Highlights
-
•
RET fusions are identified in 15 tumor types and carcinoma of unknown primary.
-
•
Different dominant RET fusion variant within each tumor type: KIF5B-RET (NSCLC), NCOA4-RET (colorectal, breast), and CCDC6-RET (well-differentiated thyroid).
-
•
RET fusion positive CRC had a higher median TMB and were commonly MSI-H.
Keywords: RET fusion, Pan-tumor survey, Next-generation sequencing, RNA sequencing, Selpercatinib, Pralsetinib, Selective RET inhibitors
Abstract
Background
RET fusions are driver alterations in cancer and are most commonly found in non-small cell lung cancer and well-differentiated thyroid cancer. However, RET fusion have been reported in other solid tumors.
Material and methods
A retrospective analysis of RET+ solid malignancies identified by targeted RNA sequencing and whole transcriptome sequencing of clinical tumor samples performed at Caris Life Science (Phoenix, AZ).
Results
As of March 22, 2022, a total of 378 RET+ solid malignancies were identified in 15 different tumor types and carcinoma of unknown primary (CUP) that underwent next-generation RNA sequencing. RET+ NSCLC and RET+ thyroid cancer constituted 66.9% and 11.1% of the RET+ solid malignancies, respectively. RET+ colorectal adenocarcinoma and RET+ breast adenocarcinoma constituted 10.1% and 2.6%, respectively. The estimated frequency of RET fusions within specific tumor types were NSCLC 0.7%, thyroid cancer 3.1%, colorectal cancer 0.2% and breast cancer 0.1%. KIF5B (46.8%) was the most common fusion partner followed by CCDC6 (28.3%) and NCOA4 (13.8%) in RET+ solid tumors. KIF5B-RET was the dominant fusion variant in RET+ NSCLC, NCOA4-RET was the dominant variant in RET+ colorectal carcinoma, and CCDC6-RET was the dominant variant in thyroid cancer. The most common single gene alterations in RET+ tumors were TP53 (34.8%), RASA1 (14.3%) and ARIAD1A (11.6%). RET+ CRC had a high median TMB of 20.0 and were commonly MSI-H.
Conclusions
RET fusions were identified in multiple tumor types. With a higher median TMB and commonly MSI-H, RET fusion positive CRC may be a unique molecular subset of CRC.
Introduction
Recurrent fusions of receptor tyrosine kinase (RTK) genes yielding constitutively active chimeras have been recognized as key actionable driver mutations in diverse solid malignancies [1]. Among the 58 human RTKs [2], there are US Food and Drug Administration (FDA) approved treatment for anaplastic lymphoma kinase (ALK), c-ROS1 (ROS1), rearranged during transfection (RET), fibroblastic growth factor receptor (FGFR2–3), and neutrophin receptor tyrosine kinase (NTRK1–3) fusion positive tumors. However, most of the US FDA approvals are tumor histologic specific: ALK (non-small cell lung cancer [NSCLC]), ROS1 (NSCLC), and FGFR2–3 (urothelial, cholangiocarcinoma). NTRK1–3 fusions was the first to obtain tumor agnostic treatment approval due to the extremely rare incidence of NTRK fusions and FDA has recently expanded approval of selpercatinib to include RET fusion positive tumors outside of NSCLC and thyroid cancers.
While these RTK fusions can be found in many solid tumor types albeit in a lower frequency (i.e. ALKoma [3], REToma [4]), the main biology of the pathological process is universal and not tumor histology-specific. Especially as FDA has recently expanded its approval on selpercatinib to include adult patients with advanced solid tumors harboring RET fusions, it is important to identify RTK fusions systemically beyond the specific tumor histologic type and to expand the horizon of patients with RTK fusion positive cancer who may benefit from targeted treatment. We must also strive to raise awareness among clinicians, pharmaceutical companies and regulatory authorities to screen and enroll these patients in future clinical trials.
In this study, we performed a large-scale pan-tumor survey of RET fusions detected by next generation RNA sequencing to identify characterize the molecular characteristics of RET+ solid tumors.
Materials and methods
Patient cohort
An institutional review board (IRB)–approved, retrospective assessment of a deidentified molecular profiling database was surveyed for solid tumors that underwent RNA based tumor profiling. From a cohort including all cases submitted to a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory (Caris Life Sciences) for comprehensive genomic profiling, all unique cases that underwent successful fusion testing for targeted RNA sequencing were identified. This study was conducted in accordance with guidelines of the Declaration of Helsinki, Belmont report, and U.S. Common rule. In keeping with 45 CFR 46.101(b)(4), this study was performed utilizing retrospective, deidentified clinical data.
Fusion detection
RET fusion was detected by either the ArcherDx fusion assay (Archer FusionPlex Solid Tumor panel) or the Illumina NovaSeq platform (Illumina, Inc., San Diego, CA) with the use of the Agilent SureSelect Human All Exon V7 bait panel (Agilent Technologies, Santa Clara, CA). For the ArcherDx fusion assay, formalin-fixed paraffin-embedded tumor samples were microdissected to enrich the sample to ≥20% tumor nuclei, and mRNA was isolated and reverse transcribed into complementary DNA (cDNA). Unidirectional gene-specific primers were used to enrich for target regions, followed by Next-Generation sequencing (Illumina MiSeq platform). For fusion detection using the Illumina NovaSeq platform, FFPE specimens underwent pathology review to diagnose percent tumor content and tumor size; a minimum of 10% of tumor content in the area for microdissection was required to enable enrichment and extraction of tumor-specific RNA. Qiagen RNA FFPE tissue extraction kit was used for extraction, and the RNA quality and quantity was determined using the Agilent TapeStation.
