Abstract
CREB-regulated transcription coactivator2 (CRTC2 or TORC2) is a transcriptional coactivator of CREB(cAMP response element binding protein), which affects human energy metabolism through cyclic adenosine phosphate pathway, Mammalian target of rapamycin (mTOR) pathway, Sterol regulatory element binding protein 1(SREBP1), Sterol regulatory element binding protein 2 (SREBP2) and other substances Current studies on CRTC2 mainly focus on glucose and lipid metabolism, relevant studies show that CRTC2 can participate in the occurrence and development of related diseases by affecting metabolic homeostasis. It has been found that Crtc2 acts as a signaling regulator for cAMP and Ca2 + signaling pathways in many cell types, and phosphorylation at ser171 and ser275 can regulate downstream biological functions by controlling CRTC2 shuttling between cytoplasm and nucleus.
Graphical abstract
Keywords: CRTC2, Glucose metabolism, Lipid metabolism
Introduction
CREB is a gluconeogenic transcription factor. In 2003, Vadim Lourenco et al. discovered a eukaryotic protein family that regulates the activity of CREB transcription factor by using genomic high-throughput screening technology for the first time (Iourgenko et al. 2003), namely CRTCs family. In mammals, there are three homologous subtypes, namely CRTC1, CRTC2, and CRTC3. All of them have highly conserved crimp-helix domains at the N-terminal in the form of highly homologous subtypes, which can bind to the alkaline Leucine zipper domain (bZIP) of CREB (Conkright et al. 2003). CRTC1 is highly expressed in the brain. CRTC2 and CRTC3 are generally expressed in tissues, of which CRTC2 is mainly distributed in muscle and liver, while CRTC3 is found in lymphocytes and lung tissues (Wang et al. 2008; Mair et al. 2011), rich in white and brown fat (Song, Altarejos et al. 2010). Three members of The CRTC family have similar module structures: they all contain an N-terminal CREB binding domain (CBD), a central regulatory domain (REG), a splicing domain (SD), and a C-terminal transactivation domain (TAD) (Altarejos and Montminy 2011). CRTC, as a CREB-regulated transcription coactivator, plays an important role in CREB-mediated transcriptional activation. CRTC is transferred from the cytoplasm to the nucleus when stimulated, and cAMP response CREB complexes on the element (CRE) promoters together activate the transcription of target genes. When DNA contains CREB, CRTC, and CREB form a 2:2 complex, CRTC interacts with CREB and DNA with its highly conserved residues (Song et al. 2018). CRTC1, as a transcription coactivator of CREB1, activates transcription through the consistent and variant cAMP response element (CRE) sites, As a coactivator of the SIK/TORC signaling pathway, activated during dephosphorylation and acting independently of CREB1 'Ser-133' phosphorylation (Sakamoto et al. 2013). CRTC3 is highly expressed in adipose tissue and is associated with insulin resistance and energy metabolism (Song, Altarejos et al. 2010). It can also promote obesity by weakening the beta-adrenergic receptor signaling pathway in fat (Song, Altarejos et al. 2010), it also plays an important role in rotenone-induced mitochondrial biogenesis (Than et al. 2011). The principal affinity determinants for CREB binding reside within the N-terminal 55 residues of CRTC2 and palindromic, variant, and half-site CREs can support moderate-affinity interactions between CREB and CRTC2. Collectively, these results demonstrate that Cys300 and Cys310 modulate CREB activity in part through opposing effects on CRTC2 recruitment (Mair, Morantte I Fau—Rodrigues et al. 2011).
CRTC2 acts as a signal regulator of cAMP and Ca2 + signaling pathways in many cell types (Screaton et al. 2004; Ch'ng et al. 2012). At present, there are many studies on CRTC2. Studies have found phosphorylation at ser 171 and ser 275. And controlled the shuttle between the cytoplasm and nucleus of CRTC2 (Horton et al. 2002; Peterson et al. 2011) Whether CRTC2 shuttles smoothly is crucial for the next biological function. CRTC2, as a transcription coactivator regulated by CREB, plays an important role in CREB-mediated transcriptional activation. In this paper, the functions of CRTC2 and its related diseases are reviewed, to provide a reference for future work.
| Genetic name | Protein name | 3d structure diagram of protein | Highly expressed site | The main physiological function | References |
|---|---|---|---|---|---|
| CRTC1 | CREB-regulated transcription coactivator 1 | Figure 1 | Brain | Involvement in depression and comorbid obesity | (Wu et al. 2006) |
| (Rossetti, Cherix et al. 2022) | |||||
| CRTC2 | CREB-regulated transcription coactivator 2 | Figure 2 | Lung | Energy metabolism | (Wu et al. 2006) |
| Muscle | |||||
| Spleen | |||||
| (Han et al. 2020) | |||||
| Ovary | |||||
| CRTC3 | CREB-regulated transcription coactivator 3 | Figure 3 | Lung | Insulin resistance and energy metabolism |
(Wu et al. 2006) (Song, |
| Ovary | |||||
| Heart | |||||
| Colon | Altarejos et al. 2010) | ||||
| Brain |
Fig. 1.
3d structure diagram of protein CRTC1.
Source: Figure to: https://www.uniprot.org/
Fig. 2.

3d structure diagram of protein CRTC2.
Source: Figure to: https://www.uniprot.org/
Fig. 3.
3d structure diagram of protein CRTC3.
Source: Figure to: https://www.uniprot.org/
Effect of CRTC2 on glucose metabolism
CRTC2 indirectly affects glucose metabolism by regulating cAMP
CRTC2, also known as cAMP response element binding protein 2, is an important regulator of glucose metabolism. In 2008, Renaud Dentin et al. discovered o-glycosyl transferase (OGT). O-glycosylation of CRTC2 transducers triggers hepatic gluconeogenesis (Dentin et al. 2008). During fasting or endoplasmic network stress, CRTC2 acts as a transcription coactivator and binds to different substances to regulate gluconeogenesis. During fasting, glucagon produced by islet activates the camp-dependent protein kinase (PKA) signaling pathway in the liver, up-regulates key enzyme genes such as phosphoenolpyruvate carboxykinase (PEPCK) and promotes gluconeogenesis in the liver (Meisner, Lamers et al. 1982, Pilkis et al. 1988, Gonzalez and Montminy 1989, Pilkis and Claus 1991, Herzig et al. 2001, Montminy et al. 2004, Koo et al. 2005). When cAMP concentration is too high due to external stimulation, high cAMP inhibits the activity of salt-Inducible kinase 2 (SIK2), dephosphorylates CRTC2, and blocks CRTC2:14–3-3 The complex activity, in turn, inhibits the extranuclear output of CRTC2, leading to the decrease of gluconeogenesis (Pilkis and Claus 1991; Screaton et al. 2004). Chandra E. Eberhard et al. found that CRTC2 is closely related to the function and proliferation of islet β cells. In β cells, glucose and cAMP signaling induces crTC2-CREb activation and dephosphorylation of CRTC2 in response to glucose and calcium-dependent phosphatase stimulation (Eberhard et al. 2013). Thereby reducing the gluconeogenesis process.
