Abstract
Introduction
A family history of inflammatory bowel disease [IBD] is the strongest risk factor for disease. However, some first-degree relatives (FDRs) will develop disease, while others will not.
Methods
Using the nationwide Danish National Patient Register, we examined risk factors in families with two or more affected FDRs. First, we compared exposures between siblings with and without IBD within the same family [within-family analysis]. Second, we compared exposures between individuals with and without IBD across all families [across-family analysis]. Exposures included sex, birth order, mode of delivery, antibiotics, personal and family history of immune-mediated diseases, gastrointestinal infections, and surgical history preceding diagnosis. Uni- and multivariable conditional logistic regression analyses were conducted.
Results
In the ‘within-family analysis’, 1669 families were included [1732 cases, 2447 controls]. Female sex (adjusted odds ratio [aOR]: 1.40, 95% confidence interval [CI] 1.23, 1.59), history of ankylosing spondylitis [aOR: 2.88, 95% CI 1.05, 7.91] and exposure to antibiotics [aOR: 1.28, 95% CI 1.02, 1.61] increased the risk for IBD. In the ‘across-family analysis’, 1254 cases and 37 584 controls were included, confirming an association with prior ankylosing spondylitis [aOR: 3.92, 95% CI 1.38, 11.12] and exposure to antibiotics [aOR: 1.29, 95% CI 1.04, 1.60]. Having two or more relatives [aOR: 6.26, 95% CI 1.34, 29.29] or a sibling with IBD [aOR: 1.36, 95% CI 1.18, 1.57] increased the risk of IBD. Appendectomy reduced the risk of ulcerative colitis [aOR: 0.32, 95% CI 0.14, 0.72].
Conclusion
In families with IBD, we identified risk factors for the unaffected FDR to develop disease. These findings provide an opportunity for counselling IBD relatives.
Keywords: IBD, family history, risk factors
1. Introduction
Inflammatory bowel disease [IBD] results from a complex relationship between genetic susceptibility, environmental exposures and intestinal microbiota changes.1,2 A family history of IBD is the strongest known risk factor for developing disease.3 Other exposures such as antibiotics,4 gastrointestinal infections,5 early life risk factors6 and concomitant immune-mediated diseases [IMIDs] have been implicated in IBD risk. However, given the rarity of IBD, and the limitations of exposure ascertainment, the impact of these factors is inconsistent across studies.7 Furthermore, while previous studies have identified non-genetic risk factors for IBD in the general population, there are limited data on the impact of these factors on those at risk for IBD. Indeed, so far it remains unknown why some first-degree relatives [FDRs] of patients with IBD go on to develop disease, while others do not. Therefore, we conducted a case-control study using data from a nationwide cohort to determine non-genetic risk factors associated with IBD risk in individuals with at least one FDR with IBD.
2. Methods
2.1. Study cohort
This was a nationwide case-control study, using the prospectively recorded, linked and continuously updated data from the Danish Civil Registration System [CRS], the Danish National Patient Registry [NPR] and the Danish National Prescription Registry.8 All individuals registered in the Danish CRS who lived in Denmark between January 1, 1977 and December 31, 2018 were included.8,9 Through a unique personal identification number [the CRS number] assigned to each resident at birth, demographic, vital status and emigration data are available. Parental data, linked with that of their offspring, are also available. The Danish NPR provides nationwide longitudinal registration of detailed administrative and clinical data, and has recorded information on all patients discharged from Danish non-psychiatric hospitals since 1977 and on psychiatric inpatients and emergency department and outpatient specialty clinics dating back to January 1, 1977 and January 1, 1995, respectively.9
IBD was identified based on the International Classification of Diseases [ICD] Eighth Revision [ICD-8] or ICD Tenth Revision [ICD-10] codes on at least two separate registrations. Diagnoses were classified according to the ICD-8 until the end of 1993 and the ICD-10 thereafter.9 These administrative algorithms have been validated in previous Danish studies.10,11 The date of IBD diagnosis was defined as the date of first IBD admission or visit. Patients were considered to have Crohn’s disease [CD] if all admissions were registered as CD and likewise for ulcerative colitis [UC]. If patients were registered with both UC and CD diagnosis codes, they were categorized as IBD, but not subcategorized further.
2.2. Family assessment
The Danish CRS was used to identify families. Only children registered with both a mother and father were included. To avoid inclusion of non-biological parents, the following algorithm was used: if a person was registered with more than one mother, then the first registered mother was considered; likewise, if a person was registered with more than one father, then the first registered father was considered. Individuals with missing data on the first registered parent were excluded. Family bonds of parents, children and siblings were included. Maternal and paternal half-siblings were excluded.
