Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a treatable form of pulmonary hypertension and right heart failure. CTEPH (group 4 pulmonary hypertension) is caused by persistent organized thromboembolic obstruction of the pulmonary arteries from incompletely resolved acute pulmonary embolism. CTEPH also may present without prior VTE history, which can contribute to its underrecognition. The true incidence of CTEPH is unclear, but is estimated to be approximately 3% after acute pulmonary embolism. scintigraphy is the best screening test for CTEPH, with CT scan imaging and other advanced imaging methods now playing a larger role in disease detection and confirmation. Perfusion defects on scintigraphy in the setting of pulmonary hypertension are suggestive of CTEPH, but pulmonary angiography and right heart catheterization are required for confirmation and treatment planning. CTEPH potentially is curative with pulmonary thromboendarterectomy surgery, with mortality rates of approximately 2% at expert centers. Advances in operative techniques are allowing more distal endarterectomies to be performed successfully with favorable outcomes. However, more than one-third of patients may be considered inoperable. Although these patients previously had minimal therapeutic options, effective treatments now are available with pharmacotherapy and balloon pulmonary angioplasty. Diagnosis of CTEPH should be considered in all patients with suspicion of pulmonary hypertension. Treatments for CTEPH have advanced with improvements in outcomes for both operable and inoperable patients. Therapy should be tailored based on multidisciplinary team evaluation to ensure optimal treatment response.
Key Words: balloon pulmonary angioplasty, chronic thromboembolic pulmonary hypertension, pulmonary endarterectomy, pulmonary hypertension, Riociguat
Chronic thromboembolic pulmonary hypertension (CTEPH) is a distinct form of pulmonary hypertension (PH) characterized by unresolved thromboembolic occlusions of the pulmonary arteries.1,2 These chronic obstructions become organized and fibrotic and, along with concomitant remodeling of the pulmonary vasculature, can lead to progressive PH, right heart failure, and death. Since CTEPH was identified nearly 100 years ago, the diagnostic methods and therapeutic foundations of CTEPH have evolved. Advancements in surgical technique have been made, and the roles for balloon pulmonary angioplasty (BPA) and medical therapy have grown.
For this review, a literature search was conducted in PubMed using the following terms: CTEPH, chronic thromboembolic pulmonary hypertension, chronic thromboembolic disease, pulmonary thromboendarterectomy, pulmonary endarterectomy, and balloon pulmonary angioplasty. The lead and senior authors screened the search list for relevance to narrow the list to the references provided. All included references were read in full.
Epidemiology and Pathophysiology
The aftermath of acute pulmonary embolism (PE) includes a spectrum of outcomes that range from full resolution of symptoms with complete restoration of normal perfusion to residual chronic obstruction that contributes to PH and persistent cardiopulmonary limitations. After acute PE, approximately 30% to 50% of patients will show abnormal perfusion scans after 6 months of anticoagulation.3,4 Of those patients, only 10% to 15% will proceed to demonstrate CTEPH.3 Chronic thromboembolic pulmonary disease (CTEPD) without PH is an increasingly recognized entity on the spectrum of possible outcomes after PE and is characterized by the presence of persistent perfusion defects with associated symptoms, but no resting PH.
The true incidence of CTEPH is unknown, and current reported rates after PE range from 0.5% to 9%.5, 6, 7 This wide variability may reflect referral bias, study design, and screening strategies used, but in clinical practice, an incidence of approximately 3% is likely most relevant. The landmark study by Pengo et al,6 which followed up 223 patients prospectively after acute PE for up to 10 years, reported a CTEPH incidence of 3.1%. Similarly, a meta-analysis of 16 studies evaluating the incidence of CTEPH found the rate to be 3.2% in survivors of PE.5 Still, CTEPH likely remains underdiagnosed. An estimated 300,000 cases of acute PE occur annually in the United States.8 Even with conservative estimates of CTEPH, that would lead to approximately 1,500 to 3,000 new CTEPH cases per year, not including in those without a history of prior PE. However, considerably fewer CTEPH cases are being observed in the United States, with only about 0.9 pulmonary endarterectomies performed annually per 1 million adults.9
Although CTEPH is triggered by occlusion of the proximal larger pulmonary arteries, it is not the sole mechanism of PH. A component of small vessel disease or microvasculopathy also exists in CTEPH. Moser and Bloor10 initially described this in lung tissue of patients with CTEPH. They observed vascular lesions such as intimal thickening and remodeling, intimal fibrosis, and plexiform lesions, similar to those seen in patients with idiopathic pulmonary arterial hypertension. The proposed mechanism for this is the redistribution of blood flow away from the obstructed vascular beds toward open, nonoccluded vasculature, resulting in increased flow and endothelial shear stress in those areas. However, this vasculopathy was observed in both the obstructed and unobstructed vascular beds. Further studies identified anastomoses between the systemic and pulmonary circulation via hypertrophied bronchial arteries and vasa vasorum, which now are thought to be key in the development of arteriopathy distal to obstructed vasculature.11 Although these collateral vessels help to maintain perfusion to lung tissue distal to thrombotic obstructions, the exposure of the pulmonary circulation to the high pressures of the systemic circulation can induce vascular remodeling, leading to arteriopathy.
