Abstract
Abstract
Cancer is a cellular impairment disorder characterized by the loss of cell cycle regulation leading to aberrant cell proliferation. Cell–cell communication plays a crucial role in cell signaling which is highly disrupted in various malignancies. Tight junctions (TJs) are major proteins that regulate the proper communication, and the dysregulation of TJ proteins makes these tumor cells more aggressive, leading to tumor invasion and metastasis. Hence targeting TJs might be a novel insight towards addressing these highly invasive, metastatic tumors. Due to the prohibitive costs of treatments, side effects, and development of resistance, herbal medications comprising bioactive ingredients have become more popular for various human ailments. Unfortunately, the importance of natural compounds has significantly reduced due to the development of modern synthetic techniques to formulate drugs. However, the pharmaceutical industry that adopts chemistry-based drug development in combination with high throughput synthesis have not resulted in the expected drug productivity. Hence, the focus was shifted back to natural compounds in search of novel drugs with advanced technology to isolate the biologically active compound from the natural ones. The current review delivers the importance of TJ regulation, promoting it through phytochemicals to target malignant tumor cells.
Graphical abstract
Keywords: Tumor metastasis, Invasion, Tight junction, Cancer, Phytochemicals
Introduction
Cellular abnormalities are the root cause of numerous diseases, including cancer. Checkpoints of cellular metabolism and genetic aberrations serve as therapeutic targets both in communicable and non-communicable diseases leading to dysregulation in the cell signaling processes. Cell–cell communication is crucial for the tight regulation of homeostasis and hence cells would be connected extracellularly with adjacent ones and intracellularly with various cytoskeletal molecules through cell junctions. These connections provide an integrated, structural diversity across the tissue to regulate its proper functioning. Tight junctions, desmosomes, and adheren junctions are the critical players in regulating the normal functioning of cells by providing the cell–cell intimacy. Table 1 describes the various components of cell–cell junctions.
Table 1.
Various components associated with cell–cell junctions
| Junctions | Components |
|---|---|
| Tight junctions | Occludins, claudins, junctional adhesion molecules |
| Adheren junctions | Cadherin adhesion receptors, cytoplasmic proteins |
| Gap junctions | Connexin transmembrane proteins |
| Desmosomes | Desmoglein, desmocollin |
Disruption of cell–cell interaction shows a high impact both in cell communication and cell signalling leading to serious health disorders like diabetes, hypertension, inflammation, and even cancer. The tight junctions (TJ) present between the adjacent cells, that restrict the paracellular movement of solutes and macromolecules (Kanda et al. 2017) are located near the apex of the lateral plasma membrane in mammals. These are the complex, dynamic structures that confine apical connections between epithelial cells (Monteiro et al. 2013). The TJ forms a proteinaceous seal by encircling each cell in order to regulate the diffusion of ions and solutes between the cells, an activity which is called the paracellular pathway. The TJ serves as a “fence” and a “gate” simultaneously to maintain the segregation of apical and basolateral membranes, and to regulate paracellular pathway respectively. TJ serves a crucial function in cell structure by organising the junctional complex on the apical side of the epithelial cells (Piche 2014) and regulating the movement of solutes that pass intercellularly, helping maintain cell polarity. Interestingly, the regions that are present in TJ intracellularly bind to cell signalling molecules and the cytoskeleton to regulate cell migration, proliferation, and differentiation (Hossain and Hirata 2008).
Disruption in cell communication results in an accumulation of cells accompanied with a poor presence or directly an absence of apoptosis, which is the characteristic feature of various malignant cells (Pandrangi et al. 2022a, b, c, d, e). Tumor cells invade various tissues and organs accompanied by the disruption of TJs. This is because the disrupted TJ elevates the production of matrix metalloproteinase (MMPs) that aids in the transformation of epithelial cells to mesenchymal cells, which is one of the hallmarks of tumour metastasis (Gulati et al. 2021). The present review focuses on the crucial role played by tight junctions in cancer and might give new insights targeting the cell–cell communications in drug-resistant cancers using phytochemicals as adjuvants.
Structure of tight junctions
TJs serve as a stopper between the epithelial and endothelial monolayers thereby serving as gate keepers to allow the passage of small molecules and ions (Lee et al. 2018). Followed by other cell–cell junctions TJ is localized at the apical lateral side of both epithelial and endothelial tissue (Wallez and Huber 2008), while the only exceptional case is the localisation of N-cadherin an adheren junction is clustered at the basal side of the endothelial cells (Bazzoni and Dejana 2004). TJ is formed by the organisation of multiprotein complexes that involves both transmembrane proteins and cytoplasmic proteins (Chovatiya and Medzhitov 2014).
Proteins involved in tight junction
Previous studies demonstrated that TJ molecules activate various signalling pathways in cancer. To summarise, epithelial/endothelial TJ is thought to serve the following purposes. Firstly, TJ molecules are intended to separate the apical and basolateral fluid compartments of epithelia and endothelia, accompanied with sealing intercellular space. Secondly, it serves as a mediator of cell–cell contact, thereby regulating cell polarity, differentiation, growth, and proliferation by serving as intermediates and transducers in cell signalling. Finally, they act as barriers to cell migration and motility. Claudin and occludin are the two major transmembrane protein families of tight junctions that usually exist as homodimers viz., claudin-claudin, and occludin-occludin complexes between cells. Other protein families of TJ include junctional adhesion molecules (JAMs), and cytoplasmic zonula occludins designated as ZO-1, ZO-2, and ZO-3. JAMs are type-1 proteins that comprise ectodomains that are similar to those found in immunoglobulin domains. Claudins are expressed in the human small intestine and epidermis in various isoforms. Claudin-1,2,3,4,5,7,8,12, and 18 are expressed the in human small intestine (Garcia-Hernandez et al. 2017) while claudin 6,9, and 13 are expressed in the epidermis (Krause et al. 2008). Various studies have demonstrated that the dysregulation of TJ proteins is associated with various diseases.