2.3. Study design
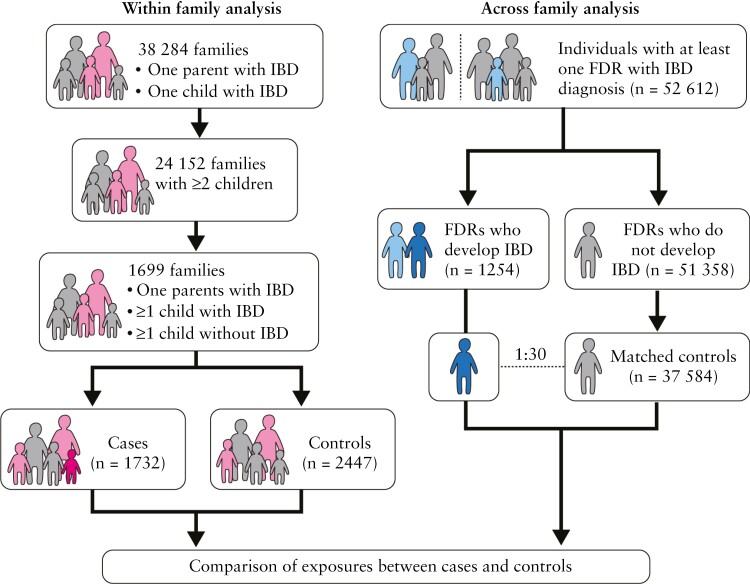
We used a matched nested case-control study design to investigate potential risk factors for IBD in families with at least two FDRs affected with IBD. For this, we pre-defined two high-risk populations [Figure 1]. First, we determined risk factors for IBD within nuclear families [within-family analysis]. For this analysis, families with at least one parent with IBD [FDR with IBD] and at least two children, of whom at least one developed IBD, were included. Cases were defined as children who developed IBD during the follow-up period, and controls were children who did not develop IBD.
Figure 1.
Study design. In the within-family analysis, families with at least one parent with IBD and one child affected were selected [cases are coloured in pink, controls in grey]. From those families, only those with at least two children, where one had IBD diagnosed, were included. Overall, there were 1699 families eligible, in whom 1732 children developed IBD [dark pink] and were compared to 2447 siblings who did not develop IBD [grey]. In the across-family analysis, individuals with at least one first-degree relative [FDR] with IBD were selected [cases are coloured in light blue]. From those individuals, 1254 developed IBD [dark blue] and were compared 1:30 to matched controls [grey]. In the within-family study children born after 1968 [the year the civil registration system was established in Denmark] were included, in order to allow us to increase sample size. In the across-family study, only children born after 1977 were included [date when the National Patient register was started].
Second, in a broader approach, we identified individuals who had at least one FDR diagnosed with IBD [mother, father, sibling or child], and from those families, we identified individuals who developed IBD during the follow-up period [cases]. These cases were compared with matched 1:30 to at-risk individuals [controls] across families [across-family analysis].
2.4. Exposures
Exposures were pre-defined based on available data [Supplementary Table 1], and included sex, birth order, mode of delivery, maternal vs paternal IBD, history of other IMIDs [iridocyclitis, asthma, type 1 diabetes, ankylosing spondylitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, multiple sclerosis, pyoderma gangrenosum, Grave’s disease, sarcoidosis, systemic lupus erythematosus, coeliac disease/dermatitis herpetiformis, primary sclerosing cholangitis, primary biliary cholangitis], depression, surgery [appendectomy, bariatric surgery or tonsillectomy] and gastroenteritis preceding IBD diagnosis. Since data on medications were only available starting in 1995, and to ensure adequate power, we chose to focus on antibiotics exposure only, which has increasingly been implicated in IBD risk.7 Using data from the National Prescription Registry, we identified antibiotic prescriptions that were prescribed prior to IBD diagnosis. We determined broad- and narrow-spectrum antibiotic exposure, and number of antibiotic courses preceding IBD diagnosis as well as maternal exposure to antibiotics during pregnancy.
2.5. Statistical analyses
2.5.1. Within-family analysis
A case [child with IBD, who has a parent with IBD] was followed from birth to IBD diagnosis date, while a control [non-IBD sibling] was followed from birth until reaching the same age as the sibling at the time of IBD diagnosis. If a control was censored [due to death or loss to follow-up] for more than 1 year before reaching the sibling’s age at IBD diagnosis date, the family was excluded, so that the siblings had similar exposure periods. We performed univariable and multivariable conditional logistic regression analyses, and adjusted the latter for sex and birth order in addition to the exposure of interest, determined a priori.
