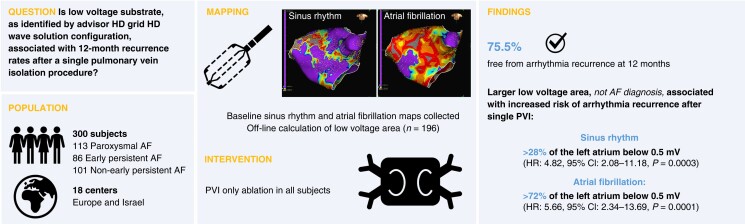
Abstract
Aims
Electro-anatomical mapping may be critical to identify atrial fibrillation (AF) subjects who require substrate modification beyond pulmonary vein isolation (PVI). The objective was to determine correlations between pre-ablation mapping characteristics and 12-month outcomes after a single PVI-only catheter ablation of AF.
Methods and results
This study enrolled paroxysmal AF (PAF), early persistent AF (PsAF; 7 days–3 months), and non-early PsAF (>3–12 months) subjects undergoing de novo PVI-only radiofrequency catheter ablation. Sinus rhythm (SR) and AF voltage maps were created with the Advisor HD Grid™ Mapping Catheter, Sensor Enabled™ for each subject, and the presence of low-voltage area (LVA) (low-voltage cutoffs: 0.1–1.5 mV) was investigated. Follow-up visits were at 3, 6, and 12 months, with a 24-h Holter monitor at 12 months. A Cox proportional hazards model identified associations between mapping data and 12-month recurrence after a single PVI procedure. The study enrolled 300 subjects (113 PAF, 86 early PsAF, and 101 non-early PsAF) at 18 centres. At 12 months, 75.5% of subjects were free from AF/atrial flutter (AFL)/atrial tachycardia (AT) recurrence. Univariate analysis found that arrhythmia recurrence did not correlate with AF diagnosis, but LVA was significantly correlated. Low-voltage area (<0.5 mV) >28% of the left atrium in SR [hazard ratio (HR): 4.82, 95% confidence interval (CI): 2.08–11.18; P = 0.0003] and >72% in AF (HR: 5.66, 95% CI: 2.34–13.69; P = 0.0001) was associated with a higher risk of AF/AFL/AT recurrence at 12 months.
Conclusion
Larger extension of LVA was associated with an increased risk of arrhythmia recurrence. These subjects may benefit from substrate modification beyond PVI.
Keywords: Atrial fibrillation, Electroanatomic mapping, Low-voltage area, Pulmonary vein isolation, Catheter ablation
Graphical Abstract
Graphical Abstract.
What’s new?
This is the first study to correlate substrate characteristics, as measured using the Advisor™ HD Grid Mapping Catheter, Sensor Enabled™ in the HD Wave Solution™ configuration, to 1-year outcomes after a single pulmonary vein isolation (PVI) procedure with a contact-force radiofrequency ablation catheter.
A larger low-voltage area in the left atrium was associated with an increased risk of recurrence of atrial fibrillation (AF), atrial flutter, or atrial tachycardia after a single PVI procedure, but AF classification of paroxysmal or persistent was not correlated with recurrence.
Introduction
Pulmonary vein isolation (PVI) is well established as the cornerstone for invasive treatment of atrial fibrillation (AF). Pulmonary vein isolation alone does not cure all AF, and a significant number of patients undergoing PVI still experience AF recurrence. As a result, additional ablation strategies have been studied to augment the long-term effectiveness of the procedure.1 One such strategy involves localization and modification of low-voltage areas (LVAs). Atrial fibrosis disturbs fibre bundle continuity and creates substrate to support the initiation and maintenance of AF, and local conduction disturbances promote non-uniform anisotropic conduction.2–4 Voltage reduction in the left atrium (LA) seems to be a diffuse process associated with fibrosis. Low-voltage areas reflect diffuse voltage reduction in the LA,5 especially in typical places like the anterior wall.6 Several studies have indicated that LA LVAs may correlate to areas of fibrotic LA substrate responsible for triggering and maintaining AF.7–9
The primary means of identifying LVAs is via electro-anatomical mapping using multi-electrode catheters. High-density multi-electrode catheters with smaller electrodes (electrode size 0.4–1 mm2) and smaller electrode spacing (1.2–6 mm) allow for the estimation of significantly larger local potentials than standard ablation catheters with large electrodes and larger spacing.7 A high-density multi-splined grid-shaped catheter, the Advisor™ HD Grid Mapping Catheter, Sensor Enabled™ (Advisor HD Grid) (Abbott, Abbott Park, IL, USA) allows for a simultaneous analysis of adjacent orthogonal bipolar signals through the HD Wave Solution™ (HDWS) configuration. Identifying and ablating LVAs, in addition to PVI for the treatment of AF, may provide benefits in certain patient populations.10–13 It is hypothesized that the Advisor HD Grid catheter with a HDWS configuration will provide high-density and high-resolution mapping for gaining improved insight into the extent of structural disease.
The aim of this study was to use the Advisor HD Grid catheter to characterize low-voltage substrate, as identified via HDWS configuration mapping in sinus rhythm (SR) and identify associations with 12-month recurrence rates after a single PVI procedure with a contact-force sensing radiofrequency (RF) ablation catheter. Secondarily, this study aimed to characterize a low-voltage LA substrate, comparing standard and HDWS configurations of the Advisor HD Grid catheter in AF and SR.
Methods
Study design
The WAVE-MAP AF study (NCT03882021) was a post-market, single-arm, multi-centre, prospective, interventional study aimed to determine correlations between pre-ablation mapping characteristics and outcomes after a single PVI-only catheter ablation of AF at 12 months post-index procedure. The conduct of the clinical study was approved by the appropriate Ethics committee of the respective clinical sites and as specified by local regulation. All patients enrolled in the study provided written informed consent.
Patient population
Eligible patients were 18 years or older, had a documented history of paroxysmal (PAF) or persistent AF (PsAF), and had a planned endocardial ablation procedure. Patients were excluded if they had long-standing PsAF or permanent AF (defined as continuous AF >12 months), prior ablation or surgery in the LA, a mitral or tricuspid valve replacement, an implantable cardiac defibrillator, or a LA appendage occluder.
Index procedure: mapping technique and ablation protocol
In each subject, investigators performed voltage mapping of the LA and followed a PVI-only ablation strategy with the EnSite Precision™ Cardiac Mapping System (Abbott, Abbott Park, IL, USA). Prior to ablation, a high-density grid-shaped catheter (Advisor HD Grid) that allows simultaneous analysis of adjacent orthogonal bipolar signals through the HDWS configuration was used to generate and record two detailed voltage maps, one each in SR and AF. When voltage mapping in SR, the protocol recommended keeping Advisor HD Grid in position for at least two cardiac cycles (five cardiac cycles recommended). Voltage mapping in AF was performed in Non-Cardiac Triggered Reference mode with segment lengths of 8 s for point collection. When mapping in AF, the protocol recommended operators hold Advisor HD Grid in place for 8 s at each anatomical location. Operators aimed, subject to their own discretion, to colour the entire LA geometry for each map. After completing the mapping protocol, operators ablated the pulmonary vein (PV) antrum to isolate the veins using a wide area circumferential ablation technique with the TactiCath™ Contact Force Ablation Catheter, Sensor Enabled™ (TactiCath SE). If isthmus-dependent flutter was confirmed by electrophysiological testing, ablation of the cavo-tricuspid isthmus (CTI) was recommended in the right atrium. Additional ablation beyond the PVs and CTI was not allowed. After each procedure, anonymized case data were exported for future analysis.