Function of tight junctions
The intestinal epithelium plays a major role in digesting the ingested food and to absorb and circulate the absorbed nutrients and dietary factors. To maintain these functions, the intestinal epithelial should interact with several barrier components, and intercellular TJs are among them. When the barrier integrity is disrupted, immune cells are robustly activated, leading to chronic inflammation of the gastrointestinal tissues. Disruption of the barrier integrity leads to the passage of inflammatory cytokines to various organs via circulation resulting in the pathogenesis of various non-intestinal disorders. The spontaneous opening and closing of a TJ is a dynamic process that contributes to the modulation of tissue permeability with respect to the variations in the chemical constituents such as type and concentration of proteolytic enzymes, ionic content, and solute, the gut microbiota composition, etc. However, vesicular trafficking of TJ proteins between the cell membrane and cytosol might lead to TJ protein destruction. Destruction of TJ proteins by vesicular trafficking might be regulated by various exogenous factors and physiological modulators (Paradis et al. 2021).
TJs act as a battleground to maintain the barrier integrity of the intestinal epithelium. The opening and closing of TJs are highly regulated. The TJ present between the adherent cells is located near the apex of the lateral plasma membrane in mammals. These are the complex, dynamic structures that confine apical connections between epithelial cells (Monteiro et al. 2013). The TJ forms a proteinaceous seal by encircling each cell in order to regulate the diffusion of ions and solutes between the cells, an activity which is called the paracellular pathway. The TJ serves as a “fence” and a “gate” simultaneously to maintain the segregation of apical and basolateral membranes, and to regulate the paracellular pathway respectively. TJs serve a crucial function in cell structure by organising the junctional complex on the apical side of the epithelial cells. They regulate the movement of solutes that pass intercellularly and maintain the cell polarity. Interestingly, the regions that are present in TJ intracellularly bind to cell signalling molecules and the cytoskeleton to regulate cell migration, proliferation, and differentiation (Chittineedi et al. 2022).
Disruption of tight junctions
Defending the body from stress stimuli caused by inflammation and infection relies heavily on TJs of the intestinal epithelial barrier. TJ homeostasis alteration is thought to induce pathogenesis of various diseases, especially cancer, as TJs are majorly involved in cell–cell interactions and cell signalling. Alteration in TJ homeostasis is influenced by factors such as proinflammatory cytokines, pathogenic bacteria, and lipopolysaccharides (LPS) (Devreotes and Horwitz 2015).
Proinflammatory cytokines
Proinflammatory cytokines like TNF-α, IL-1, and IFN promote the permeability of TJ. It has been demonstrated that both IL-1β and TNF-α are involved in suppressing TJ barrier function by activating NF-κB with a simultaneous decrease in ZO-1 protein level (Tripathy et al. 2021). Alternatively, blocking of NF-κB prevents TNF-α induced TJ barrier opening and ZO-1 downregulation. Interestingly, IL-1β treatment with Caco-2 cell monolayers suppressed occludin protein levels but did not affect the ZO-1 protein level (Wang et al. 2015).
Pathogenic bacteria and lipopolysaccharides
Toxins and pathogenic bacteria play a crucial role in maintaining the endothelial barrier. For instance, the intestinal epithelial TJ barrier is altered by certain enteric pathogenic bacteria such as E. coli and S. typhi leads to intestinal inflammation (Kumar et al. 2013). On the other hand, LPS a crucial cell wall component of various gram-negative bacteria contributes to a leaky small intestine ultimately resulting in TJ protein assembly modification. Apart from altering TJ protein assembly, LPS also leads to the altered expression of occludin and ZO-1 by inducing systemic inflammation (Maldonado et al. 2016). Hence, the TJs are to be regulated properly for the better functioning and delivery of therapeutic agents.
Role of TJs in pathophysiology of various malignancies
Tissue organization is characterised by the capability of epithelial cells to adhere with one another and with the extra cellular matrix. Cell adhesion apart from tissue organization is essential for regulating cell differentiation, gene expression, motility, and cell growth (Guo et al. 2018; Roy et al. 2022). These regulatory functions are mediated by activating signalling pathways through the formation of multimolecular complexes via cell adhesion molecules, transmembrane receptors, and cytoskeletal proteins. Evidence from recent studies demonstrate that the failure of epithelial cells to organize into TJ and establish perfect apicobasal polarity is often implicated in the development of various chronic diseases, including cancer.
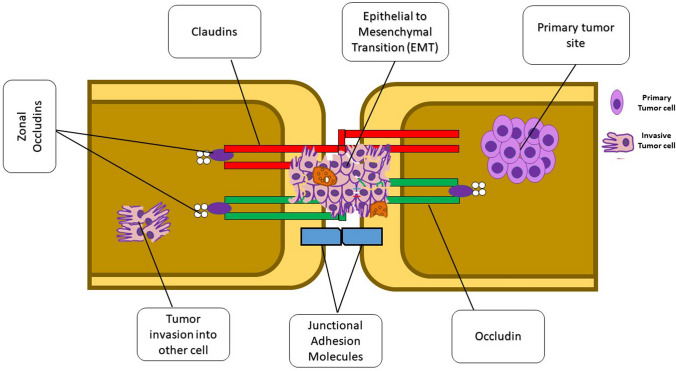
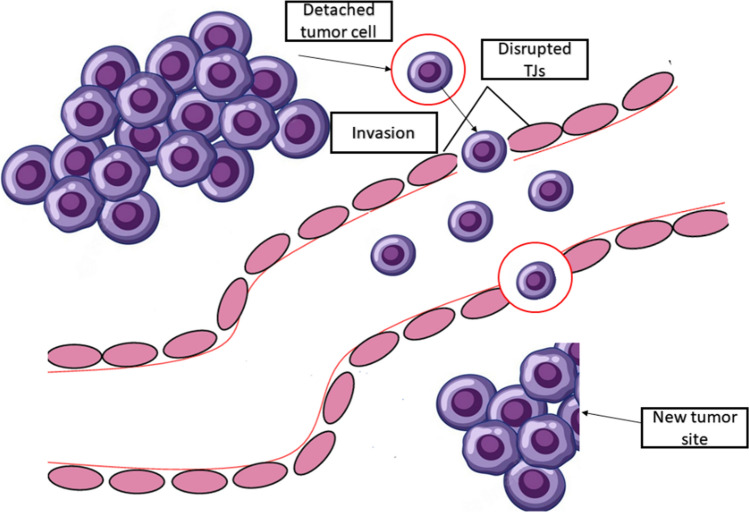
Tumor metastasis is one of the major hallmarks of cancer progression (Pandrangi et al. 2022a, b, c, d, e) and is and is often associated with the invasion of malignant tumour cells, which is associated with the dissociation of cancer cells from the primary tumor mass(Martin 2014; Malla et al. 2018). Unfortunately, the invasion is characterised by loss of cell–cell adhesion, resulting in the invasion of surrounding stroma. The key step in metastasis initiation would be the interaction and penetration in the metastasising tumour cell of the endothelium and the mesothelium. Therefore, it could be demonstrated that during metastasis, TJs act as controllers that regulate tumour cell invasion. Figure 1 describes the role of various TJ proteins involved in tumor invasion through TJ disruption (González-Mariscal et al. 2012). Studies have shown that mutations in oncogenes which result in hyper activation of the particular gene are positively correlated with increased leakiness of TJs in cancer, suggesting that an increased permeability of epithelium is associated with decreased epithelium barrier function leading to tumour development. Interestingly, Mullin et al., hypothesised that in epithelial carcinogenesis, TJ leakiness is the late event that would occur (Mullin et al. 2006). Figure 2 depicts the underlying mechanism of TJ disruption in tumour invasion and metastasis.