Risk Factors
Some characteristics of the original PE are associated with the development of CTEPH. Most significantly, unprovoked PE, a diagnostic delay of > 2 weeks, and right ventricle (RV) dysfunction at time of PE were found to be independent predictors of CTEPH.12 If the PE represented a recurrence, it also was associated with an increased risk of CTEPH, with one study showing that more than one-half of patients with CTEPH had a history of recurrent VTE.6,13 However, up to 25% of patients with CTEPH do not report a history of PE.14
Also, certain medical conditions are associated with a greater risk of CTEPH development. Although VTE history is a risk factor for CTEPH, most of the inherited thrombophilias that increase acute VTE risk are not associated with CTEPH.15 The notable exception is the presence of antiphospholipid antibodies or lupus anticoagulant, which is associated with significantly higher odds of CTEPH.13 One study demonstrated that approximately 20% of patients with CTEPH demonstrated the presence of antiphospholipid antibodies.15 Elevated factor VIII and von Willebrand factor, abnormal fibrinogen variants, and non-O blood type group also have been associated with CTEPH.16 Additionally, patients with CTEPH were more likely to have malignancy, splenectomy, and hypothyroidism.13,17 Patients with indwelling catheters or ports, infected intravascular devices, and ventriculoatrial shunts also are at higher risk of CTEPH development.13
Evaluation and Diagnosis of Suspected Chronic Thromboembolic Disease
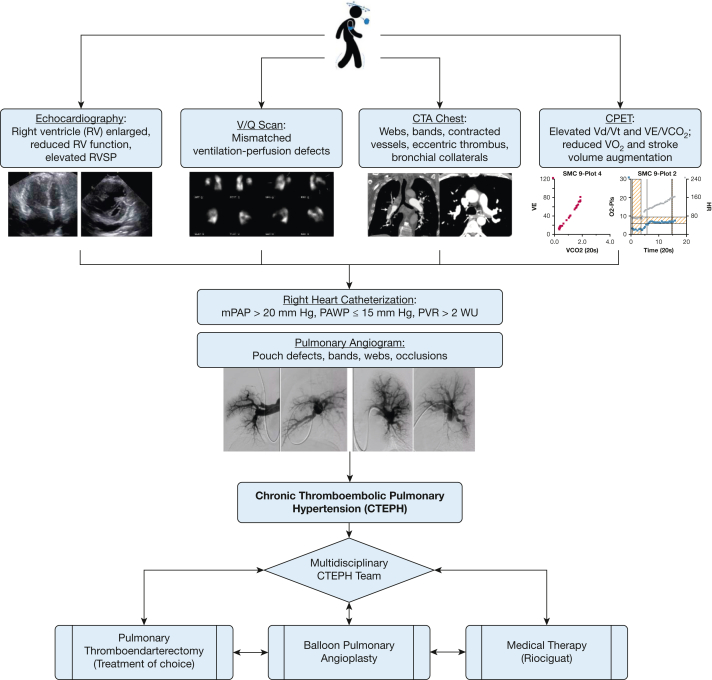
CTEPH is diagnosed when chronic thromboembolism is present in the pulmonary arteries in the setting of precapillary PH, which currently is defined as mean pulmonary artery pressure of > 20 mm Hg, pulmonary artery wedge pressure of ≤ 15 mm Hg, and pulmonary vascular resistance of > 2 Wood units (Fig 1).18 The first step in diagnosing CTEPH is considering the diagnosis in patients being evaluated for PH. The acronym SCAR—for suspect, confirm, and assess risk—has been suggested as a diagnostic tool for CTEPH and highlights that a high index of suspicion is required to diagnose CTEPH.19 In both the US and international CTEPH registries, the median time from symptom onset to diagnosis was 10 and 14 months, respectively.14,20 Delays in diagnosis negatively impact CTEPH prognosis, with longer delays associated with worse mortality.21
Figure 1.
Flowchart showing diagnostic and treatment process for CTEPH. Symptoms and signs often are nonspecific and can include dyspnea on exertion, lightheadedness, palpitations, and lower extremity edema. Echocardiography, scintigraphy, CTPA of the chest, and CPET may demonstrate abnormal findings suggestive of CTEPH. Right heart catheterization is required to confirm and determine severity of pulmonary hypertension (PH), and pulmonary angiography can confirm the pulmonary vasculature features of chronic thromboembolic disease. After CTEPH is confirmed, treatment decisions are individualized and require a multidisciplinary team consisting of a PTE surgeon, BPA specialist, PH provider, and chest radiologist with expertise in CTEPH. BPA = balloon pulmonary angioplasty; CTA = CT angiography; CTEPH = chronic thromboembolic pulmonary hypertension; CTPA = CT pulmonary angiography; mPAP = mean pulmonary artery pressure; PAWP = pulmonary artery wedge pressure; PTE = pulmonary thromboendarterectomy; PVR = pulmonary vascular resistance; RV = right ventricle; RVSP = right ventricular systolic pressure; Vd/Vt = ventilatory dead space; VE/VCO2 = ratio of minute ventilation to carbon dioxide; WU = Wood unit.
Symptoms often are nonspecific and can be seen in many other cardiopulmonary diseases. Most patients will report exertional dyspnea and progressive exercise intolerance. As RV dysfunction ensues, other signs of right heart failure, such as abdominal distention, lower extremity swelling, chest pressure, exertional lightheadedness, and syncope, can develop. Hemoptysis also can occur in CTEPH, likely related to the hypertrophied bronchial artery collateral circulation. Similarly, the physical examination findings can evolve as the disease progresses. Pulmonary flow murmurs, caused by turbulent blood flow through partially obstructed pulmonary arteries, can be heard in approximately 30% of patients.22
Chest radiography often is unrevealing, but may show enlarged central pulmonary arteries. Pulmonary function tests can show normal results in CTEPH, but mild restriction, reduced diffusion capacity, or both may be present. Cardiopulmonary exercise testing also can provide insight into the causes of dyspnea. Patients with pulmonary vascular disease have a unique signature on cardiopulmonary exercise testing that can include reduced exercise capacity, stroke volume limitation, increased dead space ventilation, and ventilatory inefficiency.23 The role of cardiopulmonary exercise testing and exercise hemodynamics in the workup of CTEPD without PH may be growing.24
Because of widespread availability of transthoracic echocardiography, this commonly provides the initial evidence of possible PH resulting from objective assessments of RV size and function, as well as estimated right ventricular systolic pressure. However, echocardiography both can overestimate and underestimate right ventricular systolic pressure, therefore missing PH in up to 30% of patients; furthermore, the echocardiogram usually will show normal findings at rest in cases of CTEPD without PH.25 Still, echocardiography remains valuable to evaluate RV function, left-sided function and valvular structures, and presence of intracardiac shunts.
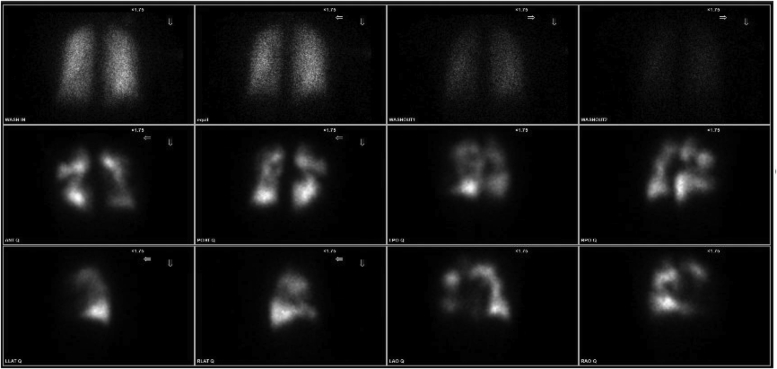
scintigraphy remains the imaging test of choice for screening for chronic thromboembolic disease.26 Although single-photon emission CT scans can be more sensitive than planar scans in detecting perfusion defects, either technique can be used for initial screening. The presence of persistent mismatched perfusion defects is a key finding in CTEPH (Fig 2). Normal scan results exclude CTEPH with a sensitivity of 96% to 97% and specificity of 90% to 95%.27 However, scintigraphy can underestimate the degree of pulmonary vascular obstruction in nonocclusive disease.28 Therefore, even the presence of a single segmental perfusion defect should alert practitioners to the possibility of CTEPH. Abnormal scan results also can be seen in other disease processes, such as acute PE, pulmonary artery tumors, pulmonary vasculitis, and pulmonary vein stenosis.29
Figure 2.