Offline map analysis: re-mapping technique
Case data were loaded onto an Ensite Precision Laptop Review Station running modified research software for retrospective analysis of low-voltage substrate. Cases were only re-evaluated if complete SR and AF map recordings were available. The PVs at the level of the lesion set and mitral annulus were removed from each model to generate more accurate and normalized measures of the surface area and volume of the LA chambers. The mitral annulus and PVs were not included in any surface area calculations. Both HDWS configuration and standard along-the-spline configuration bipolar maps were created in SR and AF.
HD Wave Solution™ configuration utilizes the unique grid electrode pattern to enable simultaneous measurement of voltage in two directions. Orthogonal bipoles are defined as a pair of perpendicular bipoles originating from a single electrode with one bipole configured across two separate splines and one bipole configured along the spline. The max voltage duplicate algorithm then used the highest amplitude electrogram (EGM) for each set of orthogonal bipoles. Standard along-the-spline configuration utilized bipoles along each of the catheter splines only (see Supplementary material online, Figure S1).
Mapping points were collected using the abs dV/dt annotation algorithm. The interior and exterior projection were set to 6 mm, so points were rejected if they were >6 mm from the geometry surface. For each map, the surface area of tissue above, in between, and below the voltage cutoffs was recorded. An upper voltage cutoff of 2.0 mV and lower voltage cutoffs ranging from 0.1 to 1.5 mV were used to analyse and compare the surface areas at different low-voltage cutoffs.
Clinical follow-up
Follow-up visits occurred at 3, 6, and 12 months. A 90-day blanking period followed the index procedure. It was recommended, not required, to stop Class I/III anti-arrhythmic drugs (AADs) after the blanking period unless there was recurrence of AF. Data collected at each follow-up visit included arrhythmia recurrence, arrhythmic medication use, and occurrence of adverse events. Twelve-lead electrocardiograms (ECGs) were collected at the 3- and 12-month follow-up visits, and a 24-h continuous ECG was recorded at the 12-month visit.
Statistical analysis
No formal hypothesis testing was performed as all endpoints were descriptive. Continuous variables are reported as mean, standard deviation (SD), and number of observations. Categorical variables are reported as proportion of observations. Survival analysis was conducted to evaluate the primary endpoint and 12-month recurrence. Subjects without events were censored at their last known event-free time point. Subjects withdrawn or otherwise lost-to-follow-up were censored at their last known visit. A Cox proportional hazards model was utilized to find the optimal low-voltage threshold and identify associations between mapping data and 12-month recurrence. The lowest Akaike information criterion (AIC) defined the best model fit.
All P-values are reported descriptively. P-values for comparison of continuous variables across subgroups were generated via a t-test or one-way analysis of variance. P-values for comparisons of categorical variables were generated via χ2 test or Fisher’s exact test if cell counts <5. Analyses were performed using SAS software (version 9.4). A P-value <0.05 indicated statistical significance.
Results
Baseline patient characteristics
Three hundred subjects were enrolled at 18 centres in Europe and Israel between 27 August 2019 and 20 October 2020. Four subjects were withdrawn following the index procedure because the operator was not able to achieve isolation of the PVs. These subjects were followed for 30 days, but none experienced any adverse events. Additionally, there were two subject deaths, neither related to Advisor HD Grid and one with unknown relationship to the study procedure. These subjects were included in the safety analysis.
Mean age was 62 ± 9.5 years with 70.3% of subjects male. Atrial fibrillation diagnosis was classified into PAF (duration <7 days), early PsAF (duration between 7 days and 3 months), and non-early PsAF (duration between 3 and 12 months). Enrolled subjects were 37.7% (113/300) PAF, 28.7% (86/300) early PsAF, and 33.7% (101/300) non-early PsAF. Subjects with PsAF were older, more often male with a history of heart failure, and exhibited larger LA compared with PAF. In addition to AF, 15.7% (47/300) of subjects had a history of typical atrial flutter (AFL). At baseline, 42.7% (128/300) of subjects were taking a Class I/III AAD, and 63.0% (189/300) were taking a Class II/IV/V AAD. Subject characteristics at baseline for each of these diagnoses are shown in Table 1.
Table 1.
Baseline characteristics
| Paroxysmal (n = 113) | Early persistent (n = 86) | Non-early persistent (n = 101) | P-value | |
|---|---|---|---|---|
| Age, mean ± SD (n) | 61.3 ± 9.6 (113) | 61.7 ± 10.4 (86) | 63.1 ± 8.6 (101) | 0.3395 |
| Sex, male | 60.2% (68/113) | 77.9% (67/86) | 75.2% (76/101) | 0.0105 |
| Height, inch, mean ± SD (n) | 68.0 ± 3.8 (113) | 68.9 ± 4.0 (86) | 68.8 ± 3.6 (101) | 0.2192 |
| Weight, lbs, mean ± SD (n) | 186.2 ± 38.5 (113) | 189.3 ± 39.4 (86) | 192.7 ± 35.3 (101) | 0.4598 |
| BMI, mean ± SD (n) | 28.2 ± 4.9 (113) | 27.9 ± 4.5 (86) | 28.6 ± 4.4 (101) | 0.6313 |
| History of heart failure | 14.2% (16/113) | 41.9% (36/86) | 29.7% (30/101) | <0.0001 |
| NYHA classification | 0.4327 | |||
| I | 18.8% (3/16) | 22.2% (8/36) | 6.7% (2/30) | |
| II | 62.5% (10/16) | 61.1% (22/36) | 53.3% (16/30) | |
| III | 12.5% (2/16) | 16.7% (6/36) | 26.7% (8/30) | |
| IV | 0.0% (0/16) | 0.0% (0/36) | 0.0% (0/30) | |
| Not evaluated | 6.3% (1/16) | 0.0% (0/36) | 13.3% (4/30) | |
| TTE performed | 99.1% (112/113) | 100.0% (86/86) | 97.0% (98/101) | 0.2605 |
| LVEF, %, mean ± SD (n) | 59.9 ± 6.9 (108) | 56.4 ± 8.9 (85) | 55.5 ± 9.2 (95) | 0.0004 |
| LAD, mm, mean ± SD (n) | 41.2 ± 12.0 (94) | 42.8 ± 10.3 (70) | 46.1 ± 13.9 (62) | 0.0464 |
| LAV, mL, mean ± SD (n) | 62.8 ± 25.7 (51) | 63.3 ± 32.7 (51) | 79.8 ± 34.6 (49) | 0.0097 |
| Evidence of left ventricular hypertrophy | 16.1% (18/112) | 17.4% (15/86) | 14.3% (14/98) | 0.8409 |
| Evidence of hypertrophic cardiomyopathy | 0.9% (1/112) | 3.5% (3/86) | 2.0% (2/98) | 0.4495 |
BMI, body mass index; LAD, left atrial diameter; LAV, left atrial volume; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation; TTE, transthoracic echocardiogram.
