Abstract
Medical device‐related pressure injuries are receiving increased attention because their social and economic costs are increasing. This study aimed to analyse the stages for each risk factor, and to assess which has a greater impact on severity. We performed a retrospective analysis of 237 patients. Severity was evaluated by pressure injury stages, and the following categories were considered as risk factors: perceptual functioning, malnutrition, reduced mobility, comorbidities, extrinsic factors, medical devices, anatomical areas, and hospital stay. The stages of pressure injury stages were more for vascular access devices than for respiratory devices. The following were related to severity: mental deterioration‐related diseases, mental status, albumin level, haemoglobin level, total cholesterol level, intensive care unit care, days of hospitalisation, and time to develop pressure injuries after admission. Decreased mental status, anaemia, hypoalbuminemia, and low total cholesterol levels were particularly critical. However, factors such as anatomical areas, age, malignancy, diabetes mellitus, diseases related to malnutrition, abnormal body mass index, immobility‐related diseases, physical restraints, and Braden scale scores were not. A different approach to the management of medical device‐related pressure injuries is necessary because they have distinctive characteristics and causative factors than other pressure injury types.
Keywords: medical device‐related pressure injury, pressure injury stages, pressure ulcers, risk factors, wounds and injuries
1. INTRODUCTION
Medical device‐related pressure injuries (MDRPI) are gradually gaining attention because of their social and economic costs. The incidence of MDRPI was 28.1% in a 2021 systemic review assessing acute hospital‐setting pressure injuries. In addition, medical device application makes patients have MDRPI 2.4 times more than those who do not. 1 , 2 Patients with MDRPI also have increased length of hospital stays (median length of stay was reported as 84.5 days versus 3.0 days for those who did not develop MDRPI in a retrospective chart review), which leads to increased medical care costs. 3
Risk assessment is indispensable in the prevention and treatment of pressure injuries including MDRPI. Research and surveys have identified more specific risk factors for MDRPI development; however, the literature is still lacking specific data about which factors have a greater influence on the severity of MDRPI.
This study aimed to analyse MDRPI severity levels for each risk factor. This will have a greater impact on the intensive management of MDRPI that has already occurred, as well as aid with the prevention of more serious stages of MDRPI. 4 , 5
2. MATERIALS AND METHODS
2.1. Sample and settings
This study involved a retrospective analysis of 237 patients with MDRPI between January and December 2020. (AJOUIRB‐DB‐2022‐533) Infants and patients whose MDRPI did not develop in an acute hospital setting were excluded from this study. Patients with unstageable injuries and deep tissue injuries were excluded.
Risk factors associated with MDRPI included perceptual functioning, malnutrition, reduced mobility, comorbidities, and extrinsic factors. Additionally, we conducted correlation tests between the severity of MDRPI, the anatomical areas of MDRPI development, specific types of implicated medical devices, days of hospitalisation, and days from admission to MDRPI development. 6
2.2. Data collection and classification
Data were collected by an in‐hospital wound care team consisting of four plastic surgeons, and three wound care nurses, as well as a patient chart review. First, MDRPIs were classified into seven types of medical devices: respiratory, urinary, vascular access, support and immobilisation, feeding and nutrition, monitoring, and anti‐embolic devices. 7 Additionally, MDRPIs were classified into four anatomical areas: face and neck, trunk and pelvis, upper extremities, and lower extremities.
Pressure injuries were recorded as per the Staging System of the National Pressure Injury Advisory Panel.
We used the Braden scale score to assess the patients' extrinsic factors, and level of consciousness was assessed as alert, drowsy, stupor, semi‐ coma, and coma. Diseases related to mental deterioration included delirium, dementia, stroke, seizure, axonal injury, DKA, sepsis, encephalopathy, drug intoxication, and cardiac arrest.
Patients with diabetes mellitus were diagnosed according to the Diabetes Diagnostic Criteria of the World Health Organisation (WHO). 8 According to the United States Social Security Administration, elderly patients were defined as those aged 65 years. Hypoalbuminemia was diagnosed as an albumin level below 3.5 g/dl. 9 WHO haemoglobin thresholds were used to diagnose anaemia in men (13 g/dl), non‐pregnant women (12 g/dl), and pregnant women (11 g/dl). 10
The lowest individual values for albumin, haemoglobin, and total cholesterol data for each patient from admission to before the development of MDRPI was used in the assessment. Participants were considered to be at high risk of malnutrition if they demonstrated: liver disease, tube feeding requirements, burns, multiple traumatic events, renal insufficiency, congenital metabolic diseases, AIDS, dysphagia, anorexia, and alcoholism. 11
Immobility‐related diseases were defined as stroke, axonal disease, Parkinson's disease, paralysis, spine/pelvic fractures, spinal stenosis, end‐stage diseases, and severe osteoarthritis.
