Abstract
Laser speckle contrast imaging or laser speckle imaging (LSI) is a noninvasive imaging technology that can detect areas of dynamic perfusion or vascular flow. Thus, LSI has shown increasing diagnostic utility in various pathologies and has been employed for intraoperative, postoperative, and long-term monitoring in many medical specialties. Recently, LSI has gained traction in clinical dermatology because it can be effective in the assessment of pathologies that are associated with increased perfusion and hypervascularity compared with that of normal tissue. To date, LSI has been found to be highly accurate in monitoring skin graft reperfusion, determining the severity of burns, evaluating neurosurgical revascularization, assessing persistent perfusion in capillary malformations after laser therapy, and differentiating malignant and benign skin lesions. LSI affords the advantage of noninvasively assessing lesions before more invasive methods of diagnosis, such as tissue biopsy, while remaining inexpensive and exhibiting no adverse events to date. However, potential obstacles to its clinical use include tissue movement artifact, primarily qualitative data, and unclear impact on clinical practice given the lack of superiority data compared with the current standard-of-care diagnostic methods. In this review, we discuss the clinical applications of LSI in dermatology for use in the diagnosis and monitoring of vascular, neoplastic, and inflammatory skin conditions.
Laser Speckle Imaging
Laser speckle imaging (LSI), first introduced in 1981, is recognized as a convenient method for visualizing blood flow within vessels. LSI often utilizes a low-intensity near-infrared laser, a low-power and long-wavelength light source, to illuminate the skin. Light is reflected toward the device’s sensor to provide information about blood flow over time, which is then captured by a camera. The image’s resulting pixel pattern, known as speckles, is subsequently analyzed by computer software to quantify the movement of these pixels. Laser speckles occur when a coherent light illuminates a surface, producing random interference effects. Such light is often from a laser beam of a particular wavelength. In LSI skin imaging, the light source is often a continuous wave laser running at near-infrared region (e.g., 785 nm), the so-called therapeutic window, where light can penetrate better and dispose less energy to tissue than visible or infrared light. The resulting laser speckle interference effect is displayed visually as a granular pattern consisting of dark and bright spots. When the speckle pattern is illuminated on a moving object, such as in-transit intravascular fluid, the flow disturbs the speckle pattern causing blurriness of laser speckles, causing partial or complete disappearance of speckles in the image of the flow-related region. LSI captures this speckle displacement, creating a quasi−real-time blood flow imaging system (Figure 1) (Aminfar et al., 2019). LSI images can be analyzed using spatial and temporal analysis, both of which are shown in Figures 2 and 3. Spatial analysis or laser speckle contrast analysis requires only one image and measures contrast in that image over pixels. Temporal analysis requires a series of multiple images and measures contrast in one pixel over the sequential images. The temporal method affords better spatial resolution because it uses single-pixel intensity over a period of time (Draijer et al., 2009). LSI has been effectively translated into medicine for the purpose of monitoring revascularization, particularly in the fields of neurosurgery and ophthalmology (Boas and Dunn, 2010). In a recent review of all clinical applications of the technology, no adverse events were reported, most likely owing to LSI’s low photon energy and illumination power (Heeman et al., 2019).
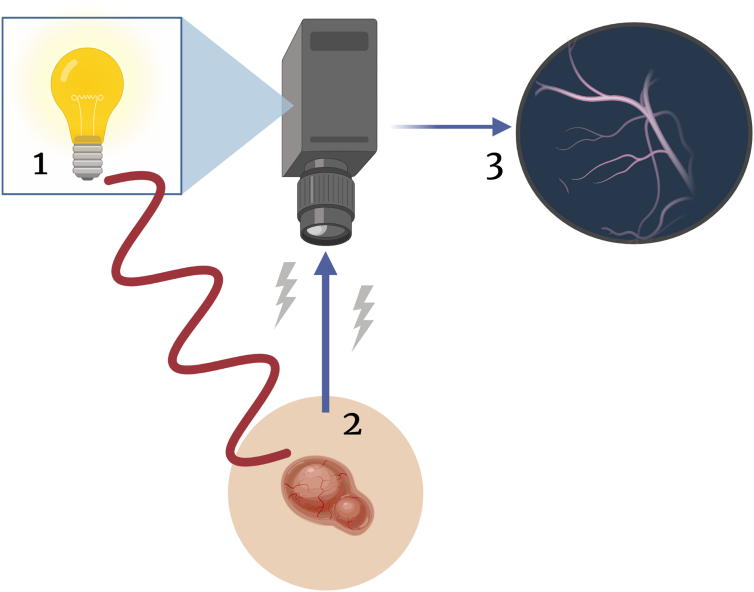
Figure 1.