2.5.2. Across-family analysis
We identified individuals who had at least one FDR diagnosed with IBD. Of these, those who developed IBD during follow-up were cases, while those who did not develop IBD were controls. For each case, we selected 30 controls matched on sex, date of birth [±30 days] and disease-free risk time. In a few instances [0.05%] fewer than 30 controls were available [lowest ratio was 1:20]. The following restrictions were applied: the IBD diagnosis date of the FDR with IBD must have occurred before the end of follow-up in order not to condition on the future; and death, emigration and loss to follow-up must have occurred after the end of follow-up. Cases could be selected as controls until the first IBD diagnosis date. Individuals could be selected as controls multiple times. If one or more FDRs were diagnosed with IBD, the FDR with the earliest date of diagnosis was selected.
We conducted univariable and multivariable conditional logistic regression analyses; the latter was adjusted for type of FDR with IBD [mother, father or sibling] and the FDR’s IBD type [CD, UC or IBD]. We also determined if there was an interaction between antibiotic exposure and type of FDR with IBD. All covariates were determined a priori. Sensitivity analyses were performed stratified by IBD sub-type [CD vs UC]. Matches were included in the analyses of the corresponding case, only.
3. Results
3.1. Study population
Overall, 63 395 patients with IBD were identified. There were 19 706 mothers and 15 620 fathers with IBD. There were 38 284 families in which at least one parent and one child had IBD, and 24 152 families with two or more children.
3.2. Within-family study cohort
Further applying our inclusion criteria, we identified 1699 families with one parent with IBD and at least one child with IBD, and one child without IBD. Therefore, the study population consisted of 4179 individuals within 1669 families corresponding to 1732 cases and 2447 controls [Table 1]. The median [interquartile range; IQR] age at IBD diagnosis was 47.6 [35.3, 59.1] years in mothers, 51.7 [38.2, 62.9] years in fathers and 26.1 [19.6, 34.2] years in the offspring. There was a median [IQR] of two [2, 3] children per family. There was a slight predominance of female offspring in cases [56.8%] and male offspring in controls [51.7%]. The distribution of the different variables studied in siblings with and without IBD is presented in Supplementary Table 2.
Table 1.
Description of study population
| Within-family analysis; n [%] | Across-family analysis; n[%] | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Population [n] | 1732 | 2447 | 1254 | 37,584 |
| Mother | 53.4% | 52.8% | 40.9% | 43.7% |
| Father | 45.7% | 46.1% | 31.1% | 34.7% |
| Crohn´s disease | 30.2% | — | 37.6% | — |
| Ulcerative colitis | 54.4% | — | 47.7% | — |
| IBD unclassified | 15.4% | — | 14.8% | — |
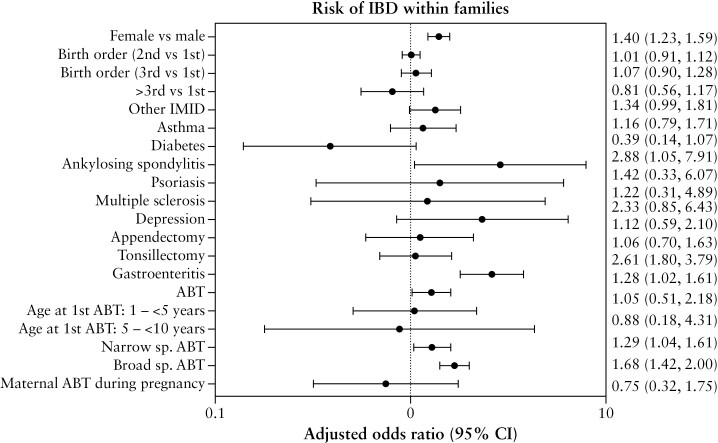
The detailed results of the univariable and multivariable conditional logistic regression are presented in detail in Supplementary Table 3. Overall, 56.8% of the children diagnosed with IBD were female as compared to 48.3% of those who did not develop IBD. In the univariable analysis, IBD diagnosis was associated with female sex (odds ratio [OR] 1.40, 95% confidence interval [CI] 1.23, 1.59), a prior diagnosis of non-IBD IMID [OR 1.35, 95% CI 1.00, 1.83] and history of any antibiotic exposure preceding IBD diagnosis [OR 1.29, 95% CI 1.03, 1.61], both narrow- and broad-spectrum antibiotics [OR 1.30, 95% CI 1.05, 1.60; and OR 1.68, 95% CI 1.43, 1.98, respectively].