2.3. Statistical analysis
A Spearman's rank‐order correlation test was conducted to analyse the correlation between dependent (MDRPI stages), and independent variables (the presence or absence of a defined risk factor). A Mann‐Whitney U test assessed the non‐parametric data. An ANOVA testing was conducted to analyse the relationship between the dependent variables as ranking variables and the independent variables of three or more groups. Statistical significance was set at P < .05. All statistical analyses were performed using R 4.0.5 (The R Foundation, Vienna, Austria).
3. RESULTS
3.1. The average analysis of MDRPI stages for types of medical devices
The types of medical devices used are listed in Table 1. MDRPIs were classified into seven types of medical devices. In total, 237 patients experienced pressure injuries. Among them, respiratory devices (32.5%) were most likely to cause pressure injuries. Next, it increased in the order of support and immobilisation (32.5%), feeding and nutrition (13.5%), vascular access (7.6%), urinary s (4.2%), anti‐embolic (3.0%), and monitoring devices (1.3%). The average MDRPI stages for the seven groups of medical devices are shown in Figure 1. The severity differences between the average MDRPI stages of the vascular access devices, and that of the respiratory devices were significant (P = .038). In other words, the MDRPI stages were higher in the group that used vascular access devices than in the group that used respiratory devices.
TABLE 1.
Total number and frequency of medical devices.
| Types of devices, N (%) | Total 237 (100) |
|---|---|
| Respiratory devices, 77 (32.5%) | |
| Bite block | 5 (2.1) |
| Dental mask | 17 (7.2) |
| ECMO | 1 (0.4) |
| Endotracheal tube | 4 (1.7) |
| HFNC | 16 (6.8) |
| Nasal cannula | 27 (11.4) |
| Oxygen mask | 6 (2.5) |
| Tracheal tube | 1 (0.4) |
| Urinary devices, 10 (4.2%) | 10 (4.2) |
| Vascular access devices, 18 (7.6%) | |
| 3‐way stopcock | 6 (2.5) |
| Arterial‐line | 3 (1.3) |
| Chest tube | 1 (0.4) |
| IV line | 5 (2.1) |
| PCD | 2 (0.8) |
| PICC | 1 (0.4) |
| Support and immobilisation devices, 93 (29.2%) | |
| Brace | 5 (2.1) |
| Endotracheal tube fastener | 6 (2.5) |
| Splint | 29 (12.2) |
| Physical restraint | 53 (22.4) |
| Feeding and nutrition devices, 32 (13.5%) | |
| NG tube | 27 (11.4) |
| PEG tube | 2 (0.8) |
| Monitoring devices, 3 (1.3%) | |
| EEG | 1 (0.4) |
| ECG | 1 (0.4) |
| Pulse oximeter | 1 (0.4) |
| Anti‐embolic devices, 7 (3.0%) | 7 (3.0) |
Abbreviations: %, Percentage; EEG, Electroencephalogram; ECG, Electrocardiogram; ECMO, Extracorporeal membrane oxygenation; HFNC, High flow nasal cannula; IV, Intravenous; N, Number; NG, Nasogastric; PCD, Percutaneous drainage; PEG, Percutaneous endoscopic gastrotomy; PICC, Peripherally inserted central catheter.
FIGURE 1.
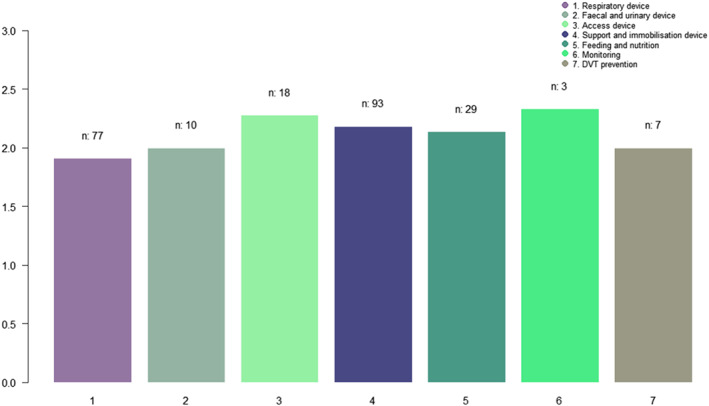
The average of MDRPI stages for seven groups of medical devices (ANOVA tests).