Cartoon rendering LSI device function. Low-intensity, near-infrared coherent light illuminates the skin surface with a targeted lesion (1). Light is reflected toward the device sensor, allowing information about blood flow over time to be captured by the camera (2). Granular pixel pattern is analyzed using ImageJ or MATLAB software (3). LSI, laser speckle imaging.
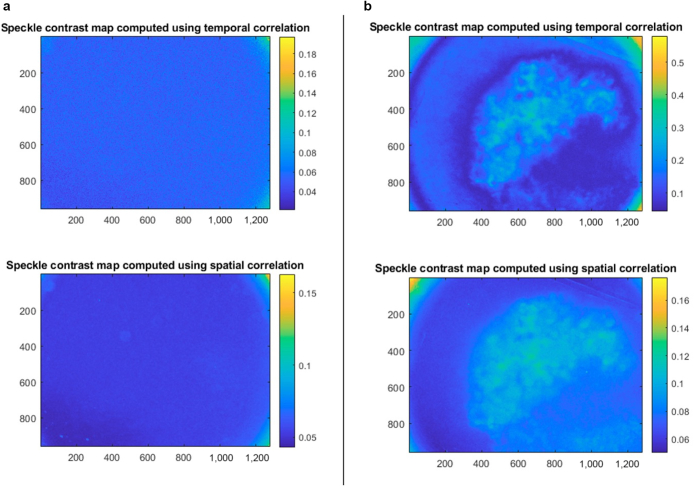
Figure 2.
Laser speckle images in normal versus lesional skin in a patient with biopsy-proven melanoma. The x- and y-axes represent pixel positions, and the color maps indicate the value of K, the local spatial variance or speckle contrast. Areas with higher blood flow have lower contrast or K. All images were captured with a laser wavelength of 785 nm and a circular aperture of 2 cm in diameter. Spatial correlation was captured over 7 × 7 pixels, and temporal correlation was analyzed over 49 images. (a) Left panel: Laser speckle images captured over normal skin in a patient seen in a dermatology clinic at the Medical University of South Carolina (Charleston, SC). Imaging of normal skin resulted in a homogeneous laser speckle pattern. (b) Right panel: Laser speckle images captured in the same patient over a lesion of cancer, confirmed with biopsy to be malignant melanoma. Imaging of melanoma shows higher blood flow around the lesion periphery than at the center of the lesion in temporal correlation as well as higher than in the control skin in a. Spatial and temporal images are similar, with improved spatial resolution reflected in the temporal correlation.
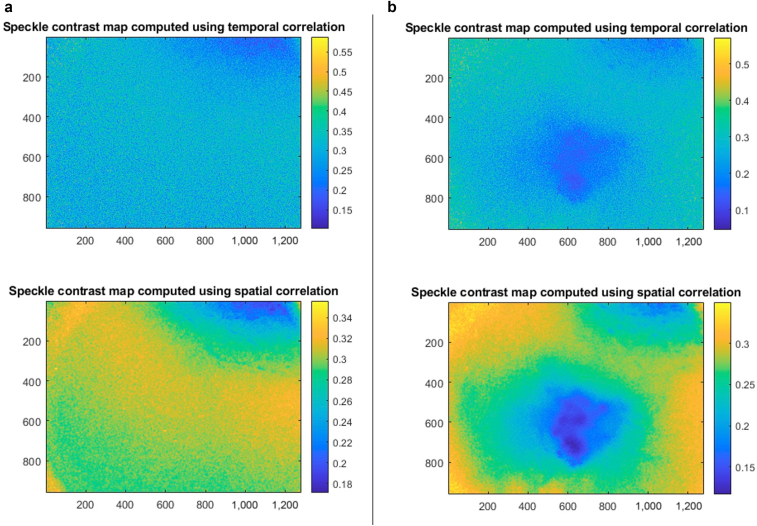
Figure 3.
