Highlights
-
•
Comorbidities associated with worse overall survival in SCLC after chemoradiation.
-
•
Multimorbidity by Age-adjusted Charlson index predictor of overall survival.
-
•
Overall survival scored by Age-adjusted Charlson index is predictor of survival independent of Karnofsky Performance Score.
-
•
Prophylactic cranial irradiation associated with favourable overall survival.
Keywords: Small cell lung cancer, Survival, Limited disease, Comorbidity, Charlson comorbidity index, CCI, SCLC
Abstract
Background
Combined, platinum-based thoracic chemoradiotherapy (TCR) is the current state-of-the-art treatment for patients with limited disease (LD) small-cell lung cancer (SCLC). There is only limited data available regarding the effect of comorbidities on survival following TRC. The purpose of this study is to assess the age-adjusted Charlson comorbidity index (ACCI) as a predictor of overall survival in LD-SCLC patients undergoing TCR.
Patients and methods
We retrospectively analyzed 367 SCLC patients diagnosed with LD-SCLC who received TCR between 2003 and 2017. We evaluated the ACCI (n = 348) as a predictor of overall survival (OS). In this cohort, 322 patients (88%) received platinum-based TCR (either cisplatin or carboplatin), and 37 (10%) patients received vincristine based TCR. Median radiation dose was 60 Gy (range 24–66 Gy). Additionally, 83% of patients (n = 303) received prophylactic cranial irradiation (PCI, 30 Gy in 2 Gy fractions). Kaplan–Meier survival analysis was performed for OS. For comparison of survival curves, Log-rank (Mantel-Cox) test was used. Univariate and multivariate Cox proportional-hazards ratios (HRs) were used to assess the influence of cofactors on OS.
Results
Patients with an ACCI > 6 had a significantly shorter OS compared with patients with an ACCI ≤ 6 (median 11 vs. 20 months; p = 0.005). Univariate analysis for OS revealed a statistically significant effect for ACCI > 6 (HR 1.7; 95% CI 1.2–2.4; p = 0.003), PCI (HR 0.5; 95% CI 0.3–0.7; p < 0.001), and Karnofsky performance status ≤ 70% (KPS) (HR 1.4; 95% CI 1.1–1.90; p = 0.015). In multivariate analysis, OS was significantly associated with PCI (HR 0.6; 95% CI 0.4–0.9; p = 0.022) and ACCI > 6 (HR 1.5; 95% CI 1.0–2.1; p = 0.049).
Conclusion
Comorbidity is significantly associated with survival in patients with LD-SCLC undergoing TCR. The ACCI may be a valuable tool to identify patients with a shorter survival and thus might be used for risk stratification and oncological decision making.
Introduction
Small cell lung cancer (SCLC) is the most aggressive form of lung cancer and is characterized by a rapid doubling time and early dissemination. The expectably favorable initial response to first-line thoracic chemoradiotherapy (TCR) is contrasted by its resistance to second-line treatments. The contemporary treatment for limited disease (LD)-SCLC patients includes cisplatin-etoposide chemotherapy in combination with radiation therapy (RT). Furthermore, prophylactic cranial irradiation (PCI) is offered to patients with response to first-line therapy [1]. SCLC is associated with tobacco exposure in the vast majority of SCLC patients [2]. There is some evidence that smoking-associated comorbidities may affect the prognosis of lung cancer and influence treatment decisions as patients with comorbidities are less likely to receive TCR [3]. The prognostic value of comorbidities in non-small-cell lung cancer (NSCLC) has been widely investigated [4], [5], [6], [7], [8] but the available data for LD-SCLC patients is limited and patients were often grouped with either NSCLC patients or extensive disease (ED) SCLC patients [9], [10], [11], [12]. The Charlson age-adjusted comorbidity index (ACCI) [13] is a widely used scoring system for assessing comorbidities and has been validated in multiple disorders [14], [15], [16], [17], [18]. The primary objective of this study was to assess the ACCI as a predictor of survival in LD-SCLC patients undergoing TCR.
