Abstract
Background and Aims:
There is significant interindividual variation in the dose of propofol required for anesthetic induction. Factors dictating this are poorly described, but understanding them would be useful for anesthetic drug dosing. It has been shown in rats and recently in humans that caffeine administration accelerates recovery from anesthesia, but no study has assessed the effect on anesthetic induction.
Material and Methods:
Forty American Society of Anesthesiologists (ASA)-I, 18–65-year-old patients, undergoing day case general anesthesia with propofol and fentanyl took part in this observational study. Total daily caffeine intake (mg) was estimated using the caffeine assessment tool and caffeine content values from the US Department of Agriculture National Nutrient Database. Pharmacokinetic–pharmacodynamic modeling was used to estimate the effect site concentration of propofol at loss of consciousness (Ce(p) LOC).
Results:
Median (interquartile range [IQR]) daily caffeine intake was 106 (51–193) mg. Ce(p) LOC was lower in those with caffeine intake greater than or equal to the median of 106 mg (median (IQR) = 0.64 μg/ml (0.51–0.72) vs. 0.70 μg/ml (0.57–1.10), P = 0.04). The effect was robust when controlling for weight-adjusted fentanyl dose, age, smoking status, and alcohol intake (F (1,34) = 4.66, P = 0.04).
Conclusion:
High daily caffeine intake is associated with lower propofol requirements for day case anesthetic induction. We propose that high daily caffeine intake may cause lower arousal levels prior to surgery due to a relative caffeine deficit caused by being nil by mouth. As such, assessment of daily caffeine intake preoperatively may aid anesthetic drug dosing.
Keywords: Anesthesia, caffeine, propofol
Introduction
There is significant interindividual variability in the behavioral response to a given dose of the anesthetic induction agent, propofol. Part of this can be explained by differences in hepatic,[1] renal,[2] and cardiac[3,4] functions, as well as differences in body weight and tissue composition.[5] However, there are still large differences in the behavioral response to the drug among individuals at a given steady-state blood concentration.[6] The factors that dictate this are poorly described, but would be useful for predicting and optimizing drug dosing in clinical anesthesia. Indeed, evidence suggests that excessive anesthetic doses are associated with higher levels of intraoperative hypotension, longer postoperative recovery times, and increased length of hospital stay.[7]
Factors that have been reported to affect the dose of anesthetic required for induction include age,[8] ethnicity,[9,10] chronic alcoholism,[11] smoking,[12] and neurological diseases including Parkinson’s disease[13] and epilepsy.[14] The effect of anxiety on anesthetic requirements has been extensively investigated and the results have been conflicting.[15,16]
Studies in rats have shown that caffeine can increase the speed of recovery from intravenous or inhalational anesthesia,[17] and a recent, albeit small, clinical trial has suggested that high doses of intravenous caffeine may accelerate recovery from inhalational anesthesia in humans.[18] No studies have assessed the effect of typical dietary caffeine intake on anesthetic requirements for any type of induction. It is known, however, that overnight deprivation of caffeine in those accustomed to regular caffeine intake can cause a morning withdrawal state with significant effects on cognitive performance and arousal.[19,20,21]
The aim of this study was to elucidate if differences in patients’ typical daily caffeine intake affect their propofol requirement for anesthetic induction in routine day case surgery.
Material and Methods
Institutional review board approval was obtained from the London Bridge Research Ethics Committee (REC reference 18/LO/0541, IRAS 228141). The study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice, and Local Regulatory Requirements, with written informed consent obtained from all participants. The study was registered before patient recruitment began (ClinicalTrials.gov NCT03453099; date of registration: 05/03/2018).
This manuscript adheres to the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting of observational studies. For this single-center, prospective, cohort study, all patients attending anesthetic preassessment clinic at a single teaching hospital between April and September 2018 were screened for eligibility. Inclusion criteria were American Society of Anesthesiologists (ASA) physical status I or II adult patients (>18 years of age), scheduled for elective day case general anesthesia, with a fentanyl and propofol-only induction regime. Exclusion criteria were administration of any induction medication other than propofol or fentanyl prior to loss of consciousness (LOC), obesity (body mass index [BMI] >29.9), pregnancy, and physician-diagnosed neurological disease, substance abuse, cardiac disease, renal disease, or hepatic disease. Potentially eligible patients who agreed to be contacted via telephone by the study team were provided with an information leaflet by the preassessment nursing team. Patients were then contacted by telephone at least 2 weeks prior to their intended operation to discuss study participation. For eligible patients interested in participating, the written consent process was completed on the morning of the operation.