Dephosphorylation of CRTC2 affects carbohydrate metabolism
Ca2+ has been found to promote the dephosphorylation of CRTC2 by activating the calmodulin-dependent phosphatase pathway (Screaton et al. 2004), CAMP signaling can also activate CRTC2 activity through the dephosphorylation of Serine/threonine protein phosphatase 3 CA or protein phosphatase 4 Ser171, resulting in its nuclear localization and increased association with CREB on the gluconeogenesis promoter (Montminy et al. 2004; Eberhard et al. 2013), thus affecting the process of gluconeogenesis. Glucagon secreted during fasting can activate calcium/calmodulin-dependent serine/threonine phosphatase by activating intracellular calcium storage and calmodulin /protein phosphatase 2B(PP2B) stimulates the dephosphorylation of CRTC2 in hepatocytes and also enhances the expression of gluconeogenic genes through the phosphorylation of Inositol 1,4, 5-triphosphate receptor (InsP3Rs) mediated by PKA to increase cytoplasmic calcium, which in turn mediates the dephosphorylation of CRTC2 (Wang et al. 2012). Phosphorylation/dephosphorylation is not the only way to turn on transcriptional activity. Studies have shown that acetylation and ubiquitination can also regulate intracellular CRTC2 activity (Liu et al. 2008; Rui 2014). For example, CRTC2 responds to glucagon translocation and is dephosphorylated by CREB Binding protein/P300 (CBP/ P300) acetylation at Ly628. However, this locus is inhibited by Constitutively photomorphogenic 1(COP1) ubiquitin modification, which increases the stability of CRTC2 (Liu et al. 2008).CRTC2 can promote hepatic amino acid catabolism to regulate glucose homeostasis Upon glucagon activation, CRTC2 can improve the availability of amino acid-derived gluconeogenic substrates, thereby increasing hepatic glucose output. CRTC2 inhibits glucagon-induced CRTC2 activation by shuttling amino acids to gluconeogenesis and reducing amino acid stimulation of pancreatic glucagon secretion.
Effect of CRTC2 on lipid metabolism
CRTC2 affects lipid metabolism through SREBP1
Sterol regulatory element binding proteins (SREBPs, including SREBP1a, SREBP1c, and SREBP2) are basic-helix-loop-helix-leucine zipper (BHLH-ZIP) transcription factors that regulate the synthesis of cholesterol and fatty acids, two components of cell membranes, as well as cellular uptake.SREBP1c primarily drives fatty acid synthesis in the liver, while SREBP1a drives both pathways in all tissues(Vergnes et al. 2016). SREBP1 is an important transcriptional regulator regulating lipid synthesis. It exists in the endoplasmic reticulum as an inactive precursor under basic conditions. After activation of an insulin signaling pathway, SREBP1 is transported from the endoplasmic reticulum to the Golgi apparatus in a COPII-dependent manner and is processed and cleaved by proteases. The mature N-SREBP1 enters the nucleus to induce the expression of genes related to lipid synthesis. In August 2015, The study of Jinbo Han et al. showed that CRTC2 regulates the processing of SREBP1 dependent on the Coat Protein Complex II (COP II) as an intermediary of mTOR signaling pathway. Two subunits of the COP complex II, Sec23A, and Sec31A, play important roles in lipid synthesis. CRTC2 competes with Sec23A, interacts with Sec31A, and interferes with the transport of SREBP1 (Han, Li et al. 2015), thus affecting lipid synthesis. In 2022, it was found that Ilexgenin A can activate AMPK and inhibit the transport of CRTC2 to the cytoplasm, preventing the maturation of SREBP1 by damaging the function of liver COP (Lu et al. 2022), and further reducing lipid synthesis.
CRTC2 affects lipid metabolism through SREBP2
Sterol regulatory element binding protein 2(SREBP2) and two rate-limiting enzymes, HMGCR and SM, are key regulators of cholesterol synthesis. SREBP2 is first synthesized in the endoplasmic reticulum as an N-terminal domain containing a basic helix-loop-helix-leucine zipper.
When the endoplasmic reticulum cholesterol concentration is depleted, SREBP-cleavage activating protein (SCAP) protein conformation is closed, making the MELADL sequence recognized by COPII vesicles. The SCAP-SREBP complex is thus cleaved from the endoplasmic neurites to the Golgi apparatus, where it is cleaved by site 1 protease(S1P) and Site 2 Protease (S2P) to release transcriptionally active N-terminal entryway (nSREBP2), thereby activating a series of downstream genes (Luo et al. 2020). In 2017, Li Y et al. demonstrated that sterol regulatory element binding protein 2(SREBP-2) participates in the mechanism of CRTC2 enhancing liver 3-hydroxyglutaryl-CoA reductase (HMGCR). CRTC2 interferes with inositol-requiring of the SREBP2 promoter by the Forkhead box Protein O1 (FOXO1) The recognition of enzyme-1(IRE1) induces the SREBP-2/HMGCR signaling pathway and further promotes the synthesis of cholesterol in the liver to regulate the transcription of SREBP-2 (Madison 2016).