Laser speckle images in normal versus lesional skin in a patient with a cherry angioma. As in Figure 2, the x- and y-axes represent pixel positions, and the color maps indicate the value of K, the local spatial variance or speckle contrast. Areas with higher blood flow have lower contrast or K. All images were captured with a laser wavelength of 785 nm and a circular aperture of 2 cm in diameter. Spatial correlation was captured over 7 × 7 pixels, and temporal correlation was analyzed over 49 images. (a) Left panel: Laser speckle images captured over normal skin. Again, imaging of normal skin resulted in a homogeneous laser speckle pattern. (b) Right panel: Laser speckle images captured in the same patient over a stable cherry angioma. Imaging of cherry angioma shows overall higher blood flow than in control skin, with pinpoint areas of highest blood flow within clumped lesional blood vessels, best appreciated in spatial correlation. Temporal correlation depicts less change in speckle contrast over time than in the contrast over one image using spatial correlation.
Applications in Dermatology
LSI is a technology with both clinical and surgical dermatologic utility. LSI is effective for evaluating vascular anomalies, cutaneous neoplasms, inflammatory dermatoses, autoimmune conditions, and dermal and subcutaneous growths through the detection of perfusion differences between lesional and perilesional skin. This imaging method has also proven to be an effective surgical tool for the planning of skin graft dissections as well as for the assessment of revascularization of skin grafts and flaps after surgery (Berggren et al., 2021; Brinca et al., 2020; Carvalho Brinca et al., 2021). Dermatologic advantages of LSI include its noninvasive nature, low cost, rapid results, and ability to produce accurate data when used simultaneously with dermoscopy (Tkaczyk, 2017; White et al., 2018). The purpose of this review is to highlight the applications of LSI in clinical dermatology. Selected studies involve investigation of the utility and efficacy of LSI for diagnostic or management purposes of three major categories of dermatologic lesions: vascular, neoplastic, and inflammatory.
Vascular anomalies
A capillary malformation, known as a nevus flammeus or port wine stain (PWS), appears clinically as a red−purple patch present from birth. PWSs are often benign birthmarks but have recently been found to be associated with high-morbidity genetic syndromes such as Sturge−Weber and Klippel−Trénaunay−Weber when present with more widespread vascular malformations (Fitzpatrick, 2018; Gangopadhyay and Tiwari, 2021). Treatment with pulsed dye laser (PDL) is the standard-of-care (SOC) approach to these congenital lesions; however, complete resolution with PDL is not commonly achieved.
Huang et al. (2009) were the first to describe the use of LSI as a tool for assessment of perfusion changes during and after treatment of PWSs with PDL. Although the study showed an overall decrease in perfusion of lesions after treatment, many speckle maps indicated areas within lesions that maintained some degree of persistent perfusion. The authors hypothesized that this persistent, undesired blood flow represented regions that received incomplete PDL therapy and suggested that increased use of real-time LSI during laser treatments could result in a more thorough treatment and improved removal of PWS lesions in the future (Huang et al., 2009).
Additional studies have since drawn similar conclusions and shown the efficacy of LSI as a noninvasive method of post-treatment monitoring of PWS birthmarks. Qiu et al. (2012) noted that the superior ability of LSI to detect microvascular blood flow changes over both space and time is a feature not afforded with lesional biopsies.
In a report of an infant aged 7 months with a magnetic resonance imaging (MRI)−confirmed arteriovenous malformation (AVM), LSI technology was used to further characterize the lesion. The authors found that LSI accurately highlighted the lesion in a similar pattern as MRI. They suggest that LSI could be preferable for initial AVM assessment in patients with contraindications to MRI or in pediatric patients owing to the lack of anesthesia required to obtain imaging (Humeau-Heurtier et al., 2017).