The primary explanatory variable was patients’ typical daily caffeine intake. On the day of surgery but prior to entering the anesthetic room, all participants completed a demographic questionnaire as well as the caffeine assessment tool (CAT),[22,23] a caffeine questionnaire previously validated for a UK population, but subsequently validated for wider population groups.[24] The questionnaire asks participants to estimate their daily and weekly intake of multiple different types of prespecified coffee, tea, energy drink, and chocolate products, in terms of standard size cups, bottles, or bars, depending on the product in question. The questionnaire requires clarification whether each product is a caffeinated or decaffeinated variety and any other caffeine-containing food or drink products not explicitly mentioned in the questionnaire can also be included using a free-text entry. Questionnaires regarding smoking status and alcohol intake were also administered. Questionnaire responses were placed in sealed envelopes until the end of the study period to ensure appropriate blinding. Caffeine questionnaire responses were converted to estimates of typical daily caffeine intake using caffeine content values from the United States Department of Agriculture (USDA) Food Content Database.[25]
The primary outcome variable was effect site propofol concentration (Ce(p)) at the point of LOC (Ce(p) LOC). In the anesthetic room, preinduction heart rate (HR) and blood pressure (BP) were recorded. At the time of commencement of anesthetic induction, a stopwatch was started and participants were asked to count backward from 100. The timing and dose of all drugs administered were recorded, as was the time of LOC, classified as the point of loss of vocal response (cessation of counting) and confirmed by loss of eye opening on verbal command.
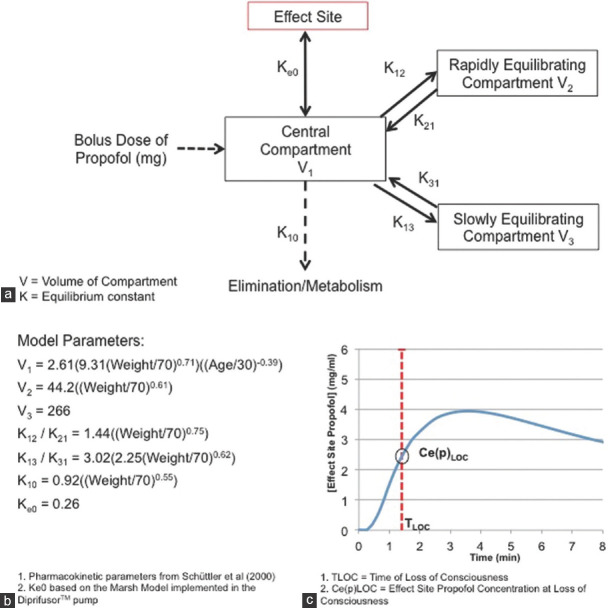
Estimates of Ce(p) across time were calculated from the timing, dose, and rate of drug administration using compartmental modeling [Figure 1]. The pharmacokinetics of propofol are known to be well described by a three-compartment model. The rate of administration of propofol was reflected by the rate of introduction of the drug into the central compartment and the model parameters used were obtained from a large observational study, which included an adjustment for bolus propofol administration.[26] Weight adjustment in this model was based on total body weight, which has not only been well validated for this model, but is also implemented in other commonly used pharmacokinetic models such as the Marsh model.[26] Moreover, all patients had their BMI calculated on the morning of surgery and no patients with a BMI >29.9 were recruited into the study. For pharmacodynamic modeling, the Ke0 (0.26 min − 1) from the Marsh model implemented in the Diprifusor™ pump (AstraZeneca, London, UK) was used. Modeling was undertaken using the Simbiology toolbox implemented in the Matlab Software Package (Mathworks Ltd 2018a). Ce(p) LOC was extracted for each participant and used as the outcome variable for analysis.
Figure 1.
Compartmental modeling of propofol. (a) Graphical representation of the model structure, involving a standard three-compartment model with an additional effect site compartment. (b) Pharmacokinetic and pharmacodynamic parameters used for the modeling. (c) Output of the model with effect site propofol concentration plotted over time and interpolation of the effect site propofol concentration at loss of consciousness
All statistical analyses were performed using the R statistical software package.[27] All continuous variables were assessed for normality using the Shapiro–Wilk normality test. All normally distributed continuous variables are presented as mean (standard deviation [SD]), continuous variables that are not normally distributed are presented as median (interquartile range [IQR]), and categorical variables are expressed as number (%). Since there is no clear definition in the literature of what constitutes high and low daily caffeine intake, for analysis, the participants were separated into two groups based on a daily caffeine intake greater than and less than the median intake. Median Ce(p) LOC was then compared between the two groups using a Mann–Whitney U test.