Methods
Patients and treatment features
We identified 367 patients in our cancer center database with histologically confirmed LD-SCLC according to the classification of the Veterans’ Affairs Administration Lung Cancer Study Group (VALG) [19] between 2003 and 2017 and received standard concurrent TCR as their first-line treatment.
The ACCI is a widely accepted measure for risk adjustment in various diseases based on the modified Charlson Comorbidity Index Score (CCI) [14], [17], [20], [10], [21], [22]. ACCI scores are calculated by the method previously reported by Charlson [13], with comorbidities being weighted and scored and additional points being added for age. Common conditions factoring into the ACCI include myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, liver disease and diabetes. The index is age-adjusted with additional score points for every decade after 40 years.
Thoracic RT was administered once daily (1 daily fraction of 2 Gy on 5 consecutive days per week), concurrently with chemotherapy. Usually, the first cycle of chemotherapy was given before radiation start with the second following concurrently with RT. Patients with complete remission (CR) or partial response (PR) after initial therapy received prophylactic cranial irradiation (PCI). Response to first-line thoracic treatment was assessed via repeated CT imaging.
In this cohort, 322 patients (88%) received platinum-based TCR (either cisplatin or carboplatin) and 37 (10%) patients received vincristine based TCR. Median radiation dose was 60 Gy (range 24–66 Gy). Additionally, 83% of patients (n = 303) received prophylactic cranial irradiation (PCI, 30 Gy in 2 Gy single fractions). Detailed patients' characteristics are shown in Table 1. ACCI was available for 348 patients (95%). Clinical, operative-, and hospital-course records were reviewed to assess comorbidities and were suitable for analysis for 337 patients (92%). Karnofsky performance status was retrospectively evaluated from the documentation up to 3 weeks before the start of TCR. All reviews were performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version. Ethics approval for the study was given from the local ethics committee (University Hospital Heidelberg).
Table 1.
Patient and treatment characteristics.
| Variable | n = 337 |
|---|---|
| Median Age in years (range) | 64 (37–93) |
| Median Karnofsky performance status % (range) | 80 (50–100) |
| Male:Female (n) | 213:137 (61:39%) |
| Median ACCI (range) | 5 (2–9) |
| Median number of pack years (range) | 40 (0–200) |
| Smoking (past and/or present) | 328 (94%) |
| Median Dose of thoracic radiotherapy (range) | 60 (24–66) |
| <45 Gy (n) | 3 (<1%) |
| 45–50 Gy (n) | 8 (2%) |
| 50–55 Gy (n) | 68 (20%) |
| 55–60 Gy (n) | 45 (13%) |
| 60–66 Gy (n) | 213 (63%) |
| Chronic obstructive pulmonary disease (n) | 110 (31%) |
| Coronary artery disease (n) | 99 (28%) |
| Type-2 diabetes (n) | 48 (13%) |
| Hypertension (n) | 233 (66%) |
Outcome evaluation
A statistical analysis was carried out using SigmaPlot™ (Systat Software GmbH, Germany) and RStudio (2022.02.3 + 492, packages “survminer”, “ggplot2”, “ggpubr”, “forester”). Univariate Cox proportional-hazards ratios (HRs) were used to assess the influence of cofactors on OS. For comparison of survival curves, the Log-rank (Mantel-Cox) test was used. OS was defined as the time from the first fraction of radiotherapy to death. Living patients were censored from survival analysis at last known contact. A p-value of < 0.05 was considered statistically significant.
All patients were seen by a physician several times during TCR and at regular follow-up intervals. After treatment, surveillance was recommended every 3 months during the first 2 years, starting with the first visit 4 – 8 weeks after completion of therapy. The visits included a detailed medical history, physical examination, and appropriate imaging procedures.
Results
The median OS from the first day of RT was 18 months. Patients with an ACCI > 6 had a significantly shorter survival compared to patient with an ACCI ≤ 6 (median 11 vs. 20 months; p = 0.005) (Fig. 1). Patients with a KPS ≤ 70 had a significantly shorter survival compared with patients with a KPS > 70 (16 vs. 20 months, p = 0.017) (Fig. 2).