Mapping data
At least one voltage map was collected in all 300 enrolled subjects, including SR maps in 271 subjects and AF maps in 255 subjects. Both SR and AF maps were collected from 222 subjects. In some cases, either SR or AF was not able to be maintained for mapping despite multiple cardioversion attempts or inductions. Cardioversions were performed during the procedure in 42.5% (48/113) of PAF subjects and 83.4% (156/187) of PsAF subjects. If performed, there were no significant differences in the number of cardioversions during the procedure for each AF diagnosis (Table 2). However, mapping time was significantly different between PAF, early PsAF, and non-early PsAF subjects, with non-early PsAF subjects having the longest mean mapping time.
Table 2.
Procedural parameters, safety, and efficacy
| Paroxysmal (n = 113) | Early persistent (n = 86) | Non-early persistent (n = 101) | All (n = 300) | P-value | |
|---|---|---|---|---|---|
| Acute procedural success | 100.0% (113/113) | 97.7% (84/86) | 98.0% (99/101) | 98.7% (296/300) | 0.2036 |
| Procedure time, min (first venous puncture to last catheter removed), mean ± SD (n) | 156.2 ± 48.0 (113) | 157.3 ± 45.9 (86) | 166.5 ± 58.8 (101) | 160.0 ± 51.4 (300) | 0.2892 |
| Mapping timea, min, mean ± SD (n) | 30.2 ± 14.6 (113) | 38.8 ± 16.5 (86) | 41.5 ± 15.1 (98) | 36.4 ± 16.1 (297) | <0.0001 |
| Fluoroscopy time, min, mean ± SD (n) | 10.2 ± 8.9 (113) | 11.6 ± 9.1 (86) | 13.7 ± 11.2 (101) | 11.8 ± 9.9 (300) | 0.0381 |
| Number of cardioversions, mean ± SD (n) | 1.8 ± 1.4 (48) | 1.5 ± 0.9 (63) | 1.9 ± 1.5 (93) | 1.7 ± 1.3 (204) | 0.0931 |
| Serious adverse event rate | 1.8% (2/113) | 2.3% (2/86) | 4.0% (4/101) | 2.7% (8/300) | 0.6689 |
Mapping time includes total time collecting maps, including both SR and AF maps.
AF, atrial fibrillation; SD, standard deviation; SR, sinus rhythm.
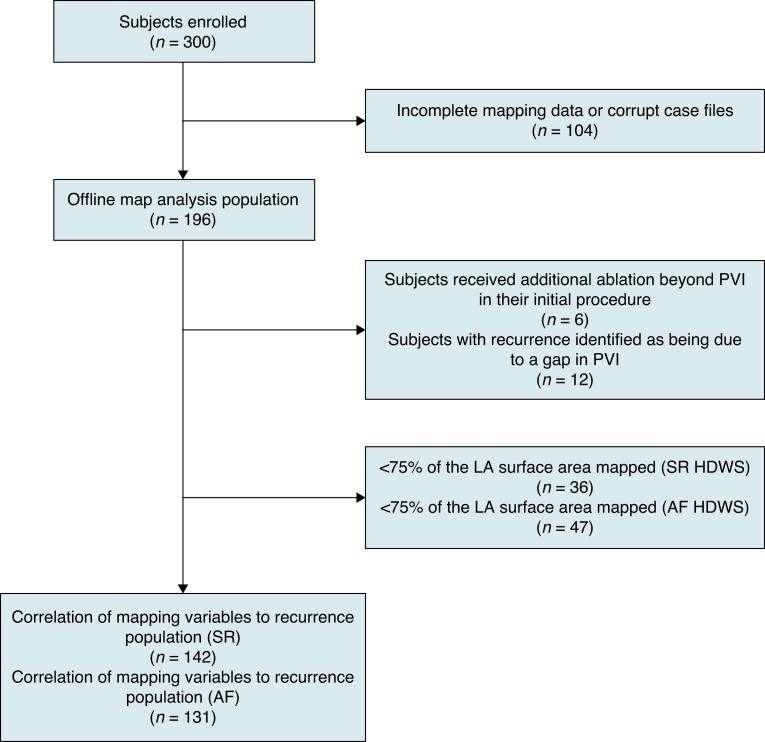
After removing subjects in which case files were corrupt or complete pre-ablation mapping data was not collected in either SR or AF, 196 subjects remained and were included in the offline map analysis (Figure 1). Sinus rhythm maps had significantly more mapping points collected compared with AF maps, but the mapped LA surface area was not significantly different (Table 3). Left atrial surface area, excluding the mitral annulus and PVs was 108.4 ± 26.5 cm2, with a mean ± SD geometry volume of 117.2 ± 39.7 cm3. On average, 83.8% of the LA surface area was mapped using HDWS configuration (mapped surface as a percentage of LA surface area) in SR compared with 81.5% in AF.
Figure 1.
Study data analysis flow chart. AF, atrial fibrillation; HDWS, HD Wave Solution; LA, left atrium; PVI, pulmonary vein isolation; SR, sinus rhythm.
Table 3.
Mapping data by rhythm during mapping and Advisor HD grid configuration
| SR maps | AF maps | All maps | P-value | |
|---|---|---|---|---|
| Mapping points collected - standard configuration | 4468.0 ± 3447.1 (195) | 1738.1 ± 1179.4 (192) | 3113.6 ± 2920.3 (387) | <0.0001 |
| Mapping points collected - HDWS configuration | 8330.7 ± 6260.0 (195) | 3193.6 ± 2130.8 (191) | 5788.8 ± 5348.0 (386) | <0.0001 |
| Mapped left atrial surface area - standard configuration (cm2) | 85.8 ± 26.2 (195) | 84.8 ± 21.7 (192) | 85.3 ± 24.0 (387) | 0.4770 |
| Mapped left atrial surface area - HDWS configuration (cm2) | 89.8 ± 25.6 (195) | 87.8 ± 22.8 (192) | 88.8 ± 24.2 (387) | 0.1674 |
| Mapped surface as a % of left atrium - standard configuration (%) | 80.2 ± 18.7 (195) | 78.7 ± 12.8 (192) | 79.4 ± 16.0 (387) | 0.4092 |
| Mapped surface as a % of left atrium - HDWS configuration (%) | 83.8 ± 16.8 (195) | 81.5 ± 13.3 (192) | 82.6 ± 15.2 (387) | 0.1332 |
AF, atrial fibrillation; HDWS, HD Wave Solution; SR, sinus rhythm.
