Abstract
Background
Abdominal aortic aneurysms (AAAs) are a relatively common vascular pathology of the elderly with high morbidity potential. Irreversible degeneration of the aortic wall leads to lethal rupture if left untreated. Nearly all AAAs contain intraluminal thrombus (ILT) to a varying degree, yet the mechanisms explaining how thrombosis is disturbed in AAA are relatively unknown. This review examined the thrombotic complications associated with AAA, the impact of thrombosis on AAA surgical outcomes and AAA pathogenesis, and the use of antithrombotic therapy in the management of this disease.
Methods
A literature search of the PubMed database was conducted using relevant keywords related to thrombosis and AAAs.
Results
Thrombotic complications are relatively infrequent in AAA yet carry significant morbidity risks. The ILT can impact endovascular aneurysm repair by limiting anatomic suitability and influence the risk of endoleaks. Many of the pathologic mechanisms involved in AAA development, including hemodynamics, inflammation, oxidative stress, and aortic wall remodeling, contain pathways that interact with thrombosis. Conversely, the ILT can also be a source of biochemical stress and exacerbate these aneurysmal processes. In animal AAA models, antithrombotic therapies have shown favorable results in preventing and stabilizing AAA. Antiplatelet agents may be beneficial for reducing risks of major adverse cardiovascular events in AAA patients; however, neither antiplatelet nor anticoagulation is currently used solely for the management of AAA.
Conclusions
Thrombosis and ILT may have detrimental effects on AAA growth, rupture risk, and patient outcomes, yet there is limited understanding of the pathologic thrombotic mechanisms in aneurysmal disease at the molecular level. Preventing ILT using platelet and coagulation inhibitors may be a reasonable theoretical target for aneurysm progression and stability; however, the practical benefits of current antithrombotic therapies in AAA are unclear. Further research is needed to demonstrate the extent to which thrombosis impacts AAA pathogenesis and to develop novel pharmacologic strategies for the medical management of this disease.
Keywords: Aortic aneurysm, Thrombosis, Intraluminal thrombus, Platelets, Coagulation
Clinical Relevance
Abdominal aortic aneurysm rupture is a leading cause of mortality in the United States. Currently, no pharmacologic or noninvasive interventions exist to prevent the development or halt the progression of this disease. The pathogenesis of abdominal aortic aneurysms is multifactorial and strongly influenced by thrombosis. The intraluminal thrombus has been gaining recent attention as an active mediator in aneurysm growth and rupture. In this review article, we will discuss the impact of thrombosis on patient outcomes, the use of antithrombotic therapy, and current understanding of the mechanisms behind pathologic thrombosis leading to the formation and maintenance of the intraluminal thrombus.
Abdominal aortic aneurysm (AAA) rupture is a leading cause of morbidity and mortality in the United States. The pathogenesis of AAA is a complex multifactorial process, involving dysregulation in inflammation, hemodynamics, and thrombosis. Destruction of the medial layer of the aorta results in irreversible pathologic remodeling, leading to progressive wall weakening, aneurysmal dilation, and eventual rupture. Each pathologic mechanism involved in the aneurysm process is strongly interconnected with thrombosis. Although all AAAs contain intraluminal thrombus (ILT) to a varying degree,1,2 there is limited understanding to the true extent that thrombosis and ILT impact aneurysmal disease. Early computational studies modeling aneurysm hemodynamics suggested that ILT may be protective by reducing aortic wall stress,3 whereas more recent correlational studies show that greater thrombus burden correlates with higher rupture risk at lower AAA diameters.4 Classically thought as an inert mass, evidence demonstrates that ILT is also biologically active and contributes to aneurysmal degeneration as a source of inflammation and other pathologic processes.5 Whether these destructive biochemical stressors outweigh the potential protective benefits of reducing mechanical wall stress has been recently discussed,5 which raises the question if preventing ILT with antithrombic therapy could be beneficial in AAA. In this review, we explore how thrombosis is disrupted in AAA, its role in aneurysm pathogenesis, and its impact on patient disease and outcomes. Furthermore, we will discuss the less known mechanisms involved in the formation, propagation, and preservation of ILT, and the current literature on antithrombotic therapy in AAA.
Methods
A literature search of the PubMed search engine MEDLINE database from inception to the present (2023) was conducted using terms related to thrombosis and AAAs. Original reports, meta-analysis, and systematic reviews were search for the terms “abdominal aortic aneurysms,” “thrombosis,” “platelets,” “coagulation,” “antiplatelet,” “anticoagulation,” “intraluminal thrombus,” “ischemia,” “embolization,” “endovascular,” “endoleak,” “hemodynamics,” “inflammation,” “hypoxic,” and “wall remodeling.” Titles and abstracts of these articles or their cited works were reviewed for relevance and suitability. Studies lacking full text were excluded. Over 300 articles were screened with 128 included.
Thrombosis-associated complications of AAA
Thrombosis-associated complications are infrequently encountered in AAA, yet often warrant aneurysm repair due to significant morbidity and mortality risks. Disseminated intravascular coagulation has been observed as a presenting symptom of AAA in roughly 3% to 4% of patients preoperatively.6 Interestingly, the consumptive coagulopathy is corrected by aneurysm repair,6 highlighting the influence of AAA on disrupted coagulation. Acute thrombosis of AAA is a deadly complication, with mortality near 50% and incidence of 0.7% to 2.8% of all surgically managed AAAs.7 Presenting symptoms include acute limb ischemia of the bilateral lower extremities,8 yet may also present as spinal cord ischemia without acute limb ischemia features,9 or even asymptomatically.10 The immediate initiation of systemic anticoagulation (AC) and emergent surgical management is critical as delays in revascularization result in high mortality, often from proximal propagation of the thrombus and occlusion of visceral arteries.7 Optimal surgical repairs include aneurysmorrhaphy and inline aortic reconstruction;7 however, extra-anatomic axillobifemoral bypass may be acceptable for high-risk patients despite a 15% risk of delayed rupture.11
Additional thrombosis-associated AAA complications include embolization of the ILT, related to thrombus friability or caused by dislodgement due to trauma or instrumentation during other procedures.12,13 Compared with other aneurysms, such as popliteal aneurysms, thromboembolism causing lower extremity ischemia is relatively infrequent, reported in 2% to 5% of patients with AAA who undergo repair.12,14 Although rare, AAA can also embolize to the lumbar arteries and present as spinal cord transient ischemic attacks,15 paralysis,16 or cauda equina syndrome.17 The risks of thromboembolism, however, may not be correlated with aneurysm diameter.14
Impact of thrombosis on AAA surgical outcomes
Thrombosis-associated consequences should be considered during AAA repair planning and assessing surgical risk related with perioperative bleeding. The presence of coagulopathies or anemias, the use of antithrombotic therapy, and the patient’s frailty should be reviewed before surgery and help guide the decision between open and endovascular repair. The location and burden of ILT alter the intraluminal anatomy of the aneurysm and can limit anatomical suitability for endovascular aneurysm repair (EVAR).
Surgical outcomes are also impacted by thrombosis, particularly with EVAR. As it has been recently shown that treatment of larger aneurysms with EVAR is associated with greater patient mortality, reintervention,18 and risks of complications,19,20 Oliveira-Pinto et al20 hypothesized that this phenomenon may be due to the impact of free luminal space. AAAs with larger intraluminal flow channels require a greater volume of new thrombus to form between the endograft and excluded aneurysm sac. New thrombus lacks the organizational strength of the laminated chronic ILT,21 allowing for greater graft mobility and potentially compromised seal when compared with smaller luminal spaces where the graft is closely abutted against chronic ILT.20 The spatial morphology of the ILT may also affect endoleaks. Thrombus located proximally at the aneurysm neck can provide challenges for EVAR by compromising proximal seal and fixation to the aortic wall.22 AAA with posterolateral and circumferential ILT have lower incidences of type 2 endoleaks, likely by preventing retrograde flow through lumbar vessels.23 Higher percentage of ILT burden, regardless of size, results in higher rates of IMA occlusion and less occurrence of type 2 endoleaks.24 However, greater thrombus area and higher ILT volume may be related to increased reintervention rates overall.25
Few studies have evaluated biomarkers in the correlation of EVAR sac thrombosis, yet the process of endovascular AAA repair itself may induce transient hypercoagulable states perioperatively. Contrast media causes endothelial cell injury, which leads to platelet activation, whereas intimal damage from wire manipulation and graft placement may increase thrombin generation and tissue factor (TF) levels.26 Elevated preoperative fibrinogen levels in AAA patients before EVAR have been correlated with aneurysm sac regression.27 Understanding the clinical impacts of AAA-associated thrombosis and ILT on surgical outcomes warrants thorough investigation of this disease component.