A-L, scans showing mismatched defects in patient with pulmonary hypertension, consistent with chronic thromboembolic disease. Top row displays 133Xe ventilation images. Bottom two rows display 99mTc macroaggregated albumin planar perfusion images.
Although CT pulmonary angiography (CTPA) is the test of choice for acute PE, it should not be the initial test for the evaluation of CTEPH. Studies have shown sensitivity rates of CTPA in diagnosing CTEPH ranging from 51% to 92%; this wide variability likely reflects provider recognition of CTEPH findings, rather than a limitation of the imaging method itself.27,30 CTPA can reveal the subtle findings suggestive of CTEPH such as intravascular webs, early vessel tapering, hypertrophied bronchial collateral circulation, and eccentric thrombus. However, these can be misinterpreted as acute PE or can be overlooked by physicians inexperienced with CTEPH.31
CTPA offers benefits of being able to assess the lung parenchyma and to evaluate for potential mimicking conditions.26 Dual-energy CT scan examinations have emerged as a promising tool because of their capability of providing evaluations of perfusion, vasculature morphologic features, and lung parenchyma simultaneously.26 The qualitative assessment of perfusion has a sensitivity for CTEPH comparable with that of scans.32 MRI also has been studied in the evaluation of CTEPH and cardiac function in PH, but is not widely available.1
Confirmation of CTEPH requires right heart catheterization and, traditionally, catheter-based pulmonary angiography.26 Right heart catheterization is required to confirm precapillary PH and can assess disease severity further, whereas pulmonary angiography is performed to obtain detailed visualization of the vasculature. Angiographic findings consistent with CTEPH include complete occlusions, so-called pouch defects, webs or bands, irregular vessel wall contour, and abrupt vessel narrowing or disappearance. Although pulmonary angiography has been referred to as the imaging gold standard in CTEPH diagnosis, it may not be required in all patients. In select patients, a high-quality CTPA with or without perfusion mapping may be sufficient for both diagnosis and evaluation of operability.
Management of CTEPH
All patients with CTEPH should undergo a multidisciplinary team evaluation to determine the optimal and individualized treatment (Fig 1).2 Treatment decisions can be subjective, and patients with CTEPH may have comorbidities that require collaborative management. The multidisciplinary team should include a pulmonary thromboendarterectomy (PTE) surgeon, BPA specialist, PH specialist, and radiologist experienced with CTEPH. The following three sections discuss more details within each treatment method.
Pulmonary Thromboendarterectomy
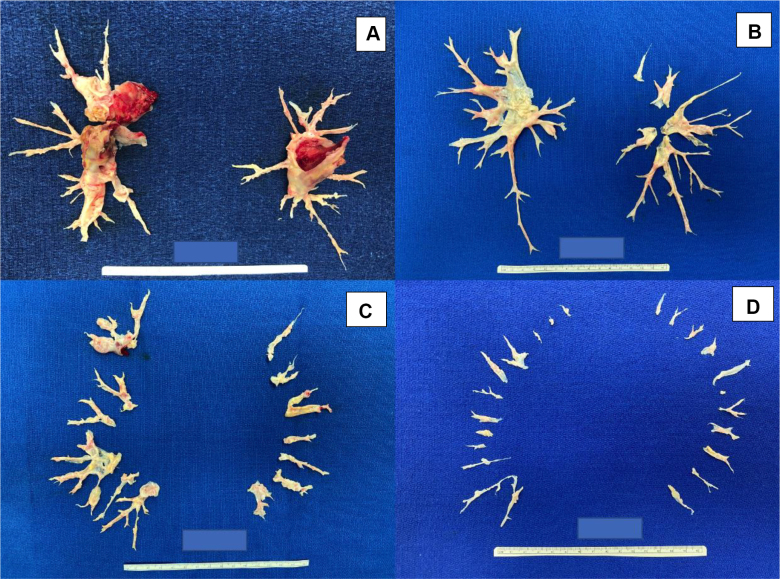
PTE surgery, also known as pulmonary endarterectomy, remains the treatment of choice for operable CTEPH. PTE offers a potential cure for CTEPH with perioperative mortality rates of approximately 2% at select experienced surgical centers.33,34 Operability assessment often is subjective and dependent on surgical and center expertise. In the US CTEPH registry, 87% of enrolled patients were deemed to be operable. However, when this was broken down by institutions, patients enrolled at the highest PTE volume center underwent surgery more commonly than at the remaining 29 centers (92% vs 69%).20 Advancements in surgical instruments and operative techniques have been made such that more distal, segmental, and subsegmental endarterectomy is being performed successfully at experienced centers.33,35 This is a more technically challenging surgery, but at expert centers, patients experience the same benefits as those with proximal disease.36 The surgical classification of disease was modified in 2016 to the University of California, San Diego, level classification, from the previous Jamieson type of clot classification. The current classification is based solely on the location of disease, with higher levels indicating more challenging surgery. It also delineates better between segmental and subsegmental disease (Fig 3).35,37 Furthermore, PTE surgery remains possible even for patients with cold agglutinins disease, sickle cell anemia, and heparin-induced thrombocytopenia but should be performed only at experienced CTEPH centers because of the complex preoperative planning required.38 Therefore, if a patient is deemed inoperable, a second opinion may be necessary because operability varies among institutions. Contrarily, several conditions can mimic CTEPH, including pulmonary artery sarcomas, vasculitis, fibrosing mediastinitis, and in situ thrombosis.29 Certain imaging findings may help to differentiate these from CTEPH, but evaluation should be carried out at an experienced center because an alternative diagnosis may vastly change management.
Figure 3.
A-D, Photographs showing pulmonary thromboendarterectomy specimens and University of California, San Diego, level classification based on level of disease: level I disease indicates obstructive material in the main pulmonary arteries (A), level II disease starts at the lobar branches (B), level III disease starts at the segmental branches (C), and level IV disease is in the subsegmental branches primarily (D). Level 0 means no disease.