Fig. 1.
Role of various TJ proteins in tumor invasion and metastasis. Claudins are the group of TJ proteins that play a critical role in tumor invasion, migration, and metastasis. Activation of claudins by phosphorylation induces synthesis of bulk quantities of matrix metalloproteinases (MMPs) which aids in tumor cell invasion by enhancing epithelial–mesenchymal transition (EMT)
Fig. 2.
Underlying mechanism of TJ disruption in tumor invasion and metastasis. TJs are more specifically disrupted in tumor tissues during the detachment of tumor cell from its primary site. Once detached from its primary site, the tumor cell enters the lymphatic system, which is characterised by the disruption of cell–cell adhesion and enters another site where it starts to proliferate leading secondary tumor
Additionally, claudin-2 which is a member of the TJ protein family has been implicated in many proliferative pathways that often dysregulated into various diseases, including cancer. Interestingly, many studies have demonstrated a positive correlation between an altered claudin-2 expression with respect to cell proliferation (Venugopal et al. 2019). It has been suggested that claudin-2 might be a pro-proliferative factor that acts as a pro-proliferative signalling pathway. On the other hand, CDKN1B a cell cycle regulator whose expression is high in quiescent cells is also interrupted by the claudin-2 which results in blocking the cell to enter the cell cycle (Rambatla et al. 2021). Since CDKN1B is a tumour suppressor that suppresses the tumour growth by blocking the cell entry, and the altered claudin-2 expression negatively regulates the expression of the CDKN1B gene (Venugopal et al. 2019). On the other hand, JAMs, a multifunctional transmembrane TJ protein belonging to the immunoglobulin superfamily, inhibited apoptosis in gastric cancer with a simultaneous proliferation of actively dividing cancer cells (Czubak-Prowizor et al. 2022). Surprisingly, inhibition of occludins an integral membrane protein localized in TJ has been implicated to inhibit apoptosis suggesting that occludin plays a crucial role in cell death signalling (Beeman et al. 2012).
Over the past few decades, numerous studies have shown that the aberrant TJ function and expression in cancer progression suggests that TJ components offer intriguing and novel targets for cancer diagnosis, detection, and therapy, resulting in an innovative therapeutic approach to treat cancer (Adams et al. 2014). Most of the commercially available antineoplastic drugs target DNA disruption with the help of topoisomerase inhibitors. Although research has been improved, splendidly indicating that target TJ components confer high invasive potential and drug resistance (Pandrangi et al. 2014b). Therefore, targeting TJ signalling with the help of natural components has been provided more interest.
Role of natural compounds in proper regulation of tight junctions
Administration of drugs is compulsory when a person suffers from an acute or chronic disease. Unfortunately, injections show a drastic impact on the patient’s quality of life (Pandrangi et al., 2022). There are other ways such as nasal, oral, or even pulmonary routes, but these encounter several obstacles. Among those obstacles, the movement of hydrophilic macromolecular drugs across the epithelia is the biggest concern. The small intestine, which absorbs the dietary nutrients, is restricted to absorb the chemotherapeutic drugs due to the presence of TJs formed by the adjacent epithelial cells forming a biological barrier. Research has been shifted towards the therapeutic efficacy of various natural compounds in regulating TJs leading to novel cancer therapeutic approaches (Pandrangi et al. 2022).
Curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadieness-3,5-dione), alternatively called diferuloylmethane, is the primary curcuminoid of Curcuma longa which is regarded as the Asian spice turmeric. Turmeric powder has been used as a traditional medicine in various parts of the world since ancient times. Studies have demonstrated that curcumin has been shown to target several signalling molecules at the cellular level and has various health benefits. Numerous studies suggest curcumin could regulate inflammatory conditions, metabolic syndrome, and other disorders, including cancer. Although there appear to be innumerable therapeutic benefits of curcumin supplementation, most of these are due to its anti-inflammatory and antioxidant effects. Numerous studies have demonstrated that curcumin is capable of lowering blood glucose levels and insulin resistance by modulating the levels of free fatty acids. To study the effect of curcumin on blood glucose levels Na and colleagues conducted randomized control trials which included 100 obese type-2 diabetic patients. These patients were prompted to intake curcumin at 300 mg/day for three months. The results were surprising, indicating that all the patients supplemented with the 300 mg/day dose of curcumin developed significantly lower levels of fasting glycemia and insulin resistance (Pivari et al. 2019).