Impacts of thrombosis on AAA pathogenesis
Hemodynamics
Numerous hemodynamic models of intact and ruptured AAAs have revealed that aneurysm morphology and wall composition cause a nonhomogeneous distribution of tensile stress, sheer stress, and pressure that impacts AAA progression and degree of ILT.28 Early hemodynamic studies analyzing the effects of ILT on biomechanical stress suggested that ILT reduces the tensile stress on the aortic wall, thus potentially providing a protective effect.3 Recent studies have found conflicting effects of ILT on hemodynamics. Interestingly, in a computational fluid dynamics model of ruptured AAAs, the location of rupture in the aneurysm sac coincided with regions of low wall sheer stress and higher deposition of ILT, contradictory to other models.29 However, there is gaining evidence that the ILT accelerates aneurysmal degeneration by actively contributing to inflammation, extracellular matrix (ECM) degeneration, and smooth muscle cell (SMC) death, and the resulting upregulated biochemical stress may outweigh any protective hemodynamic effects.30
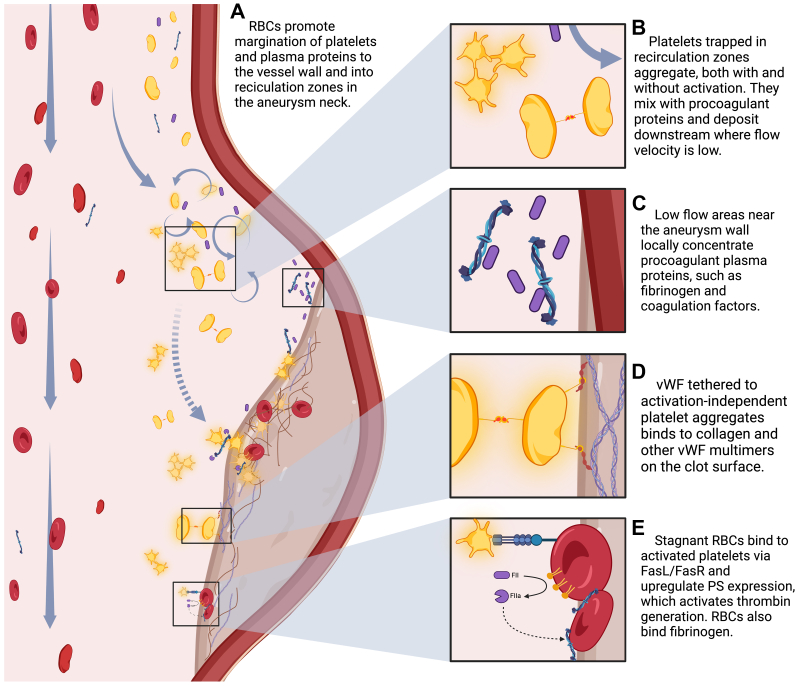
Altered AAA hemodynamics create a prothrombotic environment through nonphysiologic platelet interactions, altering local concentrations of thrombogenic proteins and disrupting the delivery of nutrients and oxygen, as seen in the Fig. Biasetti et al31,32 hypothesized a hemodynamic mechanism for thrombus formation based on aneurysm morphology and shear wall stress. Their computational model suggests that blood flow is laminar in the proximal nonaneurysmal aortic segment, yet once reaching the aneurysm neck, shear stress increases,32,33 and turbulent flow creates recirculation zones in the proximal sac.32 Shear stress promotes platelet activation,34 the production of prothrombotic microparticles,35 and even the formation of aggregates independent of activation.36 High flow velocities under these conditions alter the shape of soluble von Willebrand factor (vWF),37 allowing it to bind to platelets via glycoprotein 1bα (GP1bα) receptors, and promote cohesion by forming of membrane tethers.38 This contrasts classic primary hemostasis seen under low shear conditions, where platelets attach to subendothelial collagen and fibronectin at the site of injury, bind to vWF via the GP1bα receptor to form stable adhesions, and then become activated and aggregate.37 Interestingly, on further exposure to dynamic changes in shear stress, such as repeated accelerations and decelerations seen within recirculation zones, these platelet tethers restructure to increase their binding strength and stability, leading to further thrombus formation.39 Platelets trapped in vortical structures are exposed to longer durations of shear stress,31 which promotes activation and mixing with other aggregates and procoagulant factors.34 Along the aneurysm wall where shear rates are low,32 activated platelets bind strongly to surface receptors and existing thrombus,40 and the activation-independent platelet aggregates bind tethered vWF to ECM components, primarily collagen, and other vWF multimers.37 Decreased shear rates allow for fibrin monomer assembly into protofibrils and their lateral aggregation and crosslinking.41 Biologic activity within the thrombus and inflammatory cytokines produced by aneurysmal tissues additionally activates platelets.
Fig.
Proposed impact of hemodynamics on mechanisms of thrombosis and growth of the intraluminal thrombus (ILT). PS, Phosphatidylserine; RBC, red blood cell; vWF, von Willebrand factor.
Red blood cells (RBCs) interact with circulating platelets and promote platelet margination out of the stream trajectory to the periphery42 and into recirculation zones.40 This phenomenon increases the concentration of platelets three- to fivefold near the wall surface40, 41, 42 and explains how an increase in hematocrit may improve hemostasis.40, 41, 42, 43 In low flow or stagnant states, RBCs can provide a prothrombogenic surface for platelets through the FasL/FasR pathway,43 subsequently leading to enhanced surface expression of phosphatidylserine44 and thrombin generation. RBCs can also bind fibrinogen and therefore impact thrombus stability and structure.42
Hemodynamics also affect the exchange of circulating substances at the intraluminal surface, known as mass transfer.45 Aortic curvature and tortuosity change the flow velocity along the longitudinal arterial axis causing variations in local mass transfer rates at the wall.46 It has been hypothesized that altered mass transport of oxygen and low-density lipoprotein may result in regional hypoxia and wall damage, potentially promoting atherogenesis45 and a nidus for inflammation seen early in AAA development. Flow velocity impacts coagulation and fibrin formation by affecting the delivery of procoagulant plasma proteins and zymogens.47 Local concentrations of coagulation factors are decreased at high flow velocities and increased at low flow velocities,47 such as those within the aneurysm sac.