Analysis of covariance (ANCOVA) was then used to assess whether any difference between groups was subject to confounding by predetermined covariates of interest, namely, weight-adjusted fentanyl dose (μg/kg), age, smoking status (current smokers compared to current nonsmokers), and alcohol intake (intake twice or more per week compared to once a week or less). The significance level was defined a priori as α = 0.05. Other patient characteristics (weight, gender, ethnicity), baseline physiological variables (mean arterial pressure [MAP] and HR), the initial weight-adjusted bolus of propofol (mg/kg), the time between fentanyl and propofol administration (seconds), and the rate of propofol administration (mg/s) were all independently compared between the two groups. Two-sample Welch’s t-test was used to test for a difference between normally distributed continuous variables, Mann–Whitney U test for non-normally distributed continuous variables, and Pearson’s c² test for categorical variables, as appropriate. A meaningful power calculation could not be undertaken for this study, as there is a paucity of studies estimating Ce(p) LOC or mg/kg propofol doses using a bolus induction regime. The recruitment of 70–100 patients was based on the most similar available studies looking at the differences in weight-adjusted (mg/kg) propofol doses required for a standardized fixed end point associated with anesthetic induction.[28,29,30]
Results
There were 306 potential participants identified for the study at their preassessment clinic appointment. After further screening, 91 of these were found to be fully eligible for the study, and they verbally agreed to take part when contacted by telephone. A further 51 participants were excluded on the day of surgery, giving a total of 40 eligible participants. Reasons for exclusion at each stage of the study are listed in Figure 2. Caffeine intake was not normally distributed (W = 0.74, P = 0.00). Median (IQR) caffeine intake was 106 (51–193) mg, which was used to divide the participants into a low caffeine group (daily intake <106 mg, n = 20) and a high caffeine group (daily intake ≥106 mg, n = 20). Table 1 shows the demographic data of the participants in the two groups.
Figure 2.
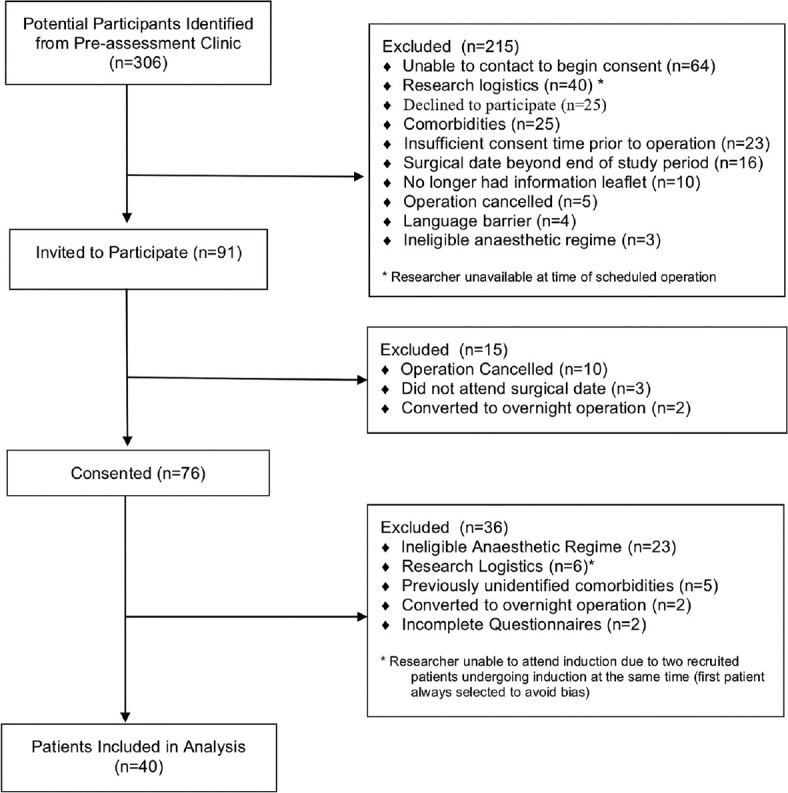
Flow diagram of patient inclusion and exclusion
Table 1.