Low-voltage area
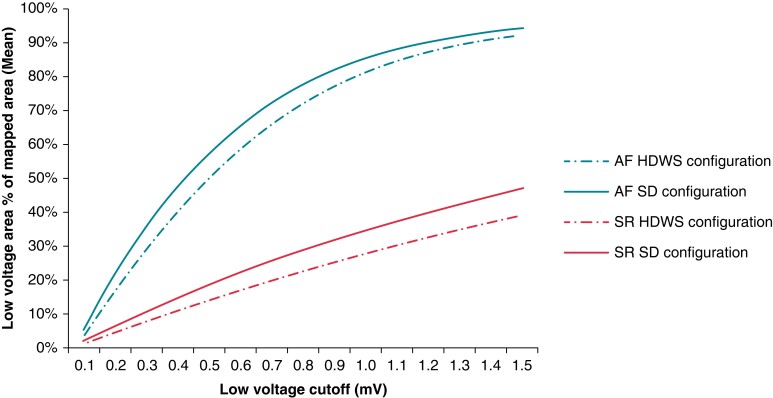
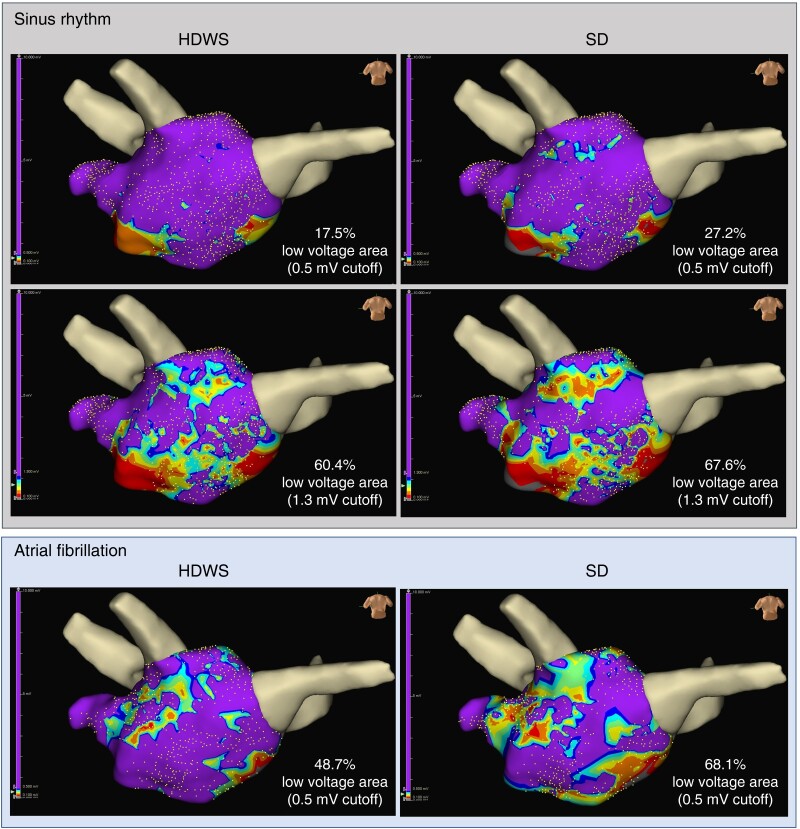
In both SR and AF, for all low-voltage cutoffs from 0.1 to 1.5 mV, the percentage of mapped LVA (mean LVA as a percentage of mapped LA surface area) was greater when standard configuration was used compared with HDWS configuration (Figure 2). Using a low-voltage cutoff of 0.5 mV in SR, mean LVA in the offline map analysis population was 16.2 and 12.8 cm2 using standard and HDWS configuration, respectively (P < 0.0001). Similarly, mean LVA using a cutoff of 0.5 mV in AF was 49.9 cm2 using the standard configuration and 45.5 cm2 using HDWS configuration (P < 0.0001). Figure 3 shows a visual example of one subject’s LVA at a low-voltage cutoff of 0.5 mV in SR and AF and 1.3 mV in SR. Due to the significant differences and the lower, more precise LVA characterization of HDWS configuration, HDWS configuration low voltage was utilized for the remaining analyses.
Figure 2.
Percentage of mapped LVA by Advisor HD Grid electrode configuration. Mean LVA as a percentage of mapped LA surface area in SR and AF for each low-voltage cutoff using HDWS configuration and Standard (SD) configuration. AF, atrial fibrillation; HDWS, HD Wave Solution; LA, left atrium; LVA, low-voltage area; SD, standard deviation; SR, sinus rhythm.
Figure 3.
Sinus rhythm and AF maps in a non-early PsAF subject. Differences in LVA between HDWS configuration (left) and standard (SD) configuration (right) using a 0.5 and 1.3 mV cutoff in SR (top) and 0.5 mV cutoff in AF (bottom). Left atrium geometry is shown in the posteroanterior view. During re-mapping, the PVs and mitral annulus were cut out at the level of the lesion set, hidden, and not included in mapping data to ensure normalization of LA geometry and surface area. The PVs are shown for visualization only. AF, atrial fibrillation; HDWS, HD Wave Solution; LA, left atrium; LVA, low-voltage area; PsAF, persistent atrial fibrillation; PV, pulmonary vein; SD, standard deviation; SR, sinus rhythm.
As expected, LVA was much lower in SR compared with AF. To understand any correlation between SR and AF LVA, the Pearson correlation coefficient was calculated for each low-voltage cutoff. There was a significant correlation between LVA under each cutoff in AF compared with the same cutoff in SR. A cutoff of 0.3 mV had the highest correlation between AF and SR LVA (r = 0.72, P < 0.0001). When comparing LVA under each cutoff in AF to the standard cutoff of 0.5 mV in SR, the highest Pearson correlation coefficient was at a low-voltage cutoff of 0.2 mV (r = 0.80, P < 0.0001). This indicates that a LVA under 0.2 mV in AF was most correlated with LVA under 0.5 mV in SR.
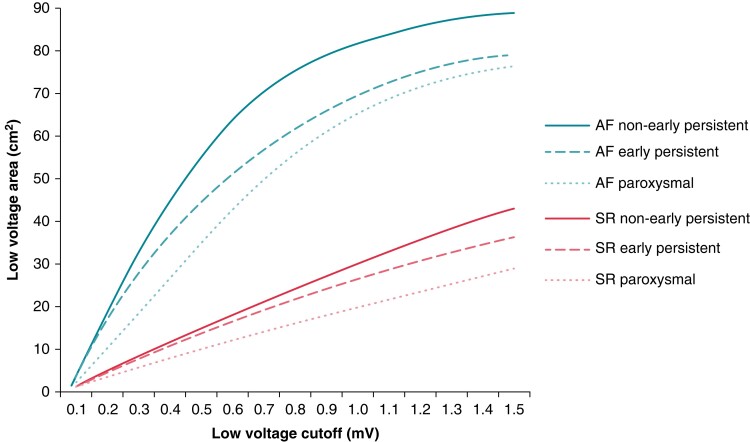
Low-voltage area at each cutoff was compared not only between HDWS configuration and standard configuration but also between diagnoses. For each low-voltage cutoff, Figure 4 shows the LVA by diagnosis in both SR and AF using HDWS configuration. Below 0.8 mV in SR, there was no significant difference between PAF, early PsAF, and non-early PsAF subjects’ LVA (P = 0.0634 at 0.7 mV). However, LVA was significantly different between the diagnoses if low-voltage cutoffs of 0.8 mV (P = 0.0498) to 1.5 mV (P = 0.0073) were used in SR. Under 0.5 mV in SR, mean LVA was 14.67 cm2 for non-early PsAF, 13.51 cm2 for early PsAF subjects, and 9.88 cm2 for PAF subjects (P = 0.1526). In AF, there was a significant difference in LVA between the three diagnoses for all low-voltage cutoffs 0.2 mV (P = 0.0065) and greater. At 0.5 mV in AF, mean LVA was 55.43 cm2 for non-early PsAF subjects, 44.39 cm2 for early PsAF subjects, and 35.14 cm2 for PAF subjects (P < 0.0001).