Literature detailing the use of LSI for the assessment of other vascular anomalies, such as venous or lymphatic malformations, is scarce, and thus further studies are required to assess the efficacy of LSI as a diagnostic and post-treatment monitoring tool for vascular malformations compared with that of current SOC imaging modalities.
Skin cancers
Tissue biopsy is the gold standard of diagnosis for skin malignancies, but this can occasionally result in unnecessary, invasive diagnostic measures in patients who are ultimately proven to have a benign skin condition (Agnew et al., 2005). Biopsy procedures have inherent risks of bleeding, infection, scarring, and disfiguration, thus assessment of suspicious skin lesions with a noninvasive modality before skin biopsy might decrease the rate of unnecessary procedures as well as procedure-related complications.
LSI has been shown to be an accurate tool in differentiating benign and malignant skin lesions. Tchvialeva et al. (2013) utilized LSI to assess five different types of benign and malignant skin lesions in vivo: basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), seborrheic keratosis (SK), and melanocytic nevus. Malignant diagnoses were confirmed with tissue biopsy. A total of 214 skin lesions were analyzed using two different wavelengths: red laser (663 nm), which differentiates SK from MM, BCC, and nevus, and blue laser (407 nm), which differentiates the speckle pattern of MM from those of SK, BCC, and SCC. Accuracy was assessed using receiver operator characteristic (ROC) analysis compared with other diagnostic methods, including Raman spectroscopy, SIAscope, multispectral imaging, SolarScan, specialized dermatologists, general dermatologists, and general practitioners. LSI was shown to have accuracy similar to that of Raman, SIAscope, SolarScan, and expert dermatologists and higher accuracy than that of general practitioners, general dermatologists, and multispectral imaging. When plotted on a ROC curve of sensitivity against 1-specificity, the area under the curve measurements for red and blue lasers were 0.87 and 0.84, respectively (Tchvialeva et al., 2013). This study suggests that LSI is a high-accuracy, noninvasive strategy for assessing skin lesions suspicious for malignancy before invasive tissue sampling.
Another proof-of-concept study was conducted by Zieger et al. (2021) in which nine patients with biopsy-confirmed BCCs were assessed with LSI to determine whether microvascular tumor blood flood differed from that of the perilesional skin. Speckle patterns were characterized by speckle size, speckle contrast, and fractional dimension. Results showed that using two specific wavelengths—450 nm (blue region of spectrum) and 515 nm (cyan region)—speckle patterns showed statistically significant differences in lesional blood flow from that of perilesional skin (P ≤ 0.05 for speckle contrast [515 nm], P ≤ 0.01 for speckle size [515 nm], P ≤ 0.001 for fractional dimension [515 nm]; no P-values specified for data at 450 nm). This study showed that LSI is a reliable method for reproducibly assessing neoplasms of uncertain behavior compared with that of normal skin in a noninvasive manner. The authors suggest that tumor margins may also be detectable using LSI technology (Zieger et al., 2021), which could be utilized during future surgical excisions.
At the Medical University of South Carolina, a customized LSI device has been built and used for lesions concerning skin cancer before tissue biopsy. The initial results showed increased perfusion in the lesional skin of neoplastic lesions as well as benign vascular lesions (Figure 2, Figure 3). Given the scarcity of literature analyzing LSI’s efficacy in the diagnosis of skin malignancies, further studies are warranted to explore LSI for this application.
Inflammatory conditions
Blood flow imaging by LSI can be used to evaluate inflammation and erythema by quantitatively measuring the velocity and hemoglobin content in the capillaries of the skin (Tkaczyk, 2017). LSI is capable of quantifying acute skin vascular permeability reactions (Kalchenko et al., 2019), including those mediated by histamine. This may be translated to characterize inflammatory dermatologic conditions associated with increased blood flow or extravasation (Lehmann et al., 2020) or to assess the effectiveness of treatment interventions utilizing therapies that have effects on blood flow, such as antihistamines and capsaicin (Bamps et al., 2020; Meyer et al., 2013). Overall, these studies suggested that LSI holds the potential to objectively assess signs of acute and chronic inflammation on the skin surface, such as in psoriasis and other inflammatory dermatoses.