Fig. 1.
Overall survival in patients with an age-adjusted Charlson comorbidity index (ACCI) > 6, compared to patients with an ACCI ≤ 6 (Kaplan-Meier’s estimation) (median 11 vs. 20 months; Log-Rank test p = 0.005).
Fig. 2.
Overall survival in patients with a Karnofsky performance score (KPS) ≤ 70%, compared to patients with an KPS > 70% (Kaplan-Meier’s estimation) (16 vs. 20 months, Log-Rank test p = 0.017).
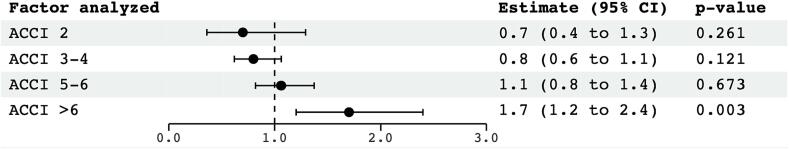
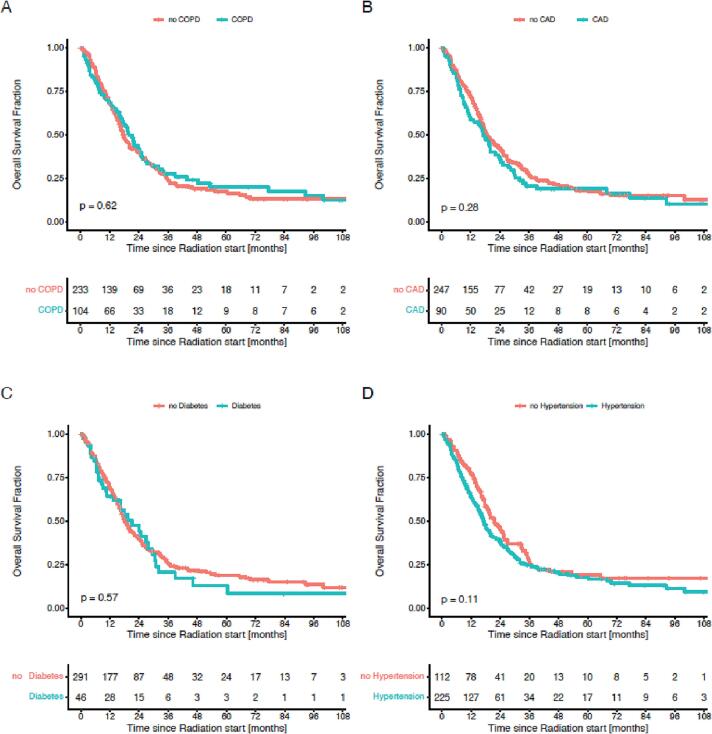
Univariate analysis for OS revealed a statistically significant effect for ACCI > 6 (HR 1.70; 95% CI 1.2–2.4; p = 0.003), PCI (HR 0.5; 95% CI 0.3–0.7; p < 0.001), and Karnofsky performance status ≤ 70% (KPS) (HR 1.4; 95% CI 1.1–1.9; p = 0.015). Age, dose of radiotherapy (>54 Gy), response to initial chemotherapy, number of pack years and smoking was not associated with a significant effect on survival. Further, there was no significant association with survival for chronic obstructive pulmonary disease (COPD), type-2 diabetes, coronary artery disease (CAD) and hypertension when evaluated separately in univariate analysis (Fig. 3).
Fig. 3.
Univariate proportional hazards regression analysis of cofactors associated with overall survival. CI = confidence interval, KPS = Karnofsky performance status, ACCI = Charlson age-adjusted comorbidity index, PR = partial remission, SD = stable disease, PCI = prophylactic cranial irradiation, COPD = chronic obstructive pulmonary disease, CAD = coronary artery disease, RT = radiotherapy.