Inflammation
The role of inflammation in the pathogenesis of AAA is well reported, as described in the excellent review by Shimizu et al,48 and is closely related to thrombosis as many of the kinase activators act on both pathways. Coagulation factors, particularly factor Xa (FXa), are gaining recognition as mediators of inflammation in cardiovascular diseases.49 Through direct stimulation or downstream effects, activated coagulation factors can promote the production of inflammatory cytokines, such as IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1), from effector cells and increase the expression of the adhesion molecules E-selectin, intracellular adhesion molecule-1, and vascular cell adhesion molecule-1 by the endothelium.50 Platelets also become activated in response to inflammation and are a source of FXa.51
Studies have used coagulation inhibitors to help clarify many of the complicated connections between inflammation and coagulation. Use of the FXa inhibitor rivaroxaban has revealed FXa’s ability to modulate inflammatory responses in multiple vascular cell types involved in AAA,52 suggesting that direct oral anticoagulants may have pleotropic effects beyond AC. Treatment of vascular SMCs (VSMCs) with rivaroxaban reduces cell migration, proliferation, and the production of the inflammatory cytokines IL-1β and TNFα in vitro.53 FXa acts on macrophages to increase mRNA expression of inflammatory molecules, IL-1β, TNFα, and MCP-1, and promotes oxidized low-density lipoprotein uptake leading to foam cell formation, all of which are attenuated by rivaroxaban.54 In fibroblasts, rivaroxaban inhibits angiotensin II (AngII)-induced cell migration and proliferation by reducing activation of the nuclear factor-κB and mitogen-activated protein kinase55 inflammatory pathways. In vascular endothelial cells and VSMCs, FXa induced senescence, which was inhibited by rivaroxaban.56 Aneurysm tissue samples cultured with rivaroxaban also exhibited significantly increased levels of the anti-inflammatory cytokine, IL-10, compared with untreated AAA samples.57
Oxidative stress
The effects of oxidative stress have been implicated to play a central role in AAA58 and may link inflammation and vascular remodeling.59 Reactive oxygen species (ROS) can be produced in response to mechanical stress or cytokines59 and contributes to AAA by inducing inflammation, SMC proliferation and apoptosis, and ECM remodeling via matrix metalloprotease (MMP) activity.59 Cytokines such as TNFα, IFN-γ, and IL-1β enhance the expression of inducible nitric oxidase synthase in VSMCs60 and macrophages,61 resulting in the production of ROS, such as superoxide and oxygen radicals.62 Once infiltrating the vascular wall, activated neutrophils express myeloperoxidase to produce hypochlorous acid from hydrogen peroxide and chloride ions, which inactivates tissue inhibitor of metalloprotease-1 and indirectly stimulates ECM degradation.63 Systemic oxidative stress markers, such as malondialdehyde, are increased in patients with AAA,64 and aneurysmal tissues demonstrate upregulated nicotinamide adenine dinucleotide phosphate oxidase expression and activity.62
Hypoxic conditions have been demonstrated to impact thrombus formation through the activation of platelets65 in both venous66 and arterial thrombosis.67 Using human AAA samples, Moñux et al57 linked FXa to oxidative stress pathways. Treatment of aneurysmal tissue with rivaroxaban significantly reduced expression of nitric oxidase synthase-2 and nicotinamide adenine dinucleotide phosphate oxidase subunits gp67- and gp91-phox compared with untreated AAA samples.57 Epidermal growth factor receptor (EGFR) activation is observed in human AAA68 and is involved with VSMC proliferation69 and the production of ROS.70 FXa acts via the EGFR pathway to induce the production of FGFR-1 receptors and the release of fibroblast growth factor.69 Thrombin also activates a G-protein coupled receptor to form MMPs, which cleave ProHIB-EGF, leading to fibroblast growth factor release.69 Treatment with the EGFR inhibitor, erlotinib, protects against the development of AAA in a mouse model using AngII plus beta-aminopropionitrile.68
Aortic wall remodeling
MMPs are a group of degradation enzymes that play a role in connective tissue remodeling by breaking down components of the ECM, such as collagen and proteoglycans, and are regulated by specific tissue inhibitors of metalloproteases. Increased MMP concentration and activity is found in both aneurysmal wall tissue and within the ILT. Aneurysmal wall tissue has higher proportions of MMPs than normal wall overall,57 whereas activated forms of MMP-9 are only evident in wall tissue that is aneurysmal.71 ILT has demonstrated to contain higher concentrations of MMPs compared with serum, specifically MMP-2, which is produced by mesenchymal cells, and MMP-9, which is secreted by proinflammatory cells such as polymorphonuclear neutrophils and macrophages.71 The ILT absorbs plasma components and traps polymorphonuclear neutrophils enriched with MMP-9 at the luminal surface.72 Neutrophils within the ILT and aneurysmal tissue both release neutrophil gelatinase-associated lipocalin, which acts to complex with MMP-9 and inhibit its degradation, thus leading to enhanced proteolytic activity.73 Beyond the ILT being a source for MMPs, they can be activated by members of the coagulation cascade and fibrinolysis. Plasminogen sequestered in the ILT is converted to plasmin by urokinase plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) secreted from the aortic wall, which then activates MMP-9 in the liquid interface.72 In addition, the presence of plasmin-α2-anti-plasmin complexes within the liquid interface72 and the association of plasminogen activators (uPA and tPA) with increased levels of active MMP-2 and MMP-974 further demonstrates interconnection between these proteolytic pathways at this interface.
Coagulopathy in AAA
Abnormalities in platelet activity, coagulation, and fibrinolysis are commonly present in patients with AAA,75 yet the true extent that clotting mechanisms impact AAA pathogenesis is largely unknown. Increased platelet activation and turnover in AAA is evident by decreased platelet counts and increased levels of GP1b in these patients.76,77 Factors involved in coagulation and fibrin turnover, such as D-dimer, thrombin-antithrombin II complexes, and fibrin degradation products, are elevated in patients with AAA,78,79 which also correlate with AAA size and tortuosity.78 Concentrations of TF are nearly doubled in AAA plasma, whereas thrombin-antithrombin II complexes are tripled80 and shown to independently predict AAA growth rates.81 Elevated levels of D-dimer also correlate with AAA diameter and ILT volume.82 Upregulated activity of the coagulation system is also evidenced by high expression of FXa in human aneurysmal tissue.57 AAA also impacts clot structure and fibrinolysis. Morphologic studies show that patients with larger AAA form thrombus that is denser with smaller pores, which is more resistant to lysis, compared with smaller AAAs and controls.83
Antithrombotic therapy in AAA
Platelet inhibitors in animal AAA models
The risks factors and multiple features of human AAAs, including progressive growth, rupture capability, location, and presence of ILT, are difficult to replicate in animal models. The two most popular AAA murine models include the AngII infusion model and topical elastase model. The AngII infusion model uses an osmotic pump inserted into hyperlipidemic mice to produce aneurysm phenotypes without surgical intervention.84 This less invasive model mimics atherosclerotic risk factors to develop rupture-capable aneurysms that involve wall inflammation and thrombosis; however, unlike human AAA, these aneurysms develop in the suprarenal abdominal aortic segment secondary to dissection or intramural hematoma, and thus, the thrombus is intramural.84 The topical elastase surgical model produces a true aneurysm augmented by the addition of lysyl oxidase inhibitor, beta-aminopropionitrile, which is gradually expanding, contains ILT, and is capable of rupture, yet the study duration is typically longer.85 Although current murine models require further refinement, some studies have been useful in further understanding the role of antithrombotic therapy in the pathophysiology of AAA.
Multiple murine models have shown antiplatelet therapy to have a beneficial impact on AAA, as demonstrated in Table I.86, 87, 88, 89 Early investigations on the role of antiplatelet agents abciximab and P2Y12 receptor inhibitor, AZD6140, used xenograft transplantation of decellularized guinea pig aortas into rats.88,89 Antiplatelet therapy revealed a reduction in aneurysm diameter in both studies,88,89 predicting that reducing the overall ILT burden also reduces the negative biological activity contributing to aneurysm progression.88 However, the xenograft transplant model does not exhibit rupture.90 Liu et al87 were the first to study the role of antithrombotic therapy in mouse AAA models. They found a link between clopidogrel and reduced wall inflammation, which was protective against aneurysm formation and progression.87
Table I.
Animal abdominal aortic aneurysm (AAA) models of antiplatelet therapy and their outcomes
| Study | Inhibitor | Model | Outcome | Proposed mechanism |
|---|---|---|---|---|
| Liu et al86 | Ticagrelor + ASA | AngII ApoE−/− mice | Decreased AAA incidence, reduced wall inflammation, MMP synthesis | Combined platelet inhibition reduces wall inflammation and elastolysis |
| Owens et al74 | ASA, clopidogrel | AngII Ldlr−/− mice; therapy started on day 28 of 70 | Reduction in rupture-induced death; reduced aortic thrombus, platelet accumulation, inflammatory cytokine production, macrophage accumulation, and MMP activity | Antiplatelet therapy reduces rupture risk in established AAA |
| Liu et al87 | Clopidogrel | AngII ApoE−/− mice | Suppressed aneurysm formation and diameter; decreased elastolysis, ROS production, macrophage infiltration, and MMPs. No change in rupture | Inhibiting platelet activation reduces inflammation-mediated AAA |
| Dai et al88 | AZD6140 (P2Y12 receptor inhibitor) | Xenograft transplant of decellularized guinea pig aortas into rats | Smaller AAA diameter, thrombus development, platelet CD41 expression, MMP-9 expression, reduced elastic fiber degradation | Antiplatelet therapy reduces negative biologic activity of ILT that contribute to AAA |
| Touat et al89 | Abciximab | Xenograft transplant of decellularized guinea pig aortas into rats | Reduced aneurysm diameter; reduced thrombus area | Prevention of ILT propagation and platelet-mediated release of procoagulant factors |
AngII, Angiotensin II; ApoE, apolipoprotein E; ASA, aspirin; ILT, intraluminal thrombus; Ldlr, low-density lipoprotein receptor; MMP, matrix metalloprotease; ROS, reactive oxygen species.
Based on the positive results of antiplatelet therapy in AAA in these three previous animal studies, Owens et al74 hypothesized that antiplatelet therapy may also be beneficial in AAA stability. Once AAAs were generated using AngII infusion, apolipoprotein E (ApoE) knockout (ApoE−/−) mice were treated with aspirin (ASA), clopidogrel, or sham.74 They found that antiplatelet therapy was protective against rupture-induced death and reduced wall recruitment of platelets and macrophages, resulting in decreased MMP-2 and MMP-9 levels, as well as lower concentrations of platelet factor 4 and plasminogen activators (tPA and uPA).74
Both clopidogrel and ticagrelor, a more novel and potent P2Y12 receptor inhibitor, have demonstrated anti-inflammatory effects in addition to their antiplatelet functions.91 Previously, a multicenter randomized controlled trial comparing ticagrelor monotherapy with sham was unable to demonstrate a reduction in aneurysm growth rates, AAA volume, or ILT volume in patients with small AAA between 35 and 49 mm.92 In a mouse model, ticagrelor was combined with ASA to evaluate the role of dual antiplatelet therapy on AAA.86 The addition of ASA with ticagrelor was shown to block AAA formation, decrease vascular inflammation, and reduce MMP synthesis in AngII-treated ApoE−/− mice,86 suggesting that dual antiplatelet therapy may be beneficial.