The technical details of the surgery are well documented, but briefly, patients undergo median sternotomy, are administered full cardiopulmonary bypass, and then their temperature is cooled to < 20 °C.37,39 After deep hypothermia is achieved, the aorta is cross-clamped and cold cardioplegia is administered. Brief periods (≤ 20 min) of circulatory arrest are required to allow for complete surgical visualization. The pulmonary artery then is incised, and endarterectomy is begun by identifying the plane between the intima and media. The dissection is continued distally until complete endarterectomy is achieved to remove the chronic obstructive material. This entire process is repeated for the contralateral pulmonary artery. In select patients and with advanced surgeon training, minimally invasive endarterectomy may be feasible.9
In addition to the usual potential complications after cardiac surgery, specific complications may arise after PTE surgery. Reperfusion pulmonary edema is a phenomenon in which newly endarterectomized and perfused areas of lung develop a high permeability edema.40 Severe cases can cause airway hemorrhage, which also can occur because of trauma during surgical dissection, friability of endarterectomized vessels, and bleeding from systemic-to-pulmonary collateral arteries. Both reperfusion pulmonary edema and airway hemorrhage are associated with increased mechanical ventilation days, length of stay, and mortality.41 No specific therapies have proven to be effective, so treatment remains supportive, including use of venovenous extracorporeal membrane oxygenation in patients with severe disease.42 Residual PH, although not defined precisely, also is associated with high perioperative mortality.43 The role and timing of rescue pharmacotherapy, BPA, or both in patients with severe disease are not defined.
Patients with CTEPH who undergo PTE surgery have improved long-term outcomes in quality of life, functional class, and exercise capacity compared with those who do not undergo surgery.44,45 Although these observations come from nonrandomized studies with confounding variables, the results remain supportive of PTE surgery. Patients who undergo surgery also were less likely to require oxygen, diuretics, or PH-targeted therapies.20 At the 1-year follow-up, patients who underwent PTE showed improved survival compared with those who did not undergo surgery; this mortality benefit was durable up to 3 years from surgery.20,44,46
Balloon Pulmonary Angioplasty
Although PTE surgery is the treatment of choice for all operable patients, up to 36% of patients are considered inoperable because of distal disease, comorbidities, or various other reasons.14,44 Although these patients previously had limited therapeutic options, BPA has emerged as an established treatment for inoperable CTEPH.
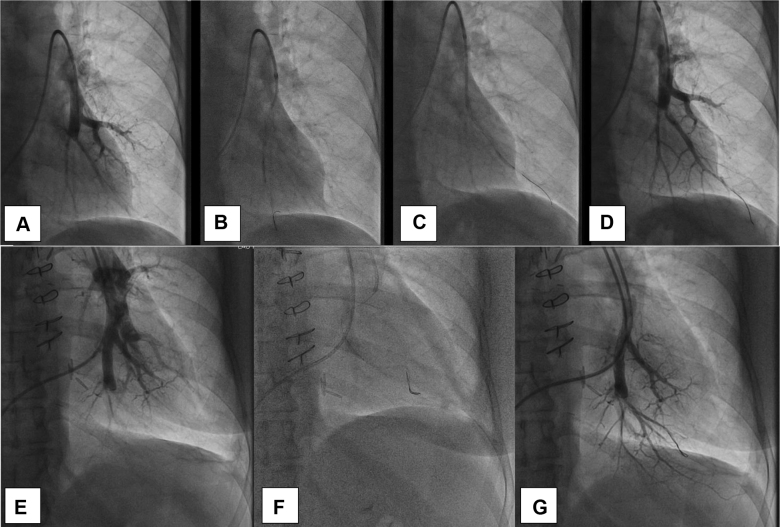
BPA is a percutaneous catheter-based intervention with vascular access obtained via the internal jugular or femoral vein. A sheath is inserted into the main pulmonary artery, through which a guide catheter then is used for selective pulmonary angiography to identify target lesions.47 A guidewire is passed across the target lesion, and balloons are used to dilate the selected area and to restore blood flow (Fig 4). Five defined categories of thromboembolic lesions exist: (1) ring-like stenosis, (2) webs, (3) subtotal occlusion, (4) total occlusion, and (5) tortuous lesions.48 Lesions that are readily amenable to BPA include webs and ring-like stenoses; subtotal and complete occlusions are more difficult to revascularize via BPA and can be performed in select patients, but with potentially higher complication rates.49,50
Figure 4.
A-G, Selective pulmonary angiograms obtained from two different patients (A-D and E-G) during balloon pulmonary angioplasty. A, E, Selective pulmonary angiograms showing occlusion of the posterior left lower lobe pulmonary artery before balloon angioplasty. B, C, F, Wire passed through the occlusion and vessels treated with balloon inflation. D, G, Selective angiograms obtained after balloon angioplasty showing improved blood flow in the left lower lobe.
The initial experiences with BPA were beset by excessively high complication rates, including vascular injury in 61% of patients, with mechanical ventilation required in 17% of patients and a mortality of 5.5% at 30 days.51 Given the high complication rates, interest in BPA waned for years until its resurgence in Japan.52,53 The Japanese centers refined the BPA technique and subsequently reported high rates of efficacy with an overall complication rate of approximately 36% and a mortality rate of 3.9%; more recent reports note a complication rate of 9% to 11% and a mortality rate of 1.8% to 2.2%.52,54,55 The most frequently reported complication resulting from BPA is vascular injury.56 The various types of pulmonary artery injury resulting from BPA can include wire perforation, high-pressure perfusion injury, pulmonary artery rupture, and pulmonary artery dissection.57 Possible management options include reversal of anticoagulation, balloon tamponade, and supportive therapy, including extracorporeal membrane oxygenation support in patients with severe cases. Similar to PTE, a learning curve accompanies BPA, as shown in the French experience, which reported improved BPA safety and efficacy with experience.55
Multiple BPA studies have demonstrated improvements in hemodynamics, functional class, and quality of life.52,54,55 Given the relatively recent renaissance of BPA, few studies have evaluated longer-term outcomes, but the available data support long-lasting effects (≥ 3 years).52,58 With BPA now an established treatment option, the challenge has moved to selecting patients appropriate for BPA. No formal criteria exist for BPA patient selection. At a high-volume PTE center, most patients selected for BPA had surgically inaccessible peripheral-appearing disease and risk factors associated with distal disease.59 However, decisions may vary based on center-specific experience and patient-specific factors.