On multivariable analysis [Figure 2], after adjusting for sex and birth order, IBD diagnosis remained significantly associated with female sex (adjusted OR [aOR] 1.40, 95% CI 1.23, 1.59). A history of gastroenteritis prior to IBD diagnosis was associated with a higher odds of IBD [OR 2.61, 95% CI 1.80, 3.79], but when excluding the 1-year period [OR 1.28, 95% CI 0.82, 1.98] or 2-year period [OR 1.15, 95% CI 0.73, 1.80] preceding IBD diagnosis the effect estimates decreased and were not statistically significant [Supplementary Table 3]. The odds of IBD were higher in those with a prior diagnosis of ankylosing spondylitis [aOR 2.88, 95% CI 1.05, 7.91], but not other IMIDs [Table S3]. Overall, 69.3% of the children who developed IBD were exposed to antibiotics, 65.9% to narrow-spectrum antibiotics and 47.1% to broad-spectrum antibiotics, as opposed to 67.0, 63.6 and 38.2% of those who did not, respectively. Antibiotic treatment [aOR 1.28, 95% CI 1.02, 1.61], comprising both narrow- [aOR 1.29, 95% 1.04, 1.61] and broad-spectrum antibiotics [aOR 1.68, 95% CI 1.42, 2.00], was associated with IBD. A higher number of antibiotic courses, broad- or narrow-spectrum, was associated with a higher odds of IBD [Supplementary Figure 1]. Birth order, mode of delivery and history of surgery, asthma or depression were not associated with IBD [Supplementary Table 3]. Maternal antibiotic exposure during pregnancy was not associated with IBD. History of surgery [appendectomy, tonsillectomy] or a prior diagnosis of depression were not associated with IBD [Supplementary Table 3].
Figure 2.
Multivariable conditional logistic regression in within-family analysis. Results are presented as adjusted odds ratios [OR; 95% CI] of the association of IBD with exposure of interest. IMID: immune-mediated diseases; ABT: antibiotic; sp: spectrum.
3.3. Across-family study cohort
We identified 52 612 individuals who had at least one FDR diagnosed with IBD. Of these, 16 602 FDRs were fathers, 21 281 were mothers and 14 729 were siblings. Overall, 1254 [2.38%] individuals developed IBD and were considered as cases. Of those who did not develop IBD during the follow-up period [51 358], 37 584 were selected as controls and matched 1:30 [99.5% match pairs] or 1:20 [0.05%] to cases. The IBD type in cases was more frequently UC [47.7%]. The FDR most commonly affected was the mother both in cases [40.9%] and in controls [43.7%], and the more common IBD type in the FDRs was UC in cases [52.3%] as well as in controls [60.0%]. There was no difference in the distribution of the number of relatives with IBD, birth year, or age at diagnosis or at end of follow-up between cases and controls. The median age at diagnosis or at censoring [IQR] was 21.4 [17.3; 26.5] years. Supplementary Table 4 summarizes the exposure distribution across cases and controls.
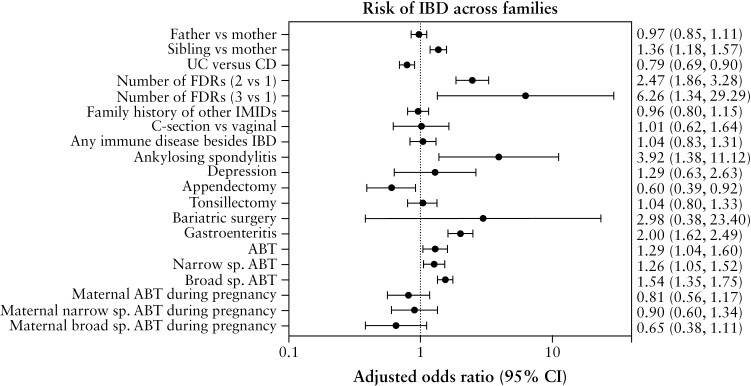
On univariable analysis [Supplementary Table 5], IBD was associated with having a sibling with IBD [OR 1.41, 95% CI 1.23, 1.63] and the odds increased with the number of FDRs affected by IBD [more than one relative: OR 2.43, 95% CI 1.83, 3.22; more than two relatives: OR 6.92, 95% CI 1.49, 32]. Additionally, IBD was associated with a prior diagnosis of ankylosing spondylitis [OR 3.87, 95% CI 1.37, 10.97] and history of any antibiotic exposure [OR 1.29, 95% CI 1.04, 1.60], including both narrow- [OR 1.27, 95% CI 1.05, 1.53] and broad-spectrum antibiotics [OR 1.54, 95% CI 1.35, 1.75]. Furthermore, for each additional antibiotic treatment the odds of IBD increased after four additional courses of antibiotic [OR 1.42, 95% CI 1.05, 1.91] and even more so with 15 courses of antibiotics [OR 2.38, 95% CI 1.81, 3.14] [Supplementary Table 5].