In multivariate analysis, OS was positively associated with PCI (HR 0.6; 95% CI 0.4–0.9; p = 0.022) and ACCI > 6 (HR 1.5; 95% CI 1.0–2.1; p = 0.049). KPS (≤70%) had no significant association with survival in multivariate analysis (Fig. 4). Since KPS and ACCI proved to be independent parameters on multivariate analysis, a subgroup analysis was performed (Fig. 5). Plotted against the remaining cohort, patients with low ACCI and high KPS hat a median overall survival of 20 months (Fig. 5A), whereas high ACCI and high KPS had a significantly lower overall survival of 8 months than the remaining cohort (Fig. 5B). Univariate analysis of the ACCI score showed a continuous trend for worse survival with higher scores with a statistical significance for scores greater 6 (Fig. 6). Comorbidities considered separately showed no significant association with OS neither in univariate analysis nor when analysed by the Log-rank test (Fig. 3, Fig. 7).
Fig. 4.
Multivariate proportional hazards regression analysis of cofactors associated with overall survival. CI = confidence interval, KPS = Karnofsky performance status, ACCI = Charlson age-adjusted comorbidity index.
Fig. 5.
Subgroup analysis of overall survival depending on Karnofsky performance score (KPS) and Charlson age-adjusted comorbidity index (ACCI). A KPS > 70% with ACCI ≤ 6 vs. the remaining cohort (Kaplan-Meier’s estimation) (15 vs. 20 months, Log-Rank test p < 0.001). B KPS > 70% with high ACCI > 6 (Kaplan-Meier’s estimation) (8 vs. 19 months, Log-Rank test p < 0.001).
Fig. 6.
Univariate proportional hazards regression analysis of individual ACCI groups associated with overall survival. CI = confidence interval, ACCI = Charlson age-adjusted comorbidity index.
Fig. 7.
Overall survival in patients in LD-SCLC depending on comorbidities: A Chronic pulmonary obstructive disease (COPD) (18 vs. 20 months, p = 0.62), B Coronary artery disease (CAD) (19 vs. 17 months, p = 0.28), C Type-2 diabetes (18 vs. 21 months, p = 0.57) and D hypertension (22 vs. 17 months, p = 0.11). P-Values were estimated using a Log-Rank test.
Discussion
The negative effect of comorbidities in general and smoking-related comorbidities in particular on survival are well recognized in patients with lung cancer [3], [4], [5], [6], [8], [23], [24], [25], [26], [27], [28], [29]. The presence of comorbidity is known to affect treatment decisions with elderly and multimorbid patients being less likely to receive standard treatment [12]. However, the effect of comorbidity on the prognosis of patients with LD-SCLC has been elusive. In this study, we retrospectively analyzed clinical data of patients with LD-SCLC to evaluate the prognostic relevance of comorbidities associated with survival. As a more manageable tool in a clinical setting, we assessed the value of the ACCI in estimating survival in patients with LD-SCLC. Patients with an ACCI > 6 had a significantly shorter survival compared with patients with an ACCI ≤ 6 indicating a subgroup of patients at high risk of early decline or death following TCR independent of KPS. In line with previous trials, our data suggests that age alone is not a prognostic factor for overall survival [12], [29], [30]. PCI was a strong prognostic factor in our cohort of patients who responded to TCR, potentially representing a selection bias since patients without therapy response or unfavorable health status may be overrepresented in the non-PCI cohort.
In a smaller series of 73 LD-SCLC patients [11], four comorbidity scales were investigated for a potential impact on survival. Significant associations were found for the CCI [31] and MRC Breathlessness Scale [32], whereas for ACCI there was a trend towards statistical significance.
