Summary
Background
Spinal cord injury (SCI) causes neural disconnection and persistent neurological deficits, so axon sprouting and plasticity might promote recovery. Soluble Nogo-Receptor-Fc decoy (AXER-204) blocks inhibitors of axon growth and promotes SCI recovery in animals. This first-in-human and randomized trial sought to determine AXER-204 safety and pharmacokinetics in chronic SCI, and secondarily its effect on recovery.
Methods
We conducted a two-part study in adults with chronic (>1 year) cervical traumatic SCI at six USA rehabilitation centers (ClinicalTrials.gov NCT03989440). In the open label first part, AXER-204 was delivered as single intrathecal doses of 3 mg, 30 mg, 90 mg, or 200 mg with primary outcomes of safety and pharmacokinetics. The randomized, parallel, double-blind second part compared six repeated intrathecal doses over 104 days of 200 mg AXER-204 versus placebo with 1:1 central randomization. The part 2 primary objectives were safety and pharmacokinetics, with a key secondary objective to assess change in International Standards for Neurological Classification of SCI (ISNCSCI) Upper Extremity Motor Score (UEMS) at Day 169 for all enrolled participants.
Findings
We treated 24 participants in part 1 (6 per dose; 18 men, 6 women), and 27 participants in part 2 (13 placebo, 14 AXER-204; 23 men, 4 women), between June 20, 2019 and June 21, 2022. There were no deaths and no discontinuations due to an adverse event. For part 2, treatment-related adverse events were of similar incidence in AXER-204 and placebo groups (10 [71%] and 9 [69%], respectively). Headache was the most common treatment-related adverse event (5 [21%] in part 1, 11 [41%] in part 2). In part 1, AXER-204 reached CSF levels exceeding those in efficacious animal studies. In part 2, mean changes from baseline to Day 169 in ISNCSCI UEMS were 1.5 (SD=3.3) for AXER-204 and 0.9 (SD=2.3) for placebo (mean difference 0.54, 95% CI: −1.48, 2.55, p=0.59).
Interpretation
This study delivers the first, to our knowledge, clinical trial of a rationally designed pharmacological treatment for neural repair in chronic SCI. AXER-204 appeared safe and reached targeted CSF concentrations with exploratory biomarkers consistent with target engagement and synaptic stabilization. Post hoc analyses indicate a need for future efficacy trials in moderate severity SCI patients without prior AXER-204 exposure.
Funding
Wings for Life, NINDS, NCATS, NIDA and ReNetX Bio.
Introduction
Traumatic spinal cord injury (SCI) occurs most frequently in motor vehicle accidents, but also in sports, military, violence, industrial and other settings. For incomplete injury and during the first year after injury, there is typically some improvement, but any change beyond 1 year post-injury is limited. In the USA, the annual incidence of SCI is about 90,000 and the prevalence exceeds 2,500,000 individuals1. Management today focuses on minimizing secondary complications, on physical therapy and on teaching compensatory function. Both cellular transplantation and electrical stimulation have been explored for SCI recovery, but these methodologies have not entered standard clinical practice. There remains no pharmaceutical intervention to enhance neural repair and neurological function.
At a cellular level, SCI causes tissue damage at one or several levels of the spinal cord, but most of the CNS remains intact. The widespread neurological deficits are largely attributable to disconnection between surviving neurons. For this reason, interventions to promote axonal growth via plasticity, sprouting or regeneration hold the potential to improve function in patients with chronic SCI, although this hypothesis has not been tested in previous randomized, double-blind, placebo-controlled trials. Failure of axon growth in the adult mammalian CNS is known to depend on both intrinsic cell autonomous growth state and extracellular environmental factors2–4. Multiple cell types contribute to the inhibitory milieu of the adult CNS, including oligodendrocytes, and nonclinical studies have shown that three oligodendrocyte-derived proteins, Nogo (Rtn4A), MAG and OMgp, participate in axon growth inhibition and that all can act via a receptor, NgR1 (Rtn4R), associated with the neuronal membrane5–9. Deletion of NgR1 expression from uninjured adult mice restores juvenile levels of neural plasticity for ocular dominance10, for acoustic preference11,12, for fear extinction13 and for cortical dendritic spine turnover14 without impaired function. Importantly, genetic deletion of these inhibitors, or their receptor, allows greater recovery from a range of mouse CNS injuries15–21.
A soluble decoy fragment of NgR1 containing the ligand-binding domain fused to the Fc domain of IgG1 has been developed as AXER-20418,19,22–24. This protein biologic blocks the three oligodendrocyte ligands, thereby protecting the cognate receptor on neurons from activation, which would otherwise suppress axon growth. The efficacy of intrathecal AXER-204, or a rat orthologue, has been demonstrated in multiple models of rat CNS injury and in non-human primate SCI. The data include evidence for axon growth and functional recovery after acute, subacute and chronic administration after spinal hemisection or contusion18,22,24, dorsal root crush25,26, optic nerve crush18,19, glaucoma19, and stroke17. Moreover, animal toxicology work has revealed a broad safety margin between efficacious and tolerated doses23.
Our trial initiates clinical development of AXER-204 for SCI recovery. We studied individuals with either complete or incomplete chronic cervical traumatic SCI, as this is the most prevalent group of SCI patients, and their deficits in hand function have a major impact on daily living27. The natural history of change in neurological function over time in the chronic state shows little variation, allowing statistical power to detect small but clinically meaningful changes27. We therefore aimed to assess the safety and pharmacokinetics of AXER-204 in participants with chronic cervical SCI.
Methods
Study design
The Protocol and Statistical Analysis Plan (SAP) are in the Supplemental Material. Study RNX-AX204–101 was a sequential 2-part study. Part 1 followed an open-label single-ascending dose design. Six patients were treated at each of 4 doses (3, 30, 90 and 200 mg), with the decision to escalate to the next higher dose group made by sponsor, investigators, and medical monitor.
Part 2 had a randomized, double-blind, placebo-controlled, repeat dose design was used. Following review of part 1 data and DSMB approval, the AXER-204 dose was 200 mg on days 1, 21, 42, 63, 84 and 104. Follow up evaluations were on days 169 and 253.
The study was conducted at five centers for part 1: Ohio State University, University of Southern California, Shepherd Center, Shirley Ryan AbilityLab and Thomas Jefferson University. For part 2, the same 5 centers plus Spaulding Rehabilitation Hospital participated. Institutional Review Board approval was obtained for each site.
Participants
For both parts, eligible patients were aged 18 to 65 years with a non-penetrating traumatic SCI that occurred at least 1 year prior. Patients had neurological impairment of upper extremities evidenced by 1) bilateral International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) upper extremity motor score (UEMS)28 between 4 and 36 points inclusive, and 2) bilateral Graded Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP, University Health Network, Toronto, ON, CA)29 prehension ability score between 4 and 17 points inclusive. An MRI scan confirming chronic SCI and CSF space spanning the lesion was required. For patients with no function caudal to the injury, remaining tissue was required. Patients from part 1 could be evaluated for part 2, provided 6 months had elapsed after part 1. Dual enrollment facilitated recruitment and was allowed because the long COVID delay between parts was hypothesized to create a new stable baseline. Gender information was by self-report. Written informed consent was obtained for all individuals before screening. Full enrollment details are in Suppl. Table S1.
Randomization and masking
Part 1 did not include placebo, was open-label and not randomized. In part 2, eligible patients were randomly assigned (1:1) to AXER-204 or placebo (isotonic phosphate buffered saline) based on a schedule prepared by a statistician. On Day 1, the patient identification number was entered in a central electronic system and a unique treatment code linked to the randomization schema assigned. Randomization was stratified in blocks of 4 by pretreatment American Spinal Injury Association Impairment Scale (AIS) grade (A-B versus C-D) and receipt of study drug in part 1 (Yes or No).
The blind was to be maintained for all blinded personnel (including investigators and patients) through Day 169. Only DSMB members, and specific designated unblinded personnel had access to unblinded information prior to database lock. Unblinded pharmacists prepared prefilled dose syringes. After Day 169 and approval of data by the medical monitor, designated sponsor staff and external contractors were unblinded for secondary efficacy assessment. The blind was maintained for site investigators, outcome assessors and patients through Day 253.
Procedures
The AXER-204 investigational product was produced under GMP22,23.
In part 1 and 2, screening was completed within 84 days from consent, and screening laboratory tests were completed within 28 days prior to Day 1. MRIs were evaluated centrally. If a patient consented to provide biobank samples, then CSF, serum and anonymized demographics were stored for future research. Biobank participation was not required. Prospective patients were required to follow medication restrictions listed in Exclusion Criteria (Suppl. Table S1).
