-7. [DOI] [PubMed] [Google Scholar]
- Zeng Q, et al. Britanin Exhibits Potential Inhibitory Activity on Human Prostate Cancer Cell Lines Through PI3K/Akt/NF-κB Signaling Pathways. Planta Med. 2020;86(18):1401–1410. doi: 10.1055/a-1211-4656. [DOI] [PubMed] [Google Scholar]
- Zhan K, et al. Fetuin B overexpression suppresses proliferation, migration, and invasion in prostate cancer by inhibiting the PI3K/AKT signaling pathway. Biomed Pharmacother. 2020;131:110689. doi: 10.1016/j.biopha.2020.110689. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. [Chinese medicinal compound CFF-1 induces the apoptosis and cycle-arrest of prostate cancer cells via the PI3K/AKT/FOXO1 signaling pathway] Zhonghua Nan Ke Xue. 2017;23(9):828–837. [PubMed] [Google Scholar]
- Zhang J, et al. Rapamycin Antagonizes BCRP-Mediated Drug Resistance Through the PI3K/Akt/mTOR Signaling Pathway in mPRα-Positive Breast Cancer. Front Oncol. 2021;11:608570. doi: 10.3389/fonc.2021.608570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, et al. LncRNA PCBP1-AS1-mediated AR/AR-V7 deubiquitination enhances prostate cancer enzalutamide resistance. Cell Death Dis. 2021;12(10):856. doi: 10.1038/s41419-021-04144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H et al (2021c) [Betaine induces apoptosis of C4-2B prostate cancer cells via inhibiting PI3K/AKT/NF-κB signaling pathway]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 37(6):513–519 [PubMed]
- Zhong H, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- Zhou S, et al. MET inhibition enhances PARP inhibitor efficacy in castration-resistant prostate cancer by suppressing the ATM/ATR and PI3K/AKT pathways. J Cell Mol Med. 2021;25(24):11157–11169. doi: 10.1111/jcmm.17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WB, Xiao N, Liu XJ. Dietary flavonoid tangeretin induces reprogramming of epithelial to mesenchymal transition in prostate cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Oncol Lett. 2018;15(1):433–440. doi: 10.3892/ol.2017.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, et al. Dihydroartemisinin suppresses glycolysis of LNCaP cells by inhibiting PI3K/AKT pathway and downregulating HIF-1α expression. Life Sci. 2019;233:116730. doi: 10.1016/j.lfs.2019.116730. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. Pinocembrin Inhibits the Proliferation and Metastasis of Breast Cancer via Suppression of the PI3K/AKT Signaling Pathway. Front Oncol. 2021;11:661184. doi: 10.3389/fonc.2021.661184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, et al. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021;286:120046. doi: 10.1016/j.lfs.2021.120046. [DOI] [PubMed] [Google Scholar]
- Zhu L, et al. PCDHB17P/miR-145-3p/MELK/NF-κB Feedback Loop Promotes Metastasis and Angiogenesis of Breast Cancer. Front Oncol. 2021;11:660307. doi: 10.3389/fonc.2021.660307. [DOI] [PMC free article] [PubMed] [Google Scholar]