Medical Therapy
The backbone in medical therapy for CTEPH is lifelong anticoagulation to protect from new thrombi. Traditionally, warfarin, a vitamin K antagonist, has been the anticoagulant of choice. However, direct oral anticoagulants (DOACs) increasingly are being used.60 A recent study evaluating the use of DOACs vs non-DOACs before PTE found that patients who were receiving DOACs were twice as likely to have associated acute or subacute thrombi at the time of surgery.61 Similarly, when use of DOAC vs vitamin K antagonist was evaluated after PTE, higher recurrent VTE rates in the DOAC group were noted.62 Although large prospective trials are still needed, these retrospective analyses raise concern about the efficacy of DOACs in CTEPH.
Multiple studies have evaluated use of PH medical therapies in CTEPH (Table 1).63, 64, 65, 66, 67 Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase Stimulator Trial 1 (CHEST-1) was the first positive study, leading to approval of riociguat for the treatment of inoperable or persistent or recurrent CTEPH after PTE.67 This study was randomized, placebo controlled, multicenter, and had an operability adjudication process that had to be satisfied before patient enrollment. Despite riociguat being the only US Food and Drug Administration-approved therapy for select patients with CTEPH, off-label use of this and other PH therapies occurs frequently in patients with CTEPH, regardless of operability.68 In the original international CTEPH registry, which was conducted before the approval of riociguat, 38% of patients were receiving PH therapies at the time of enrollment.14 In the more recent international and US CTEPH registries, 36% and 44% of patients, respectively, were receiving PH therapies at the time of enrollment, with only 37% and 65% of those, respectively, being riociguat.20,46 Although medical therapy is used frequently, no clear benefit exists for pretreatment of patients with CTEPH before surgery, and this was associated with a significant delay in referral to PTE.68
Table 1.
Selected Randomized Controlled Studies of Pulmonary Hypertension Therapeutics in CTEPH
| Study | Intervention | No. of Patients | No. of Weeks | Primary Outcome | Other Outcomes and Conclusions |
|---|---|---|---|---|---|
| BENEFiT63 | Bosentan (vs placebo) | 157 | 16 | 6MWD (+2.9 m vs +0.8 m; P = .55); PVR (–146 dynes vs +30 dynes; P < .0001) | No significant difference in FC or time to clinical worsening. Operability was adjudicated centrally retrospectively. |
| CHEST-167 | Riociguat (vs placebo) | 261 | 16 | 6MWD (+39 m vs –6 m; P < .001) | Improved PVR (–226 dyne vs +23 dyne; P < .001), FC, mPAP, NT-proBNP with riociguat. Prospective operability adjudication. First pharmacotherapy with indication for CTEPH, approved worldwide. |
| MERIT-165 | Macitentan (vs placebo) | 80 | 16 | PVR (–206 dynes vs –86 dynes; P = .04) | Improved 6MWD (+35 m vs +1 m; P = .03), NT-proBNP, cardiac output with macitentan. Background therapy (PDE5i, oral or inhaled prostanoids) in 61%. First study with combination therapy for CTEPH (but notably riociguat not allowed). |
| CTREPH64 | Subcutaneous treprostinil (30 ng/kg/min vs 3 ng/kg/min) | 105 | 24 | 6MWD (+45 m vs +4 m; P = .002) | Improved PVR (–214 dyn vs +73 dyn; P < .0001), mPAP, cardiac output, NT-proBNP with higher-dose treprostinil. Technically operable patients included. Approved in Europe. |
| Ogo et al66 | Selexipag (vs placebo) | 78 | 20 | PVR (–98 dynes vs –5 dynes; P = .006) | Improved cardiac index and Borg dyspnea scores with selexipag. No significant difference in mPAP, 6MWD, or FC. 62% receiving background riociguat and 53% previously received BPA. Approved in Japan. |
6MWD = 6-min walk distance; BPA = balloon pulmonary angioplasty; BENEFiT = Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension; CHEST-1 = A Study to Evaluate Efficacy and Safety of Oral BAY63-2521 in Patients With CTEPH. (CHEST-1); CTEPH = chronic thromboembolic pulmonary hypertension; CTREPH = Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension; FC = functional class; MERIT-1 = Clinical Study to Assess the Efficacy, Safety and Tolerability of Macitentan in Subjects With Inoperable Chronic Thromboembolic Pulmonary Hypertension; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro–brain natriuretic peptide; PDE5 = phosphodiesterase type 5 inhibitor; PVR = pulmonary vascular resistance.
Medical therapy in CTEPH remains an area with active research and clinical trials. The Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH) study led to the approval of subcutaneous treprostinil in select European countries.64 A small Japanese study with selexipag led to its approval in Japan for inoperable or for persistent or recurrent CTEPH after PTE.66 However, the Study to Find Out if Selexipag is Effective and Safe in Patients With Chronic Thromboembolic Pulmonary Hypertension When the Disease is Inoperable or Persistent/Recurrent After Surgery and/or Interventional Treatment (SELECT)69 evaluating add-on therapy with selexipag was terminated early because of lack of efficacy at an interim analysis. A Study to Evaluate Efficacy and Safety of Macitentan 75 mg in Inoperable or Persistent/Recurrent Chronic Thromboembolic Pulmonary Hypertension (MACiTEPH)70 is an ongoing phase 3 study of macitentan (with higher dose of 75 mg/d) after the positive phase 2 Clinical Study to Assess the Efficacy, Safety and Tolerability of Macitentan in Subjects With Inoperable Chronic Thromboembolic Pulmonary Hypertension (MERIT-1).65
Management of CTEPD Without PH at Rest
Patients with CTEPD without PH are symptomatic despite not having PH at rest. The mechanism of dyspnea and poor exercise tolerance may be heterogenous, including elevation of ventilatory dead space because of pulmonary arterial obstruction, elevation of pulmonary pressures with exercise, right ventricular dysfunction with exercise, or a combination thereof.24,71,72 Patients with CTEPD without PH have been treated with both PTE and BPA.73,74 However, the risk to benefit ratio of such interventions is not clear because the natural history of CTEPD without PH remains unknown.1 Although CTEPD without PH is likely more prevalent than CTEPH, more studies are needed to define further the natural course of this disease process, as well as optimal management strategies.