Due to their potential to suppress various diseases and chronic inflammation despite their poor bioavailability, it has been hypothesised that curcumin might play an essential role in regulating systemic inflammation in intestinal epithelial TJ permeability. Numerous studies have been proposed to elucidate the potent role of curcumin in regulating TJ barrier function. To evaluate the role of curcumin in TJ permeability regulation, researchers have induced CaCo2 cells to stimulate the release of proinflammatory cytokines such as TNF-α, IL-1β. The release of these proinflammatory cytokines enhanced the TJ permeability by activating NF-κB. However, when these cells were treated with curcumin, the proinflammatory cytokine that deteriorated TJ barrier function was abolished. Moreover, the treatment with curcumin suppresses the leptin signalling pathway in CaCo2 BBe monolayers, resulting in the prevention of apical leptin-impaired TJ (Kim and Kim 2014). Interestingly, in proinflammatory cytokine-induced TJ alteration, many researchers have used curcumin to inhibit the NF-κB signalling pathway that alters TJ permeability by inhibiting inhibitor-κB (I-κB) kinase followed by the stabilization of I-κB. On the other hand, the IL-1β mediated activation of p38 MAP kinase results in activation of MLCK, which phosphorylates myosin light chain, resulting in altered TJ structure and elevated intestinal permeability. However, curcumin also targets the IL-1β mediated TJ impairment by inhibiting the IL-1β/p38 signalling cascade (Pisano et al. 2016).
Quercetin
Quercetin, a flavonoid is a class of secondary metabolite found in various plants and has various biological properties such as anticarcinogenic, anti-inflammatory, antiviral, antioxidant, etc. (Hurjui et al. 2021). Quercetin, which comes under the category of flavonoids, has been shown to regulate tight junctions because of their potent roles in various health ailments (Taïlé et al. 2021). It is well known that tight junctions play a crucial role in maintaining cell–cell communication and regulates the flow of small molecules and ions. In diabetic patients, there is a high risk of attaining cerebrovascular complications (Vafadar et al. 2020). The reason behind this is hyperglycaemia, which is the hallmark of diabetes, and which alters the cerebral endothelial cellular functions that are interconnected with tight junctions. They comprise three transmembrane proteins called occludins, claudins, and junctional adhesion molecules (JAMs). Surprisingly, hyperglycaemia also upregulates occludin, claudin, ZO-1 & ZO-2, and tight junction protein synthesis to promote the adhesion on cerebral endocytes (Taïlé et al. 2021). Interestingly, NF-κB is also associated with the production of cell adhesion molecules and hence hyperglycaemia-induced inflammation is associated with an overproduction of these cell adhesion molecules, resulting in the promotion of adherence and trans-endothelial migration (Chikati et al. 2018).
Due to their antioxidant potency and ability to inhibit wide range of kinases including kinases involved in cancer cell growth, proliferation, and metastasis, quercetin is a potent molecule to induce cancer cell death by generating oxidative stress. Studies have shown that in the ascites cells of Dalton lymphoma-carrying rats, quercetin has induced receptor-mediated apoptosis. Additionally, Maurya A.K and co-workers proved that quercetin is capable of inhibiting protein kinase C activity, which is a key player in regulating cancer progression (Mullin et al. 2006).
The role of quercetin in TJ integrity has been investigated in various studies. In the absence of pro-inflammatory cytokines, quercetin has been reported to improve TJ barrier function in Caco-2 cell lines. Surprisingly, there was a significant increase in the expression of claudin-4 when Caco-2 cells were treated with 200 µM quercetin for 24 h. Unfortunately, there is no significant elevation of other TJ proteins such as occludins, claudin-1, claudin-3, and claudin-7. However, it is interesting to remark that the administration of quercetin elevated the trans epithelial electrical resistance (TER) while suppressing the paracellular marker lucifer yellow flux. TER could be defined as the measurable unit that detects the capacity of TJ to regulate the flow of ions and small molecules through the paracellular pathway (González-Mariscal et al. 2012). Therefore, an elevation of TER indicates a logarithmic increase in TJ function to permit the passage of small molecules and ions. In order to assess the beneficial role of quercetin on TJ cellular mechanisms, several protein kinase inhibitors were used. Among all the protein kinase inhibitors utilised, staurosporine, and H7 demonstrated quercetin’s protective role in regulating the TJ barrier. Interestingly, it has been demonstrated that a 100µM concentration of quercetin enhanced the TJ integrity by diminishing PKC through subsequent modulation of various TJ-related proteins including claudins, occludins, and zonal occludins, by suppressing PKCδ. All these results show that quercetin mediates TJ barrier integrity by suppressing various protein kinases.
Berberine
Berberine comes under the group of isoquinoline alkaloids. It is a quaternary ammonium salt enriched in roots, rhizomes, and stem bark of numerous plants under the Berberis genera. The protective role of berberine has been investigated in the mouse model comprising endotoxemia; the study showed that intragastric pre-treatment with berberine partially prohibited the ultrastructural damage of TJ by reversing the LPS-facilitated redistribution of occludin, ZO-1, claudin-1, claudin-4 in colon epithelium. Interestingly, when the rats with type-2 diabetes were pre-treated with berberine for 9 weeks, it significantly enriched the disruption of intestinal permeability and pro-inflammatory intestinal fluctuations, thereby protecting said rats from the adverse effects of the intestinal mucosal barrier. L, Gu et al. have demonstrated that berberine is non-toxic to human epithelial cells and is shown to tighten TJ barriers. It is worth noting, as well, that a study conducted by Ma et al. TJ reflected that permeability is regulated by NF-κB, whose activation is negatively regulated by berberine. Surprisingly, berberine which positively correlates with TJ integrity, was not able to induce subcellular localization of occludin, which is a major component of TJ and a key regulator of TJ barrier function even at high doses (Gu et al. 2009). Furthermore, berberine has been shown to diminish the pro-inflammatory cytokine-induced increase in intestinal epithelial TJ permeability (Li et al. 2010).