On multivariable analysis [Figure 3, Supplementary Table 5], after adjusting for sex, age, follow-up time, FDR with IBD and FDR’s IBD type, future IBD diagnosis was associated with having a sibling with IBD [aOR 1.36, 95% CI 1.18, 1.57], and having one or more FDRs with IBD [more than one FDR: aOR 2.47, 95% CI 1.86, 3.28; more than two FDRs: aOR 6.26, 95% CI 1.34, 29.29]. Having an FDR with UC was associated with a lower risk of developing IBD, compared to having an FDR with CD [aOR 0.79, 95% CI 0.69, 0.90]. Being the third-born [aOR 0.81, 95% CI 0.67, 0.99], compared to being the first-born, was associated with a lower risk of IBD development. While there was no association between non-IBD IMIDs and IBD overall, ankylosing spondylitis was associated with a higher risk of IBD [aOR 3.92, 95% CI 1.38, 11.12]. A prior history of gastroenteritis was associated with IBD [aOR 2.00, 95% CI 1.62, 2.49], but this finding was not statistically significant upon excluding the 1- and 2-year periods preceding disease diagnosis. A history of any antibiotic exposure [aOR 1.29, 95% CI 1.04, 1.60], including both narrow- [aOR 1.26, 95% CI 1.05, 1.52] and broad-spectrum antibiotics [aOR 1.54, 95% CI 1.35, 1.75], and in a dose-dependent manner [more than three additional antibiotic treatments: aOR 1.41, 95% CI 1.04, 1.90] was associated with IBD [Supplementary Figure 2]. Appendectomy was associated with a reduced odds of IBD [aOR 0.60, 95% CI 0.39, 0.92]. Mode of delivery, history of tonsillectomy, asthma, depression or a family history of non-IBD IMID were not associated with a higher risk of IBD.
Figure 3.
Multivariable conditional logistic regression in across-family analysis. Results are presented as adjusted odds ratios [OR; 95% CI] of the association of IBD with exposure of interest. IMID: immune-mediated diseases; ABT: antibiotic; sp: spectrum.
3.4. Stratified analysis by IBD type
We conducted univariate and multivariate stratified analyses by IBD type for all the exposures studied the within-family analysis [CD—Supplementary Table 6; UC Supplementary Table 7] and across families [CD—Supplementary Table 8; UC Supplementary Table 9].
In the within-family analysis [Supplementary Table 6], CD diagnosis was associated with female sex [adjusted OR 1.56, 95% CI 1.23, 1.98]. Both in the within- [Supplementary Tables 6 and 7] and across-family analysis [Supplementary Tables 8 and 9], similar trends as in the non-stratified analysis were observed for history of antibiotic exposure and increased risk for CD [within-family analysis: aOR 1.03, 95% CI 1.01, 1.05; across-family analysis: aOR 1.02, 95% CI 1.00, 1.03] and UC [within families: aOR 1.02, 95% CI 1.01, 1.04; across families: aOR 1.02, 95% CI 1.01, 1.02]. As previously observed in the non-stratified analysis, the risk seems to be higher especially with broad-spectrum antibiotics, with a trend for significant associations for a higher number of antibiotic courses, especially if broad-spectrum [Supplementary Tables 6 and 8].
Similar results as for the non-stratified analyses were also observed for gastroenteritis and increased risk for CD and UC, through all analyses. Likewise, an increased number of affected FDRs increased the risk for CD or UC. In the across-family analysis, with a larger number of individuals included, having a sibling was associated with CD [aOR 1.48, 95% CI 1.18, 1.86] and with UC [aOR 1.30, 95% CI 1.05, 1.61]. While in the across-family analysis in CD cases, having an FDR with UC as opposed to CD was associated with a lower risk of developing CD [aOR 0.26, 95% CI 0.48, 0.79]; in UC cases, the opposite was observed, and having a UC FDR increased the risk of UC [aOR 2.83, 95% CI 2.08, 3.87]. Finally, other history of IMIDs was associated with an increased risk of UC [aOR 23.0, 95% CI 3.69, 143.41] but not with CD.