Baseline characteristics of high and low caffeine groups
| Variable | High caffeine group (≥106 mg) | Low caffeine group (<106 mg) | Statistical comparison |
|---|---|---|---|
| Group size (n)± | 20 (50%) | 20 (50%) | - |
| Gender± | |||
| Male | 11 (55%) | 8 (40%) | χ2=0.40, P=0.53 |
| Female | 9 (45%) | 12 (60%) | - |
| Ethnicity± | |||
| Caucasian | 15 (75%) | 12 (60%) | χ2=0.46, P=0.50 |
| Other | 5 (25%) | 8 (40%) | - |
| Weight (kg)* | 80.2 (11.9) | 67.5 (13.0) | t=3.22, P=0.00 |
| Preinduction MAP (mmHg)* | 95.6 (11.9) | 97.7 (10.0) | t=-0.61, P=0.55 |
| Preinduction HR (bpm)† | 66.5 (12.3) | 79.0 (25.0) | W=139, P=0.10 |
| Daily caffeine intake (mg)† | 194 (92.0) | 50.2 (47.1) | - |
±Number (%), *Mean (SD), †Median (IQR). MAP=Mean arterial, HR=Heart rate, bpm=Beats per minute, IQR=Interquartile range, pressure, SD=Standard deviation
Ce(p) LOC was also not normally distributed (W = 0.77, P = 0.00). Ce(p) LOC was significantly lower in the high caffeine group (median (IQR) = 0.64 μg/ml (0.51–0.72) vs. 0.70 μg/ml (0.57–1.10), W = 124.5, P = 0.04) [Figure 3]. Due to the highly skewed nature of the Ce(p) LOC variable, simple mathematical transformations did not produce a normally distributed variable. Weight-adjusted fentanyl dose (W = 0.89, P = 0.00) and age (W = 0.94, P = 0.04) were also not normally distributed. As such, nonparametric ANCOVA on ranks was undertaken, with adjustment for ranked weight-adjusted fentanyl, ranked age, smoking status, and alcohol intake. Caffeine group was found to be significantly associated with ranked-Ce(p) LOC (F (1,34) = 4.58, P = 0.04). None of the other covariates was significantly associated with ranked-Ce(p) LOC [Table 2]. In separate independent analyses, there was no significant difference in gender, ethnicity, preinduction HR, or preinduction MAP between the two caffeine groups. The high caffeine group did have a higher mean weight (80.2 (11.9) vs. 67.5 (13.0) kg, t = 3.22, P = 0.003) [Table 1], but the pharmacokinetic modeling of Ce(p) LOC specifically adjusted for patient weight and further propofol requirements would normally be higher, not lower, in patients of higher weight.
Figure 3.
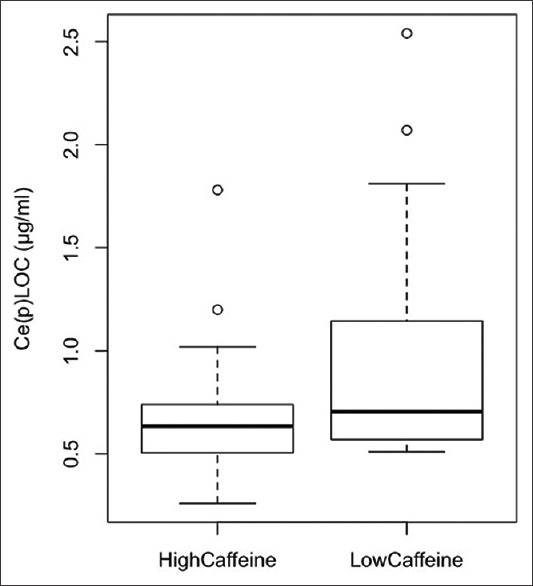
Boxplot of the difference in median Ce(p)LOC between high and low caffeine groups. Ce(p)LOC = effect site concentration of propofol at loss of consciousness
Table 2.