For part 1, patients underwent a lumbar puncture (LP) and received their single dose of study drug via intrathecal lumbar slow bolus infusion on Day 1. Patients remained in-clinic until Day 4. Specified blood and CSF samples were obtained. Patients returned to clinic on Days 8, 15, and 29. On Days 8 and 29, CSF and blood samples were collected. Vital signs, AEs, and general health were assessed at each clinic follow-up. Initiation of the next higher dose group occurred after 6 patients in the prior cohort completed the 3-day in-clinic period and 2 patients completed their Day 29 visit.
In part 2, baseline assessments and initial treatment occurred on Day 1. Patients returned to Clinic on Day 21 for safety, efficacy, and pharmacokinetic assessments and for their second dose. Thereafter, patients returned at Days 42, 63, 84, and 104 for safety, efficacy, and pre-dose pharmacokinetic assessments and investigational product administration.
CSF was collected pre-dose at each dosing visit during a single LP. Blood for pharmacokinetics was collected pre-dose within 4 hours. Blood was also collected for pharmacokinetics at 4 hours post-dose at specified visits. For dosing visits that included neurological exams and questionnaires (e.g., ISNCSCI30, GRASSP29, SCIM III self-care and mobility (Loewenstein Rehabilitation Medical Center, Ra’anana, Israel)31), these assessments were prior to dosing.
Following dosing, patients remained in clinic for 4 hours. Follow-up visits occurred at Days 169 and 253.
Outcomes
In part 1 and part 2, evaluation of safety, tolerability, and pharmacokinetics of AXER-204 was the primary objective. For part 2, change from baseline of the ISNCSCI28 UEMS at Day 169 was the key secondary outcome for efficacy. A three point UEMS change has been considered an anchor point for evaluating minimal clinically important differences32. Other secondary outcomes were change from baseline in GRASSP Prehension Performance29, SCIM III self-care31 and Patient Graded Impression of Change (PGIC), each at Day 169.
Safety and tolerability measures included general examination, vital signs, routine blood chemistry and hematology, ECG, Ashworth spasticity scale, and Brief Pain Inventory. These outcomes were assessed in all patients treated with at least 1 dose.
Pharmacokinetic tests were performed on CSF and serum in all patients who received at least 1 dose of AXER-204. The lower limit of detection was 160 ng/mL. Anti-drug antibody responses were assessed in serum.
In part 2, the ISNCSCI exam for SCI28 was assessed at screening and Days 1, 21, 63, 104, 169 and 253. From the ISNCSCI, change from baseline in the bilateral UEMS was the key secondary outcome. The GRASSP test with scores assessing upper extremity strength as well as fine motor skills29 and the SCIM III subscores for self-care and mobility31 were measured at screening and Days 1, 63, 104, 169 and 253 with change from baseline as additional secondary outcomes. From the GRASSP, the Prehension Performance score was used for analyses. At Days 1, 169 and 253, a Patient Graded Impression of Change (PGIC) was collected as an additional secondary outcome. The CUE-Q for upper limb function33, the ISAFSCI34, the SF-36 v2 Health survey35, the Neuro-QOL v1.0 – Upper Extremity Function36 and other subscores of the ISNCSCI were exploratory efficacy endpoints.
Proteomic biomarker analysis of part 1 CSF was an exploratory outcome, as described in Supplementary Materials.
In part 1, exploratory efficacy measures were collected at screening and Day 29: (1) ISNCSCI exam28; (2) GRASSP test29; and (3) SCIM III subscores for self-care and mobility31.
Statistical analyses
The part 1 sample size was derived empirically from previous studies for other treatments and to achieve adequate exposure and assess safety. In part 1 and 2, safety endpoints were summarized using descriptive statistics.
For part 2, the key secondary outcome was change from baseline in bilateral ISNCSCI UEMS to Day 169. The primary analysis was based upon a Mixed Model Repeated Measures (MMRM) using a Modified Intent-to-Treat (mITT) set of all randomized patients with at least 1 dose and 1 post-baseline assessment. At completion, the mITT equaled all randomized patients. Pre-study planning to detect a 4 point ISNCSCI UEMS improvement with SD=3, alpha=0.05 and power=90% indicated that 26 completers were required. Allowing for drop out, an enrollment of about 32 was planned. When enrollment reached 27, no dropouts had occurred and the part 1 UEMS SD was <3, so enrollment ceased.
The MMRM included treatment (AXER-204, placebo), post-baseline visits (Days 21, 63, 104, 169), treatment-by-visit interaction, AIS grade (A-B versus C-D), and prior receipt of study drug in Part 1 (yes, no) as the fixed categorical effects, the baseline bilateral UEMS as a covariate, and treatment-by-baseline UEMS interaction. An unstructured covariance structure was used to model within-patient errors. Comparisons between treatments at each visit were performed with Day 169 as primary. The adjusted means for each treatment group and the estimated treatment differences with 95% CI for differences and p-values for treatment comparisons. Details are in the Supplemental Materials, and the same tests were used for other secondary endpoints.
The following preplanned subgroups were analyzed for change from baseline in ISNCSCI UEMS: AIS grade (A versus B-C-D), Received study drug in part 1 (Yes versus No), AIS grade (A-B versus C-D), and Time since injury (≤5 years versus >5 years).
We included a post hoc analysis of secondary outcomes for patients meeting both the “AIS grade (B-C-D)” plus “Did not receive study drug in part 1” criteria. Because post hoc inspection of the data showed that least square means were about 1 point lower than the arithmetic means, a repeated measures ANOVA test was utilized. Normality was confirmed by Shapiro-Wilk test. Sphericity was not assumed and Geisser-Greenhouse correction was applied.
Role of the Funding Source
The funders (Wings for Life Foundation and National Institutes of Health) had no role in study design, data collection and interpretation, decision to publish, or preparation of the manuscript.
Results
This study was completed between June 20, 2019 and June 21, 2022. Between part 1 and 2, there was a 3 month pause due to COVID-19. AXER-204 was administered to 24 patients in part 1, with 4 groups of 6 patients receiving AXER-204 at doses of 3, 30, 90, and 200 mg (Figure 1). During the subsequent part 2, 14 patients received up to 6 doses of AXER-204 at 200 mg and 13 patients received placebo (Figure 1). Of the 27 patients in part 2, nine had received AXER-204 in part 1. All patients in part 2 completed the study, although 1 patient stopped AXER-204 treatment after 3 doses having experienced nonserious Grade 2 headaches. One 90 mg patient in part 1 was unable to complete Day 29 visit due to COVID-19 travel restrictions.
Figure 1. Enrollment and Randomization of Participants.
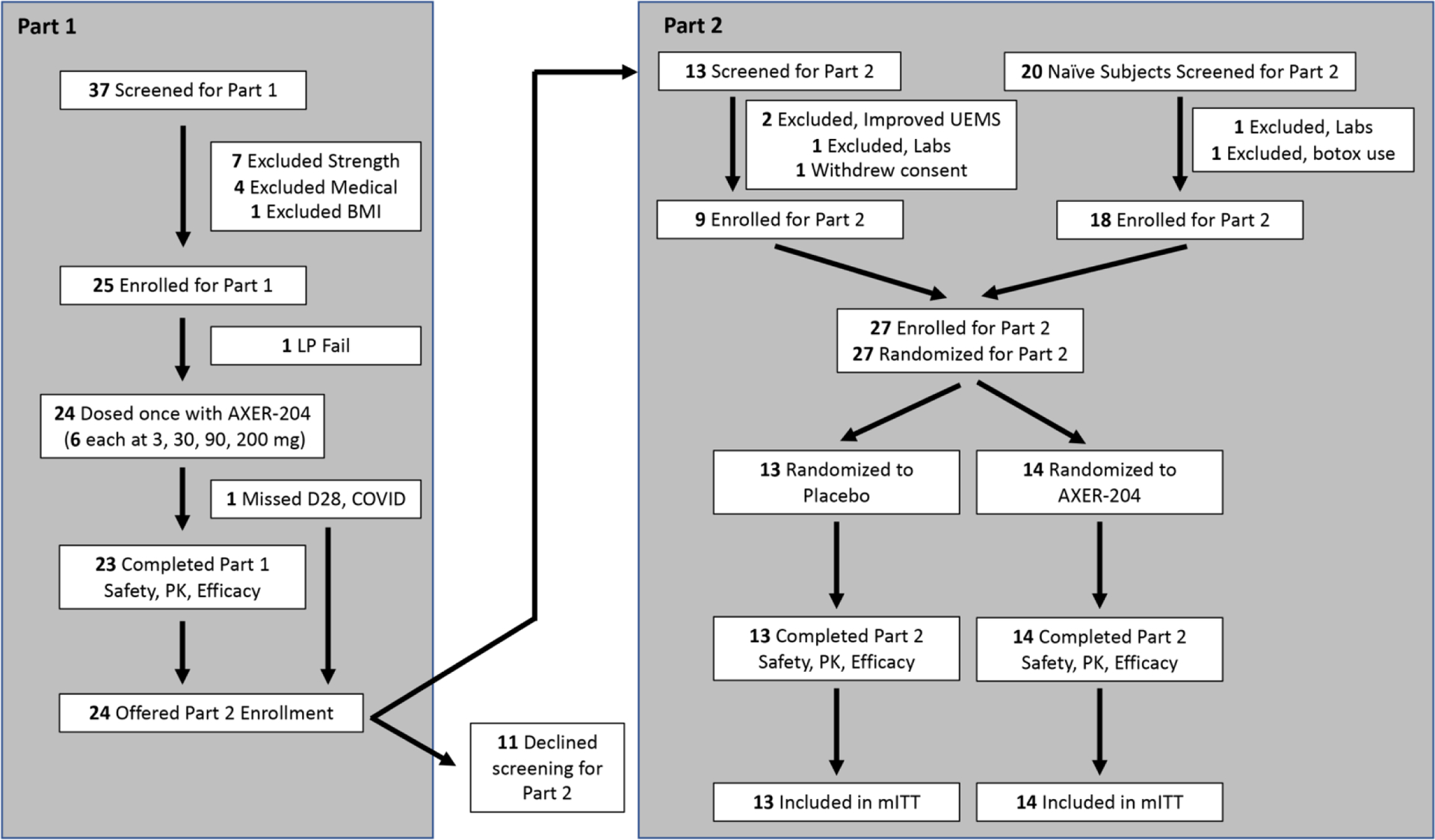
Diagram reflects the disposition of all individuals screened and enrolled in each part of the study.