4. Discussion
The strongest risk factor for IBD is having a family history of IBD, especially in FDRs. While many environmental risk factors have been implicated in IBD risk in the general population, these have not been characterized in those at increased risk of IBD. In this nationwide and population-based case-control study, we report the impact of exposures and clinical risk factors in familial IBD. In the within-family analysis, we found that, compared to unaffected siblings, individuals with IBD were more likely to be women, have a history of ankylosing spondylitis and a history of antibiotic use prior to IBD diagnosis. The association was stronger with an increasing number of antibiotic courses and with exposure to broad-spectrum antibiotics. In the across-family analysis, in which controls were not required to be siblings, the above associations held, with similar magnitude of estimates. IBD risk was higher if the affected FDR was a sibling, and if there were two or more FDRs affected with IBD. Additionally, appendectomy was associated with a reduced risk of UC but not CD. In this at-risk population, birth order, mode of delivery, depression, tonsillectomy, family history of immune-mediated diseases, immune-mediated diseases other than ankylosing spondylitis, and maternal exposure to antibiotics during pregnancy were not associated with future IBD diagnosis.
Our two-step analysis allowed for study of environmental risk factors within and across families at risk for IBD and confirmed that factors implicated in IBD risk in the general population are also relevant in a selected at-risk population. We found an aOR of 1.40 for females to develop disease in those with a maternal or paternal history of IBD. This risk was also observed when stratifying the analysis by IBD type. This finding is interesting given the sex-based differences in IBD risk described in population-based cohorts.12,13 Indeed, in Western populations females have a reportedly lower risk of CD during childhood, until the age of 10–14 years, but increasing thereafter.12 To our knowledge, this is the first time that within a family with a parent with IBD, the sex of the offspring has been linked with IBD risk.
While several studies have shown that IMIDs tend to coexist, the individual impact of each immune-mediated disease is less clear. Few studies have shown that a preceding IMID may increase the risk of IBD14 and may affect disease course.15 In the setting of a family history of IBD, ankylosing spondylitis increased the risk of future IBD, albeit with wide confidence intervals, and not reaching significance in the stratified analysis by IBD type. In line with an increased risk of immune-mediated disease [IMIDs] being associated with a future risk of IBD, we observed an increased risk of UC with the presence of other IMIDs. The inconsistency of risk estimates across analyses may be due to low prevalence of these conditions and warrants future studies. Nonetheless, FDRs of those with IBD who present with ankylosing spondylitis or other immune-mediated conditions may warrant close monitoring or a lower threshold of suspicion for IBD if gastrointestinal symptoms ensue.
Through the across-family analysis we further explored the impact of IBD relatives’ features on IBD risk. Individuals with a sibling with IBD and those with more than two relatives with IBDs, especially if diagnosed with CD, had an increased risk of IBD. This is in line with previous studies, where the reported adjusted incidence risk ratio for CD and UC increases with increasing number of FDRs affected.3
Appendectomy was associated with a lower risk of IBD, and in the sensitivity-adjusted multivariable analysis this remained significant for UC but not for CD. This is in line with the prior observation that appendectomy performed for appendicitis in FDRs could reduce the risk of UC,16 suggesting that the mechanisms linking appendicitis, appendectomy and UC merit further mechanistic studies.
Gastroenteritis was associated with future risk of IBD, both in within- and across-family analyses, and when stratifying by disease type, but when we excluded the 1 or 2 years preceding diagnosis, the association was no longer significant. This suggests that symptoms attributed to gastroenteritis may in fact have been the initial presentation of IBD. A previous study showed that the association of IBD with a prior infection due to gastrointestinal pathogens is stronger close to the time of diagnosis.17 It is also plausible that the sudden disruption of gastrointestinal microbiota due to an infection may have been the trigger for disease initiation.
We describe an important impact of antibiotic exposure in those at risk for developing IBD. Several studies have identified an association between antibiotic exposure and risk of IBD.4,18,19 In our study, antibiotic exposure, both narrow- and broad-spectrum, was positively associated with IBD, with a clear trend towards higher risk for those exposed to multiple antibiotic courses. When excluding the 1- or 2-year period preceding IBD diagnosis after the antibiotic was given for gastroenteritis, no significant associations were found; it is possible that antibiotic courses [especially broad-spectrum courses] given very close in time before the IBD diagnosis for gastroenteritis were prescribed because of early symptoms and signs of the later diagnosed IBD. When stratifying exposure by type of IBD, we observed a positive association that was most consistent with CD development, and a more consistent signal for exposure to broad-spectrum antibiotics. Our results are consistent with a recent population-based cohort study that reported a dose-dependent relationship between antibiotic exposure and IBD.20 Re-evaluation of antibiotic prescription strategies and minimizing antibiotic use may lower the risk of IBD and act as a primary prevention strategy.