Multimodality Treatment
Although PTE, BPA, and medical therapy are discussed as unique entities, the reality is that treatment of CTEPH now often involves a multimodal approach. As more distal endarterectomies are being performed successfully, operability has become even more subjective, with access to expert surgical vs BPA centers influencing choice of mechanical treatment. Recognizing the potential for complementary methods to tackle the mechanical component of CTEPH, combination PTE and BPA surgeries have been performed in selected patients with high-risk hemodynamics with unilateral operable and contralateral inoperable disease.75 The rationale for a hybrid procedure is to achieve maximal reduction in RV afterload in those with severe hemodynamics and asymmetric disease. All patients in the case series showed pulmonary vascular resistance of between 850 and 1,630 dynes/s/cm5 and underwent successful unilateral endarterectomy with contralateral angioplasty during the rewarming phase of cardiopulmonary bypass. Reports also have been made of a stepwise approach for asymmetric disease, with upfront unilateral BPA followed by PTE in the contralateral lung.76 However, the series from Poch et al59 reported nearly 1 of 5 patients undergoing BPA first having undergone PTE surgery to address the surgical component before treating the more distal residual disease with BPA in combination with medical therapy.
Availability of effective PH therapies and advances with BPA have benefited patients with inoperable CTEPH.77 The recently published Riociguat Versus Balloon Pulmonary Angioplasty in Non-operable Chronic thromboEmbolic Pulmonary Hypertension (RACE) trial randomized inoperable patients with CTEPH either to first-line BPA or riociguat, with the option to cross over if not meeting predefined goals by 26 weeks.78 Patients treated with first-line BPA showed a significant reduction in pulmonary vascular resistance compared with those treated initially with riociguat, but experienced a higher rate of adverse events related to BPA. By the end of 52 weeks, both groups showed similar improvements in hemodynamics. This landmark trial highlights the additive and incremental benefits of multimodal therapy in CTEPH and the concept of a personalized approach such as pretreatment with riociguat in patients with significant PH before BPA. Although an appreciation of the complementary role of medical therapy is evolving, questions remain. Upfront combination therapy is the current dogma for pulmonary arterial hypertension treatment, but whether this is preferable to riociguat monotherapy for inoperable CTEPH before BPA is unclear. It is hoped that the Initial Dual Oral Combination Therapy Versus Standard-of-care Initial Oral Monotherapy Prior to Balloon Pulmonary Angioplasty in Patients With Inoperable Chronic Thromboembolic Pulmonary Hypertension (IMPACT-CTEPH) trial79 will provide additional insight.
Unanswered Questions
In this era of multimodal CTEPH treatment, new questions continue to arise as the synergy among PTE, BPA, and medical therapy continues to evolve. Furthermore, despite advances in the treatment of CTEPH, many gaps remain in our understanding, with numerous unanswered questions and challenges (Table 2). From basic understanding of the link between acute PE and CTEPH to more precise definitions of treatment goals, the field has more work ahead. For the time being, the importance of expert referral and a multimodal approach to patient assessment and CTEPH treatment cannot be overemphasized.
Table 2.
Clinical Gaps in Chronic Thromboembolic Pulmonary Hypertension
| Topic | Problem | Potential Approach |
|---|---|---|
| CTEPD without PH |
|
|
| Pulmonary embolism intervention |
|
|
| Operability |
|
|
| Follow-up after intervention |
|
|
| Operable CTEPH |
|
|
| Inoperable CTEPH |
|
|
BPA = balloon pulmonary angioplasty; CTEPH = chronic thromboembolic pulmonary hypertension; MDT = multidisciplinary team; PH = pulmonary hypertension; PTE = pulmonary thromboendarterectomy.
Conclusions
CTEPH is a unique form of PH (group 4 PH) characterized by unresolved thrombotic occlusions combined with often concomitant and a complex small vessel component leading to PH. The current approach to evaluation and treatment requires expertise with the multidisciplinary team working together to provide optimal and potential combination treatment strategies. Follow-up of these patients remains critical and an area in need of careful attention going forward as the treatment options continue to evolve.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: M. M. M. is a consultant to Bayer, Janssen, and Wexler. N. H. K. is a consultant to Bayer, Janssen, Merck, United Therapeutics, Polarean, Pulnovo; a member of the speaker’s bureau of Bayer and Janssen; and receives research support from Altavant, Gossamer Bio, and the International CTEPH Association. None declared (J. Y., E. M.).
References
- 1.Delcroix M., Torbicki A., Gopalan D., et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57(6) doi: 10.1183/13993003.02828-2020. [DOI] [PubMed] [Google Scholar]
- 2.Kim N.H., Delcroix M., Jais X., et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01915-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez O., Helley D., Couchon S., et al. Perfusion defects after pulmonary embolism: risk factors and clinical significance. J Thromb Haemost. 2010;8(6):1248–1255. doi: 10.1111/j.1538-7836.2010.03844.x. [DOI] [PubMed] [Google Scholar]
- 4.Pesavento R., Filippi L., Palla A., et al. Impact of residual pulmonary obstruction on the long-term outcome of patients with pulmonary embolism. Eur Respir J. 2017;49(5) doi: 10.1183/13993003.01980-2016. [DOI] [PubMed] [Google Scholar]
- 5.Ende-Verhaar Y.M., Cannegieter S.C., Noordegraaf A.V., et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.01792-2016. [DOI] [PubMed] [Google Scholar]
- 6.Pengo V., Lensing A.W.A., Prins M.H., et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 7.Valerio L., Mavromanoli A.C., Barco S., et al. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study. Eur Heart J. 2022;43(36):3387–3398. doi: 10.1093/eurheartj/ehac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:E139–E596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 9.Madani M.M. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: state-of-the-art 2020. Pulm Circ. 2021;11(2) doi: 10.1177/20458940211007372. 20458940211007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moser K.M., Bloor C.M. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103(3):685–692. doi: 10.1378/chest.103.3.685. [DOI] [PubMed] [Google Scholar]
- 11.Dorfmüller P., Günther S., Ghigna M.R., et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J. 2014;44(5):1275–1288. doi: 10.1183/09031936.00169113. [DOI] [PubMed] [Google Scholar]
- 12.Klok F.A., Dzikowska-Diduch O., Kostrubiec M., et al. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J Thromb Haemost. 2016;14:121–128. doi: 10.1111/jth.13175. [DOI] [PubMed] [Google Scholar]