Psoriasis
Skin microvasculature changes are implicated in the pathogenesis of psoriasis and correlate with increased lesion perfusion. In a 2021 pilot study, Schaap et al. (2022) utilized the Handheld Perfusion Imager, a device powered by LSI, to examine microvascular skin perfusion in six adult patients with unstable plaque psoriasis, as defined by a recent expansion of psoriatic plaques. In a total of 110 lesions, the LSI device was employed to evaluate perilesional perfusion and perfusion inhomogeneity as a way of predicting psoriatic lesion expansion (Schaap et al., 2022). Results of a mixed multinomial logistic regression model showed that increased perilesional perfusion was predictive of psoriatic plaque expansion after 2 weeks compared with lesion stability (OR = 9.90, 95% confidence interval [CI] = 4.61−21.28; P < 0.001) and lesion reduction (OR = 10.85, 95% CI = 4.80−24.52; P < 0.001). Perfusion inhomogeneity was a weaker but still statistically significant predictor of lesion expansion at 2 weeks than stability (OR = 2.39, 95% CI = 1.11−5.14; P = 0.027). From these results, it was concluded that LSI has potential applications in assessing stable, improving, or expanding disease course in patients with psoriasis. However, further studies are required to determine specific uses in clinical practice (Schaap et al., 2022). LSI measurements are found to be reliable in detecting psoriatic plaques using both handheld and mounted devices (Chizari et al., 2021).
Autoimmune conditions
Systemic lupus erythematosus
Patients with systemic lupus erythematosus (SLE) are known to have alterations in both macro and microcirculation; thus, a 2021 study evaluated skin microvascular function in patients with SLE using LSI. The investigators found that patients with SLE exhibited blunted microvascular reactivity during reperfusion periods compared with healthy controls, even in the absence of cardiovascular (CV) disease or CV risk factors (Koletsos et al., 2021). These findings suggest that skin microvascular dysfunction is present in SLE independent of CV dysfunction (Koletsos et al., 2021). In the future, LSI may be used as an objective, noninvasive method of evaluating early signs of vascular damage in cutaneous SLE lesions.
Future directions
Assessment of skin roughness
Increases in skin roughness have been shown to correlate with cutaneous malignancy (del Carmen López Pacheco et al., 2005). In addition to the dermatologic uses afforded by recognition of increased perfusion as previously described, LSI has also been shown to discern the differences in skin surface roughness, making it a potential tool in the noninvasive assessment of skin cancers. In a study with 60 female volunteers, skin roughness of normal skin was assessed with LSI and the PRIMOS (Phaseshift Rapid In-vivo Measurement Of Skin) device, which creates a high-resolution and three-dimensional image of the skin surface. The study showed that LSI was able to detect a significant difference in skin roughness across age groups, although not quite to the degree of the PRIMOS technology. The authors propose that the ability of LSI to measure skin roughness will have substantial clinical applications, such as detecting precancerous skin lesions, which are often associated with increased skin roughness (Zieger et al., 2021).
Other potential applications
It has been suggested that handheld LSI is capable of producing reliable measurements of test reactions in allergy patch testing (Tkaczyk, 2017). In the future, LSI may be used for the objective assessment of cutaneous allergic reactions. LSI has also been shown to detect decreased perfusion in digital ulcers of patients with systemic sclerosis (Marjanovic et al., 2020) and increased vascularity in lesions of localized scleroderma (Vanhaecke et al., 2022), eczema (Bonnekoh et al., 2021; Fluhr et al., 2018), and keloids (Chen et al., 2021; Li et al., 2022; Liu et al., 2016; Yang et al., 2021). Additional research is needed to investigate whether these conditions may represent areas of future clinical utility for LSI.