Next-generation RNA sequencing
The whole transcriptome sequencing has previously been described. Briefly, next generation sequencing (NGS) was performed on genomic DNA isolated from formalin-fixed paraffin-embedded (FFPE) tumor samples using the NextSeq platform (Illumina, Inc., San Diego, CA). A custom-designed SureSelect XT assay was used to enrich 592 whole-gene targets (Agilent Technologies, Santa Clara, CA). All variants were detected with > 99% confidence based on allele frequency and amplicon coverage, with an average sequencing depth of coverage of > 500 and an analytic sensitivity of 5%. Genetic variants identified were interpreted by board-certified molecular geneticists and categorized as ‘pathogenic,’ ‘presumed pathogenic,’ ‘variant of unknown significance,’ ‘presumed benign,’ or ‘benign,’ according to the American College of Medical Genetics and Genomics (ACMG) standards. When assessing mutation frequencies of individual genes, ’pathogenic,’ and ‘presumed pathogenic’ were counted as mutations while ‘benign’, ‘presumed benign’ variants and ‘variants of unknown significance’ were excluded.
Homologous recombination-related (HRR) genes determination
A combination of multiple test platforms was used to determine the mismatch repair deficiency (dMMRP) status of the tumors profiled, including MSI fragment analysis (FA, Promega, Madison, WI), IHC (MLH1, M1 antibody; MSH2, G2191129 antibody; MSH6, 44 anti-body; and PMS2, EPR3947 antibody [Ventana Medical Systems, Inc., Tucson, AZ, USA]) and NGS (for tumors tested with NextSeq platform, 7000 target microsatellite loci were examined and compared to the reference genome hg19 from the University of California). The three platforms generated highly concordant results as previously reported and in the rare cases of discordant results, the MSI or MMR status of the tumor was determined in the order of FA, IHC and NGS [5].
PD-L1 expression
Immunohistochemistry (IHC) was performed on full formalin-fixed paraffin-embedded (FFPE) sections of glass slides. Slides were stained using automated staining techniques, per the manufacturer's instructions, and were optimized and validated per CLIA/CAO and ISO requirements. The primary antibodies used against PD-L1 were 22c3 (pharmDx, Dako) and tumor proportion score (TPS) was calculated as the number of PD-L1 staining cells tumor cells divided by the total viable tumor cells, multiplied by 100. The tumor was considered positive if TPS ≥ 1% (high PD-L1 expression if TPS ≥ 50%).
Tumor mutation burden (TMB)
TMB was measured by counting all non-synonymous missense, nonsense, inframe insertion/deletion and frameshift mutations found per tumor that had not been previously described as germline alterations in dbSNP151, Genome Aggregation Database (gnomAD) databases or benign variants identified by Caris geneticists. A cutoff point of >=10 mutations per MB was used based on the KEYNOTE-158 pembrolizumab trial [6], which showed that patients with a TMB of ≥ 10 mt/MB across several tumor types had higher response rates (RR) than patients with a TMB of <10 mt/MB. Caris Life Sciences is a participant in the Friends of Cancer Research TMB Harmonization Project [7].
Results
Distribution and frequency of RET fusion positive (RET+) tumors
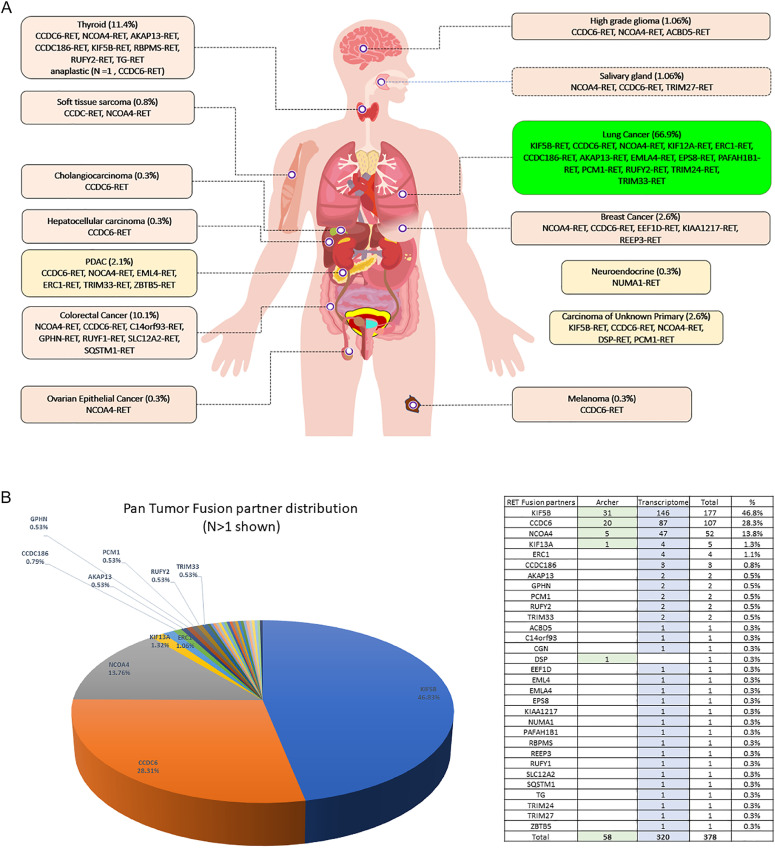
A total of 378 RET+ solid tumors were identified. The majority (84.7%, 320/378) were identified by WTS and the rest were identified by targeted NGS RNA sequencing (ARCHER). The most common RET+ sold tumors was NSCLC (66.9%, 253/378), followed by thyroid cancer (11.1%, 42/378), colorectal adenocarcinoma (10.1%, 38/378), breast cancer (2.6%, 10/378) and CUP (2.6%, 10/378) (Fig. 1A). The estimated frequency of RET+ samples within the specific tumor types was about 0.7% (253 out of ∼38,000) for NSCLC, 3.1% for thyroid cancer (42 out of ∼1300), 0.2% for colorectal carcinoma (CRC) (38 out of ∼23,000), and 0.1% for breast cancer (10 out of ∼16,000). The clinical pathologic characteristics of the RET+ patients by tumor types are listed in Table 1.