- 13.Bonderman D., Wilkens H., Wakounig S., et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33(2):325–331. doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 14.Pepke-Zaba J., Delcroix M., Lang I., et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 15.Wolf M., Boyer-Neumann C., Parent F., et al. Thrombotic risk factors in pulmonary hypertension. Eur Respir J. 2000;15(2):395–399. doi: 10.1034/j.1399-3003.2000.15b28.x. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes T., Auger W., Fedullo P. Epidemiology and risk factors for chronic thromboembolic pulmonary hypertension. Thromb Res. 2018;164:145–149. doi: 10.1016/j.thromres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Bonderman D., Jakowitsch J., Adlbrecht C., et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2005;93(3):512–516. doi: 10.1160/TH04-10-0657. [DOI] [PubMed] [Google Scholar]
- 18.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022;10 doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 19.Kim N.H., Delcroix M., Jenkins D.P., et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Kerr K.M., Elliott C.G., Chin K., et al. Results from the United States Chronic Thromboembolic Pulmonary Hypertension Registry: enrollment characteristics and 1-year follow-up. Chest. 2021;160(5):1822–1831. doi: 10.1016/j.chest.2021.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klok F.A., Barco S., Konstantinides S.V., et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH Registry. Eur Respir J. 2018;52(6):1801687. doi: 10.1183/13993003.01687-2018. [DOI] [PubMed] [Google Scholar]
- 22.Keiler E., Kerr K.M., Kim N.H., Poch D.S., Papamatheakis D.G., Fernandes T.M. Pulmonary artery flow murmurs in patients with operable chronic thromboembolic pulmonary hypertension are associated with hemodynamics and level of disease. Am Thorac Soc Int Conf Meet Abstr. 2020:A2039. [Google Scholar]
- 23.Weatherald J., Farina S., Bruno N., Laveneziana P. Cardiopulmonary exercise testing in pulmonary hypertension. Ann Am Thorac Soc. 2017;14:S84–S92. doi: 10.1513/AnnalsATS.201610-788FR. [DOI] [PubMed] [Google Scholar]
- 24.McGuire W.C., Alotaibi M., Morris T.A., Kim N.H., Fernandes T.M. Chronic thromboembolic disease: epidemiology, assessment with invasive cardiopulmonary exercise testing, and options for management. Struct Hear. 2021;5(2):120–127. [Google Scholar]
- 25.Augustine D.X., Coates-Bradshaw L.D., Willis J., et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018;5(3):G11–G24. doi: 10.1530/ERP-17-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopalan D., Delcroix M., Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160108. doi: 10.1183/16000617.0108-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tunariu N., Gibbs S.J.R., Win Z., et al. Ventilation–perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48(5):680–684. doi: 10.2967/jnumed.106.039438. [DOI] [PubMed] [Google Scholar]
- 28.Ryan K.L., Fedullo P.F., Davis G.B., Vasquez T.E., Moser K.M. Perfusion scan findings understate the severity of angiographic and hemodynamic compromise in chronic thromboembolic pulmonary hypertension. Chest. 1988;93(6):1180–1185. doi: 10.1378/chest.93.6.1180. [DOI] [PubMed] [Google Scholar]
- 29.Narechania S., Renapurkar R., Heresi G.A. Mimickers of chronic thromboembolic pulmonary hypertension on imaging tests: a review. Pulm Circ. 2020;10(1) doi: 10.1177/2045894019882620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J., Fang W., Lv B., et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun. 2012;33(5):459–463. doi: 10.1097/MNM.0b013e32835085d9. [DOI] [PubMed] [Google Scholar]
- 31.Rogberg A.N., Gopalan D., Westerlund E., Lindholm P. Do radiologists detect chronic thromboembolic disease on computed tomography? Acta Radiol. 2019;60(11):1576–1583. doi: 10.1177/0284185119836232. [DOI] [PubMed] [Google Scholar]
- 32.Masy M., Giordano J., Petyt G., et al. Dual-energy CT (DECT) lung perfusion in pulmonary hypertension: concordance rate with V/Q scintigraphy in diagnosing chronic thromboembolic pulmonary hypertension (CTEPH) Eur Radiol. 2018;28(12):5100–5110. doi: 10.1007/s00330-018-5467-2. [DOI] [PubMed] [Google Scholar]
- 33.Madani M.M., Auger W.R., Pretorius V., et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94(1):97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins D.P., Tsui S.S., Taghavi J., Kaul P., Ali J., Ng C. Pulmonary thromboendarterectomy—the Royal Papworth experience. Ann Cardiothorac Surg. 2022;11(2):128–132. doi: 10.21037/acs-2021-pte-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madani M.M. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016;12(4):213–218. doi: 10.14797/mdcj-12-4-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Armini A.M., Morsolini M., Mattiucci G., et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148:1005–1012. doi: 10.1016/j.jtcvs.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 37.Jamieson S.W., Kapelanski D.P. Pulmonary endarterectomy. Curr Probl Surg. 2000;37(3):165–252. doi: 10.1016/s0011-3840(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 38.Gernhofer Y.K., Banks D.A., Golts E., Pretorius V. Novel use of cangrelor with heparin during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia who require cardiovascular surgery: a case series. Semin Thorac Cardiovasc Surg. 2020;32(4):763–769. doi: 10.1053/j.semtcvs.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Madani M.M., Jamieson S.W. Technical advances of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Semin Thorac Cardiovasc Surg. 2006;18(3):243–249. doi: 10.1053/j.semtcvs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Levinson R.M., Shure D., Moser K.M. Reperfusion pulmonary edema after pulmonary artery thromboendarterectomy. Am Rev Respir Dis. 1986;134(6):1241–1245. doi: 10.1164/arrd.1986.134.6.1241. [DOI] [PubMed] [Google Scholar]
- 41.Bates D.M., Fernandes T.M., Duwe B.V., et al. Efficacy of a low-tidal volume ventilation strategy to prevent reperfusion lung injury after pulmonary thromboendarterectomy. Ann Am Thorac Soc. 2015;12(10):1520–1527. doi: 10.1513/AnnalsATS.201503-142OC. [DOI] [PubMed] [Google Scholar]
- 42.Thistlethwaite P.A., Madani M.M., Kemp A.D., Hartley M., Auger W.R., Jamieson S.W. Venovenous extracorporeal life support after pulmonary endarterectomy: indications, techniques, and outcomes. Ann Thorac Surg. 2006;82(6):2139–2145. doi: 10.1016/j.athoracsur.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Mayer E., Jenkins D., Lindner J., et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Delcroix M., Lang I., Pepke-Zaba J., et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133(9):859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 45.Quadery S.R., Swift A.J., Billings C.G., et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2018;52(3) doi: 10.1183/13993003.00589-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guth S., D’armini A.M., Delcroix M., et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH registry. ERJ Open Res. 2021;7(3):00850–2020. doi: 10.1183/23120541.00850-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmud E., Patel M., Ang L., Poch D. Advances in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Pulm Circ. 2021;11(2):1–9. doi: 10.1177/20458940211007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takashi K., Aiko O., Katsumasa M., et al. Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ Cardiovasc Interv. 2016;9(10) doi: 10.1161/CIRCINTERVENTIONS.115.003318. [DOI] [PubMed] [Google Scholar]