Due to mutual risk factors, cardiovascular and metabolic comorbidities are common in SCLC patients with an increasing prevalence [9], [33]. Hence, oncological treatment decisions need to be individualized appropriately and patients at risk need careful monitoring of possible side effects of TCR, both requiring an attentive effort of coordination among multiple disciplines. Using a variety of different scores, the prognostic significance of comorbidities and their effect on survival in SCLC has been investigated in previous studies without consistent results [9], [11], [12], [10], [30], [34], [35], [36]. This inconsistency may have emerged from the wide application of the original CCI [31] which was developed in 1987. As the ACCI, the original version does not take into account the severity of the comorbidities such as COPD or CAD. In contrast to the CCI however, by scoring of age, the ACCI does factor in an interrelated patient attribute inherently associated with survival. Further explanations for previous inconsistencies may be based on small study cohorts and the combined analysis of ED and LD patients [35]. A Dutch population-based study indicated that the presence of cardiovascular morbidity, hypertension and diabetes in patients with LD-SCLC may exclude them from receiving combined chemoradiation. Challenging possible preconceptions about the frailty of patients, our data does not support this exclusion from the standard of care based on particular comorbidities alone. A combination of multiple severe comorbidities in older patients might explain why the ACCI scoring procedure was a prognostic significance in the present cohort. Other studies have highlighted additional parameters such as inflammatory markers which may lead to the validation of composite predictors in the future [37].
Interestingly, age and comorbidities did not influence survival when analysed individually, suggesting that the investigated factors by themselves should not exclude standard concurrent TCR in LD-SCLC. Multimorbidity as represented by a higher ACCI seems to be more relevant in LD-SCLC.
Our study has the inherent limitations of any retrospective approach. The presence of comorbidities (e.g. COPD and CAD) may be underreported. In addition, patients who did not receive combined TCR, due to either low performance status or contraindications regarding chemotherapy, were not analysed in this study which may present a selection bias. As patients were treated with a conventional daily radiotherapy, it is worth mentioning that hyperfractionated twice daily radiotherapy remains a favorable alternative. Furthermore, there is a possible bias regarding the use of PCI as we excluded patients who were rated unfit for TCR and therefore likely no candidates for PCI either. This might overestimate the OS benefit of PCI in our cohort. Regarding PCI dose, 25 Gy in 10 fractions rather than 30 Gy in 15 fractions is currently considered standard of care [38]. One major strength of the present study is the large study cohort as well as the homogenous treatment strategy in patients with LD-SCLC regarded suitable for TCR. Notably, neither the CCI nor the ACCI was designed for lung cancer patients and further investigations might be necessary to develop a validated, lung cancer-specific scale for comorbidities.
Conclusion
Multimorbidity as scored by the ACCI is negatively associated with survival in patients with LD-SCLC treated with combined TCR. Individual comorbidities and age alone were not associated with a worse overall survival. The ACCI (>6) emerged as a relevant prognostic factor and indicates a subgroup of patients with significantly shorter survival. The ACCI may be an effective and easily manageable tool for clinicians to balance the risks of over- and undertreatment among LD-SCLC patients with significant comorbidities. Further prospective studies should validate the use of ACCI for treatment stratification.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Heidelberg University young investigator grants to DB, LK, RES and JHR.
Parts of this analysis were presented at the 24. German Radiation Oncology Conference (DEGRO) 2018 as a poster presentation. The poster abstract was published in “Abstracts” in Strahlenther Onkol. 2018 Jun;194.