Demographic characteristics were similar in parts 1 and 2, and between part 2 groups (Table 1). Mean age was 38.8 (SD=13.6) years in part 1 and 38.0 (SD=13.6) in part 2, and most were male and white. Mean time from injury was 51.5 (SD=54.9) months in part 1, and 107.8 (SD=97.2) in part 2. Patients with complete loss of motor and sensory function below the injury (AIS grade A) were comparable between part 1 and 2 (46% and 41%, respectively), with no difference between groups in part 2. In part 2, nearly all patients received the 6 scheduled doses, with a mean of 5.7 and 5.9 doses for AXER-204 and placebo, and mean treatment durations of 102.6 and 104.5 days, respectively.
Table 1:
Summary of Demographic and Baseline Characteristics – Part 1 and 2 Safety Population
| Part 1 | Part 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| AXER-204 | AXER-204 200 mg N = 14 |
Placebo N = 13 |
Total N = 27 |
|||||
| 3 mg N = 6 |
30 mg N = 6 |
90 mg N = 6 |
200 mg N = 6 |
Total N = 24 |
||||
| Age (years) | ||||||||
| Mean (SD) | 31.7 (9.1) | 44.8 (115) | 37.3 (16.0) | 41.2 (16.2) | 38.8 (13.6) | 41.1 (13.8) | 34.5 (13.2) | 38.0 (13.6) |
| Gender, n (%) | ||||||||
| Female | 1 (17%) | 0 | 1 (17%) | 4 (67%) | 6 (25%) | 1 (7%) | 3 (23%) | 4 (15%) |
| Male | 5 (83%) | 6 (100%) | 5 (83%) | 2 (33%) | 18 (75%) | 13 (93%) | 10 (77%) | 23 (85%) |
| Race, n (%) | ||||||||
| Asian | 2 (33%) | 0 | 0 | 0 | 2 (8%) | 1 (7%) | 0 | 1 (4%) |
| Black or African American | 1 (17%) | 1 (17%) | 1 (17%) | 1 (17%) | 4 (17%) | 1 (7%) | 2 (15%) | 3 (11%) |
| White | 3 (50%) | 5 (83%) | 5 (83%) | 5 (83%) | 18 (75%) | 12 (86%) | 11 (85%) | 23 (85%) |
| Ethnic, n (%) | ||||||||
| Hispanic or Latino | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 1 (8%) | 2 (7%) |
| Not Hispanic or Latin | 6 | 6 | 6 | 6 | 24 | 13 (93%) | 12 (92%) | 25 (93%) |
| Time from injury (months)a | ||||||||
| Mean (SD) | 52.7 (46.2) | 42.8 (29.3) | 65.6 (95.1) | 44.9 (37.8) | 51.5 (54.9) | 119.2 (115.2) | 95.5 (76.2) | 107.8 (97.2) |
| Median (IQR) | 36.2 (40.5) | 34.1 (44.2) | 32.5 (19.2) | 32.6 (20.0) | 35.6 (32.5) | 60.4 (102.8) | 69.5 (51.1) | 62.3 (81.0) |
| Minimum | 15.5 | 12.5 | 13.2 | 17.6 | 12.5 | 31.8 | 28.9 | 28.9 |
| Maximum | 137.6 | 83.1 | 258.5 | 119.2 | 258.5 | 397.0 | 307.4 | 397.0 |
| AIS, n (%) | ||||||||
| A | 4 (67%) | 2 (33%) | 3 (50%) | 2 (33%) | 11 (46%) | 6 (43%) | 5 (39%) | 11 (41%) |
| B | 0 | 0 | 2 (33%) | 2 (33%) | 4 (17%) | 4 (29%) | 4 (31%) | 8 (30%) |
| C | 1 (17%) | 2 (33%) | 0 | 1 (17%) | 4 (17%) | 1 (7%) | 3 (23%) | 4 (15%) |
| D | 1 (17%) | 2 (33%) | 1 (17%) | 1 (17%) | 5 (21%) | 3 (21%) | 1 (8%) | 4 (15%) |
| Neurological level of injury, n (%) | ||||||||
| C1 | 0 | 0 | 0 | 1 (17%) | 1 (4%) | 0 | 2 (15%) | 2 (7%) |
| C2 | - | - | - | - | - | 1 (7%) | 0 | 1 (4%) |
| C3 | 0 | 1 (17%) | 1 (17%) | 0 | 2 (8%) | 2 (14%) | 0 | 2 (7%) |
| C4 | 1 (17%) | 1 (17%) | 1 (17%) | 1 (17%) | 4 (17%) | 4 (29%) | 3 (23%) | 7 (26%) |
| C5 | 3 (50%) | 3 (50%) | 1 (17%) | 0 | 7 (29%) | 1 (7%) | 4 (31%) | 5 (19%) |
| C6 | 2 (33%) | 0 | 3 (50%) | 2 (33%) | 7 (29%) | 6 (43%) | 3 (23%) | 9 (33%) |
| C7 | 0 | 1 (17%) | 0 | 2 (33%) | 3 (13%) | 0 | 1 (8%) | 1 (4%) |
| Cause of SCI, n (%) | ||||||||
| Vehicular | - | - | - | - | - | 5 (36%) | 3 (23%) | 8 (30%) |
| Violence | - | - | - | - | - | 0 | 0 | 0 |
| Sports | - | - | - | - | - | 7 (50%) | 4 (31%) | 11 (41%) |
| Falls | - | - | - | - | - | 1 (7%) | 3 (23%) | 4 (15%) |
| Medical/surgical | - | - | - | - | - | 0 | 0 | 0 |
| Other | - | - | - | - | - | 1 (7%) | 3 (23%) | 4 (15%) |
| Receipt of study drug in part 1, n (%) | ||||||||
| Yes | - | - | - | - | - | 5 (36%) | 4 (31%) | 9 (33%) |
| No | 9 (64%) | 9 (69%) | 18 (67%) | |||||
Abbreviations: AIS = American Spinal Injury Association (ASIA) Impairment Scale; BMI = body mass index; Max = maximum; Min = minimum; N = number of patients in Safety Population in each treatment group; n = number of patients with valid observations; SCI = spinal cord injury; SD = standard deviation
Time from injury (months) = (Date of first dose of study treatment – Date of injury)/30.4375.
Note: Percentages were calculated based on N. Baseline was defined as the last nonmissing value before the first dose of study treatment.
For part 2, treatment-related adverse events were of similar incidence in AXER-204 and placebo groups (10 [71%] and 9 [69%], respectively, Table 2). In both parts 1 and 2, headache was the most frequent Treatment-Emergent Adverse Event (TEAE), reported in 12 patients (50%) and 19 patients (70%), respectively (Suppl. Tables S2–4). The incidence of headache appeared to be related to dose in part 1, while in part 2 headache was reported for more patients in the AXER-204 group than in placebo (13 [93%] and 6 [46%], respectively), suggesting that AXER-204 treatment is associated with headache. Nevertheless, the majority of headaches were considered to be related to LP due to their positional nature (Suppl. Table S6). Headaches deemed treatment-related were reported in 6 [43%] of the AXER-204 group and 5 [39%] of the placebo group in part 2 (Table 2), and 5 [21%] of part 1 participants (Suppl. Table S5). No Grade 3 or 4 headache was reported. One patient in the AXER-204 group had a Serious Adverse Event of Grade 2 headache with moderate pain affecting activity but not selfcare, which was considered related to the LP procedure rather than study drug.
Table 2.
