Abstract
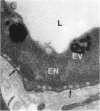
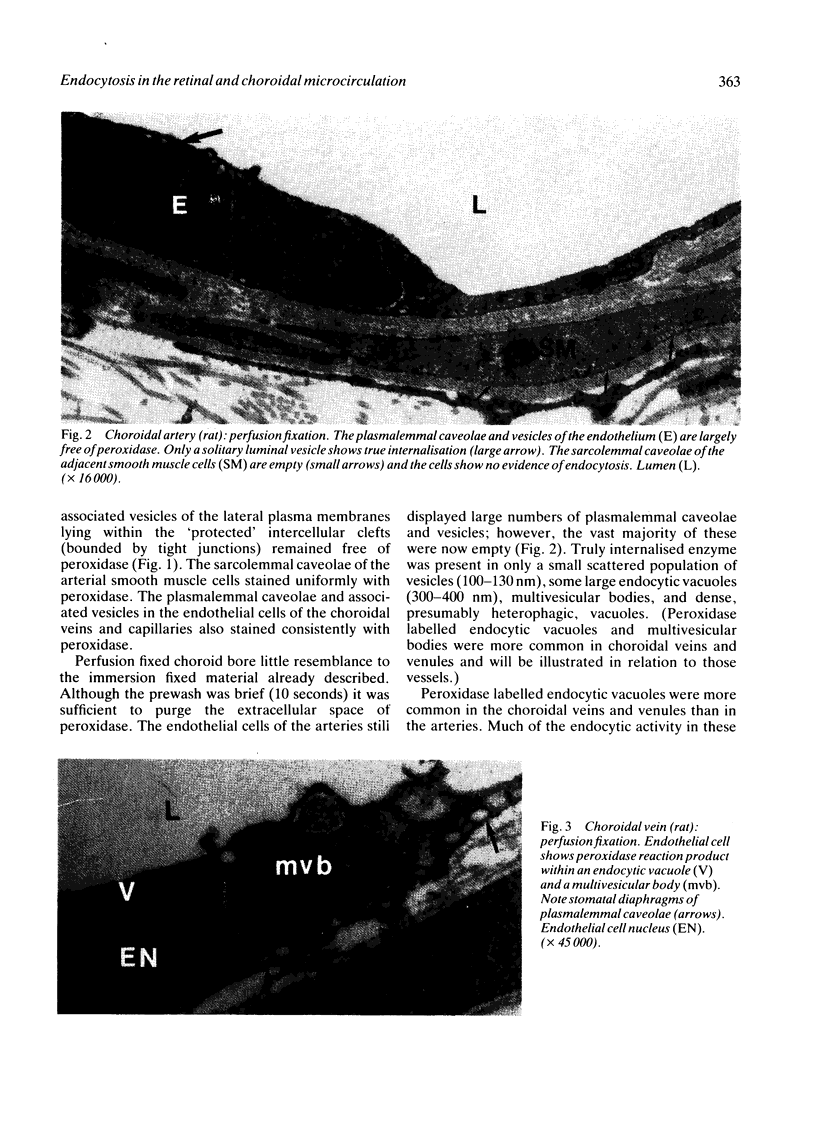
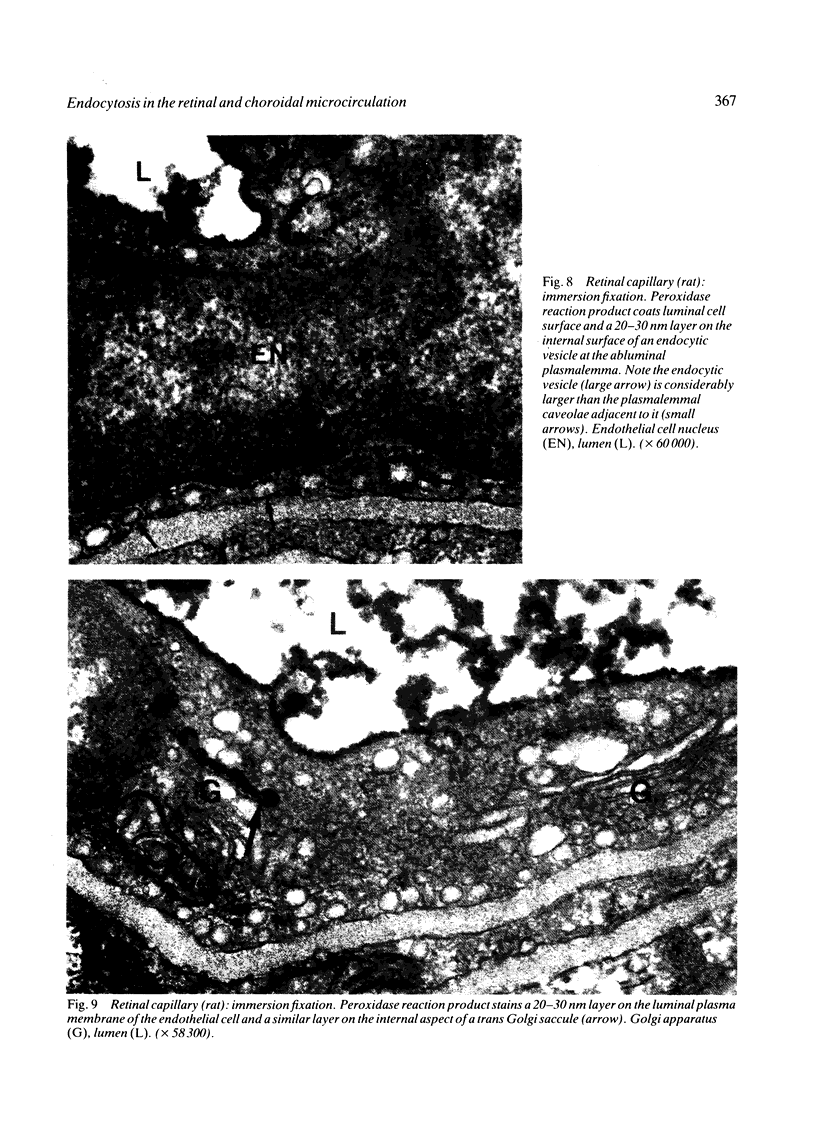
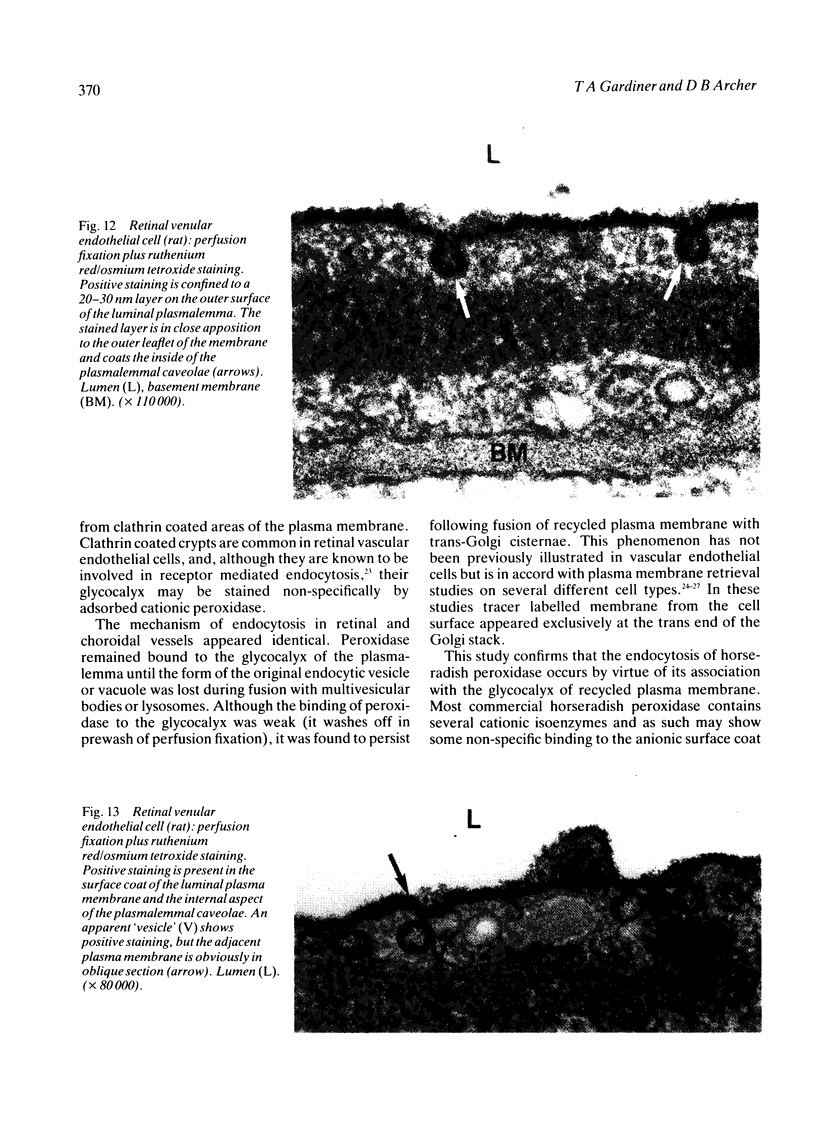
The endocytosis of horseradish peroxidase (HRP) by the vascular cells of retinal and choroidal blood vessels was compared in immersion and perfusion fixed eyes from individual rats. The mechanisms of endocytosis of HRP appeared identical in both retinal and choroidal vessels. The bulk of internalised tracer occurred in macropinosomes 300-400 nm in diameter. Tracer was localised to a 20-30 nm layer on the internal aspect of the limiting membrane. This layer was coincident with the glycocalyx of the luminal plasma membrane as revealed by ruthenium redosmium tetroxide staining. Horseradish peroxidase was also internalised by a small scattered population of vesicles (100-130 nm in diameter). The size of these vesicles suggested that they may have arisen from clathrin coated regions of the plasma membrane. It is suggested that the endocytosis of HRP in retinal and choroidal vascular endothelium occurs as a function of plasma membrane recycling. Horseradish peroxidase may also be internalised as a 'contaminant' of the glycocalyx in coated pits involved in receptor mediated endocytosis. The smooth 80 nm plasmalemmal caveolae of the retinal and choroidal vascular endothelial cells did not appear to participate either in absorptive endocytosis or vesicular transport.