Limitations
Although LSI remains an up-and-coming tool for the noninvasive assessment of skin lesions with increased vascularity, several limitations to its clinical use exist. The technology can have significant movement artifacts, particularly when used through a handheld device, which may require correction (Chizari et al., 2021; Heeman et al., 2019). This could potentially limit use of the imaging in pediatrics or other populations that may have difficulty remaining motionless during assessment. There is also evidence of multiple scattering of speckles with LSI, which necessitates calibration (Briers et al., 2013). In addition, the speckle pattern data produced are largely qualitative (Heeman et al., 2019), and investigators have used different algorithms or measurements to assess speckle pattern changes objectively within the discussed studies. A consensus on the most accurate method of objectively quantifying image differences would be beneficial. Finally, given that many of the dermatologic conditions discussed are diagnosed either clinically or with established SOC diagnostic tests, it is unclear how LSI would change the scope of clinical practice for the management of these conditions, particularly without diagnostic superiority data compared with current diagnostics. It is the authors’ opinion that LSI’s utility in providing a means of monitoring lesions during or after treatment is a promising application of the technology.
Conclusions
LSI has shown promise as a noninvasive, accurate method of diagnosing and monitoring multiple dermatologic pathologies, including vascular, neoplastic, and inflammatory skin conditions (Table 1). Additional benefits of the imaging modality include low cost and lack of adverse effects. However, some limitations of the technology exist, and most clinical studies of LSI in dermatology are limited to single lesion types with small patient populations. Future studies with larger sample sizes are necessary to further assess the clinical utility of this technology.
Table 1.
Summary of Potential Clinical Uses of LSI in Dermatology
| Dermatologic Diagnosis | Potential Uses of LSI |
|---|---|
| Vascular anomalies | |
| Port wine stains | Monitoring of treatment success in lesions after PDL therapy Real-time assessment of lesions during PDL therapy |
| Arteriovenous malformations | Initial imaging assessment, without a requirement for anesthesia in children |
| Venous and lymphatic malformations | Potential for initial imaging assessment, without a requirement for anesthesia in children; further studies are needed |
| Neoplastic lesions | |
| Skin cancers | Initial imaging assessment of lesions of malignancy before invasive tissue biopsy, specifically differentiating malignant melanoma from other benign nevi or nonmelanoma skin cancers Tumor margin assessment during surgical excisions |
| Inflammatory conditions | |
| Psoriasis | Prediction of disease progression/treatment response (stable, improving, or expanding) |
| Cutaneous lupus | Evaluation of early signs of microvascular damage |
Abbreviations: LSI, laser speckle imaging; PDL, pulsed dye laser.
Data availability statement
No datasets were generated or analyzed during this study.
ORCIDs
Courtney Linkous: http://orcid.org/0000-0001-7885-7475
Angel D. Pagan: http://orcid.org/0000-0002-4913-6705
Chelsea Shope: http://orcid.org/0000-0002-2944-4380
Laura Andrews: http://orcid.org/0000-0001-8873-230X
Alan Snyder: http://orcid.org/0000-0001-9115-626X
Tong Ye: http://orcid.org/0000-0003-1017-6969
Manuel Valdebran: http://orcid.org/0000-0002-2502-7256
Conflict of Interest
The authors state no conflict of interest.