Anticoagulation in animal AAA models
Coagulation factors have demonstrated a pathologic capability to progress vascular disease.93,94 FXa is critical to thrombus formation as the convergence point of the intrinsic and extrinsic coagulation pathways,95 and levels of FXa in aortic tissue strongly correlate with AAA diameter in murine models.94 Murine studies have used AC to block the negative impact of coagulation on VSMC homeostasis, wall remodeling, and inflammation in AAA (see Table II).96, 97, 98
Table II.
Animal abdominal aortic aneurysm (AAA) models of anticoagulation and their outcomes
| Study | Drug | Model | Outcome | Proposed mechanism |
|---|---|---|---|---|
| Searle et al96 | Factor XIIa monoclonal antibody 3F7 | AngII ApoE−/− mice | Reduced rupture, reduced aneurysm size, increased collagen deposition, reduced proinflammatory cytokines | Inhibition of FXIIa may stabilize AAA by increasing collagen synthesis and decreasing inflammation |
| Ding et al52 | Rivaroxaban | AngII ApoE−/− mice, therapy started on day 14 of 28; CaCl2 | Reduced aortic diameter; reduced MMP-2 and MMP-9 expression; downregulation in mRNA of IL-1β, IL-6, IL-8, MCP-1; reduced immunostaining for CD45, CD68 | Anti-inflammatory property reduces elastolysis |
| Moran et al97 | Factor XIIa neutralizing antibody (ch3F7-mG1L-aFXII) | AngII ApoE−/− mice | Reduced AAA diameter, reduction in circulating kallikrein and bradykinin | Inhibition of the FXII/kallikrein pathway reduces AAA expansion |
| Allen-Redpath et al98 | Rivaroxaban | AngII ApoE−/− mice | Reduced incidence of AAA | Anticoagulation blocks procoagulant effects of phospholipids in AAA |
| Moran et al94 | Fondaparinux, enoxaparin | AngII ApoE−/− mice, therapy started on day 14 of 28 | Fondaparinux reduced FXa, PAR-2, MMP-2, Smad2/3; reduced aneurysm | Inhibition of PAR-2 receptor signaling reduces elastolysis |
AngII, Angiotensin II; ApoE, apolipoprotein E; FXa, factor Xa; MCP, monocyte chemotactic protein-1; MMP, matrix metalloprotease; PAR, protease-activated receptor.
FXa acts as a VSMC mitogen,99 stimulating VSMC proliferation independently of PDGF99 and thrombin69 by activating a family of G protein-coupled receptors, known as protease-activated receptors (PARs).100 Four different PARs have been identified in vascular remodeling, fibrosis,101 angiogenesis, and inflammation,94 whereas PAR-1 and PAR-2 have been explored in aortic aneurysms in mice and humans.94,102 The PAR-1 receptor is canonically activated by thrombin, FXa, plasmin, and activated protein C, and noncanonically by MMPs, and is upregulated in human thoracic aneurysms.102 FXa independently activates PAR-2 to phosphorylate intracellular Smad2/3, a member of the TGF-β signaling pathway involved in matrix remodeling of AAAs.94,103 Murine studies reveal that AC therapy can reduce the downstream effects of PAR activation. In an AngII AAA model using ApoE−/− mice, administration of the selective FXa inhibitor fondaparinux reduced levels of FXa, PAR-2, MMP-2, and Smad2/3 phosphorylation, which also resulted in a reduction in aortic aneurysm severity.94 Additional mitogenic and cell migratory responses to FXa in VSMCs may be related to lipid signaling through the sphingosine-1-phosphate (S1P) pathway.93 FXa activation of PAR-1 induces the expression of sphingosine kinase, increasing the synthesis of S1P93 from sphingomyelin. Intracellularly, S1P levels enhance cell proliferation, while extracellularly, it can bind to spingosine-1-phosphate receptors (S1PRs) on VSMCs or endothelial cells to regulate VSMC migration, proliferation, differentiation, and vessel permeability.93 In human AAA tissue, S1PRs are upregulated.104 The use of S1PR inhibitors may be protective against aortic dissection and rupture in mice;105 however, their effect on AAA requires further investigation.
As Moran et al94 found AC to reduce wall fibrinolysis in formed aneurysms, Ding et al52 hypothesized that inhibiting coagulation after aneurysm formation may also impact wall inflammation. They found that treatment of AngII-infused ApoE−/− mice with high doses of rivaroxaban initiated on day 14 of the 28-day study significantly reduced maximal aortic diameter, compared with treatment with low-dose rivaroxaban or vehicle.52 Aortic wall inflammation was decreased, as evident by reduced CD45 immunostaining for leukocytes and CD68 immunostaining for macrophages, as well as reversal of upregulated mRNA levels of IL-1, IL-6, IL-8, and MCP-1.106 Rivaroxaban also impacted aortic wall remodeling as MMP-2 and MMP-9 expression was significantly reduced.106 To further validate their results, they repeated the study in a CaCl2-induced AAA mouse model.106 Similarly, rivaroxaban also reduced AAA growth, the infiltration of leukocytes and macrophages into the wall, and mRNA levels of inflammatory cytokines, while also improving elastic fiber fragmentation.106
The role of other coagulation factors, such as activated factor XII (FXIIa), in AAA pathogenesis is also under investigation. After primary hemostasis, activated platelets release polyphosphate, an inorganic polymer that binds to zymogen FXII and activates it to FXIIa.107 This initiates the intrinsic (or contact activation) pathway of the coagulation cascade, while also promoting a proinflammatory response through activation of the kallikrein-kinin system.97 As FXIIa is both prothrombotic and proinflammatory, Moran et al97 hypothesized that inhibition of FXII in its activated form may impact AAA. Treatment with FXIIa-blocking antibody 3F7 resulted in significantly lowered plasma kallikrein, bradykinin, and MMP-9 levels, reduced ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, 13) activity and EGFR phosphorylation, and resulted in smaller AAA diameters in an AngII murine model.97 Allen-Redpath et al98 discovered that rivaroxaban could block the procoagulant effects of phospholipids in AAA. Searle et al96 found that the inhibition of FXIIa with 3F7 monoclonal antibody resulted in a fibroprotective effect against AAA growth and rupture. Inhibiting FXIIa resulted in smaller-diameter AAAs with greater amounts of collagen, which were also more stable than sham.96 In addition, treatment with 37F monoclonal antibody downregulated circulating levels of inflammatory cytokines and chemoattractants, such as IL-1β, MCP-1, and CXCL1, and reduced lipid deposition and necrotic core size in atherosclerotic plaques.96 They concluded that targeting FXIIa may result in stabilization of both atherosclerotic plaques and aneurysmal wall tissue.96
Antithrombotic therapy on AAA patient outcomes
Current guidelines recommend the use of ASA in patients with AAA for reduction in major adverse cardiovascular events,108 rather than reducing AAA-specific thrombotic complications. ASA use is associated with decreased ILT volumes,109 likely by reducing ILT clot lysis times, and increasing clot permeability while decreasing clot density.110 The beneficial effects of antiplatelet therapy on aneurysm-related outcomes may be limited. Retrospective studies of patients with AAA taking ASA or P2Y12 inhibitors show that antiplatelet therapy may reduce patient mortality overall,74 but may also be associated with higher mortality in the setting of rupture.111 Fortunately, the use of antiplatelet agents ASA and clopidogrel as monotherapy has not been demonstrated to affect endoleak.112, 113, 114 Previous studies have shown that the use of ASA monotherapy correlates with aneurysm shrinkage after EVAR;115 however, the use of multiagent antiplatelet therapy failed to demonstrate aneurysm shrinkage.116
Despite promising data in animal models, prophylactic AC is not clinically recommended in asymptomatic AAA patients without other indications for AC. Retrospective studies may suggest some benefit to AC by altering ILT and impacting AAA events, yet are inconclusive on whether AC impacts endovascular outcomes. In a recent prospective cross-sectional study, Skov et al117 followed 309 patients with small asymptomatic infrarenal AAAs according to their antithrombotic therapy regimen for 5 years. Using three-dimensional contrast-enhanced ultrasound and adjusting for AAA volume and diameter, they found that patients on AC had lower ILT volumes than patients on antiplatelet agents or not receiving antithrombotic therapy.117 However, information regarding baseline ILT volume and thickness before antithrombotic therapy or aneurysm morphology was not available,117 and clinical outcomes were not evaluated in this study. A prospective cohort study of 1161 patients with asymptomatic unrepaired AAAs showed that those receiving therapeutic AC had a reduced risk of AAA-related events (AAA repair or mortality from rupture), yet no reduction in major adverse cardiovascular events.118 Previous literature regarding the impact of AC on endoleak is controversial. A retrospective analysis of the Society for Vascular Surgery Vascular Quality Initiative database of nearly 30,000 patients who underwent EVAR found a twofold higher incidence of late endoleaks in patients on AC 1 year after repair and were also more likely to have sac growth compared with those not anticoagulated.114 Although some studies have reported similar increases in endoleaks with AC,119,120 particularly type II,119, 120, 121, 122, 123 others report that the use of AC has no impact on endoleak,112,113,124 and stopping AC after EVAR significantly increases mortality at 1 year.114
Conclusion and future directions
The pathophysiologic mechanisms in AAA are complex yet interconnected with thrombosis and hemostasis. Insult to the aortic wall from cardiovascular risk factors, such as smoking and hypertension, can lead to the formation of atherosclerotic lesions. In AAA, the atheromatous response to injury becomes dysregulated, resulting in exacerbated levels of inflammation, wall remodeling, and eventual weakening and dilation. ILT could be viewed as a failed compensatory mechanism. An attempt to protect the wall from hemodynamic stress comes at the cost of harboring proinflammatory and proteolytic mediators and the scaffolding for thrombus turnover. The activity levels of these pathologic processes combined with the modification of risk factors (ie, aging, continued smoking or quitting, management of hypertension, etc) impact aneurysm progression and stability. The influence of thrombosis in AAA is evident by the presence of ILT in nearly all aneurysms, numerous thrombotic complications, and its influence on surgical options and medical management. Surgery remains the only therapy in AAA, necessitating the development of pharmacologic therapies in this disease.