Treatment-related Treatment-Emergent Adverse Events in Part 2 Safety Population.
| System Organ Class Preferred Term | AXER-204 200 mg (N = 14) n (%) |
Placebo (N = 13) n (%) |
Total (N = 27) n (%) |
|---|---|---|---|
| Patients with any SAEs | 0 | 0 | 0 |
| Patients with any AEs | 10 (71%) | 9 (69%) | 19 (70%) |
| Nervous system disorders | 8 (57%) | 8 (62%) | 16 (59%) |
| Headache | 6 (43%) | 5 (39%) | 11 (41%) |
| Paresthesia | 2 (14%) | 4 (31%) | 6 (22%) |
| Muscle spasticity | 2 (14%) | 1 (8%) | 3 (11%) |
| Pleocytosis | 2 (14%) | 0 | 2 (7%) |
| Cerebrospinal fluid leakage | 0 | 1 (8 %) | 1 (4%) |
| Neuropathy peripheral | 1 (7%) | 0 | 1 (4%) |
| Musculoskeletal and connective tissue disorders | 3 (21%) | 2 (15%) | 5 (19%) |
| Back pain | 1 (7%) | 1 (8%) | 2 (7%) |
| Muscle tightness | 1 (7%) | 1 (8%) | 2 (7%) |
| Muscular weakness | 1 (7%) | 0 | 1 (4%) |
| Myalgia | 1 (7%) | 0 | 1 (4%) |
| Neck pain | 1 (7%) | 0 | 1 (4%) |
| Gastrointestinal disorders | 1 (7%) | 2 (15%) | 3 (11%) |
| Constipation | 0 | 1 (8%) | 1 (4%) |
| Nausea | 0 | 1 (8%) | 1 (4%) |
| Odynophagia | 1 (7%) | 0 | 1 (3.7%) |
| Investigations | 3 (21%) | 0 | 3 (11%) |
| Blood pressure increased | 1 (7%) | 0 | 1 (4%) |
| Body temperature decreased | 1 (7%) | 0 | 1 (4%) |
| CSF white blood cell count increased | 1 (7%) | 0 | 1 (4%) |
| General disorders and administration site conditions | 1 (7%) | 0 | 1 (4%) |
| Fatigue | 1 (7%) | 0 | 1 (4%) |
| Injury, poisoning and procedural complications | 0 | 1 (8%) | 1 (4%) |
| Autonomic dysreflexia | 0 | 1 (8%) | 1 (4%) |
| Skin and subcutaneous tissue disorders | 0 | 1 (8%) | 1 (4%) |
| Blister | 0 | 1 (8%) | 1 (4%) |
| Vascular disorders | 1 (7%) | 0 | 1 (4%) |
| Labile hypertension | 1 (7%) | 0 | 1 (4%) |
Abbreviations: SAE = serious adverse event; AE = adverse event; MedDRA = Medical Dictionary for Regulatory Activities; N = number of patients in Safety Population in each treatment group; n = number of patients with valid observations
Notes: Percentages were calculated based on N. All AEs included in the table are treatment-related treatment-emergent adverse events. Treatment-emergent AEs were events with a start date on or after the date of first dose of study treatment, or with a start date prior to the date of first dose of study treatment whose severity worsened on or after the date of first dose of study treatment. Treatment-emergent AEs were limited to those events that occurred within 28 days after the last visit. Treatment-related AEs included AEs considered by the investigator as definitely, probably, or possibly related to study treatment or with unknown/missing relationship to study treatment. Patients with more than 1 AE within a particular SOC were only counted once in that SOC. Patients with more than one AE within a particular PT were only counted once in that PT. The table is displayed in descending overall frequency by SOC and in descending overall frequency by PT within SOC, and then alphabetically. Adverse events were coded using the MedDRA Dictionary (Version 22.0).
In general, TEAEs were most frequent in the system organ classes of nervous system disorders and musculoskeletal and connective tissue disorders. While nervous system disorders events appeared to show some dose dependency in part 1 and had a greater incidence with AXER-204 than placebo in part 2, musculoskeletal and connective tissue disorders did not. A summary of TEAEs reported as being LP-related events showed headache was the most frequent in both groups, with additional LP-related events including pleocytosis, back pain, paresthesia, muscle tightness and muscle spasticity (Suppl. Table S6). Many TEAEs reported as being LP-related were also reported as being treatment-related; headache was LP-related in 13 patients (93%) of the AXER-204 group, and treatment-related in 6 patients (43%).
In part 1, no patients experienced a Serious Adverse Event (SAE). In part 2, there were 4 SAEs in 4 patients with AXER-204 and 4 SAEs in 2 patients with placebo. Some SAEs were events that are often seen in SCI patients, such as urinary tract infection and decubitus ulcer. Most SAEs were not considered to be related to treatment; 1 treatment-related SAE was reported (constipation) in the placebo group. No patients discontinued due to an AE.
Safety laboratory parameters showed no clinically relevant hematology or clinical chemistry finding. Descriptive results from CSF analysis in part 2 showed no clinically relevant changes. Six patients in the AXER-204 group experienced TEAEs of pleocytosis. In each pleocytosis instance, more than 80% of cells were lymphocytes and the maximal count in any participant was 37 cells/μL. Each pleocytosis resolved spontaneously on subsequent LPs.
Vital signs and ECG showed no safety issue. Episodes of autonomic dysreflexia was reported in 1 patient (4%) during part 1, and 3 patients (11%) during part 2, all in the placebo group. History of autonomic dysreflexia was recorded for 7 patients (29%) in part 1, and for 5 patients (36%) in the AXER-204 group and 2 (15%) in the placebo group of part 2. One placebo patient in part 2 had 4 episodes of Grade 3 autonomic dysreflexia during the study. Most patients had abnormal baseline ECG. No abnormal ECG result during the study was considered clinically significant. Mild pyrexia was observed in 5 patients of part 1, but none in part 2, and these resolved within 6 hours with acetaminophen.
During part 1, maximum CSF AXER-204 was observed at 24 h, the first sampling (Suppl. Fig. S1A, S1C). Due to the 24 h delay after dosing, the maximal CSF concentration reported for the 200 mg dose (mean=412,000 ng/mL, SD=129,000) and exposure values (mean=12,300,000 h*ng/mL, SD=8,180,000) are likely underestimated. The mean half-life in CSF after 200 mg AXER-204 was 12.5 hours (SD=7.08). There was no evidence of nonlinearity of CSF and serum exposure across doses, although between-patient variability was observed. Serum AXER-204, reflecting systemic exposure, was generally nonquantifiable at 3 and 30 mg doses (Suppl. Fig. S1B, S1C), with mean maximal levels after 200 mg of 641 ng/mL (SD=173).
In part 2, CSF AXER-204 concentrations were below the limit of quantitation (160 ng/mL) in samples obtained immediately pre-dose (also 21 days after the preceding dose) across all treatment days, with the exception of results in 2 patients: 798 ng/mL and 258 ng/mL were observed at Day 63 and Day 104, respectively. This excludes CSF accumulation of AXER-204.
Serum concentrations of AXER-204 in part 2 were generally undetectable at pre-dose across treatment days, with the exception of 1 result, 576 ng/mL at Day 63, indicating no systemic accumulation. At 4 hours post-dose, serum concentrations were quantifiable in most patients with mean AXER-204 concentrations at different dose Days ranging from 235 ng/mL (SD=246) to 530 ng/mL (SD=855), consistent with part 1 data.
No serum anti-drug antibody responses were detected in part 1. Four patients in part 2 had low titer anti-drug antibodies, with a single patient displaying a response at more than two time points (Suppl. Table S7) that was unlikely to have impacted outcomes.
Efficacy was exploratory in part 1. Amongst 17 patients with data after single 30, 90 or 200 mg AXER-204 doses, 5 patients had an ISNCSCI UEMS increase at Day 29 of 3 points or greater (Figure 2A). For 6 patients with 3 mg AXER-204, designed to be sub-efficacious, no patients displayed such an increase. Of 13 part 1 patients rescreened for part 2, two individuals improved such that strength exceeded inclusion criteria.
Figure 2. Upper Extremity Motor Score from Parts 1 and 2.