Fig. 1.
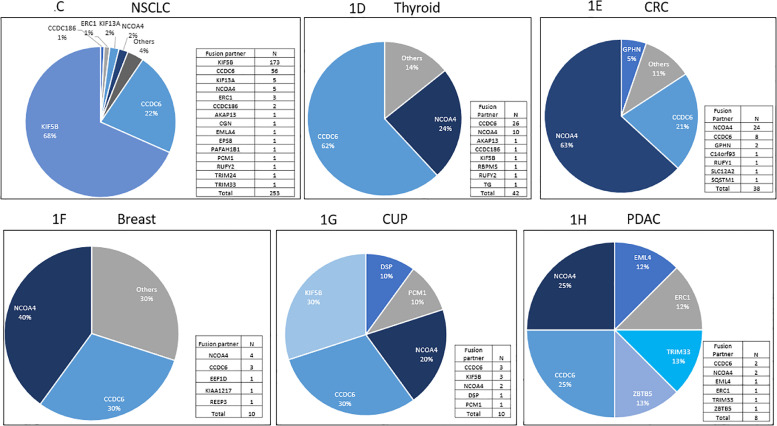
A: Distribution of RET fusion positive tumors by tumor type (N = 378) B: Distribution of fusion partners of RET+ solid tumors C: Distribution of fusion partners among RET+ NSCLC D: Distribution of fusion partners among RET+ well-differentiated thyroid carcinoma, E: Distribution of fusion partners among RET+ colorectal adenocarcinoma F: Distribution of fusion partners among RET+ breast adenocarcinoma G: Distribution of fusion partners among RET+ CUP H: Distribution of fusion partners among RET+ pancreatic carcinoma.
Table 1.
Clinical pathologic characteristics of the RET+ patients by tumor types.
| NSCLC | Colorectal | Thyroid | Breast | CUP | Pancreatic | |
|---|---|---|---|---|---|---|
| N | 253 | 38 | 42 | 10 | 10 | 8 |
| Age | ||||||
| Median (range) | 66 (27->89) | 72.5 (34–88) | 81.0 (9–84) | 59.5 (35–75) | 71.5 (41–87) | 68.5 (55–81) |
| Mean (SD) | 64.7 (12.02) | 67.9 (12.7) | 51.1 (21.1) | 58.8 (15.7) | 68.8 (13.9) | 67.3 (9.0) |
| Sex (%) | ||||||
| Male | 113 | 15 | 14 | 0 | 6 | 5 |
| Female | 140 | 23 | 28 | 10 | 4 | 3 |
| Fusion partner | ||||||
| KIF5B- | 173 | 0 | 1 | 0 | 0 | 0 |
| CCDC6- | 56 | 8 | 26 | 3 | 3 | 2 |
| NCOA4- | 5 | 24 | 10 | 4 | 2 | 2 |
| ERC1- | 3 | 0 | 0 | 0 | 0 | 1 |
| KIF13A- | 5 | 0 | 0 | 0 | 0 | 0 |
| GPHN- | 0 | 2 | 0 | 0 | 0 | 0 |
| Sequencing methods | ||||||
| Targeted RNA (Archer) | 44 | 2 | 3 | 3 | 2 | 0 |
| WTS | 209 | 36 | 39 | 7 | 8 | 8 |
| Mean junction read (SD) | 45.2 (42.6) | 22.7 (26.8) | 18.7 (35.9) | 16.7 (10.8) | 48.8 (52.1) | 38.4 (80.9) |
| PD-L1 expression (22C3) | ||||||
| <1% | 70 | NA | NA | 2 | NA | NA |
| 1–49% | 71 | NA | NA | 2 | NA | NA |
| >= 50% | 92 | NA | 1 | 0 | NA | NA |
| Unknown | 20 | 38 | 41 | 6 | 10 | 8 |
| TMB | ||||||
| 0–5 | 174 | 9 | 39 | 6 | 7 | 6 |
| >5–10 | 55 | 4 | 0 | 4 | 2 | 2 |
| >10 | 4 | 22 | 1 | 0 | 1 | 0 |
| Unknown | 20 | 3 | 2 | 0 | 0 | 0 |
| Microsatellite | ||||||
| Stable | 244 | 14 | 41 | 10 | 9 | 8 |
| Unstable | 1 | 24 | 0 | 0 | 1 | 0 |
Molecular characteristics of RET+ fusions
The most common fusion partners in all tumor types were KIF5B (46.8%) followed by CCDC6 (28.3%) and NCOA4 (13.8%) (Fig. 1B). The vast majority (97.4%, 368/378) of the fusion breakpoint occurred at exon 12 of the RET. The other fusion breakpoint occurred at exon 11 (1.9%, 7/378), exon 9 (0.5%, 2/378) and one fusion (0.3%) breakpoint at exon 10 of RET (Table 2).