References
- 1.Aupérin A., et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger D.S., Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24:4526–4527. doi: 10.1200/JCO.2006.07.3841. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey S.D., Howlader N., Etzioni R.D., Donato B. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Ball D., Thursfield V., Irving L., Mitchell P., Richardson G., Torn-Broers Y., et al. Evaluation of the Simplified Comorbidity Score (Colinet) as a prognostic indicator for patients with lung cancer: A cancer registry study. Lung Cancer. 2013;82(2):358–361. doi: 10.1016/j.lungcan.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Iachina M., Jakobsen E., Møller H., Lüchtenborg M., Mellemgaard A., Krasnik M., et al. The Effect of Different Comorbidities on Survival of Non-small Cells Lung Cancer Patients. Lung. 2015;193(2):291–297. doi: 10.1007/s00408-014-9675-5. [DOI] [PubMed] [Google Scholar]
- 6.Alexander M., et al. The Influence of Comorbidity and the Simplified Comorbidity Score on Overall Survival in Non-Small Cell Lung Cancer—A Prospective Cohort Study. J Thorac Oncol. 2016;11:748–757. doi: 10.1016/j.jtho.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Ahn D.H., et al. Influence of Medical Comorbidities on the Presentation and Outcomes of Stage I-III Non–Small-Cell Lung Cancer. Clin Lung Cancer. 2013;14:644–650. doi: 10.1016/j.cllc.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colinet B., Jacot W., Bertrand D., Lacombe S., Bozonnat M.-C., Daurès J.-P., et al. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson’s index. Br J Cancer. 2005;93(10):1098–1105. doi: 10.1038/sj.bjc.6602836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarts M.J., et al. Comorbidity in Patients With Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin Lung Cancer. 2015;16:282–291. doi: 10.1016/j.cllc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Ludbrook J.J.S., et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. Int J Radiat Oncol Biol Phys. 2003;55:1321–1330. doi: 10.1016/s0360-3016(02)04576-5. [DOI] [PubMed] [Google Scholar]
- 11.Kaesmann L., Janssen S., Schild S.E., Rades D. Value of Comorbidity Scales for Predicting Survival After Radiochemotherapy of Small Cell Lung Cancer. Lung. 2016;194(2):295–298. doi: 10.1007/s00408-016-9857-4. [DOI] [PubMed] [Google Scholar]
- 12.Janssen-Heijnen M.L.G., et al. Negligible influence of comorbidity on prognosis of patients with small cell lung cancer: A population-based study in the Netherlands. Crit Rev Oncol Hematol. 2007;62:172–178. doi: 10.1016/j.critrevonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Yang C.-C., Chen P.-C., Hsu C.-W., Chang S.-L., Lee C.-C., Chien M.-H. Validity of the Age-Adjusted Charlson Comorbidity Index on Clinical Outcomes for Patients with Nasopharyngeal Cancer Post Radiation Treatment: A 5-Year Nationwide Cohort Study. PLoS One. 2015;10(1):e0117323. doi: 10.1371/journal.pone.0117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal J., Pond G.R., Galsky M.D., Hendricks R., Small A., Tsao C.-K., et al. Association of the Charlson comorbidity index and hypertension with survival in men with metastatic castration-resistant prostate cancer. Urol Oncol Semin Orig Investig. 2014;32(1):36.e27–36.e34. doi: 10.1016/j.urolonc.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Radovanovic D., Seifert B., Urban P., Eberli F.R., Rickli H., Bertel O., et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart. 2014;100(4):288–294. doi: 10.1136/heartjnl-2013-304588. [DOI] [PubMed] [Google Scholar]
- 17.Tian Y., Jian Z., Xu B., Liu H. Age-adjusted Charlson comorbidity index score as predictor of survival of patients with digestive system cancer who have undergone surgical resection. Oncotarget. 2017;8(45):79453–79461. doi: 10.18632/oncotarget.18401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson M., Wells M.T., Ullman R., King F., Shmukler C., Catapano A. The Charlson Comorbidity Index Can Be Used Prospectively to Identify Patients Who Will Incur High Future Costs. PLoS One. 2014;9(12):e112479. doi: 10.1371/journal.pone.0112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep. 1973;3(4):31–42. [PubMed] [Google Scholar]