Author Contributions
Conceptualization: CS; Data Curation: CL, ADP; Methodology: CS; Project Administration: CS; Supervision: TY, MV; Writing - Original Draft Preparation: CL, ADP; Writing - Review and Editing: CS, LA, AS, TY, MV
corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2023.100187
References
- Agnew K.L., Gilchrest B.A., Bunker C.B. Health Press Limited; Abingdon, United Kingdom: 2005. Fast facts. Skin Cancer. [Google Scholar]
- Aminfar A., Davoodzadeh N., Aguilar G., Princevac M. Application of optical flow algorithms to laser speckle imaging. Microvasc Res. 2019;122:52–59. doi: 10.1016/j.mvr.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Bamps D., Macours L., Buntinx L., de Hoon J. Laser speckle contrast imaging, the future DBF imaging technique for TRP target engagement biomarker assays. Microvasc Res. 2020;129 doi: 10.1016/j.mvr.2019.103965. [DOI] [PubMed] [Google Scholar]
- Berggren J., Castelo N., Tenland K., Dahlstrand U., Engelsberg K., Lindstedt S., et al. Reperfusion of free full-thickness skin grafts in periocular reconstructive surgery monitored using laser speckle contrast imaging. Ophthalmic Plast Reconstr Surg. 2021;37:324–328. doi: 10.1097/IOP.0000000000001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas D.A., Dunn A.K. Laser speckle contrast imaging in biomedical optics. J Biomed Opt. 2010;15 doi: 10.1117/1.3285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnekoh H., Vera C., Abad-Perez A., Radetzki S., Neuenschwander M., Specker E., et al. Topical inflammasome inhibition with disulfiram prevents irritant contact dermatitis. Clin Transl Allergy. 2021;11 doi: 10.1002/clt2.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briers D., Duncan D.D., Hirst E., Kirkpatrick S.J., Larsson M., Steenbergen W., et al. Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 2013;18 doi: 10.1117/1.JBO.18.6.066018. [DOI] [PubMed] [Google Scholar]
- Brinca A., Pinho A., Vieira R. Laser speckle contrast imaging for assessment of human skin graft microcirculation. J Eur Acad Dermatol Venereol. 2020;34:e491–e493. doi: 10.1111/jdv.16408. [DOI] [PubMed] [Google Scholar]
- Carvalho Brinca A.M., de Castro Pinho A., Costa Vieira R.J.D. Blood perfusion of random skin flaps in humans-in vivo assessment by laser speckle contrast imaging. Dermatol Surg. 2021;47:1421–1426. doi: 10.1097/DSS.0000000000003164. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang M., Yu N., Zhang W., Long X., Wang Y., et al. Heterogeneous features of keloids assessed by laser speckle contrast imaging: a cross-sectional study. Lasers Surg Med. 2021;53:865–871. doi: 10.1002/lsm.23331. [DOI] [PubMed] [Google Scholar]
- Chizari A., Schaap M.J., Knop T., Boink Y.E., Seyger M.M.B., Steenbergen W. Handheld versus mounted laser speckle contrast perfusion imaging demonstrated in psoriasis lesions. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Carmen López Pacheco M., da Cunha Martins-Costa M.F., Zapata A.J., Cherit J.D., Gallegos E.R. Implementation and analysis of relief patterns of the surface of benign and malignant lesions of the skin by microtopography. Phys Med Biol. 2005;50:5535–5543. doi: 10.1088/0031-9155/50/23/008. [DOI] [PubMed] [Google Scholar]
- Draijer M., Hondebrink E., van Leeuwen T., Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci. 2009;24:639–651. doi: 10.1007/s10103-008-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick J., High W.A. Elsevier; Philadelphia, PA: 2018. Urgent care dermatology: symptom-based diagnosis. [Google Scholar]
- Fluhr J.W., Zuberbier T., Darlenski R. Noninvasive measures in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2018;18:417–424. doi: 10.1097/ACI.0000000000000476. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay A.N., Tiwari P. In: Vascular malformations. Khanna A.K., Tiwary S.K., editors. Springer; Singapore, Singapore: 2021. Capillary malformation; pp. 73–82. [Google Scholar]
- Heeman W., Steenbergen W., van Dam G., Boerma E.C. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt. 2019;24:1–11. doi: 10.1117/1.JBO.24.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.C., Tran N., Shumaker P.R., Kelly K., Ross E.V., Nelson J.S., et al. Blood flow dynamics after laser therapy of port wine stain birthmarks. Lasers Surg Med. 2009;41:563–571. doi: 10.1002/lsm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau-Heurtier A., Martin L., Bazeries P., Abraham P., Henni S. Laser speckle contrast imaging of skin changes in arteriovenous malformation. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005931. [DOI] [PubMed] [Google Scholar]