Table 2.
| Fusion partner | Fusion partner exon | RET exon | Anaplastic Thyroid Carcinoma | Breast Carcinoma | Cancer of Unknown Primary | Cholangiocarcinoma | Colorectal Adenocarcinoma | High Grade Glioma | Liver Hepatocellular Carcinoma | Lung Non-small cell lung cancer (NSCLC) | Melanoma | Neuroendocrine tumors | Ovarian Surface Epithelial Carcinomas | Pancreatic Adenocarcinoma | Salivary Gland Tumors | Soft Tissue Tumors | Thyroid Carcinoma | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACBD5 | 9 | 12 | 1 | 1 | ||||||||||||||
| AKAP13 | 27 | 12 | 1 | 1 | 2 | |||||||||||||
| C14orf93 | 4 | 12 | 1 | 1 | ||||||||||||||
| CCDC186 | 8 | 12 | 1 | 1 | ||||||||||||||
| 16 | 12 | 1 | 1 | 2 | ||||||||||||||
| CCDC6 | 1 | 12 | 1 | 3 | 2 | 1 | 5 | 1 | 1 | 51 | 1 | 2 | 1 | 2 | 25 | 96 | ||
| 2 | 12 | 1 | 3 | 1 | 3 | 8 | ||||||||||||
| 8 | 12 | 1 | 1 | 2 | ||||||||||||||
| 1 and intron 1 | 12 | 1 | 1 | |||||||||||||||
| CGN | 16 | 12 | 1 | 1 | ||||||||||||||
| DSP | 23 | 11 | 1 | 1 | ||||||||||||||
| EEF1D | 8 | 12 | 1 | 1 | ||||||||||||||
| EML4 | 12 | 12 | 1 | 1 | ||||||||||||||
| EMLA4 | 18 | 12 | 1 | 1 | ||||||||||||||
| EPS8 | 12 | 12 | 1 | 1 | ||||||||||||||
| ERC1 | 3 | 12 | 1 | 1 | ||||||||||||||
| 15 | 12 | 1 | 1 | |||||||||||||||
| 17 | 12 | 1 | 1 | 2 | ||||||||||||||
| GPHN | 8 | 12 | 2 | 2 | ||||||||||||||
| KIAA1217 | 11 | 12 | 1 | 1 | ||||||||||||||
| KIF13A | 18 | 12 | 5 | 5 | ||||||||||||||
| KIF5B | 14 | 11 | 1 | 1 | ||||||||||||||
| 15 | 11 | 1 | 1 | |||||||||||||||
| 12 | 3 | 140 | 1 | 144 | ||||||||||||||
| 16 | 12 | 15 | 15 | |||||||||||||||
| 22 | 12 | 4 | 4 | |||||||||||||||
| 23 | 12 | 8 | 8 | |||||||||||||||
| 24 | 9 | 1 | 1 | |||||||||||||||
| 11 | 1 | 1 | ||||||||||||||||
| 12 | 1 | 1 | ||||||||||||||||
| 16 and 15 | 12 | 1 | 1 | |||||||||||||||
| NCOA4 | 6 | 12 | 1 | 1 | 1 | 2 | 1 | 6 | ||||||||||
| 7 | 12 | 3 | 1 | 2 | 1 | 8 | 15 | |||||||||||
| 8 | 11 | 1 | 1 | |||||||||||||||
| 12 | 5 | 1 | 6 | |||||||||||||||
| 9 | 12 | 1 | 17 | 1 | 3 | 2 | 24 | |||||||||||
| NUMA1 | 22 | 9 | 1 | 1 | ||||||||||||||
| PAFAH1B1 | 5 | 12 | 1 | 1 | ||||||||||||||
| PCM1 | 28 | 11 | 1 | 1 | ||||||||||||||
| 12 | 1 | 1 | ||||||||||||||||
| RBPMS | 6 | 12 | 1 | 1 | ||||||||||||||
| REEP3 | 5 | 12 | 1 | 1 | ||||||||||||||
| RUFY1 | 14 | 12 | 1 | 1 | ||||||||||||||
| RUFY2 | 9 | 12 | 1 | 1 | 2 | |||||||||||||
| SLC12A2 | 16 | 12 | 1 | 1 | ||||||||||||||
| SQSTM1 | 3 | 10 | 1 | 1 | ||||||||||||||
| TG | 47 | 11 | 1 | 1 | ||||||||||||||
| TRIM24 | 9 | 12 | 1 | 1 | ||||||||||||||
| TRIM27 | 3 | 12 | 1 | 1 | ||||||||||||||
| TRIM33 | 11 | 12 | 1 | 1 | 2 | |||||||||||||
| ZBTB5 | 2 | 12 | 1 | 1 | ||||||||||||||
| Total | 1 | 10 | 10 | 1 | 38 | 4 | 1 | 253 | 1 | 1 | 1 | 8 | 4 | 3 | 42 | 378 |
Junctional read is the number of RNA reads that contained the fusion breakpoint. The mean junctional read per tumor sample was 53.5% +/- 10.4 standard deviation (SD). The mean junctional read per tumor sample was 45.2% +/- 42.6 standard deviation (SD) in NSCLC. The mean junctional read for thyroid cancer and colorectal cancer were 18.7% +/- 35.9 SD and 22.7% +/- 26.8, respectively.
The mean tumor mutation burden (TMB) for all RET+ tumors was 7.66 +/- 2.89 (SD). As RET+ CRC had an overall high TMB, the mean TMB in CRC not counting RET+ CRC was 7.48 +/- 13.80 (SD).
The most common single gene genomic alterations in RET+ solid tumors were TP53 mutations at 34.8% (120/345) followed by RASA1 (14.3%, 1/7) and ARID1A at 10.8% (27/250). The list of frequency of gene alterations are listed in Supplemental table 1 and Supplemental figure 1 and 2. The rest of the single gene genomic alterations occurred at a frequency < 15% (Supplemental table 2 and Supplemental figure 2). The relatively sparse spectrum for co-alterations may imply the lower genomic complexity of RET+ tumors and one could speculate that relationship to high responsiveness to RET+ tyrosine kinase inhibitors with the possible exception of RET+ CRC.