3.2. Analysis of MDRPI stages for anatomical areas
The distributions are listed in Table 2. The most common site of MDRPI was the face and neck (47.7%), followed by the upper extremities (31.2%), lower extremities (13.1%), and trunk and pelvis (8.0%). There were no significant differences in the MDRPI stage according to the anatomical area (P = 0.94) in (Figure 2).
TABLE 2.
Total number and frequency of anatomical areas.
| Anatomical areas, N (%) | Total 237 (100) |
|---|---|
| Face & neck, 113 (47.7%) | |
| Ear | 54 (22.8) |
| Lip | 14 (5.9) |
| Neck | 3 (1.3) |
| Nose | 39 (16.5) |
| Scalp | 3 (1.3) |
| Trunk & pelvis, 19 (8.0%) | |
| Abdomen | 2 (0.8) |
| Back | 4 (1.7) |
| Buttock | 1 (0.4) |
| Chest | 7 (3.0) |
| Genitalia | 1 (0.4) |
| Iliac crest | 2 (0.8) |
| Trochanteric area | 2 (0.8) |
| Upper extremity, 74 (31.2%) | |
| Elbow | 2 (0.8) |
| Forearm | 8 (3.4) |
| Hand | 28 (11.8) |
| Shoulder | 1 (0.4) |
| Wrist | 35 (14.8) |
| Lower extremity, 31 (13.1%) | |
| Ankle | 13 (5.5) |
| Foot | 5 (2.1) |
| Heel | 3 (1.3) |
| Knee | 2 (0.8) |
| Thigh | 8 (3.4) |
FIGURE 2.
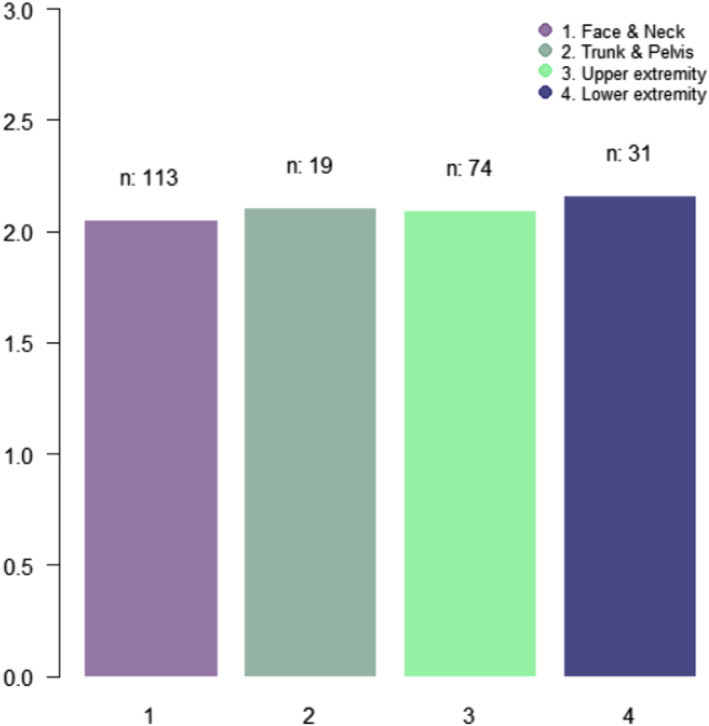
The average of MDRPI stages for four groups of anatomical areas (ANOVA tests).
3.3. Analysis of MDRPI risk factors
The average MDRPI stages for the two groups were analysed using the Mann–Whitney U test (Table 3). There were statistically significant differences between patients with, and without the following risk factors: anaemia, hypoalbuminemia, and mental deterioration‐related diseases (P < .05).
TABLE 3.