Nonparametric ANCOVA on ranks
| Variable | Sum of squares | Degrees of freedom | F | P |
|---|---|---|---|---|
| Caffeine group | 570 | 1 | 4.66 | 0.04* |
| Weight-adjusted fentanyl (rank) | 15 | 1 | 0.13 | 0.72 |
| Age (rank) | 438 | 1 | 3.58 | 0.07 |
| Smoking group | 135 | 1 | 1.10 | 0.30 |
| Alcohol group | 6 | 1 | 0.04 | 0.83 |
| Residuals | 4159 | 122 | ||
*Statistical significance. The significant variable is in bold to standout from the non-significant results. ANCOVA=analysis of covariance
Also, there was no significant difference in the weight-adjusted initial bolus dose of propofol between the groups (mean (SD) of high caffeine group = 2.79 (0.89) mg/kg, low caffeine group: 2.87 (0.56) mg/kg; t = −0.33, P = 0.74), the time between fentanyl and propofol administration (median (IQR) of high caffeine group: = 0.83 min (0.33–1.48), low caffeine group: 0.78 min (0.39–1.85); W = 190, P = 0.79), or the rate of administration of the main propofol bolus (median (IQR) of high caffeine group: = 4.67 (3.33–8.71) mg/s, low caffeine group: 3.91 (3.12–6.04) mg/s; W = 238, P = 0.32). Further, three patients in the low caffeine group required at least one additional bolus of propofol to be adequately anesthetized, while this was not required in any of the patients in the high caffeine group.
Discussion
Our results suggest that high daily caffeine intake is associated with lower propofol requirements for anesthetic induction. We propose that this is due to these patients, who are used to higher levels of exogenous caffeine, experiencing a lower state of arousal prior to surgery as a result of being nil by mouth.
Our results represent the first evidence that typical variation in exogenous caffeine intake can affect anesthetic induction in humans. It is in keeping with previous evidence suggesting that recovery from anesthesia is expedited by caffeine administration in both rats and humans. It is also in keeping with the widely reported observations of lower arousal after overnight caffeine deprivation in those who are used to regular daily caffeine intake. Indeed, pharmacological evidence suggests the half-life of caffeine is around 6 h,[31] meaning serum caffeine levels would be significantly reduced after the nil-by-mouth period recommended for elective day case surgery.
It should be noted that this study was observational and as such, has a number of limitations, most notably that the high caffeine group had a higher mean weight than the low caffeine group. However, several factors suggest this should not materially affect the conclusions that can be drawn. Firstly, propofol requirements would normally be expected to be higher, not lower, in patients of higher weight. Secondly, the pharmacokinetic modeling of Ce(p) LOC specifically accounts for the weight of each patient. Finally, there was no significant difference in the weight-adjusted initial propofol dose administered, which suggests that the anesthetist administering the anesthetic had appropriately accounted for the patients’ weight difference when planning propofol dosing.
Since this was an observational study, we also cannot completely exclude differences between the groups in terms of as yet undiagnosed subclinical organ dysfunction (e.g., fatty liver disease), which may have affected propofol requirements, but the age profile and exclusion of lifestyle factors such as obesity should have mitigated against this.
While the compartmental modeling used in this study is based on a very large well-validated dataset, clearly the effect site propofol concentrations are estimates and cannot match the accuracy of true blood propofol concentration measurements. Nonetheless, the validity of the result is qualitatively supported by the fact that none of the high caffeine group required further boluses of propofol to be adequately anesthetized compared to three members of the low caffeine group.
The gold standard for observational assessment of caffeine intake is a weekly food diary as this minimizes reporter bias. However, a food diary was not practical for this study as the consent process was finalized on the day of surgery. Further, the use of caffeine questionnaires has shown good concordance with food diaries and is considered to provide meaningful estimates of daily caffeine intake.[23] Regarding the measurement of the point of LOC, the use of loss of verbal response and eye opening on command in this study has been supplemented in some studies by processed electroencephalography (EEG)-based metrics, such as bispectral (BIS) index monitoring. However, EEG monitoring is not currently a part of normal day case general anesthetic practice in the UK for bolus administration of propofol, and therefore was not employed for the study. In addition, the time delay required for EEG waveform analysis means it will not accurately reflect the point of LOC when using a bolus induction regime.
It is noteworthy that the exclusion rate in this study after verbal agreement to participate (56%) was higher than anticipated. However, a significant number of exclusions were due to surgical cancellations or clinically necessary alterations in the way the anesthetic was delivered. As such, this merely reflects the observational nature of the study and the unpredictability of clinical practice.
Finally, the median difference in Ce(p) LOC between the two groups is modest at 0.06 mcg/ml and is smaller than the differences reported with neurological disease and heavy smoking. There are significant methodological differences limiting direct comparison with the studies, but, nevertheless, it would perhaps be expected that variations in patients’ exogenous caffeine intake would have a more moderate effect on propofol requirements than formal neurological pathology or, indeed, heavy smoking, which is known to induce hepatic enzyme activity.