For global genomic alterations, loss of heterozygosity (LOH) occurred at 12.9% (9/70) followed by recombination-related genes (HRR) mutations at 11.9% (8/67) but number of samples tested was fewer than most of the single gene alterations.
Gene amplification among RET+ solid tumors were rare with MDM2 (3.7%), followed by MYC (2.5%), and CDK4 (2.0%) (Supplemental table 3 and Supplemental figure 3).
RET+ NSCLC
RET+ NSCLC was the most common tumor types (66.9%). The most common RET fusion variant in NSCLC was KIF5B-RET at (68%) followed by CCDC6-RET at 22% (Fig. 1C). While not expressed in normal lung tissue, KIF5B-RET can be highly expressed in lung cancer tissue [8]. Based on a metanalysis including a total of over 8000 patients from 13 studies, the KIF5B-RET NSCLC genotype appear to have unique clinical features including higher frequencies in female and younger patients with no clear correlation to smoking history [9]. While there was no significant difference between sex, those with RET fusion NSCLC was significantly younger with a median age of 66 versus 69 in RET fusion positive versus negative NSCLC (p<0.01, data not shown). Of note, there were a total of 7 cases of concurrent EGFR mutatations (3 cases of L747_T751delinsP, 2 cases of E746_A750 del, 1 case each of V774N and E746_T751delinsA).
RET+ thyroid cancer
RET+ thyroid cancer was the second most common RET+ solid tumors identified in this study. The most common fusion partner was CCDC6 in RET+ thyroid cancer (62%) and the only tumor type with CCDC6 as the most common fusion partners (Fig. 1D). Of note, there were no concurrent BRAF mutations or NTRK fusions with RET+ thyroid cancers. Out of the 42 RET+ thyroid cancers, the majority was papillary (n = 38) followed by thyroid NOS (n = 3) and medullary (n = 1). There was one case of anaplastic thyroid cancer with the fusion partner of CCDC6 which may have transformed from RET+ well differentiated thyroid cancer.
RET+ colorectal cancer (CRC)
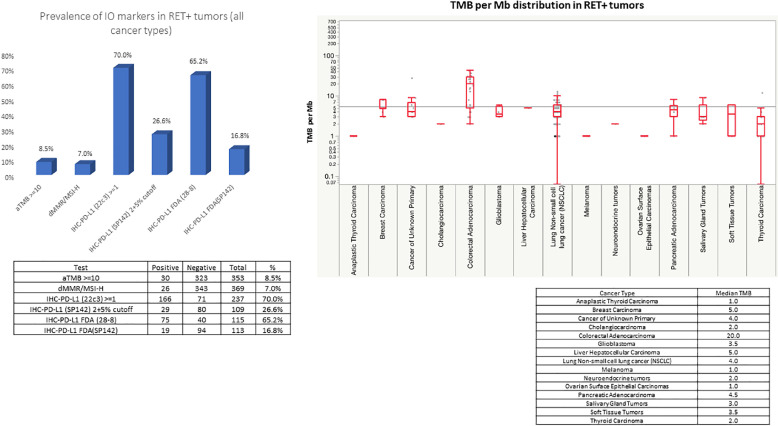
RET+ colorectal cancer constituted the third most common tumor types among RET+ solid tumors. The most common RET fusion variant in CRC was NCOA4-RET at (63%) followed by CCDC6-RET at 21% (Fig. 1E). Of note, there were one case each of concurrent BRAF and KRAS mutations with RET+ CRC. In contrast to NSCLC where RET+ was associated with younger age, those with RET fusion positive CRC was significantly older with a median age of 72.5 versus 62 in RET fusion positive versus negative CRC (p<0.01, data not shown). While there was no significant difference between sex and RET status in CRC (data not shown). The median TMB of RET+ CRC on the other hand was high at 20.0 (Fig. 2A). Additionally, 63.2% (24/38) of the RET+ CRC was MSI-H. Twenty-one out of 22 TMB high (>10mt/base) RET+ CRC had MSI-H. Of the 7 RET+ CRC patients that underwent HRR testing, 4 were positive. All 4 of these HRR positive RET+ CRC patients had high TMB and were MSI-H. No apparent correlation between RET fusion partner and MSI status were observed with NCOA4-RET (62.5%, 15/24) in MSI-H versus (64.3, 9/14) MSI-stable (MSI-S). The mean junctional read of RET fusion was 22.7% +/- 26.8 SD, which was lower compared to RET+ NSCLC.
Fig. 2.
A: Box and whiskers plot of TMB among the major RET+ solid tumors.
RET+ breast cancer
A total of 10 RET+ breast cancer samples were identified at a frequency of about 0.1%. The average age of the patients were 59.5 years old which was the youngest among the major tumor types identified harboring RET fusions (Table 1). The median TMB for these 10 breast cancer tumors was 5.0 (Fig. 2A). Out of these patients, 63% (5/8) were ER positive, 38% (38) were PR positive, while notably none had HER2 over-expression/amplification (0%, 0/8). Although with limited numbers, NCOA4- was the most common fusion partner but CCDC6-, REEP3, EEF1D and KIAA1217 fusion partners were also identified (Fig. 1F).
RET+ CUP
A total of 10 RET+ CUP were identified. Median age was 71.5 (Table 1). The most common fusion partners were KIF5B- and CCDC6- (Fig. 1G). This may imply that in at least some of these cases of CUP, the primary site of tumor may have derived from NSCLC or thyroid, as KIF5B- was the most common fusion partner for NSCLC and CCDC6- was that of thyroid cancer.