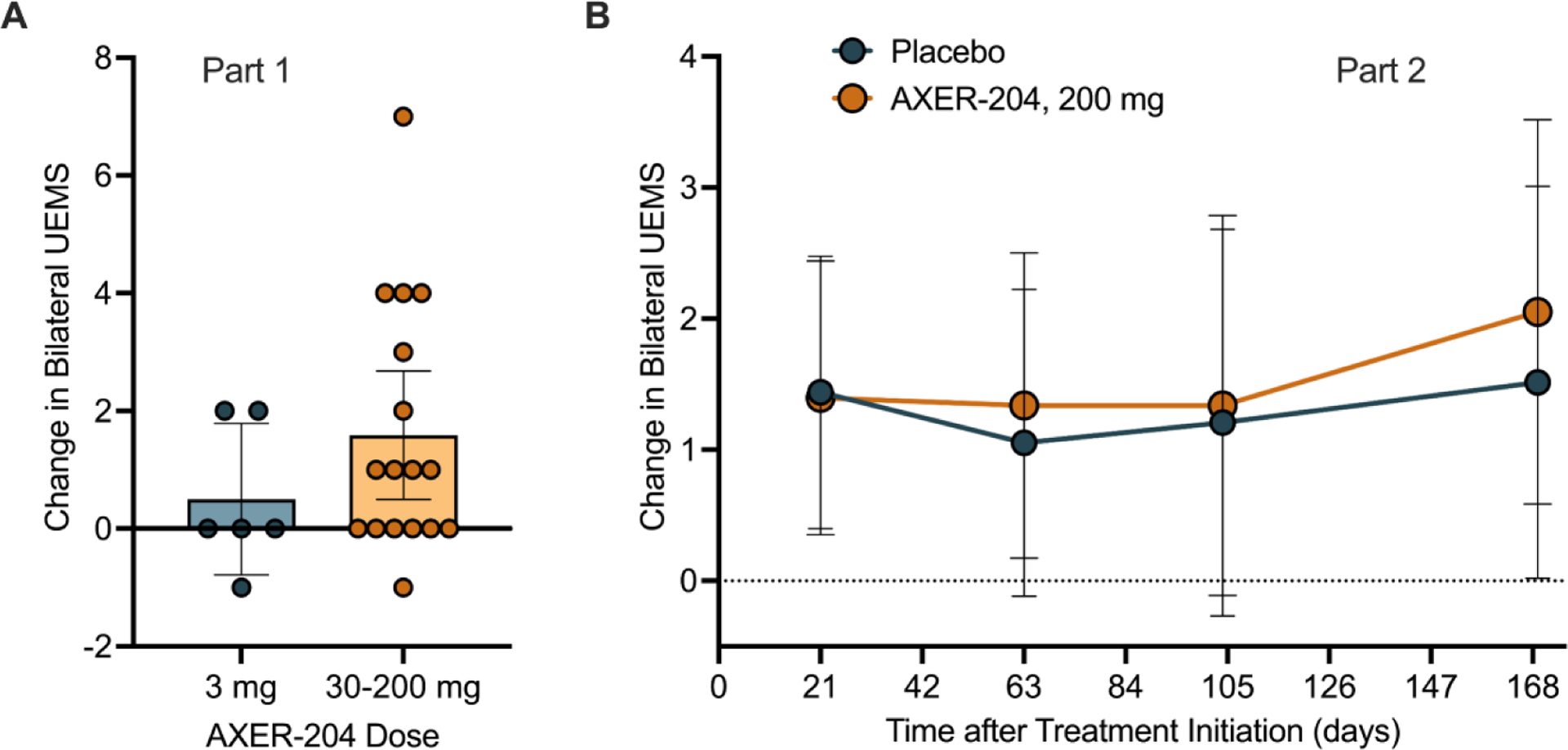
(A) Change from baseline in bilateral UEMS of the ISNCSCI examination is plotted for part 1. Bars reflect mean with ± 95% CI for indicated individuals.
(B) Change from baseline in bilateral UEMS of the ISNCSCI examination by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups. Figure shows least squares mean ± 95% CI for n =14 for AXER-204 and n=13 for placebo. Baseline was defined as the last non-missing value before the first dose of study treatment. By MRMM, non-significant.
In part 2, baseline bilateral ISNCSCI UEMS scores were similar for the AXER-204 group (26.6, SD=5.4) and the placebo group (23.9, SD=5.6), with mean changes to Day 169 of 1.5 (SD=3.3) and 0.9 (SD=2.3), respectively (LS mean difference of 0.54, 95% CI: −1.48, 2.56, p = 0.59) (Figure 2B). Scores from individual participants are illustrated in Suppl. Fig. S2A, B.
No statistically significant difference between groups was observed for other secondary endpoints, GRASSP Prehension Performance, SCIM III self-care, and PGIC response (Figure 3A–C). Exploratory efficacy endpoints, including parameters from the ISNCSCI, GRASSP, and SCIM III assessments, as well as several patient-reported outcomes and assessment of autonomic function the ISAFSCI, showed no evidence of clinically meaningful changes in patients treated with AXER-204 and no statistically significant difference between groups. Specifically, pinprick sensation from the ISNCSCI show no significant difference in patients receiving AXER-204 versus placebo (Figure 3D).
Figure 3. Secondary, Exploratory and Subgroup Efficacy Measures from Part 2.
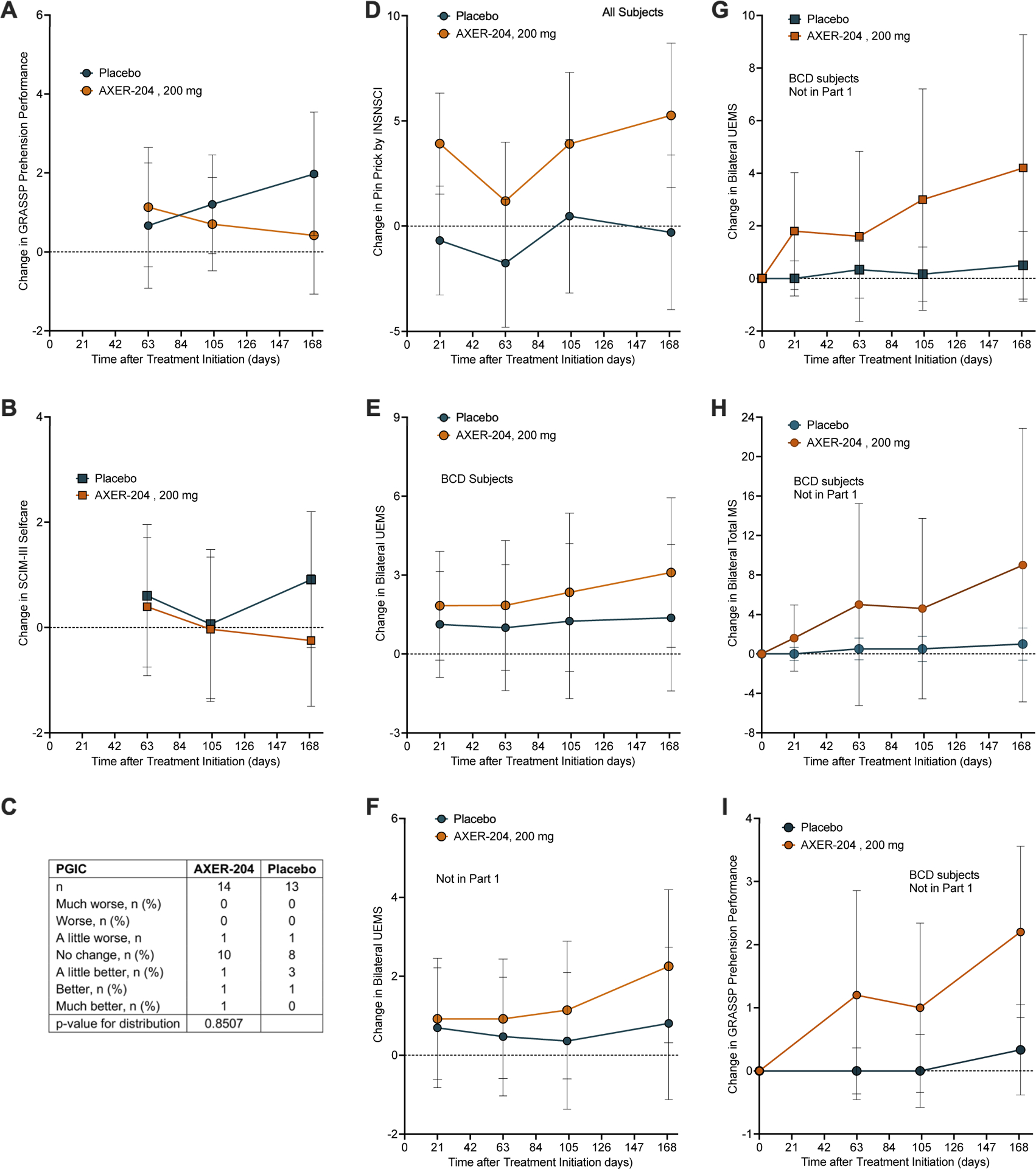
(A) Change from baseline in bilateral GRASSP Prehension Performance score by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups. Figure shows least squares mean ± 95% CI for n =14 for AXER-204 and n=13 for placebo. By MRMM, non-significant.
(B) Change from baseline in SCIM III self-care by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups. Figure shows least squares mean ± 95% CI for n =14 for AXER-204 and n=13 for placebo. By MRMM, non-significant.
(C) Patient Global Impression of Change (PGIC) from baseline to Day 169 for part 2 groups.
(D) Change from baseline in bilateral Pin Prick sensory score of the ISNCSCI examination by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups is plotted. The least squares mean ± 95% CI is plotted for n =14 for AXER-204 and n=13 for placebo. By MRMM, non-significant.