Mann–Whitney U test for variables affecting the severity of MDRPI.
| Variables | Severity (MDRPI stages, Mean ± SD) | P | |
|---|---|---|---|
| Yes | No | ||
| Anaemia | 2.16 ± 0.82 | 1.44 ± 0.58 | <.05* |
| Hypoalbuminemia | 2.18 ± 0.81 | 1.39 ± 0.57 | <.05* |
| Mental deterioration‐related diseases | 2.16 ± 0.82 | 1.88 ± 0.81 | <.05* |
| ICU care | 2.17 ± 0.85 | 1.91 ± 0.74 | <.05* |
| Diseases high risk of malnutrition | 2.13 ± 0.86 | 1.97 ± 0.69 | .29 |
| Elderly | 2.03 ± 0.80 | 2.07 ± 0.85 | .75 |
| Malignancy | 2.10 ± 0.81 | 2.06 ± 0.80 | .79 |
| Diabetes mellitus | 2.14 ± 0.88 | 2.06 ± 0.80 | .67 |
| Immobility related diseases | 2.12 ± 0.85 | 2.04 ± 0.80 | .54 |
| Physical restraint | 2.11 ± 0.78 | 2.03 ± 0.90 | .29 |
However, there were no significant differences between patients with mental deterioration‐related diseases, patients at elevated risk of malnutrition, older patients, with malignancy, diabetes mellitus, immobility‐related diseases, ICU care, physical restraints, and patients without mental deterioration (P > .05).
3.4. Correlation analysis for MDRPI risk factors
Mental status, total cholesterol, albumin, haemoglobin, days of hospitalisation, days from admission to MDRPI development, BMI, age, and Braden scale score were analysed using the Spearman's rank‐order correlation test (Table 4).
TABLE 4.
Spearman's rank‐order correlation test between variables and MDRPI stages.
| Variables | R (Correlation coefficient) | P |
|---|---|---|
| Mental status | 0.18 | <.05* |
| Haemoglobin | −0.23 | <.05* |
| Albumin | −0.30 | <.05* |
| Total cholesterol | −0.22 | <.05* |
| Days of hospitalisation | 0.13 | <.05* |
| Days from admission to MDRPI | 0.25 | <.05* |
| Age | .79 | |
| BMI | .92 | |
| Braden scale score | .96 |
There were statistically significant correlations between the following variables and MDRPI stage: mental status (R = 0.18), total cholesterol (R = −0.22), albumin (R = −0.30), haemoglobin (R = −0.23), days of hospitalisation (R = 0.13), and days from admission to MDRPI (R = 0.25) (P < .05).
In contrast, there were no significant correlations FOR age, BMI, Braden scale score, and MDRPI stage (P > .05).
3.5. Multiple linear regression analysis for MDRPI risk factors
Multiple linear regression analysis was performed on the statistically significant variables in the average and correlation analyses.
Anaemia was selected as a preferential variable between the two categories of anaemia and haemoglobin. Similarly, hypoalbuminemia was used as the favoured variable between hypoalbuminemia and albumin level.
Days from admission to the MDRPI were selected between the days from admission to the MDRPI (R = 0.25) and days of hospitalisation (R = 0.13).
Finally, multiple linear regression analysis was conducted using seven variables: mental status, mental deterioration‐related diseases, ICU care, anaemia, hypoalbuminemia, total cholesterol, and days from admission to MDRPI. Four MDRPI risk factors were identified: altered mental status (OR = 1.214), anaemia (OR = 3.501), hypoalbuminemia (OR = 5.301), and total cholesterol (OR = 0.995). These results are presented in Table 5.
TABLE 5.
Multiple linear regression analysis (Backward elimination) for MDRPI.
| Standard error | Beta | OR | P | |
|---|---|---|---|---|
| Mental status | 0.064 | 0.194 | 1.214 | <.05** |
| Anaemia | 0.490 | 1.253 | 3.501 | .01** |
| Hypoalbuminemia | 0.475 | 1.668 | 5.301 | <.05** |
| Total cholesterol | 0.003 | −0.005 | 0.995 | .13** |
P value < .1573 is considered statistically significant in AIC criteria.