Figure 4.
Low-voltage area by diagnosis. Mean LVA (HDWS configuration) for low-voltage cutoffs in SR and AF displayed per AF diagnosis. AF, atrial fibrillation; HDWS, HD Wave Solution; LVA, low-voltage area; SR, sinus rhythm.
Procedural results
Acute procedural success was 98.7% (296/300). Four subjects that did not achieve acute procedural success were withdrawn. Total procedure time, measured as the time from the first venous puncture to last catheter removed, was not significantly different between AF diagnoses (Table 2). Mean procedure time was 160.0 ± 51.4 min, which included 36.4 ± 16.1 min of mapping (includes both SR and AF map collection) and 11.8 ± 9.9 min of fluoroscopy time. Non-early PsAF subjects had the most fluoroscopy time while PAF subjects had the least. Most subjects, 68.0% (204/300), required a cardioversion during the procedure and some subjects required multiple cardioversions.
Safety
Despite the requirement to complete multiple maps, serious adverse events occurred in only 2.7% of subjects. No adverse events were related to Advisor HD Grid. There were two deaths, one from chronic obstructive pulmonary disease and one from endocarditis. Neither was related to Advisor HD Grid.
Primary effectiveness endpoint
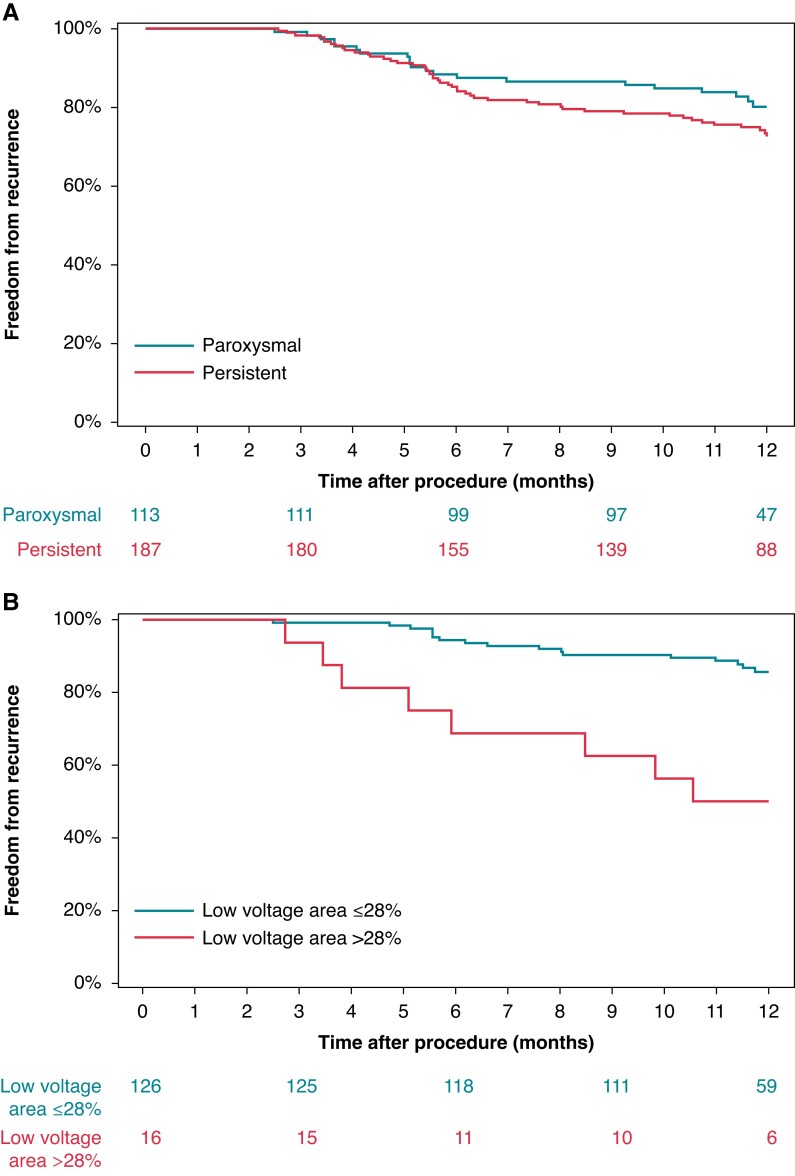
One year success, defined as freedom from AF/AFL/AT after removal from AADs as assessed from the end of the 3-month blanking period to 12 months following a single ablation procedure, was achieved in 57.2% of all subjects, 61.1% of PAF and 54.9% of PsAF subjects. Overall, 75.5% of all subjects were free from AF/AFL/AT recurrence following a single ablation procedure at 12 months, including, 80.2% of PAF and 72.6% of PsAF subjects (Figure 5A). A total of 29 repeat ablation procedures were performed in 28 subjects, 3 of which were during the blanking period.
Figure 5.
Freedom from recurrence by diagnosis and LVA. Freedom from AF/AFL/AT recurrence through 12-months with respect to (A) AF diagnosis and (B) LVA (0.5 mV low-voltage cutoff) mapped in HDWS configuration during SR. AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; HDWS, HD Wave Solution; LVA, low-voltage area; SR, sinus rhythm.
Correlation of demographic and mapping variables to recurrence
Univariate analysis found no statistically significant correlation between evaluated demographic variables (age, sex, CHA2DS2-VASc score, hypertension, body mass index, LA diameter, LA volume, left ventricular ejection fraction, or AF diagnosis) and recurrence. However, LVA was significantly associated with recurrence. This was true for LVA under the standard low-voltage cutoff of 0.5 mV when mapping was performed in either AF (P = 0.0004) or SR (P = 0.0226). Using a low-voltage cutoff of 0.5 mV, a Cox proportional hazards model determined the percent LVA (LVA as a percentage of LA surface area) with the best correlation to recurrence in SR and AF. The percent LVA with the lowest AIC demonstrated the best model fit. Prior to analysis, subjects who received additional ablation beyond PVI in their initial procedure (N = 6) and subjects with recurrence identified as being due to a gap in the PVI (N = 12) were excluded. Cases with <75% of the LA surface area mapped were also excluded (Figure 1). To ensure the precision and accuracy of the model, the model only included percent LVA categories (possibly ranging from 1 to 100%) in which there were at least 10 subjects above and below the cutoff. In SR, the best model fit corresponded to >28% LVA under 0.5 mV [hazard ratio (HR): 4.82, 95% confidence interval (CI): 2.08–11.18; P = 0.0003]. Using a low-voltage cutoff of 0.5 mV, subjects with LVA >28% of the LA in SR were associated with the highest risk of recurrence at 1 year (Figure 5B). In AF, the best model fit was >72% LVA under 0.5 mV (HR: 5.66, 95% CI: 2.34–13.69; P = 0.0001).
Using these definitions of low voltage, 23/195 (11.8%) subjects had >28% low voltage under 0.5 mV in SR and 15/192 (7.8%) subjects had >72% low voltage under 0.5 mV in AF. Ten subjects had both >28% low voltage in SR and >72% low voltage in AF.