RET+ pancreatic cancer
A total of 8 RET+ pancreatic adenocarcinoma was identified. These were all KRAS wild type pancreatic adenocarcinomas. The average age was 68.5 years old (Table 1). The median TMB was 4.5. The number of samples were limited and the most common fusion partner identified was NCOA4- and CCDC6- (Fig. 1H).
Other RET+ solid tumors
Four RET+ glioblastoma (two with CCDC6- and one each of NCOA4- and ACBD5-), four RET+ salivary gland cancer (two NCOA4- and one each of CCDC6-and TRIM27-), three RET+ soft tissue tumors (two CCDC6- and one NCOA4-) were identified. A single RET fusion variant was detected in an anaplastic thyroid carcinoma (CCDC6-RET), a cholangiocarcinoma (CCDC6-RET), one hepatocellular carcinoma (CCDC6-RET), melanoma (CCDC6-RET), neuroendocrine tumor (NUMA1) and in ovarian epithelial carcinoma (NCOA4-RET).
Discussion
Our pan-tumor RET fusion survey identified RET fusions in 15 distinct tumor types including CUP. While 78% of the RET fusions were identified in NSCLC and well-differentiated thyroid cancers, the remaining RET fusion positive tumors consisted of colorectal adenocarcinoma, which was the third most common RET+ solid tumor in our survey, followed by breast adenocarcinoma, CUP, pancreatic adenocarcinoma, salivary gland carcinoma, glioblastoma, soft tissue tumors, hepatocellular carcinoma, cholangiocarcinoma, neuroendocrine tumor and ovarian tumors. Additionally, besides well-differentiated thyroid carcinoma, one case of anaplastic thyroid carcinoma was also identified.
Given the recent FDA approval to expand the use of selpercatinib to RET+ solid tumors outside of the more common NSCLC and thyroid cancers [10], [11], [12], [13], it is of particular importance to raise awareness of RTK fusions in solid tumors.
Although the most common fusion partner to RET was KIF5B accounting for close to half of the fusion partners of the RET fusion identified, there seemed to be a tumor specific dominance of one fusion partner: KIF5B for RET+ NSCLC, CCDC6 for RET+ thyroid and NCOA4 for RET+ colon and breast cancer. In the 10 RET+ CUP patients, there were 3 each of KIF5B and CCDC6. Thus, there could be a potential for using fusion partners as a hint to identify tumor of unknown origin, if there were to be ambiguity in the tumor tissue of origin.
Colorectal adenocarcinoma was the third most common RET+ solid tumor in our survey. Interestingly, Chan and colleagues demonstrated that the ectopic expression of a novel variant of the NCOA4-RET fusion gene promoted cell proliferation in vitro and in vivo, and the growth was suppressed by RET kinase inhibitors, showing that receptor tyrosine kinase fusions could act as a significant alternative driver in the development of colorectal cancer [14].
One of the important findings in this report was that RET+ CRC has a very high tumor mutation burden and a high proportion with MSI-High (Fig. 2A). This observation extends the finding of NTRK+ CRC also having a high TMB [15]. Similarly, Yakirevich and colleagues reported 9 cases of colorectal cancer harboring ALK gene fusions which predominantly involved the proximal colon and often exhibited MSI [16]. While we await further evaluation on the association of kinase fusions with microsatellite instability in colorectal cancers, this finding cautions us to confirm the MSI status when a fusion is detected or vice versa. This issue may also have treatment implications. The efficacy of selpercatinib in tumors other than lung or thyroid was reported recently by Subbiah and colleagues and demonstrated the low RR of RET+ CRC which was at 20.0% (n = 10) when compared to their total cohort of RR 43.9% (n = 41) [17].
This could be explained at least in part by the tumor heterogeneity of CRC and the fact that as shown in our study, RET+ CRC tend to be associated with MSI high status, which is known for poor prognosis [18]. Although the small number of patients makes the assessment challenging, one must be cautious on the “blanket” use of RET TKIs in this setting of RET+ CRC, where perhaps immunotherapy and other options should also be considered.
In our survey, breast cancer followed colon cancer with 10 cases of RET fusions. In a large survey of RET alterations among 9693 breast adenocarcinoma that utilized hybrid-capture DNA sequencing, 8 RET fusions were identified (CCDC6-RET [N = 6], NCOA4-RET [N = 1], RASGEF1A-RET [N = 1] [19]). It appears that the majority of the RET+ breast cancer were ER negative and negative for HER2 amplification which was consistent with our survey. RET fusions in breast adenocarcinoma are thought to be oncogenic and indeed, one patient had a rapid clinical and radiographic response to cabozantinib, a multi-target RET kinase inhibitor [19].
While there were only 8 cases of RET positive pancreatic cancer in our survey, identification of potentially targetable alterations would be of significant value given the grave prognosis of pancreatic cancer. Utilizing FISH, Chou and colleagues have reported the identification of RET gene rearrangements in 3 out of 36 (8.3%) pure pancreatic acinar cell in which one of them was CCDC6-RET [20]. In our survey, while most of the histology reported were “pancreatic ductal adenocarcinoma”, it is possible that there may have been a classification error at the time of test requisition form completion and some of the patients may have had “pancreatic acinar cell carcinoma”, which appears to have a different biology when compared to the traditional ductal adenocarcinomas [21].