Interestingly, in endotoxemic mice, the administration of berberine partially attenuated intestinal epithelial TJ barrier dysfunction. When distal ileum and colon were subjected to IHC the results demonstrated that berberine improves morphological changes facilitated by LPS. A study conducted by Qiuke Hou et al., suggested that berberine modulates epithelial TJs by restoring the damage caused to the structural integrity of colon epithelium, downregulating NF-κB, myosin light chain kinase (MLCK) which facilitate cell movement and migration by modulating membrane tension, tumor necrosis factor receptor associated factor (TRAF) which mediates signal transduction expression with simultaneous upregulation of occludin, claudin, and ZO-1 expression (Chen et al. 2015). On the other hand, TJ dysfunction contributing to diarrhoea and inflammatory bowel disease is mediated by TNF-α. TNF-α does this by suppressing claudin-1 expression while elevating claudin-2 levels. However, the TNF-α mediated claudin dysregulation was reversed by berberine. It is well evident that NF-κB, TRAF, and TNF-α are the critical drivers of tumour progression, and literature suggests that berberine plays a crucial role in suppressing carcinogenesis mediated by TNF-α, which is considered one of the major regulators of cancer cell proliferation.
In addition to this, because of its ability to interact with nucleic acids, berberine is considered to possess potent antineoplastic properties. It is well known that topoisomerase inhibitors are the potent anticancer drugs that are being approved and used clinically. Interestingly, berberine has showed its potentiality to inhibit topoisomerases as well as telomerases by specifically binding and stabilizing DNA triplexes, finally accounting for their antiproliferative properties. Furthermore, when berberine was administrated in human A549 lung cancer, cell lines resulted in modification of microtubule-associated protein-1 light chain-3 (LC-3) accompanied with tumour shrinkage in the mice model (Lakhanpal et al. 2016; Hou et al. 2019). Studies have shown that berberine in combination with cisplatin and evodiamine elevated the cytotoxic effects of the anti-cancer drugs and resulted in cancer cell death in various cancers (Tan et al. 2011).
Genistein
Genistein, chemically regarded as 4′,5,7 trihydroxy isoflavone, is one of the major constituents of the soybean. Genistein resembles human oestrogen stereo-chemically and has a diphenol structure. Genistein plays a vital role in numerous biological pathways by targeting various molecules such as protein tyrosine kinases, topoisomerases, ABC transporters, etc., which are the sole mediators for tumour invasion, proliferation, and drug-resistance (Pandrangi et al. 2014a). With the discovery of oestrogenic properties, genistein has become one of the largest interests as a potential drug supplement for treating various diseases such as obesity, osteoporosis, metabolic syndromes, cancer, etc.
It is a well-known fact that phosphorylation of TJ proteins is associated with TJ structure and function and genistein has been reported in numerous studies to regulate TJ proteins and their integrity. Surprisingly, the TJ barriers would be opened when the intestinal cells interact with enteric bacteria such as E. coli and S. typhimurium. However, the administration of 300µM genistein blocked the invasion of enteric bacteria by preventing the TJ barrier opening (Lee et al. 2018). Another study demonstrated that genistein enhanced TJ barrier dysfunction induced by oxidative stress associated with occludin phosphorylation suppression (Rao et al. 2002).
In vitro studies support the effectiveness of genistein which induces oxidative stress as a potent chemotherapeutic agent against liver cancer. As a result of its potent activity on apoptosis and cell cycle regulation, genistein has shown to be a promising approach to affect hepatocellular carcinoma. By downregulating matrix metalloproteinase 9 (MMP-9), epidermal growth factor receptor (EGFR), and consequent suppression of NF-κB, genistein promotes anti-invasive and anti-metastatic properties. Interestingly, genistein inhibited cell proliferation and induced apoptosis in the human gastric cancer cell line BGC-823 in a dose-dependent and time-dependent manner. Additionally, genistein targets and attenuates the PI3K/Akt pathway resulting in suppression of colon cancer growth and proliferation.
Capsaicin
Capsaicin, chemically referred to as trans-8-methyl-N-vanillyl-6-nonenamide, is a naturally-occurring bioactive ingredient found in hot chilli peppers of the genus Capsicum. Because of the presence of the vanillyl group, capsaicin is considered a vanilloid, a proto-alkaloid with nitrogen located in the side chain. The role of capsaicin in TJ permeability has been extensively studied on CaCo2, a human colon cancer derived epithelial cell line, widely used as an intestinal epithelial model. Studies have demonstrated the effect of capsaicin on TJ permeability in CaCo2 cell lines. Interestingly, research has indicated that cofilin, a family of actin-binding proteins, is associated with TJ opening when dephosphorylated. However, opening of TJ was induced in capsaicin-treated CaCo2 cell lines associated with dephosphorylation of cofilin. Additionally, treatment with capsaicin also altered the F-actin structure associated with TJ protein localization (Shiobara et al. 2013). However, Tomoko Shiobara et al., showed that exposure of CaCo2 cells with capsaicin resulted in a significant decrease in occludin numbers, but there was no change in TJ protein localization. The results from their study suggested that TJ opening could be mediated by two mechanisms, which could be the subcellular actin distribution associated with modulations in the polymerisation of actin filaments and the other possible mechanism could be a reduction in the TJ occludin concentration. Finally, they confirmed that capsaicin-induced occludin downregulation coupled with actin alteration affects the TJ integrity, resulting in TJ opening in a dose-dependent manner. Interestingly, all these studies concluded that capsaicin induces TJ barrier opening in various mechanisms (Dai et al. 2018).
Recent studies have implicated the role of capsaicin in inhibiting tumour progression and inducing tumour cell death. It is very interesting to note that capsaicin could restrict the progression of tumour cells without inducing its cytotoxicity effects on normal healthy cells. Capsaicin was proposed to be a novel therapeutic agent for cancer therapy, as it would not affect the normal cells, while effectively killing the tumour cells (Nagumo et al. 2008). However, the role of capsaicin in anti-cancer activity was still in debate as it plays a dual role serving as a carcinogen as well as cancer-preventing agent. Interestingly, the majority of the studies showed the anti-tumour potentiality of capsaicin rather than its tumorigenicity capacity (Zou et al. 2017).