While 0.5 mV is a common low-voltage cutoff used clinically, a univariate Cox proportional hazards model evaluated each low-voltage cutoff from 0.1 to 1.5 mV and its ability to predict recurrence after a single PVI. In AF, 0.5 mV had the lowest AIC and thus the best model fit to predict recurrence as described above. In SR, univariate analysis found the low-voltage cutoff with the lowest AIC and best model fit was 1.3 mV. At 1.3 mV, the percent LVA most associated with recurrence was >60% of the LA under 1.3 mV (HR: 4.37, 95% CI: 1.89–10.15; P = 0.0006). Supplementary material online, Figure S2 displays percent LVA under each low-voltage cutoff that is most associated with recurrence as well as the associated HR.
Subgroup analysis: impact of LVA presence on recurrence
The correlation of mapping variables to recurrence population (SR) (N = 142) were reassigned into two groups: those with LVA (>1% LA surface area under 0.5 mV in SR) and those without LVA (≤1% of LA surface area under 0.5 mV in SR). Using these definitions, 12/142 (8.5%) subjects had no LVA, including 5 PAF and 7 PsAF subjects. Freedom from recurrence at 12 months in subjects with LVA was 81.1% (95% CI: 73.0–86.9%) compared with 90.0% (95% CI: 47.3–98.5%) in those without LVA (P = 0.3387) (see Supplementary material online, Figure S3).
Discussion
This is the first study utilizing Advisor HD Grid to characterize the LA and determine if these measurements can predict AF recurrence after a single PVI-only ablation procedure. This study found that larger HDWS configuration-defined LVA in either AF or SR was associated with an increased risk of recurrence at 1 year. However, AF classification of PAF or PsAF was not correlated with recurrence. Further, this study confirmed that HDWS configuration provides smaller, more precise LVA characterization compared with standard configuration, in both SR and AF.
While PVI alone does not completely cure all AF, substrate modification beyond PVI has shown mixed results. Several studies have shown some benefit identifying LVAs and ablating those targets in addition to PVI for PsAF, but mapping is critical to identify which subjects have LVAs and may benefit from further substrate modification.14,15 In this study, the amount of LVA substrate in the LA was more predictive of recurrence after PVI than a diagnosis of PsAF vs. PAF. The amount of LVA substrate, as identified by Advisor HD Grid, may better discern which subjects may benefit from additional substrate modification. In this study, LVA was generally greater in non-early PsAF subjects compared with early PsAF and PAF subjects, but this was only true in AF and in SR at low-voltage cutoffs above 0.8 mV. At the clinical standard low-voltage cutoff of 0.5 mV in SR, there was no statistical difference in the extent of LVA. Therefore, it may not be advised to use AF classification alone as a surrogate for the amount of LVA and the need for substrate modification beyond PVI.
Clinically, a low-voltage cutoff of 0.5 mV is commonly used, but the literature explores a variety of low-voltage cutoffs. Lower voltage cutoffs of 0.2–0.45 mV have been postulated to accurately identify scar16 and the presence of low voltage <0.4 mV in PAF subjects has been predictive of recurrence.17 While higher cutoffs for low voltage are not typically used clinically, studies have demonstrated the impact of mildly affected LVA (defined as >0.5–1.1 mV) on recurrence. Yagishita et al.18 found that the presence of mildly affected LVA was an independent predictor of recurrence after a PVI-only ablation. These areas were also highly associated with abnormal EGMs. Lin et al.19 also demonstrated an association between abnormal EGMs and areas with bipolar voltage ≤1.3 mV in patients with PsAF and long-standing AF. However, these studies mostly used traditional mapping catheters. With Advisor HD Grid, we found that >28% of the LA below 0.5 mV in SR was predictive of recurrence. However, the best model fit in SR was using a cutoff of 1.3 mV and >69% of the LA. As expected, as the low-voltage cutoff increases, so does the percent LVA under that cutoff to predict recurrence. These results suggest it could be more advantageous to characterize subjects by amount of low-voltage substrate in the LA, rather than diagnosis, for the purpose of identifying the optimal treatment approach.20
Low-voltage areas are identified via electro-anatomical mapping using multi-electrode catheters. When collecting points using non-grid mapping catheters, a single bipole is considered for any given mapping point acquisition. This makes voltage measurements susceptible to inaccuracies resulting from misalignment of the bipole with the propagating wavefront.21 High-density multi-electrode catheters with smaller electrodes and smaller electrode spacing allow for estimation of significantly larger local potentials than standard ablation catheters with large electrodes and spacing.10,22 During comparison of high-density atrial voltage mapping with multipolar Lasso circular mapping catheter (CMC) and Advisor HD Grid, average atrial voltage was significantly lower for the CMC (CMC global LA voltage was 0.75 × Advisor HD Grid).5 Saito et al.23 also revealed that bipolar voltage amplitude estimated by Advisor HD Grid was significantly larger than PentaRay catheter. Similarly, smaller LVAs were seen using Advisor HD Grid when compared with either linear duodecapolar catheter24 or CMC maps in the same patients.25 These differences in low-voltage measurements may contribute to the mixed results of studies identifying and ablating LVAs in addition to PVI.
Ultra-high-density mapping strategies like those utilized in this study may increase mapping time. However, insight into patient substrate may better inform therapy decisions intraprocedurally. Additionally, Advisor HD Grid has been shown to collect significantly more mapping points in a significantly shorter amount of time compared with a CMC.25
As demonstrated here, final estimation of voltage amplitude is also influenced by the electrode configuration of the multipolar mapping catheter. The Advisor HD Grid catheter HDWS configuration allows for simultaneous analysis of adjacent orthogonal bipolar signals and selection of the EGM with the highest amplitude, thus helping to correct for the directional influence of wavefront propagation on bipolar signal amplitude. When compared with standard linear-only bipole configurations, HDWS configuration consistently identifies significantly smaller, more precise LVAs.24–26
As expected, LVA was more prevalent in AF compared with SR maps, which correlates with published literature showing lower LA voltages during AF.27 This study also found a correlation between low voltage under cutoffs in SR compared with AF. Yagishita et al.28 reported a significant linear bipolar voltage correlation between SR and AF and suggested that a similar extent of LA scar could be identified in either SR or AF by adjusting the voltage cutoff. In this study, 10 subjects had sufficient low voltage under our predictive cutoffs in both SR and AF. Some subjects achieved the low-voltage threshold predictive of recurrence in only SR or AF. To truly understand the differences and similarities between AF and SR maps, the location of low voltage in both maps is important. While beyond the scope of this study, future studies may explore this topic.
Studies evaluating individualized substrate ablation of LVA or delayed enhancement magnetic resonance imaging (MRI) identified atrial fibrosis have mixed results.29–35 Recent results from the DECAAF II study found no significant difference in arrhythmia recurrence between MRI-guided fibrosis ablation plus PVI in PsAF subjects and PVI alone.36,37 However, fibrosis identified on MRI is not necessarily equivalent to LVA.8,38 The STABLE-SR-II trial found that LVA ablation in PsAF subjects did not improve success rates above PVI alone.37 Maps were collected after PVI using either the Lasso or PentaRay catheter. Targeted LVA was defined as 0.1–0.4 mV, but transitional zones (0.4–1.3 mV) were also defragmented. So, additional targeted ablation was done compared with other studies. Other studies found that significantly more patients in PVI plus LVA ablation groups were free from recurrence compared with controls.14,15,39,40 The ERASE-AF study recently found that ablation of LVA significantly improved outcomes in patients with PsAF.39 Maps were acquired using any multipolar mapping catheter prior to ablation. Targeted LVA was quantified using a threshold of 0.5 mV and strict ablation endpoints of bidirectional block or loss of capture were required. Differences in the results between the two recent randomized controlled trials may be due to different definitions of LVA, differences in ablation endpoints, and higher density arrhythmia recurrence monitoring in the ERASE-AF study (75% of subjects had an implantable cardiac monitor). While some studies have found that subjects with low voltage may benefit from additional substrate modification beyond PVI to achieve long-term results, precise techniques for substrate modification and their impact on long-term results need to be understood.