4. DISCUSSION
Risk factors associated with MDRPI include perceptual functioning, malnutrition, reduced mobility, comorbidities, and extrinsic factors. 6 Mental status was analysed as a risk factor from the perspective of sensory perception and reduced mobility, while serum albumin, haemoglobin, and cholesterol levels were analysed as risk factors from the perspective of malnutrition. 12 Physical restraints are associated with reduced mobility. Diabetes mellitus was analysed as a risk factor related to hypofunction of sensory perception and nutritional imbalances, complicated by comorbidities such as neuropathy. 13 , 14
Compressive, shearing, and frictional forces contribute to the development of pressure injuries and MDRPI in particular. In obese patients, these forces increase over the bony prominences especially, leading to pressure ulcers. This study demonstrated that there was no significant relationship between obesity and MDRPI severity. This is because MDRPI is not associated with pressure gradients over bony prominences of dependent positions, which are usually affected by increased body weight. 15 , 16 , 17
Malignancy and age were analysed from the perspective of reduced mobility, malnutrition, and comorbidities associated with the MDRPI. ICU care also involves the possibility of altered sensory perception because of mental deterioration. 18 , 19 Our results suggest that a causal relationship between the number of days from admission to MDRPI development and severity stage was greater than the overall length of hospital stay.
As there was no significant correlation between the Braden scale score and the MDRPI stage in this study. Our research suggests that while the Braden scale score is a powerful tool for evaluating pressure injury, it is not a useful tool not for evaluating MDRPI specifically.
Nutrition plays a significant role in the treatment of pressure injuries. 20 However, there was no significant difference in the severity of MDRPI according to the presence or absence of a higher risk of malnutrition. There were significant correlations with haemoglobin, albumin, and total cholesterol levels which suggest that specific biochemical data, such as haemoglobin, albumin, and total cholesterol levels, could be a more useful tool for evaluating the severity of MDRPI than the nutritional status of patients.
This study had a few limitations. As a result of our relatively small study group, it was difficult to adequately statistically analyse all 22 anatomical areas of the body initially assessed in this study. Therefore, we divided the anatomical areas into four groups. Similarly, for devices causing MDRPI, we classified them into seven categories. In the future, it would be immensely useful to conduct an analysis of more specific anatomical areas and devices in a study with a larger patient population.
There was a significant difference in the occurrence of intraoperative MDRPI according to the glucose level. In this study, there was no difference in the MDRPI severity according to the presence or absence of DM. This may be related to the degree of diabetes control in each patient. In future studies, it will be necessary to analyse glucose or HbA1c levels and to note whether patients have pre‐existing neuropathy. In this study, we found that patients with risk factors, such as decreased mental status, hypoalbuminemia, anaemia, reduced total cholesterol, malnutrition, ICU care, and prolonged hospital stays, were more likely to have severe stages of MDRPI than those who did not. In particular, decreased mental status, anaemia, hypoalbuminemia, and total cholesterol levels were critical risk factors for the development of severe MDRPI. In conclusion, intensive care of patients with these risk factors is needed to prevent the development and progression of MDRPI. A different approach to the management of MDRPI is necessary because it has distinctive characteristics and etiological factors when compared with other types of pressure injuries.
4.1. Limitation
As a result of lack of patient population, it was difficult to significantly analyse all 22 anatomical areas of body initially classified in this study. Therefore, we divided anatomical areas into four areas. Similarly, for devices causing MDRPI, we classified devices as seven categories. In the future, there is a need to conduct an analysis for more definitive areas and devices in a study with a larger patient population.
It is known that there is a significant difference in the occurrence of intraoperative MDRPI according to the glucose level. 21 However, in this study, there was no difference in severity according to the presence or absence of DM. This is thought to be the result of the degree of diabetes control in each patient. Instead, in additional studies, it is necessary to analyse glucose level or HbA1c and whether patients have neuropathy or not.
5. CONCLUSION
In this study, we found that patients with risk factors such as decreased mental status, hypoalbuminemia, anaemia, reduced total cholesterol, malnutrition, ICU care, and prolonged hospital stays were more likely to have severe stages of MDRPI than those who did not. Especially decreased mental status, anaemia, hypoalbuminemia, and total cholesterol were critical. In conclusion, intensive care in patients with these risk factors is needed to prevent the development and progression of MDRPI. And the different approach to the management of MDRPI is necessary because MDRPI has different characteristics and affecting factors compared with pressure injury.