Full text
PDF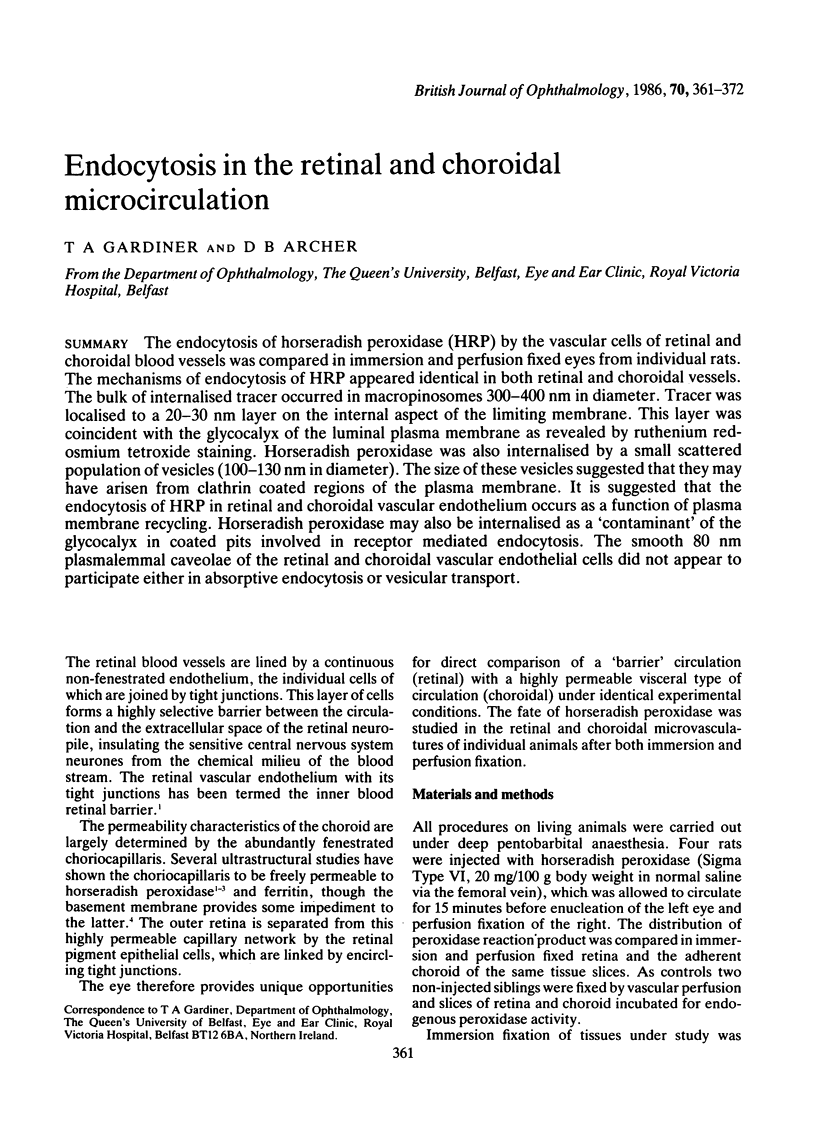








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke O. Electron microscopical observations on the surface coating of human blood platelets. J Ultrastruct Res. 1968 Jul;24(1):51–69. doi: 10.1016/s0022-5320(68)80016-4. [DOI] [PubMed] [Google Scholar]
- Bernstein M. H., Hollenberg M. J. Fine structure of the choriocappillaris and retinal capillaries. Invest Ophthalmol. 1965 Dec;4(6):1016–1025. [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Palade G. E. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968 May;37(2):244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. R., Palade G. E. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968 May;37(2):277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M., Hagman P., Crone C. The three-dimensional organization of plasmalemmal vesicular profiles in the endothelium of rat heart capillaries. Microvasc Res. 1983 May;25(3):358–368. doi: 10.1016/0026-2862(83)90025-0. [DOI] [PubMed] [Google Scholar]
- Bundgaard M. Vesicular transport in capillary endothelium: does it occur? Fed Proc. 1983 May 15;42(8):2425–2430. [PubMed] [Google Scholar]
- Crowther R. A., Finch J. T., Pearse B. M. On the structure of coated vesicles. J Mol Biol. 1976 Jun 5;103(4):785–798. doi: 10.1016/0022-2836(76)90209-6. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Editorial: The primary glomerular filtration barrier--basement membrane or epithelial slits? Kidney Int. 1975 Oct;8(4):197–211. doi: 10.1038/ki.1975.103. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Recovery of surface membrane in anterior pituitary cells. Variations in traffic detected with anionic and cationic ferritin. J Cell Biol. 1978 Jun;77(3):R35–R42. doi: 10.1083/jcb.77.3.r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J. Three-dimensional organization of plasmalemmal vesicles in endothelial cells. An analysis by serial sectioning of frog mesenteric capillaries. J Ultrastruct Res. 1980 Oct;73(1):9–20. doi: 10.1016/0022-5320(80)90111-2. [DOI] [PubMed] [Google Scholar]
- Gabella G. Cellular structures and electrophysiological behaviour. Fine structure of smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):7–16. doi: 10.1098/rstb.1973.0004. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V., Farquhar M. G. Luminal membrane retrieved after exocytosis reaches most golgi cisternae in secretory cells. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5073–5077. doi: 10.1073/pnas.74.