Our study has multiple strengths. We analysed multiple exposures using a nationwide population-based data.21 Previous studies have demonstrated that these data accurately capture IBD diagnosis.8,10,21 Despite the well-known risk of IBD conferred by having an affected FDR, no studies have assessed non-genetic risk factors in an at-risk population. We chose not to focus on a single exposure, but rather took a comprehensive approach looking at multiple risk factors. Using a two-step study design, we were able to identify relevant risk factors in a smaller population represented by the at-risk siblings, which we then confirmed in a larger population where all at-risk FDRs were compared with those FDRs with disease. Importantly our results align with previous published data identifying risk factors for IBD; in an umbrella review of meta-analyses, Piovani et al. found that previous exposure to antibiotics was associated with an increased risk of CD, with a dose–response relationship, appendectomy was protective against UC, while C-section or tonsillectomy were not associated with IBD.7
Our study is not without limitations. We focused on factors previously reported in the literature. We were unable to obtain data on potentially relevant variables such as tobacco smoking, diet or breastfeeding, as they are not available through administrative coding. We had a higher than expected number of IBD unclassified, which relates to our strict definition of CD and UC based on the administrative coding. We cannot exclude potential for misclassification of or for under-reporting of some of the variables analysed, as not all exposures have been previously validated in the NPR. Given the observational nature of the data and the multiple variables analysed, it is possible that we were not powered to detect differences between cases and controls for some of the variables; nonetheless, our findings are in line with previous literature and are plausible. Likewise, we did not account for multiple testing, given the explorative study design, and our results need to be interpreted with that in mind. Nonetheless, the fact that we found similar results in the within- and across-family analyses reduces the risk of fortuitous findings. Further, early life exposures such as mode of delivery were not captured in a consistent way in the NPR, limiting the analysis of these potential risk factors in the within-family cohort. Potential limitations of family linkage obtained through the CRS include the impossibility to distinguish between biological and adoptive relatives, or the impossibility to verify donor semen insemination; however, we believe that the magnitude of this misclassification is likely to be low.22 Prescription data were only available after 1995, limiting our analysis on the impact of medications to antibiotics. Given our association with female gender in the within-family analysis, it would be interesting to look at contraception and hormone replacement therapy, but due to the limitations of the National Prescription registry, we would be limited in power. Finally, although it was not the primary aim of our study, it would be interesting to look into phenotypic concordance among familial cases; however, the ascertainment of disease location in administrative coding is imperfect,23 and several previous studies have not shown a clear concordance between disease location or disease severity in pairs of relatives with CD or UC.24
In summary, we report on the impact of non-genetic exposures in families with IBD. While we need to acknowledge that the importance of a given risk factor depends on how common this risk factor is, we believe that our findings provide an opportunity for counselling patients and families with IBD, and may help to identify individuals at higher risk for IBD who could be more intensively monitored for disease development or targeted for IBD prevention strategies.
Supplementary Material
Acknowledgments
We would like to thank and acknowledge and Academic Medical Illustrator Jill Gregory, CMI, FAMI, for her wonderful help with study design figure.
Contributor Information
Joana Torres, Division of Gastroenterology, Hospital Beatriz Ângelo, Loures, Portugal; Division of Gastroenterology, Hospital da Luz, Lisboa, Portugal; The Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Catarina Gomes, Division of Gastroenterology, Hospital Beatriz Ângelo, Loures, Portugal.
Camilla B Jensen, Copenhagen Phase IV Unit (Phase4CPH), Department of Clinical Pharmacology and Center for Clinical Research and Prevention, Copenhagen University Hospital Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Manasi Agrawal, The Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Center for Molecular Prediction of Inflammatory Bowel Disease (PREDICT), Department of Clinical Medicine, Aalborg University, Copenhagen, Denmark.
Francisco Ribeiro-Mourão, Pediatrics Department, Centro Materno Infantil do Norte – Centro Hospitalar e Universitário do Porto, Porto, Portugal; Instituto de Ciências Biomédicas Abel Salazar, University of Porto, Porto, Portugal.
Tine Jess, Center for Molecular Prediction of Inflammatory Bowel Disease (PREDICT), Department of Clinical Medicine, Aalborg University, Copenhagen, Denmark; Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark.
Jean-Frédéric Colombel, The Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kristine H Allin, Center for Molecular Prediction of Inflammatory Bowel Disease (PREDICT), Department of Clinical Medicine, Aalborg University, Copenhagen, Denmark.