Analyzing the complex interactions between platelets, coagulation factors, and the other pathologic mechanisms involved in AAA may provide sources for future investigation and potential therapies. A platelet receptor newly identified in AAA, olfactory receptor 2L13, has shown intriguing results as its activation restricted AAA growth and reduced MMP-2 levels.125 Inhibiting downstream targets of platelet and FXa activation, such as PARs or S1PRs, rather than coagulation factors are attractive therapeutic strategies for managing thrombosis in patients with AAA without impacting bleeding risks. Touat et al89 showed that TF-containing microparticles were significantly elevated in AAA plasma, which was hypothesized to account for the higher procoagulant activity found at the luminal ILT surface, but has yet to be analyzed in AAA models.
Although significant research has been conducted on the impact of ILT on AAA, little is known whether the cells within aneurysmal aortic walls contribute to a prothrombotic local environment. Our group has demonstrated the role of necroptosis and smooth muscle death in AAA pathogenesis and progression.126,127 We have recently found that cells undergoing necroptosis upregulate their production of extracellular vesicles,128 which has inspired our ongoing investigation of whether necroptotic VSMCs within aortic aneurysms contribute to ILT by secreting thrombogenic extracellular vesicles.
The use of antithrombotic therapies in AAA has demonstrated some promising results in animal models in inhibiting aneurysm formation, slowing aneurysm progression, and stabilizing the aneurysm sac from rupture. Understanding the detrimental role of ILT and perturbed clotting mechanisms in AAA, inhibiting platelets and coagulation factors are reasonable theoretical targets. However, real-world complications associated with hemorrhage and difficulty reversing antithrombotic agents limit their current application in human aneurysmal disease. Antiplatelet therapy may be useful in reducing major adverse cardiovascular events in this high cardiovascular risk population considering that there is a minimal impact on long-term endovascular outcomes, and AC may be warranted in AAA occlusion or thromboembolic events.
Author Contributions
Conception and design: JB, BL
Analysis and interpretation: JB, JM, BL
Data collection: JB
Writing the article: JB
Critical revision of the article: JB, JM, BL
Final approval of the article: JB, JM, BL
Statistical analysis: JB
Obtained funding: JB, BL
Overall responsibility: BL
Acknowledgments
Fig image was created with BioRender.com.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
This investigation was supported by the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) under 1R01HL149404-01A1 (BL), and the Ruth L. Kirschstein National Research Service Award T32HL007936 to the University of Wisconsin-Madison Cardiovascular Research Center (JB).
References
- 1.Hans S.S., Jareunpoon O., Balasubramaniam M., Zelenock G.B. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J Vasc Surg. 2005;41:584–588. doi: 10.1016/j.jvs.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Labruto F., Blomqvist L., Swedenborg J. Imaging the intraluminal thrombus of abdominal aortic aneurysms: techniques, findings, and clinical implications. J Vasc Interv Radiol. 2011;22:1069–1075. doi: 10.1016/j.jvir.2011.01.454. [DOI] [PubMed] [Google Scholar]
- 3.Di Martino E., Mantero S., Inzoli F., et al. Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: experimental characterisation and structural static computational analysis. Eur J Vasc Endovasc Surg. 1998;15:290–299. doi: 10.1016/s1078-5884(98)80031-2. [DOI] [PubMed] [Google Scholar]
- 4.Haller S.J., Crawford J.D., Courchaine K.M., et al. Intraluminal thrombus is associated with early rupture of abdominal aortic aneurysm. J Vasc Surg. 2018;67:1051–1058.e1. doi: 10.1016/j.jvs.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 5.Boyd A.J. Intraluminal thrombus: innocent bystander or factor in abdominal aortic aneurysm pathogenesis? JVS Vasc Sci. 2021;2:159–169. doi: 10.1016/j.jvssci.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Bustamante A., Jimeno A. Disseminated intravascular coagulopathy in aortic aneurysms. Eur J Intern Med. 2005;16:551–560. doi: 10.1016/j.ejim.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Suliman A.S., Raffetto J., Seidman C.S., Menzoian J.O. Acute thrombosis of abdominal aortic aneurysms: report of two cases and review of the literature. Vasc Endovasc Surg. 2003;37:71–75. doi: 10.1177/153857440303700110. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Chan Y.C., Cui D., Cheng S.W. Acute thrombosis of an infrarenal abdominal aortic aneurysm presenting as bilateral critical lower limb ischemia. Vasc Endovasc Surg. 2021;55:186–188. doi: 10.1177/1538574420954297. [DOI] [PubMed] [Google Scholar]
- 9.Wong S.S., Roche-Nagle G., Oreopoulos G. Acute thrombosis of an abdominal aortic aneurysm presenting as cauda equina syndrome. J Vasc Surg. 2013;57:218–220. doi: 10.1016/j.jvs.2012.06.092. [DOI] [PubMed] [Google Scholar]
- 10.Vasdekis S.N., Mastoraki S., Lazaris A., Moulakakis K.G. An unusual case of acute thrombosis of abdominal aortic aneurysm without acute limb ischemia. Aorta (Stamford) 2018;6:31. doi: 10.1055/s-0038-1636991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz R.A., Nichols W.K., Silver D. Is thrombosis of the infrarenal abdominal aortic aneurysm an acceptable alternative? J Vasc Surg. 1986;3:448–455. doi: 10.1067/mva.1986.avs0030448. [DOI] [PubMed] [Google Scholar]
- 12.Baxter B.T., McGee G.S., Flinn W.R., McCarthy W.J., Pearce W.H., Yao J.S. Distal embolization as a presenting symptom of aortic aneurysms. Am J Surg. 1990;160:197–201. doi: 10.1016/s0002-9610(05)80306-x. [DOI] [PubMed] [Google Scholar]
- 13.Nigro G., Giovannacci L., Engelberger S., Van den Berg J.C., Rosso R. The challenge of posttraumatic thrombus embolization from abdominal aortic aneurysm causing acute limb ischemia. J Vasc Surg. 2011;54:840–843. doi: 10.1016/j.jvs.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Schermerhorn M. A 66-year-old man with an abdominal aortic aneurysm: review of screening and treatment. JAMA. 2009;302:2015–2022. doi: 10.1001/jama.2009.1502. [DOI] [PubMed] [Google Scholar]
- 15.Ates I., Kaplan M., Özçalık M., Yılmaz N. Transient ischemic attacks of spinal cord due to abdominal aortic aneurysm thrombus. Ann Vasc Surg. 2016;30:307.e7–307.e9. doi: 10.1016/j.avsg.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Fairhead J.F., Phillips D., Handa A. Embolic spinal cord infarction as a presentation of abdominal aortic aneurysm. J R Soc Med. 2005;98:59–60. doi: 10.1258/jrsm.98.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zrihni Y., Kukkonen T., Thaveau F., Girsowicz E., Georg Y., Chakfe N. Ischemic cauda equina syndrome due to spinal embolization as a first manifestation of an infrarenal abdominal aortic aneurysm. World J Cardiovasc Surg. 2015;5:58. [Google Scholar]
- 18.de Guerre L.E., Dansey K., Li C., et al. Late outcomes after endovascular and open repair of large abdominal aortic aneurysms. J Vasc Surg. 2021;74:1152–1160. doi: 10.1016/j.jvs.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Schanzer A., Greenberg R.K., Hevelone N., et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123:2848–2855. doi: 10.1161/CIRCULATIONAHA.110.014902. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira-Pinto J., Ferreira R.S., Oliveira N.F., et al. Total luminal volume predicts risk after endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2020;59:918–927. doi: 10.1016/j.ejvs.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Cornelissen S.A., Verhagen H.J., van Herwaarden J.A., Vonken E.P., Moll F.L., Bartels L.W. Lack of thrombus organization in nonshrinking aneurysms years after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2012;56:938–942. doi: 10.1016/j.jvs.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Gitlitz D.B., Ramaswami G., Kaplan D., Hollier L.H., Marin M.L. Endovascular stent grafting in the presence of aortic neck filling defects: early clinical experience. J Vasc Surg. 2001;33:340–344. doi: 10.1067/mva.2001.110522. [DOI] [PubMed] [Google Scholar]