CRTC2 affects lipid metabolism by regulating autophagy
The transcription factor EB (TFEB) is a member of the Basic helix-loop-Helix Leucine-Zipper (BHLH-Zip) transcription factor (MIT family) (Li et al, 2017a, b). Studies have shown that TFEB plays an important role in organelle biogenesis and metabolism.TFEB has been shown to bind with a lot of autophagy gene promoter regions, induce autophagy occurs and autophagic lysosome fusion, TFEB expression can lead to most autophagy substrate degradation and increase protein (such as long life protein) (Steingrimsson et al. 2004), and lipid droplets and the removal of the damaged mitochondria (Settembre, Di Malta et al. 2011). The transcription factors in regulating cell organelle specificity plays a role in autophagy, such as lipid and mitochondrial autophagy. These results suggest that the transcription factor also plays a role in regulating organelle-specific autophagy, such as lipid and mitochondrial autophagy. TFEB has also been found to induce lysosomal extracellular interactions (Nezich et al. 2015). Studies have shown that TFREB is a key activator of autophagy/lysosomal genes, CREB can regulate lipophagy and other processes by regulating the expression of TFREB (Steingrimsson et al. 2004; Medina et al. 2011), And then regulate lipid autophagy and other processes. In 2014, Sunmi Seok et al. identified Farnesoid X Receptor(FXR) and CREB as transcriptional regulators of autophagy gene network through experiments, In terms of mechanism, REB up-regulates autophagy genes by recruiting coactivator CRTC2, such as Atg7, Ulk1, and Tfeb (Settembre, De Cegli et al. 2013), to promote lipophagy and other processes. Previous studies have shown that mTOR inhibits autophagy by inhibiting TFEB (Herzig and Shaw 2018). At the same time, CRTC2 is closely related to some autophagy factors. At present, the research on CRTC2 is mainly in metabolism, and how CRTC2 regulates autophagy still needs to be explored.
CRTC2 and metabolism-related diseases
In the body, the stability and balance of cellular metabolism levels are crucial. Genetic mutations or the functional impact of major digestive organs such as the pancreas and liver or the environment with important metabolic functions can cause metabolic diseases.
Diabetes
In healthy people, blood sugar levels are stabilized by a balance between glucose consumption and glucose production in peripheral tissues, of which about 90% occurs in the liver, so stable and appropriate blood glucose concentration is essential for body function. One-time hyperglycemia (such as after meals and exercise, etc.) will not cause serious harm to the human body, but long-term hyperglycemia can lead to pathological changes in body tissues and organs. Glucose production in the liver is considered a first-line target for the treatment of diabetes (Rines et al. 2016). Type 2 diabetes is often accompanied by other comorbidities that lead to death from many chronic health conditions, including cardiovascular disease, stroke, and kidney disease (Forbes and Cooper 2013). Therefore, blood glucose control is very important.
CRTC2 can affect blood sugar. In 2018, Xiang Zhu et al. found that glucagon-treated primary hepatocytes of mice with maternally expressed gene 3(MEG3) knocked out reduced gluconeogenic gene expression. Subsequent experiments showed that MEG3 acts as a ceRNA in primary hepatocytes, CRTC2 is upregulated by targeted Mir-302A-3p, Meg3-mediated pyrolysis chromatography 1α Phosphoenolpyruvate carboxykinase (1αPEPCK), on the other hand, regulates Mir-302A-3p to achieve the CRTC2 axis of hepatic gluconeogenesis in the G6PC pathway(Zhu et al. 2019). Therefore, CRTC2 can be affected by MEG3 to regulate gluconeogenesis, and Glp-1 can regulate blood glucose levels by affecting (pro-) insulin synthesis, inducing insulin secretion, and inhibiting glucagon secretion (Mueller et al. 2019). In 2018, the experimental data of Ji-Hyun Lee et al. showed that glucagon-like peptide 1(GLP-1) secreted by L cells is dependent on the CREB/CRTC2 pathway, and the CREB/CRTC2 pathway has a regulatory effect on oxidative phosphorylation and cellular respiration of L cells. Animal models show that small intestinal CRTC2-knockout mice show impaired glucose metabolism, thus concluding that the CREB/ CrTC2-dependent transcription pathway plays an important role in regulating glucose homeostasis through the production of GLP-1 by L cells at the levels of transcription, maturation, and extracellular action (Lee et al. 2018).
In, Chang Wang et al (2020). found through experiments that salt-inducible kinase 1 (SIK1) can regulate the CRTC2-mediated gluconeogenesis pathway in both physiological and pathological conditions induced by high glucose (Wang, Song et al. 2020). Thyroid-stimulating hormone (TSH) regulates hepatic gluconeogenic enzymes through CREB: CRTC2 complex promotes hepatic gluconeogenic expression through dephosphorylation and regulates hepatic CRTC2 through TSHR/cAMP/PKA pathway (Li, Wang et al. 2017b). It further affects gluconeogenesis. In 2021, Bruno NE et al. found that mice with CRTC2 overexpression selectively reduced fat content and overeating during alternate-day fasting (ADF). Through a series of studies, CRTC2 can regulate mTOR signaling pathway transduction and protein synthesis attenuates the effects of fasting on mitochondrial function, and can reverse the catabolic effect of glucocorticoids on skeletal muscle and improve the metabolism of glucose-tolerant carbohydrates (Bruno, Nwachukwu et al. 2021).
Suppressor of MEK NULL (SMEK) overexpression can increase hepatic gluconeogenic gene expression and promote blood glucose elevation, deletion of SMEK protein can reduce hyperglycemia and enhance CRTC2 phosphorylation on the other hand. Therefore, it was suggested that the Suppressor of MEK NULL (SMEK)/ Protein Phosphatase 4 Catalytic Subunit (PP4C) protein was involved in the regulation of liver glucose metabolism through dephosphorylation of CRTC2(Yoon et al. 2010). Small heterodimer Partner (SHP) can inhibit crTC2-mediated CREB transcriptional activity and inhibit the binding of CRTC2 and CREB. It also inhibits the activity of phosphoenolpyruvate carboxykinase (PEPCK) gene promoter by regulating CREB-Previvor. The existing clinical drug metformin can inhibit hepatic gluconeogenesis mediated by CRTC2 by inducing the expression of the SHP gene, to achieve the purpose of lowering blood glucose (Lee et al. 2010). Similarly, studies have shown that Pin can bind and induce the translocation of CRTC2 to the cytosol, which can inhibit the transcriptional activity of cAMP response elements and thus inhibit glucose metabolism. In the liver, insulin can lead to 14-3-3 protein association and nuclear rejection and degradation of CRTC2 by phosphorylation of serine at CRTC2 171. However, glucagon can induce dephosphorylation and translocation of CRTC2 from cytoplasm to nucleus, forming CREBCBPCRTC2 complex and inducing gluconeogenesis (Nakatsu et al. 2010). In 2021, a novel hepatic gluconeogenesis regulator, Sam68, was found to be upregulated in diabetic patients and two diabetic mouse models. Subsequent studies showed that Sam68 can interact with CRTC2 to inhibit CRTC2 degradation and maintain CRTC2 protein levels to enhance glucagon signaling, thereby promoting gluconeogenesis (Qiao, Zhou et al. 2021). In 2022, Brazilian green propolis was found to reduce fasting glucose levels in obese mice by disrupting the formation of the CREB/CRTC2 transcription complex, further studies showed that artemisinin C (APC) in propolis was an inhibitor of CREB-CRTC2 interaction, and APC was not toxic, studies have shown that CREB/CRTC2 is a suitable target for anti-metabolic syndrome drugs, but whether CREB/CRTC2 can be a target for the treatment of hyperglycemia remains to be studied (Chen, Wang et al. 2022). In high-fat-fed mice, elevated P300 protein levels increased gluconeogenic gene expression by increasing CRTC3 protein levels (He 2021). In the presence of fasting, the body stimulates the release of glucagon to maintain blood sugar stability, and increased glucagon signaling dephosphorylates CRTC2 (Luo et al. 2012). CRTC2 translocates from the cytoplasm to the nucleus, where it binds to phosphorylated CREB and activates the transcription of the gluconeogenic regulator PPARγ coactivator 1α (PGC-1α) and two enzymes that catalyze the gluconeogenic rate-rate-stepping step: phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (Dentin et al. 2008; Wang et al. 2012). When eating is resumed, the body modulates the insulin pathway to promote ubiquitination and degradation of CRTC2, thus terminating the gluconeogenesis process (Dentin, Liu et al. 2007). Therefore, strategies targeting CRTC2, CREB/CRTC2 complex, may be effective in the treatment of diabetes.
Diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is one of the major complications of diabetes. At the very beginning, diabetic cardiomyopathy was described as a pathophysiological state of heart failure in the absence of coronary artery disease, hypertension, and heart valvular disease (Jia et al. 2018). In the early stage of diabetic cardiomyopathy, the structure and function of the heart have changed to a certain extent. The conditions causing the changes include impaired insulin metabolic signal, impaired glucose uptake, excessive insulin in the environment, mitochondrial dysfunction, and increased uptake of non-esterified fatty acid (NEFA) in the myocardium (Adeghate and Singh 2014; Jia et al. 2016). MEG3 overexpression has been found in AC16 cells (human cardiomyocytes) induced by high glucose, and the down-regulation of MEG3 can inhibit the apoptosis of AC16 cells induced by high glucose. MEG3 acts as a competitive endogenous RNA (ceRNA) that negatively regulates Mir-145 in human cardiomyocytes treated with high glucose (Chen, Zhang et al. 2019). CRTC2 can be regulated by MEG3 (Zhu et al. 2019), Therefore, it can be speculated that the connection among CREB, MEG3, and CRTC2 may provide a basis for future treatment of DCM. Heart function deteriorated in T2DM mice when autophagy was inhibited with lysosomal inhibitors, whereas enhanced autophagy ameliorated diabetic cardiomyopathy (Wang et al. 2014). In DCM, the activity of the lysosomal proteolytic enzyme decreased, which was associated with the decrease in TFEB expression (Trivedi et al. 2016). TFEB supports the regulation of gene transcription in several stages of autophagy (Puertollano, Ferguson et al. 2018), therefore, CREB can affect TFEB by regulating TFREB expression (Settembre, De Cegli et al. 2013), regulation of autophagy indirectly affects diabetic cardiomyopathy. In a series of experiments, glP-1 (a glucagon-stimulating agent) protects cardiac function by inhibiting the ROCK/PPARα pathway, thereby ameliorating lip toxicity in diabetic cardiomyopathy (Wu, Wang et al. 2018). Glp-1 infusion improved left ventricular ejection fraction and functional status after 5 weeks in diabetic patients with chronic heart failure (Sokos et al. 2006). CRTC2 can reduce the expression of Mir-34A, thus inducing the expression of PPARα in the liver (Han, Choi et al. 2017). Therefore, CRTC2 can indirectly protect cardiac function by up-regulating the expression of PPARα and affecting the generation of GLP-1.
Atherosclerosis
With the development of society, the Lord from the west of atherosclerosis gradually turned into a global disease, research has shown that hyperlipidemia is a major cause of atherosclerosis, hyperlipidemia is characterized by the change of the spectrum, plasma lipoproteins including low high-density lipoprotein cholesterol concentration, high triglyceride levels, and qualitative modified small density and low-density lipoprotein cholesterol (Hdl-c) (Austin et al. 1988).
Vascular endothelial cells are the continuous cell wall in the cardiovascular system and the natural vessel for blood (Gimbrone 1987). One of the important factors in the local and systemic manifestations of atherosclerotic cardiovascular disease (ACVD) is the dysfunction of the endothelium of the great artery wall (Gimbrone and Garcia-Cardena 2013). Experiments have shown that CRTC2 is highly expressed in endothelial cells and is related to the formation of blood vessels. Under the condition of ischemia, CRTC2 can protect the integrity of endothelial cells through P190RhoGAP-a, and the cAMP pathway can also stabilize the endothelial barrier function, to maintain the physiological function of blood vessels (Kanki et al. 2020). The concept that vascular smooth muscle cells (VSMCs) exist in a transitional phase between atherosclerotic smooth muscle and foam cells in humans was first proposed with the help of electron microscopy in 1961(Geer et al. 1961). With the development of The Times, the phenotypic plasticity of vascular smooth muscle cells in atherosclerosis has become a consensus (Miano et al. 2021). It has been shown that phosphorylation at SER133 of CREB and nuclear translocation of CRTC play a dual role in the mitosis of VSMCs. Overexpression of CRTC2 significantly increased CREB activity and inhibited the proliferation of VSMC, while silencing of CRTC2 inhibited CREB activity and reversed the anti-filamentation of adenosine A2B hand agonist splitting (Hudson, Kimura et al. 2018). Atherosclerosis is a chronic inflammatory disease of the artery wall driven and maintained by the proliferation and activation of immune cells (Winkels et al. 2018). Macrophages play an important role in immune cells, both in humans and mice, the classically activated macrophages, namely M1 macrophages, are the first phenotype characterized as pro-inflammatory. High expression of M1 pro-inflammatory protein contributes to plaque growth and instability, while alternately activated macrophages (M2) play a preventive role in the progression of atherosclerosis in humans and mice(Yang et al. 2020). Experimental result demonstrates macrophage CREB/CRTC pathway promotes M2 polarization, therefore, CRTC2, as a subfamily of CRTCs, can prevent the progression of atherosclerosis by promoting M2 polarization. In addition, adipose tissue plays an important role in energy metabolism, excess calories, and lipid release. In diet-induced obesity (DIO), dilated adipose tissue becomes inflamed and dysfunctional. Dysfunctional adipose tissue releases pro-inflammatory adipocytokines and free fatty acids that cause vascular inflammation and oxidative stress, exacerbating atherosclerosis throughout the body (Steinberg and Baron 2002; Kloeting and Blueher 2014). Experiments have found that mTORC1 of adipocytes can activate COX-2/PG signal of adipocytes through CRTC2/COX-2/PG dependent paracrine mechanism to protect cells from diet-induced obesity (Zhang et al. 2018). Subsequent studies showed that knockdown of CRTC2, which acts upstream of NFκB and promotes cytokine gene expression in adipose tissue, improved fat function in obese patients (Yoon, Liu et al. 2021).CRTC2 can regulate the metabolism of glucolipids in the whole body and also reduce the permeability of cells. When the metabolism of glucolipids in the body is disturbed, the content of lipids in the blood vessels will increase and atherosclerosis will occur easily. When the atherosclerotic plaque develops to a certain extent, that is, it leads to the ischemia of the blood vessels. CRTC2-CREB can regulate the transcription of p190RhoGAP-A expression in vascular endothelial cells, resulting in decreased barrier function of endothelial cells, and further aggravate the degree of atherosclerosis.