- Kalchenko V., Meglinski I., Sdobnov A., Kuznetsov Y., Harmelin A. Combined laser speckle imaging and fluorescent intravital microscopy for monitoring acute vascular permeability reaction. J Biomed Opt. 2019;24:1–4. doi: 10.1117/1.JBO.24.6.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsos N., Gkaliagkousi E., Lazaridis A., Triantafyllou A., Anyfanti P., Dolgyras P., et al. Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology (Oxford) 2021;60:2834–2841. doi: 10.1093/rheumatology/keaa722. [DOI] [PubMed] [Google Scholar]
- Lehmann S., Deuring E., Weller K., Scheffel J., Metz M., Maurer M., et al. Flare size but not intensity reflects histamine-induced itch. Skin Pharmacol Physiol. 2020;33:244–252. doi: 10.1159/000508795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang M., Long X., Wang X. Relative perfusion index: an objective, quantitative and noninvasive method for evaluating the severity of keloids. Lasers Surg Med. 2022;54:1071–1081. doi: 10.1002/lsm.23579. [DOI] [PubMed] [Google Scholar]
- Liu Q., Wang X., Jia Y., Long X., Yu N., Wang Y., et al. Increased blood flow in keloids and adjacent skin revealed by laser speckle contrast imaging. Lasers Surg Med. 2016;48:360–364. doi: 10.1002/lsm.22470. [DOI] [PubMed] [Google Scholar]
- Marjanovic E., Moore T.L., Manning J.B., Dinsdale G., Wilkinson S., Dickinson M.R., et al. Systemic sclerosis-related digital calcinosis; a pilot study of cutaneous oxygenation and perfusion. Rheumatology (Oxford) 2020;59:3573–3575. doi: 10.1093/rheumatology/keaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Gorbach A.M., Liu W.M., Medic N., Young M., Nelson C., et al. Mast cell dependent vascular changes associated with an acute response to cold immersion in primary contact urticaria. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Zhou Y., Gu Y., Ang Q., Zhao S., Wang Y., et al. Monitoring microcirculation changes in port wine stains during vascular targeted photodynamic therapy by laser speckle imaging. Photochem Photobiol. 2012;88:978–984. doi: 10.1111/j.1751-1097.2012.01153.x. [DOI] [PubMed] [Google Scholar]
- Schaap M.J., Chizari A., Knop T., Groenewoud H.M.M., van Erp P.E.J., de Jong E.M.G.J., et al. Perfusion measured by laser speckle contrast imaging as a predictor for expansion of psoriasis lesions. Skin Res Technol. 2022;28:104–110. doi: 10.1111/srt.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchvialeva L., Dhadwal G., Lui H., Kalia S., Zeng H., McLean D.I., et al. Polarization speckle imaging as a potential technique for in vivo skin cancer detection. J Biomed Opt. 2013;18 doi: 10.1117/1.JBO.18.6.061211. [DOI] [PubMed] [Google Scholar]
- Tkaczyk E. Innovations and developments in dermatologic non-invasive optical imaging and potential clinical applications. Acta Derm Venereol. 2017;Suppl:5–13. doi: 10.2340/00015555-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaecke A., Schonenberg-Meinema D., De Schepper S., Bergkamp S.C., Leone M.C., Middelkamp-Hup M.A., et al. Rarities in rare: illuminating the microvascular and dermal status in juvenile localised scleroderma. a case series. Clin Exp Rheumatol. 2022;40:12–18. doi: 10.55563/clinexprheumatol/2vm1pz. [DOI] [PubMed] [Google Scholar]
- White S.M., Valdebran M., Kelly K.M., Choi B. Simultaneous blood flow measurement and dermoscopy of skin lesions using Dual-Mode dermascope. Sci Rep. 2018;8 doi: 10.1038/s41598-018-35107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu L., Yang R., Ding X., Li Y., Liu H., et al. Blood perfusion in hypertrophic scars and keloids studied by laser speckle contrast imaging. Skin Res Technol. 2021;27:789–796. doi: 10.1111/srt.13020. [DOI] [PubMed] [Google Scholar]
- Zieger M., Kaatz M., Springer S., Riesenberg R., Wuttig A., Kanka M., et al. Multi-wavelength, handheld laser speckle imaging for skin evaluation. Skin Res Technol. 2021;27:486–493. doi: 10.1111/srt.12959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during this study.