Both the STABLE-SR-II and ERASE-AF reported greater 12-month success after PVI in subjects without low voltage compared with subjects with low voltage. This is comparable to the trend seen in this study and the significant correlation reported between LVA and recurrence. However, this study reports a lower percentage of subjects without LVA compared with ERASE-AF and STABLE-SR-II. This may be due to differences in mapping protocols or different methods used to calculate LVA and subjects that did not have LVA. In this study, LVA included all LA surface area below the low-voltage cutoff (including electrically inert tissue). The PVs and mitral annulus were not included in the LA surface area calculation. This study also excluded subjects in which <75% of the LA surface area was mapped.
No studies to date have correlated substrate characteristics, as measured using HDWS configuration mapping, to outcomes. This study provides insight into how these substrate characteristics can identify subjects who may not require ablation beyond PVI to achieve a successful result and avoid the potential risk of unnecessary ablation. These results may help design future studies of the Advisor HD Grid catheter to further investigate treatment algorithms and recommended treatment approaches based on HDWS configuration mapping of baseline substrate. Future analyses of additional variables and new technologies such as omnipolar mapping may further identify appropriate subjects for ablation beyond PVI and beneficial substrate modification techniques.
Limitations
This study was a prospective, single-arm, non-randomized, interventional study with the inherent limitations to this study design. There are several additional limitations. In some subjects, a rhythm was not inducible or sustainable, so both SR and AF maps could not be collected. Map analysis included subjects with both SR and AF maps. Discontinuation of AADs after the blanking period (unless there was recurrence) was recommended, but this was not mandatory, since deviations could have occurred from standard of care. This may have impacted our primary effectiveness endpoint, since AAD usage resulted in an endpoint failure. This did not impact freedom from recurrence analysis. Finally, 12-month effectiveness may be overestimated by missing brief or asymptomatic recurrence episodes during follow-up. Continuous 24-h Holter monitoring was collected only at the 12-month visit.
Conclusions
Using a standard low-voltage cutoff of 0.5 mV on both SR and AF maps, a larger percent LVA was associated with an increased risk of recurrence. In SR, the low-voltage cutoff under which larger LVA was most associated with recurrence was 1.3 mV. Using Advisor HD Grid, the HDWS configuration identified significantly smaller, more precise areas of low voltage. Exceeding low-voltage thresholds defined by Advisor HD Grid in this study may necessitate additional substrate modification beyond PVI.
Supplementary Material
Contributor Information
Zdenek Starek, International Clinical Research Center, St. Anne’s University Hospital Brno, Pekarska 664/53, Brno 60200, Czech Republic; First Department of Internal Medicine/Cardioangiology, St. Anne’s Hospital, Masaryk University, Pekarska 664/53, Brno 60200, Czech Republic.
Andrea Di Cori, Second Division of Cardiovascular Diseases, Cardiac-Thoracic and Vascular Department, New Santa Chiara Hospital, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy.
Timothy R Betts, Department of Cardiology, John Radcliffe Hospital, Oxford, UK.
Gael Clerici, Cardiology Department, Rhythmology Unit, Centre Hospitalier Universitaire de La Reunion, La Reunion, France.
Daniel Gras, Department of Cardiology, Hopital Prive du Confluent, Nantes, France.
Evgeny Lyan, Department of Cardiology, Section of Electrophysiology, Herz-und Gefäßzentrum Bad Bevensen, Bad Bevensen, Germany.
Paolo Della Bella, Arrhythmia Unit and Electrophysiology Laboratories, Ospedale San Raffaele, Milano, Italy.
Jingyun Li, Abbott, Saint Paul, MN, USA.
Benjamin Hack, Abbott, Saint Paul, MN, USA.
Laura Zitella Verbick, Abbott, Saint Paul, MN, USA.
Philipp Sommer, Department for Electrophysiology, Herz-und Diabetes Zentrum NRW, Bad Oeynhausen, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was supported by Abbott.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Interv Card Electrophysiol 2017;50:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nattel S, Masahide H. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014;63:2335–45. [DOI] [PubMed] [Google Scholar]
- 3. Müller-Edenborn B, Chen J, Allgeier J, Didenko M, Moreno-Weidmann Z, Neumann FJet al. Amplified sinus-P-wave reveals localization and extent of left atrial low-voltage substrate: implications for arrhythmia freedom following pulmonary vein isolation. Europace 2020;22:240–9. [DOI] [PubMed] [Google Scholar]
- 4. Ravelli F, Masè M, Cristoforetti A, Avogaro L, D'Amato E, Tessarolo Fet al. Quantitative assessment of transmural fibrosis profile in the human atrium: evidence for a three-dimensional arrhythmic substrate by slice-to-slice histology. Europace 2023;25:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamaguchi T, Otsubo T, Takahashi Y, Nakashima K, Fukui A, Hirota Ket al. Atrial structural remodeling in patients with atrial fibrillation is a diffuse fibrotic process: evidence from high-density voltage mapping and atrial biopsy. J Am Heart Assoc 2022;11:e024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang XH, Li Z, Mao JL, Zang MH, Pu J. Low voltage areas in paroxysmal atrial fibrillation: the prevalence, risk factors and impact on the effectiveness of catheter ablation. Int J Cardiol 2018;269:139–44. [DOI] [PubMed] [Google Scholar]
- 7. Malcolme-Lawes LC, Juli C, Karim R, Bai W, Quest R, Lim PBet al. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: a 2-center study. Heart Rhythm 2013;10:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Arentz T, Cochet H, Müller-Edenborn B, Kim S, Moreno-Weidmann Zet al. Extent and spatial distribution of left atrial arrhythmogenic sites, late gadolinium enhancement at magnetic resonance imaging, and low-voltage areas in patients with persistent atrial fibrillation: comparison of imaging vs. electrical parameters of fibrosis and arrhythmogenesis. Europace 2019;21:1484–93. [DOI] [PubMed] [Google Scholar]
- 9. Caixal G, Alarcón F, Althoff TF, Nuñez-Garcia M, Benito EM, Borràs Ret al. Accuracy of left atrial fibrosis detection with cardiac magnetic resonance: correlation of late gadolinium enhancement with endocardial voltage and conduction velocity. Europace 2021;23:380–8. [DOI] [PubMed] [Google Scholar]
- 10. Sim I, Bishop M, O’Neill M, Williams SE. Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol 2019;56:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blandino A, Bianchi F, Grossi S, Biondi-Zoccai G, Conte MR, Gaido Let al. Left atrial substrate modification targeting low-voltage areas for catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Pacing Clin Electrophysiol 2017;40:199–212. [DOI] [PubMed] [Google Scholar]