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta H., Johnston W. H., Stanley T. M. Sialoglycoproteins and filtration barriers in the glomerular capillary wall. J Ultrastruct Res. 1975 Jun;51(3):354–376. doi: 10.1016/s0022-5320(75)80100-6. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773–1783. [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. The structure and properties of the cell surface coat. Int Rev Cytol. 1976;45:291–382. doi: 10.1016/s0074-7696(08)60081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHESI V. T., BARRNETT R. J. The demonstration of enzymatic activity in pinocytic vesicles of blood capillaries with the electron microscope. J Cell Biol. 1963 Jun;17:547–556. doi: 10.1083/jcb.17.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren L., Olsson Y. A sensitive method for histochemical demonstration of horseradish peroxidase in neurons following retrograde axonal transport. Brain Res. 1978 Jun 16;148(2):279–294. doi: 10.1016/0006-8993(78)90720-5. [DOI] [PubMed] [Google Scholar]
- Pelletier G. Secretion and uptake of peroxidase by rat adenohypophyseal cells. J Ultrastruct Res. 1973 Jun;43(5):445–459. doi: 10.1016/s0022-5320(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Peyman G. A., Spitznas M., Straatsma B. R. Peroxidase diffusion in the normal and photocoagulated retina. Invest Ophthalmol. 1971 Mar;10(3):181–189. [PubMed] [Google Scholar]
- Popescu L. M., Diculescu I., Zelck U., Ionescu N. Ultrastructural distribution of calcium in smooth muscle cells of guinea-pig taenia coli. A correlated electron microscopic and quantitative study. Cell Tissue Res. 1974;154(3):357–378. doi: 10.1007/BF00223732. [DOI] [PubMed] [Google Scholar]
- Shakib M., Rutkowski P., Wise G. N. Fluorescein angiography and the retinal pigment epithelium. Am J Ophthalmol. 1972 Aug;74(2):206–218. doi: 10.1016/0002-9394(72)90536-3. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of muscle capillaries to exogenous myoglobin. J Cell Biol. 1973 May;57(2):424–452. doi: 10.1083/jcb.57.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. H., Jew J. Y. An improved method for perfusion fixation of neural tissues for electron microscopy. Tissue Cell. 1975;7(3):407–418. doi: 10.1016/0040-8166(75)90015-4. [DOI] [PubMed] [Google Scholar]
- Zacks S. I., Saito A. Uptake of exogenous horseradish peroxidase by coated vesicles in mouse neuromuscular junctions. J Histochem Cytochem. 1969 Mar;17(3):161–170. doi: 10.1177/17.3.161. [DOI] [PubMed] [Google Scholar]