- 23.Whaley Z.L., Cassimjee I., Novak Z., et al. The spatial morphology of intraluminal thrombus influences type II endoleak after endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg. 2020;66:77–84. doi: 10.1016/j.avsg.2019.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls J., Kirkham E.N., Haslam L., Paravastu S.C., Kulkarni S.R. Significance of preoperative thrombus burden in the prediction of a persistent type II and reintervention after infrarenal endovascular aneurysm repair. J Vasc Surg. 2022;75:1912–1917. doi: 10.1016/j.jvs.2021.12.069. [DOI] [PubMed] [Google Scholar]
- 25.Sirignano P., Menna D., Capoccia L., et al. Preoperative intrasac thrombus load predicts worse outcome after elective endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2015;26:1431–1436. doi: 10.1016/j.jvir.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Davies R.S., Abdelhamid M., Wall M.L., Vohra R.K., Bradbury A.W., Adam D.J. Coagulation, fibrinolysis, and platelet activation in patients undergoing open and endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2011;54:865–878. doi: 10.1016/j.jvs.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Rašiová M., Koščo M., Špak Ľ., et al. Higher preprocedural fibrinogen levels are associated with aneurysm sac regression after EVAR. Vasa. 2019;48:347–354. doi: 10.1024/0301-1526/a000783. [DOI] [PubMed] [Google Scholar]
- 28.Inzoli F., Boschetti F., Zappa M., Longo T., Fumero R. Biomechanical factors in abdominal aortic aneurysm rupture. Eur J Vasc Surg. 1993;7:667–674. doi: 10.1016/s0950-821x(05)80714-5. [DOI] [PubMed] [Google Scholar]
- 29.Boyd A.J., Kuhn D.C., Lozowy R.J., Kulbisky G.P. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg. 2016;63:1613–1619. doi: 10.1016/j.jvs.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Kazi M., Thyberg J., Religa P., et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 31.Biasetti J., Hussain F., Gasser T.C. Blood flow and coherent vortices in the normal and aneurysmatic aortas: a fluid dynamical approach to intra-luminal thrombus formation. J R Soc Interf. 2011;8:1449–1461. doi: 10.1098/rsif.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biasetti J., Gasser T.C., Auer M., Hedin U., Labruto F. Hemodynamics of the normal aorta compared to fusiform and saccular abdominal aortic aneurysms with emphasis on a potential thrombus formation mechanism. Ann Biomed Eng. 2010;38:380–390. doi: 10.1007/s10439-009-9843-6. [DOI] [PubMed] [Google Scholar]
- 33.Soudah E., Ng E., Loong T.H., Bordone M., Pua U., Narayanan S. CFD modelling of abdominal aortic aneurysm on hemodynamic loads using a realistic geometry with CT. Comput Math Methods Med. 2013;2013:472564. doi: 10.1155/2013/472564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raz S., Einav S., Alemu Y., Bluestein D. DPIV prediction of flow induced platelet activation—comparison to numerical predictions. Ann Biomed Eng. 2007;35:493–504. doi: 10.1007/s10439-007-9257-2. [DOI] [PubMed] [Google Scholar]
- 35.Holme P.A., Ørvim U., Hamers M.J., et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol. 1997;17:646–653. doi: 10.1161/01.atv.17.4.646. [DOI] [PubMed] [Google Scholar]
- 36.Ruggeri Z.M., Orje J.N., Habermann R., Federici A.B., Reininger A.J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108:1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggeri Z.M., Mendolicchio G.L. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell M.J., Westein E., Nesbitt W.S., Giuliano S., Dopheide S.M., Jackson S.P. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109:566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 39.Nesbitt W.S., Westein E., Tovar-Lopez F.J., et al. A shear gradient–dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 40.Fogelson A.L., Neeves K.B. Fluid mechanics of blood clot formation. Annu Rev Fluid Mech. 2015;47:377. doi: 10.1146/annurev-fluid-010814-014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neeves K.B., Illing D., Diamond S.L. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–1352. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrnes J.R., Wolberg A.S. Red blood cells in thrombosis. Blood. 2017;130:1795–1799. doi: 10.1182/blood-2017-03-745349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klatt C., Krüger I., Zey S., et al. Platelet-RBC interaction mediated by FasL/FasR induces procoagulant activity important for thrombosis. J Clin Invest. 2018;128:3906–3925. doi: 10.1172/JCI92077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noh J., Lim K., Bae O., et al. Procoagulant and prothrombotic activation of human erythrocytes by phosphatidic acid. Am J Physiol Heart Circ Physiol. 2010;299:H347–H355. doi: 10.1152/ajpheart.01144.2009. [DOI] [PubMed] [Google Scholar]
- 45.Steinman D.A., Vorp D.A., Ethier C.R. Computational modeling of arterial biomechanics: insights into pathogenesis and treatment of vascular disease. J Vasc Surg. 2003;37:1118–1128. doi: 10.1067/mva.2003.122. [DOI] [PubMed] [Google Scholar]
- 46.Qiu Y., Tarbell J.M. Numerical simulation of oxygen mass transfer in a compliant curved tube model of a coronary artery. Ann Biomed Eng. 2000;28:26–38. doi: 10.1114/1.251. [DOI] [PubMed] [Google Scholar]
- 47.Onasoga-Jarvis A.A., Puls T.J., O’Brien S.K., Kuang L., Liang H.J., Neeves K.B. Thrombin generation and fibrin formation under flow on biomimetic tissue factor-rich surfaces. J Thromb Haemost. 2014;12:373–382. doi: 10.1111/jth.12491. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu K., Mitchell R.N., Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 49.Posma J.J., Grover S.P., Hisada Y., et al. Roles of coagulation proteases and PARs (protease-activated receptors) in mouse models of inflammatory diseases. Arterioscler Thromb Vasc Biol. 2019;39:13–24. doi: 10.1161/ATVBAHA.118.311655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senden N.H., Jeunhomme T.M., Heemskerk J.W., et al. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161:4318–4324. [PubMed] [Google Scholar]
- 51.Ahmad S.S., Scandura J.M., Walsh P.N. Structural and functional characterization of platelet receptor-mediated factor VIII binding. J Biol Chem. 2000;275:13071–13081. doi: 10.1074/jbc.275.17.13071. [DOI] [PubMed] [Google Scholar]
- 52.Ding Y., Li X., Zhou M., et al. Factor Xa inhibitor rivaroxaban suppresses experimental abdominal aortic aneurysm progression via attenuating aortic inflammation. Vasc Pharmacol. 2021;136 doi: 10.1016/j.vph.2020.106818. [DOI] [PubMed] [Google Scholar]
- 53.Hara T., Fukuda D., Tanaka K., et al. Inhibition of activated factor X by rivaroxaban attenuates neointima formation after wire-mediated vascular injury. Eur J Pharmacol. 2018;820:222–228. doi: 10.1016/j.ejphar.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 54.Hara T., Fukuda D., Tanaka K., et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242:639–646. doi: 10.1016/j.atherosclerosis.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 55.Hashikata T., Yamaoka-Tojo M., Namba S., et al. Rivaroxaban inhibits angiotensin II-induced activation in cultured mouse cardiac fibroblasts through the modulation of NF-κB pathway. Int Heart J. 2015;56:544–550. doi: 10.1536/ihj.15-112. [DOI] [PubMed] [Google Scholar]
- 56.Sanada F., Muratsu J., Otsu R., S, et al. Local production of activated factor X in atherosclerotic plaque induced vascular smooth muscle cell senescence. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-17508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moñux G., Zamorano-León J.J., Marqués P., et al. FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms. Br J Clin Pharmacol. 2017;83:2661–2670. doi: 10.1111/bcp.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintana R.A., Taylor W.R. Cellular mechanisms of aortic aneurysm formation. Circ Res. 2019;124:607–618. doi: 10.1161/CIRCRESAHA.118.313187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guzik B., Sagan A., Ludew D., et al. Mechanisms of oxidative stress in human aortic aneurysms—association with clinical risk factors for atherosclerosis and disease severity. Int J Cardiol. 2013;168:2389–2396. doi: 10.1016/j.ijcard.2013.01.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teng X., Zhang H., Snead C., Catravas J.D. Molecular mechanisms of iNOS induction by IL-1β and IFN-γ in rat aortic smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C144–C152. doi: 10.1152/ajpcell.2002.282.1.C144. [DOI] [PubMed] [Google Scholar]