Other important phytochemicals
Kaempferol, a yellow compound tetrahydroxyflavone, represents one of the most common aglycone flavonoids, which is regarded as a form of a glycoside. Various in vitro and in vivo studies on kaempferol and their derivatives have demonstrated that these glycosides exhibit numerous health-promoting effects, including anti-inflammatory and antioxidant benefits, which are associated with maintaining TJ membrane integrity. Investigations on the role of kaempferol in modulating TJ functions have demonstrated that when 100 µM of kaempferol was exposed to Caco-2 cells, an increased expression of TJ-related proteins such as occludins, claudins (1,3, and 4), ZO-1 has been observed (Aghazadeh et al. 2021). On the other hand, Qu et al. (2021) demonstrated that Kaempferol regulates the gut microbiota and other metabolites through its immunoregulatory effects, particularly in murine models characterised with intestinal microbiota dysbiosis, a hallmark of ulcerative colitis, thereby downregulating LPS-mediated TLR4-NF-κB signalling (Qu et al. 2021). Since NF-κB signalling plays a critical role in tumour cell proliferation, it could be suggested that kaempferol supplementation could induce tumour cell death by modulating TJs membrane integrity.
Epigallocatechin-3-gallate (EGCG) is the active compound present in green tea isolated from Camellia sinensis, which is used as a traditional beverage, particularly in India. Due to its high anti-oxidant potency, EGCG has been used as an anti-cancer, anti-inflammatory, and anti-diabetic agent. Interestingly, studies done by Lagha and Grenier (2019) demonstrated that tea polyphenols which comprise both EGCG and theaflavins (a component of black tea), have successfully attenuated the TNF-α mediated gingival epithelial barrier dysfunction, suggesting that EGCG could modulate TJ barrier dysfunction (Lagha and Grenier 2019). As shown in Fig. 2, TJ disruption is the major driver of tumour metastasis, as it makes a way to the detached tumour cells by interrupting the adhesions present between cell–cell. Recent studies demonstrate that EGCG could potentially prevent EMT through Nrf-2 activation. When the Nrf-2 pathway has been activated, it elevates catalase, which is accompanied by increased intracellular ROS. It is well established that EMT is characterised by the presence of both epithelial markers, which include E-cadherin, occludin, ZO-1, etc., as well as mesenchymal markers, such as vimentin and fibronectin. Studies suggest that pre-treatment of cells with EGCG could regulate both epithelial and mesenchymal markers at their basal levels (Kanlaya et al. 2016).
Piperine, on the other hand, is also a traditional spice used in various foods. Piperine is the biologically-active compound present in pepper. It has numerous health benefits and has been used as traditional medicine for nose and throat infections. Studies have demonstrated that curcumin, when used in combination with piperine, enhances the bioavailability of curcumin, thereby facilitating its therapeutic effects. However, piperine could also be used individually because of its high antioxidant capacity. A recent study by Wang et al. (2021) demonstrates that piperine improves obesity through repairing intestinal barrier injury, as well as lowering intestinal fatty acid absorption (Wang et al. 2021). Rooting evidence suggests that obesity is one of the risk factors associated with cancer incidence. Hence, obesity-induced cancer might be treated with piperine.
Gingerol is an active component present in the rhizome of Zingiber officinale and has numerous health benefits due to its high anti-inflammatory, anti-oxidant, and anticancer-promoting activities. Since TJ membrane integrity is lost in various malignancies, leading to the increased risk of secondary ailments accompanied by invasiveness and metastasis, this could be overcome by the therapeutic effects of gingerol. Recent studies by Kim et al. (2014) have demonstrated that gingerol could potentially suppress the invasion and metastasis by regulating TJ-related proteins such as claudin-4, occludin, ZO-1, E-cadherin, etc. through ERK pathway inhibition (Kim and Kim 2013). Interestingly, studies by Zhong et al. (2019) have demonstrated that the relation between tumour progression/metastasis is interconnected. They performed proteomic analysis, which suggested that gingerol mediates the direct binding of VEGFa leading to VEGFR2 activation, a key regulator of angiogenesis. Activated VEGFR2 regulates F-actin cytoskeleton remodelling in endothelial cells by recruiting VE-cadherin/β-catenin/actin, thereby promoting the normalisation of tumour microvessels, which enhances the cytotoxic effects of the novel anti-neoplastic drugs, thereby halting tumour progression (Zhong et al. 2019).
Discussion and conclusion
TJ regulates the paracellular transport of various substances such as ions and small molecules through the intestinal epithelium and is associated with the physical barrier function allowing the passage of essential ions and small molecules. This is aided by the presence of intracellular space in the plasma membrane of adjacent cells. Numerous studies have revealed the role of TJ permeability in pathogenesis of chronic diseases and suggested that TJ dysfunction is associated with inflammatory and metabolic diseases. Hence maintaining proper TJ integrity is likely to be effective strategy to yield better cancer prognosis. Phytochemicals are coming into the light of research due to the presence of numerous active ingredients that are capable of fighting against various diseases including cancer through modulating TJ integrity. However, although these plant-derived bioactive compounds such as quercetin, berberine, genistein, capsaicin, curcumin, and many more natural compounds, have been shown to be effective in enhancing the TJ integrity via TJ proteins and inflammatory signalling pathways, further molecular studies are needed to confirm the effectiveness of natural compounds on TJ permeability and integrity which might lead to the development of preventive medicine and therapeutic agents against these chronic diseases.
Acknowledgements
SLP gratefully acknowledges DBT (BT/PR30629/BIC/101/1093/2018), New Delhi; UGC (Ref No: No.F.30–456/2018 (BSR), SERB (Ref No.: PDF/2015/000867), and GITAM-RSG for the financial support. PC gratefully acknowledges DBT (BT/PR30629/BIC/101/1093/2018), New Delhi, for the Junior research fellowship.
Author contributions
Wrote the manuscript: PC and SLP; figures and tables: PC and JANM; Review and Editing: JANM, GJM, and SNSL; data compiling: GJM and SNSL; Conceptualized the study: SLP. Overall supervision of the study: SLP.