In conclusion, CRTC2 can promote the polarization of M2, inhibit the proliferation of VSMC, regulate the generation of adipocytes and improve the occurrence and development of atherosclerosis by protecting the integrity of vascular endothelium.
Heart failure
Heart failure (HF) refers to the dysfunction of the systolic function and/or diastolic function of the heart, which cannot fully discharge the venous blood volume from the heart, resulting in blood stasis in the venous system and insufficient blood perfusion in the arterial system, thus causing the metabolic reprogramming of cardiac circulation disorder syndrome. It is the early manifestation of the pathogenesis of heart failure (van Bilsen, van Nieuwenhoven et al. 2009).
Studies have shown that heart failure is associated with hyperlipidemia (Luo et al. 2020). In 2010, Clemens Witten Becher et al. found and validated two lipid metabolites and several lipemic patterns as potential novel biomarkers of heart failure risk. Lipid spectroscopy can capture molecular changes predisposed to heart failure (Wittenbecher et al. 2021). In 2011, Hui-Ying Lim et al. stimulated the Drosophila sterol regulatory element binding protein (dSREBP) pathway by reducing phosphatidylethanolamine (PE) levels to simulate the effects of cholesterol deficiency in vertebrates. The results showed that in EAS2 drosophila, abnormal dSREBP signaling can lead to obesity and heart defects, and both EAS2 and m-DSREBP overexpressed hearts showed structural defects, suggesting that the dysregulation of phospholipid signaling that changes SREBP activity can promote the progression of drosophila heart dysfunction (Lim et al. 2011). Aberrant insulin metabolic signaling also reduces insulin-stimulated coronary endothelial nitric oxide synthase (NO) activity and NO production, increases intracellular Ca2+/ Ca2+ sensitization, and decreases sarcoplasmic Ca2+ uptake, leading to heart failure (Jia et al. 2016).
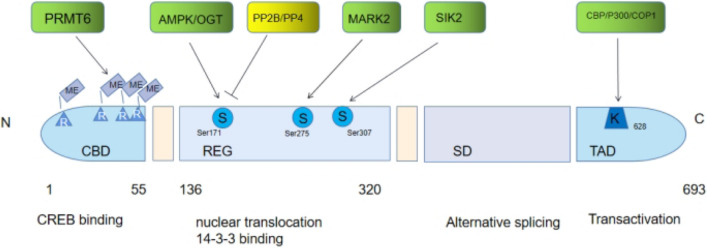
CRTC2 regulates lipid metabolism through SREBP1 and SREBP2. The expression of CRTC2 is high in endothelial cells, and CRTC2 can protect the integrity of endothelial cells through P190RhoGAP-a in the condition of ischemia. The cAMP pathway can also stabilize endothelial barrier function, to maintain vascular physiological function (Kanki et al. 2020). Endothelial cells can regulate myocardial fibrosis, hypertrophy, blood vessel formation, apoptosis, autophagy, and contraction (Marti et al. 2012). CRTC2 can affect the structure and active site of CRTC2 in heart failure through SREBP and its effect on endothelial cells as illustrated in Figure 4.
Fig. 4.
The schematic structure of the CRTC2: CRTC2 is constituted of a CREB-binding domain(CBD), a central regulatory region (REG), a splicing domain (SD), and a transactivation domain (TAD). PRMT6, protein arginine methyltransferase; AMPKadenosine monophosphate-activated protein kinase; OGT, O-linked β-N-acetylglucosamine transferase; PP2B, protein phosphatase; PP4, protein phosphatase 4;MARK2,microtubule affinity-regulating kinase 2;SIK2,salt-inducible kinase 2;CBP,CREB-binding protein;COP1,constitutive photomorphogenic 1; N,amino-terminus;R,arginine;S,serine;K,lysine;C,carboxy-terminus; Me,AS-Dimethylation
With the aggravation of heart failure, the myocardial blood supply gradually decreases. When the myocardium is in a state of ischemia, P190RhoGAP-a is activated, which affects the angiogenesis, reduces the endothelial barrier function, aggravates heart failure, and further decreases the blood supply, forming a vicious cycle (Kanki et al. 2020). Therefore, CRTC2 can affect the structure and active site of CRTC2 in heart failure through SREBP and its effect on endothelial cells. In recent years, the concept of metabolic heart failure has attracted wide attention. CRTC2, as a factor regulating the metabolism of glucose and lipid throughout the body, can lead to the change of metabolic substrate, which may provide convenience for the following research.
The role of CRTC2 in skeletal muscles and muscular disorders
Recent studies have shown that CRTC2 may play a role in skeletal muscle and muscle diseases. After CRTC2 induction, whole animal and skeletal muscle metabolism improved.Crtc2 can also significantly down-regulate myogenin, a major driver of muscle atrophy during fasting. The researchers came to their conclusion after a series of experiments, CRTC2 induced anabolic effects on protein synthesis supporting the retention of muscle mass during the fast.Crtc2 enhanced the expression of ribosomal genes that promote ribosome assembly and genes that control mitochondrial protein quality, such as Spg7, Pink1, RhoT2, and Chchd3. But prevented ADF-dependent downregulation of Minos1, a central component of the inner mitochondrial layer.Crtc2 also upregulated the expression of Tefm, mitochondrial transcription elongation factor, and Bola3, which is involved in the electron transport chain assembly (Bruno, Nwachukwu et al. 2021). Other findings suggest that ER stress and mTOR act synergistically in myotubes to reduce PGC-1α levels through a novel mechanism involving the reduction of CRTC2 nuclear levels (Montori-Grau et al. 2022). Decreased CREB/CRTC signaling contributes to the reduction of PGC-1α expression during muscle atrophy (Kumar, Rahnert et al. 2016). Crtc2 overexpression mice were shown to have increased muscle fiber cross-sectional area and increased intramuscular triglyceride and glycogen content. Furthermore, maximal exercise capacity was enhanced after induction of Crtc2 expression in transgenic mice. In striated muscle, Crtc2 overexpression led to significant increases in the mass of the gastrocnemius and soleus muscle relative to age- and weight-matched Dox-treated wild-type (WT) littermates.Crtc2 reduces metabolic stress and improves the exercise capacity of skeletal muscle (Rahnert et al. 2016).CRTC2 is abundantly expressed in skeletal muscle. When CRTC2 is overexpressed, the expression of genes related to mitochondrial mass is stimulated, the cross-sectional area of muscle fibers in mice is increased, and the metabolic stress is reduced to increase the exercise capacity of mice. In conclusion, the functional roles of CRTC2 in skeletal muscle remain to be elucidated.