- 61.Xia Y., Roman L.J., Masters B.S.S., Zweier J.L. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 62.Miller F.J., Jr., Sharp W.J., Fang X., Oberley L.W., Oberley T.D., Weintraub N.L. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- 63.Kim H.W., Blomkalns A.L., Ogbi M., et al. Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am J Physiol Heart Circ Physiol. 2017;313:H1168–H1179. doi: 10.1152/ajpheart.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papalambros E., Sigala F., Georgopoulos S., et al. Malondialdehyde as an indicator of oxidative stress during abdominal aortic aneurysm repair. Angiology. 2007;58:477–482. doi: 10.1177/0003319707305246. [DOI] [PubMed] [Google Scholar]
- 65.Qiao J., Arthur J.F., Gardiner E.E., Andrews R.K., Zeng L., Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018;14:126–130. doi: 10.1016/j.redox.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gutmann C., Siow R., Gwozdz A.M., Saha P., Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci. 2020;21:1918. doi: 10.3390/ijms21061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ambrosio G., Tritto I., Golino P. Reactive oxygen metabolites and arterial thrombosis. Cardiovasc Res. 1997;34:445–452. doi: 10.1016/s0008-6363(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 68.Obama T., Tsuji T., Kobayashi T., et al. Epidermal growth factor receptor inhibitor protects abdominal aortic aneurysm in a mouse model. Clin Sci. 2014;128:559–565. doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 69.Rauch B.H., Millette E., Kenagy R.D., Daum G., Clowes A.W. Thrombin-and factor Xa–induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circ Res. 2004;94:340–345. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- 70.Seshiah P.N., Weber D.S., Rocic P., Valppu L., Taniyama Y., Griendling K.K. Angiotensin II stimulation of NAD (P) H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 71.Sakalihasan N., Delvenne P., Nusgens B.V., Limet R., Lapière C.M. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J Vasc Surg. 1996;24:127–133. doi: 10.1016/s0741-5214(96)70153-2. [DOI] [PubMed] [Google Scholar]
- 72.Fontaine V., Jacob M., Houard X., et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol. 2002;161:1701–1710. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Folkesson M., Kazil M., Zhu C., et al. Presence of NGAL/MMP-9 complexes in human abdominal aortic aneurysms. Thromb Haemost. 2007;98:427–433. [PubMed] [Google Scholar]
- 74.Owens A.P., III, Edwards T.L., Antoniak S., et al. Platelet inhibitors reduce rupture in a mouse model of established abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:2032–2041. doi: 10.1161/ATVBAHA.115.305537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siennicka A., Zuchowski M., Kaczmarczyk M., Cnotliwy M., Clark J.S., Jastrzębska M. Tissue factor levels and the fibrinolytic system in thin and thick intraluminal thrombus and underlying walls of abdominal aortic aneurysms. J Vasc Surg. 2018;68:30S–37S. doi: 10.1016/j.jvs.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 76.Milne A.A., Adam D.J., Murphy W.G., Ruckley C.V. Effects of asymptomatic abdominal aortic aneurysm on the soluble coagulation system, platelet count and platelet activation. Eur J Vasc Endovasc Surg. 1999;17:434–437. doi: 10.1053/ejvs.1998.0790. [DOI] [PubMed] [Google Scholar]
- 77.Cameron S.J., Russell H.M., Owens A.P., III Antithrombotic therapy in abdominal aortic aneurysm: beneficial or detrimental? Blood. 2018;132:2619–2628. doi: 10.1182/blood-2017-08-743237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamazumi K., Ojiro M., Okumura H., Aikou T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg. 1998;175:297–301. doi: 10.1016/s0002-9610(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 79.Parry D.J., Al-Barjas H.S., Chappell L., Rashid T., Ariëns R., Scott D. Haemostatic and fibrinolytic factors in men with a small abdominal aortic aneurysm. J Br Surg. 2009;96:870–877. doi: 10.1002/bjs.6632. [DOI] [PubMed] [Google Scholar]
- 80.Kotschy M., Witkiewicz W., Grendziak R., Dubis J., Zapotoczny N., Kotschy D. Selected clotting factors in blood of patients with abdominal aortic aneurysms. Kardiol Pol. 2012;70:574–579. [PubMed] [Google Scholar]
- 81.Sundermann A.C., Saum K., Conrad K.A., et al. Prognostic value of D-dimer and markers of coagulation for stratification of abdominal aortic aneurysm growth. Blood Adv. 2018;2:3088–3096. doi: 10.1182/bloodadvances.2017013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan Y., Ke X., Yi Z., et al. Plasma D-dimer as a predictor of intraluminal thrombus burden and progression of abdominal aortic aneurysm. Life Sci. 2020;240 doi: 10.1016/j.lfs.2019.117069. [DOI] [PubMed] [Google Scholar]
- 83.Scott D.J.A., Prasad P., Philippou H., et al. Clot architecture is altered in abdominal aortic aneurysms and correlates with aneurysm size. Arterioscler Thromb Vasc Biol. 2011;31:3004–3010. doi: 10.1161/ATVBAHA.111.236786. [DOI] [PubMed] [Google Scholar]
- 84.Daugherty A., Manning M.W., Cassis L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E–deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu G., Su G., Davis J.P., et al. A novel chronic advanced stage abdominal aortic aneurysm murine model. J Vasc Surg. 2017;66:232–242.e4. doi: 10.1016/j.jvs.2016.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X., Weng Y., Lou J., Chen X., Du C., Tang L. Combinational therapy with aspirin and ticagrelor alleviates vascular inflammation and angiotensin II-driven abdominal aortic aneurysm formation in mice. Austin J Cardiovasc Dis Atherosclerosis. 2022;9:1048. [Google Scholar]
- 87.Liu O., Jia L., Liu X., et al. Clopidogrel, a platelet P2Y12 receptor inhibitor, reduces vascular inflammation and angiotensin II induced-abdominal aortic aneurysm progression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dai J., Louedec L., Philippe M., Michel J., Houard X. Effect of blocking platelet activation with AZD6140 on development of abdominal aortic aneurysm in a rat aneurysmal model. J Vasc Surg. 2009;49:719–727. doi: 10.1016/j.jvs.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 89.Touat Z., Ollivier V., Dai J., et al. Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution. Am J Pathol. 2006;168:1022–1030. doi: 10.2353/ajpath.2006.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allaire E., Guettier C., Bruneval P., Plissonnier D., Michel J. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994;19:446–456. doi: 10.1016/s0741-5214(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 91.Thomas G.M., Brill A., Mezouar S., et al. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost. 2015;13:1310. doi: 10.1111/jth.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wanhainen A., Mani K., Kullberg J., et al. The effect of ticagrelor on growth of small abdominal aortic aneurysms—a randomized controlled trial. Cardiovasc Res. 2020;116:450–456. doi: 10.1093/cvr/cvz133. [DOI] [PubMed] [Google Scholar]
- 93.Böhm A., Flößer A., Ermler S., et al. Factor-Xa-induced mitogenesis and migration require sphingosine kinase activity and S1P formation in human vascular smooth muscle cells. Cardiovasc Res. 2013;99:505–513. doi: 10.1093/cvr/cvt112. [DOI] [PubMed] [Google Scholar]
- 94.Moran C.S., Seto S., Krishna S.M., et al. Parenteral administration of factor Xa/IIa inhibitors limits experimental aortic aneurysm and atherosclerosis. Sci Rep. 2017;7:1–12. doi: 10.1038/srep43079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruf W., Dorfleutner A., Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 96.Searle A.K., Chen Y., Wallert M., et al. Pharmacological inhibition of Factor XIIa attenuates abdominal aortic aneurysm, reduces atherosclerosis, and stabilizes atherosclerotic plaques. Thromb Haemost. 2022;122:196–207. doi: 10.1055/a-1663-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moran C.S., Seto S., Biros E., et al. Factor XII blockade inhibits aortic dilatation in angiotensin II-infused apolipoprotein E-deficient mice. Clin Sci. 2020;134:1049–1061. doi: 10.1042/CS20191020. [DOI] [PubMed] [Google Scholar]