Jung YK, Hahn HM, Park DH. Factors influencing the severity of medical device‐related pressure injuries: Pressure injury staging comparison. Int Wound J. 2023;20(7):2735‐2741. doi: 10.1111/iwj.14147
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device related pressure ulcers in hospitalized patients. Int Wound J. 2010;7(5):358‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brophy S, Moore Z, Patton D, O'Connor T, Avsar P. What is the incidence of medical device‐related pressure injuries in adults within the acute hospital setting? A systematic review. J Tissue Viability. 2021;30(4):489‐498. [DOI] [PubMed] [Google Scholar]
- 3. Schindler CA, Barrette R, Sandock A, Kuhn E. Medical device‐related pressure injuries associated with electroencephalogram leads in a tertiary care children's hospital: a retrospective chart review. Wound Manage Prev. 2021;67(9):25‐32. [PubMed] [Google Scholar]
- 4. Gefen A. How much time does it take to get a pressure ulcer? Integrated evidence from human, animal, and in vitro studies. Ostomy Wound Manage. 2008;54(10):26‐28. [PubMed] [Google Scholar]
- 5. Hughes R. NHS 2010–2015: from good to great. Br J Healthcare Assistants. 2010;4(1):36‐37. [Google Scholar]
- 6. Mehta C, Ali M, Mehta Y, George JV, Singh MK. MDRPU‐an uncommonly recognized common problem in ICU: a point prevalence study. J Tissue Viability. 2019;28(1):35‐39. [DOI] [PubMed] [Google Scholar]
- 7. Gefen A, Alves P, Ciprandi G, et al. Device‐related pressure ulcers: SECURE prevention. J Wound Care. 2022;31(Sup3a):S1‐S72. [DOI] [PubMed] [Google Scholar]
- 8. Organization WH . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. Geneva, Switzerland: WHO Press; 2006. [Google Scholar]
- 9. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(3):193‐199. [DOI] [PubMed] [Google Scholar]
- 10. de Benoist B, Cogswell M, Egli I, McLean E. Worldwide Prevalence of Anaemia 1993–2005; WHO Global Database of Anaemia. 2008. [DOI] [PubMed]
- 11. Stratton RJ, Green CJ, Elia M. Disease‐Related Malnutrition: an Evidence‐Based Approach to Treatment. Cambridge, MA: Cabi; 2003. [Google Scholar]
- 12. Venzin RM, Kamber N, Keller WC, Suter PM, Reinhart WH. How important is malnutrition? A prospective study in internal medicine. Eur J Clin Nutr. 2009;63(3):430‐436. [DOI] [PubMed] [Google Scholar]
- 13. Association AD . Nutrition recommendations and principles for people with diabetes mellitus. Diabetes Care. 2000;23:S43. [PubMed] [Google Scholar]
- 14. Mohan M, Thombre D, Das A, Subramanian N, Chandrasekar S. Reaction time in clinical diabetes mellitus. Indian J Physiol Pharmacol. 1984;28(4):311‐314. [PubMed] [Google Scholar]
- 15. VanGilder C, MacFarlane G, Meyer S, Lachenbruch C. Body mass index, weight, and pressure ulcer prevalence: an analysis of the 2006–2007 international pressure ulcer prevalence™ surveys. J Nurs Care Qual. 2009;24(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 16. Reuler JB, Cooney TG. The pressure sore: pathophysiology and principles of management. Ann Intern Med. 1981;94(5):661‐666. [DOI] [PubMed] [Google Scholar]
- 17. Lustig A, Margi R, Orlov A, Orlova D, Azaria L, Gefen A. The mechanobiology theory of the development of medical device‐related pressure ulcers revealed through a cell‐scale computational modeling framework. Biomech Model Mechanobiol. 2021;20(3):851‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Argiles J. Cancer‐associated malnutrition. Eur J Oncol Nurs. 2005;9:S39‐S50. [DOI] [PubMed] [Google Scholar]
- 19. Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76(4):296‐302. [DOI] [PubMed] [Google Scholar]
- 20. Munoz N, Posthauer ME, Cereda E, Schols J, Haesler E. The role of nutrition for pressure injury prevention and healing: the 2019 international clinical practice guideline recommendations. Adv Skin Wound Care. 2020;33(3):123‐136. [DOI] [PubMed] [Google Scholar]
- 21. Ma LY, Chen HL, Gu HY, Hua L, Gao XM. Analysis of the clinical features and risk factors of device‐related pressure injuries in the operating room. Int Wound J. 2023;20(3):706‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