Conclusion and prospects
In recent years, the research on the CRTC family has been continuing, and CRTC2, as a member of the CRTC family, has attracted more attention CRTC2 can regulate the metabolism of glucose and lipids through dephosphorylation by regulating cAMP, SREBP1, and SREBP2. CRTC2 can participate in related metabolic processes together with CREB as a CREB/CRTC complex and can also be a member of the pathway to regulate the synthesis of lipids and sugars the regulation of CRTC2 on metabolism can be a target for the treatment of mutability-related diseases. Most of the studies on the regulation of glucolipid metabolism by CRTC2 focus on the translocation of cytoplasm to the nucleus, while few studies on other aspects are conducted. In the future, studies on CRTC2 in other cells, such as cardiomyocytes and monocytes, should be carried out, and not on CRTC2 individuals, such as CREB Studies on the overall role of/CRTC2 complex, as well as studies on CRTC1 and CRTC3 should also continue to explore the role of CRTC2 in metabolism, which is conducive to the prevention and treatment of metabolism-related diseases. The diseases: diabetes, diabetic cardiomyopathy Atherosclerosis, heart failure, skeletal muscles, and muscular disorders mentioned in the article, There is no direct evidence that CRTC2 levels change, but there is a lot of indirect evidence that regulating CRTC2 has an effect on these diseases, or on how these diseases occur. Recently, a kinase selection has shown that Nemo-like Kinase (NLK) inhibits CRTC2-CREB—and FoxO1-dependent gluconeogenesis and phosphorylates CRTC2 to promote proteasome degradation (Ji, Wang et al. 2021). Therefore, the study of the CRTC2 upstream signal is also helpful to provide a new method for the treatment of metabolic diseases.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81670429, 91839103 to ZSJ), International Joint Laboratory for Arteriosclerotic Disease Research of Hunan Province (2018WK4031), Special Funding for Construction of Innovative Provinces in Hunan Province (2020SK2105, to ZSJ) and “Double First-Class” project for innovative Group of Basic Medicine, University of South China (to ZSJ).
Funding
National Natural Science Foundation of China, 81670429, zhisheng jiang, 91839103, zhisheng jiang, International Joint Laboratory for MicroNano Manufacturing and Measurement Technologies, 2018WK4031, zhisheng jiang, Special Funding for Construction of Innovative Provinces in Hunan Province, 2020SK2105, zhisheng jiang, “Double First-Class” project for innovative Group of Basic Medicine, University of South China.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev. 2014;19(1):15–23. doi: 10.1007/s10741-013-9388-5. [DOI] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260(13):1917–1921. doi: 10.1001/jama.1988.03410130125037. [DOI] [PubMed] [Google Scholar]
- Bruno NE, Nwachukwu JC, Hughes DC, Srinivasan S, Hawkins R, Sturgill D, Hager GL, Hurst S, Sheu S-S, Bodine SC, Conkright MD, Nettles KW. Activation of Crtc2/Creb1 in skeletal muscle enhances weight loss during intermittent fasting. Faseb J. 2021 doi: 10.1096/fj.202100171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang Z, Zhu D, Zhao W, Li F. 2019. Long non-coding RNA MEG3 serves as a ceRNA for microRNA-145 to induce apoptosis of AC16 cardiomyocytes under high glucose condition. Biosci Rep. [DOI] [PMC free article] [PubMed]
- Chen Y, Wang J, Wang Y, Wang P, Zhou Z, Wu R, Xu Q, You H, Liu Y, Wang L, Zhou L, Wu Y, Hu L, Liu H, Liu Y. A propolis-derived small molecule ameliorates metabolic syndrome in obese mice by targeting the CREB/CRTC2 transcriptional complex. Nat Commun. 2022 doi: 10.1038/s41467-021-27533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng TH, Uzgil B, Lin P, Avliyakulov NK, O'Dell TJ, Martin KC. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell. 2012;150(1):207–221. doi: 10.1016/j.cell.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12(2):413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo S-H, Hedrick S, Vargas T, Heredia J, Yates J, III, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449(7160):366. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319(5868):1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- Eberhard CE, Fu A, Reeks C, Screaton RA. CRTC2 is required for beta-cell function and proliferation. Endocrinology. 2013;154(7):2308–2317. doi: 10.1210/en.2012-2088. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- Geer JC, McGill HC, Jr, Strong JP. The fine structure of human atherosclerotic lesions. Am J Pathol. 1961;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone MA., Jr Vascular endothelium: nature's blood-compatible container. Ann N Y Acad Sci. 1987;516:5–11. doi: 10.1111/j.1749-6632.1987.tb33025.x. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22(1):9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Han J, Li E, Chen L, Zhang Y, Wei F, Liu J, Deng H, Wang Y. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature. 2015;524(7564):243. doi: 10.1038/nature14557. [DOI] [PubMed] [Google Scholar]
- Han H-S, Choi BH, Kim JS, Kang G, Koo S-H. Hepatic Crtc2 controls whole body energy metabolism via a miR-34a-Fgf21 axis. Nat Commun. 2017 doi: 10.1038/s41467-017-01878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H-S, Kwon Y, Koo S-H. Role of CRTC2 in metabolic homeostasis: key regulator of whole-body energy metabolism? Diabetes Metab J. 2020;44(4):498–508. doi: 10.4093/dmj.2019.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. Alterations of gut microbiota by overnutrition impact gluconeogenic gene expression and insulin signaling. Int J Mol Sci. 2021;22(4):2121. doi: 10.3390/ijms22042121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Investig. 2002;109(9):1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C, Kimura TE, Duggirala A, Sala-Newby GB, Newby AC, Bond M. Dual role of CREB in the regulation of VSMC proliferation: mode of activation determines Pro- or anti-mitogenic function. Sci Rep. 2018 doi: 10.1038/s41598-018-23199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, Orth AP, Miraglia L, Meltzer J, Garza D, Chirn G-W, McWhinnie E, Cohen D, Skelton J, Terry R, Yu Y, Bodian D, Buxton FP, Zhu J, Song C, Labow MA. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci USA. 2003;100(21):12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y-X, Wang Y, Li P-L, Cai L, Wang X-M, Bai L, Liu Z, Tian H, Tian S, Zhang P, Zhang X-J, Cheng X, Yuan Y, She Z-G, Hu Y, Li H. A kinome screen reveals that Nemo-like kinase is a key suppressor of hepatic gluconeogenesis. Cell Metab. 2021;33(6):1171. doi: 10.1016/j.cmet.2021.04.006. [DOI] [PubMed] [Google Scholar]
- Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki H, Sasaki T, Matsumura S, Kawano T, Todo K, Okazaki S, Nishiyama K, Takemori H, Mochizuki H. CREB coactivator CRTC2 Plays a crucial role in endothelial function. J Neurosci. 2020;40(49):9533–9546. doi: 10.1523/JNEUROSCI.0407-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeting N, Blueher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15(4):277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- Koo S-H, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rahnert JA, Zheng B, Hudson MB, Woodworth-Hobbs ME, Price SR. Glucocorticoids Alter CRTC-CREB signaling in muscle cells: impact on PGC-1α expression and atrophy markers. PLoS ONE. 2016;11(7):e0159181. doi: 10.1371/journal.pone.0159181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-M, Seo W-Y, Song K-H, Chanda D, Kim YD, Kim D-K, Lee M-W, Ryu D, Kim Y-H, Noh J-R, Lee C-H, Chiang JYL, Koo S-H, Choi H-S. AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB center dot CRTC2 complex by orphan nuclear receptor small heterodimer partner. J Biol Chem. 2010;285(42):32182–32191. doi: 10.1074/jbc.M110.134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Wen X, Cho H, Koo S-H. CREB/CRTC2 controls GLP-1-dependent regulation of glucose homeostasis. FASEB J. 2018;32(3):1566–1578. doi: 10.1096/fj.201700845R. [DOI] [PubMed] [Google Scholar]
- Li Y, Song Y, Zhao M, Guo Y, Yu C, Chen W, Shao S, Xu C, Zhou X, Zhao L, Zhang Z, Bo T, Xia Y, Proud CG, Wang X, Wang L, Zhao J, Gao L. A novel role for CRTC2 in hepatic cholesterol synthesis through SREBP-2. Hepatology. 2017;66(2):481–497. doi: 10.1002/hep.29206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang L, Zhou L, Song Y, Ma S, Yu C, Zhao J, Xu C, Gao L. Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol Cell Endocrinol. 2017;446:70–80. doi: 10.1016/j.mce.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Lim H-Y, Wang W, Wessells RJ, Ocorr K, Bodmer R. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes Dev. 2011;25(2):189–200. doi: 10.1101/gad.1992411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, III, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–U274. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ma J, Li P, Liu B, Wen X, Yang J. Ilexgenin A restrains CRTC2 in the cytoplasm to prevent SREBP1 maturation via AMP kinase activation in the liver. Br J Pharmacol. 2022;179(5):958–978. doi: 10.1111/bph.15369. [DOI] [PubMed] [Google Scholar]
- Luo Q, Viste K, Urday-Zaa JC, Kumar GS, Tsai W-W, Talai A, Mayo KE, Montminy M, Radhakrishnan I. Mechanism of CREB recognition and coactivation by the CREB-regulated transcriptional coactivator CRTC2. Proc Natl Acad Sci USA. 2012;109(51):20865–20870. doi: 10.1073/pnas.1219028109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Yang H, Song B-L. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- Madison BB. Srebp2: a master regulator of sterol and fatty acid synthesis. J Lipid Res. 2016;57(3):333–335. doi: 10.1194/jlr.C066712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470(7334):404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60(16):1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21(3):421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner HM, Lamers WH, van Lelyveld PH, Hanson RW. Cyclic AMP and the regulation of gene expression of rat cytosolic phosphoenolpyruvate carboxykinase (GTP) Progr Clin Biol Res. 1982;102:241–250. [PubMed] [Google Scholar]
- Miano JM, Fisher EA, Majesky MW. Fate and state of vascular smooth muscle cells in atherosclerosis. Circulation. 2021;143(21):2110–2116. doi: 10.1161/CIRCULATIONAHA.120.049922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M, Koo SH, Zhang X. The CREB family: key regulators of hepatic metabolism. Ann Endocrinol. 2004;65(1):73–75. doi: 10.1016/S0003-4266(04)95634-X. [DOI] [PubMed] [Google Scholar]
- Montori-Grau M, Aguilar-Recarte D, Zarei M, Pizarro-Delgado J, Palomer X, Vazquez-Carrera M. Endoplasmic reticulum stress downregulates PGC-1alpha in skeletal muscle through ATF4 and an mTOR-mediated reduction of CRTC2. Cell Commun Signal. 2022;20(1):53. doi: 10.1186/s12964-022-00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschoep MH. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu Y, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Horike N, Yoneda M, Ohno H, Tsuchiya Y, Kamata H, Tahara H, Isobe T, Nishimura F, Katagiri H, Oka Y, Fukushima T, Takahashi S-I, Kurihara H, Uchida T, Asanoa T. Pin1 associates with and induces translocation of CRTC2 to the cytosol, thereby suppressing cAMP-responsive element transcriptional activity. J Biol Chem. 2010;285(43):33018–33027. doi: 10.1074/jbc.M110.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezich CL, Wang C, Fogel AI, Youle RJ. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J Cell Biol. 2015;210(3):435–450. doi: 10.1083/jcb.201501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis SJ, Claus TH. Hepatic gluconeogenesis/glycolysis: regulation and structure/function relationships of substrate cycle enzymes. Annu Rev Nutr. 1991;11(1):465–515. doi: 10.1146/annurev.nu.11.070191.002341. [DOI] [PubMed] [Google Scholar]
- Pilkis SJ, Claus TH, El-Maghrabi MR. The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis. Adv Second Messenger Phosphoprotein Res. 1988;22:175–191. [PubMed] [Google Scholar]