- 98.Allen-Redpath K., Aldrovandi M., Lauder S.N., et al. Phospholipid membranes drive abdominal aortic aneurysm development through stimulating coagulation factor activity. Proc Natl Acad Sci. 2019;116:8038–8047. doi: 10.1073/pnas.1814409116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bretschneider E., Braun M., Fischer A., Wittpoth M., Glusa E., Schrör K. Factor Xa acts as a PDGF-independent mitogen in human vascular smooth muscle cells. Thromb Haemost. 2000;84:499–505. [PubMed] [Google Scholar]
- 100.McLean K., Schirm S., Johns A., Morser J., Light D.R. FXa-induced responses in vascular wall cells are PAR-mediated and inhibited by ZK-807834. Thromb Res. 2001;103:281–297. doi: 10.1016/s0049-3848(01)00330-9. [DOI] [PubMed] [Google Scholar]
- 101.Borensztajn K., Peppelenbosch M.P., Spek C.A. Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol Med. 2008;14:429–440. doi: 10.1016/j.molmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Shin S.J., Hang H.T., Thang B.Q., et al. Role of PAR1-Egr1 in the initiation of thoracic aortic aneurysm in Fbln4-deficient mice. Arterioscler Thromb Vasc Biol. 2020;40:1905–1917. doi: 10.1161/ATVBAHA.120.314560. [DOI] [PubMed] [Google Scholar]
- 103.Krishna S.M., Seto S.W., Jose R.J., et al. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin ii–infused apolipoprotein-e–deficient mouse. Arterioscler Thromb Vasc Biol. 2015;35:389–398. doi: 10.1161/ATVBAHA.114.304732. [DOI] [PubMed] [Google Scholar]
- 104.Qu Z., Cheuk B.L., Cheng S.W. Differential expression of sphingosine-1-phosphate receptors in abdominal aortic aneurysms. Mediators Inflamm. 2012;2012:643609. doi: 10.1155/2012/643609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan G., Liao M., Dai Y., et al. Inhibition of sphingosine-1-phosphate receptor 2 prevents thoracic aortic dissection and rupture. Front Cardiovasc Med. 2021;8:748486. doi: 10.3389/fcvm.2021.748486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding Y., Shan Y., Zhou M., et al. Amount of intraluminal thrombus correlates with severe adverse events in abdominal aortic aneurysms after endovascular aneurysm repair. Ann Vasc Surg. 2020;67:254–264. doi: 10.1016/j.avsg.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Müller F., Mutch N.J., Schenk W.A., et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aboyans V., Bauersachs R., Mazzolai L., et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: a consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur Heart J. 2021;42:4013–4024. doi: 10.1093/eurheartj/ehab390. [DOI] [PubMed] [Google Scholar]
- 109.Balceniuk M.D., Trakimas L.E., Aghaie C., et al. Aspirin use is associated with decreased radiologically-determined thrombus sac volume in abdominal aortic aneurysms. Vascular. 2018;26:440–444. doi: 10.1177/1708538118762214. [DOI] [PubMed] [Google Scholar]
- 110.Bailey M.A., Aggarwal R., Bridge K.I., et al. Aspirin therapy is associated with less compact fibrin networks and enhanced fibrinolysis in patients with abdominal aortic aneurysm. J Thromb Haemost. 2015;13:795–801. doi: 10.1111/jth.12872. [DOI] [PubMed] [Google Scholar]
- 111.Wemmelund H., Jørgensen T.M., Høgh A., Behr-Rasmussen C., Johnsen S.P., Lindholt J.S. Low-dose aspirin and rupture of abdominal aortic aneurysm. J Vasc Surg. 2017;65:616–625.e4. doi: 10.1016/j.jvs.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 112.Wild J.B., Dattani N., Stather P., Bown M.J., Sayers R.D., Choke E. Effect of anticoagulation and antiplatelet therapy on incidence of endoleaks and sac size expansions after endovascular aneurysm repair. Ann Vasc Surg. 2014;28:554–559. doi: 10.1016/j.avsg.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 113.Lal B.K., Zhou W., Li Z., et al. Predictors and outcomes of endoleaks in the Veterans Affairs Open versus Endovascular Repair (OVER) trial of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1394–1404. doi: 10.1016/j.jvs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Flohr T.R., Snow R., Aziz F. The fate of endoleaks after endovascular aneurysm repair and the impact of oral anticoagulation on their persistence. J Vasc Surg. 2021;74:1183–1192.e5. doi: 10.1016/j.jvs.2021.04.033. [DOI] [PubMed] [Google Scholar]
- 115.Álvarez Marcos F., Llaneza Coto J.M., Franco Meijide F.J., et al. Effect of antiplatelet therapy on aneurysmal sac expansion associated with type II endoleaks after endovascular aneurysm repair. J Vasc Surg. 2017;66:396–403. doi: 10.1016/j.jvs.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 116.Aoki A., Suezawa T., Sangawa K., Tago M. Effect of type II endoleaks and antiplatelet therapy on abdominal aortic aneurysm shrinkage after endovascular repair. J Vasc Surg. 2011;54:947–951. doi: 10.1016/j.jvs.2011.03.269. [DOI] [PubMed] [Google Scholar]
- 117.Skov R.A.C., Eiberg J.P., Rouet L., et al. Anticoagulants and reduced thrombus load in abdominal aortic aneurysms assessed with three-dimensional contrast-enhanced ultrasound examination. J Vasc Surg. 2022;77:143–149. doi: 10.1016/j.jvs.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 118.Golledge J., Jenkins J., Bourke M., Bourke B., Singh T.P. Association of oral anticoagulation prescription with clinical events in patients with an asymptomatic unrepaired abdominal aortic aneurysm. Biomedicines. 2022;10:2112. doi: 10.3390/biomedicines10092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seike Y., Tanaka H., Fukuda T., et al. Influence of warfarin therapy on the occurrence of postoperative endoleaks and aneurysm sac enlargement after endovascular abdominal aortic aneurysm repair. Interactive Cardiovasc Thorac Surg. 2017;24:615–618. doi: 10.1093/icvts/ivw383. [DOI] [PubMed] [Google Scholar]
- 120.De Rango P., Verzini F., Parlani G., et al. Safety of chronic anticoagulation therapy after endovascular abdominal aneurysm repair (EVAR) Eur J Vasc Endovasc Surg. 2014;47:296–303. doi: 10.1016/j.ejvs.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Abularrage C.J., Crawford R.S., Conrad M.F., et al. Preoperative variables predict persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg. 2010;52:19–24. doi: 10.1016/j.jvs.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 122.Bobadilla J.L., Hoch J.R., Leverson G.E., Tefera G. The effect of warfarin therapy on endoleak development after endovascular aneurysm repair (EVAR) of the abdominal aorta. J Vasc Surg. 2010;52:267–271. doi: 10.1016/j.jvs.2010.02.290. [DOI] [PubMed] [Google Scholar]
- 123.Lazarides M.K., Georgiadis G.S., Charalampidis D.G., Antoniou G.A., Georgakarakos E.I., Trellopoulos G. Impact of long-term warfarin treatment on EVAR durability: a meta-analysis. J Endovasc Ther. 2014;21:148–153. doi: 10.1583/13-4462R.1. [DOI] [PubMed] [Google Scholar]
- 124.Johnson M.S., Chiang J., Eldrup-Jorgensen J., Clark D.E., Healey C.T. Effect of chronic oral anticoagulation with warfarin on the durability and outcomes of endovascular aortic aneurysm repair. J Vasc Surg. 2013;58:319–323. doi: 10.1016/j.jvs.2012.12.082. [DOI] [PubMed] [Google Scholar]
- 125.Morrell C.N., Mix D., Aggarwal A., et al. Platelet olfactory receptor activation limits platelet reactivity and growth of aortic aneurysms. J Clin Invest. 2022;132:e152373. doi: 10.1172/JCI152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Q., Liu Z., Ren J., Morgan S., Assa C., Liu B. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116:600–611. doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou T., Wang Q., Phan N., et al. Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models. Cell Death Dis. 2019;10:1–15. doi: 10.1038/s41419-019-1468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gupta K., Brown K.A., Hsieh M.L., et al. Necroptosis is associated with Rab27-independent expulsion of extracellular vesicles containing RIPK3 and MLKL. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]