Declarations
Institutional Review Board Statement
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams BD, Kasinski AL, Slack FJ (2014) Aberrant regulation and function of microRNAs in cancer. Curr Biol 2014 Aug 18;24(16):R762-76. 10.1016/j.cub.2014.06.043 [DOI] [PMC free article] [PubMed]
- Aghazadeh T, Bakhtiari N, Rad IA, Ramezani F. Formulation of kaempferol in nanostructured lipid carriers (Nlcs): a delivery platform to sensitization of mda-mb468 breast cancer cells to paclitaxel. Biointerface Res Appl Chem. 2021;11:14601–14591. doi: 10.33263/BRIAC116.1459114601. [DOI] [Google Scholar]
- Bazzoni G, Dejana E (2004) Endothelial cell-to–cell junctions: molecular organization and role in vascular homeostasis. 10.1152/physrev.00035.2003.-Intercellular [DOI] [PubMed]
- Beeman N, Webb PG, Baumgartner HK (2012) Occludin is required for apoptosis when claudin–claudin interactions are disrupted. 10.1038/cddis.2012.14 [DOI] [PMC free article] [PubMed]
- Chen C, Lu M, Pan Q, et al. Berberine improves intestinal motility and visceral pain in the mouse models mimicking diarrhea-predominant irritable bowel syndrome (IBS-D) symptoms in an opioid-receptor dependent manner. PLoS ONE. 2015 doi: 10.1371/JOURNAL.PONE.0145556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikati R, Pandrangi LS, Gundampati R, et al. Molecular studies on evaluation of phytol as cytoskeleton targeting element in cancer. Int J Sci Eng Res. 2018;9:1978–1992. [Google Scholar]
- Chittineedi P, Pandrangi SL Mohiddin GJ et al (2022) Concomitant therapy of Aq. theobroma extract and doxorubicin reduces stemness and induces ferroptosis in therapeutic resistant cervical cancer cells. J Carcinog Mutagen S32:001
- Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubak-Prowizor K, Babinska A, Swiatkowska M (2022) The F11 receptor (F11R)/junctional adhesion molecule-A (JAM-A) (F11R/JAM-A) in cancer progression. 477:79–98. 10.1007/s11010-021-04259-2 [DOI] [PMC free article] [PubMed]
- Dai N, Ye R, He Q et al (2018) Capsaicin and sorafenib combination treatment exerts synergistic anti-hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. 10.3892/or.2018.6754. Oncol Rep [DOI] [PMC free article] [PubMed]
- Devreotes P, Horwitz AR (2015) Signaling networks that regulate cell migration. Cold Spring Harb Lab Press. 10.1101/cshperspect.a005959 [DOI] [PMC free article] [PubMed]
- Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann NY Acad Sci. 2017 doi: 10.1111/nyas.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mariscal L et al (2012) Tight Junctions, Current Frontiers and Perspectives in Cell Biology. Available at: www.intechopen.com.
- Gu L, Li N, Li Q, et al. The effect of berberine in vitro on tight junctions in human Caco-2 intestinal epithelial cells. Fitoterapia. 2009;80:241–248. doi: 10.1016/j.fitote.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Gulati R, Naik Ramavath M, Satya Mahesh Kumar Metta V, Latha Pandrangi S (2021) Exploring the CRISPR/Cas9 system in targeting drug resistant cancer stem cells 25:1583–6258
- Guo W, Wang P, Liu Z-H, Ye P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int J Oral Sci. 2018 doi: 10.1038/ijos.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain Z, Hirata T. Molecular mechanism of intestinal permeability: Interaction at tight junctions. Mol Biosyst. 2008;4:1181–1185. doi: 10.1039/B800402A. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhu S, Zhang C, et al. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed Pharmacother. 2019;118:109206. doi: 10.1016/J.BIOPHA.2019.109206. [DOI] [PubMed] [Google Scholar]
- Hurjui LL, Maria Hartan R, Andrei Hurjui I et al (2021) Quercetin in health and disease
- Kanda Y, Yamasaki Y, Sasaki-Yamaguchi Y, et al. TRPA1-dependent reversible opening of tight junction by natural compounds with an α,β-unsaturated moiety and capsaicin OPEN. Sci Rep. 2017 doi: 10.1038/s41598-018-20526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanlaya R, Khamchun S, Kapincharanon C, Thongboonkerd V (2016) Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. 10.1038/srep30233 [DOI] [PMC free article] [PubMed]
- Kim SO, Kim MR. [6]-Gingerol prevents disassembly of cell junctions and activities of MMPs in invasive human pancreas cancer cells through ERK/NF-κB/Snail signal transduction pathway. Evid Based Complement Altern Med. 2013 doi: 10.1155/2013/761852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CY, Kim KH. Curcumin prevents leptin-induced tight junction dysfunction in intestinal Caco-2 BBe cells. J Nutr Biochem. 2014;25:26–35. doi: 10.1016/J.JNUTBIO.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta Biomembr. 2008;1778:631–645. doi: 10.1016/J.BBAMEM.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Kumar GR, Chikati R, Pandrangi SL, et al. Molecular docking and dynamics simulations of A.niger RNase from Aspergillus niger ATCC26550: for potential prevention of human cancer. J Mol Model. 2013;19:613–621. doi: 10.1007/s00894-012-1587-9. [DOI] [PubMed] [Google Scholar]
- Lagha AB, Grenier D. Tea polyphenols protect gingival keratinocytes against TNF-α-induced tight junction barrier dysfunction and attenuate the inflammatory response of monocytes/macrophages. Cytokine. 2019;115:64–75. doi: 10.1016/J.CYTO.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Lakhanpal M, Singh LC, Rahman T, et al. Study of single nucleotide polymorphisms of tumour necrosis factors and HSP genes in nasopharyngeal carcinoma in North East India. Tumor Biol. 2016;37:271–281. doi: 10.1007/s13277-015-3767-6. [DOI] [PubMed] [Google Scholar]
- Lee B, Moon KM, Kim CY (2018) Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J Immunol Res [DOI] [PMC free article] [PubMed]
- Li N, Gu L, Qu L, et al. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur J Pharm Sci. 2010;40:1–8. doi: 10.1016/j.ejps.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Maldonado RF, ´ A-Correia IS, Valvano MA, Whitfield CE. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection One sentence summary: the authors review modifications of lipopolysaccharide structure and biosynthetic pathways that occur upon bacterial adaptation to chronic respiratory and gastrointestinal infections. FEMS Microbiol Rev. 2016;007:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla RR, Pandrangi S, Kumari S, et al. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia Pac J Clin Oncol. 2018;14:383–391. doi: 10.1111/ajco.12869. [DOI] [PubMed] [Google Scholar]