- 49.Gerges C., Friewald R., Gerges M., et al. Efficacy and safety of percutaneous pulmonary artery subtotal occlusion and chronic total occlusion intervention in chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2021;14(8):E010243. doi: 10.1161/CIRCINTERVENTIONS.120.010243. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda N., Kubota S., Okazaki T., et al. The predictors of complications in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Catheter Cardiovasc Interv. 2019;93(6):E349–E356. doi: 10.1002/ccd.28133. [DOI] [PubMed] [Google Scholar]
- 51.Feinstein J.A., Goldhaber S.Z., Lock J.E., Ferndandes S.M., Landzberg M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103(1):10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa A., Satoh T., Fukuda T., et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10(11):004029. doi: 10.1161/CIRCOUTCOMES.117.004029. [DOI] [PubMed] [Google Scholar]
- 53.Mizoguchi H., Ogawa A., Munemasa M., Mikouchi H., Ito H., Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077. [DOI] [PubMed] [Google Scholar]
- 54.Olsson K.M., Wiedenroth C.B., Kamp J.C., et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49(6) doi: 10.1183/13993003.02409-2016. [DOI] [PubMed] [Google Scholar]
- 55.Brenot P., Jaïs X., Taniguchi Y., et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.02095-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ejiri K., Ogawa A., Fujii S., Ito H., Matsubara H. Vascular injury is a major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2018;11(12):005884. doi: 10.1161/CIRCINTERVENTIONS.117.005884. [DOI] [PubMed] [Google Scholar]
- 57.Inami T., Kataoka M., Shimura N., et al. Incidence, avoidance, and management of pulmonary artery injuries in percutaneous transluminal pulmonary angioplasty. Int J Cardiol. 2015;201:35–37. doi: 10.1016/j.ijcard.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 58.Inami T., Kataoka M., Yanagisawa R., et al. Long-term outcomes after percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circulation. 2016;134(24):2030–2032. doi: 10.1161/CIRCULATIONAHA.116.024201. [DOI] [PubMed] [Google Scholar]
- 59.Poch D.S., Mahmud E., Patel M., et al. Patient selection for balloon pulmonary angioplasty: six-year results from a high volume PTE surgical center. Pulm Circ. 2022;12(4) doi: 10.1002/pul2.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konstantinides S.V., Meyer G., Bueno H., et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 61.Jeong I., Alotaibi M., Fernandes T.M., et al. Direct oral anticoagulants in patients with chronic thromboembolic pulmonary hypertension and the presence of recent thrombus during pulmonary endarterectomy. Pulm Circ. 2022;12(3):e12110. doi: 10.1002/pul2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bunclark K., Newnham M., Chiu Y.D., et al. A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J Thromb Haemost. 2020;18(1):114–122. doi: 10.1111/jth.14649. [DOI] [PubMed] [Google Scholar]
- 63.Jaïs X., D’Armini A.M., Jansa P., et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension. BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 64.Sadushi-Kolici R., Jansa P., Kopec G., et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med. 2019;7(3):239–248. doi: 10.1016/S2213-2600(18)30367-9. [DOI] [PubMed] [Google Scholar]
- 65.Ghofrani H.A., Simonneau G., D’Armini A.M., et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10):785–794. doi: 10.1016/S2213-2600(17)30305-3. [DOI] [PubMed] [Google Scholar]
- 66.Ogo T., Shimokawahara H., Kinoshita H., et al. Selexipag for the treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;60(1):2101694. doi: 10.1183/13993003.01694-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghofrani H.-A., D’Armini A.M., Grimminger F., et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 68.Jensen K.W., Kerr K.M., Fedullo P.F., et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120(13):1248–1254. doi: 10.1161/CIRCULATIONAHA.109.865881. [DOI] [PubMed] [Google Scholar]
- 69.National Institutes of Health Clinical Center . National Institutes of Health; 2018. A study to find out if Selexipag is effective and safe in patients with chronic thromboembolic pulmonary hypertension when the disease is inoperable or persistent/recurrent after surgery and/or interventional treatment (SELECT). NCT03689244. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03689244 Updated July 7, 2022. [Google Scholar]
- 70.National Institutes of Health Clinical Center . National Institutes of Health; 2020. A study to evaluate efficacy and safety of Macitentan 75 mg in inoperable or persistent/recurrent chronic thromboembolic pulmonary hypertension (MACiTEPH). NCT04271475. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04271475 Updated March 24, 2023. [Google Scholar]
- 71.Claeys M., Claessen G., La Gerche A., et al. Impaired cardiac reserve and abnormal vascular load limit exercise capacity in chronic thromboembolic disease. JACC Cardiovasc Imaging. 2019;12(8, part 1):1444–1456. doi: 10.1016/j.jcmg.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 72.van Kan C., van der Plas M.N., Reesink H.J., et al. Hemodynamic and ventilatory responses during exercise in chronic thromboembolic disease. J Thorac Cardiovasc Surg. 2016;152(3):763–771. doi: 10.1016/j.jtcvs.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 73.Taboada D., Pepke-Zaba J., Jenkins D.P., et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J. 2014;44(6):1635–1645. doi: 10.1183/09031936.00050114. [DOI] [PubMed] [Google Scholar]
- 74.Wiedenroth C.B., Olsson K.M., Guth S., et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ. 2018;8(1):1–6. doi: 10.1177/2045893217753122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiedenroth C.B., Liebetrau C., Breithecker A., et al. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Hear Lung Transplant. 2016;35(5):591–596. doi: 10.1016/j.healun.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 76.Mercier O., Dubost C., Delaporte A., et al. Pulmonary thromboendarterectomy: the Marie Lannelongue Hospital experience. Ann Cardiothorac Surg. 2022;11(2):143–150. doi: 10.21037/acs-2021-pte-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taniguchi Y., Jaïs X., Jevnikar M., et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J Hear Lung Transplant. 2019;38(8):833–842. doi: 10.1016/j.healun.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Jaïs X., Brenot P., Jevnikar M., et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med. 2022;10(10):961–971. doi: 10.1016/S2213-2600(22)00214-4. [DOI] [PubMed] [Google Scholar]
- 79.National Institutes of Health Clinical Center . National Institutes of Health; 2021. Initial dual oral combination therapy versus standard-of-care initial oral monotherapy prior to balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension (IMPACT-CTEPH). NCT04780932. ClinicalTrials.gov.https://www.clinicaltrials.gov/ct2/show/NCT04780932 Updated October 25, 2022. [Google Scholar]