Johan Burisch, Gastrounit, Medical Division, Hvidovre Hospital, University of Copenhagen, Hvidovre, Denmark; Copenhagen Center for Inflammatory Bowel Disease in Children, Adolescents and Adults, Hvidovre Hospital, University of Copenhagen, Denmark.
Funding
This work was funded by Janssen Portugal as an Investigator Initiated Study.
Conflict of Interest
The authors have no competing conflicts of interest to disclose.
Author Contributions
J.T., C.G., C.B.J., K.A. and J.B. conceived and designed the study. C.B.J. and K.A. conducted data analysis. All authors contributed to data interpretation. J.T., C.G. and M.A. wrote the manuscript. All authors read the manuscript and provided critical comments, and approved the final version.
Data Availability Statement
Deidentified summarized data collected for the study will be made available on reasonable request to the corresponding author.
References
- 1. Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moller FT, Andersen V, Wohlfahrt J, et al. Familial risk of inflammatory bowel disease: a population-based cohort study 1977–2011. Am J Gastroenterol 2015;110:564–71. [DOI] [PubMed] [Google Scholar]
- 4. Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol 2014;109:1728–38. [DOI] [PubMed] [Google Scholar]
- 5. Axelrad JE, Olen O, Askling J, et al. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case-control study. Clin Gastroenterol Hepatol 2019;17:1311. [DOI] [PubMed] [Google Scholar]
- 6. Agrawal M, Sabino J, Frias-Gomes C, et al. Early life exposures and the risk of inflammatory bowel disease: systematic review and meta-analyses. EClinicalMedicine 2021;36:100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 2019;157:647–59.e4. [DOI] [PubMed] [Google Scholar]
- 8. Lo B, Vind I, Vester-Andersen MK, et al. Validation of ulcerative colitis and Crohn’s disease and their phenotypes in the Danish National Patient Registry using a population-based cohort. Scand J Gastroenterol 2020;55:1171–5. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fonager K, Sørensen HT, Rasmussen SN, et al. Assessment of the diagnoses of Crohn’s disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol 1996;31:154–9. [DOI] [PubMed] [Google Scholar]
- 11. Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980–2013: a nationwide cohort study. Aliment Pharmacol Ther 2017;45:961–72. [DOI] [PubMed] [Google Scholar]
- 12. Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-based differences in incidence of inflammatory bowel diseases – pooled analysis of population-based studies from Western Countries. Gastroenterology 2018;155:1079–89.e3. [DOI] [PubMed] [Google Scholar]
- 13. Shah SC, Khalili H, Chen CY, et al. Sex-based differences in the incidence of inflammatory bowel diseases-pooled analysis of population-based studies from the Asia-Pacific region. Aliment Pharmacol Ther 2019;49:904–11. [DOI] [PubMed] [Google Scholar]
- 14. Lo CH, Khalili H, Lochhead P, et al. Immune-mediated diseases and risk of Crohn’s disease or ulcerative colitis: a prospective cohort study. Aliment Pharmacol Ther 2021;53:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol 2019;17:2704–12.e3. [DOI] [PubMed] [Google Scholar]
- 16. Nyboe Andersen N, Gortz S, Frisch M, et al. Reduced risk of UC in families affected by appendicitis: a Danish national cohort study. Gut 2017;66:1398–402. [DOI] [PubMed] [Google Scholar]
- 17. Axelrad JE, Olén O, Askling J, et al. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case-control study. Clin Gastroenterol Hepatol 2019;17:1311–22.e7. [DOI] [PubMed] [Google Scholar]
- 18. Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Virta L, Auvinen A, Helenius H, et al. Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease—a nationwide, register-based Finnish case-control study. Am J Epidemiol 2012;175:775–84. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen LH, Örtqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol 2020;5:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bager P, Gortz S, Feenstra B, et al. Increased risk of inflammatory bowel disease in families with tonsillectomy: a Danish national cohort study. Epidemiology 2019;30:256–62. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. [DOI] [PubMed] [Google Scholar]
- 23. Kim ES, Tarassishin L, Eisele C, et al. Longitudinal changes in fecal calprotectin levels among pregnant women with and without inflammatory bowel disease and their babies. Gastroenterology 2021;160:1118–30.e3. [DOI] [PubMed] [Google Scholar]
- 24. Santos MPC, Gomes C, Torres J. Familial and ethnic risk in inflammatory bowel disease. Ann Gastroenterol 2018;31:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified summarized data collected for the study will be made available on reasonable request to the corresponding author.