- Martin TA. The role of tight junctions in cancer metastasis. Semin Cell Dev Biol. 2014;36:224–231. doi: 10.1016/J.SEMCDB.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Monteiro AC, Sumagin R, Rankin CR et al (2013) JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. 10.1091/mbc.E13-06-0298 [DOI] [PMC free article] [PubMed]
- Mullin JM, Laughlin KV, Ginanni N et al (2006) Increased Tight junction permeability can result from protein kinase C activation/translocation and act as a tumor promotional event in epithelial cancers [DOI] [PubMed]
- Nagumo Y, Han J, Bellila A, et al. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem Biophys Res Commun. 2008;377:921–925. doi: 10.1016/j.bbrc.2008.10.071. [DOI] [PubMed] [Google Scholar]
- Pandrangi SL, Chikati R, Chauhan PS, et al. Effects of ellipticine on ALDH1A1-expressing breast cancer stem cells-An in vitro and in silico study. Tumor Biol. 2014;35:723–737. doi: 10.1007/s13277-013-1099-y. [DOI] [PubMed] [Google Scholar]
- Pandrangi SL, Raju Bagadi SA, Sinha NK, et al. Establishment and characterization of two primary breast cancer cell lines from young Indian breast cancer patients: mutation analysis. Cancer Cell Int. 2014;14:1–20. doi: 10.1186/1475-2867-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrangi SL, Chalumuri SS, Garimella S. Emerging therapeutic efficacy of alkaloids as anticancer agents. Ann Rom Soc Cell Biol. 2022;26:64–74. [Google Scholar]
- Pandrangi SL, Chittineedi P SS, Chalumuri SS, et al. Role of intracellular iron in switching apoptosis to ferroptosis to target therapy-resistant cancer stem cells. Molecules. 2022;27:3011. doi: 10.3390/MOLECULES27093011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrangi SL, Chittineedi P, Chikati R, et al. Role of lipoproteins in the pathophysiology of breast cancer. Membr (Basel) 2022;12:532. doi: 10.3390/membranes12050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrangi SL, Chittineedi P, Chikati R, Lingareddy JR (2022d) Role of dietary iron revisited: in metabolism, ferroptosis and pathophysiology of cancer. 12:974–985 [PMC free article] [PubMed]
- Pandrangi SL, Shree Chalumuri S, Chittineedi P et al (2022e) Therapeutic potential of nyctanthes arbor-tristis on cancer and various diseases. Ann. Romanian Soc. Cell Biol 26(01):1690–1701
- Paradis T, Bègue H, Basmaciyan L, et al. Molecular sciences review tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int J Mol Sci. 2021 doi: 10.3390/ijms22052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche T. Tight junctions and IBS—the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil. 2014;26:296–302. doi: 10.1111/NMO.12315. [DOI] [PubMed] [Google Scholar]
- Pisano M, Palomba A, Tanca A, et al. Protein expression changes induced in a malignant melanoma cell line by the curcumin analogue compound D6. BMC Cancer. 2016;16:1–11. doi: 10.1186/s12885-016-2362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivari F, Mingione A, Brasacchio C, Soldati L (2019) Curcumin and type 2 diabetes mellitus: prevention and treatment. 10.3390/nu11081837 [DOI] [PMC free article] [PubMed]
- Qu Y, Li X, Xu F, et al. Kaempferol alleviates murine experimental colitis by restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB Axis. Front Immunol. 2021 doi: 10.3389/fimmu.2021.679897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambatla PK, Pandrangi SL, Rentala S, Sireesha V (2021) A study on the expression of CCL5, CXCR4 and angiogenic factors by prostate cancer stem cells. 25:1020–1028
- Rao RK, Basuroy S, Rao VU et al (2002) Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress [DOI] [PMC free article] [PubMed]
- Roy R, Garimella SV, Pandrangi SL (2022) Targeting the key players of DNA Repair Pathways as Cancer Therapeutics
- Shiobara T, Usui T, Han J et al (2013) The reversible increase in tight junction permeability induced by capsaicin is mediated via cofilin-actin cytoskeletal dynamics and decreased level of occludin. 10.1371/journal.pone.0079954 [DOI] [PMC free article] [PubMed]
- Tan W, Li Y, Chen M, Wang Y (2011) Berberine hydrochloride: anticancer activity and nanoparticulate delivery system. 10.2147/IJN.S22683 [DOI] [PMC free article] [PubMed]
- Taïlé J, Patché J, Veeren B, Gonthier MP. Hyperglycemic condition causes pro-inflammatory and permeability alterations associated with monocyte recruitment and deregulated nfκb/pparγ pathways on cerebral endothelial cells: evidence for polyphenols uptake and protective effect. Int J Mol Sci. 2021;22:1–20. doi: 10.3390/ijms22031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy AS, Vishwakarma S, Trimbake D et al (2021) Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: biomarkers of SARS-CoV-2 infection. 166:3301–3310. 10.1007/s00705-021-05247-z [DOI] [PMC free article] [PubMed]
- Vafadar A, Shabaninejad Z, Movahedpour A, et al. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020 doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal S, Anwer S, Szászi K. Claudin-2: roles beyond permeability functions. Mol Sci. 2019 doi: 10.3390/ijms20225655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta - Biomembr. 2008;1778:794–809. doi: 10.1016/J.BBAMEM.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang F, Ma J, Wang KS, et al. Blockade of TNF-α-induced NF-κB signaling pathway and anti-cancer therapeutic response of dihydrotanshinone I. Int Immunopharmacol. 2015;28:764–772. doi: 10.1016/J.INTIMP.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Wang F, Ma J, Wang KS, et al. Blockade of TNF-α-induced NF-κB signaling pathway and anti-cancer therapeutic response of dihydrotanshinone I. Int Immunopharmacol. 2015;28:764–772. doi: 10.1016/J.INTIMP.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang Y, Wang X et al (2021) Piperine improves obesity by repairing intestinal barrier function and inhibiting fatty acid absorption. 10.21203/rs.3.rs-416340/v1 [DOI] [PubMed]
- Zou K, Li Z, Zhang Y, et al. Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol Sin. 2017;38:157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]