Despite these limitations, the results do suggest that high daily caffeine intake is associated with lower propofol requirements for anesthetic induction during routine day case surgery. Larger multicenter studies are now required to verify this result and clarify the magnitude of the clinical effect. Confirmation of a significant clinical effect could lead to typical daily caffeine intake being incorporated into routine preoperative assessment and allow anesthetists to better optimize the dose of propofol administered for induction. Ultimately, this could minimize the risk of known complications of excessive anesthetic administration.
Financial support and sponsorship
National Institute of Health Research (NIHR) Imperial Biomedical Research Centre (BRC) Bursary
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vaja R, McNicol L, Sisley I. Anaesthesia for patients with liver disease. Contin Educ Anaesth Crit Care Pain. 2010;10:15–9. [Google Scholar]
- 2.Goyal P, Puri GD, Pandey CK, Srivastva S. Evaluation of induction doses of propofol:comparison between endstage renal disease and normal renal function patients. Anaesth Intensive Care. 2002;30:584–7. doi: 10.1177/0310057X0203000506. [DOI] [PubMed] [Google Scholar]
- 3.Nath SS, Tripathi M, Banerjee S. Propofol and fentanyl take longer for induction of anesthesia in aortic regurgitation:A case-controlled prospective study. J Cardiothorac Vasc Anesth. 2014;28:290–4. doi: 10.1053/j.jvca.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Adachi YU, Satomoto M, Higuchi H, Watanabe K. The determinants of propofol induction time in anesthesia. Korean J Anesthesiol. 2013;65:121–6. doi: 10.4097/kjae.2013.65.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingrande J, Brodsky JB, Lemmens HJ. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth Analg. 2011;113:57–62. doi: 10.1213/ANE.0b013e3181f6d9c0. [DOI] [PubMed] [Google Scholar]
- 6.Chennu S, O'Connor S, Adapa R, Menon DK, Bekinschtein TA. Brain connectivity dissociates responsiveness from drug exposure during propofol-induced transitions of consciousness. PLoS Comput Biol. 2016;12:e1004669. doi: 10.1371/journal.pcbi.1004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie K, Short TG. Anesthetic depth and long-term survival:An update. Can J Anaesth. 2016;63:233–40. doi: 10.1007/s12630-015-0490-0. [DOI] [PubMed] [Google Scholar]
- 8.Akhtar S, Heng J, Dai F, Schonberger RB, Burg MM. A retrospective observational study of anesthetic induction dosing practices in female elderly surgical patients:Are we overdosing older patients? Drugs Aging. 2016;33:737–46. doi: 10.1007/s40266-016-0394-x. [DOI] [PubMed] [Google Scholar]
- 9.Dahaba AA, Zhong T, Lu HS, Bornemann H, Liebmann M, Wilfinger G, et al. Geographic differences in the target-controlled infusion estimated concentration of propofol:Bispectral index response curves. Can J Anesth. 2011;58:364–70. doi: 10.1007/s12630-011-9453-2. [DOI] [PubMed] [Google Scholar]
- 10.Lampotang S, Lizdas DE, Derendorf H, Gravenstein N, Lok B, Quarles JP. Race-specific pharmacodynamic model of propofol-induced loss of consciousness. J Clin Pharmacol. 2016;56:1141–50. doi: 10.1002/jcph.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassoulaki A, Farinotti R, Servin F, Desmonts JM. Chronic alcoholism increases the induction dose of propofol in humans. Anesth Analg. 1993;77:553–6. doi: 10.1213/00000539-199309000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Lysakowski C, Dumont L, Czarnetzki C, Bertrand D, Tassonyi E, Tramèr MR. The effect of cigarette smoking on the hypnotic efficacy of propofol. Anaesthesia. 2006;61:826–31. doi: 10.1111/j.1365-2044.2006.04747.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu XP, Yu XY, Wu X, Hu XW, Chen JC, Li JB, et al. Propofol requirement for induction of unconsciousness is reduced in patients with Parkinson's disease:A case control study. Biomed Res Int. 2015;2015:953729. doi: 10.1155/2015/953729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi EM, Choi SH, Lee MH, Ha SH, Min KT. Effect-site concentration of propofol target-controlled infusion at loss of consciousness in intractable epilepsy patients receiving long-term antiepileptic drug therapy. J Neurosurg Anesthesiol. 2011;23:188–92. doi: 10.1097/ANA.0b013e31820a4f75. [DOI] [PubMed] [Google Scholar]