The prevalence of RET fusions were relatively low in this large cohort. One of the limitation of this study is the fact that there may be selection bias in those who were offered molecular testing. For example, it is possible that patients who were locally tested positive for KRAS mutant colorectal cancer or BRAF mutant thyroid cancer were not offered broad molecular testing and this may have skewed the overall incidence of RET fusions in certain tumor types in our study. Another limitation of this study is the lack of detailed clinical information regarding the timing of when the molecular analysis was performed (i.e. stage, pre vs post treatment evaluation). Outcomes were inferred based on time from tissue collection to date of last contact. In reality, NGS was performed at varying time points during the course of the disease and treatments. Although we were unable to distinguish if RET fusions were baseline characteristics prior to any treatment or if it actually reflected acquired resistance, there were a total of 7 cases of concurrent EGFR mutatations (3 cases of L747_T751delinsP, 2 cases of E746_A750 del, 1 case each of V774N and E746_T751delinsA), impling the possibility of RET fusions as a resistance mechanism.
Despite these limitations, we were able to determine the characteristics of RET fusions in a tumor agnostic manner. While Zhou and colleagues have also published on pan-tumor RET alterations using the cbioportal genomic database, they captured RET alterations including mutations and amplifications in addition to the fusions [22]. Finally, a more detailed examination of the clinical effects of other co-existing mutations along with underlying biological and molecular mechanism to account for the differences in survival outcomes of various RET fusions is eagerly awaited.
In conclusion, we believe that highly actionable alteration notable in multiple tumor types continues to highlight the need for broad panel testing for advanced cancers including RNA NGS and WES and tissue agnostic treatment approaches.
Executive summary of pan tumor RET fusion survey
-
(1)
15 different tumor types and CUP with in-frame RET fusions as detected by NGS RNA sequencing
-
(2)
31 different fusion partners in RET fusion solid tumors
-
(3)
A total of 378 RET+ solid tumors were identified. The majority (84.7%, 320/378) were identified by WTS and the rest were identified by targeted NGS RNA sequencing (ARCHER).
-
(4)
The majority of RET fusions were seen in the following tumor types: NSCLC (66.9%), thyroid cancer (11.1%), colorectal (10.1%)
-
(5)
The estimated frequency of RET fusions within specific tumor types were NSCLC 0.7%, thyroid cancer 3.1%, colorectal cancer 0.2% and breast cancer 0.1%
-
(6)
Three major fusion partners: KIF5B (46.8%), CCDC6 (28.3%), NCOA4 (13.8%). Although KIF5B is the most common fusion partner, it is mostly found in NSCLC while CCDC6 and NCOA4 are identified in almost all RET fusion positive (RET+) solid tumors
-
(7)Different dominant fusion variants among major tumor types.
-
(a)KIF5B-RET (NSCLC)
-
(b)NCOA4-RET (colorectal, breast)
-
(c)CCDC6-RET (Thyroid)
-
(a)
-
(8)
High PD-L1 expression ≥50% (22C3) accounted for 36.4% of RET+ NSCLC
-
(9)
RET+ CRC had a high median TMB and were commonly MSI-H.
CRediT authorship contribution statement
Misako Nagasaka: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Danielle Brazel: Formal analysis, Investigation, Writing – review & editing. Yasmine Baca: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Joanne Xiu: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Mohammed Najeeb Al-Hallak: Formal analysis, Investigation, Writing – review & editing. Chul Kim: Formal analysis, Investigation, Writing – review & editing. Jorge Nieva: Formal analysis, Investigation, Writing – review & editing. Jeffrey J. Swensen: Formal analysis, Investigation, Writing – review & editing. David Spetzler: Formal analysis, Investigation, Writing – review & editing. Wolfgang Michael Korn: Formal analysis, Investigation, Writing – review & editing. Mark A. Socinski: Formal analysis, Investigation, Writing – review & editing. Luis E. Raez: Formal analysis, Investigation, Writing – review & editing. Balazs Halmos: Formal analysis, Investigation, Writing – review & editing. Sai-Hong Ignatius Ou: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
There was no funding allocated for this research and there are no direct conflicts of interest. Potential COI from all authors are listed below. MN is on the advisory board for AstraZeneca, Daiichi Sankyo, Takeda, Novartis, EMD Serono, Janssen, Pfizer, Eli Lilly and Company, Bayer and Genentech; consultant for Caris Life Sciences (virtual tumor board); speaker for Blueprint Medicines, Janssen, Mirati and Takeda; and reports travel support from AnHeart Therapeutics. DB has no disclosures. YB, JX, JJS and DS are employees and shareholders of Caris Life Sciences. MNA discloses the following: Speaker Bureau: IPSEN, AstraZeneca, Guardant Health. External advisory board: CTI-Facts (CRO company). CK served as a consultant for Novartis, Janssen, Astrazeneca, Sanofi, PierianDx, Diffuse pharmaceuticals, Mirati, Jazz Pharmaceuticals, and Arcus Biosciences, and received research funding (to institution) from AstraZeneca, Bristol-Myers Squibb, Novartis, Genentech, Janssen, Regeneron, Debiopharm, Karyopharm, and Blueprint Medicines. JN discloses the following: Consulting: Aadi Biosciences, Astra Zeneca, Bristol Myers Squibb, Fujirebio, G1 Therapeutics, Genentech, Mindmed, Naveris, Takeda, Western Oncolytics., Research Support: Genentech, Merck, Intellectual Property: Cansera and Ownership Interests: Cansera, Epic Sciences, Indee Bio, Quantgene. WMK has stock ownership of Caris Life Sciences. MS has received honoraria from AstraZeneca, Bayer, Roche, Celgene, BMS, Genentech, Novartis and Lilly. MS is a consultant for Genentech and Novartis and has received research support from AstraZeneca, Roche and Takeda. LER has received research support from BMS, Astra-Zeneca, Roche, Pfizer, Merck, Velos, Guardant Health, Natera, Genentech, Bio Alta. BH has grants or contracts from Boehringer Ingelheim, Astra Zeneca, Merck, BMS, Advaxis, Amgen, AbbVie, Daiichi, Pfizer, GSK, Beigene, Janssen, has received consulting fees from Veracyte and has been on monitoring or advisory boards for Astra Zeneca, Boehringer Ingelheim, Apollomics, Janssen, Takeda, Merck, BMS, Genentech, Pfizer, Eli-Lilly, TPT, Arcus and Merus. SHIO has stock ownership and was on the scientific advisory board of Turning Point Therapeutics Inc (until Feb 28, 2019), is a member of the SAB of Elevation Oncology, and has received speaker honorarium from Merck, Roche/Genentech, Astra Zeneca, Takeda/ARIAD and Pfizer; has received advisory fees from Roche/Genentech, Astra Zeneca, Takeda/ARIAD, Pfizer, Foundation Medicine Inc, Spectrum, Daiichi Sankyo, Jassen/JNJ, and X-Covery.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101744.