- Puertollano R, Ferguson SM, Brugarolas J, Ballabio A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. The EMBO J. 2018 doi: 10.15252/embj.201798804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao A, Zhou J, Xu S, Ma W, Boriboun C, Kim T, Yan B, Deng J, Yang L, Zhang E, Song Y, Ma YC, Richard S, Zhang C, Qiu H, Habegger KM, Zhang J, Qin G. Sam68 promotes hepatic gluconeogenesis via CRTC2. Nat Commun. 2021 doi: 10.1038/s41467-021-23624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnert JA, Zheng B, Hudson MB, Woodworth-Hobbs ME, Price SR. Glucocorticoids Alter CRTC-CREB signaling in muscle cells: impact on PGC-1alpha expression and atrophy markers. PLoS ONE. 2016;11(7):e0159181. doi: 10.1371/journal.pone.0159181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rines AK, Sharabi K, Tavares CDJ, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15(11):786–804. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti C, Cherix A, Guiraud LF, Cardinaux J-R. New insights into the pivotal role of CREB-regulated transcription coactivator 1 in depression and comorbid obesity. Front Mol Neurosci. 2022 doi: 10.3389/fnmol.2022.810641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L. Energy Metabolism in the Liver. Compr Physiol. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Norona FE, Alzate-Correa D, Scarberry D, Hoyt KR, Obrietan K. Clock and Light Regulation of the CREB Coactivator CRTC1 in the Suprachiasmatic Circadian Clock. J Neurosci. 2013;33(21):9021–9027. doi: 10.1523/JNEUROSCI.4202-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119(1):61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia-Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332(6036):1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Tuong H, Carissimo A, Palmer D, Klisch TJ, Wollenberg AC, Di Bernardo D, Chan L, Irazoqui JE, Ballabio A. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15(6):647. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Cardiac Fail. 2006;12(9):694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, Hogan MF. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468(7326):933–939. doi: 10.1038/nature09564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhai L, Valencia Swain J, Chen Y, Wang P, Chen L, Liu Y, Xiang S. Structural insights into the CRTC2-CREB complex assembly on CRE. J Mol Biol. 2018;430(13):1926–1939. doi: 10.1016/j.jmb.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45(5):623–634. doi: 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- Than TA, Lou H, Ji C, Win S, Kaplowitz N. Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J Biol Chem. 2011;286(25):22047–22054. doi: 10.1074/jbc.M111.240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi PC, Bartlett JJ, Perez LJ, Brunt KR, Legare JF, Hassan A, Kienesberger PC, Pulinilkunnil T. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. BBA-Mol Cell Biol L. 2016;1861(12):1893–1910. doi: 10.1016/j.bbalip.2016.09.004. [DOI] [PubMed] [Google Scholar]
- van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81(3):420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Chin RG, de Aguiar T, Vallim LG, Fong TF, Osborne SG, Young KR. SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression. J Lipid Res. 2016;57(3):410–421. doi: 10.1194/jlr.M064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Goode J, Best J, Meltzer J, Schilman PE, Chen J, Garza D, Thomas JB, Montminy M. The insulin-regulated CREB coactivator TORC promotes stress resistance in Drosophila. Cell Metab. 2008;7(5):434–444. doi: 10.1016/j.cmet.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485(7396):128–U166. doi: 10.1038/nature10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yang Q, Sun Y-Y, Xing Y-F, Wang Y-B, Lu X-T, Bai W-W, Liu X-Q, Zhao Y-X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J Cell Mol Med. 2014;18(8):1599–1611. doi: 10.1111/jcmm.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Song D, Fu J, Wen X. SIK1 regulates CRTC2-mediated gluconeogenesis signaling pathway in human and mouse liver cells. Front Endocrinol. 2020 doi: 10.3389/fendo.2020.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba A-E, Zernecke A, Pramod AB, Ghosh AK, Michel NA, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K, Wolf D. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122(12):1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbecher C, Eichelmann F, Toledo E, Guasch-Ferre M, Ruiz-Canela M, Li J, Aros F, Lee C-H, Liang L, Salas-Salvado J, Clish CB, Schulze MB, Martinez-Gonzalez MA, Hu FB. Lipid profiles and heart failure risk results from two prospective studies. Circ Res. 2021;128(3):309–320. doi: 10.1161/CIRCRESAHA.120.317883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1 alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA. 2006;103(39):14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Wang K, Wang W, Wen Z, Wang P, Liu L, Wang DW. Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPAR alpha pathway. Aging Cell. 2018;17(4):e12763. doi: 10.1111/acel.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yuan H-Q, Hao Y-M, Ren Z, Qu S-L, Liu L-S, Wei D-H, Tang Z-H, Zhang J-F, Jiang Z-S. Macrophage polarization in atherosclerosis. Clin Chim Acta. 2020;501:142–146. doi: 10.1016/j.cca.2019.10.034. [DOI] [PubMed] [Google Scholar]
- Yoon Y-S, Lee M-W, Ryu D, Kim JH, Ma H, Seo W-Y, Kim Y-N, Kim SS, Lee CH, Hunter T, Choi CS, Montminy MR, Koo S-H. Suppressor of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C) is a key regulator of hepatic gluconeogenesis. Proc Natl Acad Sci USA. 2010;107(41):17704–17709. doi: 10.1073/pnas.1012665107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y-S, Liu W, Van de Velde S, Matsumura S, Wiater E, Huang L, Montminy M. Activation of the adipocyte CREB/CRTC pathway in obesity. Commun Biol. 2021 doi: 10.1038/s42003-021-02735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Luo Y, Wang C, Ding X, Yang X, Wu D, Silva F, Yang Z, Zhou Q, Wang L, Wang X, Zhou J, Boyd N, Spafford M, Burge M, Yang XO, Liu M. Adipose mTORC1 suppresses prostaglandin signaling and beige adipogenesis via the CRTC2-COX-2 pathway. Cell Rep. 2018;24(12):3180–3193. doi: 10.1016/j.celrep.2018.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Li H, Wu Y, Zhou J, Yang G, Wang W, Kang D, Ye S. CREB-upregulated lncRNA MEG3 promotes hepatic gluconeogenesis by regulating miR-302a-3p-CRTC2 axis. J Cell Biochem. 2019;120(3):4192–4202. doi: 10.1002/jcb.27706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chen Y, Zhang Z, Zhu D, Zhao W, Li F. 2019. Long non-coding RNA MEG3 serves as a ceRNA for microRNA-145 to induce apoptosis of AC16 cardiomyocytes under high glucose condition. Biosci Rep. [DOI] [PMC free article] [PubMed]