- 15.Maranets I, Kain ZN. Preoperative anxiety and intraoperative anesthetic requirements. Anesth Analg. 1999;89:1346–51. doi: 10.1097/00000539-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Morley AP, Papageorgiou CH, Marinaki AM, Cooper DJ, Lewis CM. The effect of pre-operative anxiety on induction of anaesthesia with propofol. Anaesthesia. 2008;63:467–73. doi: 10.1111/j.1365-2044.2007.05402.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Fong R, Mason P, Fox AP, Xie Z. Caffeine accelerates recovery from general anesthesia. J Neurophysiol. 2014;111:1331–40. doi: 10.1152/jn.00792.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong R, Wang L, Zacny JP, Khokhar S, Apfelbaum JL, Fox AP, et al. Caffeine accelerates emergence from isoflurane anesthesia in humans:A randomized, double-blind, crossover study. Anesthesiology. 2018;129:912–20. doi: 10.1097/ALN.0000000000002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James JE, Rogers PJ. Effects of caffeine on performance and mood:withdrawal reversal is the most plausible explanation. Psychopharmacology (Berl) 2005;182:1–8. doi: 10.1007/s00213-005-0084-6. [DOI] [PubMed] [Google Scholar]
- 20.Rogers PJ, Heatherley SV, Mullings EL, Smith JE. Faster but not smarter:Effects of caffeine and caffeine withdrawal on alertness and performance. Psychopharmacology (Berl) 2013;226:229–40. doi: 10.1007/s00213-012-2889-4. [DOI] [PubMed] [Google Scholar]
- 21.Barry RJ, Rushby JA, Wallace MJ, Clarke AR, Johnstone SJ, Zlojutro I. Caffeine effects on resting-state arousal. Clin Neurophysiol. 2005;116:2693–700. doi: 10.1016/j.clinph.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Boylan SM, Cade JE, Kirk SF, Greenwood DC, White KL, Shires S, et al. Assessing caffeine exposure in pregnant women. Br J Nutr. 2008;100:875–82. doi: 10.1017/S0007114508939842. [DOI] [PubMed] [Google Scholar]
- 23.The Caffeine and Reproductive Health (CARE) Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction:A large prospective observational study. BMJ. 2010;340:c2331. doi: 10.1136/bmj.a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudolph E, Farbinger A, Konig J. Determination of the caffeine contents of various food items within the Austrian market and validation of a caffeine assessment tool (CAT) Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:1849–60. doi: 10.1080/19440049.2012.719642. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Agriculture, Agricultural Research Service. FoodData Central, 2019. Available from: fdc.nal.usda.gov. [Google Scholar]
- 26.Schüttler J, Ihmsen H. Population pharmacokinetics of propofol:A multicenter study. Anesthesiology. 2000;92:727–38. doi: 10.1097/00000542-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team (2013). R Foundation for Statistical Computing, Vienna, Austria. R: A language and environment for statistical computing. Available from: http://www.R-project.org/
- 28.Ashay N, Wasim S, Bharati A. Propofol requirement for insertion of I-gel versus laryngeal mask airway:A comparative dose finding study using Dixon's up-and-down method. J Anaesthesiol Clin Pharmacol. 2015;31:324–8. doi: 10.4103/0970-9185.161666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Nishikawa T. Propofol requirement for insertion of cuffed oropharyngeal airway versus laryngeal mask airway with and without fentanyl:A dose-finding study. Br J Anaesth. 2003;90:14–20. [PubMed] [Google Scholar]
- 30.Burlacu CL, Gaskin P, Fernandes A, Carey M, Briggs L. A comparison of the insertion characteristics of the laryngeal tube and the laryngeal mask airway:A study of the ED50 propofol requirements. Anaesthesia. 2006;61:229–33. doi: 10.1111/j.1365-2044.2005.04442.x. [DOI] [PubMed] [Google Scholar]
- 31.Statland BE, Demas TJ. Serum caffeine half-lives. Healthy subjects vs. patients having alcoholic hepatic disease. Am J Clin Pathol. 1980;73:390–3. doi: 10.1093/ajcp/73.3.390. [DOI] [PubMed] [Google Scholar]