Appendix. Supplementary materials
References
- 1.Schram A.M., Chang M.T., Jonsson P., et al. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017;14:735–748. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blume-Jensen P., Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 3.Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2(6):495–502. doi: 10.1158/2159-8290.CD-12-0009. Jun. [DOI] [PubMed] [Google Scholar]
- 4.Kohno T., Tabata J., Nakao T. REToma: a cancer subtype with a shared driver oncogene. Carcinogenesis. 2020;41(2):123–129. doi: 10.1093/carcin/bgz184. Apr 22. [DOI] [PubMed] [Google Scholar]
- 5.Vanderwalde A., Spetzler D., Xiao N., et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746–756. doi: 10.1002/cam4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marabelle A., Fakih M., Lopez J., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. Oct 21. [DOI] [PubMed] [Google Scholar]
- 7.Merino D.M., McShane L.M., Fabrizio D., et al. Establishing guidelines to harmonize tumour mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the friends of cancer research TMB harmonization project. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000147. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrew I.S., Michael P.C., Keith A.C., et al. Large-scale analysis of the human and mouse transcriptomes. Proc. Natl. Acad. Sci. U. S. A. 2002;99(7):4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong X., Yang L., Chen C., Liu Z. KIF5B-RET fusion gene and its correlation with clinicopathological and prognostic features in lung cancer: a meta-analysis. OncoTargets Ther. 2019;12:4533–4542. doi: 10.2147/OTT.S186361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.drugs.com/newdrugs/fda-approves-lilly-s-retevmo-selpercatinib-first-only-ret-inhibitor-adults-advanced-metastatic-5901.html, accessed May 29, 2023.
- 11.Bradford D., Larkins E., Mushti S.L., et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin. Cancer Res. 2021;27(8):2130–2135. doi: 10.1158/1078-0432.CCR-20-3558. Apr 15. [DOI] [PubMed] [Google Scholar]
- 12.Kim J., Bradford D., Larkins E., et al. FDA approval summary: pralsetinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin. Cancer Res. 2021;27(20):5452–5456. doi: 10.1158/1078-0432.CCR-21-0967. Oct 15. [DOI] [PubMed] [Google Scholar]
- 13.Della Corte C.M., Morgillo F. Rethinking treatment for RET-altered lung and thyroid cancers: selpercatinib approval by the EMA. ESMO Open. 2021;6(1) doi: 10.1016/j.esmoop.2020.100041. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan A.W., Pan Y., Tong J.H., et al. Receptor tyrosine kinase fusions act as a significant alternative driver of the serrated pathway in colorectal cancer development. J. Pathol. 2020;251(1):74–86. doi: 10.1002/path.5418. May. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Li Z., Ou Q., et al. NTRK fusion positive colorectal cancer is a unique subset of CRC with high TMB and microsatellite instability. Cancer Med. 2022;11(13):2541–2549. doi: 10.1002/cam4.4561. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yakirevich E., Resnick M.B., Mangray S., et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin. Cancer Res. 2016;22(15):3831–3840. doi: 10.1158/1078-0432.CCR-15-3000. Aug 1. [DOI] [PubMed] [Google Scholar]
- 17.Subbiah V., Wolf J., Konda B., et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion positive solid tumours other than lung of thyroid tumours (LIBRETTO-001): a phase 1/2, open label, basket trial. Lancet Oncol. 2022;23(10):1261–1273. doi: 10.1016/S1470-2045(22)00541-1. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pietrantonio F., Di Nicolantonio F., Schrock A.B., et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann. Oncol. 2018;29:1394–1401. doi: 10.1093/annonc/mdy090. [DOI] [PubMed] [Google Scholar]
- 19.Paratala B.S., Chung J.H., Williams C.B., et al. RET rearrangements are actionable alterations in breast cancer. Nat. Commun. 2018;9(1):4821. doi: 10.1038/s41467-018-07341-4. Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou A., Brown I.S., Kumarasinghe M.P., et al. RET gene rearrangements occur in a subset of pancreatic acinar cell carcinomas. Mod. Pathol. 2020;33(4):657–664. doi: 10.1038/s41379-019-0373-y. Apr. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt C.M., Matos J.M., Bentrem D.J., et al. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J. Gastrointest. Surg. 2008;12:2078–2086. doi: 10.1007/s11605-008-0705-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L., Li J., Zhang X., Xu Z., Yan Y., Hu K. An integrative pan cancer analysis of RET abberations and their potential clinical implications. Sci. Rep. 2022;12(1):13913. doi: 10.1038/s41598-022-17791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.