(E) Preplanned subgroup analysis for BCD participants of change in bilateral UEMS from the ISNCSCI examination. The least squares mean ± 95% CI is plotted for n=8 for AXER-204 and n=8 for placebo. By MRMM, non-significant.
(F) Preplanned subgroup analysis for participants not enrolled in part 1 of change in bilateral UEMS from the ISNCSCI examination. The least squares mean ± 95% CI is plotted for n=9 for AXER-204 and n=9 for placebo. By MRMM, non-significant.
(G) For BCD participants not enrolled in part 1, a post hoc analysis of change from baseline in bilateral UEMS from ISNCSCI examination. Data are arithmetic mean ± 95% CI for n=6 for AXER-204 and n=5 for placebo. By repeated measures ANOVA, time*group (P = 0.026), time (P = 0.032), and group (P = 0.072).
(H) For BCD participants not enrolled in part 1, a post hoc analysis of change from baseline in total MS from ISNCSCI examination. Data are arithmetic mean ± 95% CI for n=6 for AXER-204 and n=5 for placebo. By repeated measures ANOVA, non-significant.
(I) For BCD participants not enrolled in part 1, a post hoc analysis of change from baseline in GRASSP Prehension Performance and total MS of the ISNCSCI examination. Data are arithmetic mean ± 95% CI for n=6 for AXER-204 and n=5 for placebo. By repeated measures ANOVA, non-significant.
Planned subgroup analyses evaluated ISNCSCI UEMS in subgroups based on whether patients had received prior treatment with AXER-204 in Part 1, in subgroups based on baseline AIS grade, and in subgroups based on time since injury. These analyses showed no statistically significant difference between treatment groups. Visual inspection of results from those with AIS Grade of B, C, or D (Figure 3E), and those who had not received prior AXER-204 treatment (Figure 3F) led to post-hoc exploration.
Post-hoc analyses were conducted for the secondary outcomes in the small subgroup of patients meeting both of two criteria: AIS grade of B, C, or D plus no prior treatment with AXER-204 (n=6 for AXER-204 and n=5 for placebo). Repeated measures ANOVA indicated a statistically significant and clinically relevant effect on change from baseline in ISNCSCI UEMS for time*group (P = 0.026), time (P = 0.032), but no significant effect (P = 0.072) for the AXER-204 treated group compared to placebo (Figure 3G). A similar pattern was seen in post-hoc analysis of ISNCSCI total motor score (Figure 3H) and for the GRASSP Prehension Performance (Figure 3I).
Changes in the CSF proteome after AXER-204 were assessed to evaluate biomarker evidence for target engagement. By mass spectrometry, 685 distinct proteins were detected (Figure 4A, Suppl. Table S8, Suppl. Fig. S3). A subset of proteins showed significant alterations of abundance from baseline to one day after 200 mg AXER-204 (red in Figure 4A). RTN4R as a component of AXER-204 was strongly upregulated (Fig. 4A, Suppl. Table S9). Overall, more significantly down-regulated than up-regulated proteins were detected (Fig. 4A, Suppl. Table S8, S9). A heatmap of protein level changes as a function of time and dose reveals a pattern than mimics AXER-204 levels (Fig. 4B) consistent with a biological effect of AXER-204 rather than LP procedure.
Figure 4. CSF Biomarker Changes Induced by AXER-204.
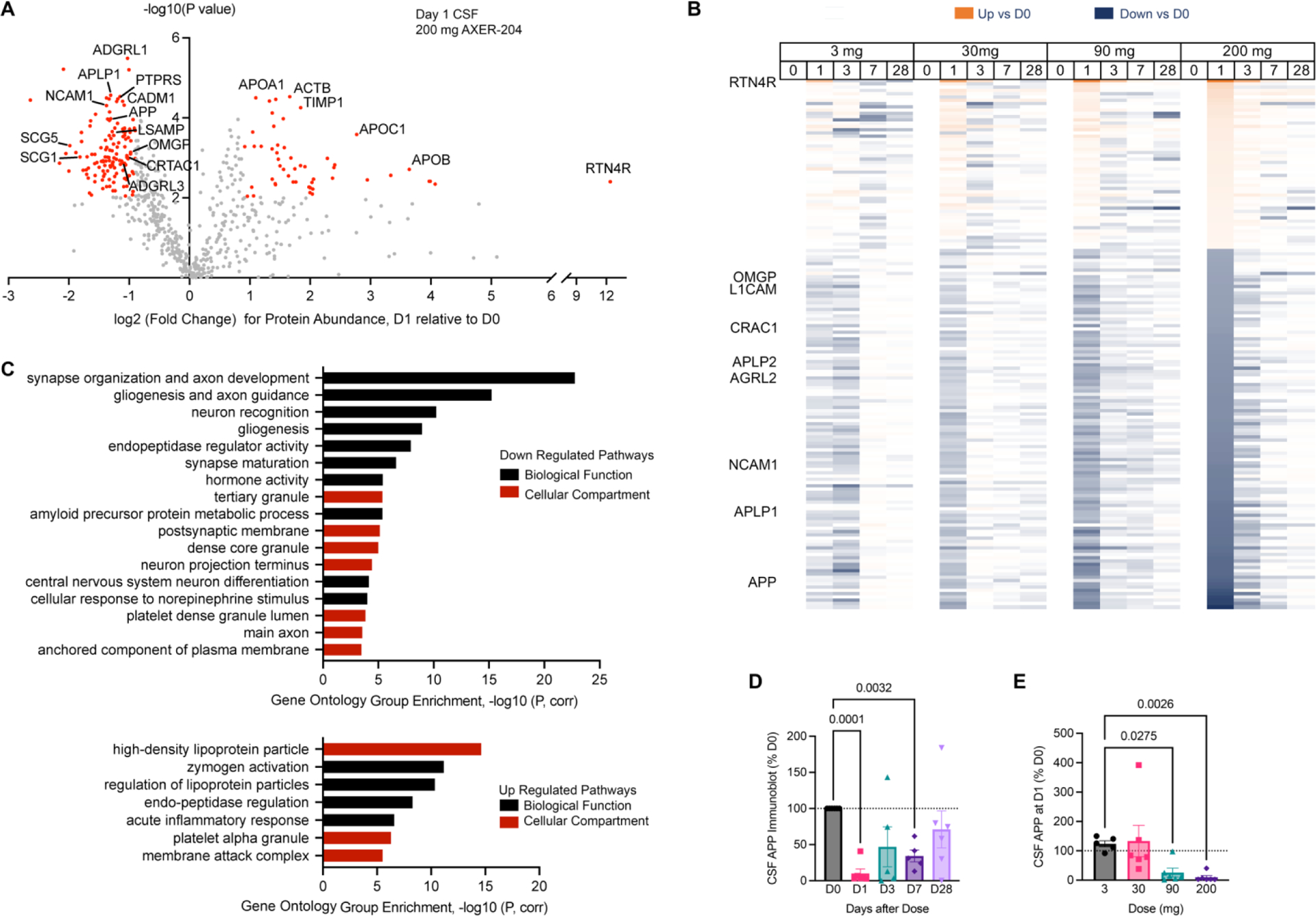
(A) Protein composition by unbiased mass spectrometry-based proteomics for all part 1 CSF samples. 685 proteins were identified with at least two peptides mapped. Values for Day 1 samples are normalized by Day 0 from the same individual receiving 200 mg dose. For each identified protein, the -log10(P value) is plotted as a function of the log2(Fold Change), and the red dots reflect those for which |log2(Fold Change)| >0.9 and False Discovery Rate P value <0.05 corrected by Benjamini-Hochberg step-down (Q=12%, and uncorrected P value of 0.01). The gray dots reflect unidentified proteins that did not meet these thresholds.
(B) Heatmap of protein levels across dose and time, showing values for up-regulated or down-regulated proteins meeting the same cutoffs as in A for 200 mg Day 1 dose are illustrated. For each protein, the values were normalized to the pre-dose value as baseline. Each box represents the average from 6 individual samples, and the intensity of the color is a linear reflection of the fold change from Day 0.
(C) Gene set enrichment of proteins significantly down-regulated or up-regulated by AXER-204 from A and B, analyzed by ClueGo44 in Cytoscape. Gene Ontology term clusters for either Gene Ontology Biological Function or Gene Ontology Cellular Compartment are shown, and Bonferroni-corrected P value for enrichment is plotted.
(D, E) Quantitation of APP level in CSF from immunoblots as a function of time after 200 mg (D) or of dose at Day 1 (E). Data are normalized by Day 0 values from the same individual, graphed as mean ± SEM. To compare the effect of AXER-204 as a function of time, data were analyzed by repeated measure ANOVA with a mixed effects model versus Day 0. D1, P=0.0001 To assess AXER-204 as a function of dose, a Kruskal-Wallis test versus the 3 mg values was used. P values <0.05 are plotted. D1, P=0.0001; D3, P=0.28; D7 P=0.0032, D28, P=0.65; 30 mg, >0.99; 90 mg, P=0.028; 200 mg, P=0.0026. Each dot represents a different CSF sample.