- 20.Koppie T.M., Serio A.M., Vickers A.J., Vora K., Dalbagni G., Donat S.M., et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112(11):2384–2392. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 21.Wu C.-C., Hsu T.-W., Chang C.-M., Yu C.-H., Lee C.-C. Age-Adjusted Charlson Comorbidity Index Scores as Predictor of Survival in Colorectal Cancer Patients Who Underwent Surgical Resection and Chemoradiation. Medicine (Baltimore) 2015;94:e431. doi: 10.1097/MD.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J.W., Koh D.H., Jang W.S., Lee J.Y., Cho K.S., Ham W.S., et al. Age-adjusted Charlson Comorbidity Index as a prognostic factor for radical prostatectomy outcomes of very high-risk prostate cancer patients. PLoS One. 2018;13(6):e0199365. doi: 10.1371/journal.pone.0199365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N., Singh P.S., Aggarwal A.N., Behera D. Comorbidity Assessment Using Charlson Comorbidity Index and Simplified Comorbidity Score and Its Association With Clinical Outcomes During First-Line Chemotherapy for Lung Cancer. Clin Lung Cancer. 2016;17(3):205–213.e1. doi: 10.1016/j.cllc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Grose D., Morrison D.S., Devereux G., Jones R., Sharma D., Selby C., et al. The impact of comorbidity upon determinants of outcome in patients with lung cancer. Lung Cancer. 2015;87(2):186–192. doi: 10.1016/j.lungcan.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Kravchenko J., Berry M., Arbeev K., Lyerly H.K., Yashin A., Akushevich I. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer. 2015;88(1):85–93. doi: 10.1016/j.lungcan.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam K.M.M., Jiang X., Anggondowati T., Lin G., Ganti A.K. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol Biomarkers Prev. 2015;24:1079–1085. doi: 10.1158/1055-9965.EPI-15-0036. [DOI] [PubMed] [Google Scholar]
- 27.Sandfeld-Paulsen B., Meldgaard P., Aggerholm-Pedersen N. Comorbidity in Lung Cancer: A Prospective Cohort Study of Self-Reported versus Register-Based Comorbidity. J Thorac Oncol. 2018;13:54–62. doi: 10.1016/j.jtho.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Kocher F., Fiegl M., Mian M., Hilbe W. Cardiovascular Comorbidities and Events in NSCLC: Often Underestimated but Worth Considering. Clin Lung Cancer. 2015;16:305–312. doi: 10.1016/j.cllc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Asmis T.R., et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. 2008;26:54–59. doi: 10.1200/JCO.2007.12.8322. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd F.A., et al. Treatment of Small Cell Lung Cancer in the Elderly. J Am Geriatr Soc. 1994;42:64–70. doi: 10.1111/j.1532-5415.1994.tb06075.x. [DOI] [PubMed] [Google Scholar]
- 31.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Stenton C. The MRC breathlessness scale. Occup Med. 2008;58(3):226–227. doi: 10.1093/occmed/kqm162. [DOI] [PubMed] [Google Scholar]
- 33.Van Leersum N.J., et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995–2010. Int J Cancer. 2013;132:2157–2163. doi: 10.1002/ijc.27871. [DOI] [PubMed] [Google Scholar]
- 34.Halvorsen T.O., et al. Comorbidity and outcomes of concurrent chemo- and radiotherapy in limited disease small cell lung cancer. Acta Oncol (Madr) 2016;55:1349–1354. doi: 10.1080/0284186X.2016.1201216. [DOI] [PubMed] [Google Scholar]
- 35.Kuo Y.-W., et al. The Prognostic Value of the Simplified Comorbidity Score in the Treatment of Small Cell Lung Carcinoma. J Thorac Oncol. 2011;6:378–383. doi: 10.1097/JTO.0b013e3181fd4107. [DOI] [PubMed] [Google Scholar]
- 36.Eaton B.R., Kim S., Marcus D.M., Prabhu R., Chen Z., Ramalingam S.S., et al. Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer. 2013;119(21):3753–3760. doi: 10.1002/cncr.28267. [DOI] [PubMed] [Google Scholar]
- 37.Bernhardt D., et al. Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer Manag Res. 2018 doi: 10.2147/CMAR.S180990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suwinski R., et al. Prophylactic cranial irradiation in SCLC. Transl Lung Cancer Res. 2021;10(4):2071–2078. doi: 10.21037/tlcr-2020-rtm-05. [DOI] [PMC free article] [PubMed] [Google Scholar]