- 12. Seewöster T, Kosich F, Sommer P, Bertagnolli L, Hindricks G, Kornej J. Prediction of low-voltage areas using modified APPLE score. Europace 2021;23:575–80. [DOI] [PubMed] [Google Scholar]
- 13. Kosiuk J, Dinov B, Kornej J, Acou W-J, Schönbauer R, Fiedler Let al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm 2015;12:2207–12. [DOI] [PubMed] [Google Scholar]
- 14. Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners Jet al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 2016;9:e002962. [DOI] [PubMed] [Google Scholar]
- 15. Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer Pet al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace 2018;20:1766–75. [DOI] [PubMed] [Google Scholar]
- 16. Kapa S, Desjardins B, Callans DJ, Marchlinski FE, Dixit S. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:1044–52. [DOI] [PubMed] [Google Scholar]
- 17. Vlachos K, Efremidis M, Letsas KP, Bazoukis G, Martin R, Kalafateli Met al. Low-voltage areas detected by high-density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2017;28:1393–402. [DOI] [PubMed] [Google Scholar]
- 18. Yagishita A, Sparano D, Cakulev I, Gimbel JR, Phelan T, Mustafa Het al. Identification and electrophysiological characterization of early left atrial structural remodeling as a predictor for atrial fibrillation recurrence after pulmonary vein isolation. J Cardiovasc Electrophysiol 2017;28:642–50. [DOI] [PubMed] [Google Scholar]
- 19. Lin Y, Yang B, Garcia FC, Ju W, Zhang F, Chen Het al. Comparison of left atrial electrophysiologic abnormalities during sinus rhythm in patients with different type of atrial fibrillation. J Interv Card Electrophysiol 2014;39:57–67. [DOI] [PubMed] [Google Scholar]
- 20. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter Set al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi T, Fukui A, Node K. Bipolar voltage mapping for the evaluation of atrial substrate: can we overcome the challenge of directionality? J Atr Fibrillation 2019;11:2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huemer M, Qaiyumi D, Attanasio P, Parwani A, Pieske B, Blaschke Fet al. Does the extent of left atrial arrhythmogenic substrate depend on the electroanatomical mapping technique: impact of pulmonary vein mapping catheter vs. ablation catheter. Europace 2016;19:1293–301. [DOI] [PubMed] [Google Scholar]
- 23. Saito J, Yamashita K, Numajiri T, Gibo Y, Usumoto S, Narui Set al. Grid-mapping catheters versus PentaRay catheters for left atrial mapping on ensite precision mapping system. J Cardiovasc Electrophysiol 2022;33:1405–11. [DOI] [PubMed] [Google Scholar]
- 24. Jiang R, Beaser AD, Aziz Z, Upadhyay GA, Nayak HM, Tung R. High-density grid catheter for detailed mapping of sinus rhythm and scar-related ventricular tachycardia: comparison with a linear duodecapolar catheter. JACC Clin Electrophysiol 2020;6:311–23. [DOI] [PubMed] [Google Scholar]
- 25. Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto Ket al. Left atrial voltage mapping with a direction-independent grid catheter: comparison with a conventional circular mapping catheter. J Cardiovasc Electrophysiol 2019;30:2834–40. [DOI] [PubMed] [Google Scholar]
- 26. Okubo K, Frontera A, Bisceglia C, Paglino G, Radinovic A, Foppoli Let al. Grid mapping catheter for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2019;12:e007500. [DOI] [PubMed] [Google Scholar]
- 27. Ndrepepa G, Schneider MA, Karch MR, Weber S, Schreieck J, Zrenner Bet al. Impact of atrial fibrillation on the voltage of bipolar signals acquired from the left and right atria. Pacing Clin Electrophysiol 2003;26:862–9. [DOI] [PubMed] [Google Scholar]
- 28. Yagishita A, De Oliveira S, Cakulev I, Gimbel JR, Sparano D, Manyam Het al. Correlation of left atrial voltage distribution between sinus rhythm and atrial fibrillation: identifying structural remodeling by 3-D electroanatomic mapping irrespective of the rhythm. J Cardiovasc Electrophysiol 2016;27:905–12. [DOI] [PubMed] [Google Scholar]
- 29. Hwang J, Park HS, Han S, Lee CH, Kim IC, Cho YKet al. Ablation of persistent atrial fibrillation based on high density voltage mapping and complex fractionated atrial electrograms: a randomized controlled trial. Medicine (Baltimore) 2021;100:e26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nery PB, Alqarawi W, Nair GM, Sadek MM, Redpath CJ, Golian Met al. Catheter ablation of low-voltage areas for persistent atrial fibrillation: procedural outcomes using high-density voltage mapping. Can J Cardiol 2020;36:1956–64. [DOI] [PubMed] [Google Scholar]
- 31. Yang G, Yang B, Wei Y, Zhang F, Ju W, Chen Het al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2016;9:e003382. [DOI] [PubMed] [Google Scholar]
- 32. Huang D, Li JB, Zghaib T, Gucuk Ipek E, Balouch M, Spragg DDet al. The extent of left atrial low-voltage areas included in pulmonary vein isolation is associated with freedom from recurrent atrial arrhythmia. Can J Cardiol 2018;34:73–9. [DOI] [PubMed] [Google Scholar]
- 33. Junarta J, Siddiqui MU, Riley JM, Dikdan SJ, Patel A, Frisch DR. Low-voltage area substrate modification for atrial fibrillation ablation: a systematic review and meta-analysis of clinical trials. Europace 2022;24:1585–98. [DOI] [PubMed] [Google Scholar]
- 34. Yagishita A, Gimbel JR, De Oliveira S, Manyam H, Sparano D, Cakulev Iet al. Long-term outcome of left atrial voltage-guided substrate ablation during atrial fibrillation: a novel adjunctive ablation strategy. J Cardiovasc Electrophysiol 2017;28:147–55. [DOI] [PubMed] [Google Scholar]
- 35. Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto Ket al. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO trial. J Am Heart Assoc 2020;9:e015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher Let al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang G, Zheng L, Jiang C, Fan J, Liu X, Zhan Xet al. Circumferential pulmonary vein isolation plus low-voltage area modification in persistent atrial fibrillation. JACC Clin Electrophysiol 2022;8:882–91. [DOI] [PubMed] [Google Scholar]
- 38. Eichenlaub M, Mueller-Edenborn B, Minners J, Figueras i Ventura RM, Forcada BR, Colomer AVet al. Comparison of various late gadolinium enhancement magnetic resonance imaging methods with high-definition voltage and activation mapping for detection of atrial cardiomyopathy. Europace 2022;24:1102–11. [DOI] [PubMed] [Google Scholar]
- 39. Huo Y, Gaspar T, Schonbauer R, Wojcik M, Fiedler L, Roithinger Fet al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evidence 2022;1:1–10. [DOI] [PubMed] [Google Scholar]
- 40. Uetake S, Maruyama M, Kobayashi N, Arai T, Miyauchi Y. Efficacy of electrical isolation of the left atrial posterior wall depends on the existence of left atrial low-voltage zone in patients with persistent atrial fibrillation. Heart Vessels 2022;37:1757–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.