For down-regulated proteins, the most significantly enriched gene ontology (GO) pathways clustered under “synapse organization and axon development” and related terms (Figure 4C, Suppl. Table S10). Other enriched pathways included “dense core granule” and “endopeptidase regulator”. Up-regulated proteins were fewer and dominated by a “high density lipoprotein particle” and “zymogen activation” pathways (Suppl. Table S11).
For down-regulated proteins mapping to “synapse organization and axon development”, there were multiple protein-protein interactions (Suppl. Fig. S4). Most of these proteins are recognized as synaptic adhesion proteins, including APP, APLP1, L1CAM (CHL1), NCAM1, NRCAM, CDH2, CDH4, LPHN1 (ADGRL1), LPH3 (ADGRL3), SLITRK1 and PTPRS. Predominant up-regulated protein pathways were also assessed for protein-protein interactions with multiple interactions detected (Suppl. Fig. S5).
The synaptic adhesion proteins regulated by AXER-204 were assessed for dependence on time of dosing and dose level. Amongst the APP family, similar time and dose dependent down-regulation was observed for APP, APLP1 and APLP2 in mass spectrometric analyses (Suppl Fig. S6A–F). Because APP is a precursor to Amyloid-ß peptide of Alzheimer’s disease and CNS trauma, we assessed Amyloid-ß40 and Amyloid-ß42 by ELISA, but the CSF level did not change (Suppl Fig. S6G, H). The dose and time dependence pattern for other members of the synaptic organization group parallels the APP pattern (Suppl. Fig. S7, S8).
To validate mass spectrometric quantitation of APP and APLP1, we used immunoblot (Figure 4D, E, Suppl. Fig. S9). A signal reflecting the secreted amino terminal fragment of APP and APLP1 is detected by CSF immunoblot and decreases very substantially one day after AXER-204. Quantitation validates significant time and dose dependent reductions of APP and APLP1 (Fig. 4D, E; Suppl. Fig. S9, S10).
Discussion
This study reports the first-in-human assessment of a NgR1-based therapeutic for promoting neural repair after chronic traumatic cervical SCI. Intrathecal administration of AXER-204 was tolerated and safe at the maximal feasible dose of 200 mg in the open-label phase, and this dose was safe and tolerated in the randomized repeat dose part of the study. Headache was the most frequent adverse event and attributed to either AXER-204 or lumbar puncture.
Pharmacokinetic measures revealed high CSF levels of AXER-204 with minimal systemic exposure and no accumulation during repeat dosing. The CSF level reached by 200 mg dosing exceeded that in animal efficacy studies with successful stimulation of axon growth and recovery of neurological function22,23. While the half-life of CSF AXER-204 was about 12 hours, and AXER-204 dropped to CSF levels below the detection limit by 7 days, AXER-204 residence in CNS tissue is substantially longer in animal analyses22.
There was no statistically significant difference in the change of ISNCSCI UEMS or other SCI outcome measures across all patients. However, in a post-hoc subgroup analyses of patients with incomplete injury (AIS grade B-C-D) and those not previously treated with AXER-204 in part 1, ISNCSCI UEMS improved by 4 points and ISNCSCI Total Motor Score improved by 9 points in the AXER-204 group, whereas there was no change in these measures for the placebo group. This suggests that incomplete, as opposed to complete, injury is more amenable to therapy designed to promote axonal growth and neural plasticity. We hypothesize that there is a ceiling for benefit from AXER-204, such that part 1 participation exhausted a substantial fraction of that benefit prior to baseline measurements for part 2. Future larger randomized studies designed to explore AXER-204 efficacy to enhance neurological function in patients matching these criteria will be required.
Unique aspects of this study are the patient population and mechanistic hypothesis. Improved neurological function via neural repair in the chronic condition has not been the basis for previous randomized, double-blind, placebo-controlled trials in SCI to our knowledge. Thus, we provide baseline data regarding variability of outcomes in a multicenter trial and support the design of future trials. Antibodies directed against two ligands of NgR1, (MAG and Nogo-A (RTN4A)) have been brought to early-stage clinical trials for subacute ischemic stroke37, subacute SCI38 and ALS39. AXER-204 has a broader scope of action by blocking both inhibitors, as well as other NgR1 ligands. While the antibodies have a more limited mechanism of action, their administration was safe and tolerated, supporting our primary conclusion for AXER-204.
NgR1 has a widespread CNS distribution and animal data indicate that blocking this pathway is beneficial for glaucoma19, ischemic stroke17, multiple sclerosis40 and Alzheimer’s disease41. The safety of repeated intrathecal NgR-Fc in people with SCI suggests potential use in other indications.
Time and dose dependent CSF protein changes were observed after AXER-204 administration. Three proteins reported to interact directly with RTN4R are downregulated, OMgp (OMG)9, APP42, and CTRAC1 (CRAC1, LOTUS)43. Most prominent was a decrease in CSF levels of proteins associated with synaptic organization and axon development, including multiple synaptic adhesion proteins. The decrease occurred within one day, the measured species were soluble CSF fragments of membrane-associated proteins, and none is recognized as an immediate early gene. Therefore, we hypothesize that altered protein metabolism and distribution are responsible, rather than altered transcription or translation, and that these reduced CSF levels are coupled with elevated intact protein in tissue. Since deletion of NgR1 expression in mice promotes stabilization of new dendritic spines13,14, the data are consistent with AXER-204 preventing baseline destruction of newly formed spines to facilitate neural network plasticity. Animal studies have not yet explored the effect of AXER-204 on synaptic protein metabolism, so this will be an important area for future work.
Several factors limit this study. While the wide range of injury severity provides broad safety data, the potential benefit for incomplete SCI may have been obscured. The dual participation of some patients in part 1 and 2 complicated the efficacy analysis in the randomized part 2. Repeat dosing at more frequent intervals might provide more complete blockade of NgR1 ligands in CNS tissue. The pharmacokinetic and proteomic results are from single dose analyses, and therefore may not reflect multiple dose therapy.
AXER-204 was safe and well tolerated. Pharmacokinetic analysis detected CSF values exceeding efficacious animal doses with little systemic exposure and no accumulation. Analysis of efficacy in the randomized, double-blind, placebo-controlled part 2 of the study showed no evidence of a clinically meaningful change in any planned analysis, but post hoc analyses suggest clinically meaningful benefit of AXER-204 in treatment-naïve patients with neurologically incomplete SCI. The CSF proteome of participants receiving AXER-204 revealed prominent CSF decreases in synaptic adhesion proteins, potentially reflecting stabilization of new synaptic structures and supporting the proposed mechanism of action.
Supplementary Material
Research in context.
Evidence before this study
We systematically searched PubMed through May 10, 2023 for “NgR1 OR RTN4R” and “spinal cord injury” and “clinical trial”. There are no approved medical treatments to promote neural repair and recovery after spinal cord trauma in the subacute or chronic period. Apart from surgical stabilization and general medical management in the acute period, standard of care in the subacute and chronic state after spinal cord trauma is focused on physical therapy and supportive therapies. Devices, neurostimulation, and stem cell transplantation approaches are in development, but no pharmacological approach is known to have a proven disease-modifying benefit for neurological deficits. Animal studies have shown endogenous protein inhibitors of synaptic plasticity, axon sprouting and axon regeneration limit recovery from spinal cord trauma. Multiple glial inhibitors, including MAG, Nogo-A (RTN4A) and OMgp, act by binding to neuronal NgR1 (RTN4R). As a soluble decoy, AXER-204 blocks all NgR1 ligands that limit axonal growth and synaptic plasticity, and in multiple animal models the decoy protein supports axon growth and recovery of neurological function as a neural plasticity agonist. No previous studies have assessed the effect of targeting NgR1 (RTN4R) in human. Antibodies targeting single ligands of NgR1, either anti-MAG or anti-Nogo-A (RTN4A), have been shown to be safe and well tolerated, but efficacy has been lacking or unclear in early-stage clinical trials.
Added value of this study
This study provides the first evidence on the effects of pharmacological blockade of NgR1 function in humans. The soluble decoy AXER-204 was safe and well tolerated at doses that match animal efficacious doses. There was little systemic exposure and no accumulation on repeat dosing. Secondary analysis of efficacy in the randomized, double-blind, placebo-controlled part showed no significant change in planned analyses, but post hoc analyses suggested a potentially clinically meaningful benefit in treatment-naïve patients with neurologically incomplete spinal cord injuries. Separate from AXER-204 effects, the data expand knowledge of the natural history of chronic cervical SCI and demonstrate the feasibility of completing randomized trials in this population. The CSF proteome of participants receiving AXER-204 revealed prominent CSF decreases in synaptic adhesion proteins, potentially reflecting stabilization of new synaptic structures, and supporting the proposed mechanism of action.
Implications of all available evidence
The safety of AXER-204, a soluble decoy for NgR1, in human at pharmacologically active CSF levels facilitates future trials with this drug. While the planned efficacy analysis showed no benefit across all participants, the encouraging data from post hoc analysis of a subset coupled with biomarker evidence for altered synaptic adhesion protein levels, support further study of AXER-204 in the incomplete SCI population, as well as in other indications.
Acknowledgments
We wish to thank the study participants for making these findings possible through their enthusiasm and dedication to completing the study procedures. We also thank the contribution of insightful discussions with Drs. Daniel Lammertse, Andrew Blight and Armin Curt to the Protocol design. The authors acknowledge assistance from Amy Bartlett, Carol Eskay, Raquel Minarsch, Dr. Eric Bourekas, Dr. D. Michele Basso, Dr. Aruna Ganju, Casey Kandilakis, Freda Michelle Tidwell and other members of the study team. We thank Scott Slough, a medical writer employed by Premier Research International, for writing portions of the Clinical Study Report, from which certain text was included here. The authors thank Ann Hollmann for overall logistical support including coordination of rater training. This work was supported by grants from Wings for Life, NINDS (R44NS118974) and NCATS BrIDGs program (1 X01 TR000313-01) to ReNetX Bio, and by grants from NINDS (R35NS097283) and NIA (R01AG034924) to SMS at Yale. TTL was supported by the Yale/NIDA Neuroproteomics Center at Yale (DA018343). We also thank the Keck MS & Proteomics Resource at Yale School of Medicine for providing the necessary mass spectrometers and the accompany biotechnology tools funded in part by the Yale School of Medicine and by the Office of The Director, National Institutes of Health (S10OD02365101A1, S10OD019967, and S10OD018034). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
GM, CA, CH, GB, and ES are employees of ReNetX Bio, Inc., which holds rights to develop AXER-204. SMS is a founder of, is a consultant for, and holds equity interest in ReNetX Bio, Inc., as well as being an Inventor on NgR1 intellectual property licensed from Yale to ReNetX Bio. AEF is a principal investigator for Medical Imaging and Data Resource Center and serves on the Board of Radiological Society of North America. RZ serves on boards of Myomo, Onecare.ai, NanoDiagnostics, J Neurotrauma and Frontiers in Neurology. JS, DPL, DC, CL, RM, XW, JL, WW and TL declare no competing interests.
Data sharing
The Study Protocol and Statistical Analysis Pan are available in the Supplemental Material. Biospecimens from CSF and serum are made available through International Spinal Cord Injury (SCI) Biobank (www.sci-biobank.org). Included with these specimens, deidentified demographic data will be made available.
References
- 1.Injury GBDTB, Spinal Cord Injury C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18(1): 56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng B, Tuszynski MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nature reviews 2023. [DOI] [PubMed] [Google Scholar]
- 3.Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ 2009; 48: 339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Z, Jin Y. Intrinsic Control of Axon Regeneration. Neuron 2016; 90(3): 437–51. [DOI] [PubMed] [Google Scholar]
- 5.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 2000; 403(6768): 439–44. [DOI] [PubMed] [Google Scholar]
- 6.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001; 409(6818): 341–6. [DOI] [PubMed] [Google Scholar]
- 7.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 2002; 297(5584): 1190–3. [DOI] [PubMed] [Google Scholar]
- 8.Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol 2014; 27: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KC, Koprivica V, Kim JA, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 2002; 417(6892): 941–4. [DOI] [PubMed] [Google Scholar]
- 10.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 2005; 309(5744): 2222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang EJ, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proc Natl Acad Sci U S A 2012; 109 Suppl 2(Suppl 2): 17213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalish BT, Barkat TR, Diel EE, Zhang EJ, Greenberg ME, Hensch TK. Single-nucleus RNA sequencing of mouse auditory cortex reveals critical period triggers and brakes. Proc Natl Acad Sci U S A 2020; 117(21): 11744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhagat SM, Butler SS, Taylor JR, McEwen BS, Strittmatter SM. Erasure of fear memories is prevented by Nogo Receptor 1 in adulthood. Mol Psychiatry 2016; 21(9): 1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron 2013; 77(5): 859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 2003; 38(2): 187–99. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 2004; 44(3): 439–51. [DOI] [PubMed] [Google Scholar]
- 17.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci 2004; 24(27): 6209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Duffy P, McGee AW, et al. Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann Neurol 2011; 70(5): 805–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Lin J, Arzeno A, et al. Intravitreal delivery of human NgR-Fc decoy protein regenerates axons after optic nerve crush and protects ganglion cells in glaucoma models. Invest Ophthalmol Vis Sci 2015; 56(2): 1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink KL, Strittmatter SM, Cafferty WB. Comprehensive Corticospinal Labeling with mu-crystallin Transgene Reveals Axon Regeneration after Spinal Cord Trauma in ngr1−/− Mice. J Neurosci 2015; 35(46): 15403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci 2010; 30(20): 6825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Yigitkanli K, Kim CY, et al. Human NgR-Fc decoy protein via lumbar intrathecal bolus administration enhances recovery from rat spinal cord contusion. J Neurotrauma 2014; 31(24): 1955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhou T, Maynard GD, et al. Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury. Brain 2020; 143(6): 1697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol 2006; 60(5): 540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey PA, Lee DH, Qian F, Weinreb PH, Frank E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci 2009; 29(19): 6285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Hasan O, Arzeno A, Benowitz LI, Cafferty WB, Strittmatter SM. Axonal regeneration induced by blockade of glial inhibitors coupled with activation of intrinsic neuronal growth pathways. Exp Neurol 2012; 237(1): 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steeves JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45(3): 206–21. [DOI] [PubMed] [Google Scholar]
- 28.Krassioukov A. Introducing the revised International Standards on documentation of remaining Autonomic Function after SCI (ISAFSCI). J Spinal Cord Med 2012; 35(4): 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalsi-Ryan S, Chan C, Verrier M, et al. The graded redefined assessment of strength sensibility and prehension version 2 (GV2): Psychometric properties. J Spinal Cord Med 2019; 42(sup1): 149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirshblum SC, Waring W, Biering-Sorensen F, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011; 34(6): 547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itzkovich M, Gelernter I, Biering-Sorensen F, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil 2007; 29(24): 1926–33. [DOI] [PubMed] [Google Scholar]
- 32.Marino RJ, Sinko R, Bryden A, et al. Comparison of Responsiveness and Minimal Clinically Important Difference of the Capabilities of Upper Extremity Test (CUE-T) and the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP). Top Spinal Cord Inj Rehabil 2018; 24(3): 227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marino RJ, Patrick M, Albright W, et al. Development of an objective test of upper-limb function in tetraplegia: the capabilities of upper extremity test. Am J Phys Med Rehabil 2012; 91(6): 478–86. [DOI] [PubMed] [Google Scholar]
- 34.Wecht JM, Krassioukov AV, Alexander M, et al. International Standards to document Autonomic Function following SCI (ISAFSCI): Second Edition. Top Spinal Cord Inj Rehabil 2021; 27(2): 23–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30(6): 473–83. [PubMed] [Google Scholar]
- 36.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012; 78(23): 1860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cramer SC, Enney LA, Russell CK, Simeoni M, Thompson TR. Proof-of-Concept Randomized Trial of the Monoclonal Antibody GSK249320 Versus Placebo in Stroke Patients. Stroke 2017; 48(3): 692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucher K, Johns D, Maier D, et al. First-in-Man Intrathecal Application of Neurite Growth-Promoting Anti-Nogo-A Antibodies in Acute Spinal Cord Injury. Neurorehabil Neural Repair 2018; 32(6–7): 578–89. [DOI] [PubMed] [Google Scholar]
- 39.Meininger V, Genge A, van den Berg LH, et al. Safety and efficacy of ozanezumab in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2017; 16(3): 208–16. [DOI] [PubMed] [Google Scholar]
- 40.Petratos S, Ozturk E, Azari MF, et al. Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation. Brain 2012; 135(Pt 6): 1794–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JH, Widi GA, Gimbel DA, Harel NY, Lee DH, Strittmatter SM. Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer’s transgenic mice. J Neurosci 2006; 26(51): 13279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Gimbel DA, GrandPre T, et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci 2006; 26(5): 1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato Y, Iketani M, Kurihara Y, et al. Cartilage acidic protein-1B (LOTUS), an endogenous Nogo receptor antagonist for axon tract formation. Science 2011; 333(6043): 769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009; 25(8): 1091–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic acids research 2021; 49(D1): D605–D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Study Protocol and Statistical Analysis Pan are available in the Supplemental Material. Biospecimens from CSF and serum are made available through International Spinal Cord Injury (SCI) Biobank (www.sci-biobank.org). Included with these specimens, deidentified demographic data will be made available.


